Abstract

Drug–polymer conjugation is a simple and efficient approach to synthesizing new, effective, and potent antimicrobial agents to counter the problem of microbial resistance. In the present study, a PEGylated dopamine ester (PDE) was synthesized using the PEGylation process and synthesis of PDE was confirmed by Fourier-transform infrared spectroscopy, elemental analysis (CHNS–O), and atomic force microscopy techniques. Later, the antimicrobial activity of PDE was assessed against four strains of bacteria (Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus vulgaris; Gram (−)) and two fungi (Aspergillus niger and Aspergillus fumigatus) by the agar well diffusion method. The minimum inhibitory concentration (MIC) of PDE was also determined by the broth dilution method against bacteria. PDE showed significant zones of inhibition ranged from 21 to 27 mm for bacteria and 16 to 20 mm for fungi under study, which were much higher than those for dopamine hydrochloride. MIC values of PDE showed its potential antimicrobial property.
1. Introduction
Over the past few decades, microbial resistance against antibiotics has emerged as a major health concern with World Health Organization reporting that most of the of the bacterial strains have acquired resistance to commonly used antibiotics especially in the developing countries.1 This grave global public health threat has set an agenda for researchers to develop new compounds or modify the existing materials with effective and potent antimicrobial properties.
Nowadays, newer strategies have been developed to synthesize more efficient materials having enhanced antimicrobial property to counter pathogenic microbial strains.2 Many researchers reported various modified antimicrobial agents having silver,3 titanium,4 copper,5 iron,6 phenols,7 aldehydes,8N-halamines,9 etc. However, due to their serious disadvantages such as high residual toxicity, environmental hazards, etc., focus has been shifted to polymeric systems, which can act as a support to other antimicrobial agents with enhanced antimicrobial properties or themselves act as strong antimicrobial agents.10 The latter include antimicrobial polymers (AMPs), which have numerous active groups, high efficacy, diminutive toxicity, broad spectrum applicability, easy introduction, and simple modification of structural and functional properties.11,12 The availability of the large number of active groups creates high local concentration, which in turn improves antimicrobial properties many times.13 Moreover, AMPs can be easily tailored by polymer analogous reactions including metathesis reactions to enhance their efficacy.14,15
Furthermore, modification of the existing drugs by drug–polymer conjugation through various chemical reactions such as acrylation, acetylation, or quaternization reactions depends upon the nature or potency of the active functional groups present on the bioactive polymer.16 One way forward is functionalization of the existing drugs such as methyl DOPA,17 promethazine,18 chlorpromazine,19 diclofenac,20 oxyphedrine,21 dicyclomine,22 and dopamine hydrochloride23 as these possess antimicrobial activity in addition to their predesigned pharmacological actions.
In view of the above discussion, the rationale of the present research is to design a novel, effective, and potent antibacterial and antifungal agent by functionalization of dopamine hydrochloride using the PEGylation process and compare its antimicrobial properties with those of dopamine hydrochloride and some existing drugs as references. Surface modification and functionalization of antibiotics or bioactive compounds via a greener route is a new strategy that can enhance the antimicrobial property of the antimicrobial agents. Poly(ethylene glycols) (PEGs) are highly biocompatible and safe polymers reported in drug delivery.24 Various bioactive compounds especially polymers having active functional groups can be covalently attached to different drugs via a greener approach. In the literature, it is reported that various dopamine-containing compounds exhibit antibacterial activities25−28 and dopamine also had been used to synthesize numerous herbicides and drugs.29,30 Dopamine ester derivatives have been reported to have better antibacterial properties than those of dopamine.31 These characteristics make dopamine or its derivatives a suitable drug for surface modification using a polymer system to develop an effective antimicrobial agent. Hence, the polydopamine coatings of dopamine for surface modification of various materials, formed by single-step autoxidation of dopamine, have recently emerged as an attractive methodology and become a very hot field of scientific technological innovation.32 The self-polymerization of dopamine requires alkaline conditions in the presence of oxygen.33 Although dopamine itself has very moderate efficacy to kill the pathogens, its functionalization improves the efficiency against the pathogens.32
2. Results and Discussion
2.1. Modification of Dopamine to a PEGylated Dopamine Ester (PDE)
The vicinal hydroxyl groups (−OH) of dopamine were functionalized with free −COOH groups of PEG diacid, which resulted in the formation of ester linkage (−COO−) between dopamine and PEG diacid via acid esterification i.e., in the presence of H2SO4, presented in Scheme 1. There was a formation of ester moiety in the PDE with the removal of water molecule. Dopamine undergoes self-polymerization in the presence of oxygen in an alkaline environment. However, at acidic pH (<7), the self-polymerization is not possible without any initiators or catalysts and it needs high temperature and pressure. Hence, under the reported synthetic conditions, dopamine does not undergo self-polymerization in the presence of oxygen.34
Scheme 1. Synthesis of PDE.
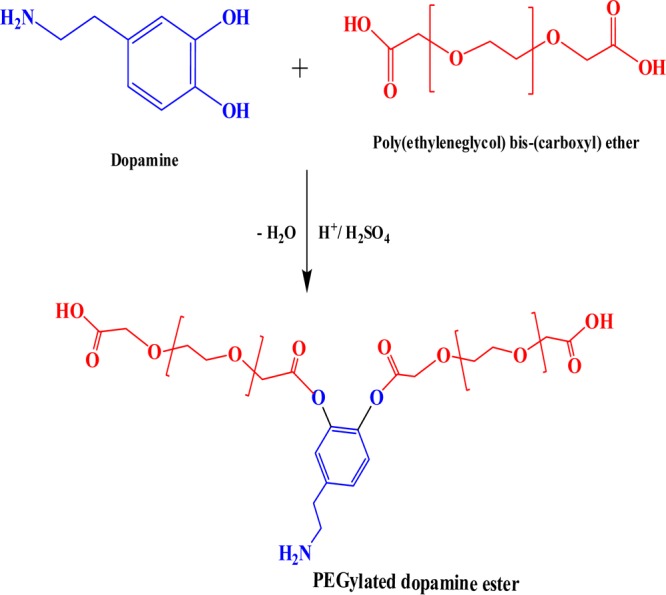
Thus, a dangling structure with highly biocompatible PEG and bioactive DOPA moieties on sides is generated.
2.2. Characterization of Modified Dopamine (PDE)
2.2.1. Elemental Analysis (CHNS–O)
In the case of dopamine hydrochloride, the elemental composition has been reported to be: carbon (50.67%), hydrogen (6.38%), nitrogen (7.39%), and oxygen (18.69%).35 CHNS–O analysis of PDE is presented in Figure 1a, wherein the concentration of oxygen is 31.32%, which confirmed that the addition of the PEG diacid polymer to dopamine molecules increased the oxygen concentration, but it was lower than 34.77% in the CHNS–O analysis of PEG diacid (Figure 1b). This confirmed the removal of water molecules in the esterification process because of the decreased oxygen concentration in the dopamine ester than in PEG diacid.
Figure 1.

(a) CHNS–O analysis of PDE and (b) PEG diacid.
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR) and Raman Spectrum Analysis
FTIR spectra of dopamine and PDE were recorded to confirm the formation of ester linkage in dopamine. The peaks observed in the FTIR spectra of dopamine were at 3346 cm–1 (amine N–H stretching), 3270 cm–1 (phenol O–H stretching), 3050.77 cm–1 (aromatic C–H stretching), 2916.77 cm–1 (alkyl C–H stretching), 1616 cm–1 (amine N–H bending), 1519 cm–1 (aromatic C=C stretching), 1245 cm–1 (amine C–N stretching), 1230 cm–1 (phenol C–O stretching) (Figure 2).36 The FTIR spectrum of PDE showed peaks at 1720 cm–1 (ester C=O stretching) and 1120 cm–1 (ester C–O stretching) with basic peaks of dopamine, which confirmed the successful incorporation of the ester moiety in dopamine to form PDE (Figure 2).
Figure 2.

(a) FTIR spectra of dopamine and PDE. (b) Raman spectra of PDE.
Figure 2b shows the Raman spectrum of PDE. The two bands at around 1300–1400 and 1500–1600 cm–1 (D and G bands), due to the catechol group, in the Raman spectrum of dopamine disappeared in the Raman spectrum of PDE, which depicts the utilization of the −OH group for the formation of dopamine ester.37 The band at 1100 cm–1 in PDE was attributed to the CH twisting, NH twisting, and CN stretching.38
2.2.3. Atomic Force Microscopy (AFM) Analysis
AFM is a versatile and powerful microscopy technology that can generate images at atomic resolution with Å scale. AFM topographic images of PDE show spherical granules having size of particles on microscale, shown in Figure 3. The microspherical particles with rough surfaces have increased contact between the microbial cell and PDE, which increases the killing efficiency of PDE toward bacteria.
Figure 3.
AFM images of PDE.
2.3. Antimicrobial Studies
In this study, four bacteria (Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus vulgaris) and two fungi (Aspergillus niger and Aspergillus fumigatus), all of pathogenic nature,39−43 were selected for evaluating the antimicrobial activity of PDE and precursor dopamine as the reference and ampicillin as the standard drug control. These microorganisms were allowed to grow on agar plates, and the zone of inhibition was measured after an incubation period of 24 and 48 h for bacteria and fungi, respectively, under optimum conditions of growth. Ampicillin was used as a standard antibacterial and antifungal drug. The antimicrobial activity assay of PDE was carried out by the minimum inhibitory concentration (MIC) method (Figure 4). Images of antibacterial and antifungal activity of dopamine, PDE, and ampicillin revealed that PDE exhibits potent antimicrobial activity than dopamine and control ampicillin.
Figure 4.
Images of antibacterial and antifungal activity of dopamine, PDE, and ampicillin (product in the pictures indicates PDE).
2.3.1. Zone of Inhibition
The diameter of zone of inhibition was found to be microorganism-dependent, and crucial structure–activity relationship was seen from the trends in the antimicrobial action of PDE. PDE (1.0 μL) in 1.0 mL of water was taken to evaluate its antibacterial activity and found to be more active against Gram (+) bacteria than Gram (−) bacteria. This can be better explained on the basis of difference in their cell wall compositions. In addition to the peptidoglycan in the cell walls, Gram (−) bacteria have an additional outer membrane, contain higher lipid contents, and are more hydrophilic than Gram (+) bacteria; thus, they are less susceptible to antibiotics. On the other hand, the wall of fungal cell is more complex than the bacterial cell wall and comprises chitin, glucans, and polysaccharides. Therefore, due to structure complexity of the fungal cell wall, lesser diameter of zone of inhibition was observed as compared to that in bacteria. Dopamine does not show antimicrobial activity up to 5 mg/mL to test organisms (Figure 4). Thus, the ester linkage is established as the necessary structural factor for antimicrobial activity.
The mechanism of antimicrobial action of PDE is suggested via cell wall lysis as is the case with biocidal amines. Cell wall lysis is associated with the attachment and penetration of antimicrobial agents, PDE in this case, through cell membranes and results in cell death by disruption or release of intracellular fluid.6,26,32 Therefore, extent of interaction directly influences the killing efficiency. The PDE has microspherical particles with a rough surface, as shown by the AFM studies, which facilitates effective interaction between PDE and bacterial cell membranes, which is further enhanced by the PEGylation of dopamine because of the introduction of synthesized ester of lipophilic nature, and PDE kills various microbes effectively than pristine dopamine. PDE showed prominent zones of inhibition of 22.25 ± 1.75, 27.00 ± 1.00, and 21.00 ± 1.25, 24.50 ± 1.75 mm against Gram (+) bacteria (B. subtilis and S. aureus) and Gram (−) bacteria (P. aeruginosa and P. vulgaris), respectively, and 20 ± 1.00 and 16.25 ± 1.00 mm against A. niger and A. fumigatus, respectively. No zone of inhibition was seen with dopamine hydrochloride against the reported bacteria and fungi. Moderate zones of inhibition of 18.25 ± 1.25 and 21.25 ± 1.00 mm were shown by ampicillin against B. subtilis and S. aureus, respectively (Table 1, Figure 4).
Table 1. Antimicrobial Activity of Ampicillin, Dopamine, and PDE with Zone of Inhibitiona (mm).
| Bacterial Study | |||
|---|---|---|---|
| bacteria | ampicillin (mm) | dopamine (mm) | PDE (mm) |
| B. subtilis | 18.25 ± 1.25 | 22.25 ± 1.75 | |
| S. aureus | 21.25 ± 1.00 | 27.00 ± 1.00 | |
| P. aeruginosa | 21.00 ± 1.25 | ||
| P. vulgaris | 24.50 ± 1.75 | ||
| Fungal Study | |||
|---|---|---|---|
| fungus | ampicillin (mm) | dopamine (mm) | PDE (mm) |
| A. niger | 20 ± 1.00 | ||
| A. fumigatus | 16.25 ± 1.00 | ||
Diameter in mm (excluding the diameter of the well).
2.3.2. MIC Studies
MIC is defined as the lowest amount (mg/L) of antimicrobial agent required to impede the visible growth of microorganisms under defined growth conditions. In the present protocol, we used the non-fluorescent resazurin (purple blue) dye as a redox indicator. The cytochrome reductases of live or active microbial cells left in Sterile 96-well microtitration plates reduce the resazurin dye to fluorescent resorufin (pink) dye, giving a direct measure of the metabolic activity of bacterial cells.44 Hence, if there is no reduction of dye by bacteria, then the color remains as such i.e., no growth of bacterial cells and vice-versa. MIC values of PDE were determined for selected pathogenic bacterial strains and fungi. S. species, P. vulgaris, B. cereus, P. aeruginosa, and Salmonella typhi were found to be the most sensitive to PDE with the MIC value of 0.156/100 μL. The MIC value for Escherichia coli, S. aureus, and Klebsiella pneumoniae was found to be 0.312/100 μL, and dopamine hydrochloride showed the MIC value of 10 mg/100 μL only against P. vulgaris and Benthesicymus cereus (Tables 2, 3 and Figure 5). A. niger and A. fumigatus both were found to be highly sensitive to PDE with MIC values 0.078/100 and 0.156/100 μL, respectively, and for dopamine hydrochloride, MIC values were found to be 2.5/100 μL for A. niger and 5.0/100 μL for A. fumigatus, shown in Table 4 and Figure 6.
Table 2. MIC of PDE Against Pathogenic Bacterial Strainsa.
| PDE (μL/100 μL) | E. coli | S. aureus | K. pneumoniae | S. spp | P. vulgaris | B. cereus | P. aeruginosa | S. typhi |
|---|---|---|---|---|---|---|---|---|
| 10.0 | – | – | – | – | – | – | – | – |
| 5.0 | – | – | – | – | – | – | – | – |
| 2.5 | – | – | – | – | – | – | – | – |
| 1.25 | – | – | – | – | – | – | – | – |
| 0.625 | – | – | – | – | – | – | – | – |
| 0.312 | – | – | – | – | – | – | – | – |
| 0.156 | + | + | + | – | – | – | – | – |
| 0.078 | + | + | + | + | + | + | + | + |
| 0.039 | + | + | + | + | + | + | + | + |
| 0.019 | + | + | + | + | + | + | + | + |
| MIC(μL/100 μL) | 0.312 | 0.312 | 0.312 | 0.156 | 0.156 | 0.156 | 0.156 | 0.156 |
(+) indicates bacterial growth; (−) indicates bacterial growth inhibition.
Table 3. MIC of Dopamine Against Pathogenic Bacterial Strainsa.
| dopamine (μL/100 μL) | E. coli | S. aureus | K. pneumoniae | S. spp | P. vulgaris | B. cereus | P. Aeruginosa | S. typhi |
|---|---|---|---|---|---|---|---|---|
| 10.0 | + | + | + | + | – | – | + | + |
| 5.0 | + | + | + | + | + | + | + | + |
| 2.5 | + | + | + | + | + | + | + | + |
| 1.25 | + | + | + | + | + | + | + | + |
| 0.625 | + | + | + | + | + | + | + | + |
| 0.312 | + | + | + | + | + | + | + | + |
| 0.156 | + | + | + | + | + | + | + | + |
| 0.078 | + | + | + | + | + | + | + | + |
| 0.039 | + | + | + | + | + | + | + | + |
| 0.019 | + | + | + | + | + | + | + | + |
| MIC(μL/100 μL) | nil | nil | nil | nil | 10.0 | 10.0 | nil | nil |
(+) indicates bacterial growth; (−) indicates bacterial growth inhibition.
Figure 5.
Images showing MIC values of PDE (top) and dopamine (bottom) against pathogenic bacterial strains.
Table 4. MIC Values of PDE and Dopamine Against Fungal Strainsa.
| PDE (μL/100 μL) | A. niger | A. fumigatus | dopamine (mg/100 μL) | A. niger | A. fumigatus |
|---|---|---|---|---|---|
| 10.0 | – | – | 10.0 | – | – |
| 5.0 | – | – | 5.0 | – | – |
| 2.5 | – | – | 2.5 | – | + |
| 1.25 | – | – | 1.25 | + | + |
| 0.625 | – | – | 0.625 | + | + |
| 0.312 | – | – | 0.312 | + | + |
| 0.156 | – | – | 0.156 | + | + |
| 0.078 | – | + | 0.078 | + | + |
| 0.039 | + | + | 0.039 | + | + |
| 0.019 | + | + | 0.019 | + | + |
| MIC (μL/100 μL) | 0.078 | 0.156 | MIC (mg/100 μL) | 2.5 | 5.0 |
(+) indicates bacterial growth; (−) indicates bacterial growth inhibition.
Figure 6.
Image showing the MIC value of dopamine ester (PDE) and dopamine against fungal strains.
2.3.3. Comparison of Antimicrobial Activity of PDE and Ampicillin
Figure 7 shows a comparison of PDE with respect to the standard drug, ampicillin. PDE showed good and higher activity than that of the standard drug, ampicillin (control).
Figure 7.
Antifungal and antibacterial activity of PDE versus ampicillin (control).
3. Conclusions
In this study, a new bioactive compound of dopamine (PDE) was synthesized by the PEGylation process, in which polymer chains of poly(ethylene glycol) were covalently attached to dopamine hydrochloride. Furthermore, synthesis of PDE was successfully confirmed by FTIR, elemental analysis, and AFM techniques. Later, antimicrobial activity of PDE was evaluated against pathogenic bacteria and fungi. Results obtained from the agar well diffusion method revealed that PDE showed strong antimicrobial activity against bacteria and fungi under study, and these results were close to those of the control. However, dopamine hydrochloride did not show any zone of inhibition against bacteria and fungi. Moreover, P. vulgaris and P. aeruginosa were resistant to the control antibiotic, but their growth was significantly affected by PDE. In view of the aforesaid, PDE is an efficient and potent antimicrobial agent having MIC values of 0.156/100 and 0.312/100 μL in case of bacteria and 2.5/100 and 5.0/100 μL in case of fungi and can be used in cosmetics, as a food packaging material, and in pharmaceutical industries.
4. Experimental Section
4.1. Materials
Dopamine hydrochloride and sodium hydrogen carbonate (NaHCO3) (Hi media, India), PEG-bis (carboxymethyl) ether (Sigma-Aldrich, Germany), sulfuric acid (H2SO4, Rankem, India), peptone, sodium chloride (NaCl), galactose, calcium chloride (CaCl2), Tween-80, and resazurine dye (Sigma-Aldrich, Germany) were of analytical grade and used as received. Beef extract, nutrient agar, potato dextrose agar, yeast extract (Hi media, India), and test organisms including Gram (+) and Gram (−) strains of bacteria (B. subtilis, S. aureus, P. aeruginosa, P. vulgaris, E. coli, K. pneumoniae, S. ssp, B. cereus, and S. typhi) and two fungal cultures (A. niger and A. fumigatus) were obtained from the Department of Biotechnology, Himachal Pradesh University, Shimla, India.
4.2. Synthesis of PEGylated Dopamine Ester (PDE)
Dopamine hydrochloride (1.0 g) was mixed with 2.0 mL of PEG-bis(carboxymethyl) ether in a flask placed in a water bath at 40–50 °C for 20 h, and 5–6 drops of H2SO4 were added to accelerate the reaction. After the completion of the reaction, PDE was formed, which was then washed by dropwise addition of 10% solution of NaHCO3 until effervescence ceased, which indicated complete removal of the acid. It was then dried at 50 °C in the oven to evaporate water.
4.3. Antimicrobial Assay
The antimicrobial activity of PDE was determined by the agar well diffusion method, and the lowest concentration of the test material that inhibited the visible growth of microbes i.e., MIC, was calculated using the broth dilution method.45
4.3.1. Agar Well Diffusion Method
The bactericidal and fungicidal effects of dopamine and PDE were tested against two Gram (+) (B. subtilis and S. aureus) two Gram (−) (P. aeruginosa, P. vulgaris) bacteria and two fungi (A. niger and A. fumigatus). Antimicrobial susceptibility was tested on solid agar–agar media in petriplates.46 For this, 5.0 g of beef extract, 1.0 g of yeast extract, 2.5 g of NaCl, and 5.0 g of peptone were added in 500 mL of distilled water. The pH of the mixture solution was maintained at 7.5. To this solution, 13.0 g of agar was added and autoclaved at 121 °C for 45–60 min to obtain nutrient agar media, which was then poured onto petriplates. In each nutrient agar plate, different bacterial cultures were spread. Thereafter, three wells were punctured into each agar plate and ampicillin (0.025 g/500 μL), PDE (10 μL/10 mL), and dopamine (1 mg/1 mL) were poured into each well. In each well, 100 μL dopamine, PDE, and ampicillin were added separately. The resulting uniform circular areas as zones of inhibition around the wells without the growth of test bacteria were observed and measured after incubation at 37 °C for 24 h.
For the fungal production media, 3.9 g of potato dextrose agar was added in 100 mL of distilled water and then autoclaved at 121 °C for 45–60 min. Then, the same procedure was followed as reported above for the bacterial study.
4.3.2. Broth Dilution Method for Determination of the MIC of Dopamine and PDE
To make nutrient broth for bacterial culture, 5.0 g of beef extract, 10.0 g of yeast extract, 2.5 g of NaCl, and 5.0 g of peptone were mixed in 500 mL of distilled water and then autoclaved at 121 °C for 45–60 min. Sterile 96-well microtitration plates were used. Each well was filled with 100 μL of nutrient broth and 10 μL of test solution: dopamine (1 mg/1 mL) and PDE (10 μL/10 mL). Broth dilution (2-fold) was achieved in each horizontal row by transferring 10 μL of test solution from first well to the next and similarly in subsequent wells so that each well had 10 μL of test solution in serially descending concentrations.47 Then, 10.0 μL of different bacterial cultures was added in each row one by one. For the purpose of testing, 10.0 μL of indicator (resazurine dye) was added in each well (0.027 g/4 mL).48 One entire vertical row of negative control contains only nutrient broth and bacteria (no antibiotic and no synthesized material). Next to negative control, an entire vertical row with all test bacteria had antibiotics (+ve control) instead of synthesized material was examined. If the color of the dye changes to pink, that means the growth of bacteria is there, and if the color is purple, then the growth of bacteria is inhibited by the testing sample, i.e., PDE.
For production media for fungus, 3.6 g of peptone, 3.0 g of galactose, 1.0 g of NaCl, 0.2 g of CaCl2, and 2.0 mL of Tween-80 were added in 200 mL of distilled water and the pH of the mixture solution was maintained at 10. Then, the solution was autoclaved at 121 °C for 45–60 min. After this, the fungus was added into this production media and left for 3 days for proper growth. Then, the same procedure followed as for the MIC of bacteria.
Acknowledgments
The authors acknowledge the facilities provided by the Department of Chemistry, Himachal Pradesh University, under the UGC-SAP.
The authors declare no competing financial interest.
References
- WHO . Antimicrobial Resistance: Global Report on Surveillance. ISBN: 978 92 4 156474 8, 2014.
- Chen S.; Li L.; Zhao C.; Zheng J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. 10.1016/j.polymer.2010.08.022. [DOI] [Google Scholar]
- Mao C.; Xiang Y.; Liu X.; Cui Z.; Yang X.; Yeung K. W. K.; Pan H.; Wang X.; Chu P. K.; Wu S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. 10.1021/acsnano.7b03513. [DOI] [PubMed] [Google Scholar]
- He S.; Zhou P.; Wang L.; Xiong X.; Zhang Y.; Deng Y.; Wei S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc., Interface 2014, 11, 20140169 10.1098/rsif.2014.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel F.; Finke B.; Zietz C.; Bader R.; Weltmann K.-D.; Polak M. Antimicrobial surface modification of titanium substrates by means of plasma immersion ion implantation and deposition of copper. Surf. Coat. Technol. 2014, 256, 52–58. 10.1016/j.surfcoat.2014.01.027. [DOI] [Google Scholar]
- Lin S.; Liu X.; Tan L.; Cui Z.; Yang X.; Yeung K. W. K.; Pan H.; Wu S. Porous Iron-Carboxylate Metal-Organic Framework: A Novel Bio platform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. 10.1021/acsami.7b04810. [DOI] [PubMed] [Google Scholar]
- Fragopoulou E.; Nomikos T.; Karantonis H. C.; Apostolakis C.; Pliakis E.; Samiotaki M.; Panayotou G.; Antonopoulou S. Biological activity of acetylated phenolic compounds. J. Agric. Food Chem. 2007, 55, 80–89. 10.1021/jf0627221. [DOI] [PubMed] [Google Scholar]
- Bisignano G.; Laganà M. G.; Trombetta D.; Arena S.; Nostro A.; Uccella N.; Mazzanti G.; Saija A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. 10.1111/j.1574-6968.2001.tb10611.x. [DOI] [PubMed] [Google Scholar]
- Bastarrachea L. J.; Goddard J. M. Antimicrobial coatings with dual cationic and N-halamine character: Characterization and biocidal efficacy. J. Agric. Food Chem. 2015, 63, 4243–4251. 10.1021/acs.jafc.5b00445. [DOI] [PubMed] [Google Scholar]
- Weintraub S.; Shpigel T.; Harris L. G.; Schuster R.; Lewis E. C.; Lewitus D. Y. Astaxanthin-based polymers as new antimicrobial compounds. Polym. Chem. 2017, 8, 4182–4189. 10.1039/C7PY00663B. [DOI] [Google Scholar]
- Kenawy E. R.; Worley S. D.; Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- Chauhan G. S.; Hyeon J.; Sharma R. K. Tailoring effect of alkyl chain length and counter anion on antimicrobial behavior of 4–vinyl pyridine–based cationic polymers. Anti-Infect. Agents 2015, 13, 78–86. 10.2174/2211352513666141215214442. [DOI] [Google Scholar]
- Dogra P.; Dharela R.; Chauhan G. S.; Gupta R.; Azmi W. Structure-property relationship in antimicrobial polymers synthesized by chemo-enzymatic route. Proc. Chem. 2012, 4, 208–215. 10.1016/j.proche.2012.06.029. [DOI] [Google Scholar]
- Shandil Y.; Chauhan G. S.; Kumar P. Antimicrobial properties of bio-inspired poly (4-vinyl-2-pyridone) and its N-alkylated cationic derivatives. Polym. Int. 2017, 66, 119–125. 10.1002/pi.5252. [DOI] [Google Scholar]
- Sharma S. K.; Chauhan G. S.; Gupta R.; Ahn J. H. Tuning anti-microbial activity of poly (4-vinyl 2-hydroxyethyl pyridinium) chloride by anion exchange reactions. J. Mater. Sci.: Mater. Med. 2010, 21, 717–724. 10.1007/s10856-009-3932-9. [DOI] [PubMed] [Google Scholar]
- Dogra P.; Chauhan G. S. Functionalization of tetracycline and evaluation of its antibacterial activity including against resistant bacteria. Med. Chem. 2014, 11, 86–93. 10.2174/1573406410666140507094435. [DOI] [PubMed] [Google Scholar]
- Dastidar S. G.; Mondal U.; Neogi S.; Chakrabarty A. N. Antibacterial property of methyl-DOPA and development of cross-resistances in m-DOPA mutants. Indian J. Med. Res. 1986, 84, 142–147. [PubMed] [Google Scholar]
- Chakrabarty A. N.; Acharya D. P.; Neogi D.; Dastidar S. G. Drug interaction of some non-conventional antimicrobial chemotherapeutic agents with special reference to promethazine. Indian J. Med. Res. 1989, 89, 233–237. [PubMed] [Google Scholar]
- Amaral L.; Lorian V. Effects of chlorpromazine on the cell envelope proteins of Escherichia coli. Antimicrob. Agents Chemother. 1991, 35, 1923–1924. 10.1128/AAC.35.9.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annadurai S.; Basu S.; Ray S.; Dastidar S. G.; Chakrabarty A. N. Antimicrobial activity of the antiinflammatory agent diclofenac sodium. Indian J. Exp. Biol. 1998, 36, 86–90. [PubMed] [Google Scholar]
- Mazumdar K.; Dutta N. K.; Kumar K. A.; Dastidar S. G. In vitro and In vivo synergism between tetracycline and the cardiovascular agent oxyfedrine HCl against common bacterial strains. Biol. Pharm. Bull. 2005, 28, 713–717. 10.1248/bpb.28.713. [DOI] [PubMed] [Google Scholar]
- Karaka P.; Kumar K. A.; Basu L. R.; Dasgupta A.; Ray R.; Dastidar S. G. Experimental analysis of antimicrobial action of dicyclomine hydrocloride. Biol. Pharm. Bull. 2004, 27, 2010–2013. 10.1248/bpb.27.2010. [DOI] [PubMed] [Google Scholar]
- Maji S.; Maji H. S.; Chakraborty P.; Sujata G. D. Potential of Dopamine Hydrochloride as a Novel Antimicrobial Agent. Int. J. Biomed. Pharm. Sci. 2010, 4, 70–75. [Google Scholar]
- Jeswani G.; Alexander A.; Saraf S.; Saraf S.; Qureshi A.; Ajazuddin Recent approaches for reducing hemolytic activity of chemotherapeutic agents. J. Controlled Release 2015, 211, 10–21. 10.1016/j.jconrel.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Pająk M.; Kańska M. Synthesis of isotopomers of dopamine labeled with deuterium or tritium. J. Label Compd. Radiopharm. 2006, 49, 1061–1067. 10.1002/jlcr.1123. [DOI] [Google Scholar]
- Li J.; Tan L.; Liu X.; Cui Z.; Yang X.; Yeung K. W. K.; Chu P. K.; Wu S. Balancing Bacteria-Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/ Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263. 10.1021/acsnano.7b05620. [DOI] [PubMed] [Google Scholar]
- Raza Z. A.; Rehman A.; Anwar F.; Usman A. Development and antibacterial performance of silver nanoparticles incorporated polydopamine–polyester-knitted fabric. Bull. Mater. Sci. 2016, 39, 391–396. 10.1007/s12034-016-1180-4. [DOI] [Google Scholar]
- Li M.; Liu X.; Xu Z.; Yeung K. W. K.; Wu S. Dopamine Modified Organic-Inorganic Hybrid Coating for Antimicrobial and Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 33972–33981. 10.1021/acsami.6b09457. [DOI] [PubMed] [Google Scholar]
- Xie J.; Xu C.; Kohler N.; Hou Y.; Sun S. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced nonspecific uptake by macrophage cells. Adv. Mater. 2007, 19, 3163–3166. 10.1002/adma.200701975. [DOI] [Google Scholar]
- Patel H. S.; Patel V. C. Polyimides containing s-triazine ring. Des. Monomers Polym. 2001, 4, 369–380. 10.1163/156855501753210844. [DOI] [Google Scholar]
- Sellami M.; Châari A.; Aissa I.; Bouaziz M.; Gargouri Y.; Miled N. Newly synthesized dopamine ester derivatives and assessment of their antioxidant, antimicrobial and hemolytic activities. Process Biochem. 2013, 48, 1481–1487. 10.1016/j.procbio.2013.07.022. [DOI] [Google Scholar]
- Su L.; Yu Y.; Zhao Y.; Liang F.; Zhang X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking assisted Method. Sci. Rep. 2016, 6, 24420 10.1038/srep24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.; Fan H.; Wang L.; Jin Z. Oxidative Self-Polymerization of Dopamine in an Acidic Environment. Langmuir 2015, 31, 11671–11677. 10.1021/acs.langmuir.5b02757. [DOI] [PubMed] [Google Scholar]
- Chen T. P.; Liu T.; Su T. L.; Liang J. Self-Polymerization of Dopamine in Acidic Environments without Oxygen. Langmuir 2017, 33, 5863–5871. 10.1021/acs.langmuir.7b01127. [DOI] [PubMed] [Google Scholar]
- Koç Z. E.; Aladag M. O.; Uysal A. Synthesis of novel dopamine derived multifunctional ligands from cyanuric chloride: structural and antimicrobial studies. EXCLI. J. 2013, 12, 396–403. [PMC free article] [PubMed] [Google Scholar]
- Durgadas C. V.; Sharma C. P.; Sreenivasan K. Fluorescent and superparamagnetic hybrid quantum clusters for magnetic separation and imaging of cancer cells from blood. Nanoscale 2011, 3, 4780–4787. 10.1039/c1nr10900f. [DOI] [PubMed] [Google Scholar]
- Liu W.; Li Y.; Meng X.; Liu G.; Hu S.; Pan F.; Wu H.; Jiang Z.; Wang B.; Li Z.; Cao X. Embedding dopamine nanoaggregates into a poly(dimethylsiloxane) membrane to confer controlled interactions and free volume for enhanced separation performance. J. Mater. Chem. A 2013, 1, 3713. 10.1039/c3ta00766a. [DOI] [Google Scholar]
- Ciubuc J. D.; Bennet K. E.; Qiu C.; Alonzo M.; Durrer W. G.; Manciu F. S. Raman computational and experimental studies of dopamine detection. Biosensors 2017, 7, 43. 10.3390/bios7040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. Y. C.; Davis J. S.; Eichenberger E.; Holland T. L.; Fowler V. G. Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations and management. Clin. Microbiol. Rev. 2015, 28, 603–661. 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena K. D.; Gerba C. P. Risk assessment of Pseudomonas aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. 10.1007/978-1-4419-0032-6_3. [DOI] [PubMed] [Google Scholar]
- Trivedi M. K.; Branton A.; Trivedi D.; Nayak G.; Mondal S. C.; Jana S. Phenotyping and Genotyping Characterization of Proteus vulgaris after biofield treatment. Int. J. Genet. Genomics 2015, 3, 66–73. 10.11648/j.ijgg.20150306.12. [DOI] [Google Scholar]
- Hohl T. M.; Feldmesser M. Aspergillus fumigatus: Principles of pathogenesis and host defense. Eukaryotic Cell 2007, 6, 1953–1963. 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person A. K.; Chudgar S. M.; Norton B. L.; Tong B. C.; Stout J. E. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J. Med. Microbiol. 2010, 59, 834–838. 10.1099/jmm.0.018309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshikh M.; Ahmed S.; Funston S.; Dunlop P.; McGaw M.; Marchant R.; Banat I. M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M.; Sadiki M.; Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangirian H.; Haron M. D. J.; Ismail M. H. S.; Moghaddam R. R.; Hejri L. A.; Abdollahi Y.; Rezayi M.; Vafaei N. Well Diffusion Method for Evaluation of Antibacterial Activity of Copper Phenyl Fatty Hydroxamate Synthesized From Canola and Palm Kernel Oils. Digest J. Nanomat. Biostructures 2013, 8, 1263–1270. [Google Scholar]
- Tudela J. L. R.; Barchiesi F.; Bille J.; Chryssanthou E.; Estrella C. M.; Denning D.; Donnelly J. P.; Dupont B.; Fegeler W.; Moore C. B.; Richardson M.; Verweij P. E. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, 1–8. 10.1046/j.1469-0691.2003.00789.x.12691538 [DOI] [Google Scholar]
- Mann C. M.; Markham J. L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]







