Abstract
Background.
Heart failure (HF) is associated with cognitive impairment. However, we know little about the time course of cognitive change after HF diagnosis, the importance of comorbid atrial fibrillation (AF), or the role of ejection fraction (EF). We sought to determine associations of incident HF with rates of cognitive decline and whether these differed by AF status or reduced versus preserved EF.
Methods and Results.
Participants were 4,864 men and women aged ≥ 65 without a history of HF and free of clinical stroke in the Cardiovascular Health Study, a community-based prospective cohort study in the United States, with cognition assessed annually from 1989/90 through 1998/99. We identified 496 participants with incident HF by review of hospital discharge summaries and Medicare claims data, with adjudication according to standard criteria. Global cognitive ability was measured by the Modified Mini-Mental State Examination (3MSE). In adjusted models, 5-year decline in model-predicted mean 3MSE score was 10.2 points (95% CI: 8.6, 11.8) after incident HF diagnosed at age 80, compared with mean 5-year decline of 5.8 points (95% CI: 5.3, 6.2) from age 80 to 85 without HF. The association was stronger at older ages than at younger ages, did not vary significantly in the presence versus absence of AF (p = 0.084), and did not vary significantly by reduced versus preserved EF (p = 0.734).
Conclusions.
Decline in global cognitive ability tends to be faster after HF diagnosis than without HF. Clinical and public health implications of this finding warrant further attention.
Keywords: Clinical studies, epidemiology, heart failure
INTRODUCTION
Heart failure (HF) is a common chronic condition in older adults and is associated with cognitive impairment and dementia,1–3 which lead to difficulties in self-care and higher risks for hospitalization and mortality.4–7 Our understanding of the time course of cognitive change in HF is limited, because most prior longitudinal studies of cognitive decline in HF were based on small groups of participants with HF, had less than one year of follow-up, lacked non-HF comparison groups, or had inadequate adjustment for vascular risk factors.8,9 A few longitudinal studies with follow-up beyond one year and non-HF comparison groups provide some evidence that HF is associated with faster cognitive decline.10–13 However, those studies, too, were limited by including relatively small groups of participants, combining people who had a history of HF at baseline with those who had incident HF diagnosed during follow-up without accounting for time elapsed since HF diagnosis, or grouping together cognitive test scores obtained before and after incident HF diagnosis to estimate cognitive trajectories. Therefore, there is a need for results from larger long-term studies of participants with incident heart failure, with statistical modeling that differentiates between cognitive test scores obtained before versus after heart failure diagnosis, in order to estimate how quickly cognition declines after incident HF diagnosis.
HF often manifests with concomitant atrial fibrillation (AF). In a previous analysis we showed that incident AF was associated with faster cognitive decline in older adults.14 Would HF and AF interact synergistically in relation to long-term cognitive decline? Data to address this question are sparse. In several cross-sectional studies of people with HF, those with concomitant AF had higher odds of cognitive impairment than those without AF.15 However, prior longitudinal studies did not assess whether long-term cognitive trajectories in HF differed according to AF status.
Another gap in our understanding of HF and long-term cognitive decline relates to left ventricular ejection fraction (EF). Would HF with reduced EF have a stronger relationship with cognitive decline than HF with preserved EF? Data to address this issue are limited. Cross-sectional data suggest that cognitive performance in people with HF varies according to whether EF is reduced or preserved.16,17 However, prior longitudinal studies did not compare long-term cognitive trajectories in people with HF who had reduced versus preserved EF.
To address these knowledge gaps, we sought to determine associations of incident HF and duration of HF with long-term cognitive trajectories and whether these associations differed by presence versus absence of AF or by reduced versus preserved EF. We used cognitive measures obtained longitudinally in a large community-based cohort of older adults in which newly diagnosed HF was identified during follow-up, and statistical models that differentiated between cognitive test scores obtained before and after incident HF diagnosis. Our goal was to produce estimates of average cognitive trajectories after incident HF diagnosis which would allow people with newly diagnosed HF, their families, and clinicians to anticipate the average rate at which cognitive decline may occur.
METHODS
Design, setting, and participants
The Cardiovascular Health Study (CHS), a community-based longitudinal study, was conducted to identify risk factors for and consequences of cardiovascular diseases in older adults.18 CHS included 5,888 men and women aged 65 and above enrolled in 1989/90 and 1992/93 in Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Allegheny County, Pennsylvania. For this analysis, participants were followed through 1998/99. Prevalent cardiovascular disease and risk factors were ascertained at enrollment.19 Updated risk factor data and functional measures including cognitive performance were collected at annual clinic visits through 1998/99. Incident cardiovascular events were ascertained throughout follow-up and adjudicated according to standard criteria.20,21 Participants were included in this analysis if they did not have prevalent HF at enrollment, had at least one cognitive assessment obtained at enrollment or during follow-up, and had complete covariate data (Supplemental Figure 1). Participants who had a history of clinical stroke at enrollment were excluded, and participants were censored at occurrence of incident clinical stroke, because stroke is known to be associated with more rapid cognitive decline, and therefore we wanted to focus on cognitive trajectories in people free of clinical stroke. Institutional review boards at the coordinating center at University of Washington and each field center approved the study, and participants gave written informed consent. The institutional review board at Brigham Young University approved the use of these data for this analysis. Requests to access the dataset from qualified researchers trained in human subjects confidentiality protocols may be sent to the Collaborative Health Studies Coordinating Center (https://www.uwchscc.org) at the University of Washington, Seattle, WA.
Assessment of HF and EF
History of HF prior to enrollment was identified by participant report and verified by medical record review.19 During follow-up, hospitalizations were identified by participant report and discharge diagnoses from Medicare data.20 Following these reports, incident HF was identified and confirmed by review of outpatient or inpatient medical records and diagnostic tests.20–23 The CHS events committee adjudicated incident HF on the basis of appropriate symptoms and signs of HF in the medical record, pulmonary edema on chest X-ray or other supporting clinical findings, or medication prescribed for HF.20–23 After a confirmed incident HF diagnosis, a participant was considered to have HF throughout the remainder of follow-up for this analysis.
Approximately 50% of participants who developed incident HF had left ventricular EF data at the time of HF diagnosis available from medical record review (Supplemental Figure 1), derived from echocardiography, cardiac catheterization, multi-gated acquisition scanning, or other modalities. For this analysis, we classified EF <45% as reduced EF, and EF ≥45% as preserved EF. We did not include EF data in this analysis for participants who did not develop incident HF.
Assessment of AF
AF was identified by electrocardiograms obtained at annual clinic visits and hospital discharge diagnosis codes.19,24,25
Assessment of cognitive function
Global cognitive function was assessed longitudinally using the 100-point Modified Mini-Mental State Examination (3MSE).26 The 3MSE was administered annually up to 9 times per participant from 1990/91 through 1998/99. The 3MSE includes questions assessing memory, orientation, fluency, problem solving, and ability to follow instructions, yielding a global cognitive score between zero (worst) and 100 (best). During 1995/96 to 1998/99, for participants not able to complete an in-person exam, telephone-based measures of global cognitive performance were obtained, including the Telephone Interview for Cognitive Status (TICS) and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).27,28 These were used to estimate missing 3MSE scores using equations previously developed for CHS data (Supplemental Figure 1).29
Processing speed was assessed longitudinally using the Digit Symbol Substitution Test (DSST).30 The DSST was administered annually up to 10 times per participant from 1989/90 through 1989/99. The DSST consists of a visual motor task in which, over 90 seconds, a person matches symbols with correct digits according to a key, yielding a measure of information processing speed on a scale of zero (worst) to 90 (best).
Assessment of other variables
Age, birth year, sex, race, years of education, and use of cigarettes and alcohol were determined by participant report. Medication use was determined by annual medication inventory.31 Creatinine, hemoglobin, and glucose were measured in blood samples.32 Blood pressure was recorded as the average of two measurements. Body mass index was calculated from measured height and weight. Chronic obstructive pulmonary disease was identified by participant report. Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2based on serum creatinine and the CKD-EPI equation.33 Anemia was defined as hemoglobin <13 g/dL for men and <12 g/dL for women.34 Stroke and coronary heart disease were identified by participant report or proxy report, confirmed by medical record review, and adjudicated according to standard criteria.19–21,35 Diabetes was defined as use of insulin or oral hypoglycemic medications, or fasting serum glucose ≥126 mg/dL or non-fasting serum glucose ≥200 mg/dL. Hypertension was defined as self-reported physician-diagnosed hypertension plus use of antihypertensive medication or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Stroke, coronary heart disease, AF, diabetes, and hypertension status were initially defined at enrollment and then updated during follow-up. A participant was considered to have diabetes through the end of follow-up after the first observed elevated glucose or reported use of antidiabetic medication. All other covariates were defined at enrollment and considered non-time-varying.
Statistical analysis
We developed statistical models separately for each cognitive function test (3MSE and DSST). We used linear mixed models with age as the time scale to model the relationship of incident HF with longitudinal cognitive trajectory.36 Incident HF was a time-varying variable, coded as absent (0) at the beginning of follow-up for all participants, then recoded to present (1) part of the way through follow-up at the age of HF diagnosis for participants who experienced incident HF. We considered intercept, age, and age-squared to be random at the participant level and included them as random effects with a spatial exponential covariance structure. We included incident HF and time elapsed since incident HF diagnosis as time-varying fixed effects; and the following four multiplicative interaction terms of HF variables with age variables as fixed effects: incident HF × age, incident HF × age-squared, time elapsed since incident HF diagnosis × age, and time elapsed since incident HF diagnosis × age-squared. Thus, average cognitive performance with no prior history of HF at any given age was based on cognitive test scores obtained from participants with no prior history of HF at that age, including those participants who went on to experience incident HF at older ages (Supplemental Figure 1). We also included other variables listed above as fixed effects to adjust for potential confounding. To determine whether the cognitive trajectory across age differed significantly by incident HF, we used an F test to simultaneously test the four interaction terms, thereby comparing our primary model as specified above with a simpler model that did not include interactions of HF variables with age variables. We graphed model-predicted cognitive trajectories according to age and HF status, and calculated model-predicted five-year declines in cognitive performance without of HF and after incident HF diagnosis.
To explore whether the association of incident HF with cognitive decline differed according to presence versus absence of AF, we augmented the models described above with multiplicative interaction terms for AF with incident heart failure and age variables as fixed effects, including four two-way interaction terms: AF × incident HF, AF × time elapsed since incident HF diagnosis, AF × age, and AF × age-squared; and four three-way interaction terms: AF × incident HF × age, AF × incident HF × age-squared, AF × time elapsed since incident HF diagnosis × age, and AF × time elapsed since incident HF diagnosis × age-squared. We used two different F tests to assess this model. First, to determine whether cognitive trajectories estimated from our primary model differed overall by AF status, we simultaneously tested all eight interaction terms involving AF, thereby comparing this model with our primary model. Second, to determine whether the differences in cognitive trajectory across age by incident HF further differed significantly by AF status, we simultaneously tested the four three-way interaction terms involving AF, HF, and age variables, thereby comparing this model with an intermediate model that included only two-way interactions of AF with HF and age variables but no three-way interactions. We graphed model-predicted cognitive trajectories and calculated model-predicted five-year declines in cognitive performance, similar to the primary analysis but stratified by AF status.
To explore the association of EF with rate of cognitive decline after HF diagnosis, we classified each participant with HF as having reduced EF, preserved EF, or unknown EF. For these models, we included only post-HF cognitive test scores; used time since incident HF diagnosis as the time scale instead of age; included intercept and time as random effects; included EF category and EF category × time as fixed effects; and adjusted for age at incident HF diagnosis, sex, race, and education as fixed effects. We considered a time-squared term but omitted it because it did not significantly improve the estimate of average cognitive test score trajectory over time; a linear term for time since incident HF diagnosis was sufficient. Using this simpler model, we estimated model-predicted five-year declines in cognitive performance after incident HF diagnosis for each EF category.
In a sensitivity analysis we omitted participants who had only one cognitive assessment and analyzed only participants who had multiple cognitive assessments over time. In another sensitivity analysis we omitted 3MSE scores estimated from telephone-based cognitive assessments and analyzed only directly measured 3MSE scores.
Statistical analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.2.4 (R Development Core Team, www.r-project.org). Dr. Thacker had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
RESULTS
Incident HF and participant characteristics
Incident HF was identified in 496 (10.2%) of 4,864 participants over a mean of 6.4 years of follow-up (maximum of 8 years) in 3MSE analyses, and in 389 (7.8%) of 4,969 participants over a mean of 6.5 years of follow-up (maximum of 9 years) in DSST analyses. A greater number of incident HF cases were identified in 3MSE analyses than for DSST analyses because follow-up for 3MSE analyses was extended into older ages for some participants by estimating missing 3MSE scores using telephone-based cognitive measures (see above and Supplemental Figure 1). Participants who developed incident HF were on average older, more often male, of white race, and at higher cardiovascular risk than those who did not develop incident HF (Table 1 and Supplemental Table 1).
Table 1.
Characteristics at study enrollment and incident conditions by incident heart failure (HF) for participants included in analysis of Modified Mini-Mental State Examination (3MSE)*
| Characteristic† | Participants diagnosed with incident HF during follow-up (N = 496) |
Participants not diagnosed with incident HF during follow-up (N = 4,368) |
|---|---|---|
| Age, y, mean (SD) | 75.7 (6.0) | 73.7 (5.3) |
| Birth year, mean (SD) | 1915 (6.0) | 1917 (5.4) |
| Male, % | 50.4 | 40.6 |
| Black race, % | 11.7 | 14.5 |
| Years of education through 12th grade, mean (SD) | 10.7 (2.3) | 11.1 (1.9) |
| Any education beyond 12th grade, % | 40.1 | 44.3 |
| Former smoking, % | 43.8 | 41.7 |
| Current smoking, % | 12.5 | 11.6 |
| Any current alcohol use, % | 43.1 | 52.7 |
| Drinks/week, mean (SD) | 4.4 (7.0) | 5.1 (8.7) |
| Beta-blocker use, % | 16.5 | 12.1 |
| Angiotensin converting enzyme inhibitor use, % | 7.1 | 5.9 |
| Systolic blood pressure, mm Hg, mean (SD) | 140.9 (22.5) | 135.3 (21.2) |
| Body mass index, kg/m2, mean (SD) | 27.5 (5.1) | 26.5 (4.5) |
| Chronic kidney disease, % | 44.0 | 36.7 |
| Chronic obstructive pulmonary disease, % | 15.9 | 12.1 |
| Anemia, % | 8.5 | 7.3 |
| Diabetes | ||
| At enrollment, % | 24.4 | 13.1 |
| Incident, % | 7.5 | 4.3 |
| Hypertension | ||
| At enrollment, % | 65.5 | 55.6 |
| Incident, % | 14.9 | 16.4 |
| Coronary heart disease | ||
| At enrollment, % | 30.8 | 14.9 |
| Incident, % | 30.4 | 7.0 |
| Atrial fibrillation | ||
| At enrollment, % | 5.2 | 1.6 |
| Incident, % | 33.3 | 6.5 |
The 4,864 participants included in this table (496 + 4,368) had at least one 3MSE score and were included in regression models for 3MSE.
All percentages in the table are column percentages. Mean drinks per week was calculated among participants who reported any current alcohol use. For diabetes, hypertension, coronary heart disease, and atrial fibrillation, percentages of participants with the condition present at enrollment and of those with the incident condition occurring during follow-up were mutually exclusive and therefore sum to the total percentage of participants with the condition either present at enrollment or occurring during follow-up (mean of 6.4 years). Incident diabetes, hypertension, coronary heart disease, or atrial fibrillation may have occurred before or after incident HF diagnosis.
Modified Mini-Mental State Examination
We analyzed 34,854 3MSE scores obtained from 4,864 participants, representing >90% of potential 3MSE scores that could have been obtained had there been no censoring and no missed exams. A summary of 3MSE scores not obtained or not analyzed due to censoring or missed exams is given in Supplemental Figure 1. Model-predicted trajectories of mean 3MSE score are illustrated in Figure 1 Panel A. With no prior history of HF (solid curve), mean 3MSE declined steeply after age 75. Model-predicted trajectories of mean 3MSE score after incident HF diagnosed at ages 70, 75, 80, and 85 are shown in four dashed curves. Mean 3MSE score declined faster after incident HF diagnosis relative to no prior history of HF. For example, decline in mean 3MSE score from age 80 to age 85 was 10.2 points (95% CI: 8.6, 11.8) after incident HF diagnosed at age 80, compared with mean decline of 5.8 points (95% CI: 5.3, 6.2) from age 80 to age 85 without HF (Table 2). We observed age-related differences in the association of incident HF with mean 3MSE score: older age at HF diagnosis was associated with larger differences in trajectories of mean 3MSE score after HF diagnosis relative to no prior history of HF. Model coefficients and standard errors are in Supplemental Table 2.
Figure 1. Model-predicted trajectories of mean Modified Mini-Mental State Examination (3MSE) score and Digit Symbol Substitution Test (DSST) score with no prior history of heart failure (HF) or after incident HF.
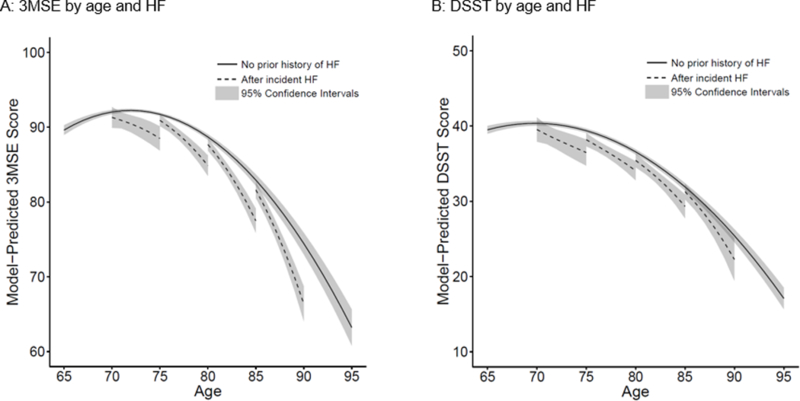
Panel A: Model-predicted trajectories of 3MSE score. Panel B: Model-predicted trajectories of DSST score.
In each graph, solid curve represents model-predicted mean cognitive test score trajectory with no prior history of heart failure (HF). Four dashed curves represent model-predicted mean cognitive test score trajectories after incident HF diagnosed at ages 70, 75, 80, and 85 years. Shading represents 95% confidence bands. Trajectories are adjusted for birth year, sex, race, education, cigarette smoking, alcohol use, beta-blocker use, angiotensin converting enzyme inhibitor use, systolic blood pressure, body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, diabetes, hypertension, coronary heart disease, and atrial fibrillation.
F test P = 0.011 for whether 3MSE trajectory across age differed by incident HF.
F test P = 0.479 for whether DSST trajectory across age differed by incident HF.
Table 2.
Model-predicted five-year declines in mean Modified Mini-Mental State Examination (3MSE) score, by age and incident heart failure (HF)
| Predicted five-year decline (95% confidence interval)* | ||||
|---|---|---|---|---|
| Heart failure† | From age 70 to age 75 |
From age 75 to age 80 |
From age 80 to age 85 |
From age 85 to age 90 |
| No HF diagnosed before or during five-year interval | 0.3 (0.0, 0.6) | 3.0 (2.8, 3.3) | 5.8 (5.3, 6.2) | 8.5 (7.7, 9.2) |
| Incident HF diagnosed at beginning of five-year interval | 2.8 (0.7, 4.9) | 6.1 (4.6, 7.5) | 10.2 (8.6, 11.8) | 15.2 (13.1, 17.4) |
| Difference (incident HF minus no HF) | 2.5 (0.4, 4.6) | 3.0 (1.6, 4.4) | 4.4 (2.8, 6.0) | 6.7 (4.6, 8.8) |
Adjusted for birth year, sex, race, education, cigarette smoking, alcohol use, beta-blocker use, angiotensin converting enzyme inhibitor use, systolic blood pressure, body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, diabetes, hypertension, coronary heart disease, and atrial fibrillation. Participants were censored at the development of clinically recognized stroke.
F test P = 0.011 for whether 3MSE trajectory across age differed by incident HF.
Digit Symbol Substitution Test
We analyzed 34,769 DSST scores obtained from 4,969 participants, representing >84% of potential DSST scores that could have been obtained had there been no censoring and no missed exams. A summary of DSST scores not obtained or not analyzed due to censoring or missed exams is given in Supplemental Figure 1. Model-predicted trajectories of mean DSST score are illustrated in Figure 1 Panel B. Rate of decline in mean DSST score with advancing age was not significantly different after incident HF relative to no prior history of HF. For example, decline in mean DSST score from age 80 to age 85 was 6.1 points (95% CI: 4.4, 7.8) after incident HF diagnosed at age 80, compared with mean decline of 4.7 points (95% CI: 4.4, 5.0) from age 80 to age 85 without HF (Table 3), a statistically non-significant difference. We did not observe age-related differences in the association of incident HF with mean DSST score; differences in trajectories of mean DSST score after HF diagnosis relative to no prior history of HF were similar across the age range. Model coefficients and standard errors are in Supplemental Table 2.
Table 3.
Model-predicted five-year declines in mean Digit Symbol Substitution Test (DSST) score, by age and incident heart failure (HF)
| Predicted five-year decline (95% confidence interval)* | ||||
|---|---|---|---|---|
| Heart failure† | From age 70 to age 75 |
From age 75 to age 80 |
From age 80 to age 85 |
From age 85 to age 90 |
| No HF diagnosed before or during five-year interval | 1.0 (0.8, 1.2) | 2.8 (2.6, 3.0) | 4.7 (4.4, 5.0) | 6.5 (6.0, 6.9) |
| Incident HF diagnosed at beginning of five-year interval | 3.1 (0.8, 5.4) | 4.1 (2.7, 5.5) | 6.1 (4.4, 7.8) | 9.0 (6.3, 11.8) |
| Difference (incident HF minus no HF) | 2.1 (−0.2, 4.4) | 1.3 (−0.1, 2.7) | 1.5 (−0.2, 3.2) | 2.5 (−0.2, 5.3) |
Adjusted for birth year, sex, race, education, cigarette smoking, alcohol use, beta-blocker use, angiotensin converting enzyme inhibitor use, systolic blood pressure, body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, diabetes, hypertension, coronary heart disease, and atrial fibrillation. Participants were censored at the development of clinically recognized stroke.
F test P = 0.479 for whether DSST trajectory across age differed by incident HF.
Interaction of AF with incident HF
Model-predicted trajectories of mean 3MSE score in the presence or absence of AF are shown in Figure 2 Panels A and B. Overall, 3MSE trajectories were significantly different in the presence of AF versus absence of AF (p = 0.027); this overall difference was explained by mean model-predicted 3MSE score declining more quickly with age in the presence of AF than in its absence, regardless of incident HF, especially at older ages (Figure 2 Panel A). However, the magnitude of accelerated decline in 3MSE score after incident HF diagnosis was not significantly different in the presence versus absence of AF (p = 0.084; for magnitudes of these differences see Table 4). Overall trajectories of DSST score were not significantly different by AF status (p = 0.339), nor were differences in decline in DSST score by incident HF significantly different by AF status (p = 0.505; Figure 2 Panels C and D; Table 4).
Figure 2. Model-predicted trajectories of mean Modified Mini-Mental State Examination (3MSE) score and Digit Symbol Substitution Test (DSST) score with no prior history of heart failure (HF) or after incident HF, by atrial fibrillation (AF) status.
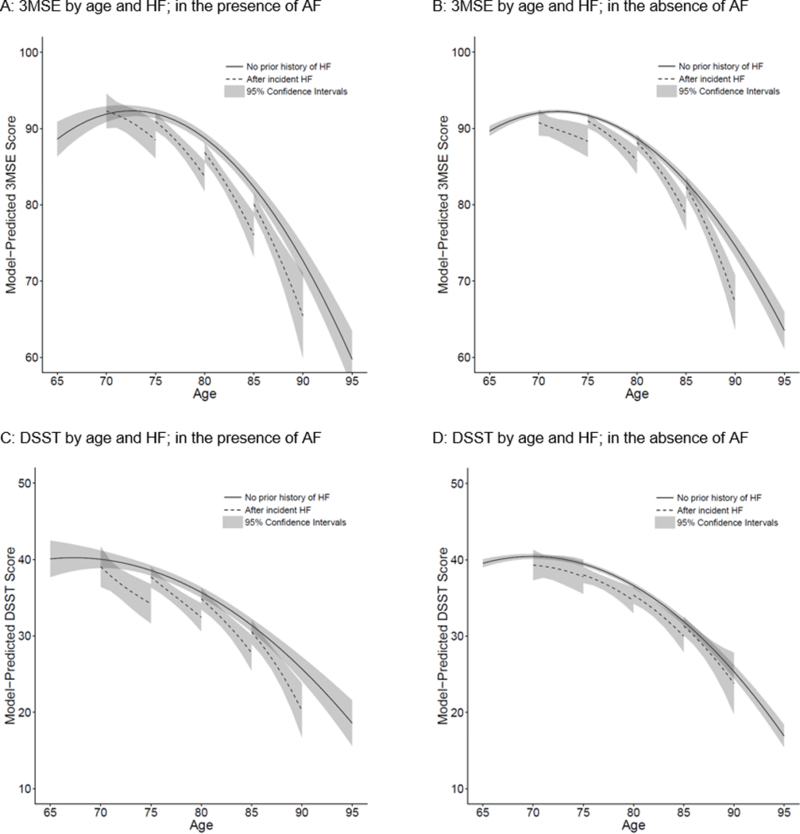
Panel A: Model-predicted trajectories of 3MSE score in the presence of AF. Panel B: Model-predicted trajectories of 3MSE score in the absence of AF. Panel C: Model-predicted trajectories of DSST score in the presence of AF. Panel D: Model-predicted trajectories of DSST score in the absence of AF.
In each graph, solid curve represents model-predicted mean cognitive test score trajectory with no prior history of heart failure (HF). Four dashed curves represent model-predicted mean cognitive test score trajectories after incident HF diagnosed at ages 70, 75, 80, and 85 years. Shading represents 95% confidence bands. Trajectories are adjusted for birth year, sex, race, education, cigarette smoking, alcohol use, beta-blocker use, angiotensin converting enzyme inhibitor use, systolic blood pressure, body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, diabetes, hypertension, and coronary heart disease.
F test P = 0.027 for whether 3MSE trajectories differed overall by AF status.
F test P = 0.084 for whether the difference in 3MSE trajectory by incident HF further differed by AF status.
F test P = 0.339 for whether DSST trajectories differed overall by AF status.
F test P = 0.505 for whether the difference in DSST trajectory by incident HF further differed by AF status.
Table 4.
Model-predicted five-year decline in mean Modified Mini-Mental State Examination (3MSE) score and Digit Symbol Substitution Test (DSST) score, by age, incident heart failure (HF), and atrial fibrillation (AF) status
| Predicted five-year decline (95% confidence interval) * | ||||
|---|---|---|---|---|
| Heart failure | From age 70 to age 75 |
From age 75 to age 80 |
From age 80 to age 85 |
From age 85 to age 90 |
| 3MSE by age and HF; in the presence of AF† | ||||
| No HF diagnosed before or during five-year interval | 0.0 (−0.9, 0.8) | 3.2 (2.7, 3.7) | 6.4 (5.7, 7.2) | 9.7 (8.4, 10.9) |
| Incident HF diagnosed at beginning of five-year interval | 3.8 (0.5, 7.1) | 7.2 (5.0, 9.3) | 10.8 (7.8, 13.8) | 14.7 (9.2, 20.1) |
| Difference (incident HF minus no HF)‡ | 3.8 (0.4, 7.3) | 4.0 (1.8, 6.2) | 4.4 (1.3, 7.4) | 5.0 (−0.5, 10.5) |
| 3MSE by age and HF; in the absence of AF† | ||||
| No HF diagnosed before or during five-year interval | 0.3 (0.1, 0.6) | 3.0 (2.7, 3.3) | 5.7 (5.2, 6.2) | 8.4 (7.6, 9.1) |
| Incident HF diagnosed at beginning of five-year interval | 2.4 (−0.3, 5.1) | 5.2 (3.4, 7.0) | 9.4 (7.3, 11.6) | 15.2 (11.9, 18.4) |
| Difference (incident HF minus no HF)‡ | 2.1 (−0.6, 4.8) | 2.2 (0.3, 4.0) | 3.7 (1.6, 5.9) | 6.8 (3.6, 10.0) |
| DSST by age and HF; in the presence of AF§ | ||||
| No HF diagnosed before or during five-year interval | 1.5 (0.6, 2.4) | 2.9 (2.4, 3.4) | 4.3 (3.7, 4.9) | 5.7 (4.7, 6.8) |
| Incident HF diagnosed at beginning of five-year interval | 4.9 (1.3, 8.5) | 5.3 (3.2, 7.4) | 7.1 (4.5, 9.7) | 10.3 (6.4, 14.1) |
| Difference (incident HF minus no HF)|| | 3.4 (−0.3, 7.1) | 2.4 (0.2, 4.6) | 2.8 (0.2, 5.4) | 4.6 (0.6, 8.5) |
| DSST by age and HF; in the absence of AF§ | ||||
| No HF diagnosed before or during five-year interval | 1.0 (0.8, 1.2) | 2.8 (2.6, 3.0) | 4.7 (4.4, 5.0) | 6.6 (6.1, 7.0) |
| Incident HF diagnosed at beginning of five-year interval | 1.5 (−1.5, 4.5) | 3.4 (1.5, 5.2) | 5.4 (3.1, 7.6) | 7.5 (3.6, 11.5) |
| Difference (incident HF minus no HF)|| | 0.6 (−2.5, 3.6) | 0.5 (−1.3, 2.4) | 0.7 (−1.6, 2.9) | 1.0 (−3.0, 4.9) |
Adjusted for birth year, sex, race, education, cigarette smoking, alcohol use, beta-blocker use, angiotensin converting enzyme inhibitor use, systolic blood pressure, body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, diabetes, hypertension, and coronary heart disease.
F test P = 0.027 for whether 3MSE trajectories differed overall by AF status.
F test P = 0.084 for whether the difference in 3MSE trajectory by incident HF further differed by AF status.
F test P = 0.339 for whether DSST trajectories differed overall by AF status.
F test P = 0.505 for whether the difference in DSST trajectory by incident HF further differed by AF status.
HF with reduced, preserved, or unknown EF
After HF diagnosis, rates of cognitive decline by EF category were not significantly different (Table 5). Precision of these estimates was poor, due to relatively sparse data within each EF category.
Table 5.
Model-predicted five-year decline in mean Modified Mini-Mental State Examination (3MSE) score and Digit Symbol Substitution Test (DSST) score after incident heart failure (HF) diagnosis, by left ventricular ejection fraction (EF) category
| Predicted five-year decline (95% confidence interval)* | ||
|---|---|---|
| EF category | 3MSE analysis† | DSST analysis‡ |
| Reduced EF | 5.8 (2.3, 9.3) | 4.6 (1.7, 7.6) |
| Preserved EF | 7.7 (4.6, 10.8) | 4.9 (2.1, 7.6) |
| Unknown EF | 6.8 (4.2, 9.5) | 7.3 (4.9, 9.8) |
Adjusted for age at incident HF diagnosis, sex, race, and education.
F test P = 0.734 for the difference in 3MSE trajectories by EF category.
F test P = 0.280 for the difference in DSST trajectories by EF category.
Sensitivity analyses
Results were similar when we omitted participants who had only one cognitive assessment, or when we omitted 3MSE scores estimated from telephone-based cognitive assessments.
DISCUSSION
In this longitudinal study of older adults, participants who developed incident HF experienced faster average decline in global cognitive ability than their peers of the same age who had no prior history of HF. Differences between the rates of decline in 3MSE score after incident HF diagnosis versus with no prior history of HF were larger at older ages than at younger ages. We are unable to conclude that there is an association of incident HF with decline in psychomotor processing speed. The association of incident HF with decline in DSST score was smaller in magnitude than the association with decline in 3MSE score, fell short of statistical significance, and did not vary significantly by age.
Four previous studies with follow-up longer than one year compared cognitive trajectories in adults with versus without HF (Supplemental Table 3).10–13 Their results did not support a consistent profile of cognitive deficits in people with HF, but rather suggested that an association of HF with cognitive decline can be detected by some neuropsychological tests when a variety of tests are employed. Our study agreed with one prior study12 in finding a statistically significant association of HF with faster decline in global cognitive ability, and with three prior studies10–12 in finding that the association of HF with rate of decline in processing speed (DSST) was small in magnitude and not statistically significant.
Our study has several methodologic strengths. First, we excluded participants who had prevalent HF at study enrollment, modelled cognitive trajectories after incident HF diagnosis, and used time-varying variables for incident HF and time elapsed since incident HF. Second, we excluded participants who had a history of clinically recognized stroke, and censored participants when they developed clinical stroke, allowing us to rule out the possibility that accelerated cognitive decline after HF diagnosis is explained merely by a higher rate of clinical stroke. Third, we used interaction terms between age and HF variables in our statistical models, enabling us to observe that the association of incident HF with 3MSE trajectory differed by age. Fourth, we conducted a large study with long follow-up, allowing us to and graph model-predicted trajectories with excellent precision.
In our previously published work on AF and cognitive decline,14 our model predicted that people diagnosed with incident AF at age 80 would experience on average a 9- to 12-point decline in 3MSE score over the next five years, whereas people the same age without AF would experience only a 6- to 7-point decline over five years. Similarly, in this new analysis we observed that people diagnosed with incident HF at age 80 would be expected to show on average a 9- to 12-point decline over five years, compared with an average 5- to 6-point decline without HF. To build on this understanding that both AF and HF are independently associated with cognitive decline, in the present work we took a further step, using more complex models to assess whether AF and HF would interact synergistically to hasten cognitive decline. For 3MSE, when we assessed the possibility of an interaction of AF with incident HF, we found that average cognitive performance declined faster, overall, in the presence of AF, as we would expect from our prior work.14 However, we did not see evidence of synergism between AF and HF; rather, we observed that the magnitude of the association of incident HF with rate of cognitive decline was not significantly different in the presence versus absence of AF. Our results are congruent with previously published cross-sectional data showing that HF patients who also had AF were more likely to be cognitively impaired,15 but this observation may be explained by independent effects of AF and HF, rather than by an interaction of AF with HF.
We observed similar degrees of cognitive decline after HF diagnosis among participants with reduced EF, those with preserved EF, and those with unknown EF. Although we hypothesized that reduced EF would be associated with faster cognitive decline than preserved EF on the basis of previously published research,16,17 our results did not support this hypothesis. Unfortunately, we lacked precision to detect significant differences in rates of cognitive decline after incident HF diagnosis by EF category. Therefore, answering this question will require further investigation in other settings.
Our study has limitations. First, we did not have tests of specific cognitive domains other than psychomotor processing speed. Second, we had low statistical power to investigate whether EF category was associated with different cognitive trajectories. Third, our results may have been influenced by non-random loss to follow-up (see Supplemental Figure 1). Individuals who have cognitive impairment or HF are more likely to miss cognitive assessments than those who are cognitively intact and free of HF, leading to underestimated average rates of cognitive decline.37 Fourth, shared risk factors for HF and cognitive decline such as genetics, patterns of physical activity or diet, and severity or duration of diabetes and hypertension may have confounded the association of HF with cognitive change, even in our adjusted models. Fifth, our data were collected in the 1990s and are therefore ≥20 years old. However, the high-quality study design, community-based sample, data collection, length of follow-up, and statistical analysis make these data ideal for studying the epidemiology of incident heart failure and long-term cognitive decline.
It is biologically plausible that HF could cause cognitive decline. Neuroimaging studies suggest that many brain regions involved in cognition sustain injury in HF.38 Commonly cited mechanisms by which HF is thought to reduce cognitive ability are cerebral hypoperfusion and cerebral microemboli.4,5,39 However, those explanations may not be sufficient . Subclinical left ventricular dysfunction is associated with neuropsychological dysfunction and subclinical vascular brain injury, suggesting relatively subtle mechanisms.40–42 Additional mechanisms could include systemic inflammation, oxidative damage, homocysteinemia, elevated B-type natriuretic peptide, thiamine deficiency, neurohormonal dysfunction, altered cellular metabolism, and impaired cerebrovascular reactivity.1,5,38,43–46 Alternatively, HF may be a marker of underlying non-specific influences on cognitive ability such as poor general health, overall burden of illness, hospitalization, multimorbidity, polypharmacy, or fatigue.46
Based on our results, older adults who were diagnosed with HF were more likely than their peers with no prior history of HF to show global cognitive deficits or processing speed deficits, even when HF was first diagnosed. People with HF then experienced, on average, more rapid subsequent decline in global cognitive performance than people who had no prior history of HF. This association was not significantly different by AF status or by EF category. It remains to be seen whether early and consistent optimal HF management can counteract or prevent associated decline in cognitive function in people with HF.
Supplementary Material
What is new?
Global cognitive ability declined significantly faster after newly diagnosed (incident) heart failure (HF) relative to no prior history of HF, in the absence of clinical stroke.
The faster rate of decline after incident HF was evident across all ages between 70 and 90 years, and was significantly more pronounced at older ages.
The association of incident HF with more rapid cognitive decline did not differ significantly by whether participants also had concomitant atrial fibrillation.
The rate of cognitive decline after incident HF did not differ significantly by whether ejection fraction was reduced or preserved.
What are the clinical implications?
Clinicians may use our estimates of cognitive trajectories to anticipate average rates of cognitive decline that may occur in their populations of patients who have newly diagnosed HF.
Acknowledgments
SOURCES OF FUNDING
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC85084, N01HC35129, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Heckbert was supported by R01 HL127659 from NHLBI. Dr. Thacker has received a Clinical Research Loan Repayment Program award from NHLBI.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 2.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.M268. [DOI] [PubMed] [Google Scholar]
- 3.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Dodson JA, Chaudhry SI. Geriatric conditions in heart failure. Curr Cardiovasc Risk Rep. 2012;6:404–410. doi: 10.1007/s12170-012-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. doi: 10.1016/j.ijcard.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 6.Dodson JA, Truong T- TN, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126:120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murad K, Goff DC Jr, Morgan TM, Burke GL, Bartz TM, Kizer JR, Chaudhry SI, Gottdiener JS, Kitzman DW. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: the Cardiovascular Health Study. JACC Heart Fail. 2015;3:542–550. doi: 10.1016/j.jchf.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes. 2013;6:451–460. doi: 0.1161/CIRCOUTCOMES.113.000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alosco ML, Garcia S, Spitznagel MB, van Dulmen M, Cohen R, Sweet LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Cognitive performance in older adults with stable heart failure: longitudinal evidence for stability and improvement. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2014;21:239–256. doi: 10.1080/13825585.2013.818616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelm C, Dahl A, Broström A, Mårtensson J, Johansson B, Strömberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs. 2012;21:994–1003. doi: 0.1111/j.1365-2702.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 11.Alwerdt J, Edwards JD, Athilingam P, O’Connor ML, Valdés EG. Longitudinal differences in cognitive functioning among older adults with and without heart failure. J Aging Health. 2013;25:1358–1377. doi: 10.1177/0898264313505111. [DOI] [PubMed] [Google Scholar]
- 12.Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: the Heart-Mind Study. Int Psychogeriatr. 2012;24:38–47. doi: 10.1017/S1041610211001657. [DOI] [PubMed] [Google Scholar]
- 13.Verhaegen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the berlin aging study. Health Psychol. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- 14.Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myserlis PG, Malli A, Kalaitzoglou DK, Kalaitzidis G, Miligkos M, Kokkinidis DG, Kalogeropoulos AP. Atrial fibrillation and cognitive function in patients with heart failure: a systematic review and meta-analysis. Heart Fail Rev. 2017;22:1–11. doi: 10.1007/s10741-016-9587-y. [DOI] [PubMed] [Google Scholar]
- 16.Bratzke-Bauer LC, Pozehl BJ, Paul SM, Johnson JK. Neuropsychological patterns differ by type of left ventricle dysfunction in heart failure. Arch Clin Neuropsychol. 2013;28:114–124. doi: 10.1093/arclin/acs101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athilingam P, D’Aoust RF, Miller L, Chen L. Cognitive profile in persons with systolic and diastolic heart failure. Congest Heart Fail. 2013;19:44–50. doi: 10.1111/chf.12001. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 19.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT Jr. Study of cardiovascular health outcomes in the era of claims data. Circulation. 2016;133:156–164. doi: 10.1161/CIRCULATIONAHA.115.018610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 22.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1638–1637. doi: 10.1016/S0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 23.Schellenbaum GD, Heckbert SR, Smith NL, Rea TD, Lumley T, Kitzman DW, Roger VL, Taylor HA, Psaty BM. Congestive heart failure incidence and prognosis: case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–122. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.CIR.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 25.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 27.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 28.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. [DOI] [PubMed] [Google Scholar]
- 29.Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The Cardiovascular Health Study. Neuroepidemiology. 2009;33:55–65. doi: 10.1159/000215830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D Wechsler Adult Intelligence Scale-Revised Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- 31.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-B. [DOI] [PubMed] [Google Scholar]
- 32.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD- EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inzitari M, Studenski S, Rosano C, Zakai NA, Longstreth WT Jr, Cushman M, Newman AB. Anemia is associated with the progression of white matter disease in older adults with high blood pressure: the cardiovascular health study. J Am Geriatr Soc. 2008;56:1867–1872. doi: 10.1111/j.1532-5415.2008.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longstreth WT Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, Furberg CD. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/WNL.56.3.368: 1526-632X. [DOI] [PubMed] [Google Scholar]
- 36.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 37.Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008;19:440–447. doi: 10.1097/EDE.0b013e31816a1d31. [DOI] [PubMed] [Google Scholar]
- 38.Cermakova P, Eriksdotter M, Lund LH, Winblad B, Religa P, Religa D. Heart failure and Alzheimer′s disease. J Intern Med. 2015;277:406–425. doi: 10.1111/joim.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon JA, McMurray JJV, Quinn TJ. ‘Hearts and minds’: association, causation and implication of cognitive impairment in heart failure. Alzheimers Res Ther. 2015;7:22. doi: 10.1186/s13195-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo C, Jin Z, Homma S, Elkind MSV, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) Study. Circulation. 2013;128:1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kizer JR. Enhancing detection of subclinical end-organ damage: echocardiographic left ventricular stroke holds up a mirror to the brain. Circulation. 2013;128:1045–1047. doi: 10.1161/CIRCULATIONAHA.113.004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogren JA, Fonarow GC, Woo MA. Cerebral Impairment in Heart Failure. Curr Heart Fail Rep. 2014;11:321–329. doi: 10.1007/s11897-014-0211-y. [DOI] [PubMed] [Google Scholar]
- 44.Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart Fail Rev. 2016;21:661–673. doi: 10.1007/s10741-016-9568-1. [DOI] [PubMed] [Google Scholar]
- 45.Kure CE, Rosenfeldt FL, Scholey AB, Pipingas A, Kaye DM, Bergin PJ, Croft KD, Wesnes KA, Myers SP, Stough C. Relationships among cognitive function and cerebral blood flow, oxidative stress, and inflammation in older adult heart failure patients. J Cardiac Fail. 2016;22:548–559. doi: 10.1016/j.cardfail.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, Arnold AM. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol. 2016;45:1135–1145. doi: 10.1093/ije/dyw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


