Abstract
Removal of oral biofilms involves the use of broad-spectrum antimicrobials, which eradicate both pathogenic and protective oral commensal species. Ideal therapeutics for dental caries should be able to selectively inhibit pathogenic biofilms caused by Streptococcus mutans. S. mutans extracellular glucosyltransferases (Gtfs), particularly GtfB and GtfC, synthesize predominantly water-insoluble glucans, which contribute to the structural scaffold of biofilms. The lead stilbene identified through our docking study against the catalytic domain of GtfC is a natural product known as piceatannol, which inhibited S. mutans biofilm formation in a dose-dependent manner, with considerable selectivity over growth inhibition of S. mutans and commensal streptococci. Binding kinetic analysis of piceatannol was performed using Octet RED against both GtfB and GtfC, which produced low micromolar KD values. Piceatannol inhibited S. mutans colonization in an in vivo drosophila model and a rat model of dental caries.
Introduction
Causative relationships between Streptococcus mutans (S. mutans) and dental caries have been established over the years.1 As the primary etiological agent, S. mutans has developed multiple mechanisms to integrate into the dental biofilm (plaque) to colonize tooth surfaces. One of the prominent pathways is initiated when S. mutans produces glucans, high-molecular-weight sticky glucosyl polymers, via glucosyltransferases (Gtfs) and adheres to the tooth surfaces, which then trap other oral bacteria, food debris, and salivary components to create a cariogenic biofilm environment.2
S. mutans possesses three Gtfs, which are products of gtfB, gtfC, and gtfD genes; GtfB synthesizes mostly insoluble glucans containing more of α-1,3-linked glucans and GtfC synthesizes a mixture of soluble and insoluble glucans, whereas GtfD synthesizes predominantly soluble glucans (containing more α-1,6-linked glucans).3,4 Previous work in this field has revealed that the deletion of gtfB and gtfC genes in S. mutans markedly disrupted microcolony formation and biofilm formation.5 Biological and immunochemical properties of both these enzymes are very similar. Genes encoding GtfB and GtfC lie next to each other, have 76% amino acid sequence homology, and are subject to similar regulatory processes.6−8 Therefore, GtfB and GtfC are valid targets for the structure-based discovery of S. mutans biofilm inhibitors.
Current approaches to eradicate dental biofilms include its mechanical removal and the use of nonspecific broad-spectrum antibiotics.9 The removal of bacterial biofilms through brushing demands frequent repetition because the tooth surfaces are rapidly recolonized.10 Similarly, antimicrobial agents in mouthwashes such as chlorhexidine and delmopinol lack selectivity, affecting both pathogenic species and commensal beneficial species, and give rise to undesired side effects such as vomiting, diarrhea, addiction, or teeth discoloration.9
Numerous natural products and their derivatives have been investigated for their potential to inhibit cariogenic plaque formation. These include constituents found in cranberry, plant lectins, crude extracts of Morus alba leaves, and components found in barley coffee.11,12 Most of the reported studies suggest these agents to be effective against biofilm formation of S. mutans through varying degrees of regulation of Gtfs.11,13 In addition, several small molecules, including anthraquinones,14 apigenin,15,16tt-farnesol,17,18 chitosan,19 7-epiclusianone,20,21 α-mangostin,22 myricetin,23,24 and honokiol25 have been characterized and shown to have antibiofilm activity toward S. mutans. However, the majority of these compounds do not exhibit high selectivity against S. mutans biofilms. Chemical structures of a few of these natural products are given in Figure 1.
Figure 1.
Chemical structures of some known S. mutans biofilm and Gtf inhibitors. (a) α-Mangostin; (b) myricetin; and (c) honokiol.
Prior studies have indicated that resveratrol inhibits glycolytic acid production and Gtf activity of S. mutans, when tested using an ethyl acetate extract from Pediomelum cuspidatum root, which is composed of polydatin, resveratrol, anthraglycoside B, and emodin.26−28 Our laboratory has a long-standing interest in developing selective anti-biofilm agents that target S. mutans virulence,29 and we have recently explored the effect of small molecules against S. mutans biofilms and developed Gtf-selective inhibitors.30−32 Nevertheless, there are no reports related to stilbene’s possible effect on the virulence of dental biofilms. Because of the promise demonstrated by polyphenols in the inhibition of Gtf and the ability of S. mutans to assemble biofilms, in the present study, we have performed in silico docking on natural and synthetic polyphenols against the X-ray crystal structure33 of GtfC’s catalytic domain active site and have successfully identified low micromolar inhibitors of both glucan production and S. mutans biofilm formation.
Results and Discussion
Structure-Based Virtual Screening of Natural Polyphenols
The three-dimensional (3D) crystal structure of GtfC in the complex with acarbose has been successfully employed to develop Gtf-selective inhibitors.33 We thus performed in silico docking, using FlexX/LeadIT software package, on a database of compounds containing at least one phenolic group against the high-resolution X-ray crystal structure of GtfC (PDB code: 3AIC).33 Top scoring compounds were examined for their binding interactions with key residues such as Glu515, Ala478, Tyr430, Asp959, Leu333, Gln960, Asp477, and Asp588, druglike properties based on Lipinski’s rules, and synthetic feasibility.
Inhibition of S. mutans Biofilms by Natural and Synthetic Stilbenes
Stilbenes obtained from the National Cancer Institute (NCI) were first evaluated for their biofilm inhibitory and growth inhibitory activities using previously reported assays.34 The results are summarized in Table 1. A range of activities were observed, with several compounds being inactive (compounds 1–3 and 13) and a few demonstrating inhibition of both S. mutans growth and biofilm at the micromolar range.
Table 1. Biofilm and Growth Inhibitory Activities of Stilbenes.
Average of at least five measurements; NI: no inhibition; NA: not available.
Our studies have demonstrated that the stilbene scaffold alone does not possess a biological effect against S. mutans, as both the E and Z isomers were inactive (compounds 1 and 2). A significant effect is seen with the variation of substituents. Compounds 3 and 4 maintain the regiochemistry of the substituents but differ in their functional groups. Whereas dihydroxyl stilbene 4 has demonstrated biofilm formation and growth with IC50 values of 344 and 854 μM respectively, diamino analogue 3 did not produce any activity in either growth or biofilm formation. Similarly, compound 5 is a diamidine compound that also has an extra hydroxyl group and has demonstrated biofilm formation and growth with IC50 values of 104 and 179 μM, respectively.
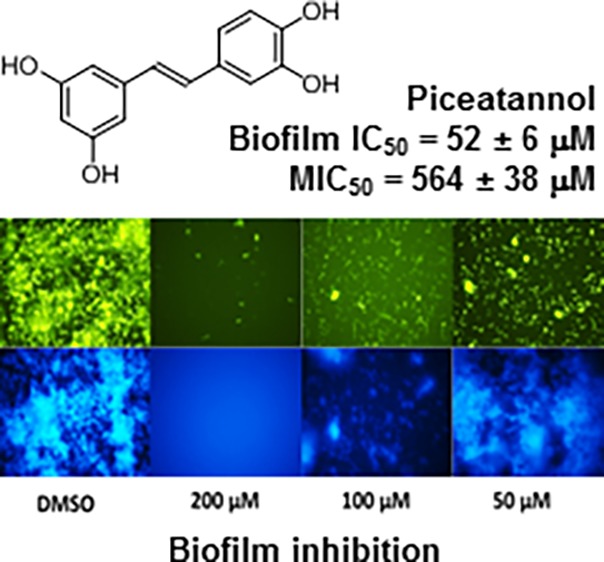
Compounds 6–9 explore the regiochemistry and the substituent effect of hydroxyl stilbenes. Compound 6 is the least active compound of this class, showing a high micromolar range activity. This compound also does not have any substituents on one of the rings. Compounds 7 and 8 are natural products, resveratrol and piceatannol, respectively. These compounds differ by the inclusion of one extra hydroxyl group. Previous studies have shown resveratrol to inhibit S. mutans biofilm.26,27,35 In comparison to that, compound 8 shows a marginally better activity toward S. mutans biofilm with a 52 μM IC50 value and also demonstrates increased (11-fold) selectivity. Compound 9 is a trimethoxy, monohydroxy analogue of piceatannol that maintains its regiochemistry. This scaffold demonstrated less biofilm inhibition, increased growth inhibition compared to piceatannol (8), and decreased selectivity, which suggests that the OH groups are important for the selectivity.
Because the glucan synthesis pathway involves the degradation of glycosidic bond in sucrose and the formation of new glucosidic bonds between glucosyl units, we were interested in exploring the effect of attaching a glucose unit to one of the active compounds. Compound 12 is a glucoside analogue of piceatannol 8, with the methylation of one of its hydroxyl groups. This compound also shows similar activity when compared to that of compound 9. However, compound 13 is a piceatannol analogue that has a glucose substituent that is not attached through a glucosidic bond, and this compound is not active against biofilm and growth. Finally, compounds 10–11 are miscellaneous scaffolds that resemble a stilbene. Of these compounds, compound 11 demonstrated considerable activity against the S. mutans biofilm. Overall, piceatannol (8) is the compound identified from this study that demonstrated good activity and selectivity toward S. mutans biofilm inhibition.
Our Lead Compound, Piceatannol, Inhibited Biofilms Selectively Over Growth
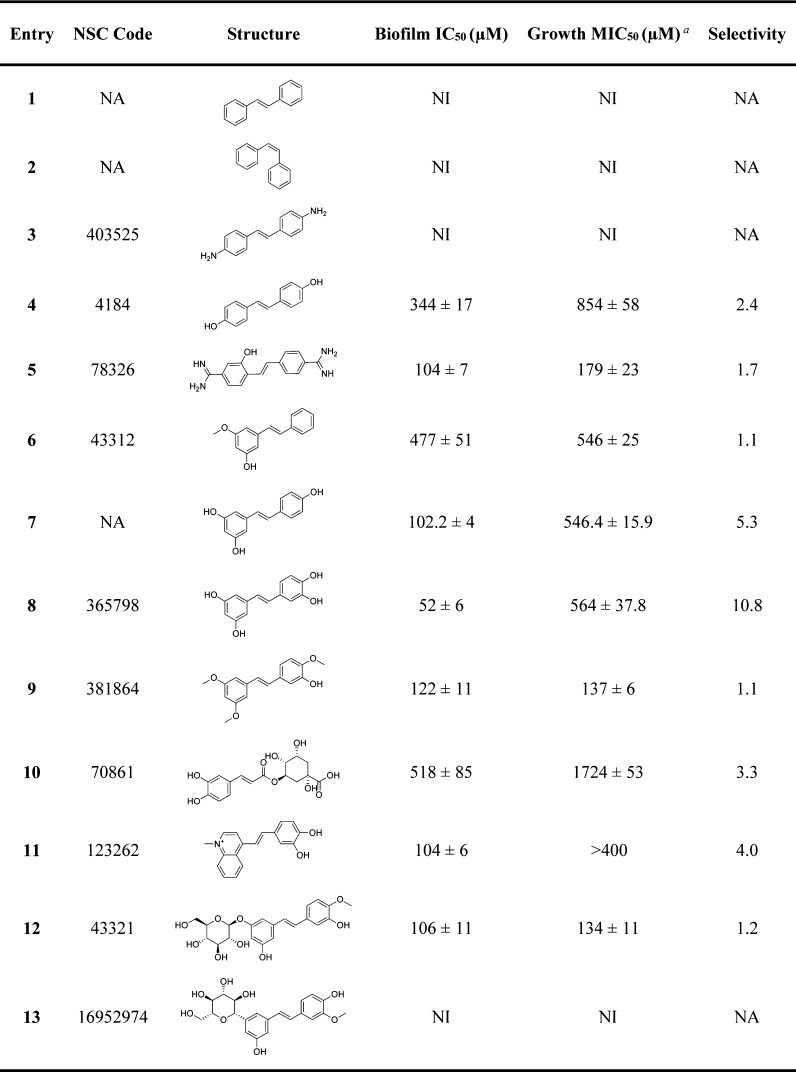
A comparison of piceatannol’s ability to inhibit S. mutans biofilm with reverastrol and E-stilbene shows our lead to be the most potent at 200 μM (Table 1 and Figure 2A). Upon further analysis of the compound’s effect on S. mutans growth, we have identified previously studied resveratrol26,27,35 and a novel agent called piceatannol to demonstrate biofilm inhibitory activity with IC50 values of 102 and 52 μM, respectively, with minimal bactericidal activity. We chose piceatannol for further analysis because of its source of existence, simplicity of structure, and ease of synthesis. Piceatannol is found in several natural sources ranging from roots of Norway spruces, seeds of the palm Aiphanes horrida, and in Gnetum cleistostachyum.36 It is also a metabolite of resveratrol, which is found in red wine, grapes, and passion fruit.37
Figure 2.
(A) Comparison of piceatannol and their structural analogues at 200 μM in the crystal violet biofilm assay. (B) Fluorescence microscopy images of the S. mutans biofilms treated with 8 (200–50 μM). Green images correspond to bacteria stained with SYTO9, whereas blue images correspond to the fluorescent glucans within the biofilm tracked by the cascade blue-labeled dextran. (C) Two-dimensional diagram of the proposed residues interacting with piceatannol. (D) Docking pose of piceatannol (blue) and acarbose (green) in the GtfC active site.
Docking Analysis of Piceatannol in the GtfC Active Site
Our docking model (Figure 2D) of piceatannol shows several key interactions. This pocket docked by the compound is at the same space occupied by acarbose, a weak inhibitor of GtfC that was cocrystallized with GtfC.33 The best docked structure, visualized by UCSF Chimera molecular modeling system, showed interactions of six amino acids: Asp909, Asp477, Glu515, His587, Asp480, and Trp517. It is already reported in the literature that binding of acarbose to Glu515 compromised the acid/base catalyst function, whereas interaction with Trp517 blocked the acceptor glycosyl moiety, explaining the inhibitory effects shown by acarbose when bound to GtfC.33 The hydroxyl functional groups interact with Asp477, Asp480, and Glu515 and have interactions with Asp909 and Trp517. The binding free energy of piceatannol predicted by FlexX software was −25 kJ/mol, indicating a stable and strong binding with the protein.
Binding and Inhibition of Gtfs by Piceatannol and Resveratrol
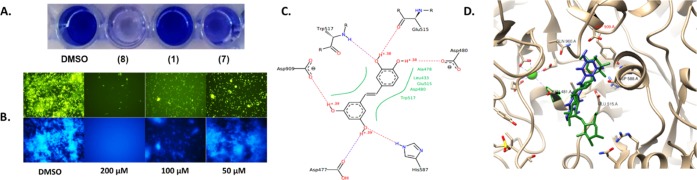
To verify the docking results, we used the Octet system to evaluate the binding of potent small-molecule compounds to GtfB and GtfC. Recombinant His-tagged GtfB and GtfC were produced, and HIS1K Biosensor was employed to capture and quantify His-tagged Gtfs for binding kinetic characterization. The kinetics of the binding of piceatannol and resveratrol with GtfB and GtfC were examined and are shown in Figure 3A–D. The best binding fits were observed with piceatannol, producing KD values of 14.6 μM (Figure 3A) and 1.58 μM (Figure 3B) for GtfB and GtfC, respectively. These data are consistent with the zymogram results, as the compound is more active against GtfC when compared to GtfB. Resveratrol was also subjected to the same analysis. Its KD values are 144 and 510 μM for GtfB and GtfC, respectively (Figure 3C,D). The piceatannol scaffold is more potent toward GtfB. All four experiments produced reliable R2 values and fit well in the 1:1 binding mode.
Figure 3.
Octet RED 96 analysis of (A) piceatannol and GtfB, (B) piceatannol and GtfC, (C) resveratrol and GtfB, and (D) resveratrol and GtfC. (E) Results of the zymogram assay conducted with serial dilution concentrations of piceatannol. (F) Results of the zymogram assay comparing effect of compound treatment on the GTF enzyme production.
In addition to quantitative analysis, we performed a zymogram assay to evaluate the inhibition of Gtf enzymatic activity qualitatively, as reported.3 Natural products, resveratrol and piceatannol, were added to the growth media initially to see if they had an effect on glucan production. The results parallel the observations seen in biofilms, as piceatannol greatly reduced glucan production when compared to the dimethyl sulfoxide (DMSO) control. Resveratrol showed marginal inhibition. To assess the dose-dependent effect, a zymogram assay was performed using same amounts of Gtf proteins in each of the lanes resolved on the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and submerged in different concentrations of the lead compound. Our results show an apparent decrease in the bottom band where GtfC produces its glucans; a decrease in the upper band is also observed in which GtfB and GtfD are comigrated, suggesting that the compound inhibits at least two, if not all three Gtfs (Figure 3E,F). The zymogram assay on resveratrol and E-stilbene paralleled the biofilm inhibition. A higher inhibition was seen with piceatannol in comparison to resveratrol, whereas the inactive E-stilbene in the biofilm assay showed no glucan inhibition. These results suggest that the biofilm inhibition by the potent compound is directly related to the inhibition of glucan production by Gtfs.
Piceatannol Does Not Inhibit the Growth of Commensal Streptococcal Species
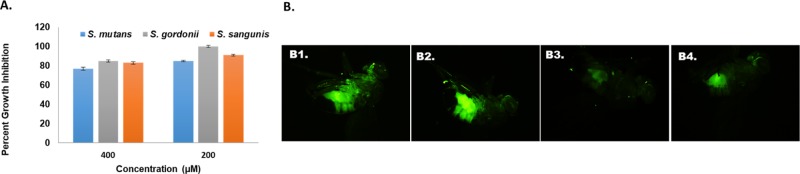
To evaluate the selectivity of piceatannol, we examined its ability to affect bacterial viability in both biofilm and planktonic cells of S. mutans. The viability of the biofilms and the planktonic cells of S. mutans were not significantly impacted by the treatment of piceatannol. Furthermore, piceatannol was used to assess its effect on the growth of other oral commensal species (Streptococcus sanguinis and Streptococcus gordonii) at concentrations ranging from 50 to 400 μM. At the biofilm IC50 value of 52 μM, less than 10% of growth of S. mutans, S. sanguinis, and S. gordonii is inhibited by piceatannol. Piceatannol inhibits S. mutans cell viability by 37% at 400 μM and has reduced toxicity to S. sanguinis and S. gordonii, decreasing the cell density by ∼15–18% (Figure 4A). Thus, piceatannol exhibits significant selectivity for biofilms.
Figure 4.
(A) Effect of piceatannol on the growth of S. mutans, S. gordonii, and S. sanguinis assessed by the alamar blue protocol. (B) Fluorescent microscopy images of S. mutans colonization in drosophila; (B1) treatment with sucrose, (B2) treatment with DMSO, (B3) ΔgtfB mutant strain, and (B4) treatment with 50 μM piceatannol.
Piceatannol Inhibited S. mutans Colonization in Vivo
The effect of piceatannol on S. mutans colonization in vivo was first evaluated using a sucrose-dependent drosophila colonization model.38 Briefly, Gfp-tagged S. mutans bacteria were used to infect flies along with the treatment of piceatannol at 50 μM in a feeding-assay (Figure 4B). DMSO was used as the negative control (Figure 4B2) and gtfB mutant, a known biofilm defective strain, was used as the positive control (Figure 4B3). The intensity of the fluorescence was measured in the guts of the flies fed with the Gfp-tagged bacteria after 7 days of infection. A significant decrease in fluorescence was observed when treated by piceatannol (Figure 4B4), producing an effect similar to that observed in the gtfB mutant (Figure 4B3). These data suggest piceatannol inhibits S. mutans colonization in vivo.
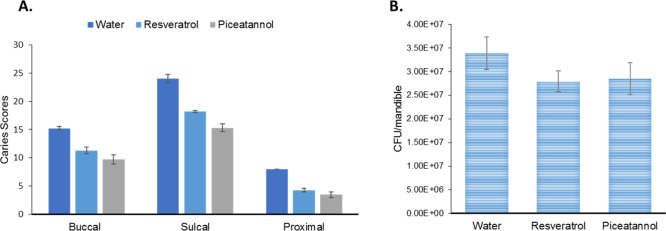
We further evaluated the in vivo efficacy of resveratrol and piceatannol using a rat model of dental caries.39,40 The treatment of both stilbenes produced a significant reduction in the buccal, sulcal, and proximal surface caries scores of the treated animals, with piceatannol demonstrating a greater reduction (Figure 5A). Although bacterial colonization appeared to be reduced by the treatment with the tested stilbenes, it was not statistically significant when compared with the control group treated with water (Figure 5B). These data suggest that both piceatannol and resveratrol selectively inhibit virulence factors, Gtfs and Gtf-mediated biofilm formation, and do not affect the bacterial growth. At the time of sacrifice and the removal of the mandible, there were no obvious differences in the oral tissue (mucosal and gingival tissues) between the treated and nontreated rats. Furthermore, because the treated rats did not lose weight over the course of the study in comparison with the control group, we believe that the natural stilbenes are safe and nontoxic.
Figure 5.
Effect of resveratrol and piceatannol treatment on the susceptibility of gnotobiotic rats to S. mutans UA159-induced dental caries. (A) Mean enamel caries scores (±standard error of the mean) of rats infected starting at ∼19 days of age and placed on Diet 305. (B) cfu/mandible determined by plating on MS plates. Resveratrol to water, p < 0.05; piceatannol to water, p < 0.01.
Conclusions and Future Perspectives
Modulation of cariogenic biofilms formed by S. mutans is a viable strategy for the prevention and treatment of dental caries. Considering the selectivity of polyphenols toward inhibiting S. mutans biofilms rather than altering its cell viability, we have explored polyphenol scaffolds in an effort to develop selective anti-biofilm agents. Here, we investigated the antimicrobial and anti-biofilm activities of a small series of stilbenes against the cariogenic bacterium S. mutans. The identified lead compound, piceatannol, exhibited anti-biofilm activity against S. mutans in the low micromolar range with a selectivity index of about 11 through the inhibition of the Gtfs, a key virulence factor of S. mutans. This compound showed marginal inhibition of the growth of S. mutans, S. sanguinus, and S. gordonii at high micromolar concentrations, suggesting its selectivity and nontoxicity. Piceatannol inhibited S. mutans-induced cariogenecity in vivo. Thus, our study demonstrated a virulence-selective therapeutic approach. This study has laid the foundation for additional preclinical and clinical studies to explore the potential of piceatannol as a new natural product-based drug that can be used for dental caries prevention and treatment.
Methods
Structure-Based 3D Database Search
The cocomplex crystal structure of GtfC and acarbose (PDB code: 3AIC)33 was utilized for the purposes of in silico docking. The active site residues were selected using the a 6.5 Å parameter of the crystallized ligand, acarbose, and residues Asp588 (H-acceptor) and Gln960 (H-donor) were used to generate a pharmacophore. We accessed the ZINC database to obtain a small library of polyphenolic small molecules and docked them using FlexX. Binding energies less than −20 kJ/mol were selected for further investigation, such as their druglike properties based on Lipinski’s rules, binding interactions with key residues, and synthetic feasibility.
Bacterial Strains and Culture Conditions
Todd-Hewitt broth (THB) agar plate and 5% CO2 in THB or in chemically defined biofilm medium supplemented with 1% sucrose were used to grow S. mutans UA159 and various Gtf mutants, S. sanguinis SK36, and S. gordonii DL1 statically at 37 °C.41
Small-molecule compounds were obtained from the NCI. Stock solutions (10 mM) were prepared in DMSO and arrayed in a 96-well format for biological screening.
S. mutans Biofilm Formation and Inhibition Assays
A well-established protocol to study S. mutans biofilm formation in 96-well flat-bottom polystyrene microtiter plates was used in triplicate.31,42 The stock solutions were prepared in 100% DMSO, and the final concentration of DMSO used in the assays was 1%. Minimum biofilm inhibitory concentration of compounds was determined by serial dilutions. The most potent of these scaffolds were progressed into further evaluations.
Inhibition of the Activity of Gtfs Determined by Zymographic Assays
A previously reported zymographic assay was utilized for the investigation of Gtf enzymatic activity.43 A 1:100 ratio of fresh 5 mL of THB with 50 μL of selective compounds at a series of concentrations was used to dilute S. mutans UA159 cultures overnight and grown to OD470 of 1.0. The final concentration of DMSO used in the assays was 1%. After the centrifugation at 4 °C, the supernatants were isolated and filtered through a 0.22 μM pore size filter membrane and dialyzed at 4 °C against 0.02 M sodium phosphate buffer (pH 6.8) with 10 μM phenylmethylsulfonyl fluoride (PMSF), followed by a second dialysis against 0.2 mM sodium phosphate containing 10 μM PMSF. Samples (4 mL) were concentrated to 40 μL by a 100K Amicon Ultra-4 centrifugal filter (Merk Millipore Ltd.).
Next, 10 μL of each concentrated culture supernatant was applied to 8% SDS-PAGE in duplicate. One gel was subjected to Coomassie blue dye for protein detection, whereas the other one was subjected to the zymographic assay, as described.43 The resultant white opaque glucan bands were visualized against a black background.
Cell Viability of S. mutans and Commensal S. gordonii and S. sanguinis
Cell viability and the small-molecule effect on it were investigated according to previous reports.42 DMSO served as the control group and provided a relative comparison for the number of colony-forming units (cfu) per milliliter for each compound at different concentrations determined after incubation for 24 h at 37 °C. The final concentration of DMSO used in the assays was 1%.
Colonization of Drosophila
Colonization of flies was performed, as described.38,44,45 Cultures of Gfp-tagged (green fluorescent protein) S. mutans UA159 grown to the middle log phase were spun down and resuspended in a solution containing 5% sucrose and 50 μM of each compound. The resuspended cells (100 μL) were aliquotted onto a sterile filter that was placed on the surface of 5 mL of solidified 5% sucrose agar in a plastic vial. Upon drying of the vials at room temperature for 30 min, the flies were introduced to the vessels. Male Canton S flies (1–3 days old) were treated with antibiotics for 2 days and starved for 3 h before addition to vials supplied with S. mutans (10–14 flies per vial). A Nikon eclipse 90i microscope, equipped with an Epi-fluorescence and NIS elements AR imaging system, was used to analyze the colonization of flies by Gfp-tagged strains, as described.38
Dental Caries Rat Model
A previously established rat model of dental caries was used to study S. mutans in vivo colonization.46,47 Fischer 344 rats used in this study were bred and maintained in Trexler isolators. At the age of 20 days, rat pups were removed from isolators and randomly assigned into five groups. Group A consisting of 3 female + 3 male rats were treated with resveratrol; group B consisting of 3 female + 3 male rats were treated with piceatannol; group C consisting of 3 female + 3 male rats were treated with water; group D consisting of 3 female + 2 male rats were not treated; group E consisting of 2 female + 3 male rats were neither treated nor infected by UA159. Rats were then infected with S. mutans UA159 for 3 consecutive days and provided a caries-promoting Diet 305, which contains 5% sucrose (TD.80406, diet with 62% corn starch, Harlan Laboratories, Madison, WI), and sterile drinking water ad libitum. The rats were then treated with stilbene, water, or not treated, respective of their study group, as described above at 100 μM twice daily for 4 weeks beginning 10 days post infection. Drinking water was withheld for 1 h after each treatment. Rats were weighed at weaning and after 45 days at the termination of the experiment. The animals were euthanized, their mandibles excised for microbiological analysis of plaque samples on MS agar plates and BAP and for scoring of caries by the method of Keyes.48 All experimental protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and in accordance with the relevant guidelines and regulations.
Octet RED Analysis
Octet RED full kinetic-binding analysis was performed for piceatannol and resveratrol against GtfB and GtfC. GtfC and GtfB were purified, as described previously.30 The Octet RED 96 system (ForteBio, Menlo Park, CA) was used to determine the rate constant, KD. Phosphate buffer with 2.5% (w/v) DMSO was used as the negative control. The dip-and-read Anti-Penta-HIS (HIS1K) Biosensor containing Penta-His antibody from Qiagen preimmobilized on a fiber optic biosensor was used to capture the Gtf proteins with high affinity and specificity. Three-fold serial dilution treatment from 200, 66.6, 22.2, 7.4, and 2.46 to 0 μM was used to study the stilbenes in phosphate buffer. Sensorgrams and the accuracy of the analysis was then calculated using the ForteBio Octet RED analysis software (ForteBio, Menlo Park, CA).
Acknowledgments
S.E.V. and H.W conceived the idea and designed the experiments; B.N. and H.Z performed the experiments; and X.C. and S.M.M. performed the rat experiment. This study was funded by the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIDCR/NIH) R03DE025058-0 to S.E.V., R01DE022350 to H.W., and F31 DE025783-01A1 to B.N. Compounds were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute.
Glossary
Abbreviations
- BAP
blood agar plate
- DMSO
dimethyl sulfoxide
- E
entgegen(trans) isomer
- Gtfs
glucosyltransferases
- IC50
the concentration of an inhibitor where the response (or binding) is reduced by half
- KD
equilibrium dissociation constant between the protein and its ligand
- MS
mannitol salt
- NCI
National Cancer Institute
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Z
Zusammen(cis) isomer
The authors declare no competing financial interest.
References
- Hamada S.; Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S.; Horikoshi T.; Minami T.; Kawabata S.; Hiraoka J.; Fujiwara T.; Ooshima T. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect. Immun. 1991, 59, 4161–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos-Graner R. O.; Napimoga M. H.; Fukushima K.; Duncan M. J.; Smith D. J. Comparative analysis of Gtf isozyme production and diversity in isolates of Streptococcus mutans with different biofilm growth phenotypes. J. Clin. Microbiol. 2004, 42, 4586–4592. 10.1128/jcm.42.10.4586-4592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J.; Koo H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. 2010, 108, 2103–2113. 10.1111/j.1365-2672.2009.04616.x. [DOI] [PubMed] [Google Scholar]
- Fears K. P.; Gonzalez-Begne M.; Love C. T.; Day D. E.; Koo H. Surface-induced changes in the conformation and glucan production of glucosyltransferase adsorbed on saliva-coated hydroxyapatite. Langmuir 2015, 31, 4654–4662. 10.1021/la504461h. [DOI] [PubMed] [Google Scholar]
- Vacca-Smith A. M.; Venkitaraman A. R.; Schilling K. M.; Bowen W. H. Characterization of glucosyltransferase of human saliva adsorbed onto hydroxyapatite surfaces. Caries Res. 1996, 30, 354–360. 10.1159/000262342. [DOI] [PubMed] [Google Scholar]
- Vacca-Smith A. M.; Bowen W. H. Binding properties of streptococcal glucosyltransferases for hydroxyapatite, saliva-coated hydroxyapatite, and bacterial surfaces. Arch. Oral Biol. 1998, 43, 103–110. 10.1016/s0003-9969(97)00111-8. [DOI] [PubMed] [Google Scholar]
- Nakano K.; Nomura R.; Nakagawa I.; Hamada S.; Ooshima T. Role of glucose side chains with serotype-specific polysaccharide in the cariogenicity of Streptococcus mutans. Caries Res. 2005, 39, 262–268. 10.1159/000084831. [DOI] [PubMed] [Google Scholar]
- Walsh T.; Oliveira-Neto J. M.; Moore D. Chlorhexidine treatment for the prevention of dental caries in children and adolescents. Cochrane Database Syst. Rev. 2015, CD008457. 10.1002/14651858.CD008457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charugundla B. R.; Anjum S.; Mocherla M. Comparative effect of fluoride, essential oil and chlorhexidine mouth rinses on dental plaque and gingivitis in patients with and without dental caries: a randomized controlled trial. Int. J. Dent. Hyg. 2015, 13, 104–109. 10.1111/idh.12094. [DOI] [PubMed] [Google Scholar]
- Koo H.; Nino de Guzman P.; Schobel B. D.; Vacca Smith A. V.; Bowen W. H. Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development. Caries Res. 2006, 40, 20–27. 10.1159/000088901. [DOI] [PubMed] [Google Scholar]
- Thimothe J.; Bonsi I. A.; Padilla-Zakour O. I.; Koo H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J. Agric. Food Chem. 2007, 55, 10200–10207. 10.1021/jf0722405. [DOI] [PubMed] [Google Scholar]
- Duarte S.; Gregoire S.; Singh A. P.; Vorsa N.; Schaich K.; Bowen W. H.; Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- Pandit S.; Song K.-Y.; Jeon J.-G. Withania somnifera attenuates acid production, acid tolerance and extra-cellular polysaccharide formation of Streptococcus mutans biofilms. Am. J. Chin. Med. 2014, 42, 157–171. 10.1142/s0192415x14500116. [DOI] [PubMed] [Google Scholar]
- Koo H.; Rosalen P. L.; Cury J. A.; Park Y. K.; Bowen W. H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. 10.1128/aac.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H.; Hayacibara M. F.; Schobel B. D.; Cury J. A.; Rosalen P. L.; Park Y. K.; Vacca-Smith A. M.; Bowen W. H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- Koo H.; Pearson S. K.; Scott-Anne K.; Abranches J.; Cury J. A.; Rosalen P. L.; Park Y. K.; Marquis R. E.; Bowen W. H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 2002, 17, 337–343. 10.1034/j.1399-302x.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- Koo H.; Schobel B.; Scott-Anne K.; Watson G.; Bowen W. H.; Cury J. A.; Rosalen P. L.; Park Y. K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasempour M.; Rajabnia R.; Gharekhani S.; Gholamhoseinnia S.; Soroorhomayoon S. Anti-Streptococcus mutans property of a chitosan: Containing resin sealant. J. Int. Soc. Prev. Community Dent. 2016, 6, 49–53. 10.4103/2231-0762.175405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata R. M.; Branco-de-Almeida L. S.; Franco E. M.; Yatsuda R.; dos Santos M. H.; de Alencar S. M.; Koo H.; Rosalen P. L. Inhibition of Streptococcus mutans biofilm accumulation and development of dental caries in vivo by 7-epiclusianone and fluoride. Biofouling 2010, 26, 865–872. 10.1080/08927014.2010.527435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-de-Almeida L. S.; Murata R. M.; Franco E. M.; dos Santos M. H.; de Alencar S. M.; Koo H.; Rosalen P. L. Effects of 7-epiclusianone on Streptococcus mutans and caries development in rats. Planta Med. 2011, 77, 40–45. 10.1055/s-0030-1250121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. T. M.; Falsetta M. L.; Hwang G.; Gonzalez-Begne M.; Koo H. alpha-Mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS One 2014, 9, e111312 10.1371/journal.pone.0111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetta M. L.; Klein M. I.; Lemos J. A.; Silva B. B.; Agidi S.; Scott-Anne K. K.; Koo H. Novel antibiofilm chemotherapy targets exopolysaccharide synthesis and stress tolerance in Streptococcus mutans to modulate virulence expression in vivo. Antimicrob. Agents Chemother. 2012, 56, 6201–6211. 10.1128/aac.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.; Hwang G.; Liu Y.; Wang Y.; Singh A. P.; Vorsa N.; Koo H. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS One 2015, 10, e0145844 10.1371/journal.pone.0145844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.; Urnezis P.; Tian M. Compressed mints and chewing gum containing magnolia bark extract are effective against bacteria responsible for oral malodor. J. Agric. Food Chem. 2007, 55, 9465–9469. 10.1021/jf072122h. [DOI] [PubMed] [Google Scholar]
- Kwon Y.-R.; Son K.-J.; Pandit S.; Kim J.-E.; Chang K.-W.; Jeon J.-G. Bioactivity-guided separation of anti-acidogenic substances against Streptococcus mutans UA 159 from Polygonum cuspidatum. Oral Dis. 2010, 16, 204–209. 10.1111/j.1601-0825.2009.01636.x. [DOI] [PubMed] [Google Scholar]
- Pandit S.; Kim H.-J.; Park S.-H.; Jeon J.-G. Enhancement of fluoride activity against Streptococcus mutans biofilms by a substance separated from Polygonum cuspidatum. Biofouling 2012, 28, 279–287. 10.1080/08927014.2012.672646. [DOI] [PubMed] [Google Scholar]
- Yim N.; Ha D. T.; Trung T. N.; Kim J. P.; Lee S.; Na M.; Jung H.; Kim H. S.; Kim Y. H.; Bae K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. 10.1016/j.bmcl.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Nijampatnam B.; Nadkarni D. H.; Wu H.; Velu S. Antibacterial and Antibiofilm Activities of Makaluvamine Analogs. Microorganisms 2014, 2, 128–139. 10.3390/microorganisms2030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Nijampatnam B.; Hua Z.; Nguyen T.; Zou J.; Cai X.; Michalek S. M.; Velu S. E.; Wu H. Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence. Sci. Rep. 2017, 7, 5974. 10.1038/s41598-017-06168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Nguyen T.; McMichael M.; Velu S. E.; Zou J.; Zhou X.; Wu H. New small-molecule inhibitors of dihydrofolate reductase inhibit Streptococcus mutans. Int. J. Antimicrob. Agents 2015, 46, 174–182. 10.1016/j.ijantimicag.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijampatnam B.; Casals L.; Zheng R.; Wu H.; Velu S. E. Hydroxychalcone inhibitors of Streptococcus mutans glucosyl transferases and biofilms as potential anticaries agents. Bioorg. Med. Chem. Lett. 2016, 26, 3508–3513. 10.1016/j.bmcl.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K.; Ito S.; Shimamura T.; Weyand S.; Kawarasaki Y.; Misaka T.; Abe K.; Kobayashi T.; Cameron A. D.; Iwata S. Crystal Structure of Glucansucrase from the Dental Caries Pathogen Streptococcus mutans. J. Mol. Biol. 2011, 408, 177–186. 10.1016/j.jmb.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Wen Z. T.; Burne R. A. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 2002, 68, 1196–1203. 10.1128/aem.68.3.1196-1203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban S.-H.; Kwon Y.-R.; Pandit S.; Lee Y.-S.; Yi H.-K.; Jeon J.-G. Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci. Fitoterapia 2010, 81, 30–34. 10.1016/j.fitote.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Yao C.-S.; Lin M.; Liu X.; Wang Y.-H. Stilbene derivatives from Gnetum cleistostachyum. J. Asian Nat. Prod. Res. 2005, 7, 131–137. 10.1080/10286020310001625102. [DOI] [PubMed] [Google Scholar]
- Seyed M. A.; Jantan I.; Bukhari S. N. A.; Vijayaraghavan K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- Peng X.; Zhang Y.; Bai G.; Zhou X.; Wu H. Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 2015, 99, 945–959. 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M.; McGhee J. R.; Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect. Immun. 1975, 12, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M.; McGhee J. R.; Shiota T.; Devenyns D. Low sucrose levels promote extensive Streptococcus mutans-induced dental caries. Infect. Immun. 1977, 16, 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C. Y.; Corliss D. A.; Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Worthington R. J.; Melander C.; Wu H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 2011, 55, 2679–2687. 10.1128/aac.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos-Graner R. O.; Napimoga M. H.; Fukushima K.; Duncan M. J.; Smith D. J. Comparative analysis of Gtf isozyme production and diversity in isolates of Streptococcus mutans with different biofilm growth phenotypes. J. Clin. Microbiol. 2004, 42, 4586–4592. 10.1128/jcm.42.10.4586-4592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani S. A.; Whiteley M.; Lee K. M.; D’Argenio D.; Manoil C.; Greenberg E. P. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 2752–2757. 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H.; Sibley C. D.; Surette M. G.; Lewenza S. Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathog. 2011, 7, e1002299 10.1371/journal.ppat.1002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley P. J.; Brady L. J.; Michalek S. M.; Bleiweis A. S. Virulence of a spaP Mutant of Streptococcus mutans in a Gnotobiotic Rat Model. Infect. Immun. 1999, 67, 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. J.; Michalek S. M.; Zhu M.; Drake D.; Qian F.; Banas J. A. Cariogenicity of Streptococcus mutans glucan-binding protein deletion mutants. Oral Health Dent. Manag. 2013, 12, 191–199. [PMC free article] [PubMed] [Google Scholar]
- Keyes P. H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 1958, 37, 1088–1099. 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]