Abstract
Small interfering RNA (siRNA), consisting a 21-mer duplex molecule, is often modified by conjugation with specific ligands to enhance its capacity for tissue-specific delivery. However, these attempts are hampered by the low permeability of negatively charged RNA molecules to enter the cell membrane. In this study, we designed and synthesized siRNA conjugates modified with cationic oligospermine and cyclic RGD (cRGD) to overcome the low-membrane permeability of siRNA. The siRNA conjugate, which contains 15 spermines and a cRGD peptide, showed sufficient gene-silencing activity at 250 nM final concentration without a transfection reagent. Under these conditions, the cationic oligospermine and cRGD–siRNA conjugate did not show any cytotoxicity.
Introduction
Oligonucleotide (ON) therapeutics, using rationally designed antisense oligonucleotides (ASONs) or small interfering RNAs (siRNAs), are promising candidates for a treatment of unmet medical needs, which could not be covered by conventional low-molecular-weight drugs.1,2 However, these ONs, especially double-stranded siRNAs, have relatively high molecular weights and negative charges derived from a phosphodiester backbone. As a result, they can hardly penetrate the negatively charged cell membrane.3 Furthermore, the ASON and siRNA composed of natural nucleosides do not exhibit tissue specificity. Therefore, the development of a target tissue-specific drug delivery system is crucial for the use of ASON or siRNA as a drug. To date, numerous chemical approaches have been reported to overcome the low membrane permeability of siRNA.4,5 Among them, a targeted tissue-specific ligand–siRNA conjugate is one promising strategy for siRNA delivery into specific cells.6−15 In particular, siRNAs conjugated with triantennary N-acetylgalactosamine (GalNAc), which has a high binding affinity to the asialoglycoprotein receptor (ASGR), have shown efficient gene-silencing activity in vivo, and clinical and preclinical trials are ongoing.13−15 Because ASGR is highly expressed in hepatocytes and shows rapid internalization and recycling, GalNAc–siRNA conjugates bound to ASGR are readily incorporated into cells and exhibit sufficient gene-silencing activity at low concentrations. However, unfortunately, the development of other type of ligand–siRNA conjugates has not been successful because of the low membrane permeability of RNA molecules. Although the development of alternative ligand–siRNA conjugates is required to expand deliverable tissues, no comparable ligand–receptor systems, similar to GalNAc–ASGR, are yet to be established. Therefore, to develop ligand–siRNA conjugates that target receptors other than ASGR, a new approach which improves potentially low cell membrane permeability of siRNA conjugate is needed.
In in vitro studies using cultured cells, it was demonstrated that cationic oligospermine conjugates neutralized the negative charge of siRNAs derived from their phosphodiester backbones, resulting in improved thermal stability, nuclease resistance, and cell membrane permeability.16−19 Although oligospermine–siRNA and AON conjugates have been synthesized and successfully transfected in vitro,20 tissue-specific delivery of oligospermine–siRNA or AON conjugates has not been achieved because of spermine’s lack of tissue specificity. Further, more than 20 spermine molecules must be conjugated to one siRNA to provide sufficient cell permeability, which sometimes causes cytotoxicity.18 Alternatively, the cyclic RGD (cRGD) peptide has a high binding affinity for integrin αVβ3, which is overexpressed on the surface of various tumor cells.21 cRGD–siRNA conjugates have been successfully transfected in vitro and in vivo by several research groups.22,23
On the basis of these reports, we designed and synthesized siRNA conjugates containing both the cationic oligospermine and the tumor-targeting cRGD peptide (Figure 1). We postulated that the cationic oligospermine modification of the cRGD–siRNA would increase its membrane permeability, thus enhancing the tumor cell-specific delivery. Furthermore, we postulated that the oligospermine and cRGD-peptide would work synergistically. As such, the amount of spermine to be incorporated into the siRNA could be reduced, thus reducing oligospermine-induced cytotoxicity.
Figure 1.
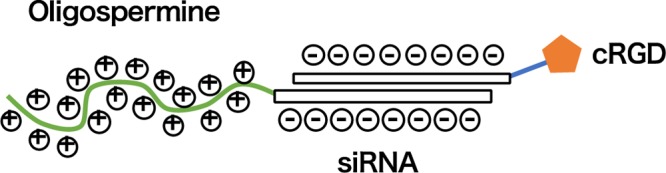
Schematic illustration of cationic oligospermine and cRGD–siRNA conjugates.
Results and Discussion
Synthesis of ON Conjugates
We designed and synthesized siRNA conjugates modified by both oligospermine and cRGD at the 3′-termini of the sense and antisense strands, respectively. RecQL1-siRNA, which silences the RecQL1 mRNA encoding RecQL1 DNA helicase, was chosen as a model siRNA. Futami and colleagues have previously shown that gene silencing of RecQL1 helicase by RecQL1-siRNA results in the following: (1) inhibits DNA repair in replicating cells, (2) induces mitotic catastrophes at the checkpoints of negative tumor cells, and (3) causes mitotic death in the tumor cell specifically.24,25 It was also reported that oligospermine conjugation at the antisense (guide) strand of the siRNA reduces the siRNA silencing activity greater than the sense (passenger) strand.19 Thus, we introduced the oligospermine at the 3′ end of the sense (passenger) strand and cRGD at that of the antisense (guide) strand, respectively (Figure 2A,B). To improve nuclease resistance and silencing activities of the siRNAs, the 2′-hydroxyl group of some pyrimidine nucleotides was modified by a methyl group (Table 1). The 2′-O methylations are known to reduce immunogenicity of siRNAs and to suppress the innate immune response.26
Figure 2.
(A) Structures of antisense RNAs conjugated to cRGD. (B) Structures of sense RNAs conjugated to cationic oligospermine. Small letters indicate 2′-O-methyl RNA and dT indicates 2′-deoxythymidine.
Table 1. Sequences of siRNAs, N/P Ratio.
| name | S/ASa | sequence (5′–3′)b | N/P |
|---|---|---|---|
| siQL1 | S | GuucAGACCACuucAGcuudTdT | |
| AS | AAGCUGAAGUGGuCuGAAcdTdT | ||
| siQL1[RGD] | S | GuucAGACCACuucAGcuudTdT | |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD | ||
| siQL1[S5/RGD] | S | GuucAGACCACuucAGcuudTdT-S5-dT | 0.42 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD | ||
| siQL1[S10/RGD] | S | GuucAGACCACuucAGcuudTdT-S10-dT | 0.77 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD | ||
| siQL1[S15/RGD] | S | GuucAGACCACuucAGcuudTdT-S15-dT | 1.03 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD | ||
| siQL1[S15] | S | GuucAGACCACuucAGcuudTdT-S15-dT | 1.05 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT | ||
| Cy3-siQL1 | S | GuucAGACCACuucAGcuudTdT | |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-Cy3 | ||
| Cy3-siQL1[RGD] | S | GuucAGACCACuucAGcuudTdT | |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD-Cy3 | ||
| Cy3-siQL1[S5/RGD] | S | GuucAGACCACuucAGcuudTdT-S5-dT | 0.41 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD-Cy3 | ||
| Cy3-siQL1[S10/RGD] | S | GuucAGACCACuucAGcuudTdT-S10-dT | 0.75 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD-Cy3 | ||
| Cy3-siQL1[S15/RGD] | S | GuucAGACCACuucAGcuudTdT-S15-dT | 1.02 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-RGD-Cy3 | ||
| Cy3-siQL1[S15] | S | GuucAGACCACuucAGcuudTdT-S15-dT | 1.03 |
| AS | AAGCUGAAGUGGuCuGAAcdTdT-Cy3 |
S denotes sense strand and AS represents antisense strand.
Small letters indicate 2′-O-methyl RNA and dT indicates 2′-deoxythymidine.
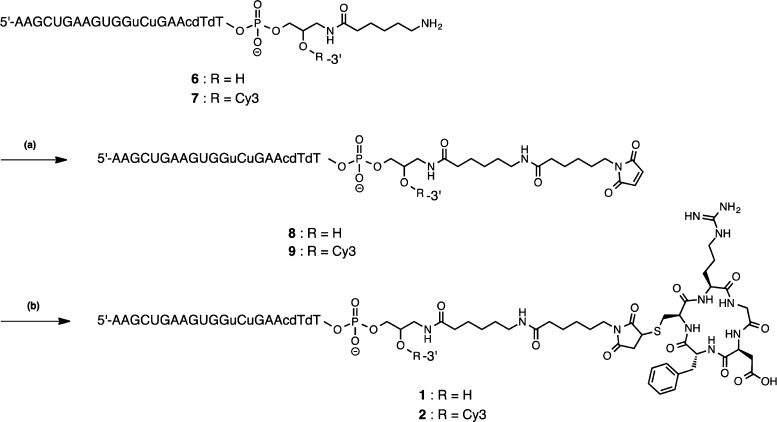
Incorporation of cRGD peptide into the antisense strand was carried out by a postsynthetic modification method (Scheme 1). Briefly, single-stranded RNA (ssRNA) 6, which has a reactive amino moiety at the 3′ end of the strand, was synthesized using an amino-modified controlled pore glass (Scheme S1). Subsequently, 6 was reacted with N-(6-maleimidocaproyloxy)succinimide (EMCS) in a phosphate buffer (pH = 7.4) at 37 °C to give a maleimide-modified ssRNA 8. A thiol-containing cRGD peptide (cRGDfC) was conjugated to 8 via a thiol–maleimide Michael addition reaction to produce cRGD conjugate 1. To assess the cellular uptake of the conjugate, a Cy3-labeled cRGD conjugate 2 was also synthesized by a similar procedure. Product purification was performed by reversed-phase high-performance liquid chromatography (RP-HPLC), and the structures of the synthesized ONs were confirmed by matrix-assisted laser desorption ionization time-of-flight/mass spectrometry (Table S2). The cationic oligospermine conjugates 3–5 were synthesized according to the procedure previously reported using a spermine amidite.27 To determine the number of spermine required for cellular uptake and examine the effects of the N/P ratio on cellular uptake, we synthesized three oligospermine conjugates with different amounts of spermine modifications. Each ssRNA conjugates were annealed to complementary RNA to form the siRNA conjugates with the oligospermine and cRGD.
Scheme 1. Synthesis of cRGD Conjugates; (a) EMCS, in a Phosphate Buffer (pH 7.4), Room Temperature, Overnight and (b) c(RGDfC), in 0.1 M triethylammonium acetate (TEAA), 50% MeCN aq, Room Temperature, Overnight.
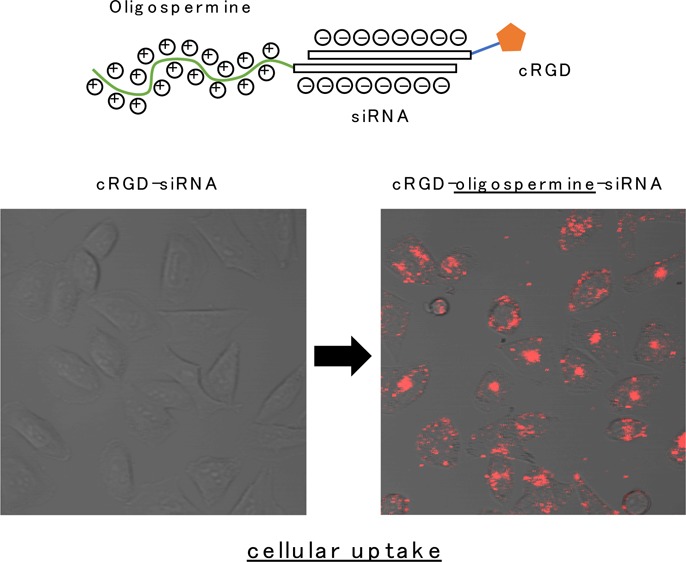
Cellular Uptake Test
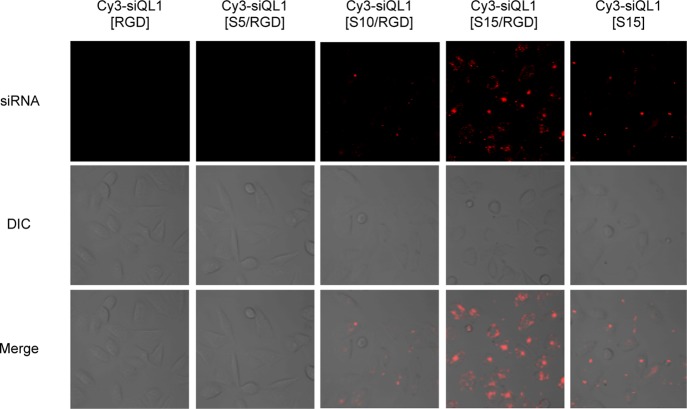
Cellular uptakes of Cy3-labeled siRNA conjugates were evaluated using confocal laser microscopy. Human melanoma A2058 cells were incubated with 200 nM of each Cy3-labeled siRNA conjugates in serum-free medium. After a 3 h incubation, accumulations of Cy3-labeled siRNA conjugates in the cells were visualized by confocal laser microscopy (Figure 3). In the siQL1[S10/RGD] and siQL1[S15/RGD] RNAs, which were modified by spermine, the accumulation of Cy3 fluorescence signal was detected. However, no fluorescence signal was seen in the unmodified spermine conjugate, siQL1[RGD]. The fluorescence intensity in the cell was dependent on the number of spermine molecules conjugated to RNA. The strongest fluorescence intensity was observed when the siQL1[S15/RGD] was used. These results indicate that the cationic oligospermine addition to cRGD–siRNA conjugates promoted cellular uptake. Furthermore, because the fluorescence intensity in A2058 cells was significantly lower in the cRGD-unmodified siQL1[S15], it is conceivable that the synergistic work of cRGD and cationic oligospermine may be crucial for the cellular uptake of siRNA conjugates.
Figure 3.
Transfection reagent-free cellular uptake of Cy3-labeled siRNA heteroconjugates in vitro. A2058 cells were treated with 200 nM of Cy3-labeled siRNA conjugates. After a 3 h incubation, Cy3-labeled siRNA conjugates were visualized by confocal laser microscopy.
Gene-Silencing Activities
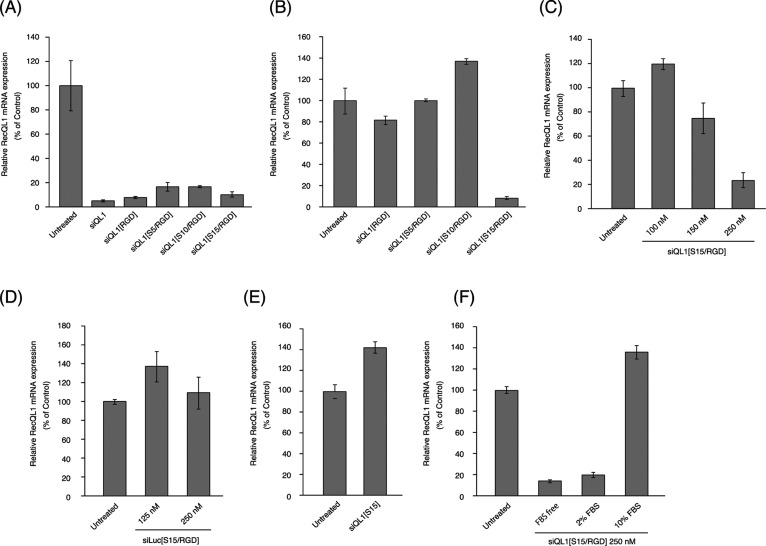
To evaluate the RNA interference (RNAi) activity of the cRGD and oligospermine-modified siRNAs, we assessed the gene-silencing activities of each siRNA conjugate in the presence of the transfection reagent Lipofectamine. Each siRNA conjugate was mixed with Lipofectamine RNAiMAX and transfected into HeLa cells at 20 nM final concentration. The relative expression levels of RecQL1 mRNA were measured by RT-qPCR. In Figure 4A, all siRNA conjugates showed greater gene-silencing activity than the unmodified siQL1. These data indicated that the modification of siRNA by cRGD and oligospermine does not disrupt the formation of the RNA-induced silencing complex and target mRNA recognition.
Figure 4.
Effect of RECQL1-siRNA conjugates on the expression of HeLa cell RECQL1 mRNA. (A) siRNA (20 nM) conjugates were transfected using Lipofecatamine RNAiMAX. (B) HeLa cells were transfected with 250 nM of siRNA conjugates without a transfection reagent. (C) Cells were transfected with 100–250 nM of siQL1[S15/RGD] without a transfection reagent. (D) Cells were transfected with 125 and 250 nM of siLuc[S15/RGD] without a transfection reagent. (E) Cells were transfected with 250 nM of siQL1[S15] without a transfection reagent. (F) siQL1[S15/RGD] were transfected without a transfection reagent in the absence or presence of FBS.
We then evaluated the gene-silencing activities of siRNA conjugates without transfection reagent under serum-free conditions. As shown in Figure 4B,C, only siQL1[S15/RGD] showed strong gene-silencing activity at 150–250 nM. The result indicates that the cationic oligospermine modification improved the cell membrane permeability of cRGD–siRNA conjugates. In addition, it was found that at least 15 spermine modifications (N/P = 1.02) were needed for efficient gene-silencing activity. Previously, it was reported that oligospermine–siRNA conjugates required more than 20 spermine modifications (N/P = 1.33) for efficient gene-silencing activity without a transfection reagent.20 Thus, the cRGD peptide addition to siRNA reduces the required number of oligospermine molecules, which sometimes cause cytotoxicity. The siLuc[S15/RGD]- targeting Renilla luciferase mRNA (Table S3), which contains 15 spermines and a cRGD-peptide, did not silence the expression of RecQL1 mRNA under similar conditions (Figure 4D). Therefore, the RNAi activity of siQL1[S15/RGD], which inhibits RecQL1 mRNA expression, appears to be supported not only by accessory conjugates but also by elaborated RNA sequences. The siQL1[S15] (contains 15 spermines only) did not show gene-silencing activity (Figure 4E). This result indicates that both oligospermine and cRGD-peptide are required for efficient gene silencing. The gene-silencing activities of siRNA conjugates were also confirmed by the Western blot analysis (Figure S5).
Next, we evaluated the effects of serum on the silencing activity of siQL1[S15/RGD]. As shown in Figure 4F, the silencing activity of siQL1[S15/RGD] decreased slightly in 2% fetal bovine serum (FBS) and was eliminated in 10% FBS conditions. It was reported that intracellular uptake of the cationic oligospermine–siRNA conjugate was inhibited by nonspecific-binding of protein to the siRNA in the serum.21 Thus, it is suggested that our siQL1[S15/RGD] also interacted with serum proteins, such that its intercellular uptake was severely reduced.
Cytotoxicity Test
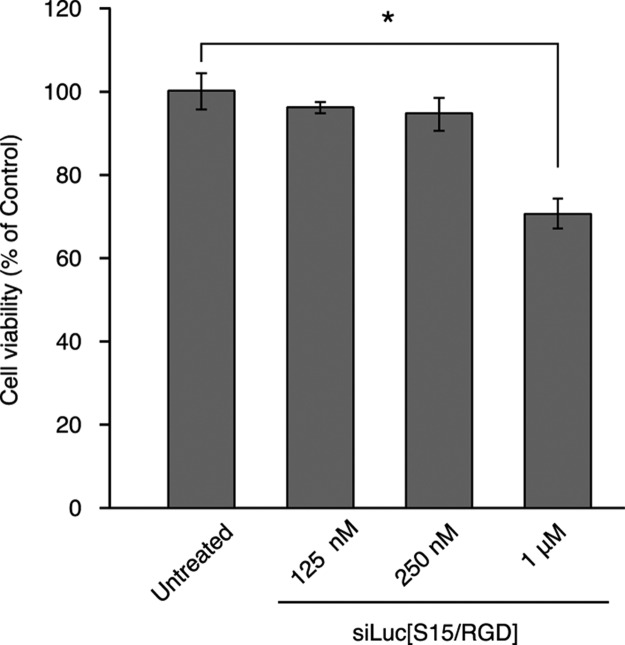
Finally, we examined the cytotoxicity of the siRNA conjugates using siLuc[S15/RGD] because siQL1-targeting RecQL1 mRNA has strong antitumor cell activity. A2058 cells were incubated with 125 nM to 1 μM of siLuc[S15/RGD] in serum-free medium. After a 48 h incubation, cytotoxicity was examined by measuring cell viabilities (Figure 5). Although slight growth inhibition was observed at 1 μM of siLuc[S15/RGD], there was no cytotoxicity at 250 nM, which is the concentration needed for transfection reagent-free gene silencing of siQL1[S15/RGD]. Although the oligospermine–siRNA conjugates show gene-silencing activities when the N/P ratio is greater than 1.33, they are sometimes cytotoxic. On the other hand, by incorporating the cRGD peptide into the oligospermine–siRNA conjugate, we succeeded in reducing the N/P ratio to 1.02 to eliminate cytotoxicity while also maintaining the gene-silencing activity.
Figure 5.
Cell viability at 48 h after siLuc[S15/RGD] transfection. Cells were transfected with 125 nM to 1 μM of siLuc[S15/RGD] without a transfection reagent.
Conclusions
In this study, we successfully synthesized novel cationic oligospermine and cRGD–siRNA conjugates. By combining cRGD peptides with a cationic 15 spermine modification (N/P = 1.02), we found that siQL1[S15/RGD] containing both 15 spermines and cRGD peptides showed sufficient gene-silencing activity at 250 nM final concentration without a transfection reagent. Conversely, the siQL1[S15] containing only 15 spermine did not show gene-silencing activity under same conditions. Collectively, these results demonstrate synergy between the oligospermine and cRGD-peptide for efficient gene silencing. It was also found that the siLuc[S15/RGD] caused no cytotoxicity at 250 nM, which is the required concentration for transfection reagent-free gene silencing of siQL1[S15/RGD]. Taken together, the oligospermine and cRGD-peptide-combined conjugation holds a great promise for efficient siRNA-mediated drug delivery to various organs and tissues beyond the liver.
Experimental Section
RNA Synthesis
Synthesis was carried out with a DNA/RNA synthesizer by phosphoramidite method. Deprotection of bases and phosphates was performed in concentrated NH4OH/EtOH (3:1, v/v) at 55 °C for 4 h. 2′-TBDMS groups were removed by TEA·3HF (Aldrich) at 65 °C for 1.5 h. The reaction was quenched with 0.1 M TEAA buffer (pH 7.0) and desalted on a Sep-Pak C18 cartridge. The deprotected ONs were separated from unprotected ONs by 20% polyacrylamide gel electrophoresis containing 7 M urea to give rise to the highly purified siRNA (Supporting Table).
Postsynthetic Modification of siRNAs Conjugated with cRGD
The cRGD-conjugated antisense strand of RECQL1-siRNA was synthesized by postsynthetic modification method. The cRGD-conjugated antisense RNA strands were purified by reversed-phase C-18 HPLC using a linear gradient of 5–50% of MeCN in 0.1 M TEAA buffer at pH 7.0. The HPLC profiles of synthesized RNA are shown in Figure S4.
Synthesis of siRNAs Conjugated with cRGD at the Antisense RNA 1
To the solution of 6 (15 nmol in a total of 100 μL) consisting 10 mM NaCl, 100 mM phosphate buffer (pH 7.4) (85 μL), and dimethyl sulfoxide (DMSO) (15 μL), 0.2 M EMCS in DMSO (12 μL) was added at the room temperature. The mixture was incubated at 37 °C overnight and maleimide-modified 8 was collected by RP-HPLC. Subsequently, 50 mM c(RGDfC) peptide (Bachem) in DMSO (2 μL) was added directly to the collected 8 solution. The mixture was then incubated at room temperature overnight and the cRGD-conjugated RNA 1 (3.48 nmol) was purified by RP-HPLC with a recovery of 23%.
Synthesis of RNA (or ONs) with cRGD Conjugates 2
To the solution of 7 (15 nmol in a total of 100 μL) consisting 10 mM NaCl, 100 mM phosphate buffer (pH 7.4) (85 μL), and DMSO (15 μL), 0.2 M EMCS in DMSO (12 μL) was added at the room temperature. The mixture was incubated at 37 °C overnight, and maleimide-modified 9 was collected by RP-HPLC. Subsequently, 50 mM c(RGDfC) peptide in DMSO (3 μL) was directly added to the collected solution containing 9. The mixture was incubated at room temperature overnight, and cRGD-conjugated RNA 2 was purified by RP-HPLC (3.03 nmol, 20%).
Cellular Uptake Test
A2058 cells (4.0 × 104 cell/mL) were placed on the 35 mm glass-bottom dish (200 μL/dish) and were grown for 24 h before transfection. Cells were transfected with 200 nM of Cy3-labeled siRNA conjugates in serum-free Opti-MEM medium. After 3 h of transfection, cells were washed by PBS, and the accumulations of Cy3-labeled siRNA conjugates in the transfected cells were visualized by confocal microscope (Zeiss LSM710).
Gene-Silencing Assay
A2058 cells (4.0 × 104 cell/mL) were transferred to 96-well plate (100 μL/well), 24 h before transfection. Cells were transfected with various concentrations of siRNA conjugates in serum-free Opti-MEM medium. After 4 h transfection, FBS was added to each well at 2% final concentration and incubated for 44 h. Cell lysis was performed using SuperPrep Cell Lysis Kit for qPCR (TOYOBO), and RNAs were extracted. Real-time PCR was performed with primers specific for target mRNA by using THUNDERBIRD SYBR qPCR Mix (TOYOBO). The sequences of primer are listed in Table S4.
Cytotoxicity Test
A2058 cells (4.0 × 104 cell/mL) were transferred to a 6-well plate (1 mL/well) 24 h before transfection. Cells were transfected with various concentrations of siRNA conjugates in serum-free Opti-MEM medium. After 4 h transfection, FBS was added to each well at 2% final concentration and further incubation for 44 h. The cell viabilities were determined by the trypan blue dye exclusion test.
Acknowledgments
This work was supported by Grant-in-Aid for JSPS Research Fellow grant number JP16J03464. This work was also supported by the Japan Agency for Medical Research and Development (AMED) through its Funding Program for Basic Science and Platform Technology Program for Innovative Biological Medicine for the development of siRNA conjugates with tissue-specific delivery functions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00850.
Experimental procedures, characterizations, and sequences of RNAs and copies of 1H and 13C NMR spectra for compound 12 and 31P NMR spectra for compound 13 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sharma V. K.; Watts J. K. Oligonucleotide therapeutics: chemistry, delivery and clinical progress. Future Med. Chem. 2015, 7, 2221–2242. 10.4155/fmc.15.144. [DOI] [PubMed] [Google Scholar]
- Crooke S. T. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017, 27, 70–77. 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy S. F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Corey D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2017, 46, 1584–1600. 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos S. A.; Jones S. W.; Perry M. M.; Williams A. E.; Erjefalt J. S.; Turner J. J.; Barnes P. J.; Sproat B. S.; Gait M. J.; Lindsay M. A. Lung Delivery Studies Using siRNA Conjugated to TAT(48–60) and Penetratin Reveal Peptide Induced Reduction in Gene Expression and Induction of Innate Immunity. Bioconjugate Chem. 2007, 18, 1450–1459. 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.; Dohmen C.; Philipp A.; Kiener D.; Maiwald G.; Scheu C.; Ogris M.; Wagner E. Synthesis and Biological Evaluation of a Bioresponsive and Endosomolytic siRNA–Polymer Conjugate. Mol. Pharmaceutics 2009, 6, 752–762. 10.1021/mp9000124. [DOI] [PubMed] [Google Scholar]
- Ming X.; Alam M. R.; Fisher M.; Yan Y.; Chen X.; Juliano R. L. Intracellular delivery of an antisense oligonucleotide via endocytosis of a G protein-coupled receptor. Nucleic Acids Res. 2010, 38, 6567–6576. 10.1093/nar/gkq534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen C.; Fröhlich T.; Lächelt U.; Röhl I.; Vornlocher H.-P.; Hadwiger P.; Wagner E. Defined Folate-PEG-siRNA Conjugates for Receptor-specific Gene Silencing. Mol. Ther.--Nucleic Acids 2012, 1, e7. 10.1038/mtna.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willibald J.; Harder J.; Sparrer K.; Conzelmann K.-K.; Carell T. Click-Modified Anandamide siRNA Enables Delivery and Gene Silencing in Neuronal and Immune Cells. J. Am. Chem. Soc. 2012, 134, 12330–12333. 10.1021/ja303251f. [DOI] [PubMed] [Google Scholar]
- Lu H.; Wang D.; Kazane S.; Javahishvili T.; Tian F.; Song F.; Sellers A.; Barnett B.; Schultz P. G. Site-Specific Antibody-Polymer Conjugates for siRNA Delivery. J. Am. Chem. Soc. 2013, 135, 13885–13891. 10.1021/ja4059525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikan M.; Osborn M. F.; Coles A. H.; Biscans A.; Godinho B. M. D. C.; Haraszti R. A.; Sapp E.; Echeverria D.; DiFiglia M.; Aronin N.; Khvorova A. Synthesis and Evaluation of Parenchymal Retention and Efficacy of a Metabolically Stable O-Phosphocholine-N-docosahexaenoyl-l-serine siRNA Conjugate in Mouse Brain. Bioconjugate Chem. 2017, 28, 1758–1766. 10.1021/acs.bioconjchem.7b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J. K.; Willoughby J. L. S.; Chan A.; Charisse K.; Alam M. R.; Wang Q.; Hoekstra M.; Kandasamy P.; Kel’in A. V.; Milstein S.; Taneja N.; O’Shea J.; Shaikh S.; Zhang L.; van der Sluis R. J.; Jung M. E.; Akinc A.; Hutabarat R.; Kuchimanchi S.; Fitzgerald K.; Zimmermann T.; van Berkel T. J. C.; Maier M. A.; Rajeev K. G.; Manoharan M. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- Matsuda S.; Keiser K.; Nair J. K.; Charisse K.; Manoharan R. M.; Kretschmer P.; Peng C. G.; Kel’in A. V.; Kandasamy P.; Willoughby J. L. S.; Liebow A.; Querbes W.; Yucius K.; Nguyen T.; Milstein S.; Maier M. A.; Rajeev K. G.; Manoharan M. siRNA Conjugates Carrying Sequentially Assembled Trivalent N-Acetylgalactosamine Linked Through Nucleosides Elicit Robust Gene Silencing In Vivo in Hepatocytes. ACS Chem. Biol. 2015, 10, 1181–1187. 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- Huang Y. Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther.--Nucleic Acids 2017, 6, 116–132. 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noir R.; Kotera M.; Pons B.; Remy J.-S.; Behr J.-P. Oligonucleotide–Oligospermine Conjugates (Zip Nucleic Acids): A Convenient Means of Finely Tuning Hybridization Temperatures. J. Am. Chem. Soc. 2008, 130, 13500–13505. 10.1021/ja804727a. [DOI] [PubMed] [Google Scholar]
- Paris C.; Moreau V.; Deglane G.; Karim L.; Couturier B.; Bonnet M.-E.; Kedinger V.; Messmer M.; Bolcato-Bellemin A.-L.; Behr J.-P.; Erbacher P.; Lenne-Samuel N. Conjugating Phosphospermines to siRNAs for Improved Stability in Serum, Intracellular Delivery and RNAi-Mediated Gene Silencing. Mol. Pharmaceutics 2012, 9, 3464–3475. 10.1021/mp300278b. [DOI] [PubMed] [Google Scholar]
- Perche P.; Nothisen M.; Bagilet J.; Behr J.-P.; Kotera M.; Remy J.-S. Cell-penetrating cationic siRNA and lipophilic derivatives efficient at nanomolar concentrations in the presence of serum and albumin. J. Controlled Release 2013, 170, 92–98. 10.1016/j.jconrel.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Nothisen M.; Bagilet J.; Behr J.-P.; Remy J.-S.; Kotera M. Structure Tuning of Cationic Oligospermine-siRNA Conjugates for Carrier-Free Gene Silencing. Mol. Pharmaceutics 2016, 13, 2718–2728. 10.1021/acs.molpharmaceut.6b00309. [DOI] [PubMed] [Google Scholar]
- Gagnon K. T.; Watts J. K.; Pendergraff H. M.; Montaillier C.; Thai D.; Potier P.; Corey D. R. Antisense and Antigene Inhibition of Gene Expression by Cell-Permeable Oligonucleotide-Oligospermine Conjugates. J. Am. Chem. Soc. 2011, 133, 8404–8407. 10.1021/ja200312y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F.; Le Breton A.; Préat V. RGD-Based Strategies To Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharmaceutics 2012, 9, 2961–2973. 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- Alam M. R.; Ming X.; Fisher M.; Lackey J. G.; Rajeev K. G.; Manoharan M.; Juliano R. L. Multivalent Cyclic RGD Conjugates for Targeted Delivery of Small Interfering RNA. Bioconjugate Chem. 2011, 22, 1673–1681. 10.1021/bc200235q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wang W.; Samarsky D.; Liu L.; Xu Q.; Zhang W.; Zhu G.; Wu P.; Zuo X.; Deng H.; Zhang J.; Wu Z.; Chen X.; Zhao L.; Qiu Z.; Zhang Z.; Zeng Q.; Yang W.; Zhang B.; Ji A. Tumor-targeted in vivo gene silencing via systemic delivery of cRGD-conjugated siRNA. Nucleic Acids Res. 2014, 42, 11805–11817. 10.1093/nar/gku831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami K.; Kumagai E.; Makino H.; Goto H.; Takagi M.; Shimamoto A.; Furuichi Y. Induction of mitotic cell death in cancer cells by small interference RNA suppressing the expression of RecQL1 helicase. Cancer Sci. 2008, 99, 71–80. 10.1111/j.1349-7006.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- Futami K.; Kumagai E.; Makino H.; Sato A.; Takagi M.; Shimamoto A.; Furuichi Y. Anticancer activity of RecQL1 helicase siRNA in mouse xenograft models. Cancer Sci. 2008, 99, 1227–1236. 10.1111/j.1349-7006.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Judge A. D.; Bola G.; Lee A. C. H.; MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006, 13, 494–505. 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]; b Robbins M.; Judge A.; Liang L.; McClintock K.; Yaworski E.; MacLachlan I. 2′-O-methyl-modified RNAs Act as TLR7 Antagonists. Mol. Ther. 2007, 15, 1663–1669. 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- Voirin E.; Behr J.-P.; Kotera M. Versatile synthesis of oligodeoxyribonucleotide-oligospermine conjugates. Nat. Protoc. 2007, 2, 1360–1367. 10.1038/nprot.2007.177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.