Abstract
The aim of the present study was to investigate the eradicating effects of 460 nm blue light (BL) on Candida albicans in vitro and in C. albicans-infected skin wounds in a mouse model. In the present study, the antifungal effects of irradiation with BL on C. albicans in vitro and in vivo were investigated. C. albicans colonies and cell numbers were investigated using the spread plate method and flow cytometry respectively following treatment with BL irradiation. In order to determine whether BL can eradicate C. albicans cells within biofilms, an in vitro C. albicans biofilm model was established, and the effect of BL was subsequently investigated using a confocal laser scanning microscope and a Live/Dead staining kit. Furthermore, a mouse skin wound infection model infected with C. albicans was established. Wound healing rates and histological examinations were determined 0, 3, 7, 10 and 14 days post-wounding. The results revealed that C. albicans was eradicated by BL in a dose-dependent manner, with a minimum fluence of 60 J/cm2. Irradiation with BL almost completely eradicated C. albicans when the light fluence was 240 J/cm2. C. albicans inside biofilms was also eradicated and biofilms were destroyed following BL irradiation at 240 J/cm2. In addition, BL was revealed to significantly suppress C. albicans infection in vivo. Irradiation with BL promoted the wound healing of C. albicans infected-skin wounds in a mouse model. In conclusion, the results of the present study demonstrated that 460 nm BL may eradicate planktonic and biofilm C. albicans in vitro, and represents a novel therapeutic strategy for the treatment of C. albicans infections in vivo.
Keywords: blue light, C. albicans, biofilm, wound infection
Introduction
Candida albicans is the major pathogen that causes hospital-acquired fungal infections in humans (1). C. albicans is an opportunistic pathogen, which is commonly found on the skin and in the urogenital tract of humans (2). Alterations in host immunity, stress, resident microbiota and other factors can induce the overgrowth of C. albicans, thus resulting in a wide range of infections, including superficial mucosal and hematogenously disseminated candidiasis (3). In addition, the widespread use of antifungal agents has resulted in the emergence of drug-resistant C. albicans among previously drug-susceptible populations (4). Research within the previous few decades has identified numerous drug resistance mechanisms. One of the main factors resulting in drug tolerance is adaptive flexibility to different environments via adherence to a surface and growth in microbial populations, which is known as biofilm development (2,5). In general, C. albicans biofilm formation is characterized by four stages: i) Cell-wall protein-mediated adherence of cells to a surface; ii) growth of the cells into a thin layer; iii) biofilm maturation via development of pseudohyphae and hyphae, and excretion of matrix material; and iv) finally, dispersal of cells from the biofilm, which may lead to colonization of new surfaces (6–9). The clinical treatment of C. albicans infection has become more difficult with the emergence of biofilms, as C. albicans cells present in biofilms are highly resistant to antifungal agents, including fluconazole, nystatin, amphotericin B and chlorhexidine (10). Therefore, there is an urgent requirement for the development of novel antifungal therapies.
In recent years, phototherapy has been suggested to represent a potential therapeutic alternative to antifungal treatment for the treatment of C. albicans biofilm infections. In addition, numerous studies have demonstrated that light with a wavelength of 400–500 nm, namely blue light (BL), exhibits marked antimicrobial effects against methicillin-sensitive Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa and Acinetobacter baumannii (11–20). However, at present, there is insufficient evidence to demonstrate that BL induces C. albicans cell death within the biofilm matrix. Considering the increasing emergence of antimicrobial-resistant microorganisms, BL may represent a novel antimicrobial therapeutic agent. BL results in bacterial cell death and exhibits far fewer adverse side effects to host cells and tissues compared with ultraviolet light, and it does not require the use of additional exogenous photosensitizers (15). However, the bactericidal mechanism of BL is still not fully understood. One particularly well-established hypothesis regarding the bactericidal mechanism of BL is that BL excites endogenous intracellular porphyrins of bacteria, resulting in production of cytotoxic reactive oxygen species that can kill bacterial cells (21–23).
In order to determine the effects of 460 nm BL irradiation on C. albicans infection, the present study aimed to investigate the efficacy of BL treatment on planktonic and biofilm C. albicans infection in vitro and in a mouse skin wound infection model in vivo. The results demonstrated that irradiation with BL effectively eradicated planktonic and biofilm C. albicans infections in a dose-dependent manner. In addition, the results revealed that BL exhibited a therapeutic effect on wounds infected with C. albicans in mice via induction of cell death.
Materials and methods
Light source
The BL source used in the present study was a 6.25 cm2 cluster of 50 light emitting diode (LED) array (Lifotronic Technology Co., Ltd., Shenzhen, China), which emitted light at a 450–470 nm spectral width and 460 nm peak emission (Fig. 1). Irradiance of light was adapted via adjustment of the distance between the LED array aperture and the C. albicans, which was determined using a power meter FieldMaxII-TO (RoHS) system (Coherent Inc., Santa Clara, CA, USA). The red-light source with a 620–640 nm spectral width was purchased from Lifotronic Technology Co., Ltd.
Figure 1.
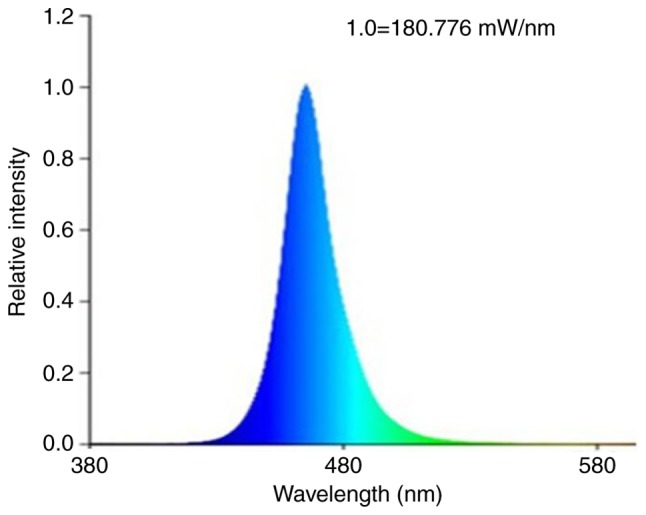
Emission spectrum of blue light.
Light exposure
The LED array was positioned 10 cm perpendicularly above each uncovered plate or wound to ensure accurate irradiation of each sample. The fluencies used are presented in Table I. A small fan was used to prevent heat accumulation during the irradiation process and the temperature on the surface of the organisms was measured every 1 min using an infrared thermometer.
Table I.
Energy fluencies used in the present study.
| Scenario | Irradiance (mW/cm2) | Illumination time (sec) | Energy fluencies (J/cm2) |
|---|---|---|---|
| Planktonic C. albicans | 60 | 200, 1,000, 2,000, 4,000 | 12, 60, 120, 240 |
| Biofilm C. albicans | 60 | 4,000 | 240 |
| C. albicans in wound | 60 | 4,000 | 240 |
C. albicans, Candida albicans.
Fungal strain and cultivation
The pathogenic strain, C. albicans ATCC 10231 was purchased from Shanghai Beinuo Life Science Bio Technology Co. Ltd. (Shanghai, China). The strain was identified by standard procedures, including gram stain and cell morphology, colony description, purity, viability and genotypic testing. The C. albicans strain was cultured overnight at 37°C under aerobic conditions with Sabouraud dextrose medium (Qingdao Hope bio-Technology Co., Ltd., Qingdao, China). Subsequently, C. albicans cells were inoculated in Sabouraud dextrose liquid medium at 37°C and were maintained in an orbital shaker at 150 rpm until the cells reached an optical density of 1.0 at 600 nm, which was equivalent to ~108 CFU/ml. Following this, the suspension was centrifuged at 300 × g and 37°C for 5 min, washed with PBS and diluted to 107 CFU/ml for subsequent analysis.
BL eradication of planktonic C. albicans in vitro
The spread plate method was used to investigate the antifungal effect of BL. C. albicans suspension (10 µl; ~105 CFU/ml) was evenly spread on Sabouraud dextrose medium plates. Uncovered plates containing C. albicans were subsequently subjected to irradiation using BL LED array or red-light LED array (energy densities, 0, 12, 60, 120 and 240 J/cm2). Subsequently, the plates were permitted to grow in a 37°C incubator for 24 h, after which images of each plate were captured and the number of colonies present was counted. The experiments were performed independently and in triplicate.
Flow cytometry was also used to determine the antifungal effects of BL. Using a micropipette, 1×107 CFU/ml C. albicans suspension (50 µl) in PBS was added to each well of a 24-well plate. Subsequently, the plates were irradiated using 0 or 240 J/cm2 energy density of BL or red light. C. albicans cells were subsequently stained using propidium iodide (PI; 100 µg/ml) for 25 min at room temperature and detected via flow cytometry. PI staining was used to investigate the number of dead cells, as it can enter membrane-damaged cells. The mortality rate of C. albicans was determined using the formula: Mortality rate=(number of cells in the R2 region/number of cells in the R1 region) ×100%. Data were analyzed using WinMDI software (version 2.9; The Scripps Research Institute, La Jolla, CA, USA). Experiments were repeated independently and in triplicate.
Phototoxicity assay using human skin fibroblasts and keratinocytes
Human skin fibroblast (HSF) Hs27 cells were purchased from Shanghai Bioleaf Biotech (Shanghai, China) and were cultured in petri dishes (diameter, 10 cm) supplemented with Dulbecco's modified Eagle's medium (Hyclone, USA) and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a 5% CO2 incubator. HaCaT cells were purchased from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China) and were cultured in petri dishes (diameter, 10 cm) with serum-free keratinocyte medium and phenol red, supplemented with recombinant epidermal growth factor (5 µg/ml) and bovine pituitary extract (50 µg/ml; all Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2 incubator. The growth medium was replaced with fresh medium every 2–3 days. Once a confluence of 80% was reached, the cells were trypsinized. Subsequently, the cell suspension was centrifuged at 300 × g and 37°C for 5 min and the cell pellet was resuspended in PBS. The HSF cell suspension (100 µl) and HaCaT cell suspension (100 µl) containing 1×106 cells/ml in media were added to 96-well plates and incubated overnight at 37°C. The 16 wells of a 96-well plate were separated into a light treatment group (8 wells) and a control group (8 wells). The wells in the light treatment group were subjected to irradiation with 240 J/cm2 BL. The wells in the control group were not subjected to any treatment. Following this, 100 µl fresh medium containing 10 µl water-soluble tetrazolium salt [Cell Counting kit-8 (CCK-8); Dojindo Molecular Technologies, Inc., Kumamoto, Japan] was added and incubated at 37°C for 2 h in the dark. The absorbance was measured at 450 nm using an Infinite M200 Pro multifunctional microplate reader (Tecan Group, Ltd., Männedorf, Switzerland). Experiments were performed in triplicate.
Establishment of a C. albicans biofilm model
A C. albicans biofilm model was established using the method described by Ramage et al (24), with slight modifications. Using a micropipette, 1×107 CFU/ml C. albicans (500 µl) and Sabouraud dextrose medium (2.5 ml) were added to laser confocal special culture plates (Cellvis, Mountain View, CA, USA). The plate was incubated at 37°C with 120 rpm agitation for 100 min. Following cell adhesion, the medium was aspirated, non-adherent cells were removed and 2.5 ml fresh medium was added. The plate was further incubated at 37°C for 3 days. The medium was replaced with fresh medium every 24 h. Following the removal of culture medium, biofilm formation was observed under an optical microscope (Nikon Corporation, Tokyo, Japan), and images were captured at 1, 2 and 3 day time intervals.
Irradiation of BL on C. albicans biofilm in vitro
Following culture for 3 days, C. albicans-exhibiting mature biofilms were divided into a control group and a light group. The control group was not subjected to any treatment, whereas the light group was subjected to irradiation using 240 J/cm2 BL. Following this, the two groups were stained using a LIVE/DEAD® Fungal Light™ Yeast Viability kit (Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for 30 min in the dark. The kit contained solutions of SYTO® 9 green-fluorescent nucleic acid stain and PI red-fluorescent nucleic acid stain. These stains differ both in their spectral characteristics as well as their ability to penetrate healthy cells. As a result, C. albicans with intact membranes were stained fluorescent green, whereas cells with damaged membranes were stained fluorescent red. The antifungal effect of BL was observed using a confocal laser scanning microscope (LSM 510; Zeiss GmbH, Jena, Germany). Images were acquired using an EC Plan-Neofluar 40×/1.30 Oil DIC M27 objective, and 488 and 555 nm laser lines for excitation of Live/Dead stain.
Establishment of mouse models bearing skin wounds infected with C. albicans
Animal experiments were approved by the Committee on Research Animal Use of Shanghai Jiao Tong University (Shanghai, China) and were performed in accordance with National Institutes of Health guidelines (25). A total of 30 adult female BALB/c nude mice (6–8 weeks old, 15–20 g) were purchased from the Experimental Animal Center at The Second Military Medical University (Shanghai, China) and were maintained under a 12-h light/dark cycle at room temperature and 40–60% relative humidity. Standard complete nutritional feeds were sterilized and drinking water was filtered to remove bacteria. Mice were able to eat, drink and move freely in a sterile isolation chamber. Mice were administered an intraperitoneal injection of 1% pentobarbital sodium (75 mg/kg; Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) prior to inoculation with C. albicans on day 0. Following disinfection using 75% alcohol, 1.0 cm2 full-thickness abrasions were made on the back surface of each mouse. A total of 30 mice were randomly divided into three groups, including: Negative control group [C. albicans (−); BL (−)], positive control group [C. albicans (+); BL (−)] and the light treatment group [C. albicans (+), BL (+)]. A total of 10 min following wound induction, the wound surfaces of mice in the positive control group and the light treatment group were treated with 20 µl suspension containing 109 CFU/ml of C. albicans by evenly smearing the suspension onto the wound surface using an inoculation loop. The skin wound was covered with sterile gauze and bound up with 3M surgical dressing to avoid scratching or chewing by the animal. A single cage was kept for each mouse. A technician monitored the mice every hour and they did not exhibit any pain or discomfort. Thus, we did not use extra drugs such as anesthetics for the mice following the wound formation.
Treatment with BL for skin wounds infected with C. albicans in a mouse model
Mice in the light treatment group were subjected to irradiation once a day using 240 J/cm2 BL at 0, 1 and 2 day time intervals. The negative control group and the positive control group were not subjected to any treatment. At 0, 1, 2 and 3 days post-irradiation, the secreta on the wound surfaces of all of the mice were collected using sterile swabs and then smeared on the surface of Sabouraud Dextrose Agar Medium plates using a sterile inoculation loop prior to BL treatment. Plates were subsequently incubated for 24 h at 37°C, after which the number of colonies present on each plate was determined. Skin wounds were covered with sterile gauze and bound with 3M surgical dressing that was punched with a sterile needle for air circulation.
Hematoxylin and eosin (H&E) staining of wound tissue
In order to observe histological alterations of the wound tissues in mice during the process of wound healing, three skin samples from wounds of mice in each group were obtained 7 days post-injury. The skin samples were fixed in 4% paraformaldehyde for 24 h at room temperature, embedded in paraffin and then cut into 4 µm sections. Sections were then stained using 5 µg/ml H&E at room temperature for 10 min; the results of which were observed and images were captured under an optical microscope (Nikon Corporation).
Wound healing measurement
The skin abrasion area of each mouse was monitored and images were captured using a digital camera at 0, 3, 7, 10 and 14 days post-injury. The average duration of time taken for wound healing, as well as the percentage of the unhealed wound, were calculated to determine the healing speed of the mouse skin wound using the following formula: % of unhealed wound=(unhealed area at each time interval/total wound area at the 0 day time interval) ×100%.
Statistical analysis
All results are presented as the means ± standard deviation and are the results of experiments repeated three times. Statistical analysis was performed using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Data were analyzed using either the Student's t-test or one-way analysis of variance followed by Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
BL eradicates planktonic C. albicans in vitro
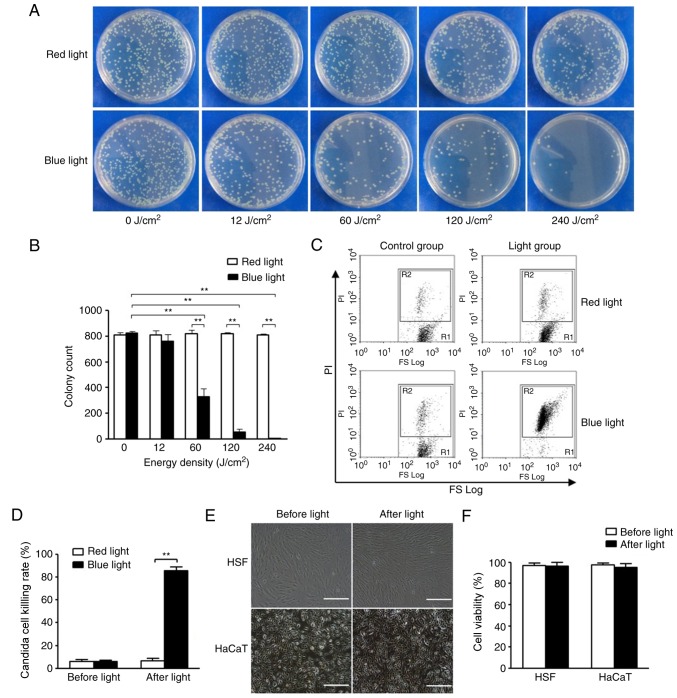
Using the spread plate method, the viability of planktonic C. albicans cells in the presence or absence of BL was determined. As revealed in Fig. 2A and B, the colony count of C. albicans was significantly decreased following treatment with BL (≥60 J/cm2) in a dose-dependent manner compared with C. albicans treated with red light. Irradiation with BL almost completely eradicated C. albicans colonies when the light fluence was 240 J/cm2. Furthermore, flow cytometry was used to further investigate the viability of C. albicans treated with or without BL. Furthermore, the flow cytometry results revealed that C. albicans treated with BL exhibited a significantly increased killing rate compared with C. albicans treated with red light (P<0.01; Fig. 2C and D). In addition, quantitative analysis of these results demonstrated that the killing rates of C. albicans exposed to 0 and 240 J/cm2 BL were 7.68±0.43 and 88.92±2.01% (P<0.01; Fig. 2D), respectively.
Figure 2.
Effects of BL on planktonic C. albicans, and HSF and HaCaT cells in vitro. (A) Spread plate method was used to investigate the effects of BL (0, 12, 60, 120 and 240 J/cm2) and red light irradiation, the results of which were (B) quantitatively analyzed. (C) Flow cytometry was performed to investigate the effects on C. albicans following irradiation with 240 J/cm2 BL and red light. R1 represents the total number of C. albicans cells and R2 represents the total number of dead C. albicans cells stained by PI, the results of which were (D) quantitatively analyzed. (E) Cell morphology of HSF and HaCaT cells following irradiation with 240 J/cm2 BL. Scale bar, 100 µm. (F) Quantitative analysis of cell viability of HSF and HaCaT cells following irradiation with 240 J/cm2 BL as determined by Cell Counting kit-8 assays. **P<0.01. BL, blue light; C. albicans, Candida albicans; HSF, human skin fibroblast; PI, propidium iodide.
In order to determine whether 240 J/cm2 BL induces phototoxicity in normal cutaneous cells, the cell viabilities of HSF and HaCaT cells following BL irradiation with 240 J/cm2 were investigated using a CCK-8 kit. As revealed in Fig. 2E and F, no marked differences in the morphology and cell viabilities of HSF and HaCaT cells were revealed (P>0.05).
BL eradicates C. albicans inside biofilms
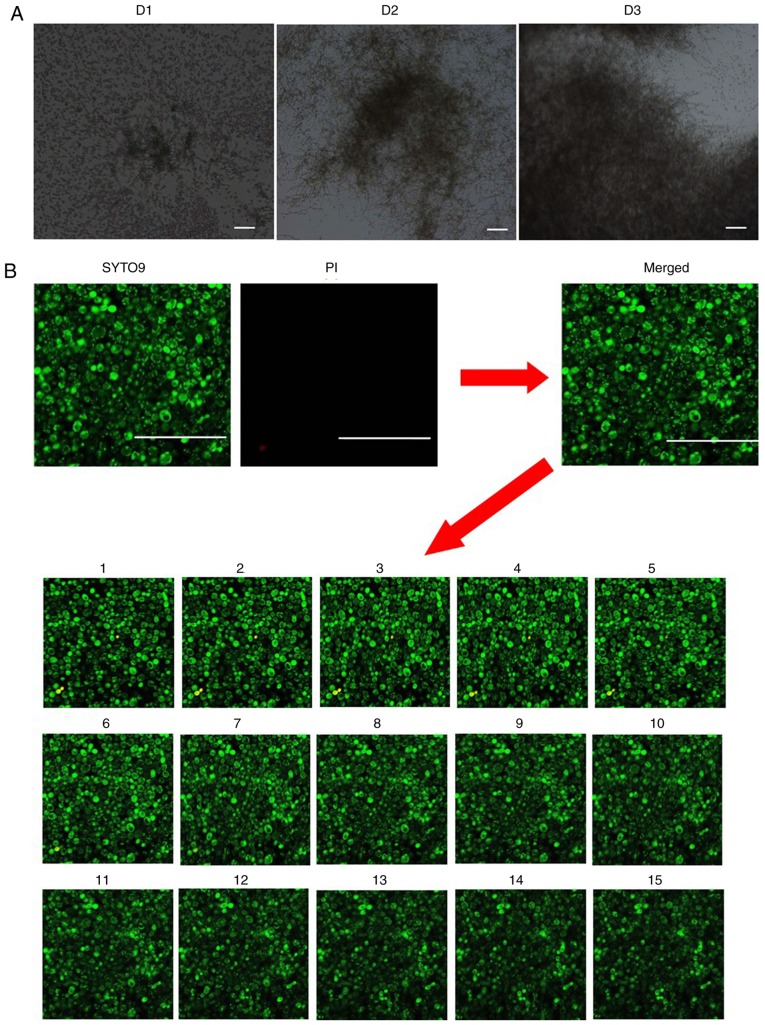
In order to determine whether BL can eradicate C. albicans within biofilms, a C. albicans biofilm model was established (Figs. 3 and 4). As revealed in Fig. 3A, C. albicans biofilm formation can be categorized into three distinct developmental phases: Early (D1; 0–24 h), intermediate (D2; 24–48 h) and maturation (D3; 48–72 h). C. albicans communities were completely encased within extracellular material during the maturation stage.
Figure 3.
Effects of blue light on C. albicans inside biofilms. (A) Optical microscope images of C. albicans biofilms grown in the wells of plates. D1 represents the early phase, D2 represents the intermediate phase and D3 maturation phase of C. albicans biofilm formation. (Magnification, ×100; scale bars, 100 µm). (B) Distribution of the dead/viable C. albicans in the biofilm of the control group as observed by confocal laser scanning microscopy (Magnification, ×400; scale bars, 50 µm). Green staining indicates live C. albicans, and red staining indicates dead C. albicans. Images 1–15 represent scanning images from the deepest layer of the biofilm to the superficial layers of the biofilm. C. albicans, Candida albicans; PI, propidium iodide.
Figure 4.
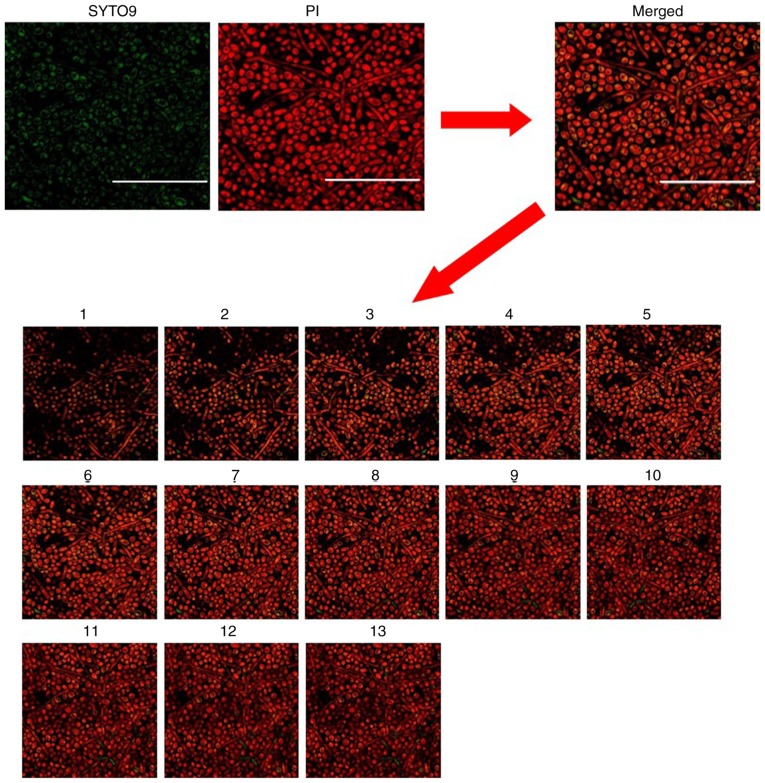
Distribution of Candida albicans cell death inside the established biofilm model following treatment with blue light, as observed by confocal laser scanning microscopy (Magnification, ×400; scale bars, 50 µm). Green pixels indicate live fungi. Red pixels indicate dead fungi. Images 1–15 represent scanning images from the deepest layer of the biofilm to the superficial layers of the biofilm. PI, propidium iodide.
The present results demonstrated that irradiation with BL at 240 J/cm2 induced mortality of C. albicans cells inside the biofilm, as seen by increased red color in the light group (Fig. 4), whereas the vast majority of fungi in the blank control group were alive (Fig. 3B) as revealed by confocal laser scanning microscopy. These results suggested that BL irradiation can eradicate C. albicans inside biofilms.
BL significantly suppresses C. albicans infection in vivo
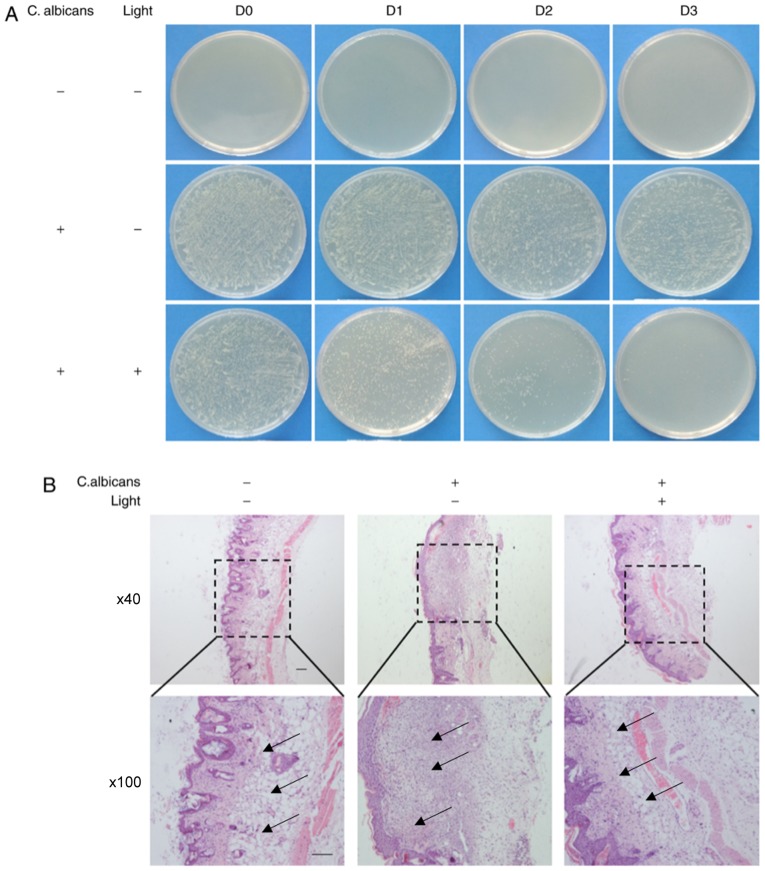
Using C. albicans-infected skin wounds in a mouse model, the effects of BL on C. albicans infection were investigated. As revealed in Fig. 5A, the number of C. albicans colonies grown from in vivo swabs following treatment with 240 J/cm2 BL was markedly decreased compared with in the positive control group at day 1. In addition, the number of C. albicans colonies grown from in vivo swabs following treatment with 240 J/cm2 BL markedly decreased in a time-dependent manner, resulting in almost no visible C. albicans colonies at the 3 day time interval (Fig. 5A).
Figure 5.
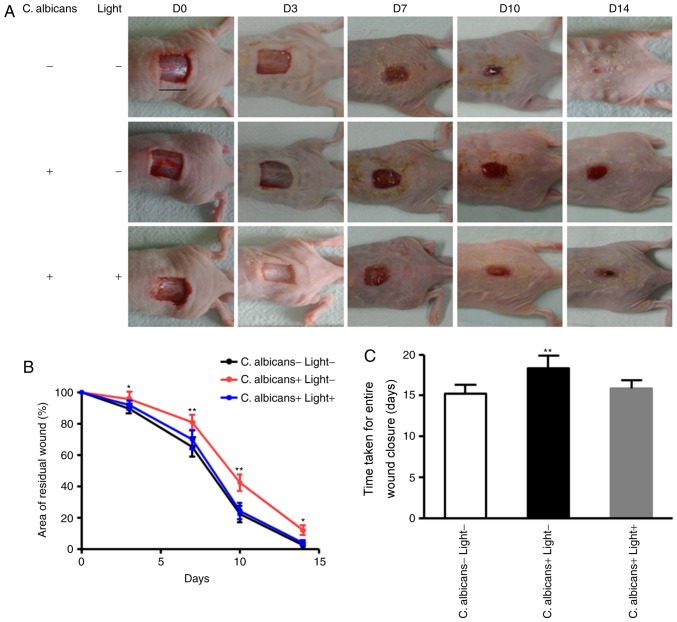
Investigation into the effects of BL on established models of mice wound healing following inoculation with C. albicans in vivo. (A) Images of spread plates of the mice wound secreta obtained from the control group (C. albicans -, Light -), the untreated C. albicans group (C. albicans +, Light-) and the C. albicans group treated with 240 J/cm2 BL (C. albicans +, Light +) at 0, 1, 2 and 3 day time intervals. (B) Hematoxylin and eosin staining of wound tissues at day 7. Black arrow: Infiltration of inflammatory cells. (Scale bars, 100 µm). BL, blue light; C. albicans, Candida albicans.
In general, the infiltration of inflammatory cells was associated with infection severity (26). The infiltration of inflammatory cells into the wounds of mice in the three groups was investigated using H&E staining. As presented in Fig. 5B, large numbers of inflammatory cells were revealed to have infiltrated into skin tissue in the positive control group compared with in the negative control group and the BL light-treated group at the 7 day time interval. These results suggested that treatment with BL may protect tissues from infection with C. albicans in mice skin wounds.
Treatment with BL promotes healing of infected skin wounds in mice
In order to determine whether BL affected the wound-healing process following infection with C. albicans, a murine skin wound infection model was established in mice, and wound-healing rates were determined. The wound-healing rates in BL-treated mice infected with C. albicans and in the negative control group were significantly increased compared with in the non-treated mice infected with C. albicans at 3, 7, 10 and 14 days (P<0.05 and P<0.01; Fig. 6A and B). The average wound closure time for the negative control group, the positive control group and BL-treated mice infected with C. albicans were 15.9±0.94, 18.3±1.49, and 15.2±1.08, respectively (Fig. 6C). These results demonstrated that irradiation with BL on C. albicans-infected wounds accelerated wound closure compared with in the positive control group. However, no significant differences in entire wound closure time were exhibited between BL-treated mice infected with C. albicans and the negative control group (P>0.05; Fig. 6C).
Figure 6.
Effects of BL irradiation on wound healing in mice. (A) Back wounds of non-treated mice infected with C. albicans, BL-treated mice infected with C. albicans and the negative control group at 0, 3, 7, 10 and 14 day time intervals. (Scale bar, 1 cm). (B) Quantification of the topical wound healing rate. Data are presented as the means ± standard deviation. (C) Duration of time taken for entire wound closure. *P<0.05, **P<0.01. BL, blue light; C. albicans, Candida albicans, D, day.
Discussion
There has been a steady increase in the incidence of fungal infections over the past several decades, and only a limited number of effective antifungal drugs remain available for treatment (27). Furthermore, the incidence of resistance to antifungal drugs is also increasing (28); therefore, there is a requirement for the development of novel therapeutic strategies for the treatment of fungal infections. In the present study, the antifungal effects of irradiation with BL on C. albicans were investigated. C. albicans represents the most common fungal infection; in the present study, C. albicans was cultured in vitro and inoculated in mice wounds. Firstly, the results demonstrated that BL effectively eradicated planktonic C. albicans in a dose-dependent manner, which was in agreement with the findings of Zhang et al (20). To the best of our knowledge, the present study is the first to demonstrate that irradiation with 460 nm BL may increase eradication of C. albicans inside biofilms. Finally, the results revealed that irradiation with BL increased wound healing rates in mice, which may be due to the eradication of C. albicans in mouse skin wounds. Therefore, the results suggested that BL may represent a novel antifungal therapeutic strategy.
Numerous studies have demonstrated that photodynamic therapy (PDT) effectively eradicates fungi (29–31). However, the necessary introduction of a photosensitizer into the targeted fungi (32) and into the infected tissues (33) represents a major disadvantage of PDT. In recent years, BL has gained increasing attention due to its intrinsic antimicrobial effect without the addition of an exogenous photosensitizer (11–20). Guffey and Wilborn (18) revealed that irradiation with 405 and 470 nm BL induces bactericidal effects on Pseudomonas aeruginosa and Staphylococcus aureus in a dose-dependent manner; however, BL has no effect on Propionibacterium acnes infection in vitro (18). Dai et al (15) demonstrated that BL exhibits a broad-spectrum antimicrobial effect against both gram-positive and gram-negative bacteria. The effective induction of bacterial cell death by BL has been well established; however, very few studies have investigated the effects of BL on fungi. In the present study, a wavelength of 460 nm BL was used to eradicate C. albicans in vitro, which was similar to the wavelength used by Zhang et al (20). The results of the present study revealed that 68.21±2.10 and 99.92±0.14% of C. albicans were eradicated by 60 and 240 J/cm2 of BL, respectively.
Biofilms are the communities of microorganisms that develop on living or inert surfaces and are enclosed within extracellular matrixes, which form complex three-dimensional structures (16,33). The present study, as well as previous studies (16,19), revealed that the biofilm matrix is almost transparent; therefore, light can penetrate through the transparent biofilm. Halstead et al (16) investigated treatment with 400 nm BL against bacteria in planktonic and biofilm growth modes in vitro. Our previous study demonstrated that 460 nm BL markedly eradicates planktonic and biofilm MRSA in vitro and in vivo in a dose-dependent manner (19). Therefore, the aim of the present study was to determine whether BL can also eradicate C. albicans in biofilms. Among the fungal pathogens that can infect humans, C. albicans is the species most frequently associated with biofilm formation and has high morbidity and mortality rates (34). Similar to bacterial biofilms, biofilm-grown C. albicans cells are highly resistant to antimicrobials (34). In order to investigate the effects of BL on C. albicans inside biofilms, a C. albicans biofilm model was established according to the method described by Ramage et al (24), with slight modifications. Using this model, it was revealed that C. albicans was able to form mature biofilms within 3 days. In addition, the results of the present study revealed that C. albicans within biofilms was markedly eradicated by 240 J/cm2 BL irradiation and the complex structures of the biofilm were destroyed. To the best of our knowledge, this is the first study to demonstrate that irradiation with BL may significantly eradicate C. albicans inside biofilms and this was also the main difference between our study and other studies on the BL inactivation of C. albicans. This result may be due to the ability of BL to penetrate through the transparent biofilm. In the present study, the inhibition of C. albicans infection following irradiation with 460 nm BL was further investigated using C. albicans-infected skin wounds in a mouse model. The amount of C. albicans present in wounds treated with one exposure of 240 J/cm2 BL was significantly suppressed compared with in the C. albicans-infected group. Furthermore, multiple treatments (240 J/cm2 per day, for 3 days) with BL revealed that treatment enhanced the eradication of C. albicans in a dose-dependent manner. In addition, the results of the present study revealed that BL decreased numbers of inflammatory cells in skin tissue and promoted wound healing in vivo. The results of the present study were in agreement with the in vivo results revealed by Zhang et al (20). However, Zhang et al (20) used a single exposure of 415 nm BL with a markedly higher dose (432 J/cm2), which was able to significantly reduce the fungal burden in infected mouse wounds (20). This difference may due to different apparatus used.
In conclusion, the results of the present study may aid the development of novel therapeutic strategies for the treatment of C. albicans wound infections. Future studies should perform experiments using large animal models, as well as human trials, to verify the results of the present study. In addition, despite BL exhibiting a therapeutic effect against C. albicans, the specific mechanism underlying this effect remains unclear. Dai et al (15) suggested that the mechanism of BL eradication of P. acnes, Helicobacter pylori and some oral bacteria involves the photo-excitation of intracellular porphyrins and the subsequent production of cytotoxic reactive oxygen species. Our previous study demonstrated that BL irradiation eradicates MRSA via induction of prophage activation (19). However, the molecular mechanisms underlying the eradication of C. albicans by BL require further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported partially by grants from The National Nature Science Foundation of China (grant no. 81501656) and The Doctoral Fund of Ministry of Education of China (grant no. 20120073110088).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CW, ZY, JD and MY designed and performed the experiments. CW, ZY, YG and YP analyzed the data. CW and ZY wrote the manuscript. JD and MY revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal experiments were approved by the Committee on Research Animal Use of Shanghai Jiao Tong University (Shanghai, China) and were performed in accordance with National Institutes of Health guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Raman N, Lee MR, Palecek SP, Lynn DM. Polymer multilayers loaded with antifungal β-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes. J Control Release. 2014;10:54–62. doi: 10.1016/j.jconrel.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulati M, Ennis CL, Rodriguez DL, Nobile CJ. Visualization of biofilm formation in candida albicans using an automated microfluidic device. J Vis Exp. 2017 Dec 14; doi: 10.3791/56743. doi: 10.3791/56743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. Practice guidelines for the treatment of candidiasis. Infectious diseases society of America. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 4.Huang M, Kao KC. Population dynamics and the evolution of antifungal drug resistance in Candida albicans. FEMS Microbiol Lett. 2012;333:85–93. doi: 10.1111/j.1574-6968.2012.02587.x. [DOI] [PubMed] [Google Scholar]
- 5.de Carvalho Dias K, Barbugli PA, de Patto F, Lordello VB, de Aquino Penteado L, Medeiros AI, Vergani CE. Soluble factors from biofilm of Candida albicans and Staphylococcus aureus promote cell death and inflammatory response. BMC Microbiol. 2017;17:146. doi: 10.1186/s12866-017-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankenship JR, Mitchell AP. How to build a biofilm: A fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, Mccormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko Y, Miyagawa S, Takeda O, Hakariya M, Matsumoto S, Ohno H, Miyazaki Y. Real-time microscopic observation of Candida biofilm development and effects due to micafungin and fluconazole. Antimicrob Agents Chemother. 2013;57:2226–2230. doi: 10.1128/AAC.02290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, Kadosh D, Lopez-Ribot JL. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra J, Mukhrjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–908. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 11.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57:1238–1245. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. 2014;209:1963–1971. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 2009;27:221–226. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- 14.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2008;40:734–737. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- 15.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15:223–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, Webber MA, Oppenheim BA. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol. 2016;82:4006–4016. doi: 10.1128/AEM.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T, Hamblin MR. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg. 2013;31:531–538. doi: 10.1089/pho.2012.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 2006;24:684–688. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Wang N, Wang C, Yao Y, Fu X, Yu W, Cai R, Yao M. 460 nm visible light irradiation eradicates MRSA via inducing prophage activation. J Photochem Photobiol B. 2017;166:311–322. doi: 10.1016/j.jphotobiol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR, Dai T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence. 2016;7:536–545. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008;92:180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 2005;81:1186–1189. doi: 10.1562/2005-04-06-RA-477. [DOI] [PubMed] [Google Scholar]
- 23.Aahkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramage G, Savilie SP, Wickes BL, López-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute for Laboratory Animal Research 2011, corp-author. Guide for the care and use of laboratory animals, 8th edition Washington (DC) National Academies Press; [Google Scholar]
- 26.Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLos One. 2015;10:e0128220. doi: 10.1371/journal.pone.0128220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowen LE, Anderson JB, Kohn LM. Evolution of drug resistance in Candida albicans. Annu Rev Microbiol. 2002;56:139–165. doi: 10.1146/annurev.micro.56.012302.160907. [DOI] [PubMed] [Google Scholar]
- 28.Rogers TR. Antifungal drug resistance: Does it matter? Int J Infect Dis. 2002;6(Suppl 1):S47–S53. doi: 10.1016/S1201-9712(02)90154-2. [DOI] [PubMed] [Google Scholar]
- 29.Lam M, Jou PC, Lattif AA, Lee Y, Malbasa CL, Mukherjee PK, Oleinick NL, Ghannoum MA, Cooper KD, Baron ED. Photodynamic therapy with Pc 4 induces apoptosis of Candida albicans. Photochem Photobiol. 2011;87:904–909. doi: 10.1111/j.1751-1097.2011.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambrechts SA, Aalders MC, Van Marie J. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob Agents Chemother. 2005;49:2026–2034. doi: 10.1128/AAC.49.5.2026-2034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai T, Arce Bilde VJ, Tegos GP, Hamblin MR. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob Agents Chemother. 2011;55:5710–5717. doi: 10.1128/AAC.05404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Bonhomme J, D'Enfert C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol. 2013;16:398–403. doi: 10.1016/j.mib.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5:608–611. doi: 10.1016/S1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.