Abstract
Starting from known p38α mitogen-activated protein kinase (MAPK) inhibitors, a series of inhibitors of the c-Jun N-terminal kinase (JNK) 3 was obtained. Altering the substitution pattern of the pyridinylimidazole scaffold proved to be effective in shifting the inhibitory activity from the original target p38α MAPK to the closely related JNK3. In particular, a significant improvement for JNK3 selectivity could be achieved by addressing the hydrophobic region I with a small methyl group. Furthermore, additional structural modifications permitted to explore structure–activity relationships. The most potent inhibitor 4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine showed an IC50 value for the JNK3 in the low triple digit nanomolar range and its binding mode was confirmed by X-ray crystallography.
Introduction
The mitogen-activated protein kinases (MAPKs) represent a family of enzymes involved in several signal transduction pathways, whose activation is part of a phosphorylation cascade triggered by diverse extracellular stimuli. Among the members of this family, the c-Jun N-terminal kinases (JNKs) mostly respond to a variety of stress stimuli such as radiation, osmotic or heat shock, oxidative insult, and proinflammatory cytokines, modulating responses such as cell survival and apoptosis.1 The JNK subfamily is encoded by the three genes jnk1, jnk2, and jnk3, which in turn give rise to 10 different isoforms through alternative splicing.2 Despite their structural homology and the partially functional redundancy, these isoforms follow a different tissue distribution pattern, JNK3 being restricted to the central nervous system, heart, and testis oppositely to the ubiquitous expression of JNK1 and 2.2,3 In addition to this, a different substrate specificity of the JNK1, 2, and 3 suggests the existence of isoform-specific roles of these enzymes, which were partially disclosed through gene knock-out studies.4 There is well-documented evidence for the critical role of the JNK subfamily members in several neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease, as well as in neuronal death derived by stroke and ischemia/reperfusion injury.3−6 Furthermore, some members of the JNKs are also involved in metabolic and inflammatory diseases, and several studies suggest that these kinases might contribute to the development and diffusion of some forms of cancer,7−9 thus emerging as particularly attractive drug targets. Despite the intense endeavor in the research of JNK inhibitors, only a scarce number of candidates have reached clinical trial phases and to date, none of them have been approved.10−12 Until early 2010s, a major challenge in the development of JNK inhibitors has been the achievement of selectivity over the closely related p38α MAPK,11 a member of the same family which, analogously to the JNKs, participates in regulating the cellular response to stress stimuli. This protein kinase was also shown to assume a key function in different inflammatory and neurodegenerative diseases13−15 and the simultaneous inhibition of JNK and p38α MAPK is assumed to obtain a synergistic effect in the treatment of some pathological conditions.16 Nevertheless, obtaining a JNK-selective inhibitor would be beneficial to fully elucidate the effective role of this protein kinase in the aforementioned pathological conditions and thereby assess its therapeutic potential. Furthermore, most of the reported clinical trials on selective p38α MAPK inhibitors have been discontinued because of the insurgence of adverse effects mostly related to liver toxicity,17 leading to the assumption the activity on the p38α MAPK to be undesired for an improved safety profile of JNK inhibitors.
Regarding the selectivity within the JNK subfamily, the achievement of JNK isoform-selective inhibitors would be desirable to dissect the contribution of the different isoforms in various pathological conditions. However, the JNK1, 2, and 3 share more than 80% sequence identity, making the development of isoform-specific inhibitors extremely challenging.
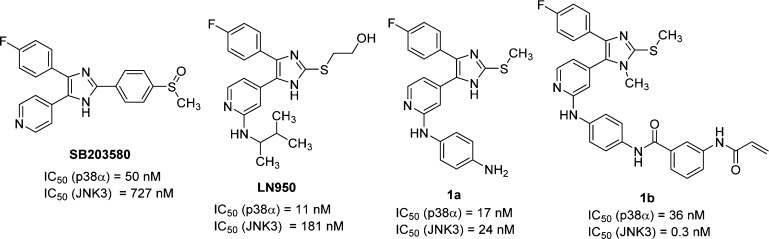
In the last decades, pyridinylimidazoles have encountered a remarkable success in the field of p38α MAPK inhibition. This class of inhibitors counts a large number of examples starting from the precursor SB203580 to the optimized compound LN950 (Figure 1), until reaching derivatives with low single digit nanomolar IC50 values (a review on this class of compounds has recently been published).18 As can be seen from Figure 1, the reported p38α MAPK inhibitors are also able to inhibit the JNK3 with IC50 values in the submicromolar range, thus offering a suitable starting point for optimization when aiming to target this enzyme. In 2016, we published compound 1a as a balanced dual JNK3/p38α MAPK inhibitor, which served as a precursor for the synthesis of a fluorescent probe used in fluorescence polarization-based binding assays.19,20 As it is evident from the biological activity of 1a in comparison to the activity of previous inhibitors, modifying the substitution pattern around the pyridinylimidazole scaffold can contribute to a shift in selectivity toward the JNK3.
Figure 1.
Tri- and tetrasubstituted pyridinylimidazoles. Data are taken from Ansideri et al.19 and Muth et al.21
Some of us have recently reported the optimization of compound 1a following a covalent inhibition approach (compound 1b), which was based on the introduction of an electrophilic moiety able to target a noncatalytic cysteine of the JNK3 that is not conserved in the closely related p38α MAPK.21 The aim of the herein presented work consists instead in the achievement of a potent and selective JNK inhibitor by structural modification of the pyridinylimidazole scaffold following the canonical concept of reversible inhibition.
Results and Discussion
Chemistry
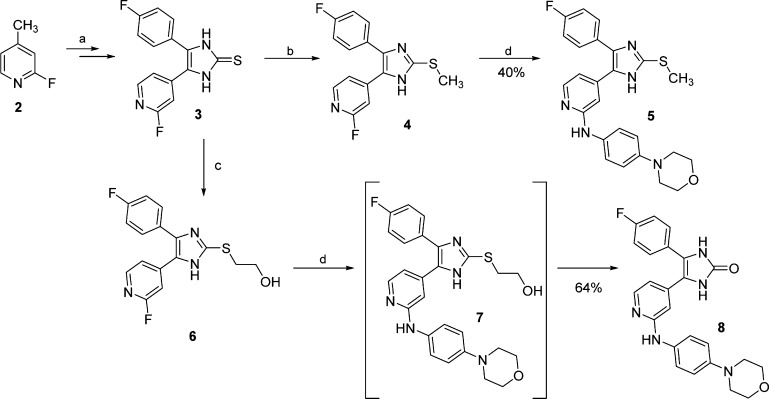
Despite the overall similarity of their structures, the herein reported compounds were synthesized following considerably diverse routes, especially with regard to the construction of the five-membered heterocyclic central core. The synthesis of compounds 5 and 8 was achieved as displayed in Scheme 1. The route leading to the common intermediate 3, starting from 2-fluoro-4-methylpyridine (2), is based on the Marckwald imidazole synthesis22 and was previously reported by Laufer et al.23 The substitution on the imidazole-C2-S position was obtained by reacting imidazole-2-thione 3 with the appropriate alkyl halide. Finally, the introduction of the 4-morpholinoaniline moiety was carried out through nucleophilic aromatic substitution in acidic conditions, this representing the final step for most of the herein presented compounds. Applying these conditions to the hydroxyethyl derivative 6 unexpectedly yielded imidazol-2-one 8, instead of imidazole 7, as a result of a previously described rearrangement.24
Scheme 1. Synthesis of Imidazole 5 and Imidazol-2-one 8.
Reagents and conditions: (a) four-step route reported by Laufer and co-workers;23 (b) MeI, K2CO3, MeOH, rt, 18 h; (c) 2-bromoethyl acetate, t-BuONa, MeOH, 55 °C, 3 h; and (d) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h.
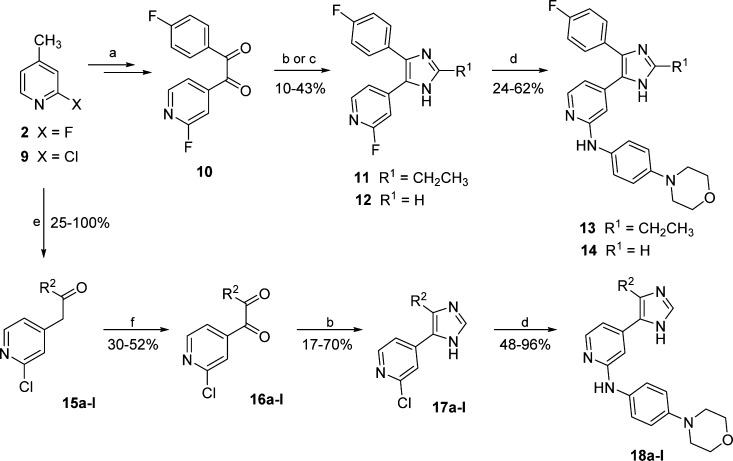
The preparation of 2,4,5-trisubstituted imidazole 13 and of 4,5-disubstituted imidazoles 14 and 18a–l is outlined in Scheme 2. The route providing α-diketone 10 starting from 2-fluoro-4-methylpyridine (2) was recently described by Ansideri et al.,19 whereas the synthesis of intermediates 16a–l was achieved following a similar approach. Ethanones 15a–l were obtained by condensation of the appropriate ethyl ester with 2-chloro-4-methylpyridine (9) and were subsequently oxidized by SeO2 to the corresponding diketones (16a–l). Microwave-assisted cyclization with formaldehyde and NH4OAc in Radzisewski conditions25 then afforded the disubstituted imidazoles 12 and 17a–l, whereas propionaldehyde and methanolic NH3 were employed to obtain the 2-ethylimidazole 11. Finally, introduction of the 4-morpholinoaniline moiety at the pyridine-C2 position, giving the final compounds 13, 14, and 18a–l, was accomplished by the aforementioned nucleophilic aromatic substitution.
Scheme 2. Synthesis of 4,5-Disubstituted Imidazoles 13, 14, and 18a–l.
Reagents and conditions: (a) route reported by Ansideri et al.;19 (b) HCHO(aq), NH4OAc, AcOH, 180 °C microwave irradiation, 2–5 min; (c) propionaldehyde, 7 M NH3 in MeOH, 80 °C, 4 h; (d) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C 16 h; (e) ethyl arylcarboxylate or ethyl alkylcarboxylate, NaHMDS, dry THF, 0 °C 1–5 h; and (f) SeO2, AcOH, 70 °C, 2–3 h; (R2 = see Table 2).
The synthesis of 4,5-disubstituted pyridinylimidazoles 38 and 39, featuring a linear alkyl group at the imidazole-C4 position, required a different strategy than the examples having aromatic or branched aliphatic moieties (14 and 18a–l). This was mainly due to the fact that alkyl esters of linear alkanoic acids did not undergo condensation with 2-chloro-4-methylpyridine (9) to give the desired ethanone intermediates.
An alternative approach to compounds 38 and 39 could also be employed for the synthesis of the 2,4(2,5)-disubstituted imidazole 43 as well as for the 2,4,5-trisubstituted imidazoles 44 and 45 (Scheme 3). This route started from the commercially available 1-(2-chloropyridin-4-yl)ethan-1-one (21) or from the acylpyridines 22 and 23, which were synthesized by Grignard reaction of the appropriate alkylmagnesium bromide with Weinreb amide 20.
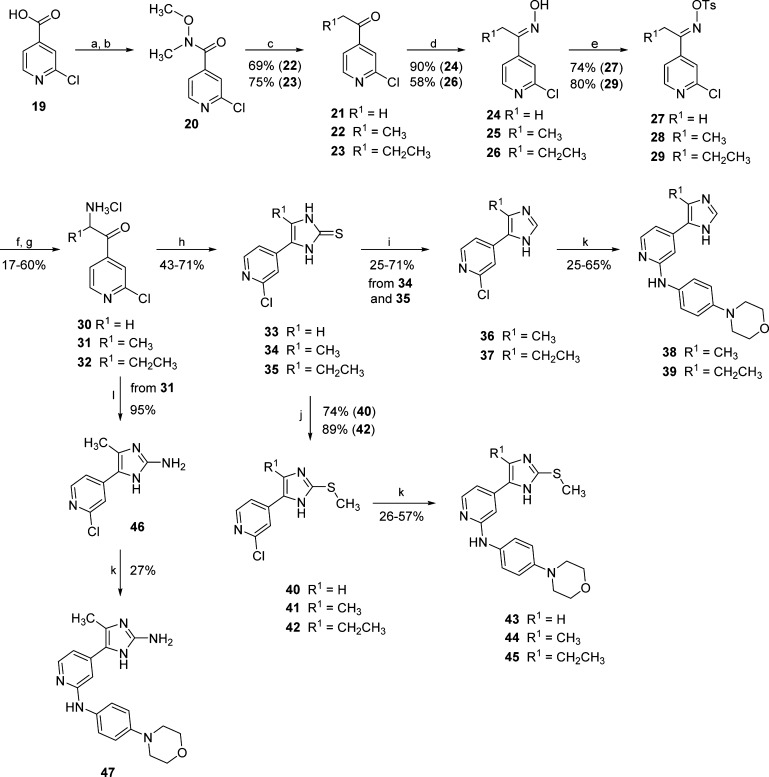
Scheme 3. Synthesis of Imidazoles 38, 39, 43–45, and 47.
Reagents and conditions: (a) SOCl2, reflux temperature, 5 h; (b) N,O-dimethylhydroxylamine hydrochloride, Et3N, dry DCM, 16 h; (c) EtMgBr or n-PrMgBr, dry THF, −10 °C, 1–3 h; (d) NH2OH·HCl, 20% NaOH(aq), MeOH, H2O, 0 °C, 1–2 h; (e) TsCl, pyridine, rt, 24–72 h; (f) EtOHabs, K, 0 °C, 2–16 h; (g) concd HCl, 50 °C, 1–4 h; (h) KSCN, MeOH, reflux temperature, 4 h; (i) H2O2, AcOH, rt, 15 min; (j) MeI, t-BuONa, MeOH, 50 °C, 0.5–3 h; (k) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h; and (l) cyanamide, EtOH, reflux temperature, 2 h.
Formation of the corresponding oximes 24–26 and following tosylation of the hydroxyl groups led to intermediates 27–29. Tosylated oximes 27–29 were then first converted into the α-aminoketones 30–32 through Neber rearrangement26 and subsequently cyclized by KSCN, yielding imidazole-2-thione derivatives 33–35. From these intermediates, it was possible to achieve the disubstituted imidazoles 36 and 37 by oxidative desulfurization27 as well as the 2-methylsulfanylimidazoles 40–42 via monomethylation. Alternatively, compound 46 displaying a 2-aminoimidazole core could be prepared by cyclization of the α-aminoketone 31 with cyanamide. Intermediates 36, 37, 40–42, and 47 were then reacted with 4-morpholinoaniline, as previously mentioned, to afford the final compounds 38, 39, 43–45, and 47, respectively.
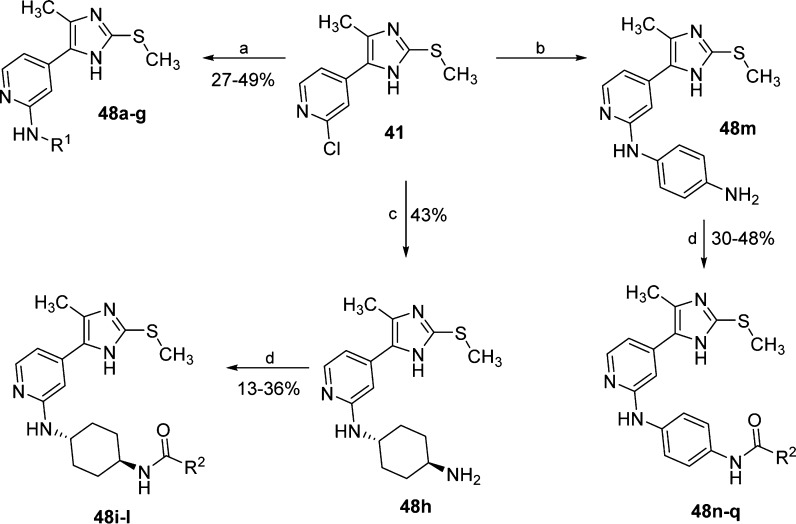
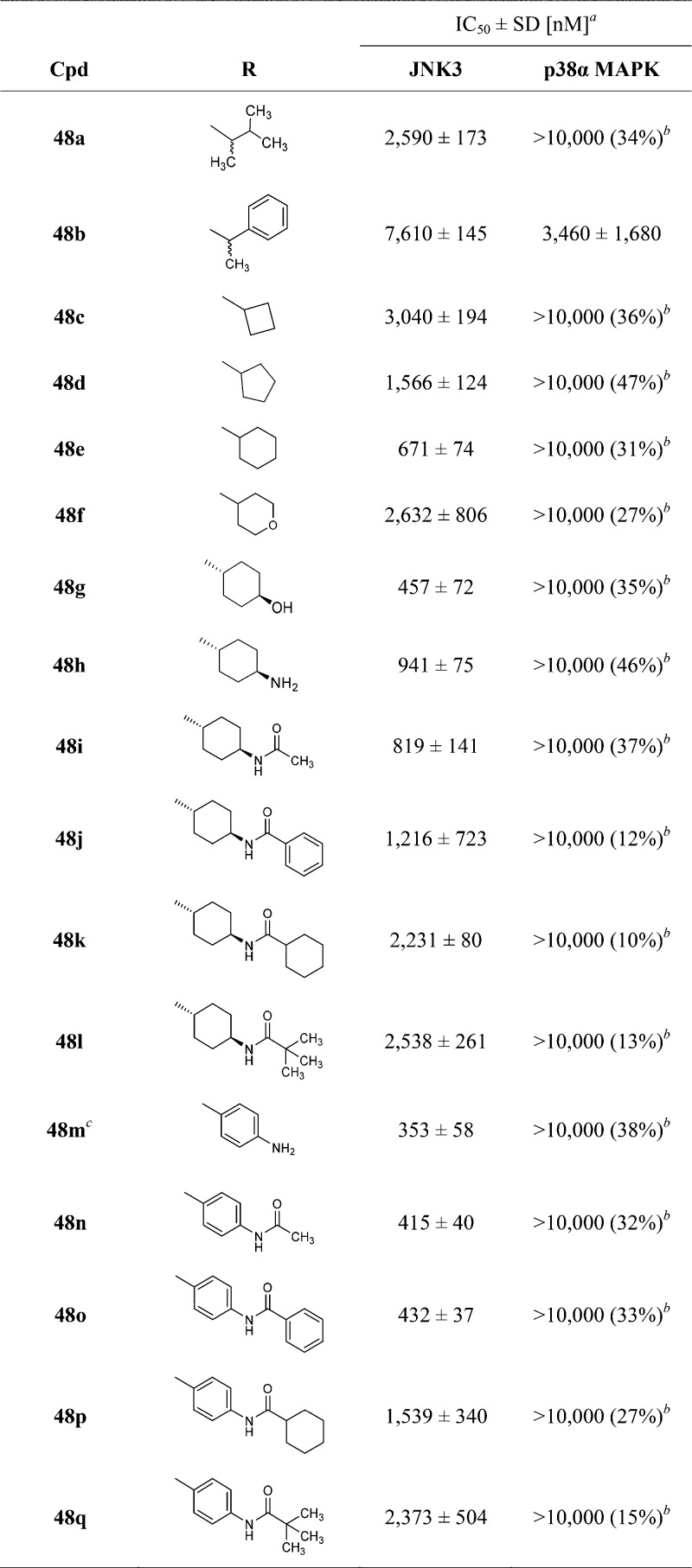
Several analogues of compound 44 featuring a different substituent at the pyridine-C2 position (compounds 48a–h and 48m, Scheme 4) could be prepared by nucleophilic aromatic substitution of synthone 41 with p-phenylendiamine, 1-phenylethanamine, or with diverse branched or cycloalkyl amines. In addition, compound 48h and the previously reported 48m(21) were coupled with different acid chlorides or anhydrides to obtain the corresponding amides 48i–l and 48n–q (Scheme 4).
Scheme 4. Synthesis of 4(5)-Methyl-2-methylsulfanyl-5-(4)pyridin-4-ylimidazoles 48a–q.
Reagents and conditions: (a) cycloalkylamine (NEAT or n-BuOH), 180 °C, 24–72 h; (b) p-phenylendiamine, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h; (c) trans-diaminocyclohexane, n-BuOH, 180 °C, 72 h; and (d) acyl chloride or anhydride, dry pyridine, rt, 16 h; (R1, R2 = see Table 6).
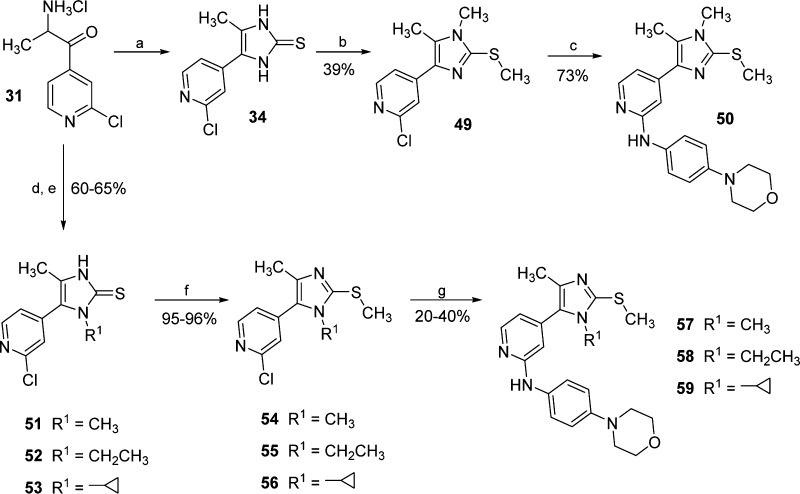
The introduction of a methyl substituent on the imidazole-N atom, providing 1,2,4,5-tetrasubstituted imidazoles 50 and 57, required a distinct approach depending on the desired N-methylated regioisomer. In fact, double nucleophilic substitution of imidazole-2-thione 34 using excess of methyl iodide almost exclusively afforded the regioisomer bearing the substituent on the N atom away from the pyridine ring (49, Scheme 5). The regioselectivity of the methylation reaction was confirmed by crystal structure analysis of intermediate 49 (see Figure S1 in the Supporting Information) and was attributed to the lower steric hindrance offered by the methyl group compared to the pyridine ring. The regioisomer 54, having the methyl group on the N atom adjacent to the pyridine ring, was instead achieved by cyclizing the α-aminoketone 31 with methyl isothiocyanate, followed by methylation of the sulfur of the resulting N1-methylimidazole-2-thione 51.
Scheme 5. Synthesis of Tetrasubstituted Imidazoles 50 and 57–59.
Reagents and conditions: (a) KSCN, MeOH, reflux temperature, 4 h; (b) MeI, t-BuONa, MeOH, 80 °C, 3 h; (c) 4-morpholinoaniline, Pd2(dba)3, Xantphos, Cs2CO3, dry 1,4-dioxane, 100 °C, 18 h; (d) alkyl isothiocyanate, Et3N, 60 °C, 16 h; (e) AcOH, 80 °C, 1 h; (f) MeI, t-BuONa, MeOH, 50 °C, 30 min; and (g) 4-morpholinoaniline, Pd2(dba)3, XPhos, Cs2CO3, dry 1,4-dioxane, 100 °C, 16 h.
This approach, adapting a procedure published by Xi et al.,27 represents an unusual route to tetrasubstituted pyridinylimidazoles and was recently reported by some of us for the preparation of tetrasubstituted imidazoles bearing two aromatic moieties at the 4 and 5 positions.28 The same method could also be employed, using the appropriate alkyl isothiocyanate, to achieve the N-ethyl- and the N-cyclopropyl-imidazole derivatives 55 and 56, respectively. Unlike the majority of the reported compounds, the introduction of the 4-morpholinoaniline moiety, yielding compounds 50 and 57–59, was carried out by palladium-catalyzed Buchwald–Hartwig aryl amination.
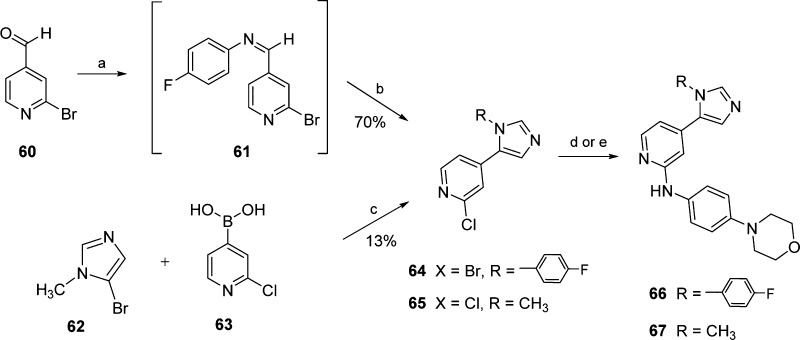
The synthesis of the 1,5-disubstituted imidazole 66, bearing an aromatic substituent on the imidazole-N1 atom, was performed starting from 2-bromoisonicotinaldehyde (60) via a two-step procedure as depicted in Scheme 6. Such a route entails the formation of the imine derivative 61 and its direct cyclization through the Van Leusen reaction29 using toluene sulfonylmethylisocyanide (TOSMIC) and K2CO3. The analogous route was unfortunately not accessible for the synthesis of the N1-methyl substituted derivative 67 because of the instability of the corresponding imine intermediate. As an alternative, the preformed N1-methyl imidazole group was introduced through Suzuki cross-coupling reaction30 between 5-bromo-1-methyl-1H-imidazole (62) and pyridinyl-boronic acid 63 (Scheme 6). The last step of both routes consisted of the introduction of the 4-morpholinoaniline moiety. In the case of the 4-fluorophenyl derivative 64, this was performed by Buchwald–Hartwig amination giving compound 66, whereas the acid-catalyzed nucleophilic aromatic substitution was employed for the synthesis of compound 67.
Scheme 6. Synthesis of 1,5-Disubstituted Imidazoles 66 and 67.
Reagents and conditions: (a) 4-fluoroaniline, AcOH, EtOH, reflux temperature, 2 h; (b) TOSMIC, K2CO3, MeOH/dimethoxyethane 2:1, reflux temperature, 3 h; (c) Pd(PPh3)4, Cs2CO3, H2O, DMF, 60 °C, 24 h; and (d) 4-morpholinoaniline, t-BuONa, Pd2(dba)3, BINAP, toluene, 80 °C, 3 h; (e) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h.
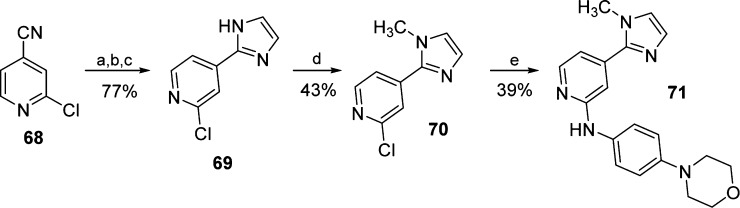
The 1,2-disubstituted imidazole derivative 71 was obtained starting from 2-chloroisonicotinonitrile (68), which was initially reacted in a one-pot procedure described by Voss et al.31 (Scheme 7). This reaction involves the formation of an imidate, followed by substitution with acetal-protected aminoacetaldehyde and final ring closure by deprotection, affording 2-(pyridine-4-yl)imidazole 69 in good yield. At last, N-methylimidazole 70 was obtained by nucleophilic substitution with methyl iodide and subsequently reacted with 4-morpholinoaniline as previously discussed, yielding compound 71.
Scheme 7. Synthesis of Imidazol-2-yl Pyridine Derivative 71.
Reagents and conditions: (a) 30% NaOMe in MeOH, MeOH, 40 °C, 1 h; (b) aminoacetaldehyde dimethylacetal, AcOH, MeOH, reflux temperature, 30 min; (c) 6 M HCl, reflux temperature, 18 h; (d) MeI, NaH, dry DMF, rt, 2 h; and (e) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h.
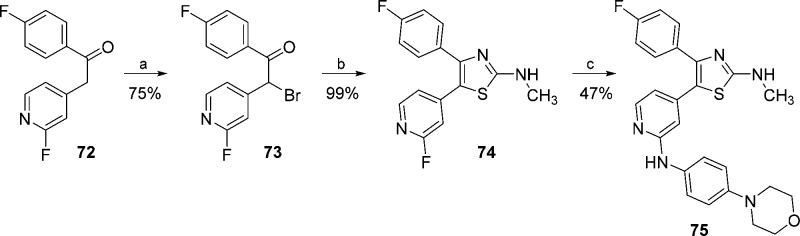
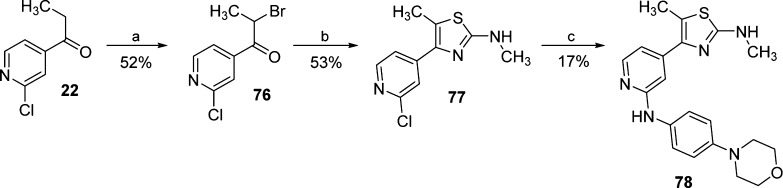
For the synthesis of compounds 75 and 78, presenting a methylaminothiazole central core, an approach related to Hantzsch thiazole synthesis32 was employed (Schemes 8 and 9). Thiazole 75 was obtained starting from 1-(4-fluorophenyl)-2-(2-fluoropyridin-4-yl)ethan-1-one (72),23 whereas compound 78 was synthesized starting from 1-pyridinyl-propan-1-one (22). Both ketones 72 and 22 were monohalogenated at the α-position under acidic conditions and then cyclized via N-methylthiourea, affording intermediates 74 and 77, respectively. Conclusively, substitution with 4-morpholinoaniline yielded the desired compounds 75 and 78.
Scheme 8. Synthesis of 2-Methylaminothiazole 75.
Reagents and conditions: (a) Br2, 30% HBr in AcOH, 75 °C, 2 h; (b) N-methylthiourea, EtOH, reflux temperature, 1 h; and (c) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h.
Scheme 9. Synthesis of 2-Methylaminothiazole 78.
Reagents and conditions: (a) Br2, HBr 30% in AcOH, 75 °C, 4 h; (b) N-methylthiourea, EtOH, reflux temperature, 1 h; and (c) 4-morpholinoaniline, 1.25 M HCl in EtOH, n-BuOH, 180 °C, 16 h.
Biological Evaluation
All synthesized inhibitors were evaluated by enzyme-linked immunosorbent assays33,34 to determine their ability to inhibit JNK3 and p38α MAPK, and the results are presented in Tables 1–4 and 6.
Table 1. Core Modifications on 4-F-Phenyl-Substituted Derivativesa.
Data of standard inhibitors SB203580 (p38α MAPK) and SP600125 (JNK3) in our in-house activity assay are included.
IC50 values are the mean of three experiments.
n = 16.
n = 20.
Table 4. Effect of Small Alkyl Substituents in the HR I.
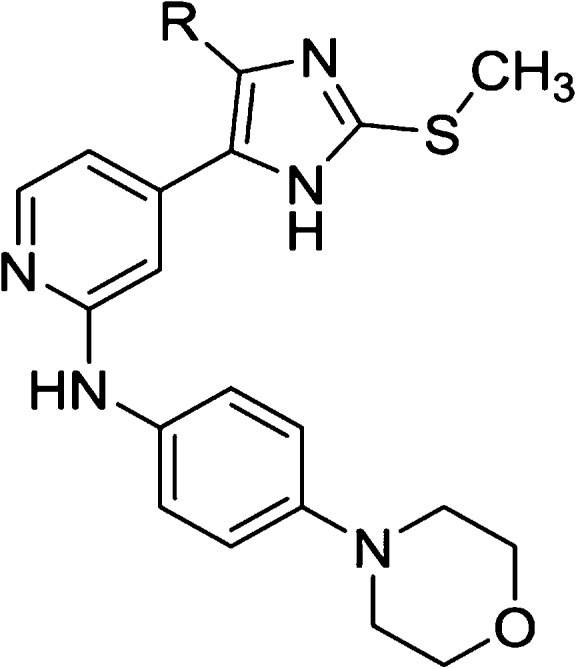
IC50 values are the mean of three experiments.
Percent inhibition at indicated concentration.
Table 6. Influence of Substituents at the Pyridine-C2 Position.
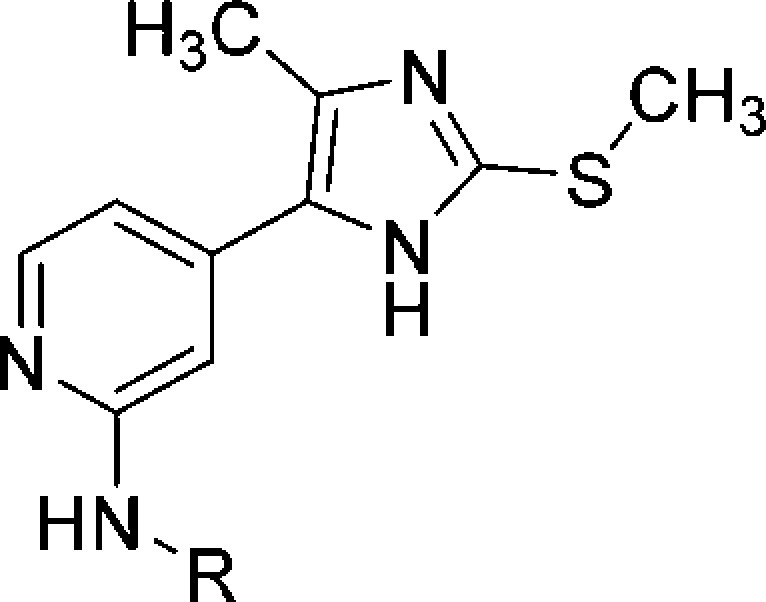
IC50 values are the mean of three experiments.
Percent inhibition at indicated concentration.
According to the ZINC patterns tool, compound 48m represents a potential pan-assay interference compound. However, this compound was synthesized as the intermediate for the preparation of inhibitors 48n–q. To estimate the impact of the amide moiety present in compounds 48n–q on the inhibition of the two kinases, the activities of 48m are listed in this table.
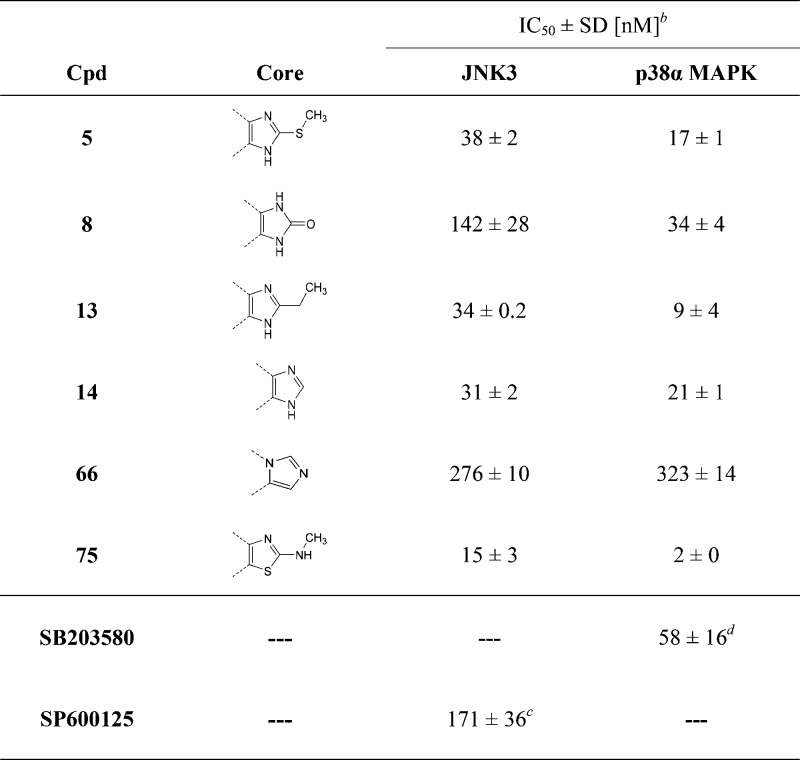
The free terminal aniline moiety of compound 1a is considered to be potentially responsible for aggregation and therefore might result in assay interference, as also pointed out by analysis through the ZINC 15 pattern tool.35 For this reason, the p-phenylendiamine moiety at the pyridine-C2 position of compound 1a was modified in a 4-morpholinoaniline group, which has already been reported as a beneficial substituent in this position.36 Resulting compound 5 (Table 1) displayed extremely close inhibition values to its analogoue 1a (1a, IC50(JNK3) = 24 nM; IC50(p38α MAPK) = 17 nM) and this moiety was, therefore, maintained constant during the investigation of other positions of the scaffold.
The first attempt, which was carried out to shift the preference of compound 5 toward the JNK3, consisted of modifying the central imidazole core together with acting on the substitution at the imidazole-C2 position (Table 1). Transformation of the methylsulfanyl group at the imidazole-C2 position into an ethyl group or removal of the same group, resulting in compounds 13 and 14, respectively, did not seem to affect the inhibitory activity on the two enzymes. Replacement of the imidazole core with an imidazol-2-one ring instead caused a decrease in the JNK3 inhibitory activity while leaving the inhibition of p38α MAPK unchanged (8: IC50(JNK3) = 142 nM; IC50(p38α MAPK) = 34 nM). The position of the two nitrogen atoms at the central imidazole core seems to be essential for the inhibition of both enzymes, as the different arrangements of substituents around the five-membered ring of 1,5-disubstituted imidazole 66 resulted in a drop in activity on both target kinases. On the other hand, exchange of 2-sulfanylimidazole with 2-methylaminothiazole (75) yielded an increase in inhibitory activity of 2.5- and 8-fold for JNK3 and p38α MAPK, respectively.
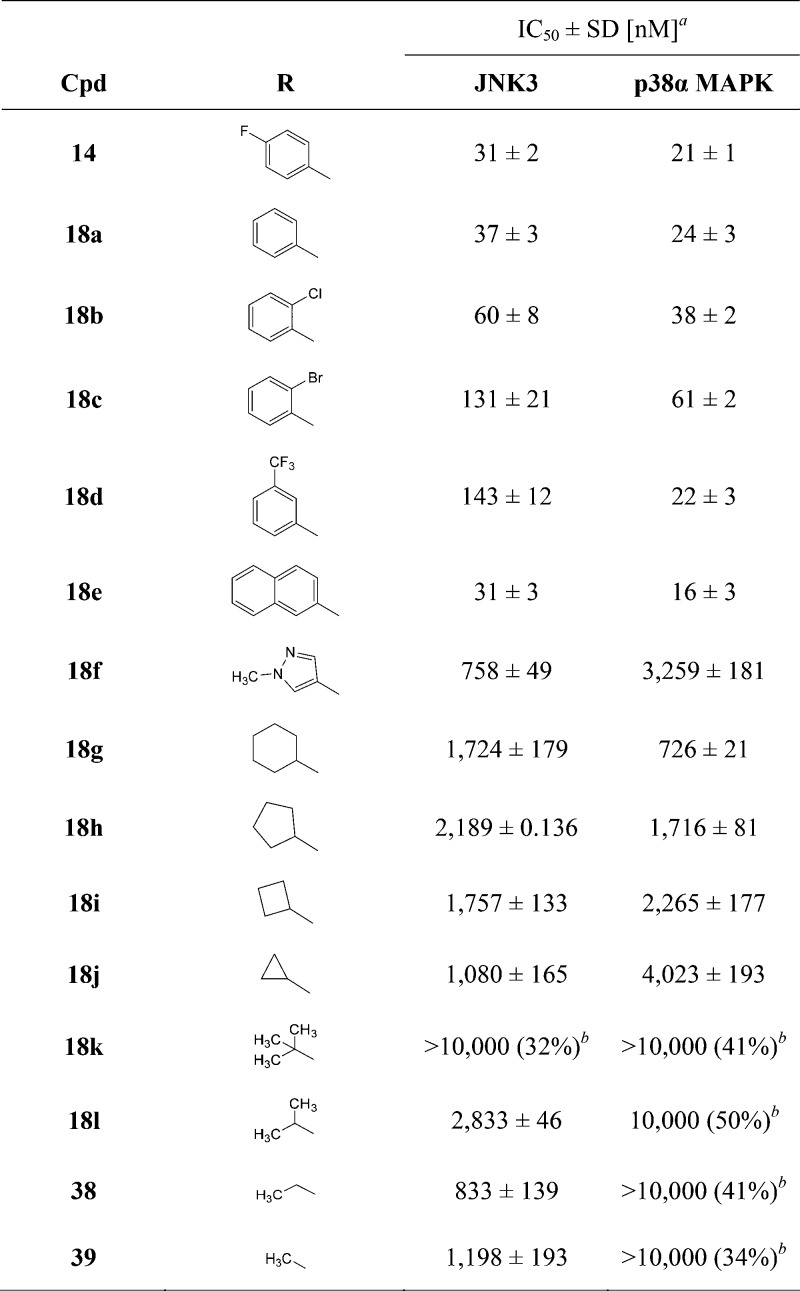
To assess the effect of the substituent located in the hydrophobic region (HR) I, the 4-fluorophenyl group was replaced by different aromatic, alkyl, and cycloalkyl moieties (Table 2). In terms of both ligand efficiency (LE) as well as lipophilic LE (LLE), the 4,5-disubstituted derivative 14 is the most efficient one out of the series of Table 1 and serves, therefore, as the optimal starting point for these modifications. Moreover, this scaffold presents a substantially equal activity compared to its S-methylated analogue 5, along with a convenient synthetic strategy, facilitating the preparation of a broad range of derivatives.
Table 2. Effect of Different Aryl and Alkyl Substituents at the Imidazole C4(5) Position.
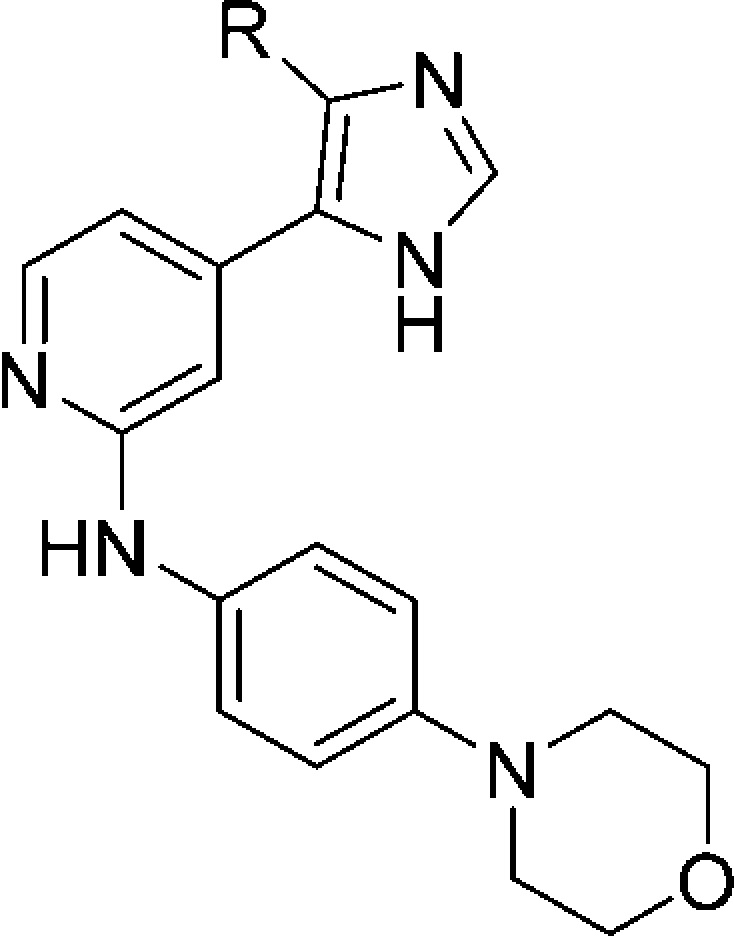
IC50 values are the mean of three experiments.
Percent inhibition at indicated concentration.
Most of the 4,5-disubstituted pyridinylimidazoles having an aromatic moiety at the imidazole-C4 position (compounds 18a–f) revealed to be potent inhibitors for both enzymes, displaying IC50 values down to the low double digit nanomolar range. In general, addressing the HR I with a phenyl or monosubstituted phenyl ring resulted in dual inhibitors displaying a slight preference toward p38α MAPK over JNK3. This trend is most distinct in the case of compound 18d having a 3-(trifluoromethyl)phenyl moiety, which presents a 6-fold higher activity for p38α MAPK than for JNK3. The only aromatic substituent stepping out of this trend was the heteroaromatic N-methylpyrazole of compound 18f, producing an overall decrease in activity on both kinases while conserving a moderate preference toward JNK3 (18f: IC50(JNK3) = 758 nM; IC50(p38α MAPK) = 3259 nM). These findings indicate that substitution on the phenyl ring is not beneficial when pursuing selectivity on JNK3 and instead seems to be counterproductive, increasing the activity on p38α MAPK. The reason behind this lack of selectivity can be intuitively explained by considering the dimensions of the hydrophobic pocket known as HR I in the two target kinases. This cleft is wider in the p38α MAPK than in JNK3 mostly because of a difference in the “gatekeeper” residue (Thr106 in p38α MAPK vs Met146 in JNK3). However, as already mentioned in some cocrystallization studies,37 aromatic moieties can induce a shift of the flexible side chain of Met146 (JNK3), thus essentially abolishing the size differences between the two pockets. As a proof of that, even the bulky 2-naphthyl group of compound 18e seems to be accommodated in the “reshaped” hydrophobic pocket of JNK3, therefore resulting in a high inhibitory potency. Moreover, attempts of substituting the ortho and meta positions of the phenyl ring, seeking for additional interactions, did not succeed and produced negative outcomes instead (compounds 18b–d).
The replacement of the aromatic ring at the imidazole-C4 position by cycloalkyl moieties resulted in a dramatic decrease in activity for both enzymes, with IC50 values in the low micromolar range. The only exception was the cyclohexyl derivative 18g that was able to interact with p38α MAPK with an IC50 value of 726 nM, 2-fold more potent than on JNK3. The inhibitory effect of compounds 18g–j on p38α MAPK, decreasing alongside the reduction of the ring size, is symptomatic of a gradually diminished capability of the cyclic group to occupy the spacious cavity of the enzyme. On the other side, JNK3 activity of derivatives 18g–i, bearing a four- to six-membered ring at the imidazole-C4 position, remained substantially constant, although significantly decreased compared to the parent compound 14. An analogous scenario occurred in the case of compounds featuring branched aliphatic groups at the same position. The isopropyl derivative 18l, analogously to the closely related 18j, showed a significant drop in activity on p38α MAPK, while conserving an IC50 value on JNK3 in the low micromolar range. On the other hand, introduction of a tert-butyl moiety (18k) resulted in a complete loss of activity on both JNK3 and p38α MAPK. Because of their flexibility and low electron density, cyclic and branched aliphatic groups are presumably unable to promote the Met146 shift and therefore cannot fit in the narrow hydrophobic back pocket of JNK3. A reasonable consequence of this would therefore consist of the flip of the imidazole ring, directing the branched or cyclic alkyl moieties away from the hydrophobic back pocket of the JNK3, thus explaining the similarity of the inhibitory activity regardless of the substituent size.
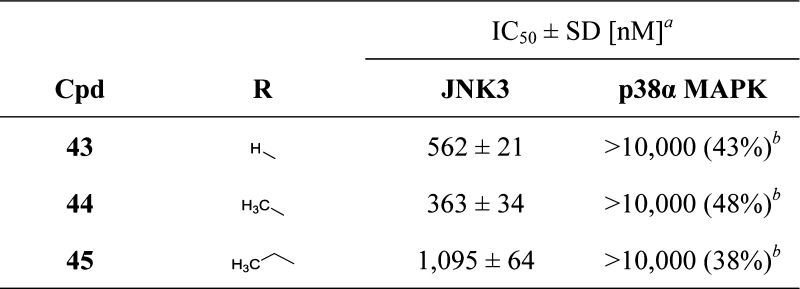
In agreement with the trend of the series, methyl- and ethyl-substituted imidazoles 38 and 39, respectively, displayed no inhibition of the p38α MAPK (IC50 > 10 μM), however, preserving activity on the JNK3. In particular, the methyl derivative 38 represented the sole compound of this series reaching a submicromolar activity on JNK3 without any remarkable effect on the p38α MAPK. Moreover, this inhibitor also represents the most efficient selective inhibitor of this series in terms of LE and LLE and was therefore chosen as the starting point for further investigations.
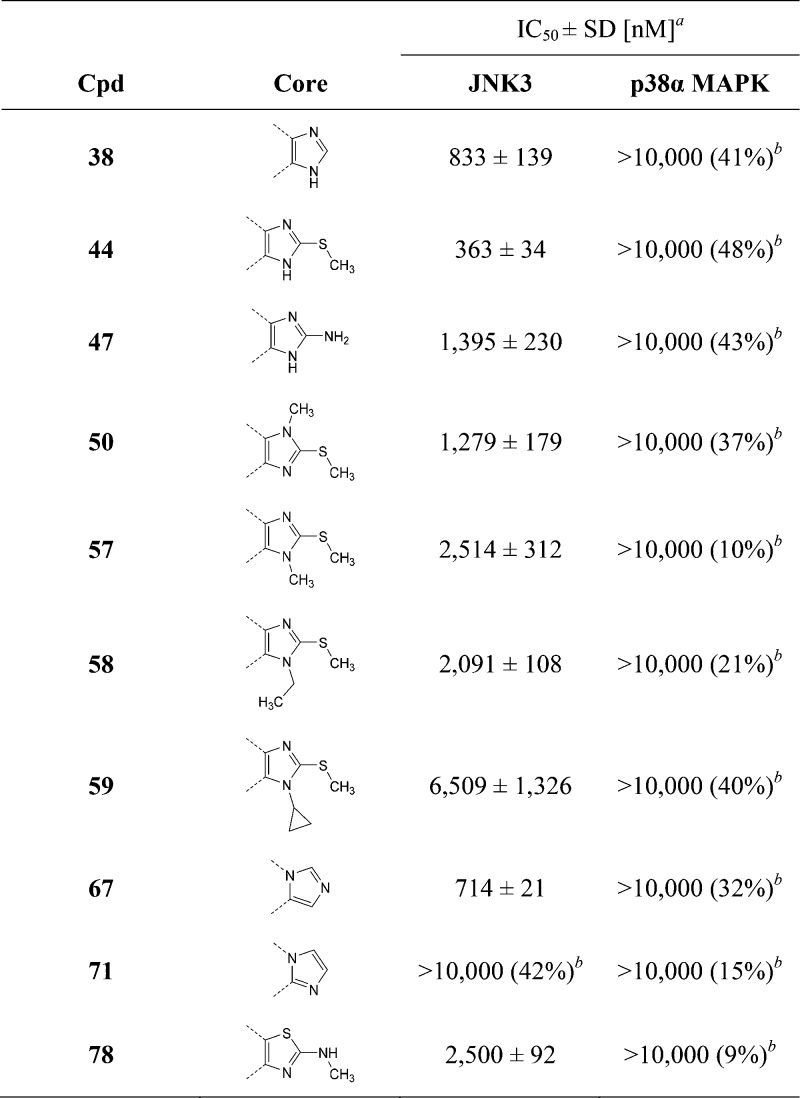
Once the methyl substituent at the imidazole-C4 position was selected, our attention was refocused on the central core (Table 3). Because of the presence of the methyl substituent, all derivatives presented in this series lost their potency on the p38α MAPK, with each one displaying an IC50 value higher or equal 10 μM. Altering the arrangement of the substituents around the imidazole ring proved beneficial in the case of the 1,5-disubstituted imidazole 67, slightly increasing its potency compared to the precursor 38, whereas it was deleterious for the 1,2-disubstituted derivative 71. Replacement of the imidazole core with a 2-aminomethyl thiazole (78) also revealed to be detrimental for the inhibitory activity. A different approach consisted of the introduction of an additional substituent on the imidazole-N atom, together with a reintroduction of the S-methyl group at the C2 position, yielding the tetrasubstituted imidazole scaffold already reported in potent dual JNK3/p38α MAPK as well as JNK3 selective inhibitors.16,21 In the case of p38α MAPK, the effect of an additional alkyl substituent on the imidazole ring has been reported to be strictly dependent on the position of the substituted N atom. Several examples have demonstrated alkylation of the imidazole-N atom away from the pyridine ring to cause a severe reduction of the activity because of the impossibility to establish a hydrogen bond with the Lys53 of the p38α MAPK.38,39 Because the same interaction has shown to also occur in the binding to JNK3 (Lys93), an analogous effect was expected on this enzyme as well and was confirmed by the remarkably reduced JNK3 inhibition by compound 50, carrying a methyl group on the distal imidazole N atom. On the other hand, because several tetrasubstituted JNK3/p38α MAPK inhibitors have been reported with an alkyl substituent on the imidazole N adjacent to the pyridine ring, we assumed this modification to be suitable with our 4-methyl substituted scaffold as well. However, derivatives 57 and 58, featuring a methyl and an ethyl substituent on the N atom proximal to pyridine, respectively, unexpectedly presented an even lower potency on JNK3 than the supposedly “wrong” regioisomer 50. The drop in activity appeared to increase with the size of the alkyl substituent, as N-cyclopropyl substituted 59 was almost 3-fold less active compared to its N-methyl analogue 57. This outcome suggests that despite not hampering the formation of a H bond with the Lys93, alkyl substituents at the imidazole N atom proximal to pyridine reduce the tightness of the binding to the JNK3 active site.
Table 3. Modification of the Core on Methyl-Substituted Derivatives.
IC50 values are the mean of three experiments.
Percent inhibition at indicated concentration.
To complete this series, starting from 4,5-disubstituted imidazole 38, the original S-methyl group or a free amino substituent was introduced at the imidazole-C2 position, affording compounds 44 and 47, respectively. Although the 2-amino imidazole derivative showed a drop in activity compared to the parent compound 38, reintroduction of the S-methyl group at the imidazole-C2 position surprisingly produced a 2-fold increase in the inhibitory potency on JNK3 (44: IC50(JNK3) = 363 nM; IC50(p38α MAPK) > 10 μM). This outcome prompted us to reconsider our previous assumption regarding the role of the 2-methylsulfanyl moiety. Although the S-methyl group exerts no influence on the inhibitory activity when the 4-fluorophenyl moiety is installed at the imidazole-C4 position, it has a significant impact in the case of 4-methyl imidazole derivatives.
In a closer evaluation concerning the influence of the alkyl chain in position 4 of the imidazole core combined with the 2-methylsulfanyl moiety in C2 position, the methyl group (44) emerged once more as the substituent presenting the optimal length to target the JNK3 HR I, when compared to the 4-unsubstituted and to the 4-ethyl derivatives 43 and 45, respectively (Table 4). Comparison of imidazoles 5 (Table 1) and 44 (Table 4) reveals the replacement of the 4-fluorophenyl ring at the imidazole-C4(5) position by a smaller methyl group to result in a 1 order of magnitude loss in JNK3 inhibition and in a complete loss of p38α MAPK inhibitory activity.
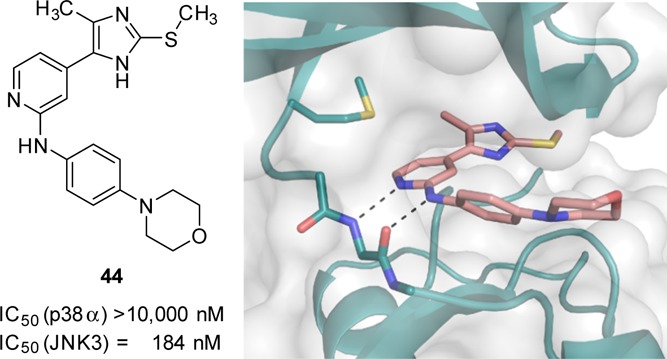
To elucidate the binding mode of the 4-methyl-substituted-5-(pyridine-4-yl)imidazole derivatives, as well as to gain insight into the role of the 2-methylsulfanyl group, crystal structures of JNK3 in complex with compounds 38 and 44 were determined (Figure 2).
Figure 2.
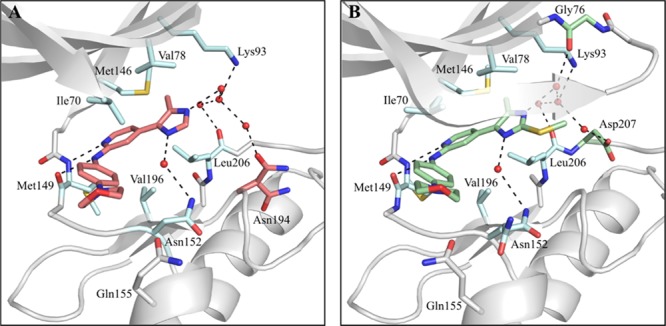
Crystal structures of JNK3 in complex with inhibitors 38 (A) and 44 (B) featuring a pyridinylimidazole scaffold. Only the JNK3 active site is shown. The protein backbone is displayed in gray. The compounds, the side chain of gatekeeper Met146, and a part of the Gly-rich loop are highlighted in stick display. Active site residues with common orientations and interactions are shown in light blue, whereas residues that differ between both complexes are highlighted in the same color as the respective inhibitor. Side chains for which multiple orientations are observed (Asn194 in complex with 38 and Asn152 in complex with 44) are shown in both orientations. Water molecules are represented as red spheres and hydrogen bonds are shown as black dashed lines.
The structures revealed a similar binding mode of the inhibitors within the adenosine 5′-triphosphate (ATP) pocket of JNK3 (Figure 2). As expected, both scaffolds interacted with the hinge region of the kinase via two hydrogen bonds involving the main chain carbonyl and backbone amine groups of Met149 and mimicking the interactions of the enzyme with ATP40 as well as with its nonhydrolyzable analogue β,γ-methyleneadenosine-5′-triphosphate (AMP–PCP, Figure S3, Supporting Information). In both structures, the imidazole-N atom distal from the pyridine ring is part of a network of water-mediated hydrogen bonds, involving the side chain of Lys93 and the main chain of Leu206. Further water-mediated hydrogen bonds in the JNK3-38 crystal structure (Figure 2A) include the side chain of Asn194, whereas in the JNK3-44 structure (Figure 2B), the backbone of Gly76 and the side chain of Asp207 are involved. The structure of JNK3 in complex with inhibitor 38 also showed that the imidazole-N atom proximal to the pyridine ring participates in a water-mediated hydrogen bond with the Asn152 side chain and the same interaction seems to be present in the JNK3-44, thus explaining the detrimental effect produced by the substitution of this position (compounds 57–59). Multiple hydrophobic interactions comprising the gatekeeper Met146 and the side chains of Ile70, Val78, Val196, and Leu206 were also observed. These interactions have been previously described by Scapin et al.37 and confer JNK3 selectivity as they cannot be formed in the larger binding pocket of p38α MAPK. The methyl group present in both inhibitors was oriented toward the HR I, which resulted in an identical orientation of the side chain of the gatekeeper residue Met146. The 4-morpholinoaniline moiety, which occupied the solvent-exposed HR II, exhibited higher flexibility and no direct interactions with JNK3, that is, this moiety likely contributes barely or not at all to the binding.
A major structural difference between the two complex structures was observed for the Gly-rich loop. In the JNK3-38 complex structure, no electron density for residues Gly71–Gly76 was visible because of high local flexibility, a phenomenon also encountered in other JNK3 crystal structures.41−43 In the JNK3-44 complex, however, the electron density for this loop was clearly defined, hinting to a structural stabilization of this region upon interaction with the 2-methylsulfanyl moiety in compound 44.
A superposition of our inhibitor complex structures with crystal structures of JNK3 bound to AMP–PCP and the dual JNK3/p38α MAPK inhibitor by Scapin et al.37 (PDB code: 1PMN) yielded insights into the structural basis for the observed selectivity of compounds 38 and 44 (Figure 3).
Figure 3.
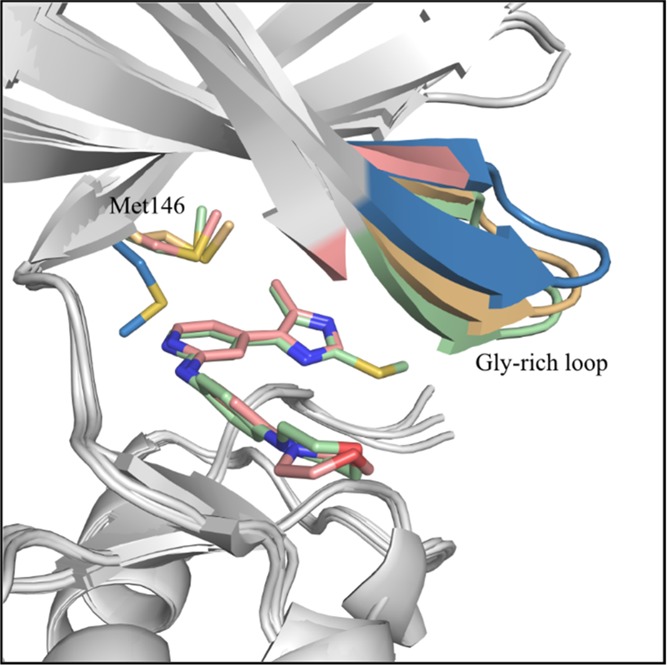
Comparison of the gatekeeper Met146 orientation and the Gly-rich loop positioning upon JNK3 inhibitor binding with other ligand-bound JNK3 structures. Overlay of the JNK3-44 complex structure (light green), the JNK3-38 complex structure (light red), the AMP–PCP-bound JNK3 structure (light orange), and the 1PMN structure reported by Scapin, et al.37 (blue). The superposition was performed using the “align” function in PyMOL. The side chains of the gatekeeper Met146 and the Gly-rich loop are highlighted. Only compounds 38 and 44 are shown for the sake of clarity.
As can be seen from this structural comparison, no movement of the gatekeeper Met146 side chain is induced by compounds 38 and 44 when compared to the AMP–PCP complex, contrary to the dual kinase inhibitor studied by Scapin et al. In the latter crystal structure, an induced fit of side chain 146 occurred to accommodate the dichlorophenyl moiety of the dual kinase inhibitor. Conversely, it appears that the methyl substituent of compounds 38 and 44 was unable to occupy the wider HR I of the p38α MAPK, while possessing the optimal length to target the respective region of JNK3. Therefore, this moiety determined the selectivity achieved over p38α MAPK, demonstrated by the activities of compounds listed in Tables 2−4. In the case of AMP–PCP and compound 44, another result of the interaction is a downward positioning of the flexible Gly-rich loop. A similar compression of the binding pocket caused by a repositioning of the Gly-rich loop was reported for a JNK3 complex crystal structure by Kamenecka et al.44 and might be a result of hydrophobic interactions and water-mediated hydrogen bonds provided by inhibitor 44, which stabilized this otherwise flexible section. Overall, as a result of inhibitor binding, the JNK3 ATP binding pocket in our crystal structures appears somewhat narrower in comparison to the p38α MAPK binding site (where the gatekeeper is Thr106), an effect that is less prominent for the dual kinase inhibitor (Figure 3) and probably responsible for the selectivity of compounds 38 and 44. With respect to the 2-fold increase in the inhibitory potency on JNK3 of compound 44 over its analogue 38, the influence of the S-methyl group on the positioning of the Gly-rich loop is the most likely structural reason for the significant gain in affinity.
An additional characterization of the two compounds 38 and 44 included the determination of the protein melting temperature (Tm) in the presence and absence of inhibitors by nano differential scanning fluorimetry (nanoDSF). This methodology consists of assessing the influence of the binding event on the stability of the target protein and is carried out by monitoring temperature-dependent changes in the intrinsic protein fluorescence as a consequence of unfolding. The corresponding curves (Figure S2, Supporting Information) exhibited a significant increase in stability of JNK3 upon inhibitor binding, as can be seen from the associated Tm values (Table 5). The Tm value of JNK3 alone was determined to be 46.3 °C and increased to 53.8 and 54.8 °C in the presence of compounds 38 and 44, respectively, which correlates well with the results concerning the inhibitory activity and stability of the Gly-rich loop.
Table 5. Determined Melting Temperatures (Tm) for JNK3 Alone and in Complex with Inhibitors 38 and 44.
| sample | Tm (°C)a |
|---|---|
| JNK3 | 46.28 ± 0.58 |
| JNK3-38 | 53.87 ± 0.04 |
| JNK3-44 | 54.83 ± 0.04 |
Data represent mean value ± SD of a single experiment performed in triplicate. nanoDSF measurements (Figure S2, Supporting Information) were conducted using Prometheus NT.48 (NanoTemper Technologies, Munich).
A further approach in the pursuit of a tighter binding with the JNK3 consisted of modifying the amino moiety at the pyridine-C2 position (Table 6).
An initial attempt was carried out by introducing α-methyl(phenyl)alkylamino moieties (compounds 48a–b) as well as cycloalkylamino groups (compounds 48c–e). The former moieties have been reported in potent p38α MAPK inhibitors, for example, LN950 (Figure 1) and ML3403,45 and were thus introduced to evaluate their effect on JNK3 inhibitory potency. In detail, these substituents were hypothesized to yield an increase in the JNK3 inhibitory activity while conserving selectivity over the p38α MAPK because of the combination with the 4-methyl substituent on the imidazole ring. However, the 3-methyl-2-butylamino group (48a) resulted in a loss of activity compared to the 4-morpholinoaniline precursor 44, although maintaining some selectivity over the p38α MAPK. Substitution with the α-methylbenzylamine, giving rise to compound 48b, was instead counterproductive as it not only caused a tremendous drop in JNK3 inhibition but also a recovery of the activity on the p38α MAPK (48b: IC50(JNK3) = 7610 nM; IC50(p38α MAPK) = 3460 nM). On the other hand, although not reaching the potency of the parent compound 44, the JNK3 inhibitory activity of compounds bearing cycloalkylamino moieties at the pyridine-C2 position (48c–e) increased alongside the size of the aliphatic ring, a trend suggesting the importance of hydrophobic interactions in this area of the molecule. Nevertheless, replacement of the cyclohexyl ring of 48e with the similar tetrahydropyranyl group (48f) yielded, unexpectedly, a remarkable loss of activity on JNK3.
A possible strategy to gain activity and selectivity on JNK3 would consist of targeting the side chain of Gln155 as this residue is replaced by a shorter Asn in the p38α MAPK.46 As suggested by the structure of the JNK3–44 complex (Figure 2), this amino acidic residue is located about 4 Å away from the 4-morpholinoaniline-N atom but cannot be reached because of the rigidity of this substituent. Moreover, the 4-morpholinoanilino moiety is only able to accept a hydrogen bond, whereas the Gln residue has the potential to act as both acceptor and donor of hydrogen bonds. For this reason, trans-4-aminocyclohexanol and trans-1,4-diaminocyclohexyl moieties were selected for compounds 48g and 48h, respectively, because of a higher flexibility and their additional capability to donate hydrogen bond interactions. In particular, the former moiety is also present in the structure of clinical candidate CC-930, wherein it is reported to interact with the aforementioned Gln155,47 and included in potent p38α MAPK inhibitors.45 Unfortunately, despite preserving the selectivity over the p38α MAPK, none of the two inhibitors 48g and 48h succeeded in overcoming the activity of the parent compound 44 on the JNK3, with the latter displaying a 3-fold drop in potency. This observation suggests an inability of the introduced moiety to form the desired interaction with the Gln155 side chain or this interaction being compensated by other factors. Additionally, it underlines the necessity of the aromatic moiety at the pyridine-C2 amino function for the binding to the JNK3. The significantly lower activity of compound 48h could also derive from the not tolerated protonation of its terminal amino functionality. With the aim to reach the Gln155 side chain by the introduction of an additional hydrogen bond acceptor, a series of amides of compound 48h and of its aromatic counterpart 48m was synthesized. This approach also permits to seek additional interactions with the enzyme HR II. Unfortunately, in neither of the two series, the introduction of amide moieties permitted to gain an inhibitory activity comparable with the precursor 44. In the series featuring a cycloaliphatic amine (48i–l), only the small acetamide derivative 48i exhibited an almost similar activity to the precursor, whereas bulkier alkyl and aromatic residues displayed a 2- to 3-fold decrease in potency. In an analogous fashion, when considering the series derived from the aromatic intermediate 48m, compounds bearing a tert-butyl or a cyclohexyl amide (48p and 48q, respectively) showed a significant drop in inhibitory activity, with IC50 values in the micromolar range. On the other hand, both inhibitors carrying an acetamido or benzamido moiety (48n and 48o, respectively) were still able to inhibit the JNK3 with a potency akin to the free amine derivative 48m. The comparison of the two amide series also supports the theory of a higher suitability of aromatic substituents at the pyridine-C2 amino position when targeting the JNK3.
Compound 44 resulted as the best inhibitor of the synthesized series and was, therefore, further investigated to achieve a comprehensive characterization. At a first instance, to evaluate the intra-JNK selectivity, compound 44 was tested on the three JNK isoforms (Table 7). As expected, compound 44 inhibited the three isoforms with a similar potency but showed a moderate preference for JNK1 and JNK3 over JNK2.
Table 7. Inhibition Data of Compound 44 on the Three JNK Isoforms.
| IC50 [nM]a | ||
|---|---|---|
| JNK1 | JNK2 | JNK3 |
| 119 | 468 | 184 |
Compound 44 was tested by Reaction Biology corporation (Malvern, PA, USA) using a radiometric assay.
Moreover, inhibitor 44 was further screened against a panel of 45 diverse kinases to achieve a preliminary evaluation of its selectivity within the kinome. Out of the kinase panel, 10 kinases (including JNK1) were inhibited more than 50% at a testing concentration of 10 μM (Table S3, Supporting Information).
Additional studies were aimed at evaluating the inhibition of the human-ether-à-go-go related gene (hERG) potassium channels as well as liver cytochromes P450 (CYP450) to highlight potential liabilities of the synthesized scaffold. As displayed in Table 8, compound 44 showed a reduced interference with the hERG channels (IC50 > 10 μM).
Table 8. Inhibitory Activity of Compound 44 on hERG Channels and on the Most Relevant CYP Isoforms.
| CYP450 inhibition [% inhibition at 10 μM] | |||||
|---|---|---|---|---|---|
| hERG inhibition [% inhibition at 10 μM] | 1A9 | 2C9 | 2C19 | 2D6 | 3A4 |
| 38.8 | 51.5 | 53.9 | 35.6 | 19.0 | 75.1 |
Regarding interaction with hepatic enzymes, compound 44 displayed low to moderate inhibition of four of the five tested isoenzymes, this representing a significantly cleaner profile in comparison with previously reported inhibitors of this class.48 However, the elevated blockage of the most abundant CYP450 isoform 3A4 still constitutes a serious limit, which needs to be solved by subsequent optimization strategies.
Finally, additional tests were performed to assess the metabolic stability of methyl-substituted pyridinylimidazole 44 upon incubation with human liver microsomes. One of the most serious limitations of previously reported 2-alkylsulfanylimidazoles is their severe metabolization consisting of oxidation of the thioether function to the corresponding sulfoxide.48 Nevertheless, in vitro assays performed on compound 44 demonstrated a substantial metabolic stability, as approximately 80% of the unmodified compound was still present after 4 h incubation (Figure S6, Supporting Information). The major metabolite formed still appears to be represented by the sulfoxide derivative (8.49%), although modifications at the 4-morpholinoaniline substituent might also be present.
Conclusions
Optimization of 4-(4-fluorophenyl)-5-(pyridin-4-yl)imidazole-based p38α MAPK inhibitors by modification of the five-membered heterocyclic core, the aryl moiety at the imidazole-C4 position, and the pyridine-C2 amino function resulted in a novel series of JNK3 inhibitors exhibiting high selectivity over the closely related p38α MAPK. Biological evaluation of the different pyridinyl-substituted five-membered rings provided valuable insights into the structure activity relationship of this scaffold with respect to JNK3 and p38α MAPK inhibitory potencies. By addressing the HR I with a small methyl group, a significant selectivity toward JNK3 was achieved. This feature is not yet reported for this class of compounds, which have been generally described as p38α MAPK inhibitors. The binding mode at the ATP binding site of the enzyme for this class of compounds was confirmed by X-ray structures of JNK3 crystals incubated with imidazoles 38 and 44. The most potent inhibitor 4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (44) inhibits the JNK3 in the low triple digit nanomolar range, is metabolically stable, and displays a slight selectivity over the JNK2 isoform. Further characterization of this inhibitor highlighted reduced interactions with the hERG channel as well with most of the tested CYP450 isoforms.
Experimental Section
Chemistry
General
All chemicals were purchased from commercial sources unless otherwise specified and used without further purification. Thin-layer chromatography (TLC) reaction controls were performed for all reactions using fluorescent silica gel 60 F254 plates (Merck) and visualized under natural light and UV illumination at 254 and 366 nm. The purities of all tested compounds were confirmed to be >95% as determined by reverse-phase high-performance liquid chromatography (HPLC) using one of the two following methods. In the case of method 1, the instrument used was a Hewlett Packard HP 1090 Series II LC equipped with a UV diode array detector (DAD) (detection at 230 and 254 nm). The chromatographic separation was performed on a Phenomenex Luna 5u C8 column (150 mm × 4.6 mm, 5 μm) at 35 °C oven temperature. The injection volume was 5 μL and the flow was 1.5 mL/min using the following gradient: 0.01 M KH2PO4, pH 2.3 (solvent A), MeOH (solvent B), 40% B to 85% B in 8 min; 85% B for 5 min; 85% to 40% B in 1 min; 40% B for 2 min; stop time 16 min. In the case of method 2, an Agilent 1100 Series HPLC system was used, equipped with a UV DAD (detection at 218, 254, and 280 nm). The chromatographic separation was performed on an XBridge C18 column (150 mm × 4.6 mm, 5 μm) and the oven temperature was set to 30 °C. The injection volume was 10 μL and the flow was 1.5 mL/min using the following gradient: 0.01 M KH2PO4, pH 2.3 (solvent A), MeOH (solvent B), 45% B to 85% B in 9 min; 85% B for 6 min; stop time 16 min. Flash column chromatography was performed using an Interchim puriFlash 430 automated flash chromatography system with Davisil LC60A 20–45 μm silica from Grace Davison and Geduran Si60 63–200 μm silica from Merck for the precolumn. Nuclear magnetic resonance (NMR) data were obtained on a Bruker ARX NMR spectrometer at 250 MHz, on a Bruker AVANCE III HD NMR spectrometer at 300 MHz, or on a Bruker AVANCE NMR spectrometer at 400 MHz at ambient temperature. Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane. All spectra were calibrated against the (residual proton) peak of the deuterated solvent used. Mass spectra were recorded on an Advion expression S electrospray ionization mass spectrometer (ESI-MS) with TLC interface.
Experimental Procedures
General Procedure for the Nucleophilic Aromatic Substitution with 4-Morpholinoaniline (General Procedure A)
In a pressure vial, the 2-halide pyridine intermediate (1 equiv) and 4-morpholinoaniline (1.5 equiv) were suspended in n-butanol (3 mL) and 1.25 M HCl in EtOH (1 equiv) was added. After tightly closing the vial, the reaction mixture was heated in a heating block at 180 °C and stirred for 18 h. After removing the solvent at reduced pressure, the residue was purified by flash column chromatography.
General Procedure for the Synthesis of Compounds 15a–l (General Procedure B)
In a three-neck round-bottom flask under anhydrous conditions, 2-chloro-4-methylpyridine (9) (1 equiv) and the appropriate ethyl ester (1 equiv) were dissolved in dry tetrahydrofuran (THF) (2 mL). After cooling the reaction mixture to 0 °C, 2 M sodium bis(trimethylsilyl)amide (NaHDMS) in dry THF (2.2 equiv) was added dropwise and the mixture was stirred at 0 °C for 1.5–5 h. After adding H2O, the aqueous phase was extracted three times with dichloromethane (DCM) or EtOAc and washed with NaCl saturated solution. The combined organic layers were dried over anhydrous Na2SO4 and the solvent was evaporated at reduced pressure. The residue was finally purified by flash column chromatography.
General Procedure for the Synthesis of Compounds 16a–l (General Procedure C)
Ethan-1-one intermediates 15a–l (1 equiv) and SeO2 (1.1 equiv) were suspended in 5–10 mL of glacial AcOH and the reaction mixture was stirred at 65 °C for 2–3 h. After cooling to room temperature (rt), the formed solid residue of Se was removed by filtration and the filtrate was diluted with EtOAc and then washed with saturated NaHCO3 solution four times. Finally, the organic phase was washed with saturated NaCl solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. The residue was purified by flash column chromatography.
General Procedure for the Synthesis of Compounds 17a–l (General Procedure D)
In a pressure vial, ethane-1,2-dione derivatives 16a–l (1 equiv) and NH4OAc (10 equiv) were suspended in 3 mL of glacial AcOH and after that a 37% aqueous solution of formaldehyde (1 equiv) was added. The reaction vessel was heated in a CEM microwave reactor at 180 °C, with an initial power of 200 W, for 2–5 min. The mixture was added dropwise to NH4OH concentrated solution at 0 °C. The suspension obtained was extracted three times with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was purified by flash column chromatography.
General Procedure for the Synthesis of Compounds 48a–h (General Procedure E)
In a pressure vial, 2-chloro-4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (41) was suspended in ≈2 mL of cycloalkylamine (in the case of solid amine, 20 equiv of amine was added and the mixture was suspended in ≈2 mL of n-butanol). The closed vial was then heated at 180 °C and stirred for 48–120 h. The reaction mixture was poured in H2O and the aqueous layer was extracted three times with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was finally purified by flash column chromatography.
General Procedure for the Synthesis of Compounds 48i–l and 48n–q (General Procedure F)
Under an argon atmosphere, trans-N1-(4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)cyclohexane-1,4-diamine (48h) or N1-(4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)benzene-1,4-diamine (48m) was dissolved in 1.5 mL of dry pyridine and after that the appropriate acid chloride or anhydride was added and the reaction mixture was stirred at rt for 16 h. The reaction mixture was poured in H2O and the aqueous layer was extracted three times with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was finally purified by flash column chromatography.
2-Fluoro-4-(4-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-5-yl)pyridine (4)23
The title compound was synthesized as described in the literature23 and analytical data were in agreement with the reported ones.
4-(4-(4-Fluorophenyl)-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (5)
The title compound was synthesized according to general procedure A starting from compound 4 (100 mg, 0.33 mmol) and 4-morpholinoaniline (88.1 mg, 0.49 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 9:1) afforded 61 mg of the desired compound (40% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.61 (s, 3H), 3.00 (br s, 4H), 3.73 (br s, 4H), 6.53–6.75 and 6.88–7.00 (m, 2H), 6.82 (d, J = 7.6 Hz, 2H), 7.09–7.42 (m, 4H), 7.43–7.61 (m, 2H), 7.82–8.12 (m, 1H), 8.55–8.84 (m, 1H), 12.65 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 15.0, 15.1, 49.4, 66.2, 106.1, 106.4, 111.4, 111.7, 115.2 (d, J = 21.2 Hz), 115.7, 115.9, 119.9, 120.3, 126.2, 126.9, 129.5 (d, J = 8.1 Hz), 130.7 (d, J = 8.0 Hz), 133.7, 134.2, 134.8, 137.9, 138.7, 141.9, 142.7, 145.6, 145.9, 147.3, 148.1, 156.7, 161.9 ppm (d, J = 244.4 Hz); MS–FAB m/z: [M] calcd for C25H24FN5OS, 461.2; found, 461.3; HPLC (method 1): tR = 5.326 min (100%).
2-((4-(4-Fluorophenyl)-5-(2-fluoropyridin-4-yl)-1H-imidazol-2-yl)thio)ethan-1-ol (6)49
The title compound was synthesized as described in the literature49 and analytical data were in agreement with the reported ones.
4-(4-Fluorophenyl)-5-(2-((4-morpholinophenyl)amino)pyridin-4-yl)-1,3-dihydro-2H-imidazol-2-one (8)
The title compound was prepared according to general procedure A starting from 6 (300 mg, 0.90 mmol) and 4-morpholinoaniline (240.6 mg, 1.35 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 85:15) afforded 200 mg of the desired compound (64% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.81–3.11 (m, 4H), 3.58–3.85 (m, 4H), 6.42–6.65 (m, 2H), 6.80 (d, J = 6.6 Hz, 2H), 7.12–7.36 (m, 4H), 7.36–7.59 (m, 2H), 7.99 (dd, J = 4.7, 2.4 Hz, 1H), 8.65 (br s, 1H), 10.64 (br s, 1H), 10.72 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.3, 66.2, 105.2, 111.9, 115.6, 115.8, 115.9 (d, J = 19.0 Hz), 119.7, 120.5, 126.2 (d, J = 2.9 Hz), 129.9 (d, J = 8.0 Hz), 133.6, 138.2, 145.9, 147.9, 153.9, 156.6, 161.7 ppm (d, J = 245.9 Hz); MS–FAB m/z: [M + H]+ calcd for C24H22FN5O2, 431.18; found, 431.30; HPLC (method 1): tR = 4.552 min (96.7%).
1-(4-Fluorophenyl)-2-(2-fluoropyridin-4-yl)ethane-1,2-dione (10)
The title compound was synthesized according to the literature and the analytical data were in agreement with the reported ones.50
4-(2-Ethyl-4-(4-fluorophenyl)-1H-imidazol-5-yl)-2-fluoropyridine (11)
To a solution of 10 (250 mg, 1.01 mmol) in MeOH (5 mL), 7 M ammonia in MeOH (2.89 mL, 20.23 mmol) and propionaldehyde (88.11 mg, 1.52 mmol) were added and the reaction mixture was heated to reflux temperature and stirred for 4 h. After cooling down, the solvent was evaporated at reduced pressure and the residue was purified by flash column chromatography (SiO2, DCM/EtOH 97:03 to 94:06), obtaining 125 mg of the desired product (43% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.28 (t, J = 7.6 Hz, 3H), 2.64–2.77 (m, 2H), 7.09 (s, 1H), 7.17–7.40 (m, 3H), 7.47–7.56 (m, 2H), 8.06 (d, J = 5.4 Hz, 1H), 12.41 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C16H13F2N3, 286.1; found, 286.0; m/z: [M – H]− calcd for C16H13F2N3, 284.1; found, 284.0; HPLC (method 2): tR = 3.680 min.
4-(2-Ethyl-4-(4-fluorophenyl)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (13)
The title compound was synthesized according to general procedure A starting from 4-(2-ethyl-4-(4-fluorophenyl)-1H-imidazol-5-yl)-2-fluoropyridine (11) (85 mg, 0.30 mmol) and 4-morpholinoaniline (80.2 mg, 0.45 mmol). The crude residue was purified twice by flash column chromatography (SiO2, DCM/EtOH 96:04 to 94:06) and (RP-C18, iso-propanol/H2O 1:1), obtaining 32 mg of the desired compound (24% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.27 (t, J = 7.6 Hz, 3H), 2.69 (q, J = 7.6 Hz, 2H) 2.96–3.06 (m, 4H), 3.70–3.79 (m, 4H), 6.63–6.92 (m, 3H), 6.97 (br s, 1H), 7.12–7.57 (m, 6H), 7.86–8.08 (m, 1H), 8.60–8.79 (m, 1H), 12.20 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 12.7, 21.2, 49.4, 66.2, 106.2, 115.9, 119.9, 120.2, 127.6, 129.6, 130.6, 134.4, 143.5, 149.6, 156.7, 162.6 ppm; MS-ESI m/z: [M + H]+ calcd for C26H26FN5O, 444.2; found, 444.2; m/z: [M – H]− calcd for C26H26FN5O, 442.2; found, 442.2; HPLC (method 2): tR = 4.960 min (98.6%).
4-(4-(4-Fluorophenyl)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (14)
The title compound was synthesized according to general procedure A starting from 2-fluoro-4-(4-(4-fluorophenyl)-1H-imidazol-5-yl)pyridine (12)19 (70 mg, 0.27 mmol) and 4-morpholinoaniline (71.3 mg, 0.40 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10) afforded 70 mg of the desired compound (62% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.93–3.07 (m, 4H), 3.66–3.79 (m, 4H), 6.63–7.00 (m, 4H), 7.12–7.41 (m, 4H), 7.42–7.60 (m, 2H), 7.81 (s, 1H), 7.89–8.13 (m, 1H), 8.57–8.80 (m, 1H), 12.53–12.78 ppm (m, 1H); MS-ESI m/z: [M + H]+ calcd for C24H22FN5O, 415.18; found, 416.2; m/z: [M – H]− calcd for C24H22FN5O, 414.2; found, 414.2; HPLC (method 2): tR = 3.692 min (97.9%).
N-(4-Morpholinophenyl)-4-(4-phenyl-1H-imidazol-5-yl)pyridin-2-amine (18a)
The title compound was synthesized according to general procedure A starting from compound 17a (100 mg, 0.39 mmol) (for the synthesis of 17a see Supporting Information) and 4-morpholinoaniline (103.4 mg, 0.58 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 138 mg of the desired compound (89% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.92–3.08 (m, 4H), 3.66–3.80 (m, 4H), 6.68 (dd, J = 5.3, 0.9 Hz, 1H), 6.82 (d, J = 9.0 Hz, 2H), 6.93 (br s, 1H), 7.30–7.53 (m, 7H), 7.82 (s, 1H), 7.97 (d, J = 5.3 Hz, 1H), 8.70 (s, H), 12.66 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.4, 66.1, 106.5, 111.8, 115.9, 120.1, 127.6, 128.1, 128.6, 132.0, 134.0, 136.0, 142.4, 145.7, 147.2, 156.6 ppm; MS-ESI m/z: [M + H]+ calcd for C24H23N5O, 398.2; found, 398.2; m/z: [M – H]− calcd for C24H23N5O, 396.2; found, 396.3; HPLC (method 1): tR = 3.513 min (99.1%).
4-(4-(2-Chlorophenyl)-1H-imidazole-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18b)
The title compound was synthesized according to general procedure A starting from compound 17b (100 mg, 0.34 mmol) (for the synthesis of 17b see Supporting Information) and 4-morpholinoaniline (90.9 mg, 0.51 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 127 mg of the desired compound (87% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.85–3.17 (m, 4H), 3.59–3.89 (m, 4H), 6.51 (d, J = 4.8 Hz, 1H), 6.81 (d, J = 8.5 Hz, 2H), 6.92 (br s, 1H), 7.28 (d, J = 7.5 Hz, 2H), 7.37–7.67 (m, 4H), 7.79–8.01 (m, 2H), 8.60 (s, 1H), 12.63 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.4, 66.2, 104.8, 110.4, 115.9, 120.1, 127.4, 129.8, 130.5, 130.6, 132.5, 133.3, 134.0, 136.0, 136.0, 142.8, 145.7, 146.1, 147.4, 156.7 ppm; MS-ESI m/z: [M + H]+ calcd for C24H22ClN5O, 432.1; found, 432.1; m/z: [M – H]− calcd for C24H22ClN5O, 430.15; found, 429.8; HPLC (method 2): tR = 3.671 min (99.4%).
4-(4-(2-Bromophenyl)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18c)
The title compound was synthesized according to general procedure A starting from compound 17c (100 mg, 0.30 mmol) (for the synthesis of 17c see Supporting Information) and 4-morpholinoaniline (80.2 mg, 0.45 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 100 mg of the desired compound (71% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.91–3.15 (m, 4H), 3.62–3.95 (m, 4H), 6.51 (d, J = 4.2 Hz, 1H), 6.82 (m, J = 8.0 Hz, 3H), 7.09–7.60 (m, 5H), 7.67–8.01 (m, 3H), 8.63–8.97 (m, 1H), 12.66 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.3, 66.1, 104.7, 110.3, 115.9, 120.4, 124.0, 127.9, 130.6, 130.8, 132.5, 132.9, 133.6, 135.8, 145.9, 145.9, 146.9, 146.9, 156.4 ppm; MS-ESI m/z: [M + H]+ calcd for C24H22BrN5O, 476.1; found, 476.0; m/z: [M – H]− calcd for C24H22BrN5O, 474.1; found, 473.9; HPLC (method 2): tR = 3.669 min (99.3%).
N-(4-Morpholinophenyl)-4-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-5-yl)pyridin-2-amine (18d)
The title compound was synthesized according to general procedure A starting from compound 17d (100 mg, 0.30 mmol) (for the synthesis of 17d see Supporting Information). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 120 mg of the desired compound (86% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.96–3.10 (m, 4H), 3.68–3.80 (m, 4H), 6.73 (d, J = 5.4 Hz, 1H), 6.80–6.92 (m, 3H), 7.34 (d, J = 8.8 Hz, H), 7.61–7.81 (m, 3H), 7.84 (br s, 1H), 7.96 (s, 1H), 7.99–8.09 (m, 1H), 8.94 (br s, 1H), 13.01 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C25H22F3N5O, 466.2; found, 465.9; m/z: [M – H]− calcd for C25H22F3N5O, 464.18; found, 463.8; HPLC (method 2): tR = 5.413 min (100%).
N-(4-Morpholinophenyl)-4-(4-(naphthalen-2-yl)-1H-imidazol-5-yl)pyridin-2-amine (18e)
The title compound was synthesized according to general procedure A starting from compound 17e (100 mg, 0.327 mmol) (for the synthesis of 17e see Supporting Information) and 4-morpholinoaniline (87.5 mg, 0.49 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 90:10) afforded 88 mg of the desired compound (60% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.79–2.96 (m, 4H), 3.61–3.80 (m, 4H), 6.53 (d, J = 9.0 Hz, 2H), 6.80 (d, J = 5.1 Hz, 1H), 6.87 (br s, 1H), 7.18 (d, J = 8.8 Hz, 2H), 7.47–7.65 (m, 3H), 7.88 (s, 1H), 7.90–8.12 (m, 5H), 8.60 (s, 1H), 12.72 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.3, 66.1, 106.0, 111.8, 115.7, 120.1, 126.2, 126.5, 126.8, 127.6, 128.0, 128.1, 132.3, 133.1, 133.7, 136.4, 145.6, 147.7, 156.6 ppm; MS-ESI m/z: [M + H]+ calcd for C28H25N5O, 448.2; found, 448.3; m/z: [M – H]− calcd for C28H25N5O, 446.2; found, 446.3; HPLC (method 2): tR = 5.541 min (98.5%).
4-(4-(1-Methyl-1H-pyrazol-4-yl)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18f)
The title compound was synthesized according to general procedure A starting from compound 17f (105.0 mg, 0.40 mmol) (for the synthesis of 17f see Supporting Information) and 4-morpholinoaniline (107.0 mg, 0.60 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 70:30) afforded 148 mg of the desired compound (92% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.92–3.09 (m, 4H), 3.65–3.79 (m, 4H), 3.88 (s, 3H), 6.83–6.93 (m, 3H), 7.08 (s, 1H), 7.45 (d, J = 8.8 Hz, 2H), 7.60 (s, 1H), 7.79 (s, 1H), 7.93 (s, 1H), 8.00 (d, J = 5.4 Hz, 1H), 8.87 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 38.6, 49.4, 66.2, 106.2, 111.3, 112.1, 115.9, 120.2, 123.4, 129.4, 131.1, 134.0, 135.6, 137.7, 142.9, 145.8, 146.7, 156.5 ppm; MS-ESI m/z: [M + H]+ calcd for C22H23N7O, 402.2; found, 402.4; m/z: [M – H]− calcd for C22H23N7O, 400.2; found, 400.5; HPLC (method 2): tR = 1.766 min (100%).
4-(4-Cyclohexyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18g)
The title compound was synthesized according to general procedure A starting from compound 17g (100 mg, 0.38 mmol) (for the synthesis of 17g see Supporting Information) and 4-morpholinoaniline (107.6 mg, 0.57 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 120 mg of the desired compound (78% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.14–1.86 (m, 10H), 2.85–2.97 (m, 1H), 2.98–3.06 (m, 4H), 3.65–3.80 (m, 4H), 6.81–6.97 (m, 4H), 7.47 (d, J = 8.9 Hz, 2H), 7.64 (s, 1H), 8.04 (d, J = 5.3 Hz, 1H), 8.72 (br s, 1H), 12.25 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 25.4, 26.0, 32.4, 34.9, 49.4, 66.2, 105.7, 111.5, 116.0, 120.5, 126.4, 127.7, 134.2, 134.5, 143.2, 145.8, 147.3, 156.9 ppm; MS-ESI m/z: [M + H]+ calcd for C24H29N5O, 404.2; found, 404.4; m/z: [M – H]− calcd for C24H29N5O, 402.2; found, 402.2; HPLC (method 2): tR = 4.730 min (100%).
4-(4-Cyclopentyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18h)
The title compound was synthesized according to general procedure A starting from compound 17h (100 mg, 0.40 mmol) (for the synthesis of 17h see Supporting Information) and 4-morpholinoaniline (107.0 mg, 0.60 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 9:1) afforded 107 mg of the desired compound (69% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.57–1.86 (m, 6H), 1.86–2.05 (m, 2H), 2.96–3.06 (m, 4H), 3.30–3.50 (m, 1H), 3.69–3.77 (m, 4H), 6.85–6.93 (m, 3H), 6.96 (s, 1H), 7.49 (d, J = 8.8 Hz, 2H), 7.70 (s, 1H), 8.05 (d, J = 5.4 Hz, 1H), 8.78 ppm (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 25.1, 32.9, 36.3, 49.5, 66.2, 106.1, 111.6, 116.0, 120.3, 130.9, 131.5, 134.3, 134.8, 142.7, 145.7, 147.2, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C23H27N5O, 390.2; found, 390.0; m/z: [M – H]− calcd for C23H27N5O, 388.2; found, 387.9; HPLC (method 2): tR = 4.071 min (99.6%).
4-(4-Cyclobutyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18i)
The title compound was synthesized according to general procedure A starting from compound 17i (100 mg, 0.43 mmol) (for the synthesis of 17i see Supporting Information) and 4-morpholinoaniline (114.0 mg, 0.64 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 77 mg of the desired compound (48% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.77–2.04 (m, 2H), 2.08–2.37 (m, 4H), 2.90–3.11 (m, 4H), 3.70–3.76 (m, 4H), 3.78–3.93 (m, 1H), 6.67–7.00 (m, 4H), 7.50 (d, J = 8.9 Hz, 2H), 7.59–7.73 (m, 1H), 8.03 (d, J = 5.0 Hz, 1H), 8.62–8.79 (m, 1H), 12.18–12.40 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 7.7, 28.6, 31.1, 49.4, 66.2, 106.0, 111.3, 116.0, 120.7, 128.0, 129.8, 133.7, 134.9, 142.3, 146.1, 146.5, 156.5 ppm; MS-ESI m/z: [M + H]+ calcd for C22H25N5O, 376.2; found, 376.1; m/z: [M – H]− calcd for C22H25N5O, 374.2; found, 373.9; HPLC (method 2): tR = 3.480 min (100%).
4-(4-Cyclopropyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18j)
The title compound was synthesized according to general procedure A starting from compound 17j (150 mg, 0.68 mmol) (for the synthesis of 17j see Supporting Information) and 4-morpholinoaniline (181.8 mg, 1.02 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 80:20) afforded 153 mg of the desired compound (62% yield); 1H NMR (250 MHz, DMSO-d6): δ 0.70–0.81 (m, 2H), 0.91–1.02 (m, 2H), 2.06 (tt, J = 8.3, 5.2 Hz, 1H), 2.92–3.10 (m, 4H), 3.60–3.84 (m, 4H), 6.88 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 5.1 Hz, 1H), 7.22 (s, 1H), 7.51 (d, J = 8.8 Hz, 2H), 7.55 (s, 1H), 8.05 (d, J = 5.6 Hz, 1H), 8.74 (s, 1H), 12.12 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 7.4, 7.5, 49.5, 66.2, 105.4, 110.9, 116.0, 120.0, 134.1, 134.5, 145.6, 147.2, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C21H23N5O, 362.2; found, 362.6; m/z: [M – H]− calcd for C21H23N5O, 360.2; found, 360.5; HPLC (method 2): tR = 2.699 min (100%).
4-(4-(tert-Butyl)-1H-imidazol-y-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18k)
The title compound was synthesized according to general procedure A starting from compound 17k (100 mg, 0.43 mmol) (for the synthesis of 17k see Supporting Information) and 4-morpholinoaniline (115.0 mg, 0.64 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 155 mg of the desired compound (96% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.20–1.35 (m, 9H), 3.04 (br s, 4H), 3.74 (br s, 4H), 6.70–6.94 (m, 4H), 7.47–7.52 (m, 2H), 8.14–8.20 (m, 1H), 9.04 (br s, 1H), 9.10 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 29.9, 31.6, 49.3, 66.1, 111.4, 114.9, 116.0, 120.6, 125.6, 133.4, 137.5, 138.7, 147.0, 156.1, 158.3, 158.7 ppm; ESI-MS m/z: [M + H]+ calcd for C22H27N5O, 378.2; found, 378.3; ESI-MS m/z: [M – H]− calcd for C22H27N5O, 376.2; found, 376.1; HPLC (method 2): tR = 2.860 min (100%).
4-(4-Isopropyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (18l)
The title compound was synthesized according to general procedure A starting from compound 17l (100 mg, 0.45 mmol) (for the synthesis of 17l see Supporting Information) and 4-morpholinoaniline (119.4 mg, 0.67 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 124 mg of the desired compound (76% yield); 1H NMR (300 MHz, methanol-d4): δ 1.31 (d, J = 7.0 Hz, 6H), 3.07–3.16 (m, 4H), 3.25–3.45 (m, 1H), 3.80–3.91 (m, 4H), 6.86–6.94 (m, 2H), 6.96–7.03 (m, 2H), 7.30–7.39 (m, 2H), 7.65 (s, 1H), 8.03 ppm (d, J = 6.2 Hz, 1H); 13C NMR (101 MHz DMSO-d6): δ 22.4, 24.6, 49.5, 66.1, 106.2, 111.6, 115.9, 119.8, 131.9, 134.4, 134.5, 134.6, 143.8, 145.5, 147.2, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C21H25N5O, 364.2; found, 364.5; m/z: [M – H]− calcd for C21H25N5O, 362.2; found, 362.3; HPLC (method 2): tR = 2.492 min (98.6%).
2-Chloro-4-(4-methyl-1H-imidazol-5-yl)pyridine (36)
Compound 34(21) (1.0 g, 4.43 mmol) was suspended in glacial AcOH (10 mL) and subsequently 30% H2O2 (602.7 mg, 17.72 mmol) was added dropwise and the reaction mixture was stirred at rt for 15 min. After adding H2O, the pH was adjusted to 8 using K2CO3 saturated solution and the aqueous phase was extracted five times with EtOAc. The combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure, affording 230 mg of the product which was used in the following step without further purification (25% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.47 (s, 3H), 7.62 (dd, J = 5.3, 1.3 Hz, 1H), 7.65 (br s, 1H), 7.69 (s, 1H), 8.33 ppm (d, J = 5.2 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.7, 118.8, 119.1, 127.9, 130.5, 134.9, 145.9, 149.8, 150.8 ppm; MS-ESI m/z: [M + H]+ calcd for C9H8ClN3, 194.0; found, 194.0; m/z: [M – H]− calcd for C9H8ClN3, 192.0; found, 191.8; HPLC (method 2): tR = 1.375 min.
2-Chloro-4-(4-ethyl-1H-imidazol-5-yl)pyridine (37)
Compound 35 (400 mg, 1.67 mmol) (for the synthesis of compound 35 see Supporting Information) was suspended in glacial AcOH (10 mL) and subsequently 30% H2O2 (227.2 mg, 6.68 mmol) was added dropwise and the reaction mixture was stirred at rt for 40 min. The reaction mixture was concentrated at reduced pressure and after that 20 mL of K2CO3 saturated solution was added. The aqueous layer was extracted five times with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure, affording 230 mg of the product which was used in the following step without further purification (71% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.22 (t, J = 7.4 Hz, 3H), 2.85 (q, J = 7.4 Hz, 2H), 7.58 (d, J = 5.0 Hz, 1H), 7.62 (s, 1H), 7.70 (s, 1H), 8.33 ppm (d, J = 5.1 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 13.4, 18.8, 119.1, 119.4, 129.7, 133.8, 135.2, 145.8, 149.9, 150.8 ppm; MS-ESI m/z: [M + H]+ calcd for C10H10ClN3, 208.0; found, 208.1; m/z: [M – H]− calcd for C10H10ClN3, 206.0; found, 205.9; HPLC (method 2): tR = 1.653 min.
4-(4-Methyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (38)
The title compound was synthesized according to general procedure A starting from compound 36 (100 mg, 0.47 mmol). The crude product was purified twice by flash column chromatography (SiO2, DCM/EtOH 90:10 to 80:20), (SiO2, EtOAc), obtaining 38 mg of the desired compound (25% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.42 (s, 3H), 2.94–3.09 (m, 4H), 3.63–3.81 (m, 4H), 6.88 (d, J = 9.0 Hz, 2H), 6.94 (d, J = 5.1 Hz, 1H), 7.07 (s, 1H), 7.51 (d, J = 9.1 Hz, 2H), 7.60 (s, 1H), 8.04 (d, J = 5.4 Hz, 1H), 8.69 (s, 1H), 12.15 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.4, 49.5, 66.2, 105.3, 110.8, 116.0, 119.7, 124.7, 133.0, 133.9, 134.6, 143.6, 145.5, 147.2, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C19H21N5O, 336.2; found, 336.2; m/z: [M – H]− calcd for C19H21N5O, 334.2; found, 334.1; HPLC (method 2): tR = 1.871 min (100%).
4-(4-Ethyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (39)
The title compound was synthesized according to general procedure A starting from compound 37 (100 mg, 0.48 mmol). The crude product was purified twice by flash column chromatography (SiO2, DCM/EtOH 90:10 to 80:20), (SiO2, EtOAc), obtaining 110 mg of the desired compound (65% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.22 (t, J = 7.5 Hz, 3H), 2.70–2.90 (m, 2H), 2.92–3.11 (m, 4H), 3.62–3.85 (m, 4H), 6.80–6.98 (m, 3H), 7.07 (br s, 1H), 7.51 (d, J = 9.0 Hz, 2H), 7.61 (s, 1H), 8.04 (d, J = 5.0 Hz, 1H), 8.70 (br s, 1H), 12.03–12.48 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 13.9, 18.5, 49.5, 66.2, 105.6, 111.0, 116.0, 119.8, 130.6, 132.5, 134.2, 134.6, 143.7, 145.5, 147.3, 156.9 ppm; MS-ESI m/z: [M + H]+ calcd for C20H23N5O, 350.4; found, 350.4; m/z: [M – H]− calcd for C20H23N5O, 348.2; found, 348.2; HPLC (method 2): tR = 1.774 min (99.4%).
2-Chloro-4-(2-(methylthio)-1H-imidazol-5-yl)pyridine (40)
Under an argon atmosphere, compound 33 (500 mg, 2.36 mmol) (for the synthesis of compound 33 see Supporting Information) and t-BuONa (454 mg, 4.72 mmol) were dissolved in dry MeOH (20 mL) and after cooling the reaction mixture to 0 °C, methyl iodide (147.5 μL, 2.36 mmol) was added and the reaction mixture was stirred at 0 °C for 30 min. The reaction mixture was then heated to 55 °C and stirred for 3 h. After cooling to rt, the solvent was evaporated at reduced pressure and H2O was added. The aqueous phase was then extracted two times with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was finally purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 90:10) giving 396 mg of the desired compound (74% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.59 (s, 3H), 7.64–7.72 (m, 1H), 7.73–7.79 (m, 1H), 8.03 (s, 1H), 8.31 (dd, J = 5.3, 1.8 Hz, 1H), 12.70 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C9H8ClN3S, 226.0; found, 225.9; m/z: [M – H]− calcd for C9H8ClN3S, 224.0; found, 223.9; HPLC (method 1): tR = 4.096 min.
2-Chloro-4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (41)
The title compound was prepared as previously described21 and analytical data were in agreement with the reported ones.
2-Chloro-4-(4-ethyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (42)
In a pressure vial, compound 35 (400 mg, 1.67 mmol) (for the synthesis of compound 35 see Supporting Information) and t-BuONa (160.5 mg, 1.67 mmol) were dissolved in dry MeOH (15 mL) and after cooling the reaction mixture to 0 °C, methyl iodide (203 μL, 3.26 mmol) was added. The vial was tightly closed and the mixture was stirred at 50 °C for 1 h. The solvent was evaporated at reduced pressure and the residue was purified by flash column chromatography (SiO2, DCM/EtOH 99:01 to 95:05), affording 378 mg of the product (89% yield); 1H NMR (300 MHz, CDCl3): δ 1.32 (t, J = 7.6 Hz, 3H), 2.63 (s, 3H), 2.89 (q, J = 7.6 Hz, 2H), 7.50 (dd, J = 5.3, 1.5 Hz, 1H), 7.64 (br s, 1H), 8.35 ppm (dd, J = 5.3, 0.4 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 13.5, 16.6, 19.4, 119.3, 120.7, 132.4, 135.1, 141.7, 145.0, 149.4, 151.9 ppm; MS-ESI m/z: [M + H]+ calcd for C11H12ClN3S, 254.0; found, 254.0; m/z: [M – H]− calcd for C11H12ClN3S, 252.0; found, 252.0; HPLC (method 2): tR = 3.575 min.
4-(2-(Methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (43)
The title compound was synthesized according to general procedure A starting from 40 (100 mg, 0.44 mmol) and 4-morpholinoaniline (117.6 mg, 0.66 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 90:10) afforded 92 mg of the desired compound (57% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.54–2.62 (m, 3H), 2.92–3.09 (m, 4H), 3.63–3.78 (m, 4H), 6.87 (d, J = 7.8 Hz, 2H), 6.96 (d, J = 4.5 Hz, 1H), 7.17 (br s, 1H), 7.53 (d, J = 7.8 Hz, 2H), 7.76 (br s, 1H), 8.00 (d, J = 4.5 Hz, 1H), 8.75 (br s, 1H), 12.34–12.62 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 15.3, 49.5, 66.2, 104.1, 109.5, 116.0, 116.8, 119.7, 120.3, 134.6, 139.2, 142.0, 145.4, 147.3, 156.9 ppm; MS–FAB m/z: [M] calcd for C19H21N5OS, 367.1; found, 367.2; HPLC (method 1): tR = 2.501 min (100%).
4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (44)
The title compound was synthesized according to general procedure A starting from 41 (100 mg, 0.42 mmol) and 4-morpholinoaniline (112.3 mg, 0.63 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 80:20) afforded 42 mg of the desired compound (26% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.39 (br s, 3H), 2.52–2.60 (m, 3H), 2.93–3.09 (m, 4H), 3.61–3.83 (m, 4H), 6.78–6.94 (m, 3H), 7.06 (br s, 1H), 7.52 (d, J = 7.8 Hz, 2H), 8.03 (d, J = 4.3 Hz, 1H), 8.74 (br s, 1H), 12.27 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 15.4, 25.4, 49.5, 66.2, 105.3, 110.5, 116.0, 119.6, 134.6, 145.4, 147.3, 156.7 ppm; MS–FAB m/z: [M + H]+ calcd for C20H23N5OS, 382.2; found, 382.3; HPLC (method 1): tR = 3.024 min (96.4%).
4-(4-Ethyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (45)
The title compound was synthesized according to general procedure A starting from compound 42 (100 mg, 0.39 mmol) and 4-morpholinoaniline (103.4 mg, 0.58 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 99:01 to 90:10) afforded 51 mg of the desired compound (33% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.21 (t, J = 7.5 Hz, 3H), 2.55 (s, 3H), 2.78 (q, J = 7.4 Hz, 2H), 2.96–3.08 (m, 4H), 3.66–3.81 (m, 4H), 6.69–6.96 (m, 3H), 7.03 (br s, 1H), 7.52 (d, J = 8.8 Hz, 2H), 8.03 (d, J = 5.3 Hz, 1H), 8.67–8.81 (m, 1H), 12.07–12.37 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 13.9, 15.3, 18.7, 49.5, 66.2, 105.5, 110.8, 116.0, 119.8, 133.2, 134.5, 139.7, 143.0, 145.5, 147.3, 151.6, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C21H25N5OS, 396.2; found, 396.3; m/z: [M – H]− calcd for C21H25N5OS, 394.2; found, 394.1; HPLC (method 2): tR = 3.499 min (97.0%).
4-(2-Chloropyridin-4-yl)-5-methyl-1H-imidazol-2-amine (46)
Cyanamide (652 mg, 15.52 mmol) was dissolved in EtOH (30 mL) and after heating at reflux temperature, compound 31 was added portionwise over 1 h and the mixture was stirred at the same temperature further for 3 h. After cooling down, the solvent was evaporated at reduced pressure and the residue was purified by flash column chromatography (SiO2, DCM/EtOH/Et3N 95:05:0 to 80:18:2), obtaining 900 mg of the desired product (95% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.37 (s, 3H), 7.53 (d, J = 4.9 Hz, 1H), 7.62 (br s, 3H), 8.42 (d, J = 5.1 Hz, 1H), 12.86 ppm (br s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 10.7, 117.6, 118.9, 119.4, 124.4, 139.2, 147.0, 150.3, 151.1 ppm; MS-ESI m/z: [M + H]+ calcd for C9H9ClN4, 209.0; found, 208.9; m/z: [M – H]− calcd for C9H9ClN4, 207.0; found, 206.9; HPLC (method 2): tR = 1.524 min.
4-(2-Amino-5-methyl-1H-imidazol-4-yl)-N-(4-morpholinophenyl)pyridin-2-amine (47)
The title compound was synthesized according to general procedure A starting from compound 46 (100 mg, 0.48 mmol) and 4-morpholinoaniline (128.3 mg, 0.72 mmol). Purification by flash column chromatography (SiO2, DCM/MeOH 99:01 to 90:10) and (SiO2, DCM/MeOH 95:05 to 80:20) afforded 46 mg of the desired compound (27% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.30 (s, 3H), 2.95–3.07 (m, 4H), 3.67–3.79 (m, 4H), 6.76 (dd, J = 5.4, 1.4 Hz, 1H), 6.83 (s, 1H), 6.89 (d, J = 9.0 Hz, 2H), 7.25 (br s, 2H), 7.50 (d, J = 9.0 Hz, 2H), 8.11 (d, J = 5.4 Hz, 1H), 8.91 (s, 1H), 12.37 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.0, 49.9, 66.7, 105.9, 110.6, 116.4, 120.1, 120.6, 122.2, 134.4, 137.5, 146.4, 147.4, 148.4, 157.2 ppm; MS-ESI m/z: [M + H]+ calcd for C19H22N6O, 351.2; found, 351.1; m/z: [M Z H]− calcd for C21H25N5OS, 349.2; found, 349.1; HPLC (method 2): tR = 1.876 min (96%).
4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(3-methylbutan-2-yl)pyridin-2-amine (48a)
The title compound was synthesized according to general procedure E starting from 41 (85 mg, 0.355 mmol) and 3-methylbutan-2-amine. The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 80:20), affording 35 mg of pure product (34% yield); 1H NMR (400 MHz, DMSO-d6): δ 0.79–0.94 (m, 6H), 1.03 (d, J = 6.6 Hz, 3H), 1.70–1.86 (m, 1H), 2.36 (br s, 3H), 2.54 (br s, 3H), 3.72–3.89 (m, 1H), 6.35 (d, J = 7.1 Hz, 1H), 6.53–6.92 (m, 2H), 7.86 (d, J = 5.3 Hz, 1H), 12.23 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 15.4, 16.7, 17.9, 19.2, 32.1, 50.5, 104.1, 108.4, 127.0, 134.5, 139.0, 142.9, 146.5, 158.6 ppm; HPLC (method 1): tR = 3.355 min (95%).
4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(1-phenylethyl)pyridin-2-amine (48b)
The title compound was synthesized according to general procedure E starting from 41 (100 mg, 417 mmol) and 1-phenylethan-1-amine. The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 80:20), affording 42 mg of pure product (31% yield); 1H NMR (400 MHz, CDCl3): δ 1.38–1.55 (m, 3H), 1.96–2.13 (m, 3H), 2.36–2.54 (m, 3H), 4.50–4.69 (m, 1H), 5.50 (br s, 1H), 6.33 (br s, 1H), 6.77 (br s, 1H), 7.08–7.34 (m, 6H), 7.84–7.98 ppm (m, 1H); 13C NMR (101 MHz, CDCl3): δ 12.2, 16.8, 24.4, 52.3, 103.5, 110.7, 125.8, 127.0, 128.7, 140.7, 142.5, 144.6, 147.1, 158.0 ppm; HPLC (method 1): tR = 2.748 min (100%).
N-Cyclobutyl-4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-amine (48c)
The title compound was synthesized according to general procedure E starting from 41 (150 mg, 0.62 mmol) and cyclobutylamine (48 h). The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10), affording 83 mg of pure product (49% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.55–1.73 (m, 2H), 1.77–1.95 (m, 2H), 2.19–2.41 (m, 5H), 2.53 (s, 3H), 4.16–4.40 (m, 1H), 6.41–6.77 (m, 3H), 7.84–7.97 (m, 1H), 12.08–12.34 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 14.7, 15.5, 30.7, 46.1, 103.3, 109.0, 126.6, 134.5, 138.7, 142.7, 147.5, 158.3 ppm; MS-ESI m/z: [M + H]+ calcd for C14H18N4S, 275.1; found, 275.0; m/z: [M – H]− calcd for C14H18N4S, 273.1; found, 273.0; HPLC (method 2): tR = 2.499 min (99%).
N-Cyclopentyl-4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-amine (48d)
The title compound was synthesized according to general procedure E starting from 41 (100 mg, 0.42 mmol) and cyclopentylamine (120 h). The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10), affording 51 mg of pure product (42% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.36–1.74 (m, 6H), 1.84–1.98 (m, 2H), 2.37 (s, 3H), 2.54 (s, 3H), 3.99–4.15 (m, 1H), 6.60–7.02 (m, 3H), 7.88 (d, J = 5.6 Hz, 1H), 12.32 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C15H20N4S, 289.1; found, 289.0; m/z: [M – H]− calcd for C15H20N4S, 287.1; found, 287.0; HPLC (method 2): tR = 3.265 min (98%).
N-Cyclohexyl-4-(4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-amine (48e)
The title compound was synthesized according to general procedure E starting from 41 (100 mg, 0.42 mmol) and cyclohexylamine (72 h). The crude residue was purified twice by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) and (SiO2, DCM/EtOH 95:05 to 90:10), affording 35 mg of pure product (27% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.11–1.32 (m, 5H), 1.35 (br s, 1H), 1.52–1.63 (m, 1H), 1.64–1.76 (m, 2H), 1.80–1.96 (m, 2H), 2.35 (br s, 3H), 2.53 (s, 3H), 3.62–3.74 (m, 1H), 6.29 (d, J = 7.6 Hz, 1H), 6.53–6.83 (m, 2H), 7.88 (d, J = 5.3 Hz, 1H), 12.19 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C16H22N4S, 303.2; found, 303.1; m/z: [M – H]− calcd for C16H22N4S, 301.2; found, 301.2; HPLC (method 2): tR = 4.347 min (100%).
4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(tetrahydro-2H-pyran-4-yl)pyridin-2-amine (48f)
The title compound was synthesized according to general procedure E starting from 41 (80 mg, 0.33 mmol) and 4-aminotetrahydropyran (120 h). The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10), affording 30 mg of pure product (30% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.33–1.48 (m, 2H), 1.87 (d, J = 10.6 Hz, 2H), 2.36 (br s, 3H), 2.53 (s, 3H), 3.40–3.45 (m, 2H), 3.76–3.99 (m, 3H), 6.29–6.86 (m, 3H), 7.90 (d, J = 4.5 Hz, 1H), 12.20 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 15.4, 32.9, 46.2, 66.0, 104.2, 108.9, 126.6, 134.4, 138.7, 142.6, 147.2, 158.4 ppm; MS-ESI m/z: [M + H]+ calcd for C15H20N4OS, 305.1; found, 305.0; m/z: [M – H]− calcd for C15H20N4OS, 303.1; found, 303.1; HPLC (method 2): tR = 1.570 min (96%).
trans-4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)cyclohexan-1-ol (48g)
The title compound was synthesized according to general procedure E starting from 41 (100 mg, 0.42 mmol) and trans-4-aminocyclohexanol (484 mg, 4.20 mmol) and adding 2 mL of n-butanol (120 h). The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 92:08 to 80:20), affording 37 mg of pure product (27% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.10–1.35 (m, 4H), 1.76–2.00 (m, 4H), 2.22–2.42 (m, 3H), 2.52 (s, 3H), 3.41 (br s, 1H), 3.61 (br s, 1H), 4.47–4.70 (m, 1H), 6.14–6.85 (m, 3H), 7.78–8.02 (m, 1H), 12.06–12.36 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 15.5, 30.6, 34.1, 48.5, 68.5, 104.1, 108.7, 126.5, 134.5, 138.6, 142.5, 147.3, 158.7 ppm; MS-ESI m/z: [M + H]+ calcd for C16H22N4OS, 319.1; found, 319.1; m/z: [M – H]− calcd for C16H22N4OS, 317.1; found, 317.2; HPLC (method 2): tR = 1.640 min (98%).
trans-N1-(4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)cyclohexane-1,4-diamine (48h)
The title compound was synthesized according to general procedure E starting from 41 (300 mg, 1.25 mmol) and trans-1,4-diaminocyclohexane (2.8 g, 25 mmol) and adding 2 mL of n-butanol (72 h). The crude residue was purified by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10), affording 172 mg of pure product (43% yield); 1H NMR (300 MHz, methanol-d4): δ 1.23–1.42 (m, 4H), 1.85–2.13 (m, 4H), 2.41 (s, 3H), 2.55 (s, 3H), 2.58–2.75 (m, 1H), 3.56–3.67 (m, 1H), 6.67 (br s, 1H), 6.73 (dd, J = 5.6, 1.5 Hz, 1H), 7.89 ppm (dd, J = 5.6, 0.6 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ 12.6, 15.9, 32.0, 35.5, 49.3, 50.4, 104.4, 109.0, 129.2, 133.0, 139.6, 142.0, 148.0, 159.2 ppm; MS-ESI m/z: [M + H]+ calcd for C16H23N5S, 318.2; found, 318.0; m/z: [M – H]− calcd for C16H23N5S, 316.2; found, 316.1; HPLC (method 2): tR = 1.234 min (100%).
N-(trans-4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)cyclohexyl)acetamide (48i)
The title compound was synthesized according to general procedure F starting from 48h (180 mg, 0.57 mmol) and acetic anhydride (116 mg, 1.14 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 90:10 to 80:20) afforded 74 mg of the desired product (36% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.14–1.35 (m, 4H), 1.74–1.87 (m, 5H), 1.90–2.06 (m, 2H), 2.26–2.41 (m, 3H), 2.53 (s, 3H), 3.43–3.57 (m, 1H), 3.63 (br s, 1H), 6.21–6.80 (m, 3H), 7.75 (d, J = 7.8 Hz, 1H), 7.84–7.98 (m, 1H), 12.11–12.32 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 15.4, 22.7, 31.2, 31.4, 47.3, 48.4, 104.2, 108.7, 126.6, 134.4, 138.7, 142.5, 147.1, 158.5, 168.1 ppm; MS-ESI m/z: [M + H]+ calcd for C18H25N5OS, 360.2; found, 360.1; m/z: [M – H]− calcd for C18H25N5OS, 358.2; found, 358.1; HPLC (method 2): tR = 1.647 min (99%).
N-(trans-4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)cyclohexyl)benzamide (48j)
The title compound was synthesized according to general procedure F starting from 48h (120 mg, 0.38 mmol) and benzoyl chloride (80 mg, 0.57 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 90:10 to 80:20) afforded 22 mg of the desired product (13% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.20–1.57 (m, 4H), 1.78–2.11 (m, 4H), 2.28–2.43 (m, 3H), 2.54 (s, 3H), 3.60–3.90 (m, 2H), 6.26–6.88 (m, 3H), 7.36–7.58 (m, 3H), 7.75–8.01 (m, 3H), 8.27 (d, J = 7.9 Hz, 1H), 12.04–12.44 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 15.4, 31.1, 31.6, 48.1, 48.6, 104.2, 108.7, 126.6, 127.2, 128.1, 130.9, 134.4, 134.8, 138.7, 142.7, 147.0, 158.5, 165.5 ppm; MS-ESI m/z: [M + H]+ calcd for C23H27N5OS, 422.2; found, 422.0; m/z: [M – H]− calcd for C23H27N5OS, 420.2; found, 420.0; HPLC (method 2): tR = 4.076 min (97%).
N-(trans-4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)cyclohexyl)cyclohexanecarboxamide (48k)
The title compound was synthesized according to general procedure F starting from 48h (120 mg, 0.38 mmol) and cyclohexane carbonyl chloride (83 mg, 0.57 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 95:05 to 80:20) afforded 29 mg of the desired product (18% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.05–1.43 (m, 9H), 1.54–1.87 (m, 7H), 1.90–2.10 (m, 3H), 2.23–2.42 (m, 3H), 2.53 (s, 3H), 3.45–3.72 (m, 2H), 6.15–6.84 (m, 3H), 7.56 (d, J = 7.6 Hz, 1H), 7.76–8.03 (m, 1H), 12.20 ppm (br s, 1H); MS-ESI m/z: [M + H]+ calcd for C23H33N5OS, 428.2; found, 428.0; m/z: [M – H]− calcd for C23H33N5OS, 426.2; found, 426.1; HPLC (method 2): tR = 5.391 min (98%).
N-(trans-4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)cyclohexyl)pivalamide (48l)
The title compound was synthesized according to general procedure F starting from 48h (120 mg, 0.38 mmol) and pivaloyl chloride (69 mg, 0.57 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 90:10 to 80:20) afforded 29 mg of the desired product (19% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.08 (s, 9H), 1.15–1.40 (m, 4H), 1.64–1.77 (m, 2H), 1.92–2.05 (m, 2H), 2.25–2.41 (m, 3H), 2.53 (s, 3H), 3.46–3.70 (m, 2H), 6.23–6.77 (m, 3H), 7.13 (d, J = 8.1 Hz, 1H), 7.89 (d, J = 4.5 Hz, 1H), 12.04–12.35 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.3, 15.5, 27.4, 31.0, 31.6, 37.8, 47.5, 48.6, 104.1, 108.6, 126.6, 134.4, 138.7, 142.6, 147.1, 158.5, 176.5 ppm; MS-ESI m/z: [M + H]+ calcd for C21H31N5OS, 402.2; found, 402.0; m/z: [M – H]− calcd for C21H31N5OS, 400.2; found, 400.0; HPLC: tR = 4.114 min (99%).
N1-(4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)benzene-1,4-diamine (48m)21
The title compound was prepared as previously described21 and analytical data were in agreement with the reported ones.
N-(4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)phenyl)acetamide (48n)
The title compound was synthesized according to general procedure F starting from 48m (100 mg, 0.32 mmol) and acetic anhydride (49 mg, 0.48 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 37 mg of the desired product (33% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.01 (s, 3H), 2.28–2.44 (m, 3H), 2.54 (s, 3H), 6.75–7.04 (m, 1H), 7.10 (s, 1H), 7.43 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 8.01–8.13 (m, 1H), 8.80–8.98 (m, 1H), 9.78 (s, 1H), 12.19–12.47 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.5, 15.6, 23.8, 105.9, 111.1, 118.4, 119.9, 127.3, 132.3, 134.2, 137.6, 139.2, 143.1, 147.3, 156.5, 167.9 ppm; MS-ESI m/z: [M + H]+ calcd for C18H19N5OS, 354.1; found, 354.1; m/z: [M – H]− calcd for C18H19N5OS, 352.1; found, 352.2; HPLC (method 2): tR = 2.035 min (98%).
N-(4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)phenyl)benzamide (48o)
The title compound was synthesized according to general procedure F starting from 48m (100 mg, 0.32 mmol) and benzoyl chloride (67 mg, 0.48 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 98:02 to 90:10) afforded 27 mg of the desired product (33% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.41 (s, 3H), 2.56 (s, 3H), 6.97 (br s, 1H), 7.14 (br s, 1H), 7.46–7.73 (m, 7H), 7.90–8.00 (m, 2H), 8.10 (d, J = 5.3 Hz, 1H), 9.01 (s, 1H), 10.12 (s, 1H), 12.29 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 14.2, 15.9, 106.4, 111.5, 118.6, 121.6, 128.0, 128.8, 129.2, 131.4, 131.7, 132.5, 135.6, 138.6, 139.9, 143.7, 147.7, 156.9, 165.5 ppm; MS-ESI m/z: [M + H]+ calcd for C23H21N5OS, 416.1; found, 415.7; m/z: [M – H]− calcd for C23H21N5OS, 414.1; found, 413.7; HPLC (method 2): tR = 4.357 min (97%).
N-(4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)phenyl)cyclohexanecarboxamide (48p)
The title compound was synthesized according to general procedure F starting from 48m (100 mg, 0.32 mmol) and cyclohexane carbonyl chloride (70 mg, 0.48 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 40 mg of the desired product (30% yield); 1H NMR (300 MHz, DMSO-d6): δ 1.11–1.50 (m, 5H), 1.56–1.85 (m, 5H), 2.22–2.36 (m, 1H), 2.40 (s, 3H), 2.55 (s, 3H), 6.94 (d, J = 5.0 Hz, 1H), 7.09 (br s, 1H), 7.47 (d, J = 9.0 Hz, 2H), 7.58 (d, J = 9.0 Hz, 2H), 8.06 (d, J = 5.4 Hz, 1H), 8.91 (s, 1H), 9.62 (s, 1H), 12.28 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.5, 15.4, 25.2, 25.4, 29.2, 44.7, 105.7, 110.9, 118.3, 119.7, 127.1, 132.5, 134.2, 137.3, 139.4, 142.8, 147.2, 156.4, 173.6 ppm; MS-ESI m/z: [M + H]+ calcd for C23H27N5OS, 422.2; found, 422.0; m/z: [M – H]− calcd for C23H27N5OS, 420.2; found, 420.1; HPLC (method 2): tR = 4.646 min (99%).
N-(4-((4-(4-Methyl-2-(methylthio)-1H-imidazol-5-yl)pyridin-2-yl)amino)phenyl)pivalamide (48q)
The title compound was synthesized according to general procedure F starting from 48m (120 mg, 0.39 mmol) and pivaloyl chloride (70 mg, 0.58 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 74 mg of the desired product (48% yield); 1H NMR (400 MHz, DMSO-d6): δ 1.22 (s, 9H), 2.41 (s, 3H), 2.55 (s, 3H), 6.96 (d, J = 5.6 Hz, 1H), 7.11 (s, 1H), 7.50 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 8.07 (d, J = 5.3 Hz, 1H), 9.00 (s, 1H), 9.07 (s, 1H), 12.35 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 11.9, 15.4, 27.3, 38.9, 105.7, 110.9, 118.2, 121.1, 128.8, 132.5, 137.3, 139.5, 142.6, 146.7, 156.2, 175.9 ppm; MS-ESI m/z: [M + H]+ calcd for C21H25N5OS, 396.2; found, 395.7; m/z: [M – H]− calcd for C21H25N5OS, 394.2; found, 393.7; HPLC (method 2): tR = 3.999 min (100%).
2-Chloro-4-(1,5-dimethyl-2-(methylthio)-1H-imidazol-4-yl)pyridine (49)
Under an argon atmosphere, compound 34 (250 mg, 1.11 mmol) and t-BuONa (213 mg, 2.22 mmol) were dissolved in dry MeOH (10 mL), and after cooling the reaction mixture to 0 °C, methyl iodide (205 μL, 3.32 mmol) was added and the reaction mixture was let to heat to rt. The reaction mixture was then heated to 80 °C and stirred for 3 h. After cooling to rt, the solvent was evaporated at reduced pressure and H2O was added. The aqueous phase was then extracted two times with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was finally purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 90:10) giving 110 mg of the desired compound (39% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.44 (s, 3H), 2.54–2.62 (m, 3H), 3.34 (s, 3H), 7.47–7.77 (m, 2H), 8.34 ppm (d, J = 4.5 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 10.5, 15.4, 30.6, 119.1, 119.4, 130.5, 132.4, 142.3, 145.6, 149.8, 150.8 ppm; HPLC (method 1): tR = 4.812 min.
4-(1,5-Dimethyl-2-(methylthio)-1H-imidazol-4-yl)-N-(4-morpholinophenyl)pyridin-2-amine (50)
Under an argon atmosphere, tris(dibenzylidenaceton)dipalladium(0) (Pd2(dba)3) (17.5 mg, 0.02 mmol) and 9,9-dimethyl-4,5-bis(diphenylphosphino)xanten (Xantphos) (22.1 mg, 0.04 mmol) were dissolved in dry 1,4-dioxane (5 mL) and stirred for 10 min. After that compound 49 (50 mg, 0.21 mmol), Cs2CO3 (138.1 mg, 0.42 mmol), and 4-morpholinoaniline (56.7 mg, 0.32 mmol) were added and the reaction mixture was heated to 100 °C and stirred for 15 h. After cooling to rt, the reaction mixture was diluted with DCM and the solid residue was removed by filtration. The filtrate was then concentrated at reduced pressure and the residue was purified by flash column chromatography (DCM/EtOH 100:0 to 90:10) giving 61 mg of the desired product (73% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.36–2.44 (m, 3H), 2.54 (m, 3H), 2.93–3.05 (m, 4H), 3.45–3.55 (m, 3H), 3.67–3.76 (m, 4H), 6.79–6.96 (m, 3H), 7.07 (s, 1H), 7.54 (d, J = 7.3 Hz, 2H), 8.00–8.09 (m, 1H), 8.76 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 10.5, 15.8, 30.5, 49.5, 66.2, 106.0, 111.0, 115.9, 119.5, 128.4, 134.4, 134.6, 140.9, 142.9, 145.4, 147.2, 156.7 ppm; MS–FAB m/z: [M] calcd for C21H25N5OS, 395.2; found, 395.3; HPLC (method 1): tR = 3.156 min (98.7%).
5-(2-Chloropyridin-4-yl)-1,4-dimethyl-1,3-dihydro-2H-imidazole-2-thione (51)
In a pressure vial, compound 31 (200 mg, 0.77 mmol) (for the synthesis of compound 31 see Supporting Information) and methyl isothiocyanate (284 mg, 3.88 mmol) were suspended in triethylamine (2 mL), and after closing the vial tightly, the reaction mixture was stirred at 60 °C for 16 h. The excess of triethylamine was evaporated at reduced pressure and the residue was suspended in glacial AcOH and stirred at 80 °C for 1.5 h. The reaction mixture was concentrated at reduced pressure and after that NaHCO3 saturated solution (20 mL) was added and the aqueous phase was extracted four times with EtOAc. The combined organic layers were washed with H2O and NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 95:05), affording 110 mg of the desired product (60% yield); 1H NMR (300 MHz, DMSO-d6): δ 2.11 (s, 3H), 3.45 (s, 3H), 7.47 (dd, J = 5.2, 1.4 Hz, 1H), 7.55–7.63 (m, 1H), 8.47 (d, J = 5.1 Hz, 1H), 12.51 ppm (br s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 9.6, 32.4, 122.7, 122.9, 123.3, 124.4, 139.5, 150.2, 150.9, 161.8 ppm; MS-ESI m/z: [M – H]− calcd for C10H10ClN3S, 238.0; found, 238.0; HPLC (method 2): tR = 2.353 min.
5-(2-Chloropyridin-4-yl)-1-ethyl-4-methyl-1,3-dihydro-2H-imidazole-2-thione (52)
The title compound was prepared following the same procedure of compound 51 starting from 31 (200 mg, 0.77 mmol) (for the synthesis of compound 31 see Supporting Information) and ethyl isothiocyanate (335.5 mg, 3.85 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 99:01 to 95:05) afforded 128 mg of the desired compound (65% yield); 1H NMR (300 MHz, CDCl3): δ 1.15 (t, J = 6.9 Hz, 3H), 2.14 (s, 3H), 4.04 (q, J = 6.7 Hz, 2H), 7.11 (d, J = 4.5 Hz, 1H), 7.21 (s, 1H), 8.46 (d, J = 4.8 Hz, 1H), 12.32 ppm (br s, 1H); 13C NMR (101 MHz, CDCl3): δ 9.6, 14.1, 40.3, 122.5, 123.2, 124.2, 124.8, 139.5, 150.4, 152.4, 160.1 ppm; MS-ESI m/z: [M – H]− calcd for C11H12ClN3S, 252.0; found, 252.0; HPLC (method 2): tR = 3.168 min.
5-(2-Chloropyridin-4-yl)-1-cyclopropyl-4-methyl-1,3-dihydro-2H-imidazole-2-thione (53)
The title compound was prepared following the same procedure of compound 51 starting from 31 (500 mg, 1.94 mmol) (for the synthesis of compound 31 see Supporting Information) and cyclopropyl isothiocyanate (962.4 mg, 9.70 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 95:05) afforded 335 mg of the desired compound (60% yield); 1H NMR (300 MHz, DMSO-d6): δ 0.43–0.53 (m, 2H), 0.79–0.96 (m, 2H), 2.09 (s, 3H), 3.17–3.29 (m, 1H), 7.49 (dd, J = 5.2, 1.4 Hz, 1H), 7.55–7.64 (m, 1H), 8.45 (d, J = 5.2 Hz, 1H), 12.42 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 6.5, 9.6, 27.0, 122.5, 122.9, 123.3, 124.4, 139.6, 149.6, 150.4, 163.9 ppm; MS-ESI m/z: [M – H]− calcd for C12H12ClN3S, 264.0; found, 264.0; HPLC (method 2): tR = 3.057 min.
2-Chloro-4-(1,4-dimethyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (54)
In a pressure vial, compound 51 (285 mg, 1.19 mmol) and t-BuONa (114.3 mg, 1.19 mmol) were dissolved in dry MeOH (15 mL), and after cooling the reaction mixture to 0 °C, methyl iodide (217 μL, 3.48 mmol) was added. The vial was tightly closed and the mixture was stirred at 50 °C for 30 min. After evaporating the solvent at reduced pressure, H2O was added and the aqueous phase was extracted four times with EtOAc. The combined organic layers were washed with H2O and NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure, giving 290 mg of the product which was used in the following step without further purification (96% yield); 1H NMR (300 MHz, CDCl3): δ 2.28 (s, 3H), 2.66 (s, 3H), 3.52 (s, 3H), 7.12 (dd, J = 5.2, 1.5 Hz, 1H), 7.22–7.24 (m, 1H), 8.44 ppm (d, J = 5.1 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 13.7, 15.8, 32.3, 122.0, 123.5, 126.8, 138.5, 141.1, 145.5, 149.9, 152.0 ppm; MS-ESI m/z: [M + H]+ calcd for C11H12ClN3S, 254.0; found, 254.0; HPLC (method 2): tR = 1.720 min.
2-Chloro-4-(1-ethyl-4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (55)
The title compound was synthesized following the same procedure of compound 54 starting from 52 (125 mg, 0.49 mmol), t-BuONa (47 mg, 0.49 mmol), and methyl iodide (90 μL, 1.44 mmol) giving 120 mg of the product, which was used in the following step without further purification (95% yield); 1H NMR (300 MHz, CDCl3): δ 1.13 (t, J = 7.2 Hz, 3H), 2.15 (s, 3H), 2.57 (s, 3H), 3.85 (q, J = 7.2 Hz, 2H), 7.06 (dd, J = 5.1, 1.5 Hz, 1H), 7.13–7.18 (m, 1H), 8.35 ppm (dd, J = 5.1, 0.5 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 13.3, 15.5, 15.6, 39.7, 122.0, 123.5, 125.6, 138.3, 141.3, 144.4, 149.7, 151.8 ppm; MS-ESI m/z: [M + H]+ calcd for C12H14ClN3S, 268.0; found, 268.0; HPLC (method 2): tR = 2.250 min.
2-Chloro-4-(1-cyclopropyl-4-methyl-2-(methylthio)-1H-imidazol-5-yl)pyridine (56)
The title compound was synthesized following the same procedure of compound 54 starting from 53 (210 mg, 0.74 mmol), t-BuONa (71.4 mg, 0.74 mmol), and methyl iodide (135 μL, 2.16 mmol) giving 200 mg of the product, which was used in the following step without further purification (95% yield); 1H NMR (300 MHz, CDCl3): δ 0.59–0.71 (m, 2H), 0.93–1.03 (m, 2H), 2.27 (s, 3H), 2.67 (s, 3H), 3.03–3.14 (m, 1H), 7.19 (dd, J = 5.2, 1.5 Hz, 1H), 7.27–7.31 (m, 1H), 8.39 ppm (d, J = 5.1 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 9.6, 13.9, 14.6, 26.1, 121.7, 123.1, 126.9, 138.1, 141.3, 148.3, 149.3, 151.5 ppm; MS-ESI m/z: [M + H]+ calcd for C13H14ClN3S, 280.1; found, 280.0; HPLC (method 2): tR = 2.763 min.
4-(1,4-Dimethyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (57)
In an argon-flushed pressure tube, compound 54 (100 mg, 0.39 mmol), 4-morpholinoaniline (105.3 mg, 0.59 mmol), Pd2(dba)3 (36.1 mg, 0.04 mmol), 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (XPhos) (37.18 mg, 0.08 mmol), and Cs2CO3 (770.2 mg, 2.36 mmol) were suspended in dry 1,4-dioxane, and after closing the vial tightly, the mixture was stirred at 100 °C for 36 h. The solvent was evaporated at reduced pressure and after that NH4Cl saturated solution was added to the residue and the aqueous phase was extracted five times with EtOAc. The combined organic layers were washed twice with H2O and NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. Finally, the residue was purified twice by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10) and (SiO2 DCM/EtOH 97:03) giving 30 mg of the desired product (20% yield); 1H NMR (300 MHz, CDCl3): δ 2.23 (s, 3H), 2.62 (s, 3H), 3.07–3.23 (m, 4H), 3.46 (s, 3H), 3.83–3.94 (m, 4H), 6.52–6.62 (m, 2H), 6.72 (br s, 1H), 6.92 (d, J = 8.3 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 8.19 ppm (d, J = 5.1 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 13.7, 15.9, 32.2, 49.6, 66.8, 107.2, 114.1, 116.7, 124.2, 128.4, 131.6, 137.5, 140.4, 144.3, 146.7, 148.5, 157.1 ppm; MS-ESI m/z: [M + H]+ calcd for C21H25N5OS, 396.2; found, 396.5; m/z: [M – H]− calcd for C21H25N5OS, 394.2; found, 394.3; HPLC (method 2): tR = 2.116 min (99.3%).
4-(1-Ethyl-4-methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (58)
Under an argon atmosphere, 4-morpholinoaniline (98.8 mg, 0.55 mmol), Pd2(dba)3 (16.94 mg, 0.02 mmol), XPhos (17.64 mg, 0.04 mmol), and Cs2CO3 (365 mg, 1.12 mmol) were placed and after that compound 55 (100 mg, 0.37 mmol) previously dissolved in 5 mL of dry 1,4-dioxane was added and the reaction mixture was stirred at 100 °C for 18 h. The solvent was evaporated at reduced pressure and after that NH4Cl saturated solution was added to the residue and the aqueous phase was extracted three times with EtOAc. The combined organic layers were washed with H2O and NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (SiO2, DCM/EtOH 100:0 to 95:05) giving 30 mg of the desired product (20% yield); 1H NMR (300 MHz, CDCl3): δ 1.16 (t, J = 7.1 Hz, 3H), 2.20 (s, 3H), 2.63 (s, 3H), 3.05–3.22 (m, 4H), 3.73–3.99 (m, 6H), 6.49–6.64 (m, 2H), 6.81–7.03 (m, 3H), 7.24 (d, J = 8.7 Hz, 2H), 8.19 ppm (d, J = 5.0 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 13.5, 15.8, 16.1, 39.8, 49.8, 66.9, 107.2, 114.6, 116.8, 124.0, 127.9, 132.3, 137.2, 140.3, 143.0, 148.3, 148.4, 157.7 ppm; MS-ESI m/z: [M + H]+ calcd for C22H27N5OS, 410.2; found, 410.1; m/z: [M – H]− calcd for C22H27N5OS, 408.2; found, 408.1; HPLC (method 2): tR = 2.427 min (98.0%).
4-(1-Cyclopropyl-4-methyl-2-(methylthio)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (59)
The title compound was synthesized following the same procedure used for the preparation of compound 58 starting from 56 (150 mg, 0.53 mmol), 4-morpholinoaniline (141.7 mg, 0.79 mmol), Pd2(dba)3 (24.7 mg, 0.03 mmol), XPhos (25.2 mg, 0.05 mmol), and Cs2CO3 (524 mg, 1.61 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 95:05) afforded 86 mg of the desired product (40% yield); 1H NMR (300 MHz, CDCl3): δ 0.63–0.72 (m, 2H), 0.87–0.97 (m, 2H), 2.22 (s, 3H), 2.65 (s, 3H), 2.91–3.02 (m, 1H), 3.09–3.19 (m, 4H), 3.80–3.93 (m, 4H), 6.63 (br s, 1H), 6.66 (d, J = 5.1 Hz, 1H), 6.81 (br s, 1H), 6.91 (d, J = 8.9 Hz, 2H), 7.24 (d, J = 8.9 Hz, 2H), 8.16 ppm (d, J = 5.1 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ 9.4, 13.8, 14.7, 26.1, 49.7, 66.8, 107.0, 114.3, 116.7, 124.0, 128.6, 132.0, 137.0, 140.7, 146.4, 147.1, 148.3, 156.9 ppm; MS-ESI m/z: [M + H]+ calcd for C23H27N5OS, 422.2; found, 422.0; m/z: [M – H]− calcd for C23H27N5OS, 420.2; found, 420.1; HPLC (method 2): tR = 2.989 min (98.0%).
2-Bromo-4-(1-(4-fluorophenyl)-1H-imidazol-5-yl)pyridine (64)
2-Bromoisonicotinaldehyde (60, 300 mg, 1.61 mmol), 4-fluoroaniline (179 mg, 1.61 mmol), and AcOH (160 μL), were dissolved in EtOH and the reaction mixture was stirred at reflux temperature for 2 h. After cooling to rt, the solvent was evaporated at reduced pressure and the residue was resuspended in a mixture 2:1 of MeOH and 1,2-dimethoxyethane (8 mL) and transferred into a three-neck round-bottom flask under an argon atmosphere. TOSMIC (471.5 mg, 2.41 mmol) and K2CO3 (445 mg, 3.22 mmol) were added and the mixture was stirred at reflux temperature for 3 h. The mixture was cooled at rt and the solvent was evaporated at reduced pressure. The residue was suspended in DCM and the organic layer was washed three times with H2O and one time with NaCl saturated solution. The organic phase was dried over anhydrous Na2SO4 and evaporated at reduced pressure. Finally, the residue was purified by flash column chromatography (SiO2, DCM to DCM/EtOH 95:05) yielding 360 mg of the desired compound (70% yield); 1H NMR (400 MHz, DMSO-d6): δ 7.03 (d, J = 4.3 Hz, 1H), 7.30–7.50 (m, 5H), 7.69 (br s, 1H), 8.08 (br s, 1H), 8.24 ppm (d, J = 4.3 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 116.6 (d, J = 22.7 Hz), 120.5, 124.6, 128.3 (d, J = 8.8 Hz), 128.4, 131.9, 132.1 (d, J = 2.9 Hz), 139.5, 141.7, 142.0, 150.3, 161.8 ppm (d, J = 246.6 Hz); MS-ESI m/z: [M + H]+ calcd for C14H9BrFN3, 318.0; found, 317.8; HPLC (method 1): tR = 5.51 min.
2-Chloro-4-(1-methyl-1H-imidazol-5-yl)pyridine (65)
Tetrakis(triphenylphosphine)palladium (367 mg, 0,317 mmol) was dissolved in dimethylformamide (DMF) (50 mL) and after that 5-bromo-1-methyl-1H-imidazole (62) (2.04 g, 12.7 mmol), (2-chloropyridin-4-yl)boronic acid (63) (1.0 g, 6.35 mmol), Cs2CO3 (4.13 g, 12.7 mmol), and H2O (228 mg, 12.7 mmol) were added and the reaction mixture was stirred at 60 °C for 24 h. The mixture was poured in H2O and the aqueous phase was extracted five times with EtOAc. The combined organic layers were washed with NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. The residue obtained was purified by flash column chromatography (SiO2, DCM/EtOH 95:05 to 90:10), affording 160 mg of the desired product (13% yield); 1H NMR (250 MHz, CDCl3): δ 3.79 (s, 3H), 7.27 (dd, J = 5.2, 1.6 Hz, 2H), 7.34 (br s, 1H), 7.38 (dd, J = 1.6, 0.6 Hz, 1H), 7.60 (br s, 1H), 8.43 ppm (dd, J = 5.2, 0.6 Hz, 1H); MS-ESI m/z: [M + H]+ calcd for C9H8BrN3, 194.0; found, 194.0; HPLC (method 2): tR = 1.162 min.
4-(1-(4-Fluorophenyl)-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (66)
Under an argon atmosphere, compound 64 (200 mg, 0.62 mmol), 4-morpholinoaniline (134.5 mg, 0.75 mmol), t-BuONa (83.8 mg, 0.87 mmol), Pd2(dba)3 (20 mg, 0.02 mmol), and 2,2′-bis(difenilfosfino)-1,1′-binaftile (BINAP) (30 mg, 0.02 mmol) were dissolved in dry toluene (15 mL) and the reaction mixture was then stirred for 3 h at 80 °C. After removing the solvent at reduced pressure, the residue was suspended in H2O and the aqueous phase was extracted with EtOAc. The combined organic layers were then dried over anhydrous Na2SO4 and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (DCM/MeOH 100:0 to 95:05), affording 62 mg of the product (24% yield); 1H NMR (400 MHz, DMSO-d6): δ 3.01 (s, 4H), 3.73 (s, 4H), 6.41–6.44 (m, 2H), 6.80–6.82 (m, 2H), 7.22–7.24 (m, 2H), 7.37–7.42 (m, 5H), 7.99 (s, 2H), 8.70 ppm (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 49.3, 66.2, 106.3, 111.8, 115.8, 116.6 (d, J = 22.0 Hz), 127.4 (d, J = 9.0 Hz), 129.9, 132.5, 133.5, 137.3, 140.8, 146.0, 147.9, 156.6, 161.6 ppm (d, J = 244.0 Hz); FAB–MS m/z: [M] calcd for C24H22FN5O, 415.2; found, 415.3; HPLC (method 1): tR = 5.08 min (100%).
4-(1-Methyl-1H-imidazol-5-yl)-N-(4-morpholinophenyl)pyridin-2-amine (67)
The title compound was synthesized according to general procedure A starting from 65 (140 mg, 0.72 mmol) and 4-morpholinoaniline (192.5 mg, 1.08 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 100:0 to 90:10) afforded 50 mg of the desired compound (35% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.97–3.07 (m, 4H), 3.69–3.79 (m, 7H), 6.78–6.85 (m, 2H), 6.89 (m, J = 9.0 Hz, 2H), 7.22 (d, J = 1.2 Hz, 1H), 7.52 (m, J = 9.0 Hz, 2H), 7.76 (br s, 1H), 8.11 (d, J = 5.1 Hz, 1H), 8.85 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 32.9, 49.4, 66.2, 106.9, 111.7, 115.9, 119.9, 128.9, 130.7, 134.0, 137.8, 141.1, 145.8, 147.8, 156.8 ppm; MS-ESI m/z: [M + H]+ calcd for C19H21N5O, 336.2; found, 336.3; m/z: [M – H]− calcd for C19H21N5O, 334.2; found, 334.3; HPLC (method 1): tR = 2.048 min (100%).
2-Chloro-4-(1H-imidazol-2-yl)pyridine (69)
To a solution of 2-chloroisonicotinonitrile (68) (2.0 g, 14.44 mmol) in MeOH (8 mL), a 30% solution of NaOMe in MeOH (260 μL, 1.44 mmol) was added and the reaction mixture was stirred at 40 °C for 1 h. After that both 2,2-dimethoxyethan-1-amine (1.56 mL, 14.44 mmol) and AcOH (1.56 mL, 27.27 mmol) were added dropwise and the mixture was stirred at reflux temperature for 30 min. After cooling to rt, the mixture was diluted with MeOH (8 mL) and then 6 N HCl solution (7.2 mL, 43.2 mmol) was added and the mixture was stirred at reflux temperature for 18 h. The solvent was evaporated at reduced pressure and after that a 10% solution of K2CO3 was added to the residue until reaching pH = 10. The precipitate obtained was filtered off and washed with H2O, affording 2.01 g of the product which was used for the following step without further purification (77% yield); 1H NMR (400 MHz, DMSO-d6): δ 7.30 (br s, 2H), 7.78–7.91 (m, 1H), 7.95 (br s, 1H), 8.36–8.49 (m, 1H), 13.03 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 118.1, 118.6, 124.8, 140.7, 141.9, 150.5, 151.1 ppm; MS-ESI m/z: [M + H]+ calcd for C8H6ClN3, 180.0; found, 179.8; m/z: [M – H]− calcd for C8H6ClN3, 178.0; found, 177.8; HPLC (method 1): tR = 2.346 min.
2-Chloro-4-(1-methyl-1H-imidazol-2-yl)pyridine (70)
Under an argon atmosphere, compound 69 (1.72 g, 9.61 mmol) was dissolved in dry DMF (20 mL) and after cooling the reaction mixture to 0 °C, NaH (231 mg, 9.61 mmol) was added and the mixture was stirred at 0 °C for 15 min. After that methyl iodide (1.61 mL, 25.6 mmol) was added dropwise and the reaction mixture was let to heat to rt and stirred for 90 min. The mixture was poured in H2O and the aqueous phase was extracted three times with DCM. The combined organic layers were dried over anhydrous Na2SO4 and the solvent was evaporated at reduced pressure. Finally, the residue was treated with a mixture of n-hexane/EtOAc 40:1 and the solid obtained was filtered off and dried in vacuo, affording 793 mg of the product which was used for the following step without further purification (43% yield); 1H NMR (400 MHz, DMSO-d6): δ 3.82–3.93 (m, 3H), 7.09 (br s, 1H), 7.40 (br s, 1H), 7.71–7.78 (m, 1H), 7.80 (br s, 1H), 8.40–8.53 ppm (m, 1H); 13C NMR (101 MHz, DMSO-d6): δ 34.8, 121.0, 121.6, 125.8, 128.7, 140.9, 142.3, 150.1, 150.8 ppm; MS-ESI m/z: [M + H]+ calcd for C9H8ClN3, 194.0; found, 193.8; HPLC (method 1): tR = 1.161 min.
4-(1-Methyl-1H-imidazol-2-yl)-N-(4-morpholinophenyl)pyridin-2-amine (71)
The title compound was synthesized according to general procedure A starting from 70 (150 mg, 0.77 mmol) and 4-morpholinoaniline (205 mg, 1.15 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 95:05) afforded 100 mg of the desired compound (39% yield); 1H NMR (400 MHz, DMSO-d6): δ 2.93–3.09 (m, 4H), 3.66–3.77 (m, 4H), 3.81 (s, 3H), 6.90 (d, J = 8.3 Hz, 2H), 6.95–7.04 (m, 2H), 7.09 (s, 1H), 7.31 (s, 1H), 7.52 (d, J = 8.1 Hz, 2H), 8.15 (d, J = 4.8 Hz, 1H), 8.89 ppm (br s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 34.6, 49.4, 66.1, 107.8, 111.9, 115.9, 120.0, 124.5, 127.9, 133.9, 138.5, 144.3, 145.8, 147.6, 156.7 ppm; FAB–MS m/z: [M] calcd for C19H21N5O, 335.2; found, 335.3; HPLC (method 1): tR = 2.359 min (100%).
2-Bromo-1-(4-fluorophenyl)-2-(2-fluoropyridin-4-yl)ethan-1-one (73)51
Compound 72(19) (1.0 g, 4.29 mmol) was dissolved in 30% HBr in AcOH (6 mL). After cooling the reaction mixture to 0 °C, Br2 (220 μL, 4.29 mmol) was added dropwise and the reaction mixture was heated for 6 h at 40 °C. After evaporating the solvent at reduced pressure, H2O was added and the pH was adjusted to 8 using NH4OH solution. The water layer was then extracted three times by DCM and the combined organic layers were dried over anhydrous Na2SO4 and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (SiO2, n-hexane/EtOAc 7:3), affording 1.0 g of the desired compound (75% yield). Analytical data were in agreement with the reported ones.51
4-(4-Fluorophenyl)-5-(2-fluoropyridin-4-yl)-N-methylthiazol-2-amine (74)
Compound 73 (513 mg, 1.64 mmol) and N-methylthiourea (148 mg, 1.64 mmol) were dissolved in EtOH and the reaction mixture was stirred at reflux temperature for 1 h. After cooling to rt, the solvent was evaporated at reduced pressure and then H2O was added. The solution was alkalized to pH = 8 and then extracted three times with DCM. The combined organic layers were dried over anhydrous Na2SO4, concentrated at reduced pressure, and the residue was purified by flash column chromatography (SiO2, DCM/EtOH 95:05), obtaining 495 mg of the desired compound (99% yield): 1H NMR (250 MHz, DMSO-d6): δ 2.89 (d, J = 4.6 Hz, 3H), 6.77 (br s, 1H), 6.93–7.02 (m, 1H), 7.14–7.29 (m, 2H), 7.40–7.52 (m, 2H), 8.04 (d, J = 5.4 Hz, 1H), 8.10 ppm (q, J = 4.8 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 30.8, 106.8 (d, J = 39.5 Hz), 114.2 (d, J = 3.7 Hz), 115.4 (d, J = 21.2 Hz), 120.4 (d, J = 3.7 Hz), 130.9 (d, J = 8.1 Hz), 131.4 (d, J = 3.7 Hz), 145.9 (d, J = 8.8 Hz), 147.6 (d, J = 16.1 Hz), 149.2, 162.0 (d, J = 245.9 Hz), 163.4 (d, J = 234.2 Hz), 168.3 ppm; MS-ESI m/z: [M + H]+ calcd for C15H11F2N3S, 304.3; found, 304.1; m/z: [M – H]− calcd for C15H11F2N3S, 302.3; found, 302.1; HPLC (method 2): tR = 7.123 min.
4-(4-Fluorophenyl)-N-methyl-5-(2-((4-morpholinophenyl)amino)pyridin-4-yl)thiazol-2-amine (75)
The title compound was synthesized according to general procedure A starting from 74 (200 mg, 0.66 mmol) and 4-morpholinoaniline (176.4 mg, 0.99 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 97:03 to 90:10) afforded 143 mg of the desired compound (47% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.87 (d, J = 4.9 Hz, 3H), 2.94–3.07 (m, 4H), 3.64–3.82 (m, 4H), 6.37 (dd, J = 5.4, 1.7 Hz, 1H), 6.54 (d, J = 1.0 Hz, 1H), 6.82 (m, J = 9.0 Hz, 2H), 7.12–7.25 (m, 2H), 7.27–7.38 (m, 2H), 7.42–7.55 (m, 2H), 7.85 (q, J = 4.6 Hz, 1H), 7.93 (d, J = 5.4 Hz, 1H), 8.69 ppm (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 30.7, 49.3, 66.1, 107.6, 112.7, 115.2 (d, J = 21.2 Hz), 115.8, 116.3, 120.0, 130.8 (d, J = 8.1 Hz), 131.8 (d, J = 2.9 Hz), 133.7, 141.0, 145.8, 146.7, 147.8, 156.7, 161.7 (d, J = 244.4 Hz), 167.3 ppm; MS-ESI m/z: [M + H]+ calcd for C25H24FN5OS, 462.2; found, 462.1; m/z: [M – H]− calcd for C25H24FN5OS, 460.2; found, 460.1; HPLC (method 2): tR = 8.518 min (98.2%).
2-Bromo-1-(2-chloropyridin-4-yl)propan-1-one (76)
1-(2-Chloropyridin-4-yl)propan-1-one (22) (3.0 g, 17.68 mmol) was dissolved in a 30% solution of HBr in AcOH (20 mL), and after cooling the mixture to 0 °C, bromine (900 μL, 17.68 mmol) was added and the reaction mixture was stirred at 45 °C for 2 h and then heated to 75 °C and stirred for additional 2 h. After evaporating the solvent at reduced pressure, H2O was added and the pH was adjusted to 9 using NH4OH solution. The aqueous phase was extracted three times with DCM and the combined organic layers were washed with H2O, dried over anhydrous Na2SO4, and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (n-hexane/EtOAc 90:10 to 80:20), affording 2.3 g of the desired product (52% yield); 1H NMR (300 MHz, CDCl3): δ 1.85 (d, J = 6.6 Hz, 3H), 5.07 (q, J = 6.6 Hz, 1H), 7.64 (dd, J = 5.1, 1.5 Hz, 1H), 7.76 (dd, J = 1.4, 0.7 Hz, 1H), 8.53 ppm (dd, J = 5.1, 0.7 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 19.5, 41.2, 120.4, 123.0, 143.2, 150.9, 152.9, 191.2 ppm; MS-ESI m/z: [M + H]+ calcd for C8H7BrClNO, 247.9; found, 248.0; m/z: [M + MeOH]+ calcd for C8H7BrClNO, 279.9; found, 280.0; HPLC (method 2): tR = 5.761 min.
4-(2-Chloropyridin-4-yl)-N,5-dimethylthiazol-2-amine (77)
Compound 76 (1.0 g, 4.0 mmol) and N-methylthiourea (362.7 mg, 4.0 mmol) were dissolved in EtOH (20 mL) and the reaction mixture was stirred at reflux temperature for 1 h. The solvent was evaporated at reduced pressure and after that the residue was suspended in H2O and the pH was adjusted to 8 using NH4OH solution. The resulting suspension was extracted three times with DCM and the combined organic layers were washed with H2O and NaCl saturated solution, dried over anhydrous Na2SO4, and concentrated at reduced pressure. Finally, the residue was purified by flash column chromatography (SiO2, DCM/EtOH 95:05) giving 510 mg of the desired product (53% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.43 (s, 3H), 2.83 (d, J = 4.9 Hz, 3H), 7.47 (q, J = 4.9 Hz, 1H), 7.61 (dd, J = 5.1, 1.5 Hz, 1H), 7.64–7.66 (m, 1H), 8.40 ppm (dd, J = 5.1, 0.7 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ 12.2, 30.5, 120.4, 121.2, 121.8, 141.0, 145.7, 149.8, 150.6, 165.5 ppm; MS-ESI m/z: [M + H]+ calcd, for C10H10ClN3S, 240.0; found, 239.9; HPLC (method 2): tR = 9.091 min.
N,5-Dimethyl-4-(2-((4-morpholinophenyl)amino)pyridin-4-yl)thiazol-2-amine (78)
The title compound was synthesized according to general procedure A starting from 77 (150 mg, 0.62 mmol) and 4-morpholinoaniline (165.8 mg, 0.93 mmol). Purification by flash column chromatography (SiO2, DCM/EtOH 95:05) afforded 42 mg of the desired compound (17% yield); 1H NMR (250 MHz, DMSO-d6): δ 2.40 (s, 3H), 2.82 (d, J = 4.9 Hz, 3H), 2.95–3.07 (m, 4H), 3.67–3.80 (m, 4H), 6.81–6.93 (m, 3H), 7.01 (br s, 1H), 7.34 (q, J = 4.6 Hz, 1H), 7.46–7.58 (m, 2H), 8.07 (d, J = 5.4 Hz, 1H), 8.78 ppm (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ 12.3, 30.6, 49.5, 66.2, 108.2, 112.6, 115.9, 117.3, 119.7, 134.4, 143.3, 143.3, 145.5, 147.2, 156.6, 165.4 ppm; MS-ESI m/z: [M + H]+ calcd for C20H23N5OS, 382.2; found, 382.2; m/z: [M – H]− calcd for C20H23N5OS, 380.2; found, 380.2; HPLC (method 2): tR = 5.343 (99.4%).
Acknowledgments
The authors gratefully acknowledge Katharina Bauer, Jens Strobach, and Daniela Müller for the biological assays of the synthesized compounds. Moreover, we thank Philipp Krause, Urs Haun, and Raphael Ceamanos-Matilla for their support in the synthesis of some of the presented derivatives. Finally, we are grateful to Dr. Georg Zocher for his support with diffraction data processing and analysis, Dr. Tobias Pflüger (NanoTemper Technologies, Munich) for his assistance with nanoDSF measurements and the beamline staff of Swiss Light Source (SLS, Villigen, Switzerland) for assistance with crystallographic data collection.
Glossary
Abbreviations
- MAPK
mitogen activated protein kinase
- JNK
c-Jun N terminal kinase
- TOSMIC
toluene sulfonylmethylisocyanide
- HR
hydrophobic region
- AMP–PCP
β,γ-methyleneadenosine-5′-triphosphate
- nanoDSF
nano differential scanning fluorimetry
- hERG
human-ether-à-go-go related gene
- CYP450
cytochrome P450
- TLC
thin layer chromatography
- HPLC
high performance liquid chromatography
- DAD
diode array detector
- NMR
nuclear magnetic resonance
- ESI-MS
electrospray ionization mass spectrometer
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00668.
Detailed procedures for the preparation of intermediates 17a–l and 33–35; crystal structure of compound 49; melting curves and method description of the nanoDSF experiments; experimental procedures for the crystallization and structure determination of JNK3 in the complex with compounds 38, 44, and AMP–PCP; selectivity screening of 44; and in vitro metabolic stability study for compound 44 (PDF)
SMILES strings of tested compounds (CSV)
Accession Codes
The atomic coordinates and structures factors of complex structures containing compounds 38 and 44 and AMP–PCP were deposited in the Protein Data Bank (PDB) with the respective accession codes 6EMH, 6EKD, and 6EQ9, respectively. The authors will release the atomic coordinates and experimental data upon article publication.
Author Present Address
§ Chemistry Department, Faculty of Science, Menofia University, Gamal Abdel-Nasser Street, 32511 Shebin El-Koum, Menofia, Egypt.
Author Present Address
∥ Department of Applied Sciences, Chemistry Branch, University of Technology-Baghdad, Sinaa’ Street, 10066, Baghdad, Iraq.
The authors declare no competing financial interest.
Supplementary Material
References
- Margutti S.; Laufer S. A. Are MAP kinases drug targets? Yes, but difficult ones. ChemMedChem 2007, 2, 1116–1140. 10.1002/cmdc.200600271. [DOI] [PubMed] [Google Scholar]
- Barr R. K.; Bogoyevitch M. A. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int. J. Biochem. Cell Biol. 2001, 33, 1047–1063. 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- Davis R. J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M. A. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. BioEssays 2006, 28, 923–934. 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- Graczyk P. P. JNK inhibitors as anti-inflammatory and neuroprotective agents. Future Med. Chem. 2013, 5, 539–551. 10.4155/fmc.13.34. [DOI] [PubMed] [Google Scholar]
- Resnick L.; Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discovery Today 2004, 9, 932–939. 10.1016/s1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- Manning A. M.; Davis R. J. Targeting JNK for therapeutic benefit: from junk to gold?. Nat. Rev. Drug Discovery 2003, 2, 554–565. 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Ebelt N. D.; Cantrell M. A.; Van Den Berg C. L. c-Jun N-Terminal Kinases Mediate a Wide Range of Targets in the Metastatic Cascade. Genes Cancer 2013, 4, 378–387. 10.1177/1947601913485413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F.; Nebreda Á. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Gehringer M.; Muth F.; Koch P.; Laufer S. A. c-JunN-terminal kinase inhibitors: a patent review (2010 - 2014). Expert Opin. Ther. Pat. 2015, 25, 849–872. 10.1517/13543776.2015.1039984. [DOI] [PubMed] [Google Scholar]
- Koch P.; Gehringer M.; Laufer S. A. Inhibitors of c-Jun N-terminal kinases: an update. J. Med. Chem. 2015, 58, 72–95. 10.1021/jm501212r. [DOI] [PubMed] [Google Scholar]
- Siddiqui M. A.; Reddy P. A. Small Molecule JNK (c-Jun N-Terminal Kinase) Inhibitors. J. Med. Chem. 2010, 53, 3005–3012. 10.1021/jm9003279. [DOI] [PubMed] [Google Scholar]
- Cuenda A.; Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta, Mol. Cell Res. 2007, 1773, 1358–1375. 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Corrêa S. A. L.; Eales K. L. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J. Signal Transduction 2012, 2012, 1. 10.1155/2012/649079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Boehm J.; Lee J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discovery 2003, 2, 717–726. 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Muth F.; Günther M.; Bauer S. M.; Döring E.; Fischer S.; Maier J.; Drückes P.; Köppler J.; Trappe J.; Rothbauer U.; Koch P.; Laufer S. A. Tetra-Substituted Pyridinylimidazoles As Dual Inhibitors of p38α Mitogen-Activated Protein Kinase and c-Jun N-Terminal Kinase 3 for Potential Treatment of Neurodegenerative Diseases. J. Med. Chem. 2015, 58, 443–456. 10.1021/jm501557a. [DOI] [PubMed] [Google Scholar]
- Genovese M. C. Inhibition of p38: Has the fat lady sung?. Arthritis Rheum. 2009, 60, 317–320. 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- Koch P.; Ansideri F. 2-Alkylsufanyl-4(5)-aryl-5(4)-heteroarylimidazoles: An Overview on Synthetic Strategies and Biological Activity. Arch. Pharm. 2017, 350, 1700258. 10.1002/ardp.201700258. [DOI] [PubMed] [Google Scholar]
- Ansideri F.; Lange A.; El-Gokha A.; Boeckler F. M.; Koch P. Fluorescence polarization-based assays for detecting compounds binding to inactive c-Jun N-terminal kinase 3 and p38α mitogen-activated protein kinase. Anal. Biochem. 2016, 503, 28–40. 10.1016/j.ab.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Ansideri F.; Dammann M.; Boeckler F. M.; Koch P. Fluorescence polarization-based competition binding assay for c-Jun N-terminal kinases 1 and 2. Anal. Biochem. 2017, 532, 26–28. 10.1016/j.ab.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Muth F.; El-Gokha A.; Ansideri F.; Eitel M.; Döring E.; Sievers-Engler A.; Lange A.; Boeckler F. M.; Lämmerhofer M.; Koch P.; Laufer S. A. Tri- and Tetrasubstituted Pyridinylimidazoles as Covalent Inhibitors of c-Jun N-Terminal Kinase 3. J. Med. Chem. 2017, 60, 594–607. 10.1021/acs.jmedchem.6b01180. [DOI] [PubMed] [Google Scholar]
- Marckwald W. Ein Beitrag zur Kenntniss der Imidazole und der Constitution des Glyoxalins. Ber. Dtsch. Chem. Ges. 1892, 25, 2354–2373. 10.1002/cber.18920250227. [DOI] [Google Scholar]
- Laufer S. A.; Wagner G. K.; Kotschenreuther D. A.; Albrecht W. Novel substituted pyridinyl imidazoles as potent anticytokine agents with low activity against hepatic cytochrome P450 enzymes. J. Med. Chem. 2003, 46, 3230–3244. 10.1021/jm030766k. [DOI] [PubMed] [Google Scholar]
- Koch P.; Laufer S. Unexpected Reaction of 2-Alkylsulfanylimidazoles to Imidazol-2-ones: Pyridinylimidazol-2-ones as Novel Potent p38α Mitogen-Activated Protein Kinase Inhibitors. J. Med. Chem. 2010, 53, 4798–4802. 10.1021/jm100161q. [DOI] [PubMed] [Google Scholar]
- Radzisewski B. Ueber Glyoxalin und seine Homologe. Ber. Dtsch. Chem. Ges. 1882, 15, 2706–2708. 10.1002/cber.188201502245. [DOI] [Google Scholar]
- Neber P. W.; Friedolsheim A. V. Über eine neue Art der Umlagerung von Oximen. Justus Liebigs Ann. Chem. 1926, 449, 109–134. 10.1002/jlac.19264490108. [DOI] [Google Scholar]
- Xi N.; Xu S.; Cheng Y.; Tasker A. S.; Hungate R. W.; Reider P. J. Regio-controlled synthesis of N-substituted imidazoles. Tetrahedron Lett. 2005, 46, 7315–7319. 10.1016/j.tetlet.2005.08.138. [DOI] [Google Scholar]
- Ansideri F.; Andreev S.; Kuhn A.; Albrecht W.; Laufer S.; Koch P. A Diverse and Versatile Regiospecific Synthesis of Tetrasubstituted Alkylsulfanylimidazoles as p38α Mitogen-Activated Protein Kinase Inhibitors. Molecules 2018, 23, 221. 10.3390/molecules23010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leusen A. M.; Wildeman J.; Oldenziel O. H. Chemistry of sulfonylmethyl isocyanides. 12. Base-induced cycloaddition of sulfonylmethyl isocyanides to carbon,nitrogen double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides. J. Org. Chem. 1977, 42, 1153–1159. 10.1021/jo00427a012. [DOI] [Google Scholar]
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- Voss M. E.; Beer C. M.; Mitchell S. A.; Blomgren P. A.; Zhichkin P. E. A simple and convenient one-pot method for the preparation of heteroaryl-2-imidazoles from nitriles. Tetrahedron 2008, 64, 645–651. 10.1016/j.tet.2007.11.009. [DOI] [Google Scholar]
- Hantzsch A.; Weber J. H. Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe). Ber. Dtsch. Chem. Ges. 1887, 20, 3118–3132. 10.1002/cber.188702002200. [DOI] [Google Scholar]
- Goettert M.; Graeser R.; Laufer S. A. Optimization of a nonradioactive immunosorbent assay for p38α mitogen-activated protein kinase activity. Anal. Biochem. 2010, 406, 233–234. 10.1016/j.ab.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Goettert M.; Luik S.; Graeser R.; Laufer S. A. A direct ELISA assay for quantitative determination of the inhibitory potency of small molecules inhibitors for JNK3. J. Pharm. Biomed. Anal. 2011, 55, 236–240. 10.1016/j.jpba.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sterling T.; Irwin J. J. ZINC 15 - Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Duckett D.; Chen W.; Ling Y. Y.; Cameron M. D.; Lin L.; Ruiz C. H.; LoGrasso P. V.; Kamenecka T. M.; Koenig M. Synthesis and SAR of novel isoxazoles as potent c-jun N-terminal kinase (JNK) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 161–164. 10.1016/j.bmcl.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapin G.; Patel S. B.; Lisnock J. M.; Becker J. W.; LoGrasso P. V. The Structure of JNK3 in Complex with Small Molecule Inhibitors. Chem. Biol. 2003, 10, 705–712. 10.1016/s1074-5521(03)00159-5. [DOI] [PubMed] [Google Scholar]
- Thaher B. A.; Koch P.; Schattel V.; Laufer S. Role of the Hydrogen Bonding Heteroatom–Lys53 Interaction between the p38α Mitogen-Activated Protein (MAP) Kinase and Pyridinyl-Substituted 5-Membered Heterocyclic Ring Inhibitors. J. Med. Chem. 2009, 52, 2613–2617. 10.1021/jm801467h. [DOI] [PubMed] [Google Scholar]
- Wagner G. K.; Kotschenreuther D.; Zimmermann W.; Laufer S. A. Identification of Regioisomers in a Series of N-Substituted Pyridin-4-yl Imidazole Derivatives by Regiospecific Synthesis, GC/MS, and1H NMR. J. Org. Chem. 2003, 68, 4527–4530. 10.1021/jo026619w. [DOI] [PubMed] [Google Scholar]
- Toledo L. M.; Lydon N. B.; Elbaum D. The structure-based design of ATP-site directed protein kinase inhibitors. Curr. Med. Chem. 1999, 6, 775–805. [PubMed] [Google Scholar]
- Kamenecka T.; Habel J.; Duckett D.; Chen W.; Ling Y. Y.; Frackowiak B.; Jiang R.; Shin Y.; Song X.; LoGrasso P. Structure-activity relationships and X-ray structures describing the selectivity of aminopyrazole inhibitors for c-Jun N-terminal kinase 3 (JNK3) over p38. J. Biol. Chem. 2009, 284, 12853–12861. 10.1074/jbc.m809430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst G. D.; Bowers S.; Sealy J. M.; Truong A. P.; Hom R. K.; Galemmo R. A.; Konradi A. W.; Sham H. L.; Quincy D. A.; Pan H.; Yao N.; Lin M.; Tóth G.; Artis D. R.; Zmolek W.; Wong K.; Qin A.; Lorentzen C.; Nakamura D. F.; Quinn K. P.; Sauer J.-M.; Powell K.; Ruslim L.; Wright S.; Chereau D.; Ren Z.; Anderson J. P.; Bard F.; Yednock T. A.; Griswold-Prenner I. Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorg. Med. Chem. Lett. 2011, 21, 315–319. 10.1016/j.bmcl.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Swahn B.-M.; Xue Y.; Arzel E.; Kallin E.; Magnus A.; Plobeck N.; Viklund J. Design and synthesis of 2′-anilino-4,4′-bipyridines as selective inhibitors of c-Jun N-terminal kinase-3. Bioorg. Med. Chem. Lett. 2006, 16, 1397–1401. 10.1016/j.bmcl.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Kamenecka T.; Jiang R.; Song X.; Duckett D.; Chen W.; Ling Y. Y.; Habel J.; Laughlin J. D.; Chambers J.; Figuera-Losada M.; Cameron M. D.; Lin L.; Ruiz C. H.; LoGrasso P. V. Synthesis, Biological Evaluation, X-ray Structure, and Pharmacokinetics of Aminopyrimidine c-jun-N-terminal Kinase (JNK) Inhibitors. J. Med. Chem. 2010, 53, 419–431. 10.1021/jm901351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer S. A.; Hauser D. R. J.; Domeyer D. M.; Kinkel K.; Liedtke A. J. Design, synthesis, and biological evaluation of novel Tri- and tetrasubstituted imidazoles as highly potent and specific ATP-mimetic inhibitors of p38 MAP kinase: focus on optimized interactions with the enzyme’s surface-exposed front region. J. Med. Chem. 2008, 51, 4122–4149. 10.1021/jm701529q. [DOI] [PubMed] [Google Scholar]
- Fricker M.; LoGrasso P.; Ellis S.; Wilkie N.; Hunt P.; Pollack S. J. Substituting c-Jun N-terminal kinase-3 (JNK3) ATP-binding site amino acid residues with their p38 counterparts affects binding of JNK- and p38-selective inhibitors. Arch. Biochem. Biophys. 2005, 438, 195–205. 10.1016/j.abb.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Krenitsky V. P.; Nadolny L.; Delgado M.; Ayala L.; Clareen S. S.; Hilgraf R.; Albers R.; Hegde S.; D’Sidocky N.; Sapienza J.; Wright J.; McCarrick M.; Bahmanyar S.; Chamberlain P.; Delker S. L.; Muir J.; Giegel D.; Xu L.; Celeridad M.; Lachowitzer J.; Bennett B.; Moghaddam M.; Khatsenko O.; Katz J.; Fan R.; Bai A.; Tang Y.; Shirley M. A.; Benish B.; Bodine T.; Blease K.; Raymon H.; Cathers B. E.; Satoh Y. Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 1433–1438. 10.1016/j.bmcl.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Heider F.; Haun U.; Döring E.; Kudolo M.; Sessler C.; Albrecht W.; Laufer S.; Koch P. From 2-Alkylsulfanylimidazoles to 2-Alkylimidazoles: An Approach towards Metabolically More Stable p38α MAP Kinase Inhibitors. Molecules 2017, 22, 1729. 10.3390/molecules22101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P.; Bäuerlein C.; Jank H.; Laufer S. Targeting the ribose and phosphate binding site of p38 mitogen-activated protein (MAP) kinase: Synthesis and biological testing of 2-alkylsulfanyl-, 4(5)-aryl-, 5(4)-heteroaryl-substituted imidazoles. J. Med. Chem. 2008, 51, 5630–5640. 10.1021/jm800373t. [DOI] [PubMed] [Google Scholar]
- Koch P.; Jahns H.; Schattel V.; Goettert M.; Laufer S. Pyridinylquinoxalines and Pyridinylpyridopyrazines as Lead Compounds for Novel p38α Mitogen-Activated Protein Kinase Inhibitors. J. Med. Chem. 2010, 53, 1128–1137. 10.1021/jm901392x. [DOI] [PubMed] [Google Scholar]
- Revesz L.; Blum E.; Di Padova F. E.; Buhl R.; Feifel R.; Gram H.; Hiestand P.; Manning U.; Rucklin G. Novel p38 inhibitors with potent oral efficacy in several models of rheumatoid arthritis. Bioorg. Med. Chem. Lett. 2004, 14, 3595–3599. 10.1016/j.bmcl.2004.03.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.