Abstract
The present study investigated the role of tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) in regulating the proliferation, migration, apoptosis and activity of matrix metalloproteinase (MMP)-2 and −9, during the development of an atherosclerotic abdominal artery aneurysm (AAA). Experiments were conducted using rabbit AAA neck (NA) smooth muscle cells (SMCs), to investigate the potential for TIMP-3 to be used as a novel stent coating in preventing aortic dilation adjacent to the AAA. The atherosclerotic AAA model was induced in New Zealand white rabbits via a 6-week high-cholesterol diet, followed by incubation of the targeted aortic region with elastase. SMCs were isolated from the aorta adjacent to the aneurysm 30 days after AAA model induction, and stimulated with 3, 10, 30 or 100 ng/ml TIMP-3. Cell proliferation was investigated using Cell Counting Kit-8 reagent, migration was examined using a Boyden chamber assay and apoptotic rate was analyzed using the Annexin V-fluorescein isothiocyanate Apoptosis Detection kit. Gelatin zymography and ELISA were used to measure the activity of MMP-2 and MMP-9, and the expression of tumor necrosis factor-α (TNF-α), respectively. Analysis of cell proliferation indicated that 10, 30 and 100 ng/ml TIMP-3 reduced cell viability. Cell migration was decreased by 10, 30 and 100 ng/ml TIMP-3. MMP-2 activity was inhibited by 10, 30 and 100 ng/ml TIMP-3, and MMP-9 activity was suppressed by 30 and 100 ng/ml TIMP-3. The protein levels of secreted TNF-α were reduced by 10, 30 and 100 ng/ml TIMP-3. The present study demonstrated the ability of 30 and 100 ng/ml TIMP-3 to attenuate migration and proliferation, and to inhibit the activity of MMP-2, MMP-9 and TNF-α secretion of NA SMCs. In conclusion, TIMP-3 may be considered a potential therapeutic drug for use in a novel drug-eluting stent, to attenuate the progressive dilation of the aortic NA.
Keywords: abdominal aortic aneurysm, smooth muscle cell, matrix metalloproteinase, tissue inhibitor of matrix metalloproteinase-3, extracellular matrix, abdominal artery aneurysm neck
Introduction
An abdominal aortic aneurysm (AAA) is a common, life threatening condition, which is associated with significant morbidity and mortality (1). Endovascular aortic aneurysm repair (EVAR) is the preferred treatment option for many patients with AAA, as it is minimally invasive, and is associated with a reduced perioperative morbidity rate and fewer complications, as compared with open surgery (2). However, as these endovascular grafts only provide mechanical hemostatic sealing between the graft and the arterial wall, and do not promote tissue healing, over time, EVAR therapy results in more complications than surgical repair. These complications include dilation of the proximal aortic neck, which may lead to graft migration, endoleaks and aneurysm rupture (3–8). The mechanism of progressive aortic neck dilatation remains unclear; however, previous research has indicated that it may result from a continuous degenerative process in the aortic wall (9,10). Atherosclerosis, inflammation and progressive extracellular matrix (ECM) degradation may serve key roles in this dilation process (11). Increased expression and activity of matrix metalloproteinases (MMPs) has been observed in aortic aneurysm tissues, and an imbalance between these MMPs, and the tissue inhibitor of matrix metalloproteinases (TIMPs) that inhibit them, may promote a phenotype of matrix degradation (12), which is associated with the development of various diseases, such AAA, arteriosclerosis obliterans and hypertension (13).
Vascular smooth muscle cells (SMCs) are the primary intrinsic cell of the aortic wall; these cells perform various biological functions through proliferation, migration and secretion of numerous cytokines, including MMPs, tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-1β (14,15). The proliferation and migration of SMCs act as critical factors in the initiation and progression of atherosclerosis (16); the secreted MMPs participate in ECM degeneration, and TNF-α, which is an important proinflammatory cytokine (17), also servesa role in the formation of AAA by stimulating the expression of adhesion molecules and MMPs. TIMP-3, as a special member of the TIMP family, is the only ECM-bound TIMP, allowing it to exert tissue-specific effects (18,19) such as regulating ECM remodeling, endothelial permeability and inflammation (20–22). Furthermore, TIMP-3 can inhibit the proliferation and migration of various cell types, including SMCs, breast cancer cells and endothelial cells (23). The present study therefore aimed to investigate whether local expression of TIMP-3 could inhibit the activity of MMP-2, MMP-9 and TNF-α, and reduce the proliferation and migration of SMCs into the proximal aorta of the AAA, thereby preventing aortic dilation adjacent to the AAA.
Materials and methods
Induction of the AAA model
The present study was approved by the Animal Experimental Ethics Committee of the China Medical University (Shenyang, China). All the animal experiments were carried under the anesthesia via an intravenous injection of 30 mg/kg sodium pentobarbital. A total of 10 male adult (~6 months old) New Zealand White rabbits (1.8–2.0 kg) (Shenyang Long yuan Economic Animal Breeding Farm, Shenyang, China) were fed a high-cholesterol diet (2% cholesterol) for 16 weeks, prior to induction of the AAA model. The rabbits housed in individual cages were allowed free water and food before and after surgery under a 12-h dark/light cycle 0.03% CO2 at room temperature The AAA model was induced as previously described (24). Briefly, rabbits were anaesthetized with an intravenous injection of 30 mg/kg sodium pentobarbital. Then the abdomen was accessed by a midline laparotomy under sterile conditions and a segment of the abdominal aortic artery (~1.5 cm long), proximal to the bifurcation, was carefully isolated from the vena cava and surrounding tissues. A sterile gauze was placed carefully around the isolated artery and 20 µl 10 U/Ul elastase solution (>30 units/mg; isoelectric point 9.5; pH 8.1–8.9; Shanghai Kayon Biological Technology Co., Ltd., Shanghai, China) was applied to the gauze and maintained for 30 min. The treated segment was then irrigated twice with physiological saline. The abdominal incision was closed with a continuous running suture, and the rabbits were placed in warming cages at ~25–26°C. To investigate whether the AAA model had been successfully induced, the rabbits underwent serial intravenous digital subtraction angiography (IVDSA) imaging 30 days after surgery. Briefly, following anesthesia, 6 ml of iodinated contrast material was administered within 3 sec by manual injection into the rabbits via a 22-gauge angiocatheter placed in the ear vein of the rabbits. Diameter of the aortic segment was measured in reference to an external sizing device (Sizing catheter; 1 cm in length; Cook Medical LLC, Bloomington, IN, USA) placed under the abdomen during IVDSA, and the formation of a successful AAA was defined as a focal dilation ratio of the elastase-incubated aorta with a diameter of ≥50% of the normal.
Primary cell isolation and identification
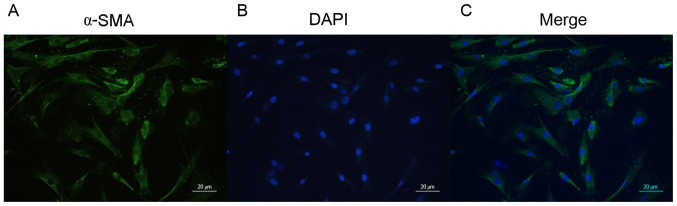
In order to minimize suffering, air embolism euthanasia procedure was performed under 30 mg/kg sodium pentobarbital an esthesia. Primary SMCs were isolated from a ~1 cm section of the AAA neck (NA) tissue, taken from a region ranging from the undilated part of the abdominal artery to the renal artery. The tissue pieces were cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), containing 20% fetal bovine serum (Hyclone; GE Healthcare), 100 U/ml penicillin and 100 mg/ml streptomycin (Nanjing Key Gen Biotech Co., Ltd., Nanjing, China), and were maintained at 37°C in a humidified 5% CO2 incubator. Following SMC migration out of the tissue sections, the cells were routinely passaged and SMCs from the third passage were identified by immunocytochemistry with anti-α-smooth muscle actin (α-SMA; clone 1A4; cat. no. A2547, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A total of 1×106 SMCs were fixed in 4% paraformaldehyde, and blocked with 5% normal goat serum (Shanghai Biyuntian Bio-Technology Co., Ltd., Shanghai, China) at room temperature for 1 h. The fixed cells were then incubated with anti-α-SMA (1:100) at 4°C overnight. Secondary antibody incubation was performed using fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (cat. no. SA00003-1; 1:50; ProteinTech Group, Inc., Chicago, IL, USA) at room temperature for 1 h. Cell nuclei were stained with DAPI according to the manufacturer protocol (cat. no. C1005; Shanghai Biyuntian Bio-Technology Co., Ltd.) Stained cells were observed using a fluorescence microscope (Olympus Corporation, Tokyo, Japan). Cells from passages 4–10 were used for further in vitro studies.
Cell proliferation assay
The proliferation of SMCs was indirectly assayed using Cell Counting Kit-8 (CCK-8) reagent (Dojindo Molecular Technologies, Inc., Rockville, MD, USA), according to the manufacturer's protocol. Briefly, cells were seeded at a density of 3×103 cells/100 µl/well in 96-well plates with 3, 10, 30, or 100 ng/ml of TIMP-3 (R&D Systems, Inc., Minneapolis, MN, USA) (4 wells/group) for 24 h at 37°C in a humidified 5% CO2 incubator. Cells cultured without TIMP-3 were used as the control group. Subsequently, 10 µl CCK-8 solution was added to each well, and the plates were incubated at 37°C for 3 h. The optical density of the wells was measured at 450 nm, using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Trans well assay
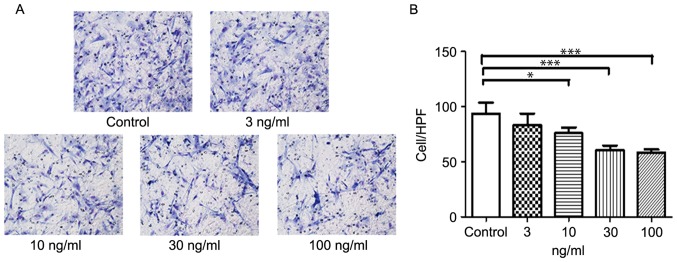
A 96-well chemo tax is chamber (Neuro Probe, Inc., Gaithersburg, MD, USA) was used for the trans well assays. The polyvinyl membrane, containing 8 µm pores, was coated with 10 µg/ml fibronectin (Sigma-Aldrich; Merck KGaA) for 2 h, and then air-dried. The lower chamber was filled with DMEM containing 0.25% bovine serum albumin (BSA) (Sigma-Aldrich; Merck KGaA), with or without 3, 10, 30 or 100 ng/ml TIMP-3. SMCs were then seeded onto the filter at 2×105 cells per well, in DMEM containing 0.25% BSA. The plates were incubated for 5 h in a humidified chamber at 37°C. The chambers were disassembled and the membranes were fixed in methanol and stained with hematoxylin and eosin. Cell nuclei were counted across ten high power fields per membrane, using a light microscope. Results were presented as the mean number of cells migrated per field.
Cell apoptosis assay
The ability of TIMP-3 to induce apoptosis in NA SMCs was determined by flow cytometry. Cells were incubated with 3, 10, 30 or 100 ng/ml TIMP-3 and stained using the Annex in V-FITC Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA), according to manufacturer's protocol. The cells were analyzed with the FACS Aria Flow Cytometer (BD Biosciences) within 1 h.
Gelatin zymography
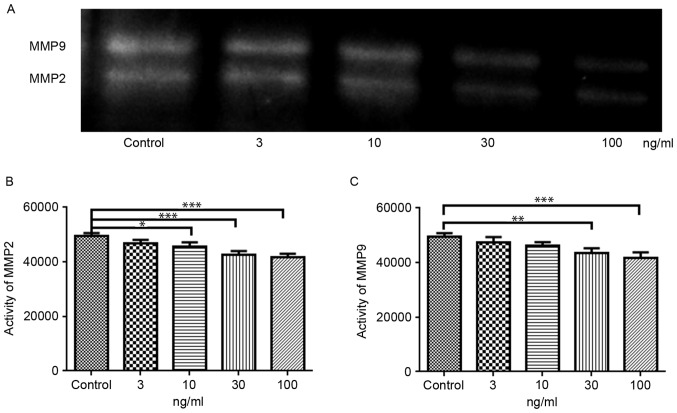
The activity of MMP-2 and MMP-9 was measured using MMP Zymography Assay kit according to the manufacturer's protocol (Applygen Technologies Inc., Beijing, China). Briefly, the NA SMCs were treated with 3, 10, 30 or 100 ng/ml TIMP-3 for 24 h, and the cells were subsequently washed with ice-cold phosphate-buffered saline and lysed with radioimmunoprecipitation assay buffer (Shanghai Biyuntian Bio-Technology Co., Ltd.). The lysates were cleared by centrifugation at 12,000 × g for 15 min, and the supernatants were collected. Protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein (25 µg) were separated by 8% SDS-PAGE containing 1 mg/ml gelatin, under non-reducing conditions. The gel was subsequently washed and incubated at 37°C for 24 h, and then stained with Coomassie brilliant blue R250. The activity of MMP-2 and MMP-9 was detected with ChemiDoc MP imaging System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the intensity of the bands was estimated using the 5.1 (Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay (ELISA) of TNF-α levels
The conditioned medium of NA SMCs was collected, and the levels of TNF-α were measured using a TNF-α ELISA kit (cat. no. E04T0008; Shanghai BlueGene Biotech Co., Ltd., Shanghai, China) according to the manufacturer's protocol. Absorbance was measured at 450 nm using a microplate reader. Samples were measured in duplicate.
Statistical analysis
Data are presented as the mean ± standard error of mean. Statistical analyses were performed using one-way analysis of variance, followed by Dunnett's multiple comparison test. All statistical analyses were performed with Prism5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Induction of the rabbit AAA model, and isolation of SMCs
The AAA model was confirmed by inspection for an aortic aneurysm, using intravenous digital subtraction angiography, 30 days after induction of AAA (Fig. 1). The NA tissue, ~1 cm long, was excised from the aortic section between the un dilated region and the renal artery. Following incubation of the NAtissue, SMC smigrated onto the surface of the culture dish ~10 days later. The cells were positively identified as SMCs by immunofluorescent staining for α-SMA (Fig. 2).
Figure 1.
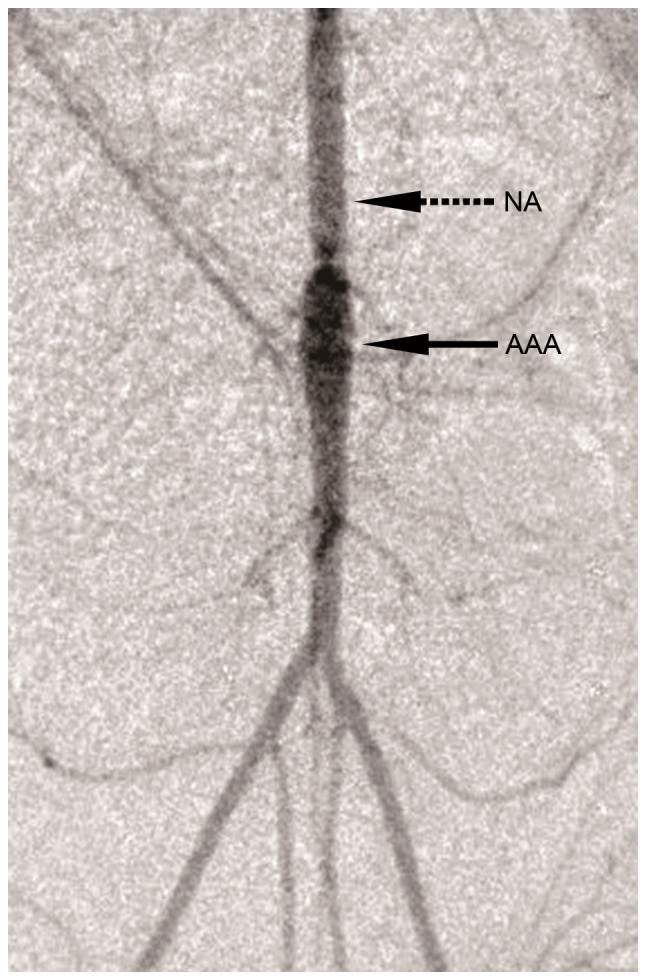
Intravenous digital subtraction angiography image of an AAA. The solid arrow indicates the AAA, the dashed arrow indicates the NA. AAA, abdominal artery aneurysm; NA, AAA neck (magnification, ×2).
Figure 2.
Immunostaining of α-SMA in abdominal artery aneurysm neck smooth muscle cells. Cells were stained for (A) α-SMA (green) and (B) counterstained with DAPI (blue). (C) Merge. Magnification, ×20; magnification bar, 20 µm. α-SMA, α-smooth muscle actin.
TIMP-3 reduced the proliferation and migration of SMCs
SMC proliferation was markedly reduced following treatment with 10 to 100 ng/ml TIMP-3; however, there was no apparent anti-proliferative effect following treatment with 3 ng/ml TIMP-3 (Fig. 3). Similarly, SMC migration was significantly decreased by 10, 30 and 100 ng/ml of TIMP-3 (Fig. 4). Analysis of cellular apoptosis revealed no significant differences among the groups (data not shown), indicating that TIMP-3 had no effect on the apoptosis of NA SMCs.
Figure 3.
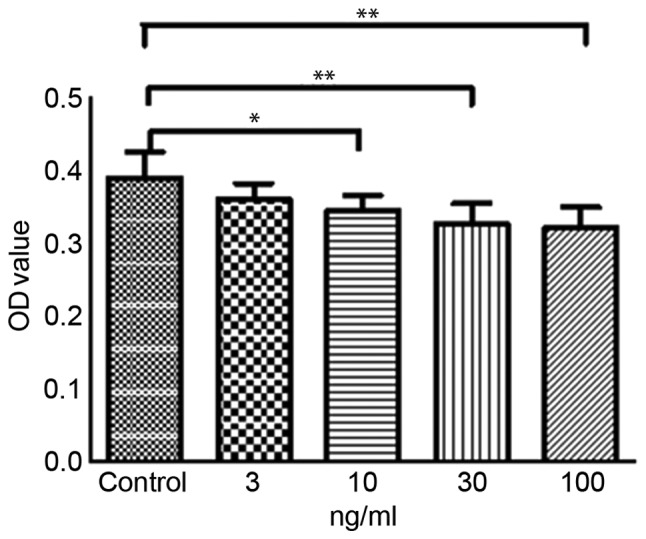
NASMC viability following treatment with TIMP-3. Cell viability of NA SMCs was analyzed following incubation with various concentrations of TIMP-3 for 24 h. Data are presented as the mean ± standard error of the mean (n=4). *P<0.05 and **P<0.01. NA, abdominal artery aneurysm neck; SMC, smooth muscle cell; TIMP-3, tissue inhibitor of matrix metalloproteinase 3; OD, optical density.
Figure 4.
Effects of TIMP-3 on the migration of abdominal artery aneurysm neck SMCs. (A) Effects of TIMP-3 on SMC migration. SMCs were incubated with TIMP-3 (3, 10, 30 and 100 ng/ml) for 5 h. (B) Histogram of the migration of SMCs following TIMP-3 treatment. Magnification, ×200. *P<0.05, ***P<0.001 vs. untreated control. SMCs, smooth muscle cells; TIMP-3, tissue inhibitor of matrix metalloproteinase 3; HPF, high power field.
Role of TIMP-3 in the activity of MMP-2 and MMP-9
Gelatin zymography analysis was used to investigate the effects of TIMP-3 on the activities of MMP-2 and MMP-9 (Fig. 5A). MMP-2 activity was significantly reduced in NA SMCs treated with 10, 30 and 100 ng/ml TIMP-3, compared with untreated control cells (Fig. 5B). Similarly, the activity of MMP-9 was significantly reduced by 30 and 100 ng/ml TIMP-3 (Fig. 5C).
Figure 5.
Effects of TIMP-3 on the activity of MMP-2 and MMP-9. (A) Activity of MMP-2 and MMP-9 was detected by gelatin zymography. (B) Activity of MMP-2 was reduced following treatment with 10, 30 and 100 ng/ml TIMP-3. (C) Activity of MMP-9 was reduced following treatment with 30 and 100 ng/ml TIMP-3. *P<0.05, **P<0.01 and ***P<0.001, vs. untreated control. MMP, matrix metalloproteinase; TIMP-3, tissue inhibitor of MMP-3.
TIMP-3 reduces secretion of TNF-α in SMCs
In order to investigate the effects of TIMP-3 on the inflammatory responses of NASMCs, the levels of TNF-α secreted into the conditioned medium were measured by ELISA. Compared with the control group, NA SMCs treated with TIMP-3 secreted less TNF-α in a concentration-dependent manner. The percentage inhibition of TNF-α secretion, exerted by TIMP-3 at concentrations of 10, 30 and 100 ng/ml, on NA SMCs was 16.72, 29.94 and 65.38%, respectively (Fig. 6).
Figure 6.
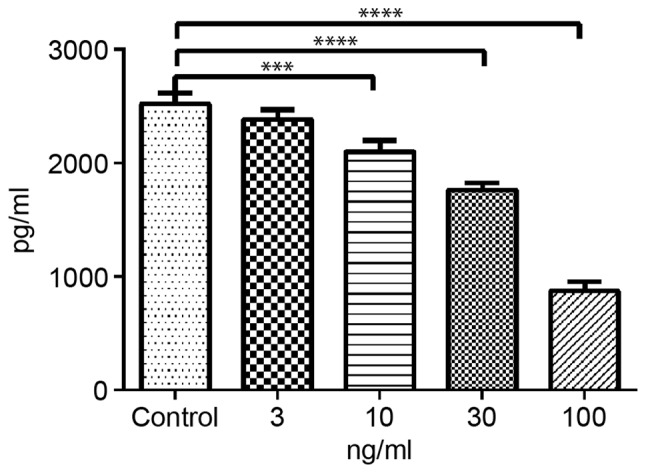
TIMP-3 reduces the expression of TNF-α in NA SMCs. NA SMCs were stimulated with 3, 10, 30 and 100 ng/ml TIMP-3, and TNF-α levels were measured by enzyme-linked immunosorbent assay. ***P<0.001, ****P<0.0001, vs. untreated control. TIMP-3, tissue inhibitor of matrix metalloproteinase 3; TNF-α, tumor necrosis factor α; NA, abdominal artery aneurysm neck; SMC, smooth muscle cell.
Discussion
AAA is a common and potentially fatal disease that has major effects on the health care of the elderly (2); atherosclerosis is an almost universal finding in the walls of these aneurysms (25). In order to imitate the pathogenesis of human AAA, the present study induced an arteriosclerosis obliterans model by feeding rabbits a high-cholesterol diet for 16 weeks, prior to induction of the AAA model. After 30 days, SMCs were isolated from the aorta adjacent to the AAA. This is the region to which endovascular stents would be attached, and are therefore at risk of long-term dilation. Previous studies (26,27) have demonstrated that the aorta proximal to the AAA demonstrates relative preservation of the aortic structure, combined with reduced damage of the medial elastic fibers and mild adventitial inflammation, in comparison with the body or center of the AAA. This region may provide insight into the early mechanisms of AAA pathogenesis, and the present study therefore investigated the impact of treating SMCs isolated from this region with various concentrations of TIMP-3, an inhibitor of the ECM-degrading enzymes MMP-2 and MMP-9.
The present study demonstrated that 10, 30 and 100 ng/ml TIMP-3 reduced the proliferation of SMCs, and 10, 30 and 100 ng/ml TIMP-3 attenuated the migration of SMCs. These results are consistent with previous research indicating that TIMP-3 may inhibit the growth of different cell types, and reduce cell proliferation and invasion (23). TIMP-3 was not found to increase the apoptotic rate of NA SMCs, however this may be due to an insufficient concentration of TIMP-3. SMC migration and proliferation are the leading factors in the progression of arteriosclerosis obliterans therefore, the inhibition of SMC migration and proliferation may present a therapeutic strategy for the treatment of atherosclerosis (28). The present study investigated this hypothesis in vitro, and the results indicated that TIMP-3 may prevent the dilation of the proximal aorta of an AAA, and therefore indicated the potential for TIMP-3 to be used for atherosclerotic therapy.
A key mechanism in the pathogenesis and progression of AAA involves proteolytic degradation of the aortic wall. Increased elastase and collagenase activity have been detected in aortic aneurysms, and have been positively correlated with alterations in the ECM composition, and in the size of the aneurysm (29). Further more, MMP-2 and MMP-9 have previously been demonstrated to be overexpressed in human and animal AAA tissue specimens (30). In the present study, 30 and 100 ng/ml TIMP-3 suppressed the activity of MMP-2 and MMP-9, and this occurred in a concentration-dependent manner. This inhibition of MMP-2 and MMP-9 activity may prevent excessive degeneration of the ECM, and therefore the resulting dilation of the aortic wall.
Another feature of human AAA is inflammation (11). TNF-α is an important proinflammatory cytokine (28), which serves a role in atherosclerosis and AAA by stimulating the expression of adhesion molecules and MMPs (31–33). Furthermore, TNF-α is involved in activation of the mitogen-activated protein kinase cascade, leading to vascular SMC migration and proliferation (31,32,34). The present study demonstrated that TNF-α expression was attenuated by 10, 30 and 100 ng/ml of TIMP-3, and this may subsequently inhibit the activity of MMP-2 and MMP-9, and thus reduce the proliferation and migration of SMCs from the atherosclerotic aorta adjacent to the AAA. Stimulated SMCs actively participate in the inflammatory cascade by secreting a range of factors including TNF-α, IL-6, IL-1β (14) and MMPs (15). The results of the present study indicated that 30 and 100 ng/ml of TIMP-3 was able to suppress the proliferation and migration of SMCs from the proximal a orta of the AAA, which may decrease the secretion of TNF-α and reduce the activity of MMPs. A schematic diagram illustrating a potential mechanism for the TIMP-3-induced attenuation of the progressive development of AAA is presented in Fig. 7.
Figure 7.
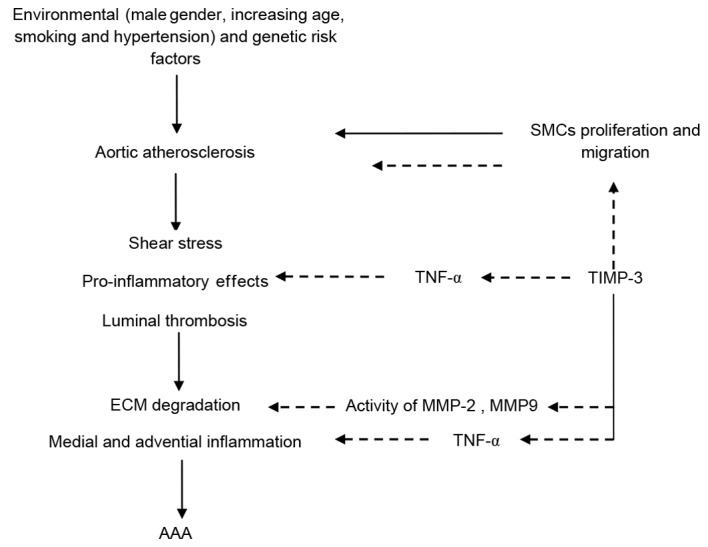
Schematic diagram of the potential mechanism of TIMP-3-attenuated AAA development. AAA, abdominal artery aneurysm; MMP, matrix metalloproteinase; TIMP-3, tissue inhibitor of MMP-3; TNF-α, tumor necrosis factor α; SMC, smooth muscle cell; ECM, extracellular matrix.
Our previous study demonstrated the use of gene transduction into the aortic wall; using plasmid-loaded cationized gelatin hydrogel-coated polyester stent grafts (35). Using this gene transfer method to deliver TIMP-3 cDNA may assist in ameliorating the atherosclerotic region of the seal zone following EVAR treatment, there by preventing the progression of the an eurysm along the aneurysmal neck.
In conclusion, the present study demonstrated that 10, 30 and 100 ng/ml TIMP-3 attenuate migration and proliferation, and to inhibit the activity of MMP-2, MMP-9 and TNF-α secretion of NA SMCs. Future research will investigate the use of a stent-graft implanted in vivo, as a gene therapy vehicle to inhibit dilation of the NA, and to improve the long-term out comes of EVAR therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81571778 and 81201168), the Science and Technology Plan Project of Liaoning Province (grant no. 2013225086) and the Key Laboratory Basic Research Project from the Education Department of Liaoning Province (grant no. LZ2014040).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HOZ, KX and HUZ have designed the experiments, and HUZ, XQ, ZL and CL performed the experiments. WZ and JL analysed the data. KX and HOZ revised the manuscript critically and provided valuable comments during revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Animal Experimental Ethics Committee of the China Medical University (Shenyang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Roggerio A, Sambiase NV, Palomino SA, de Castro MA, da Silva ES, Stolf NG, de Lourdes Higuchi M. Correlation of bacterial coinfection versus matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 expression in aortic aneurysm and atherosclerosis. Ann Vasc Surg. 2013;27:964–971. doi: 10.1016/j.avsg.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Lovegrove RE, Javid M, Magee TR, Gall RB. A meta-analysis of 21,178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br J Surg. 2008;95:677–684. doi: 10.1002/bjs.6240. [DOI] [PubMed] [Google Scholar]
- 3.Brewster DC, Jones JE, Chung TK, Lamuraglia GM, Kwolek CJ, Watkins MT, Hodgman TM, Cambria RP. Long-term outcomes after endovascular abdominal aortic aneurysm repair: The first decade. Ann Surg. 2006;244:426–438. doi: 10.1097/01.sla.0000234893.88045.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Herwaarden JA, van de Pavoordt ED, Waasdorp EJ, Vos Albert J, Overtoom TT, Kelder JC, Moll FL, de Vries JP. Long-term single-center results with AneuRx endografts for endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2007;14:307–317. doi: 10.1583/06-1993.1. [DOI] [PubMed] [Google Scholar]
- 5.Peterson BG, Matsumura JS, Brewster DC, Makaroun MS. Excluder Bifurcated Endoprosthesis Invistigators: Five-year report of a multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysms. J Vasc Surg. 2007;45:885–890. doi: 10.1016/j.jvs.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 6.White RA, Donayre CE, Walot I, Kopchok GE, Wilson EP, deVirgilio C. Regression of an abdominal aortic aneurysm after endograft exclusion. J Vasc Surg. 1997;26:133–137. doi: 10.1016/S0741-5214(97)70157-5. [DOI] [PubMed] [Google Scholar]
- 7.Rodway AD, Powell JT, Brown LC, Greenhalgh RM. Do abdominal aortic aneurysm necks increase in size faster after endovascular than open repair? Eur J Vasc Endovasc Surg. 2008;35:685–693. doi: 10.1016/j.ejvs.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Diehm N, Dick F, Katzen BT, Schmidli J, Kalka C, Baumgartner I. Aortic neck dilatation after endovascular abdominal aortic aneurysm repair: A word of caution. J Vasc Surg. 2008;47:886–892. doi: 10.1016/j.jvs.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Badran MF, Gould DA, Raza I, McWilliams RG, Brown O, Harris PL, Gilling-Smith GL, Brennan J, White D, Meakin S, Rowlands PC. Aneurysm neck diameter after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2002;13:887–892. doi: 10.1016/S1051-0443(07)61771-0. [DOI] [PubMed] [Google Scholar]
- 10.Conners MS, III, Sternbergh WC, III, Carter G, Tonnessen BH, Yoselevitz M, Money SR. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: A cautionary note. J Vasc Surg. 2002;36:476–484. doi: 10.1067/mva.2002.126561. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka A, Hasegawa T, Morimoto K, Bao W, Yu J, Okita Y, Tabata Y, Okada K. Controlled release of ascorbic acid from gelatin hydrogel attenuates abdominal aortic aneurysm formation in rat experimental abdominal aortic aneurysm model. J Vasc Surg. 2014;60:749–758. doi: 10.1016/j.jvs.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Tsarouhas K, Soufla G, Apostolakis S, Zaravinos A, Panagiotou M, Khoury M, Hassoulas JA, Tsatsakis AM, Spandidos DA. Transcriptional regulation of TIMPs in ascending aorta aneurysms. Thromb Res. 2010;126:399–405. doi: 10.1016/j.thromres.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott RA, Paderi JE, Sturek M, Panitch A. Decorin mimic inhibits vascular smooth muscle proliferation and migration. PLoS One. 2013;8:e82456. doi: 10.1371/journal.pone.0082456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saratzis A, Abbas AA, Kiskinis D, Melas N, Saratzis N, Kitas GD. Abdominal aortic aneurysm: A review of the genetic basis. Angiology. 2011;62:18–32. doi: 10.1177/0003319710373092. [DOI] [PubMed] [Google Scholar]
- 16.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994;269:9352–9360. [PubMed] [Google Scholar]
- 19.Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 20.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 1997;95:205–212. doi: 10.1161/01.CIR.95.1.205. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, III, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–1036. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 23.Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, Crombleholme TM, Cripe TP. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68:1170–1179. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi Y, Zhong H, Xu K, Ni Y, Qi X, Zhang Z, Li W. Performance of a modified rabbit model of abdominal aortic aneurysm induced by topical application of porcine elastase: 5-month follow-up study. Eur J Vasc Endovasc Surg. 2013;45:145–152. doi: 10.1016/j.ejvs.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Johnsen SH, Forsdahl SH, Singh K, Jacobsen BK. Atherosclerosis in abdominal aortic aneurysms: A causal event or a process running in parallel? The Tromso study. Arterioscler Thromb Vasc Biol. 2010;30:1263–1268. doi: 10.1161/ATVBAHA.110.203588. [DOI] [PubMed] [Google Scholar]
- 26.Golledge J, Clancy P, Moran C, Biros E, Rush C, Walker P, Norman P. The novel association of the chemokine CCL22 with abdominal aortic aneurysm. Am J Pathol. 2010;176:2098–2106. doi: 10.2353/ajpath.2010.090416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biros E, Moran CS, Rush CM, Gäbel G, Schreurs C, Lindeman JH, Walker PJ, Nataatmadja M, West M, Holdt LM, et al. Differential gene expression in the proximal neck of human abdominal aortic aneurysm. Atherosclerosis. 2014;233:211–218. doi: 10.1016/j.atherosclerosis.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Karki R, Jeon ER, Kim DW. Nelumbo nucifera leaf extract inhibits neointimal hyperplasia through modulation of smooth muscle cell proliferation and migration. Nutrition. 2013;29:268–275. doi: 10.1016/j.nut.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Saracini C, Bolli P, Sticchi E, Pratesi G, Pulli R, Sofi F, Pratesi C, Gensini GF, Abbate R, Giusti B. Polymorphisms of genes involved in extracellular matrix remodeling and abdominal aortic aneurysm. J Vasc Surg. 2012;55:171–179.e2. doi: 10.1016/j.jvs.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Riches K, Angelini TG, Mudhar GS, Kaye J, Clark E, Bailey MA, Sohrabi S, Korossis S, Walker PG, Scott DJ, Porter KE. Exploring smooth muscle phenotype and function in a bioreactor model of abdominal aortic aneurysm. J Transl Med. 2013;11:208. doi: 10.1186/1479-5876-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang YH, Yang IJ, Shin HM. Herbal formula HMC05 prevents human aortic smooth muscle cell migration and proliferation by inhibiting the ERK1/2 MAPK signaling cascade. J Nat Med. 2012;66:177–184. doi: 10.1007/s11418-011-0573-3. [DOI] [PubMed] [Google Scholar]
- 32.Goetze S, Xi XP, Kawano Y, Kawano H, Fleck E, Hsueh WA, Law RE. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension. 1999;33:183–189. doi: 10.1161/01.HYP.33.1.183. [DOI] [PubMed] [Google Scholar]
- 33.Hingorani A, Ascher E, Scheinman M, Yorkovich W, DePippo P, Ladoulis CT, Salles-Cunha S. The effect of tumor necrosis factor binding protein and interleukin-1 receptor antagonist on the development of abdominal aortic aneurysms in a rat model. J Vasc Surg. 1998;28:522–526. doi: 10.1016/S0741-5214(98)70139-9. [DOI] [PubMed] [Google Scholar]
- 34.Hultin MB, Shapiro SS, Bowman HS, Gill FM, Andrews AT, Martinez J, Eyster EM, Sherwood WC. Immunosuppressive therapy of Factor VIII inhibitors. Blood. 1976;48:95–108. [PubMed] [Google Scholar]
- 35.Zhong H, Matsui O, Xu K, Ogi T, Sanada J, Okamoto Y, Tabata Y, Takuwa Y. Gene transduction into aortic wall using plasmid-loaded cationized gelatin hydrogel-coated polyester stent graft. J Vasc Surg. 2009;50:1433–1443. doi: 10.1016/j.jvs.2009.07.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.