Abstract
Background
We conducted a prospective phase II study to examine the response rate of gross chemo-refractory breast cancer treated with concurrent capecitabine (CAP) and radiotherapy (RT).
Methods
Breast cancer patients with inoperable disease after chemotherapy, residual nodal disease after definitive surgical resection, unresectable chest wall or nodal recurrence after a prior mastectomy, or oligometastatic disease were eligible. Response by RECIST criteria was assessed after 45Gy. Conversion to operable (CTO), locoregional control, and grade≥3 toxicities were assessed. The first nine patients received CAP 825 mg/m2 BID continuously. Due to toxicity, subsequent patients received CAP only on radiation days. Kaplan-Meier analysis was used to estimate overall survival (OS) and locoregional recurrence-free survival (LRFS).
Results
From 2009–2012, 32 patients were accrued; 26 received protocol-specified treatment. Median follow-up was 12.9 months (interquartile range 7.1–42.9). Nineteen patients (73%) had partial or complete response. Fourteen patients (53.9%) experienced grade 3 non-dermatitis toxicity (7/9 continuous dosing). Three/four inoperable patients converted to operable. One-year actuarial OS in the treated cohort was 54%. The trial was stopped early after interim analysis suggested futility independent of response. Treatment was deemed futile (i.e., CTO but M1 disease immediately post-op) in 9/10 patients with triple-negative (TN) versus 6/16 with non-TN disease (p=0.014). Median OS and 1-yr LRFS among non-TN vs. TN patients was 22.8 vs. 5.1 months, and 63% vs. 20% (p=0.007).
Conclusions
Capecitabine can be safely administered on radiation days with careful clinical monitoring and was associated with encouraging response in this chemo-refractory cohort. However, patients with TN breast cancer had poor outcomes even when response was achieved. Further study in non-TN patients may be warranted.
INTRODUCTION
Locally advanced breast cancer can be painful and distressing due to high local symptom morbidity, and current therapeutic options are limited.1–4 In particular, patients with inoperative tumors and chemo-resistant disease tend to have particularly poor prognosis with current definitive therapies. Alternatively some patients develop progressive or inoperable breast cancer as a recurrence after definitive therapy for breast cancer or present or recur with distant disease and are often not surgical candidates but may benefit from palliative radiation to the primary tumor to reduce what would be otherwise significant morbidity from local disease. This study is a prospective single arm trial of radiation (RT) and capecitabine (CAP), oral chemotherapy that is also a functional radiosensitizer, to improve response rates in these patients with advanced disease after inadequate response to systemic therapy.
Although the optimal treatment strategy has not been established, preoperative therapies that render patients candidates for mastectomy can improve locoregional control and reduce morbidity. Among 38 patients treated with radiation with concurrent chemotherapy for inoperable breast cancer resistant to anthracycline-containing primary chemotherapy on five consecutive trials at our institutional without evidence of distant metastases at diagnosis, thirty-two (84%) were able to undergo mastectomy after radiotherapy with an overall survival of 46% at 5 years.5 Locoregional control was modestly improved for patients who were able to undergo mastectomy, and was 73% vs. 64% at 5 years.5 Despite the high conversion to operable rate with RT alone, the 5-year postoperative complication rate was 53%, and preoperative radiation doses equal to or higher than 54 Gy was significantly associated with complications requiring surgical revision. Therefore, pursuing an alternative strategy such as addition of capecitabine to radiation to achieve conversion to operability allowing for response with a lower radiation dose was warranted.
Capecitabine (Xeloda®) is a fluoropyrimidine carbamate that undergoes sequential conversion via a triple enzyme pathway to 5-FU with documented antineoplastic activity approved as a first line agent for the treatment of metastatic breast cancer resistant to anthracycline and taxane therapy.6 The final enzyme in the pathway is thymidine phosphorylase (TP) which is preferentially expressed in tumor cells as opposed to normal tissue cells thereby increasing the therapeutic index.7 Radiation therapy has been demonstrated to upregulate TP levels which is the final and rate limiting enzyme in the capecitabine pathway, leading to a synergistic improvement in therapeutic index when used concurrently with radiation.8,9 Use of pre-operative radiation and CAP for rectal cancer has led to significant preliminary experience at our institution using concurrent pre-operative CAP and RT for inoperable breast cancer with greater than 90% conversion to operable, and a small published phase II trial10,11 as well as a French retrospective study12 supports this approach for breast cancer patients resistant to first line chemotherapy to improve operability.
Therefore, we conducted a phase II study to prospectively examine the efficacy and toxicity of CAP and RT in a larger group of patients with gross disease in spite of systemic therapy for whom radiation was to be utilized for disease control in whom radiosensitization was desirable.
STUDY METHODS
Patient Eligibility
This study was a single center phase II study (Study Schema, Supplementary Fig 1.). Eligible subjects were women with invasive breast cancer with measurable disease that had progressed on standard chemotherapy. This includes 1) those with inoperable disease after chemotherapy, 2) patients with residual nodal disease after definitive surgical resection and 3) those with an unresectable chest wall or nodal recurrence after a prior mastectomy. Of note, in all patients, baseline imaging including ultrasound evaluation of the nodal basin was conducted with biopsy proven confirmation of nodal involvement‥ Patients with oligometastatic disease (generally ≤ 3 distant sites, but at the discretion of the treating physician) who would benefit in terms of symptom palliation (pain, drainage, or emotional duress), referred to herein as “aggressive palliation” were also eligible. Radiation intent was recorded beforehand as pre-operative, for local control in patients with residual or unresectable recurrent disease, or for palliation.
Eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, ability to swallow and retain oral medication, age 18 or older, female gender, having a histologically confirmed diagnosis of invasive breast cancer, and no contraindications to radiation treatment to a minimum dose of 50 Gy in 25 fractions (such as pregnancy, prior radiation to the volume with disease, or systemic disease in which radiation therapy is an absolute contraindication). Patients with known brain metastasis, active or uncontrolled infection, uncontrolled arrhythmia or congestive heart failure, on therapeutic anticoagulation, or who had used an investigational drug within 21 days preceding the first dose of study medication were excluded.
Objectives
The primary objective of this study was to determine the rate of response by Response Evaluation Criteria in Solid Tumors (RECIST) (Supplemental Table 1). The secondary efficacy objectives were to determine the rate of conversion to operable among relevant patients, clinical and pathologic complete response, and locoregional control of unresected nodal disease treated with definitive radiotherapy. Secondary safety objectives were to determine the rates of post-surgical wound complications and grade 3 toxicity.
Treatment
All patients were treated with preoperative, postoperative or palliative concurrent capecitabine and radiation therapy. Daily oral capecitabine 825 mg/m2 bid was initially administered continuously during the course of radiation 7 days per week. One of the two daily doses of capecitabine was taken approximately 2 hours before receiving radiotherapy. This dosing regimen was chosen based on our prior institutional experience with this dosing in patients with locally advanced rectal cancer.13 Capecitabine was dose reduced during the study to 825 mg/m2 bid 5 days a week on radiation treatment days during the course of radiation with weekend breaks for grade 3 toxicity. Toxicity was recorded at each visit using standardized Radiation Therapy Oncology Group (RTOG) criteria. In our practice, first line chemotherapy was typically a Taxol-based weekly regimen with Adriamycin/cyclophosphamide (AC) or 5-FU/epirubicin/cyclophosphamide (FEC), with the addition of Herceptin for Her2/neu positive patients.
Radiation treatment
Patients were treated to the chest wall or breast and undissected draining lymphatics with a 3D conformal approach using tangent fields with a coplanar posterior border to encompass the gross disease in the breast.14 Internal mammary nodes were treated with electrons, deep tangent technique or IMRT if normal tissue constraints could not be met without this technology.
Radiation dose was 50–57 Gray (Gy) to the initial clinical target volume (CTV, gross disease and tissue at risk for micrometastatic disease including margin around gross disease and draining regional lymphatics). Dose to potentially resectable disease was limited to 54 Gy at 2 Gy/fraction or 57 Gy at 1.8 Gy/fraction to limit surgical complications.5 Additional “boost” dose was acceptable to bring the total dose to 60–72 Gy (60–66 Gy to gross disease <1 cm, up to 72 Gy to gross disease > 1 cm) to gross target volumes (GTV) defined by the presence of gross disease on pre-treatment imaging. Treating physicians selected one of three regimens at their clinical discretion: 50–54 Gy at 2 Gy per fraction (once daily treatment) followed by optional GTV boost at 2 Gy per fraction to total dose; 57 Gy at 1.8 Gy per fraction (IMRT) with optional nested GTV dose to total not to exceed 72 Gy at 2.2 Gy per fraction; or 51 Gy at 1.5 Gy per fraction twice daily (bid) to the CTV followed by optional GTV boost at 1.5 Gy bid to a total dose of 66 Gy.15
All radiation planning was peer-reviewed in a weekly breast radiation planning quality assurance conference. Radiation to a second site (metastatic disease excluding whole brain) during or overlapping with protocol specified therapy was permitted. Definitive or palliative local therapy with surgery when indicated was performed.
Patients were taken off study for locoregional disease progression, treatment interruption longer than 1 consecutive week, significant intercurrent illness, other chemotherapy administered during study treatment, treatment noncompliance or refusal.
Imaging response
Entry primary tumor size based on RECIST criteria was evaluated by a single physician (WW) on the planning CT obtained for simulation (Supplementary Table 1). CT simulator based imaging was performed after 45 Gy and tumor size and response were assessed using RECIST criteria based on these images. Response measurement was attained after 45Gy to attain a signal for treatment response so that radiation could be stopped prior to a dose that could increase risk of surgical complications if surgery were to be indicated. PET/CT scan or ultrasound was performed in conjunction with the first follow up visit 3 months after completion of radiation therapy. Patients with sites treated definitively for gross unresected disease to a dose ≥ 60 Gy were scored as having complete response, partial response, or no response/progressive disease based on imaging and exam when evaluable.
STATISTICAL ANALYSIS
The primary endpoint was disease response (complete response or partial response) as evaluated by RECIST criteria by a single physician (WW). Those who did not complete treatment or with unknown response were considered non-responders. All analyses included patients who received at least one dose of CAP.
Secondary efficacy endpoints were rates of conversion to operable and clinical and pathologic complete response after completion of all specified therapy among patients who proceeded to surgery and locoregional control of unresected nodal disease treated to definitive radiation dose (≥ 60 Gy). Secondary safety objectives were the rate of post-surgical wound complications after pre-operative radiation and CAP and rate of grade 3 or higher toxicity (excluding acute skin toxicity). Surgical complications were assessed independently within 6 weeks of surgery. Stopping rules were based on grade 3 or higher toxicity rate, excluding acute skin toxicity, which was monitored throughout. Stopping boundaries were assessed in cohorts of 5 patients after 10 patients were enrolled initially.
The study regimen was to be considered of interest for further study only if there was a high probability that the response rate would be at least 80%.8,10,14 Predetermined stopping boundaries for efficacy were calculated. If the trial were not stopped early and 54 responses were observed among the planned 60 patients, then the 95% posterior credible interval for response rate would have been 82.7–95.2%. We calculated the actual posterior distribution of the response rate given the study was stopped early. Of 26 patients, there were 2 with CR, 17 with PR, 4 with SD, and 3 with PD. Our prior assumption was that response rate would have prior beta(1.6, 0.4). With the data, the posterior distribution for response was beta(20.6, 7.4), which yielded a 95% credible interval of 56.1% to 87.8%. The posterior probability that the response rate was more than 80% is 0.228.
Actuarial survival was analyzed and compared between patients with triple negative and non-TNBC using the Kaplan-Meier method.
RESULTS
From 2009–2012, 32 patients were accrued, and 26 received protocol specific treatment. Six patients did not receive protocol specified treatment. One patient withdrew from study due to lack of insurance clearance for protocol participation, two patients were taken off study prior to any treatment due to the severe pre-existing peripheral neuropathy. Two patients withdrew after having mild side effects (one with nausea, another with mild foot pain/diarrhea. In one patient, radiation treatment was stopped early at 55Gy due to a combination of severe pain from a progressing metastatic lesion in the sacrum and a brisk skin reaction. Only patients receiving protocol specific treatment were analyzed (n=26). The median patient age was 50 years (range, 37–83 years). Table 1 depicts the clinical setting in which patients were treated. Thirteen (50%) patients had residual nodal disease after definitive surgical resection, 4 (15%) patients had an unresectable chest wall or nodal recurrence after a prior mastectomy, 4 (15%) had inoperable disease after chemotherapy, and 5 (19%) were treated with palliative intent in the setting of oligometastatic disease. Ten (38%) patients had triple negative marker status. The median duration of patient follow up was 12.9 months (interquartile range 7.1 – 42.9).
Table 1.
Patient Characteristics, Response, and Toxicity
| Radiation Treatment Intent | N (%) |
|---|---|
| Local control in patients with residual nodal disease after definitive surgical resection | 13 (50%) |
| Local control in patients with unresectable chest wall or nodal recurrence after prior mastectomy | 4 (15%) |
| Pre-operative | 4 (15%) |
| Palliation | 5 (19%) |
| Radiation Regimen | |
| Maximum dose | |
| 50 Gy | 5 |
| 60 Gy | 2 |
| ≥66 Gy | 19 |
| Fractionation | |
| Daily | 25 |
| Twice daily (BID) | 1 |
| TNBC | |
| Yes | 10 (38%) |
| No | 16 (61%) |
| Response | |
| PR/CR | 19 (73%) |
| SD/PD | 7 (27%) |
| CTO | |
| n = 4 | 3 (75%) |
| Toxicity | |
| G3 Toxicity - overall | 14 (54%) |
| G3 toxicity - daily capecitabine (n=9) | 7 (78%) |
| G3 toxicity - weekday only capecitabine (n=17) | 7 (41%) |
| Grade IV/V toxicity | 0 (0%) |
> one grade three non-dermatitis toxicity, Gy, Gray; TNBC, triple negative breast cancer; PR/CR partial or complete response; SD/PD Stable or progressive disease; CTO convert to operable
Patients received a median radiotherapy dose of 66 Gy (range, 50–72 Gy). Of the three patients who were converted to operable (CTO), 2 out of 3 were treated up to 51Gy and one to 66Gy. The first nine patients analyzed (one patient in this initial cohort of ten withdrew after having mild nausea) received CAP 825 mg/m2 BID continuously beginning on the first day of radiotherapy. Due to observed excess grade 3 toxicity, the protocol was amended and subsequent patients received CAP only on radiation treatment days (5 days per week).
The trial was stopped early after an unplanned interim analysis prompted by slow accrual suggested futility independent of response rate. Nineteen patients (73%) had a partial or complete response. Two patients (8%) had a CR and 17 (65%) had a PR.
Four inoperable patients were treated with pre-operative radiation therapy and 3 (75%) converted to operable. Mastectomy was performed within 3–6 weeks after completion of radiation in all patients. The patient who was treated pre-operatively who did not convert to operable had oligometastatic disease on presentation and was treated with both CTO and palliative intent. None of the four patients achieved a pCR or near pCR. Two of the three patients converted to operable had no evidence of disease (NED) at last follow-up at 57 and 84 months, and the third was found to have distant metastases two weeks after surgery. One patient, who was treated to 66Gy preoperatively, developed a chest wall abscess one week after surgery which responded to needle aspiration and IV antibiotics. No wound dehiscence was observed, and no patients required surgical revision.
Treatment-related adverse events with at least possible attribution to study-specific treatment are shown in Table 2. Fourteen patients (53.9%) experienced at least one grade 3 non-dermatitis toxicity including 7/9 (78%) treated with continuous dosing. Of patients who received non-continuous CAP twice daily 5 days per week, 7/17 (41%) experienced grade 3 non-dermatitis toxicity. The most common grade 3 toxicities were gastrointestinal toxicities (diarrhea, nausea or vomiting) and hand-foot skin reaction. Among TN patients, 6/10 (60%) had grade 3 non-dermatitis toxicity, with 3 patients experiencing hand-foot skin reaction. Non-continuous CAP dosing was much better tolerated than continuous dosing. Thirteen out of 26 patients (50%) had grade 3 and higher treatment-related dermatologic toxicity.
Table 2.
Grade ≥ 3 Treatment-Related Adverse Events (NCI Common Toxicity Criteria)
| CAP continuous dosing (n) |
CAP weekday dosing (n) |
|
|---|---|---|
| Any Grade ≥ 3 Adverse Event | 7 | 7 |
| Hand-foot skin reaction | 4 | 2 |
| Diarrhea | 1 | 0 |
| Nausea | 1 | 1 |
| Vomiting | 2 | 4 |
| Dehydration | 1 | 0 |
| Fatigue | 1 | 0 |
| Neutropenia | 1 | 0 |
| Leukopenia | 1 | 0 |
| Opportunistic infection | 1 | 0 |
| Non-opportunistic infection | 2 | 0 |
| Fibrosis of deep connective tissue | 1 | 0 |
| Edema - limb | 2 | 0 |
| Edema - truncal | 2 | 0 |
| Pain - skin | 1 | 2 |
| Pain - extremity | 2 | 1 |
| Pain - chest wall | 2 | 0 |
| Pain - bone | 1 | 0 |
| Pain - stomach | 2 | 4 |
| Pain - esophageal | 1 | 1 |
| Esophagitis | 1 | 1 |
| Dyspnea | 1 | 0 |
| Thrombus | 1 | 3 |
AEs with at least possible attribution
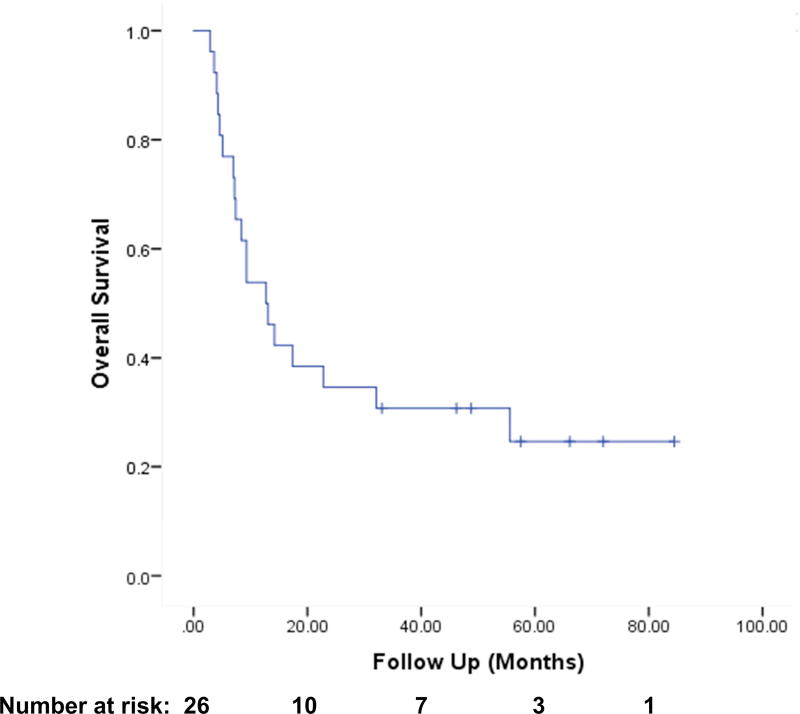
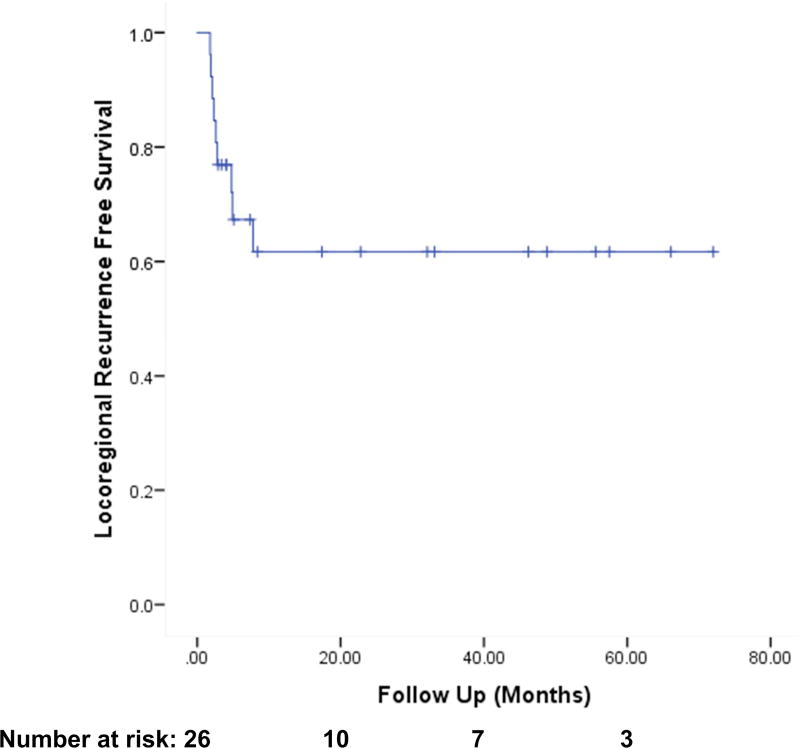
The one-year actuarial overall survival (OS) was 54% (Fig. 1). One-year actuarial locoregional recurrence free survival was 65% (Fig 2). One-year actuarial locoregional recurrence free survival among PMRT patients was 76%. The median time to progression of disease was 4.2 months.
Figure 1.
Actuarial Overall Survival among all Patients (n=26)
Figure 2.
Locoregional Recurrence Free Survival among all Patients (n=26)
Ten patients had triple negative (TN) receptor status. There was no significant difference in radiation response by receptor status (p = 0.56); however, treatment was deemed subjectively futile (i.e., converted to operable but new widespread M1 disease immediately post-op) in 9 of the 10 patients with TN disease as compared to 6 of the 16 patients with non-TN disease (P = 0.014).
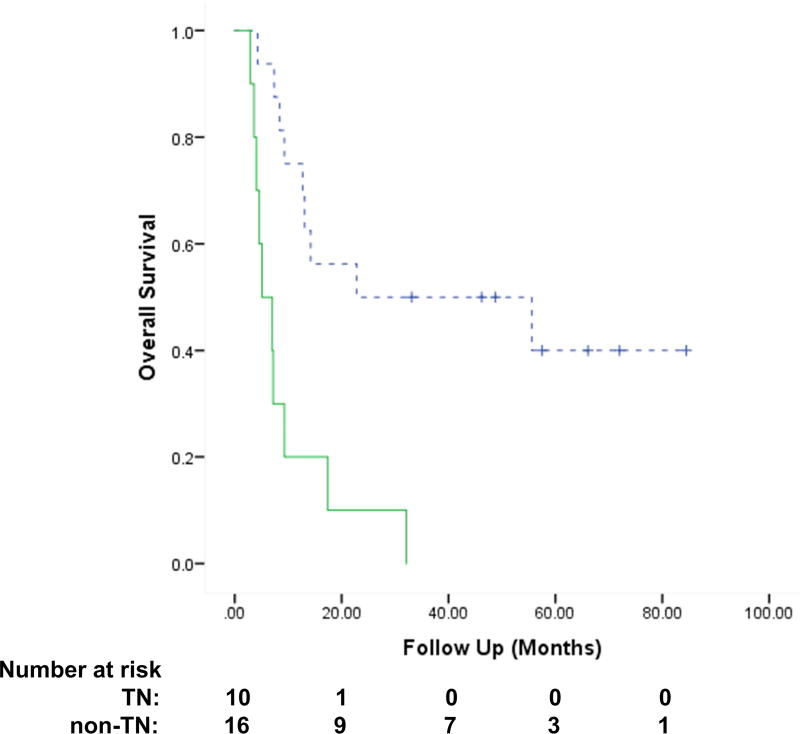
Median OS, 1-yr actuarial OS, and 1-yr LRFS among non-TN vs. TN patients were 22.8 vs. 5.1 months, 75% vs. 20% (P = 0.001) and 63% vs. 20% (P = 0.007), respectively (Fig. 3).
Figure 3.
Actuarial Overall Survival among Non-Triple Negative (TN) (n=16) vs TN pts (n=10, p < 0.001). Dotted line: non-Triple Negative Breast Cancer (TNBC), Solid line: TNBC
DISCUSSION
This study provides prospective results investigating disease response to concurrent capecitabine and radiotherapy in patients with inoperable, chemotherapy-resistant or oligometastatic disease. Local disease burden is often associated with high morbidity and distress in these patients for whom therapeutic options are limited.
Previously, capecitabine has been offered as concurrent neoadjuvant treatment with radiation at our institution. An unpublished retrospective review of 64 patients treated at our institution with concurrent radiation and capecitabine for inoperable breast cancer (inflammatory and non-inflammatory), concurrent chemoradiation with capecitabine demonstrated 88% of these patients converted to operable. The clinical complete response rate was 33%; moreover, the overall pathological CR rate was 19%. Only one patient had progressive disease. The 5-year OS, local recurrence-free survival, and distant metastasis-free survival rates were 48%, 85%, and 37%.10,15
Use of capecitabine in the neoadjuvant setting concurrent with radiation for breast cancer has been reported in other small institutional studies. Previously, Gaui et al. reported a small phase II study using CAP with radiation as second-line neoadjuvant treatment in inoperable patients who failed anthracycline-based chemotherapy.11 Concurrent CAP was given orally twice daily for 14 days and repeated every 3 weeks during the radiation therapy. Gaui et al. concluded their regimen was well-tolerated and effective, rendering 23 of 28 patients (82%) operable with median clinical tumor size decrease from 80 cm2 to 49cm2 (39%).11 Local recurrence and distant failure rates were not reported.
Bourgier et al. reported on 14 locally advanced initially operable breast cancer patients with inoperable tumor progression after neoadjuvant chemotherapy treated with rescue concurrent vinorelbine and 5FU/CAP chemoradiation.12 Chemo-refractory patients were treated with 4 cycles of a 3-weekly regimen combining vinorelbine (25 mg/m2 IV or orally 60 mg/m2; day-1 and day-8) and 5FU-based chemotherapy (either 5 days of continuous IV 5FU 750 mg/m2/day or 14 days of CAP 1800 mg/m2/day). Radiation dose and fractionation was 50 Gy in 2 Gy fractions over 5 weeks. CTO rate was 71%, and the treatment was well tolerated and postoperative complication rate was low with one grade 2 and one grade 3 complication.12
In a small Japanese series, 39 patients with T4 unresectable tumors were treated with chemoradiotherapy upfront receiving docetaxel, paclitaxel or capecitabine with radiotherapy to a median dose of 60 Gy in 30 fractions to the whole breast and axilla with a single anterior electron beam to cover tumor extension. Of 12 patients who were treated with capecitabine, 2(17%) had a complete response.16
Another small (n=20) series from the University of Louisville investigated an array of different concurrent chemotherapies in patients with recurrent or advanced breast cancer deemed not to be operative candidates. Patients were treated with chemoradiotherapy with capecitabine, paclitaxel, or cisplatin/etoposide. Twelve of 20 patients were treated with capecitabine. Among these patients, the overall clinical response rate was 100%, and 65% were judged to have had a complete clinical response.17 The most common toxicity was skin-related, with 80% experiencing Grade 2 or greater radiation dermatitis of which most cases were self-limited.
Our study investigated the efficacy and toxicity of CAP and RT in a larger, clinically broader group of patients for whom preoperative radiation was offered after lack of response to systemic therapy. Ultimately, eligibility was expanded to include patients with inoperable disease after chemotherapy, residual nodal disease after definitive surgical resection, unresectable chest wall or nodal recurrence after a prior mastectomy, or oligometastatic disease. Of patients enrolled in this study, many had more advanced local and distant disease including oligometastatic disease and recurrent, unresectable disease, making direct comparison of our study results with that from the above studies difficult. Despite the good response rate of 73% partial or complete response, the primary endpoint of our study and comparable to that published by Gaui et al., the trial was stopped early after an unplanned interim analysis prompted by slow accrual suggested futility independent of response rate.
Even in patients with local response to therapy by RECIST criteria, there were many early failures due to early distant metastasis, progression of metastatic disease, or out-of-field failure. The distant metastasis risk was very high, and would drive overall clinical status despite locoregional control. The risk of disease progression both locally and distantly seemed to be particularly high for patients with TNBC, although there was no difference in response by receptor status. Subjective futility of treatment was assessed as a global endpoint in this mixed cohort to provide context for the decision to undertake prolonged radiation with a radiosensitizer in this cohort of pre-treated patients. It addresses the reality that some may achieve an encouraging specific endpoint like conversion to operable or response, but died rapidly after surgery or achieve a local response with failure to palliate symptoms. This was due mostly to early progression of metastatic disease which was disproportionately higher in TNBC (90%) patients compared with patients without TNBC (38%). Concordantly, one-year actuarial overall survival, and 1-yr locoregional recurrence free survival were lower in TNBC patients.
It would be remiss to disregard the potential clinical benefit of the local tumor response seen in this study in the majority of treated patients to concurrent treatment with CAP and RT. The morbidity associated with uncontrolled local disease in this patient population can be very high (and associated with significant pain, drainage, or emotional duress). Palliation of local disease or conversion to operability can improve quality of life independent of other clinical outcomes. This study, consistent with our prior institutional experience, shows that the majority of patients had at least a partial response with regard to the local disease treated and conversion to operable in preoperative patients.
With respect to toxicity, concurrent CAP twice daily with weekend holidays was better tolerated than continuous CAP. The type and rate of non-skin grade III toxicities seen with noncontinuous CAP therapy were as expected from prior studies combining CAP and RT and included gastrointestinal and hand-foot syndrome. Furthermore, rates of surgical complications and wound dehiscence were not increased.
CONCLUSION
Capecitabine can be safely administered as a concurrent chemoradiation regimen on radiation treatment days with careful clinical monitoring for patients with inflammatory breast cancer, inoperable, chemo-refractory, locally recurrent, or gross residual disease after mastectomy. In this small, prospective and selected cohort, concurrent chemoradiation with capecitabine was associated with a high risk of distant disease progression among TN patients despite good radiographic response. Other treatment strategies should be considered in these patients. Further examination of concurrent chemoradiation with capecitabine in non-TN patients may be indicated.
Supplementary Material
Summary.
Capecitabine can be safely administered on radiation days and was associated with encouraging response in a chemo-refractory breast cancer cohort. However, patients with triple negative breast cancer had poor outcomes even when response was achieved. Further study of this regimen in non-triple negative patients may be warranted.
Acknowledgments
Acknowledgements and Funding: This study was funded with a National Institutes of Health NCI R01 Grant CA138239 awarded to Wendy Woodward.
NIH Funding: Yes, see funding statement above.
Disclosure statement: Dr. Smith receives grant funding from Varian Medical Systems, outside the current work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of locally advanced breast cancer-perspectives and future directions. Nat. Rev. Clin. Oncol. 2015;12:147–162. doi: 10.1038/nrclinonc.2015.13. [DOI] [PubMed] [Google Scholar]
- 2.Yalcin B. Overview on locally advanced breast cancer: defining, epidemiology, and overview on neoadjuvant therapy. Exp. Oncol. 2013;35:250–252. [PubMed] [Google Scholar]
- 3.Damast S, et al. Locoregional outcomes of inflammatory breast cancer patients treated with standard fractionation radiation and daily skin bolus in the taxane era. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:1105–12. doi: 10.1016/j.ijrobp.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Bristol IJ, et al. Locoregional Treatment Outcomes After Multimodality Management of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. 2008;72:474–484. doi: 10.1016/j.ijrobp.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang E, et al. Locoregional treatment outcomes for inoperable anthracycline-resistant breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:1225–33. doi: 10.1016/s0360-3016(02)02878-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman PA, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa T, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem. Pharmacol. 1998;55:1091–1097. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 8.Liauw SL, Benda RK, Morris CG, Mendenhall NP. Inflammatory breast carcinoma: outcomes with trimodality therapy for nonmetastatic disease. Cancer. 2004;100:920–8. doi: 10.1002/cncr.20083. [DOI] [PubMed] [Google Scholar]
- 9.Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999;5:2948–53. [PubMed] [Google Scholar]
- 10.Perkins GH, Middleton LP, Tran R, Garcia SM CM, Pusztai L, Singletary SE, Strom EA, Woodward WA, Yu T-K, O JL, Tereffe W, Whitman GJ, Huang E, Allen PK, B.T. Concurrent chemoradiation with capecitabine achieves meritable response and local control for inoperable and recurrent neoadjuvant chemotherapy refractory breast cancer. Breast Cancer Res Treat. 2007;106 SABCS abstract. [Google Scholar]
- 11.Gaui M, de FD, et al. A phase II study of second-line neoadjuvant chemotherapy with capecitabine and radiation therapy for anthracycline-resistant locally advanced breast cancer. Am. J. Clin. Oncol. 2007;30:78–81. doi: 10.1097/01.coc.0000245475.41324.6d. [DOI] [PubMed] [Google Scholar]
- 12.Bourgier C, et al. Effect of preoperative rescue concomitant FUN/XUN-based chemo-radiotherapy for neoadjuvant chemotherapy-refractory breast cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012;103:151–154. doi: 10.1016/j.radonc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan S, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 14.Woodward WA, Buchholz TA. The role of locoregional therapy in inflammatory breast cancer. Semin. Oncol. 2008;35:78–86. doi: 10.1053/j.seminoncol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Woodward WA, Debeb BG, Xu W, Buchholz TA. Overcoming radiation resistance in inflammatory breast cancer. Cancer. 2010;116:2840–5. doi: 10.1002/cncr.25173. [DOI] [PubMed] [Google Scholar]
- 16.Karasawa K, et al. The role of chemoradiotherapy in patients with unresectable T4 breast tumors. Breast Cancer Tokyo Jpn. 2013;20:254–61. doi: 10.1007/s12282-012-0336-3. [DOI] [PubMed] [Google Scholar]
- 17.Shaughnessy JN, et al. Efficacy of concurrent chemoradiotherapy for patients with locally recurrent or advanced inoperable breast cancer. Clin. Breast Cancer. 2015;15:135–42. doi: 10.1016/j.clbc.2014.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.