A library of imidazopyridine–propenone conjugates (8a–8u) were synthesized and evaluated for their antitumor activity against four human cancer cell lines.
A library of imidazopyridine–propenone conjugates (8a–8u) were synthesized and evaluated for their antitumor activity against four human cancer cell lines.
Abstract
A library of imidazopyridine–propenone conjugates (8a–8u) were synthesized and evaluated for their antitumor activity against four human cancer cell lines, namely, prostate (DU-145), lung (A549), cervical (Hela) and breast (MCF-7) cancer cell lines. These conjugates showed good to moderate activity against the tested cell lines. Among them, two conjugates (8m and 8q) showed significant antiproliferative activity against the human lung cancer cell line (A549) with IC50 values of 0.86 μM and 0.93 μM, respectively. Flow cytometry analysis revealed that these compounds arrested the cell cycle at the G2/M phase in the human lung cancer cell line (A549), inhibiting tubulin polymerization leading to apoptosis. Further, Hoechst staining, decrease in mitochondrial membrane potential and Annexin V-FITC assay suggested that the cell death was due to apoptosis induction. Overall, the present investigation demonstrated that the synthesized imidazopyridine–propenone conjugates are promising tubulin inhibitors and apoptotic inducers.
Introduction
In eukaryotic cells, microtubules are key components and essential in a variety of elemental cellular processes such as cell formation, cell division, maintenance of cell shape, regulation of motility, secretion and cytoplasmic transport, making microtubules an important target for anticancer drugs.1–3 In addition, microtubules are also involved in cell signalling pathways which are accountable for cellular apoptosis. The microtubule dynamics are regulated by different important proteins such as dynein and kinesin.4 Various reports have recognized that microtubule-dependent force is essential for chromosomal translocation and spindle formation.5 The irregular microtubule dynamics result in the obstruction of cell division at the metaphase; as a result, various efforts aimed at blocking mitosis, like inhibition of tubulin polymerization by tubulin targeting agents, have emerged as an effective approach to treat cancer.6,7 Many literature reports are available on the inhibition of tubulin polymerization with subsequent arrest of cells during mitosis leading to apoptosis.8 Microtubule polymerization inhibitors are effective in the treatment of breast, lung, ovarian and other cancers. Colchicine (I) and nocodazole (II) are prominent examples of compounds (Fig. 1) that inhibit assembly of microtubules by binding to tubulin.9,10 Among the said compounds, nocodazole shows preferential interference in the destabilization of microtubule polymerization.
Fig. 1. Chemical structures of microtubule targeting agents: colchicine (I), nocodazole (II), imidazopyridine guanylhydrazones (III), imidazopyridine–benzimidazoles (IV), aryl propenones (V) and imidazopyridine–propenone conjugates 8(a–u).
Imidazopyridine is a fused bicylic heterocycle that represents an important class of privileged scaffolds.11 This scaffold displays a broad spectrum of biological activity such as inflammation, tumour suppression, viral, apoptosis, fungal and bacterial.12 This heterocyclic core can also be found in drugs like olprinone, nicopidem, saripidem, zolpidem and zolimidine.13 Investigations demonstrated that this scaffold can be extensively used owing to its various therapeutic benefits. Many attempts to discover new drugs through creative discovery of technologies have fallen short of producing the expected results. Henceforth, privileged structure-guided scaffold re-evolution is a primary strategy to identify structurally novel chemotypes by modifying either the central core of the scaffold or the side chain of existing active compounds.14 In this regard, our group previously reported imidazopyridine–benzimidazoles15 (IV) as apoptosis inducers that inhibit tubulin polymerization by binding at the colchicine binding site on tubulin. Thus imidazopyridine motifs provide immense opportunity to exploit undescribed bioactivities by making use of readily derivatized motifs with well established synthetic protocols of imidazopyridine.
Aryl-propenones16 are a new class of compounds reported recently as potent tubulin binders which block mitotic cell division leading to apoptotic cell death. This, combined with our previous work on aryl-propenones containing molecules as tubulin polymerization inhibitors,17 prompted us to combine an aryl-propenone chain with the imidazopyridine scaffold, which may exhibit an interesting cytotoxicity profile. Our continued efforts to discover effective anticancer agents through the combination of the said two scaffolds led us to design and synthesize a new series of twenty-one imidazopyridine–propenone conjugates that consist of a hybrid molecule with three rings. In addition, a comprehensive structure–activity relationship has been established by varying the substituents on the rings. These synthesized conjugates were tested for their antiproliferative effect on the human lung cancer cell line (A549). The two most active molecules in the series (conjugates 8m and 8q) were further investigated for their ability to inhibit tubulin assembly and induce apoptosis. The results of our investigations along this direction are presented in this work.
Results and discussion
Chemistry
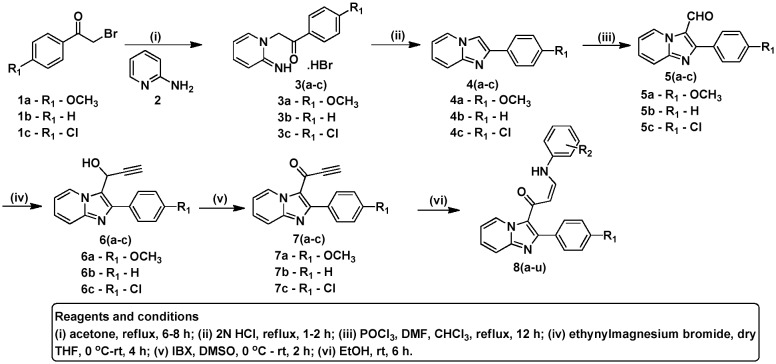
The synthesis of the imidazopyridine–propenones 8(a–u) is shown in Scheme 1 (also given in the ESI‡). To obtain 2-arylimidazopyridine 4(a–c), equimolar mixtures of substituted 2-bromoacetophenones and 2-aminopyridine were refluxed for 4–5 h, followed by addition of 2 N HCl under reflux conditions. The intermediates imidazopyridine aldehydes 5(a–c) were prepared by means of Vilsmeier–Haack reaction on the corresponding 2-arylimidazopyridine 4(a–c). These aldehydes 5(a–c) were further treated with ethynylmagnesium bromide in THF to obtain the intermediates 6(a–c), followed by oxidation with IBX in DMSO, providing the corresponding precursors 7(a–c). Subsequently, the desired compounds 8(a–u) were prepared by reaction of the corresponding precursors 7(a–c) with arylamines in ethanol. The list of substituents, along with their corresponding yields and respective melting points, are listed in Table 1.
Scheme 1. Synthesis of imidazopyridine–propenones.
Table 1. Structures of compounds 8(a–u) and their yields.
| Compound | R2 | R1 | Yield % | Melting point (°C) |
| 8a | 3,5-Dimethoxyphenyl | OCH3 | 74% | 152–154 |
| 8b | 4-Methoxyphenyl | OCH3 | 75% | 130–132 |
| 8c | 4-Bromophenyl | OCH3 | 71% | 174–176 |
| 8d | 4-Flourophenyl | OCH3 | 74% | 118–120 |
| 8e | 5-Indolyl | OCH3 | 71% | 218–220 |
| 8f | 6-Indolyl | OCH3 | 66% | 198–200 |
| 8g | 3-Quinolyl | OCH3 | 62% | 210–212 |
| 8h | 6-Quinolyl | OCH3 | 64% | 232–234 |
| 8i | 3,5-Dimethoxyphenyl | H | 78% | 151–153 |
| 8j | 4-Methoxyphenyl | H | 82% | 172–174 |
| 8k | 4-Bromophenyl | H | 75% | 160–162 |
| 8l | 3-Quinolyl | H | 65% | 188–190 |
| 8m | 6-Quinolyl | H | 69% | 204–206 |
| 8n | 3,4,5-Trimethoxyphenyl | Cl | 72% | 194–196 |
| 8o | 3,5-Dimethoxyphenyl | Cl | 73% | 120–122 |
| 8p | 4-Methoxyphenyl | Cl | 67% | 96–98 |
| 8q | 4-Bromophenyl | Cl | 79% | 208–210 |
| 8r | 5-Indolyl | Cl | 61% | 251–253 |
| 8s | 6-Indolyl | Cl | 64% | 219–221 |
| 8t | 3-Quinolyl | Cl | 69% | 229–231 |
| 8u | 6-Quinolyl | Cl | 71% | 214–216 |
Biological evaluation
Cytotoxic activity
The synthesized conjugates were evaluated for their cytotoxic potential against four human cancer cell lines, namely, prostate (DU-145), lung (A549), cervical (Hela) and breast (MCF-7) cancer cells, by employing MTT18 assay using nocodazole as a reference drug. The structure–activity relationship was analysed based on the cytotoxicity results given in Table 2. For ease of understanding, the rings in the molecule are labelled A, B and C, as shown in Fig. 2. Structural modifications were performed at the 4th position of rings B and C, respectively, whereas ring A was left as such. The IC50 values of these conjugates showed considerable cytotoxic activity ranging from 0.86–22.78 μM. The synthesized conjugates fall into three categories depending on the substituents on ring B, as follows: compounds 8(a–h) with 4-methoxy substitution, conjugates 8(i–m) with no substitution and compounds 8(n–u) with chlorine substituted at the 4th position. Among the conjugates 8(a–h), it can be observed from the data that conjugate 8g (IC50 – 1.05 μM) and conjugate 8h (IC50 – 1.21 μM) have displayed improved cytotoxicity on human lung cancer cells compared to nocodazole (IC50 – 1.39 μM). Conjugate 8c (IC50 – 1.34 μM) is equally active as nocodazole when tested on lung cancer cells. The presence of three methoxy groups in conjugate 8a has caused moderate cytotoxicity on the four tested cancer cell lines. Among conjugates 8(i–m), particularly, conjugate 8m with quinolyl substitution on ring C possessed significant activity against human lung cancer and prostate cancer cells with IC50 values of 0.86 μM and 1.07 μM, respectively. Also, conjugate 8m (IC50 – 1.21 μM) was found to exert potent cytotoxicity on Hela cells in comparison with the rest of the conjugates. Conjugate 8k (IC50 – 1.34 μM) with bromine at the 4th position of ring C exerted cytotoxicity close to that of nocodazole. An overview on the cytotoxicity results of conjugates 8(n–u) reveals that conjugate 8q, having a chlorine atom at the 4th position of ring B and a bromine atom at the 4th position of ring C, displayed an IC50 value of 0.93 μM on the human lung cancer cell line. Conjugate 8r (IC50 – 1.28 & 1.01 μM) with 5-indolyl substitution on ring C was found to display potent cytotoxicity against human prostate cancer cells and human lung cancer cells, respectively. A look at the substituents on ring B shows that the potency is maximum when the ring is unsubstituted, followed by a good profile with an electron withdrawing halogen (chlorine), and less potency with an electron donating substituent (methoxy). Similarly on ring C, heterocyclic substituents (quinolyl, indolyl) were found to be highly active, followed by withdrawing substituents (bromine, fluorine), compared to a donating substituent (methoxy) on ring C.
Table 2. IC50 (μM) values a for compounds 8(a–u) on selected human cancer cell lines.
| Compound | DU-145 b | A549 c | Hela d | MCF-7 e |
| 8a | 6.261 | 4.467 | 4.571 | 14.86 |
| 8b | 11.13 | 12.65 | 19.95 | 16.50 |
| 8c | 1.452 | 1.346 | 4.545 | 9.833 |
| 8d | 6.130 | 3.251 | 10.95 | 13.00 |
| 8e | 11.33 | 10.97 | 13.60 | 18.94 |
| 8f | 10.69 | 8.800 | 15.32 | 19.35 |
| 8g | 5.280 | 1.052 | 9.440 | 14.95 |
| 8h | 4.720 | 1.211 | 4.852 | 11.63 |
| 8i | 2.924 | 2.239 | 3.090 | 10.22 |
| 8j | 2.190 | 1.570 | 14.90 | 7.609 |
| 8k | 1.820 | 1.349 | 2.042 | 8.667 |
| 8l | 2.443 | 1.750 | 3.076 | 5.167 |
| 8m | 1.076 | 0.861 | 1.219 | 1.807 |
| 8n | 3.250 | 1.138 | 3.020 | 3.388 |
| 8o | 11.97 | 9.897 | 14.56 | 13.58 |
| 8p | 10.79 | 6.458 | 11.85 | 16.33 |
| 8q | 1.500 | 0.933 | 1.479 | 2.692 |
| 8r | 1.288 | 1.014 | 3.243 | 7.125 |
| 8s | 2.630 | 2.291 | 10.93 | 9.600 |
| 8t | 4.168 | 2.884 | 3.715 | 18.860 |
| 8u | 13.630 | 13.040 | 16.350 | 22.780 |
| Nocodazole | 1.259 | 1.393 | 1.611 | 1.086 |
a50% inhibitory concentration after 48 h of drug treatment.
bHuman prostate cancer.
cHuman lung cancer.
dHuman cervical cancer.
eHuman breast cancer.
Fig. 2. Structure–activity relationship of imidazopyridine–propenone conjugates.

Effect on cell cycle arrest
Many anticancer compounds exert their growth inhibitory effect either by arresting the cell cycle at a particular checkpoint or by induction of apoptosis or a combination of both.19,20 The screening results revealed that both the compounds 8m and 8q showed improved antiproliferative activity against the lung cancer cell line (A549). To understand that the cell growth inhibition was due to cell cycle arrest, we performed cell cycle analysis using human lung cancer (A549) cells. In this study, A549 cells were treated with compounds 8m and 8q at concentrations of 0.5 and 1 μM for 48 h. The data obtained clearly indicated that these compounds exhibited G2/M cell cycle arrest in comparison with the untreated cells. The compounds 8m and 8q showed 15.69% and 14.40% cell accumulation in the G2/M phase at 0.5 μM concentration, whereas they exhibited 30.50 and 29.84% cell accumulation at 1 μM concentration, respectively (Fig. 3 and Table 3).
Fig. 3. Flow cytometric analysis of the A549 lung cancer cell line after treatment with compounds 8m and 8q at 0.5 and 1 μM concentrations for 48 h. A: Control cells (A549); B: nocodazole (1 μM); C: 8m (0.5 μM); D: 8m (1 μM); E: 8q (0.5 μM) and F: 8q (1 μM).
Table 3. Distribution of A549 cells in various phases of the cell cycle.
| Sample | Sub G1% | G 0/G1% | S% | G 2/M% |
| A: Control (A549) | 1.11 | 90.52 | 1.92 | 7.40 |
| B: Nocodazole (1 μM) | 0.79 | 66.92 | 4.55 | 29.68 |
| C: 8m (0.5 μM) | 0.50 | 82.37 | 2.77 | 15.69 |
| D: 8m (1 μM) | 0.91 | 66.32 | 4.28 | 30.50 |
| E: 8q (0.5 μM) | 0.66 | 83.28 | 2.68 | 14.40 |
| F: 8q (1 μM) | 0.86 | 65.94 | 5.62 | 29.84 |
Effect of compounds on tubulin polymerization
In general, G2/M cell cycle arrest is strongly associated with inhibition of tubulin polymerization21 and since compounds 8m and 8q caused cell cycle arrest at the G2/M phase, it was considered of interest to investigate their microtubule inhibitory function. Tubulin subunits are known to heterodimerize and self-assemble to form microtubules in a time-dependent manner. The progression of tubulin polymerization22,23 was thus examined by monitoring the increase in fluorescence emission at 420 nm (excitation wavelength is 360 nm) in a 384-well plate for 1 h at 37 °C with and without the conjugates in comparison with the reference compound nocodazole. The test compounds 8m and 8q inhibited tubulin polymerization by 68.21% and 66.53%, respectively, whereas the reference compound (nocodazole) inhibited 66.83% of tubulin polymerization (Fig. 4). This was followed by the evaluation of the IC50 values for these conjugates, and the results are shown in Table 4. It was observed that these conjugates (8m and 8q) showed tubulin assembly inhibition with IC50 values of 1.82 and 1.93 μM, respectively.
Fig. 4. Effect of conjugates on tubulin polymerization: tubulin polymerization was monitored by means of the increasing fluorescence at 360 nm (excitation) and 420 nm (emission) for 1 h at 37 °C.
Table 4. Inhibition of tubulin polymerization (IC50) by compounds 8m and 8q.
| Compound | IC50 a ± SD (in μM) |
| 8m | 1.82 ± 0.06 |
| 8q | 1.93 ± 0.03 |
| Nocodazole | 1.98 ± 0.04 |
aConcentration of drug to inhibit 50% of tubulin assembly.
Immunohistochemistry studies on tubulin
As most antimitotic agents affect microtubules, the alterations in the microtubule network in A549 cells induced by 8m and 8q by using a fluorescence microscope were investigated. The cells were treated with 8m and 8q at 0.5 μM concentration for 48 h. The results demonstrated a well-organized microtubular network in the control cells; however, cells treated with these compounds and nocodozole resulted in disrupted microtubule organization (Fig. 5), thus confirming the inhibition of tubulin polymerization.
Fig. 5. Immunohistochemistry analysis of the microtubule network in A549 cells treated with conjugates 8m and 8q at 0.5 μM concentration for 48 h followed by staining with an antitubulin antibody and an FITC conjugated secondary antibody. A: Control (A549); B: nocodazole (0.5 μM); C: 8m (0.5 μM) and D: 8q (0.5 μM).
Hoechst staining for apoptosis
Apoptosis, with its classic characteristics of chromatin condensation and fragmented nuclei, is one of the major pathways that lead to the process of cell death. The study was performed using the A549 cell line by the Hoechst staining (H33258) method. In the study, A549 cells were treated with the test compounds at 0.5 μM concentration for 48 h. Manual field quantification of apoptotic cells based on cytoplasmic condensation, presence of apoptotic bodies, nuclear fragmentation and relative fluorescence of the test compounds (8m and 8q) revealed that the compounds exerted apoptotic activity, as evident from the increase in the percentage of apoptotic cells (Fig. 6).
Fig. 6. Hoechst staining of the A549 lung cancer cell line. A: Control cells (A549); B: nocodazole (0.5 μM); C: 8m (0.5 μM) and D: 8q (0.5 μM). The arrows indicate apoptotic cells.
Measurement of mitochondrial membrane potential (ΔΨm)
Maintenance of mitochondrial membrane potential (ΔΨm) is considered vital for mitochondrial integrity and bioenergetic function.24 Loss of mitochondrial membrane potential (ΔΨm) is a key event that takes place during drug-induced apoptosis. Mitochondrial injury by compounds 8m and 8q was evaluated by measuring the drop in mitochondrial membrane potential (ΔΨm). In this study, we investigated the involvement of mitochondria in the induction of apoptosis by these compounds. After 48 h of drug treatment with these compounds at 0.5 and 1 μM concentrations, it was observed that there was a considerable reduction in the mitochondrial membrane potential (ΔΨm) of the A549 cells, as assessed by JC-1 staining (Fig. 7).
Fig. 7. Compounds 8m and 8q trigger mitochondrial injury. The drop in membrane potential (ΔΨm) was assessed by JC-1 staining of A549 cells treated with the test compounds, and the samples were then subjected to flow cytometry analysis using a FAC scan (Becton Dickinson) in the FL1 and FL2 channels to determine the mitochondrial potential. A: Control cells (A549); B: nocodazole (1 μM); C: 8m (0.5 μM); D: 8m (1 μM); E: 8q (0.5 μM) and F: 8q (1 μM).
Annexin V-FITC for apoptosis
The apoptotic effect of 8m and 8q was further evaluated by Annexin V FITC/PI dual staining assay25 to examine the occurrence of phosphatidyl-serine externalization and also to understand whether it was due to physiological apoptosis or nonspecific necrosis. In this study, A549 cells were treated with these compounds for 48 h at 0.5 and 1 μM concentrations to examine the apoptotic effect. It was observed that compounds 8m and 8q showed good apoptotic effect against A549 cells, as shown in Fig. 8. Results indicated that compounds 8m and 8q showed 34.27% and 31.85% apoptosis at 0.5 μM concentration, whereas they exhibited 53.51% and 52.03%, respectively, at 1 μM concentration (Fig. 8 and Table 5).
Fig. 8. Annexin V-FITC staining assay. Quadrants: Upper left (necrotic cells), lower left (live cells), lower right (early apoptotic cells) and upper right (late apoptotic cells). A: Control cells (A549); B: nocodazole (1 μM); C: 8m (0.5 μM); D: 8m (1 μM); E: 8q (0.5 μM) and F: 8q (1 μM).
Table 5. Distribution of apoptotic cells in the Annexin-V FITC experiment.
| Sample | Upper left % | Upper right % | Lower left % | Lower right % |
| A: Control(A549) | 1.17 | 3.83 | 93.97 | 1.03 |
| B: Nocodazole (1 μM) | 1.11 | 16.23 | 51.48 | 31.19 |
| C: 8m (0.5 μM) | 0.75 | 11.35 | 64.98 | 22.92 |
| D: 8m (1 μM) | 1.07 | 18.52 | 45.42 | 34.99 |
| E: 8q (0.5 μM) | 1.64 | 9.94 | 66.51 | 21.91 |
| F: 8q (1 μM) | 1.38 | 18.06 | 46.59 | 33.97 |
Molecular modelling study
Molecular docking studies were performed on the two selected compounds 8m and 8q to authenticate the obtained experimental results. These compounds were successfully docked in the colchicine binding site of tubulin (PDB code: ; 3UT5)26 using the Schrödinger docking program.27 The binding mode was predicted and analysed using Glide docking which showed that these compounds bind in the colchicine binding pocket at the interface of the α- and β-chains. For 8m, the N atom in the imidazopyridine ring has shown H-bonding with the Asnα101 (1.96 Å) residue of the α-chain. Further, in the case of 8m the protein ligand complex was stabilized by hydrophobic interactions with other residues, like Alaα180, Valα181, Tyrα224, Metβ259, Leuβ255, Cysβ241, Alaβ250, Ileβ378, Ileβ318, Alaβ354, Alaβ316, Tyrβ202, Valβ238. The N atom in the imidazopyridine ring and the O atom of the carbonyl group of 8q have shown H-bondings with Asnα101 (1.97 Å) and Serα178 (2.62 Å) residues, respectively. Other residues like Valα181, Alaα180, Tyrα224, Alaβ250, Cysβ241, Leuβ255, Alaβ354, Alaβ316, Ileβ318, Ileβ378 and Metβ259 were found to be involved in hydrophobic interactions with 8q. The binding models for compounds 8m and 8q are shown in Fig. 9.
Fig. 9. Binding model of ligands at the interface of the α,β-tubulin heterodimer. Ligands are shown in ball and stick models (yellow colour). Hydrogen bonding interactions are shown as green coloured dashed lines and the residues involved are represented by thin tube models. 9A) Represents the binding mode of 8m; 9B) represents the binding mode of 8q.
Conclusion
In the present study, we synthesized 21 conjugates of imidazopyridine–propenone (8a–8u) and evaluated their antiproliferative activity against four human cancer cell lines, namely, prostate (DU-145), lung (A549), cervical (Hela) and breast (MCF-7) cancer. Among them, compounds 8m and 8q showed significant antiproliferative activity (IC50, 0.86 μM and 0.93 μM, respectively) against the human lung cancer cell line (A549). Flow cytometry analysis revealed that these conjugates caused cell cycle arrest at the G2/M phase. These conjugates (8m and 8q) exerted their cytotoxicity by inhibiting tubulin polymerization, with an IC50 value of 1.82 μM and 1.93 μM, respectively. Further, Hoechst staining, mitochondrial membrane potential and Annexin V FITC assay studies suggested that these compounds induced cell death by apoptosis in human lung cancer cells (A549). The docking studies gave an insight that these compounds bind at the colchicine site of the tubulin. Overall, the encouraging biological profiles exhibited by imidazopyridine–propenone conjugates make them promising tubulin polymerization inhibitors and apoptosis inducers.
Supplementary Material
Acknowledgments
I. B. S. acknowledges CSIR, New Delhi for the award of senior research fellowship. We also acknowledge CSIR, New Delhi, for financial support under the 12th Five Year plan project “Affordable Cancer Therapeutics (ACT)” (CSC0301).
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00043j
References
- Amos L., Walczak C. E. Org. Biomol. Chem. Curr. Opin. Cell Biol. 2004;2000;212:2153. 52. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]
- Sarger P. K., Dobles M., Tournebize R., Hyman A. A. Curr. Opin. Cell Biol. 1997;9:807. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- Honore S., Pasquier E., Braguer D., Amos L. A., Downing K. H., Nogales E. Cell. Mol. Life Sci. Org. Biomol. Chem. Curr. Opin. Struct. Biol. 2005;2004;1998;6228:3039. 2153, 785. doi: 10.1007/s00018-005-5330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Merta P. J., Lin N. H., Tahir S. K., Kovar P., Sham H. L., Zhang H. Mol. Cancer Ther. 2005;4:562. doi: 10.1158/1535-7163.MCT-04-0229. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Cell. 2003;112:467. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Howard J., Hyman A. A. Curr. Opin. Cell Biol. 2007;19:31. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Salmon E. D., Nat. Cell Biol., 2001. , E17 –E21 , , Review. Erratum in: Nat. Cell. Biol. 2001, 3, 530 . [DOI] [PubMed] [Google Scholar]
- Carlson R. O. Expert Opin. Invest. Drugs. 2008;17:707. doi: 10.1517/13543784.17.5.707. [DOI] [PubMed] [Google Scholar]
- Boyer F. D., Dubois J., Thoret S., Dau M. E., Hanna I. Bioorg. Chem. 2010;38:149. doi: 10.1016/j.bioorg.2010.03.003. [DOI] [PubMed] [Google Scholar]
- (a) Semenova M. N., Kiselyov A. S., Tsyganov D. V., Konyushkin L. D., Firgang S. I., Semenov R. V., Malyshev O. R., Raihstat M. M., Fuchs F., Stielow A., Lantow M., Philchenkov A. A., Zavelevich M. P., Zefirov N. S., Kuznetsov S. A., Semenov V. V. J. Med. Chem. 2011;54:7138. doi: 10.1021/jm200737s. [DOI] [PubMed] [Google Scholar]; (b) Flynn B. L., Gill G. S., Grobelny D. W., Chaplin J. H., Paul D., Leske A. F., Lavranos T. C., Chalmers D. K., Charman S. A., Kostewicz E., Shackleford D. M., Morizzi J., Hamel E., Jung M. K., Kremmidiotis G. J. Med. Chem. 2011;54:6014. doi: 10.1021/jm200454y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Song Y., Zhan P., Zhan Q., Liu X. Curr. Pharm. Des. 2013;19(8):1528. [PubMed] [Google Scholar]; (b) Li Z., Zhan P., Liu X. Mini-Rev. Med. Chem. 2011;11(13):1130. doi: 10.2174/138955711797655407. [DOI] [PubMed] [Google Scholar]; (c) Song Y., Zhan P., Liu X. Curr. Pharm. Des. 2013;19(40):7141. doi: 10.2174/13816128113199990505. [DOI] [PubMed] [Google Scholar]
- Dyminska L. Bioorg. Med. Chem. 2015;23:6087. doi: 10.1016/j.bmc.2015.07.045. [DOI] [PubMed] [Google Scholar]
- Bagdi A. K., Santra S., Monir K., Hajra A. Chem. Comm. 2015;51:1555. doi: 10.1039/c4cc08495k. [DOI] [PubMed] [Google Scholar]
- Song Y., Chen W., Kang D., Zhang Q., Zhan P., Liu S. Comb. Chem. High Throughput Screening. 2014;17(6):536. doi: 10.2174/1386207317666140122101631. [DOI] [PubMed] [Google Scholar]
- Kamal A., Kumar G. B., Nayak V. L., Reddy V. S., Shaik A. B., Reddy R. M. K. Med. Chem. Commun. 2015;6:606. [Google Scholar]
- Vilanova C., Oltra S. D., Murga J., Falomir E., Carda M., Horcajo M. R., Diaz J. F., Barasoain I., Marco J. A. J. Med. Chem. 2014;57:10391. doi: 10.1021/jm501112q. [DOI] [PubMed] [Google Scholar]
- Kamal A., Reddy V. S., Shaik A. B., Kumar G. B., Vishnuvardhan M. V. P. S., Polepalli S., Jain N. Org. Biomol. Chem. 2015;13:3416. doi: 10.1039/c4ob02449d. [DOI] [PubMed] [Google Scholar]
- Botta M., Armaroli S., Castagnolo D., Fontana G., Perad P., Bombardelli E. Bioorg. Med. Chem. Lett. 2007;17:1579. doi: 10.1016/j.bmcl.2006.12.101. [DOI] [PubMed] [Google Scholar]
- Chan K. T., Meng F. Y., Li Q., Ho C. Y., Lam T. S., To Y., Lee W. H., Li M., Chu K. H., Toh M. Cancer Lett. 2010;294:24. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Shen J. K., Du H. P., Yang M., Wang Y. G., Jin J. Ann. Hematol. 2009;88:52. doi: 10.1007/s00277-008-0677-3. [DOI] [PubMed] [Google Scholar]
- Kanthou C., Greco O., Stanford A., Cook I., Knight R., Benzakour O., Tozer G. Am. J. Pathol. 2004;165:1401. doi: 10.1016/S0002-9440(10)63398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K., Patel P., Zhang L., Evans H., Westwell A. D., Fischer P. M., Chan S., Martin S. Mol. Cancer Ther. 2008;7:143. doi: 10.1158/1535-7163.MCT-07-0486. [DOI] [PubMed] [Google Scholar]
- Kamal A., Srikanth Y. V. V., Shaik T. B., Khan M. N. A., Ashraf M., Reddy M. K., Kumar K. A., Kalivendi S. V. Med. Chem. Commun. 2011;2:819. [Google Scholar]
- Gonda K., Tsuchiya H., Sakabe T., Akechi Y., Ikeda R., Nishio R., Terabayashi K., Ishii K., Matsumi Y., Ashla A. A., Okamoto H., Takubo K., Matsuoka S., Watanabe Y., Hoshikawa Y., Kurimasa A., Shiota G. Biochem. Biophys. Res. Commun. 2008;370:629. doi: 10.1016/j.bbrc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Zhu H., Zhang J., Xue N., Hu Y., Yang B., He Q. Invest. New Drugs. 2010;28:493. doi: 10.1007/s10637-010-9424-4. [DOI] [PubMed] [Google Scholar]
- Ranaivoson F. M., Gigant B., Berritt S., Joullie M., Knossow M. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2012;68:927. doi: 10.1107/S0907444912017143. [DOI] [PubMed] [Google Scholar]
- Schrodinger Suite (http://www.schrodinger.com).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.