Abstract
Functional magnetic resonance imaging (fMRI) is a powerful tool used in cognitive neuroscientific research. fMRI is noninvasive, safe, and relatively accessible, making it an ideal method to draw inferences about the brain–behavior relationship. When conducting fMRI research, scientists must consider risks associated with brain imaging. In particular, the risk of potentially identifying an abnormal brain finding in an fMRI research scan poses a complex problem that researchers should be prepared to address. This article illustrates how a social constructivism decision-making model can be used as a framework to guide researchers as they develop protocols to address this issue.
Keywords: ethics, incidental findings, neuroimaging, social constructivism
Magnetic resonance imaging (MRI) is a commonly used tool in psychological research. The first studies using MRI were published in the 1990s (e.g., Kwong et al., 1992; Ogawa, Lee, Kay, & Tank, 1990, 1992), and since then research involving MRI has increased by a factor of 1,000 (Huettel, Song, & McCarthy, 2014). Functional MRI (fMRI) in particular has become a primary investigative tool used by thousands of researchers worldwide (Huettel et al., 2014). Safe, noninvasive, and relatively accessible, fMRI is a powerful tool for making inferences about the relationship between the brain and behavior.
The increase in fMRI research has led to many advances in cognitive neuroscience. Scientists have been able to map the relationship between many simple and complex behaviors and brain regions and networks. For example, in early fMRI studies, researchers showed a relationship between simple finger tapping, complex finger movement, and imagined finger movement and premotor, primary motor, and supplementary motor areas in the brain (Roa et al., 1993). As research in this field has developed, higher level processes including memory, decision making, and social cognition, among others, have been investigated using fMRI.
Over the past two decades, fMRI-oriented research has yielded many important scientific advancements in understanding human behavior. As is the case with most work involving human participants, however, ethical issues have arisen in fMRI research. Although fMRI is generally seen as a low-risk technique, there is the possibility that incidental findings (IFs) may be discovered in the research setting. An incidental finding is broadly defined as “a finding concerning an individual research participant that has potential health or reproductive importance and is discovered in the course of conducting research but is beyond the aims of the study” (Wolf et al., 2008, p. 219). Although IFs can include findings of clinical significance, such as an unexpected mass, aneurysm, bleed/stroke, evidence of current or past trauma to the brain, malformation, or anatomic evidence of dementia, they can also include findings that typically lack any clinical significance, such as normal variations in anatomical structure, benign abnormalities, or artifacts of the image itself (Hoffman & Schmucker, 2014; Wolf et al., 2008). Critically, IFs are findings that are unintended in a given research protocol and are characterized by the potential to be of importance to an individual’s health. IFs are not contingent on any resulting clinical significance following further investigation (Hoffman & Schmucker, 2014).
Research shows that IFs appear in 13%–84% of brain MRI scans, ranging in terms of clinical urgency and significance (Alphs, Schwartz, Stewart, & Yousem, 2006; Kumra, Ashtari, Anderson, Cervellione, & Kan, 2006; Wolf et al., 2008). Katzman, Dagher, and Patronas (1999) conducted a retrospective analysis of brain MRI scans from 1,000 healthy volunteers and found that of the 18% of scans showing IFs, 15.1% required no referral, 1.8% required routine referral, 1.1% required urgent referral, and 0% required immediate referral. Similarly, further work has reported that in brain imaging research overall, approximately 2%–8% of IFs have immediate clinical consequences (Illes et al., 2008; Vernooji et al., 2007). There is also evidence that the prevalence of IFs increases with participant age and detection is more likely using high-resolution MRI sequences (Illes et al., 2004b; Morris et al., 2009). The wide range in prevalence figures for IFs in neuroimaging reflects varying methods and samples across studies (Wolf et al., 2008). However, despite the variability in prevalence figures, it is largely agreed that the frequency at which researchers encounter IFs necessitates guidance regarding how to ethically manage these findings (Wolf et al., 2008).
Given the acknowledged possibility of IFs in research using MRI, the question as to how researchers should manage IFs has been heavily debated. A key problem that has been addressed is how and when to report IFs found in a research setting. Little consensus has emerged on this issue (Illes et al., 2006). In fact, creating a protocol to deal with research IFs in brain imaging studies involves many practical elements, as well as ethical principles, making the decision-making process quite complex.
Although managing IFs affects researchers in many biomedical fields, this article focuses on the challenges faced by behavioral scientists conducting neuroimaging research using MRI and fMRI techniques. Given various practical limitations, such as limitations in material resources and expertise, determining the most ethical way to handle neuroimaging IFs may be particularly complex for behavioral scientists. Although general guidelines for how researchers might handle IFs in brain MRI research have been suggested (Illes et al., 2008), few philosophically grounded ethical decision-making models have been applied to the issue or made available for scientists dealing with this problem. This article (a) outlines the current state of the ethical debate surrounding best practices for managing IFs, (b) elucidates the practical and ethical considerations involved in managing IFs, and (c) provides a novel approach to addressing IFs in neuroimaging research.
THE STATE OF THE DEBATE
According to the Working Group on Incidental Findings sponsored by the National Institutes of Health, the primary issues to consider in deciding how to handle incidental MRI findings are how to protect subject welfare and how to ensure research integrity (Illes et al., 2006). Current best practices dictate that researchers should explicitly outline how IFs will be handled in both the research proposal reviewed by the Institutional Review Board (IRB) and in the informed consent document. Anticipating IFs in research and having a prepared protocol for handling such findings in both IRB materials generally and in consent forms specifically is critical to ensuring that participant expectations align with the purpose and scope of the research being conducted (Brown & Hasso, 2008; Illes et al., 2006; Kirschen, Jaworska, & Illes, 2006).
There is a general consensus that the possibility of discovering IFs in neuroimaging research is best addressed in the research study design and in the consent document (Brown & Hasso, 2008). Brown and Hasso (2008) argued that disclosure of IFs should be addressed in the research study design, suggesting that no neuroimaging study be approved by an IRB if a protocol for reporting unexpected findings to participants and for initiating treatment for these findings is not delineated in the research proposal. Because no uniform national or international policy exists for such protocols, neuroethicists largely suggest that researchers within the neuroimaging community petition their home IRB to enforce the inclusion of such a protocol in research proposals (Brown & Hasso, 2008; Ulmer et al., 2013).
Although a general consensus exists that protocols for handling IFs should be included in research proposals and consent documents, there is less agreement on what such protocols should include. Should researchers always report IFs to participants? Should researchers never report IFs to participants? How should a research team decide when it is appropriate to seek consultation about an IF? Must research teams always include a radiologist trained to read MRI scans?
Strong arguments have been put forth supporting various approaches to managing IFs. Although some researchers endorse mandating radiologic review for all research scans and, regardless of findings, providing a copy of the radiology report to all participants (Phillips et al., 2015; Shoemaker et al., 2011), others suggest taking a more balanced approach in which not all scans receive radiologic review but IFs are also not ignored (Cramer et al., 2011). Still others argue that IFs should never be reviewed by a radiologist or reported to a participant unless the finding is “obviously life-threatening” (Royal & Peterson, 2008, p. 313). With positions on how to manage IFs varying widely across research groups and institutions, participants involved in similar research studies may receive very different treatment.
In a review by Illes and colleagues (2008), the authors outlined various options for handling IFs in cognitive neuroscience and psychology research. They provided potential ways for researchers to assess and respond to IFs, arguing that different options are appropriate for different research settings. These options range from taking no action to manage IFs beyond articulating in the consent document that brain images will not be screened for abnormalities, to routinely collecting clinical-grade images and having images screened by a trained radiologist. They concluded that what choice is most appropriate for a given research team will depend on the nature of the research and the resources available to the team.
PRACTICAL CONSIDERATIONS
The resources available to a research team play a material role in deciding how to manage IFs. Although an in-depth examination of the complexities of MRI techniques is beyond the scope of this article, a brief discussion examining how MR images are captured is warranted to illustrate how practical aspects of imaging contribute to the ethical dilemmas surrounding the management of IFs.
The scan sequences required for fMRI studies differ from those required for clinical-grade scans, and often have limited clinical utility (Royal & Peterson, 2008). In a typical fMRI research protocol, T1-weighted images and echoplanar images are most commonly collected (Royal & Peterson, 2008). T1-weighted images provide structural information about the brain, offering good contrast between gray and white matter. Although T1-weighted images offer good spatial precision, they can be used to detect little more than anatomical distortions associated with large, space-occupying lesions or hydrocephalus, limiting their use as a clinical tool (Rorden & Karnath, 2004; Royal & Peterson, 2008). Similarly, echoplanar image sequences, which offer precise temporal resolution, are virtually useless for clinical application, as they offer extremely poor spatial resolution and are highly susceptible to artifact (Royal & Peterson, 2008).
In addition to T1-weighted images and echoplanar images, researchers have access to many structural sequences and methods of analysis. For example, psychological and cognitive neuroscience researchers increasingly use techniques and methods such as diffusion weighted imaging, magnetic resonance spectroscopy, cortical thickness measurements, and voxel-based morphometry, among others, to investigate the relationship between behavior and various structural markers. Although IFs may be detectable using these techniques and methods, interpretations of these scans for clinical use is often far outside the capacity of a researcher and the scope of the research question (Cole et al., 2015).
To adequately evaluate brain tissue and glean clinically relevant information from MR scans, radiologists use protocols that consist of a combination of MR sequences. For example, a clinical protocol might include a T1-weighted image to acquire an anatomical overview; a T2-weighted image sequence that provides good pathological information; and a diffusion weighted image or a fluid-attenuated inversion recovery image, which can be used to detect hyperacute and acute cerebral infarct, respectively (Rorden & Karnath, 2004). Depending on the question at hand, other additional orthogonal acquisitions may be required as well or contrast agents may be needed (Ulmer et al., 2013). Thus, to reliably detect an abnormality and to make a reasonable clinical judgment about an IF would require collection of MR images beyond what may be necessary for research. This can be expensive and time consuming and may not be feasible (Cole et al., 2015).
In addition to the limitations of research-quality scans, which may not have good clinical utility, the decision to report IFs can be complicated by the expertise of the research team. This issue is particularly relevant for behavioral scientists. Oftentimes, fMRI research is conducted by a researcher with a PhD, who typically has not been trained to read scans for pathological findings. Not all research teams have access to collaborations with medical institutions, which may make it difficult to involve a physician on the research team. Even on teams that do involve a specifically trained and qualified physician to review abnormal brain MRI findings, these physicians do not typically run the research scans. It is common for students (undergraduate and graduate) and MRI technicians to have primary scanning responsibilities, making it less likely that abnormalities will be detected reliably in the research setting (Illes et al., 2004a). Similarly, students are often responsible for analyzing images, again making it less likely that an abnormality will be detected. These realities further contribute to the complexities of deciding how to manage IFs in neuroimaging.
ETHICAL CONSIDERATIONS
Related to and yet distinct from the practical realities and limitations that make creating a protocol to manage IFs in neuroimaging research difficult, ethical considerations add another layer of complexity to the decision-making process. The divergent approaches to managing IFs debated in the literature all take various ethical principles into consideration. The ethical issues surrounding how to manage IFs are not straightforward, however, and oftentimes the same ethical principle can be argued in support of different protocols.
At a most basic level, scientists must adhere to international and local regulations in place to protect human research participants. International ethical guidelines, such as those outlined in the Declaration of Helsinki (World Medical Association, 2013), have been developed to guide physicians and other professionals involved in research involving human participants, including any research with identifiable human material and data. According to these standards, it is the duty of the physician to promote and protect the health, well-being, and rights of participants involved in research. In particular, physicians or other professionals must “protect the life, health, dignity, integrity, right to self-determinations, privacy, and confidentiality of personal information of research subjects” (World Medical Association, 2013).
International guidelines regarding human research outline various ways in which investigators are obligated to protect participants. For example, there is international agreement that human participation in research is completely voluntary and requires informed consent. The Nuremberg Code (1949) of international ethics begins with the specification that “the voluntary consent of the human subject is absolutely critical” (p. 1). Similarly, the Council for International Organizations of Medical Sciences, which was established by the World Health Organization and the United National Educational Scientific and Cultural Organization, requires informed consent of prospective research participants (Booth, Jackson, Wardlaw, Taylor, & Waldman, 2010).
Rules and regulations at the national level converge with international regulations for protecting human research participants. For example, in the United States researchers must adhere to the Federal Policy for the Protection of Human Subjects, known as the “Common Rule,” which outlines basic provisions for informed consent, IRBs, and Compliance Assurances (Office for Human Research Protections, 2016). This law was codified in 1991 and was heavily influenced by the Belmont Report, which delineates basic ethical principles in research involving human participants, including respect for persons, beneficence, and justice (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1978; Office for Human Research Protections, 2016). Like the “Common Law” in the United States, in Europe researchers must adhere to the Additional Protocol to the Convention on Human Rights and Biomedicine Concerning Biomedical Research, which is a legal mandate requiring consent for participation in research.
Regulations pertaining more specifically to IFs in neuroimaging research have also been suggested at the national level. For example, in 2012 a working group sponsored by the National Institutes of Health that included neuroimaging, ethics, and law experts from various U.S. and Canadian agencies, compiled guidelines for researchers to use when making decisions about managing IFs (National Core for Neuroethics, 2012; Underwood, 2012). Similarly, the National Research Ethics Service in the United Kingdom provided explicit guidelines on the process of informed consent as it relates to IFs (Booth et al., 2010). These guidelines state that research participants not only should be informed of the possible risks or discomforts associated with participation in neuroimaging research but that they must also be told about the protocol to be followed if “conditions were discovered of which he or she was unaware” (National Research Ethics Service, 2011, p. 17).
Despite these regulations and guidelines, a common framework for managing IFs in neuroimaging has yet to be established. Across nations and institutions, and even within nations and institutions, standardization of protocols to manage IFs is limited because of the complexities encountered in formulating a protocol appropriate for all situations. Although policies from the United States, Canada, and Europe have served as broad guidelines for handling IFs in neuroimaging research worldwide, in some countries little guidance is provided for handling IFs in neuroimaging research (Fujita et al., 2014; Hoffman & Schmucker, 2014). For example, until recently, the only guidelines for handling IFs in Japan were provided by the Japanese Neuroscience Society, and adherence to these guidelines was limited to members of the society (Fujita et al., 2014). Recently, additional guidance on how to handle IFs in Japan has been provided, focusing on the obligation to screen all images for any clear abnormalities.
In many countries, adopting recommendations for handling IFs requires careful consideration given local laws, principles, and values (Fujita et al., 2014). Oftentimes, in addition to the legal regulations and suggested guidelines for protecting human subjects at the national level, researchers are obligated to adhere to the ethical principles mandated by their profession. For example, in the United States, the American Psychological Association’s (APA) Ethical Principles of Psychologists and Code of Conduct (APA Ethics Code) outlines overlapping and incremental ethical responsibilities that psychologists should take into account when conducting research (APA, 2010). Although many of the ethical principles and standards outlined in the APA Ethics Code are pertinent to conducting fMRI research, three ethical principles are particularly important for psychologist researchers to consider when creating a protocol to manage IFs in neuroimaging research: beneficence and nonmaleficence, respect for people’s rights and dignity, and fidelity and responsibility. The following review of these three ethical principles is not intended to be comprehensive but rather to illustrate the complex and often contradictory role that ethical considerations play as researchers evaluate how to address IFs. Although the specific ethical obligations faced by researchers in different countries and in different fields may vary, the following discussion illustrates how the consideration of ethical principles further complicates the formation of protocols for managing IFs.
Consideration of Beneficence and Nonmaleficence
The general principles outlined in the APA Ethics Code delineate aspirational guidelines for practicing psychology in line with the highest ethical ideals of the profession. The principle of beneficence and nonmaleficence, or the obligation to do good and to do no harm, is the first principle outlined in the APA Ethics Code. This principle states that it is of utmost importance for psychologists to safeguard the rights and welfare of those with whom they interact professionally, be it patients or research participants. Psychologists should aim to resolve any ethical conflicts or dilemmas that may arise in a way that will maximize benefit and minimize harm to the patient or participant involved.
In the context of managing IFs in neuroimaging research, upholding the principle of beneficence and nonmaleficence can be intricate. On the one hand, in line with the obligation to “do good,” if an abnormality that may have implications for a person’s health is detected in a scan, it is important that the person be notified of the finding, the finding be reviewed by a radiologist, and appropriate follow-up action be taken. In a study conducted by Cole et al. (2015), the researchers interviewed six key stakeholder groups affected by policies for managing IFs in neuroimaging research. The groups, which consisted of research participants, parents of child participants, investigators, IRB members, physicians, and community members, generally agreed that it is ethical for researchers to disclose IFs, given the health and emotional benefits such information may provide (Cole et al., 2015). Early detection of clinically relevant IFs could result in immediate and long-term health benefits for the participant, and could even be lifesaving (Wardlaw et al., 2015).
While reporting brain IFs may prove beneficial for a participant’s health, however, there can also be adverse consequences. Reporting IFs, especially those detected using research-quality scans, as is common in fMRI research, may put a participant at unnecessary risk, including, but not limited to, potentially reporting false-negative or false-positive findings to a participant and drawing the participant into stressful and costly follow-up procedures. Given the relatively high frequency of false-positives in neuroimaging IFs (Seki, Uchiyama, Fukushi, Sakura, & Tatsuya, 2010), researchers should be cognizant of the potential consequences of reporting IFs. Both research participants and investigators agree that researchers are obligated to prevent harm to participants, including preventing undue anxiety by reporting IFs (Cole et al., 2015).
Disclosing IFs to participants can also cause undue anxiety due to the complex nature of such results. Radiology reports are medical documents, and participants may not fully understand the meaning of the report. Research shows that returning neuroimaging IFs to participants may challenge their health literacy skills (Rancher et al., 2016). Although investigators are concerned that participants will not understand an MRI report, however, participants are generally confident in their capacity to understand such information (Phillips et al., 2015). This conflicting view between researchers and participants further complicates a researcher’s obligation to do good and to do no harm. Avoiding harm by not reporting IFs must be weighed against the risk of the IF being potentially life-threatening and against respecting the participant’s right to know about an IF.
Respect for People’s Rights and Dignity
Despite recognition that receiving information about IFs may cause harm, participants in research studies generally favor this option (Cole et al., 2015). According to the APA Ethics Code, psychologists are also obligated to respect the dignity and worth of all people. This includes respecting a person’s right to privacy, confidentiality, and self-determination.
In line with respecting people’s rights and dignity, there is unanimous agreement that protocols for managing IFs should be outlined in the informed consent process. Standard 3.10 in the APA Ethics Code holds that when psychologists conduct research, they must obtain informed consent. The informed consent document should be written in language that is reasonably understandable. Providing clear information about the steps that will be taken to address IFs supports participant autonomy. Some researchers argue that participants should be given the option to opt-in or opt-out of receiving information regarding IFs (Illes, 2006).
Data suggest that participants prefer to have autonomous control over their personal health information and feel that they have the right to know about IFs (Cole et al., 2015). In fact, even participants who consent to scanning procedures for research purposes alone still typically expect that abnormalities can be detected in a scan and that any discovered abnormalities will be disclosed to them (Chow & Drummond, 2010; Kirschen et al., 2006). Although investigators worry about the downstream consequences of reporting IFs, research indicates that, to a large degree, participates want to be told (Cole et al., 2015). Thus, it might be argued that researchers have an obligation to inform participants about any IFs.
On the other hand, although seemingly counterintuitive, especially if the knowledge of an IF could lead to beneficial outcomes, participants have the right to request not to be informed about potential IFs (Heinrichs, 2011). Therefore, in the case that a potentially harmful IF has been detected but a participant has indicated that he or she does not want to be informed about any potential IFs, the principles of nonmaleficence and respect for a person’s rights and dignity may directly conflict.
Fidelity and Responsibility
Balancing a respect for the participant’s autonomy with the benefits and risks that can be involved in disclosing IFs can be challenging. For a psychologist involved in neuroimaging research, a commitment to the principle of fidelity and responsibility outlined in the APA Ethics Code adds additional variables to the decision, particularly surrounding the question of competence. The APA Ethics Code dictates that psychologists uphold professional standards of conduct in both clinical and research settings. Psychologists should be aware of their professional and scientific responsibilities both to society and to the individuals with whom they work. Among the standards of conduct that psychologists should uphold is the competence standard (APA Ethics Code, Standard 2). Psychologists should be aware of the boundaries of their competencies and provide services and conduct research only within the bounds of their competence. Given the limited training most psychologists have in reading scans for abnormalities, in some cases, disclosing a potential IF to a participant could represent a step outside the competence of a psychologist researcher.
Even if neuroimaging research studies are not advertised as having diagnostic or therapeutic value, and even if a participant provides written informed consent to a scanning procedure for research purposes alone, it is likely that participants still assume that the investigators conducting the research are competent to detect abnormalities and will report any findings to them (Chow & Drummond, 2010; Kirschen et al., 2006). Participants entrust investigators with access to their private information (images of their brains) and, in so doing, expect that the investigator will protect their rights. Safeguards need to be put in place to ensure that participant expectations are accurate and met. If a PhD-level researcher is not competent to detect abnormalities or responsibly deliver such information, it may be inappropriate for a psychologist to report IFs to participants.
By contrast, it could be argued that the nature of the investigator–subject relationship obligates the researcher to inform participants of IFs despite limited competence (Miller, Mello, & Joffe, 2008). Miller et al. (2008) discussed the professional obligations of a physician-scientist practicing outside the ordinary context of patient care, suggesting that if
1. there is a professional (though not a standard doctor–patient) relationship; 2. there is privileged access to private information obtained legitimately via the subject’s consent to enter the relationship; and 3. information bearing on the health of the subject is discovered that is incidental to the primary goal of the relationship. (p. 276)
then the physician has a duty to respond to IFs. Just as the physician–patient relationship is professional, so too is the investigator–subject relationship (Miller et al., 2008). Miller et al. (2008) defined a professional as someone who possesses specialized knowledge, exercises appropriate discretion in his or her work, and can claim membership in a professional group with a regulatory structure and ethical code. As such, psychologist researchers possess the core qualities of a professional and should advance subject’s well-being and support their autonomy by making appropriate disclosures of IFs (Miller et al., 2008). Upholding the duty to fidelity and responsibility in neuroimaging research is quite complex.
This discussion illustrates just a few of the ethical principles that psychologists must consider when creating a protocol to manage IFs in brain imaging research. Although the current discussion focuses on ethical principles outlined in the APA Ethics Code, behavioral scientists worldwide must consider similar ethical codes and principles when deciding how to manage IFs in neuroimaging research.
Given the range and scope of principles and standards at play, weighing the costs and benefits of any given decision and settling on a conclusive course of action requires thoughtful deliberation and then action. Although specific policies and procedures for managing IFs in neuroimaging research vary across countries and institutions, there is international agreement that investigators have a responsibility to be an advocate for the participant and to inform and counsel participants on the benefits and risks of receiving information about IFs. This obligation holds both during the consent process and in the case of any actual identification of an IF (Leung, 2013).
A SOCIAL CONSTRUCTIVISM APPROACH TO RESPONDING TO IFS IN BRAIN IMAGING
Although guidelines describing how to manage abnormal brain MRI findings have been outlined in the literature, they are limited in that they typically provide guidance on steps to take once an approach to managing IFs is already assumed. These guidelines may be useful for implementing a protocol to manage Ifs, but they neglect to adequately describe how the protocol choice was reached. For example, current guidelines generally include a step in which researchers should weigh the costs and benefits of reporting an IF. However, there is little explanation on how values are weighed and the process by which the choice is determined. Rather, the decision-making process disappears into the mind of the researcher and remains mysterious (Cottone, 2001).
Recommendations for language to include in consent documents, outlines for setting up a process to investigate abnormal findings, and suggestions for reporting IFs to participants have been proposed using various approaches (e.g., Booth et al., 2010; Illes et al., 2008; Illes et al., 2006; Shoemaker et al., 2011; Wolf et al., 2008). These guidelines, however, rest on the supposition that a course of action to manage IFs has been agreed upon. Many of these guidelines use practice-based decision-making approaches, which are derived largely from experience and are intended to be used as practical guidelines (for a review, see Cottone & Claus, 2000). Although these pragmatic guidelines for implementing an approach to managing IFs can be helpful in many ways, and many researchers have called for a standard set of such guidelines, they provide an inadequate framework for guiding researchers through the actual process of making complex decisions about reporting IFs. Moreover, rigid guidelines for managing IFs may fail to account for differences in laws, principles, and ethical codes across countries and disciplines.
Because no one single value or principle will always prevail in all situations, it proves difficult to delineate a standard set of steps to managing IFs in neuroimaging research. Rather than using a practice-based decision-making model, it may be appropriate to apply a philosophically based decision-making model to the process of managing IFs. Grounding ethical decision making in theory, philosophically based models can help elucidate the decision-making process in the face of difficult decisions. In the context of deciding how to manage IFs in neuroimaging research, a social constructivism decision-making model may be particularly useful for behavioral scientists to consider.
Social Constructivism
The term social constructivism refers to an intellectual movement in the mental health field that provides a distinct view of the decision-making process, rooted purely in the relational view of reality (Cottone, 2001). From a social constructivism perspective, reality is not based on objective fact; rather, reality evolves through interpersonal interaction and agreement on what is real (Cottone, 2001). The social constructivism approach to decision making is a purely biosocial interpretation of the decision-making process.
The social constructivism model offers a theoretically unique decision-making approach. Whereas more psychologically based decision-making models portray the decision maker him-or herself as “a psychological ‘entity’ making the decision alone or within some social context,” social constructivism takes decision making out of the head of the decision maker and places it into the social context itself (Cottone, 2001, p. 40). Integrating biological theories of cognition (Maturana, 1978; Maturana & Varela, 1980) and purely social conceptions of the world (Gergen, 1985), social constructivism holds that all behavior is biologically affected and emerges through social relationships (Cottone, 2004). People are biologically predisposed in certain ways, but the relevance of these predispositions are rooted in social context. That is, motivation and intelligence relate to biosocial phenomena in which a person’s biological predisposition fits well within their social context (Cottone, 2004).
A core tenet of social constructivism is the notion that knowledge is not something that people possess somewhere in their heads but rather something people do together. Decisions are a form of knowledge, and thus always occur in interaction. Placing decision making out into the open, social constructivism moves decisions out of the intrapsychic realm of the individual decision maker and into the interpersonal sphere of the social context (Cottone, 2001). Thus, a decision is “simply an action taken within a social context deriving from biological and social forces” (Cotton, 2004, p. 7).
Application of Social Constructivism to Managing IFs in Neuroimaging
With its emphasis on the notion that decisions are a product of the social context itself, the social constructivism model of ethical decision making may be particularly useful for researchers deciding how to manage IFs in neuroimaging. Rather than focusing on identifying universal steps that can be used to address IFs in neuroimaging across various contexts, ranging from different institutions to different countries, a social constructivism approach focuses on the social dynamics in which a decision on how to manage IFs evolves. Ethical predicaments are biologically and socially compelled, existing only within the biological and social context of the time at which an action occurred (Cottone, 2004). Thus, using a model that accounts for the biosocial nature of reality is useful in the context of managing ethical choices.
The social constructivism process of ethical decision making includes obtaining information from those involved, assessing the nature of the relationships operating at that moment in time, consulting valued colleagues and professional expert opinions, negotiating when there is a disagreement, and responding in a way that allows for reasonable consensus as to what should happen (Cottone, 2001). Important to note, the steps involved in the decision-making approach of social constructivism are not linear or sequential. Many of these processes are overlapping and recapitulative.
The dynamic nature of a social constructivism approach allows for the consideration of various, and even contradictory, ethical principles in the decision-making process. In a social constructivism model, it is essential to always obtain information from those involved. Obtaining information from the players in the situation involves, first, identifying the key players and, second, assessing the nature of the relationships operating between those players at that moment in time.
In the context of managing IFs in neuroimaging research, different players can be involved at different levels and at different times. From a macro perspective, deciding how to manage IFs involves governing bodies and associations, and even more broadly, the scientific community at large. As actors in society, investigators and participants recognize certain areas of agreement within these communities. For example, the APA Ethics Code reflects what is acceptable practice in the field of psychology in the United States, and the membership of a behavioral scientist in this group indicates agreement between the researcher and this association (Cottone, 2001). Similarly, membership in the Japanese Neuroscience Society reflects a researcher’s agreement to adhere to the guidelines suggested by that organization. Furthermore, employment at a university, hospital, or research center indicates agreement to adherence to certain rules and regulations dictated in those settings. These types of macro-level relationships play a ubiquitous role in the decision-making process.
At a more microlevel—a level not altogether distinct from the macrolevel of a decision—the most obvious players involved in the interaction that constitutes decision making are the investigator and the participant. Presumably, the investigator possesses or is anticipating the possibility of possessing a piece of health information about the participant and must decide if sharing the information with be beneficial or harmful to the patient. At the same time, by agreeing to participate in the study, the participant has presumably entrusted potentially sensitive health information to the investigator and expects that investigators will act in a way that will not harm him or her. In fact, participants may expect that investigators will share any potential IFs from the research.
In the case of discovering an IF, the cost–benefit analysis of reporting the IF may look quite different from an investigator versus a participant perspective. For example, it is easy to imagine a situation in which a PhD-level investigator, untrained in reading scans for pathology, may identify what looks like a brain abnormality while the participant is in the scanner. The investigator may feel some obligation to notify the participant of this finding, but he or she may also feel unsure about the decision. As identifying brain abnormalities may be beyond the researcher’s expertise, the researcher cannot be sure that the finding is clinically significant. Reporting the IF to the participant could result in unnecessary stress for the participant and cause undue harm.
In this same scenario, the participant may want to know about the possible brain abnormality, despite the risk that it may be a false-positive and could lead to anxiety and potentially costly follow-up care. Or perhaps the participant does not want to know about the IF, for exactly those reasons. From a social constructivism perspective, an ethical decision on how to proceed can be reached only by placing the decision in the interaction between the investigator and participant. If the decision-making process remains inside the head of either the investigator or the participant, there are many opportunities for expectations to be unmet and for disagreement to emerge.
The following is an example of language typically included in neuroimaging consent forms in major university settings. The following language comes from a consent document used at a major research university and was IRB approved:
The MRI images will not be used to evaluate your health. The images obtained for the study are for specific research purposes and are not being used to find medical abnormalities. If we find something in the image that looks suspicious, we will show a diagnostic radiologist who will advise us on how to proceed. We will contact you with this information.
Incorporating this text into the consent document for neuroimaging studies is in line with current best practices in neuroimaging research, differentiating between a medical scan and a research scan and outlining a protocol as to how IFs will be managed (Illes, Desmond, Huang, Raffin, & Atlas, 2002). However, there are various ambiguities in this language, leaving space for misinterpretation and confusion. For example, although the statement begins by stating that the images will not be used for health-related purposes, it then follows that if something “looks suspicious,” a radiologist will be notified. Not only is it unclear what is meant by “something that looks suspicious,” but it is not clear whether the participant will be notified before a radiologist is consulted, if he or she will be made aware of the radiological review, or what information will be revealed to the participant when he or she is contacted. This sort of language leaves room for participant expectations to be unmet and prevents participants from electing if they want to be informed of IFs at all. Although standard language such as the preceding does move toward addressing how IFs will be managed, it may not be sufficient and may contribute to participants feeling misguided or misinformed at various points in the research process.
Only when at least two key players interact can an ethical and mutually agreed-upon decision on how to manage IFs be reached. Using a social constructivism framework, current language used in consent documents for neuroimaging studies could be modified to ensure a more transparent process. The following example illustrates the type of language that might be useful:
The images obtained for the study are for specific research purposes and are not being used to find brain abnormalities. The types of images collected in this study are not of clinical quality and are typically not useful for identifying brain abnormalities, including but not limited to findings of an unexpected mass, aneurysm, bleed/stroke, evidence of current or past trauma to the brain, malformation, or anatomic evidence of dementia. However, sometimes gross brain abnormalities can be detected. Abnormalities detected in any images acquired during the scan may or may not be clinically relevant, might be benign, or might be artifacts of the image itself.
As indicated above, the images being collected today are for research purposes only. No additional images for clinical purposes will be collected. Although the primary purpose of the MRI images is not to evaluate any potential risks to your health, we acknowledge the chance that a potential brain abnormality could be detected either during the scan session or following the session during image analysis. We would like you to indicate below by signing your initials if you:
Would like to speak with the researcher running the session about any possible brain abnormalities visible in the images at the time of the scan session_____
Would NOT like to speak with the researcher running the session about any possible brain abnormalities visible in the images at the time of the scan session _____
Would like to be contacted by the PI of this study if any possible brain abnormalities are visible in the images at the time of image analysis _____
Would NOT like to be contacted by the PI of this study if any possible brain abnormalities are visible in the images at the time of image analysis _____
If you have indicated above that you would like to speak with the researcher running the session about possible brain abnormalities visible in the images or that you would like to be contacted by the PI of this study if possible brain abnormalities are visible in the images at the time of image analysis, we will notify you of any visible potential brain abnormalities as desired. Furthermore, we will ask you if you would like the image to be sent to a diagnostic radiologist who will then contact you with the findings. You may also request a copy of the image at any time. If the researcher or PI determines that the brain abnormality seems like it may pose an immediate risk to your health, we will notify you immediately and help you arrange next steps. The protocol for managing potential brain abnormalities is subject to change and remains an open topic for discussion for the duration of your participation in this study. At any point, you may indicate to us if your preference for managing possible abnormal brain images has changed. We will also indicate to you if circumstances arise that warrant a change in the agreed upon protocol for managing any potential brain abnormalities.
This example shows how a researcher might open the discussion of deciding how to manage potential IFs. Using the consent form as a starting point, researchers can initially gauge whether a participant would or would not like to be notified of potential brain abnormalities. In the face of changing circumstances, however, using flexible language creates space for the conversation to be reopened and modified as needed over the course of the researcher–participant relationship. This flexibility is critical to adopting a social constructivism approach to managing IFs in neuroimaging. The following hypothetical scenario illustrates how a social constructivism approach to managing IFs in neuroimaging might play out:
A 35-year-old woman volunteers for a neuroimaging study. The purpose of the study is to examine the neural correlates of episodic memory retrieval. The woman is a healthy volunteer, with no history of any serious physical or mental illness and no current signs or symptoms of illness. Upon arrival for the study, the woman is given a consent form and asked to read carefully through the document. The woman elects to speak with the researcher running the session about any possible brain abnormalities visible in the images at the time of the scan session. At this point, the researcher running the study, a graduate student in the psychology department at the university, carefully explains to the woman the risks and benefits associated with being told about potential brain IFs. The researcher clearly states that he is not trained to detect brain abnormalities and that while an abnormality may be clinically significant, there is also a possibility of a false-positive. False-positive findings may cause undue stress and anxiety for the participant and may also lead to potentially costly follow-up. He explains that only about 2–8% of IFs in neuroimaging research are clinically relevant in the general population. However, despite these potential risks, there is also the chance that a possible brain IF may lead to a life-saving intervention. Given this information, he confirms that the woman still prefers to speak with him during the session about any possible brain abnormalities visible in the images at the time of the scan. After the woman confirms her preference, the researcher explains that if at any point she changes her mind or in the case of unforeseen circumstances that may arise, they will discuss her options again and modify the agreed upon protocol accordingly.
The steps involved in the social constructivism approach are flexible, dynamic, and reiterative, allowing for a decision to evolve from the social interactions of the situation. Figure 1 (modified with permission from Cottone, 2001) depicts the decision-making path a researcher operating from a social constructivism perspective might follow when deciding how to manage brain IFs in his or her research protocol. Note that all players involved in a decision-making process should also take care to consult valued colleagues and outside professional expert opinions, negotiate when there is a disagreement, and respond to any disagreement in a way that allows for reasonable consensus as to what should happen.
FIGURE 1.
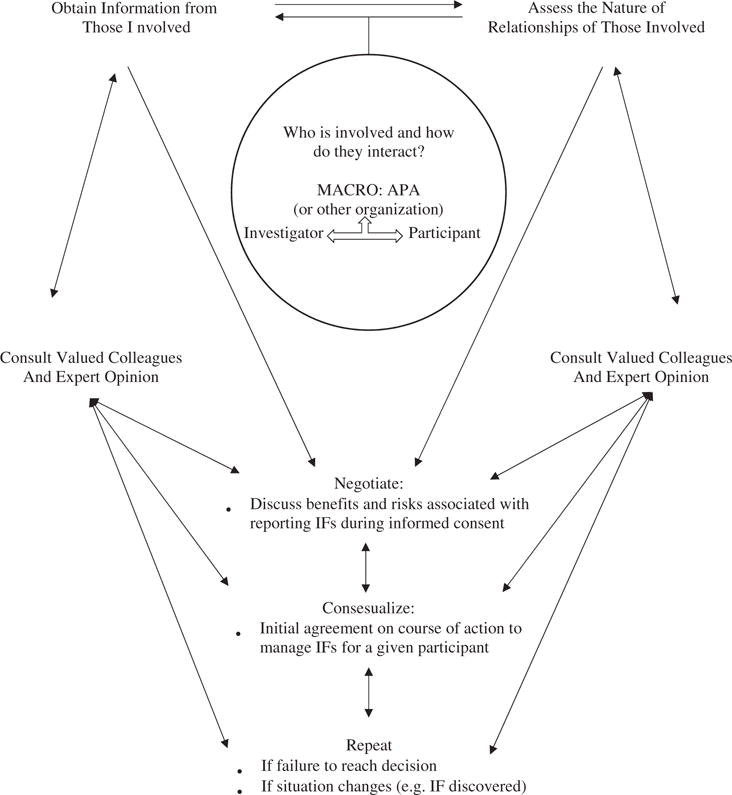
Hypothetical application of social constructivism model to the decision-making process of how to manage IFs. Adapted from Cottone, 2001.
CONCLUSIONS
For behavioral scientists conducting neuroimaging research, deciding how to manage potential IFs is a complex problem. Many practical and ethical considerations must be made when evaluating whether to report IFs. Although many researchers in the fields of cognitive neuroscience and psychology have pointed to a need for universal guidelines on this decision-making process (e.g., Illes et al., 2002; Leung, 2013), a consensus on a protocol for managing IFs has not been reached.
Applying a social constructivism ethical decision-making model to the issue of reporting IFs in brain imaging research provides a useful framework for this decision to emerge. Unlike many other proposed guidelines for managing IFs, the social constructivism model is flexible and dynamic in nature. Instead of outlining steps to implementing a protocol to manage IFs, the social constructivism approach provides a method that researchers can use as they develop such a protocol. Placing the decision on how to manage IFs in the realm of the social interaction between those involved helps account for many potential issues that may arise.
Social constructivism provides a framework for a collaboration between the researcher and participants to emerge. This collaboration allows for a decision-making strategy that transcends practical and ethical limitations. Placing the decision in the realm of the interaction between the investigator and participant provides a mechanism to develop a protocol for managing IFs that takes into account varying expectations between the individuals involved in neuroimaging research, varying legal and ethical principles across and within countries, and varying capacities to clinically interpret neuroimaging results. A social constructivism approach to managing IFs fosters conversation between researchers and participants, allowing investigators and participants to work toward decisions in line with expectations and ethics in the face of various situations. Regardless of differences in opinion on whether an IF should be disclosed, regardless of seemingly contradictory ethical principles, and regardless of rapidly changing expertise required to interpret neuroimaging results, a conversation between the researcher and participant can emerge and can be used to develop a protocol for IFs. Although this model does not provide a universal protocol for IFs per se, it does provide a framework that can be used universally.
The social constructivism model might be especially helpful in stressful times that accompany an ethical challenge, such as in deciding an approach to handling research IFs, because the model is parsimonious and does not involve complex steps or stages. The model presents a comprehensive approach to decision making, underscoring the importance of social interactions and relationships and placing the decision-making process in this sphere. The decision no longer rests entirely with the researcher, or any one individual. Rather, the decision emerges from the complex, variable, and sometimes elusive interactions between all those involved.
The current work examines how a social constructivism model might apply to managing IFs in neuroimaging. A standard protocol for managing IFs in neuroimaging has yet to emerge in the literature. Although various approaches to managing IFs have been suggested, none explicitly emphasize the importance of placing the decision-making process in the interaction between the researcher and the participant. A social constructivism approach to managing IFs in neuroimaging provides a framework for a decision-making process that transcends both the practical and ethical complexities of the situation and should be considered as researchers develop protocols for managing IFs.
References
- Alphs HH, Schwartz BS, Stewart WF, Yousem DM. Findings on brain MRI from research studies of occupational exposure to known neurotoxicants. American Journal of Roentgenology. 2006;187(4):1043–1047. doi: 10.2214/AJR.05.0421. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Ethical principles of psychologists and code of conduct. Washington, DC: Author; 2010. [Google Scholar]
- Booth TC, Jackson A, Wardlaw JM, Taylor SA, Waldman AD. Incidental findings found in “healthy” volunteers during imaging performed for research: Current legal and ethical implications. The British Journal of Radiology. 2010;83:456–465. doi: 10.1259/bjr/15877332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Hasso AN. Toward a uniform policy for handling incidental findings in neuroimaging research. American Journal of Neuroradiology. 2008;29(8):1425–1427. doi: 10.3174/ajnr.A1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Drummond KJ. Ethical considerations for normal control subjects in MRI research. Journal of Clinical Neuroscience. 2010;17(9):1111–1113. doi: 10.1016/j.jocn.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Cole C, Petree LE, Phillips JP, Shoemaker JM, Holdsworth M, Helitzer DL, Gluck J. ‘Ethical responsibility’ or ‘a whole can of worms’: Differences in opinion on incidental finding review and disclosure in neuroimaging research from focus group discussions with participants, parents, IRB members, investigators, physicians and community members. Journal of Medical Ethics. 2015;41:841–817. doi: 10.1136/medethics-2014-102552. [DOI] [PubMed] [Google Scholar]
- Cottone RR. A social constructivism model of ethical decision making in counseling. Journal of Counseling and Development: JCD. 2001;79(1):39–45. doi: 10.1002/j.1556-6676.2001.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Cottone RR. Displacing the psychology of the individual in ethical decision-making: The social constructivism model. Canadian Journal of Counselling. 2004;38(1):5. [Google Scholar]
- Cottone RR, Claus RE. Ethical decision-making models: A review of the literature. Journal of Counseling & Development. 2000;78(3):275–283. doi: 10.1002/j.1556-6676.2000.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Wu J, Hanson JA, Nouri S, Karnani D, Chuang TM, Le V. A system for addressing incidental findings in neuroimaging research. Neuroimage. 2011;55(3):1020–1023. doi: 10.1016/j.neuroimage.2010.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Hayashi Y, Tashiro S, Takashima K, Nakazawa E, Akabayashi A. Handling incidental findings in neuroimaging research in Japan: Current state of research facilities and attitudes of investigators and the general population. Health Research Policy and Systems. 2014;12(1):58. doi: 10.1186/1478-4505-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen KJ. The social constructionist movement in modern psychology. American Psychologist. 1985;40:266–275. doi: 10.1037/0003-066X.40.3.266. [DOI] [Google Scholar]
- Heinrichs B. A new challenge for research ethics: Incidental findings in neuroimaging. Journal of Bioethical Inquiry. 2011;8(1):59–65. doi: 10.1007/s11673-010-9268-9. [DOI] [Google Scholar]
- Hoffmann M, Schmücker R. Whole-body MRI screening. Berlin, Germany: Springer; 2014. The Ethics of Incidental Findings in Population-Based MRI Research; pp. 1–19. [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional magnetic resonance imaging. 3rd. Sunderland, MA: Sinauer Associates; 2014. [Google Scholar]
- Illes J. ‘Pandora’s box’ of incidental findings in brain imaging research. Nature Clinical Practice Neurology. 2006;2(2):60–61. doi: 10.1038/ncpneuro0119. [DOI] [PubMed] [Google Scholar]
- Illes J, Desmond JE, Huang LF, Raffin TA, Atlas SW. Ethical and practical considerations in managing incidental findings in functional magnetic resonance imaging. Brain and Cognition. 2002;50(3):358–365. doi: 10.1016/S0278-2626(02)00532-8. [DOI] [PubMed] [Google Scholar]
- Illes J, Kirschen MP, Edwards E, Bandettini P, Cho MK, Ford PJ, Wolf SM. Practical approaches to incidental findings in brain imaging research. Neurology. 2008;70(5):384–390. doi: 10.1212/01.wnl.0000280469.17461.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Kirschen MP, Edwards E, Stanford LR, Bandettini P, Cho MK, Michael DB. Incidental findings in brain imaging research: What should happen when a researcher sees a potential health problem in a brain scan from a research subject? Science (New York, NY) 2006;311(5762):783. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Kirschen MP, Karetsky K, Kelly M, Saha A, Desmond JE, Atlas SW. Discovery and disclosure of incidental findings in neuroimaging research. Journal of Magnetic Resonance Imaging. 2004a;20(5):743–747. doi: 10.1002/jmri.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes J, Rosen AC, Huang L, Goldstein RA, Raffin TA, Swan G, Atlas SW. Ethical consideration of incidental findings on adult brain MRI in research. Neurology. 2004b;62(6):888–890. doi: 10.1212/01.WNL.0000118531.90418.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. Journal of the American Medical Association. 1999;282(1):36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Jaworska A, Illes J. Subjects’ expectations in neuroimaging research. Journal of Magnetic Resonance Imaging. 2006;23(2):205–209. doi: 10.1002/(ISSN)1522-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Anderson B, Cervellione KL, Kan LI. Ethical and practical considerations in the management of incidental findings in pediatric MRI studies. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(8):1000–1006. doi: 10.1097/01.chi.0000222786.49477.a8. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences. 1992;89(12):5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. Incidental findings in neuroimaging: Ethical and medicolegal considerations. Neuroscience Journal. 2013 doi: 10.1155/2013/439145. Article 439145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana HR. Biology of language: The epistemology of reality. In: Miller GA, Lenneberg E, editors. Psychology and biology of language and thought. New York, NY: Academic Press; 1978. pp. 27–63. [Google Scholar]
- Maturana HR, Varela FJ. Autopoiesis and cognition: The realization of the living. Boston, MA: Reidel; 1980. [Google Scholar]
- Miller FG, Mello MM, Joffe S. Incidental findings in human subjects research: What do investigators owe research participants? The Journal of Law, Medicine & Ethics. 2008;36(2):271–279. doi: 10.1111/j.1748-720X.2008.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT, Weber F, Lee YC, Tsushima Y, Salman RAS. Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. British Medical Journal. 2009;339:b3016. doi: 10.1136/bmj.b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research-the national commission for the protection of human subjects of biomedical and behavioral research. Washington, DC: U.S. Government Printing Office; 1978. (DHEW Publication No OS 78-0012). [Google Scholar]
- National Core for Neuroethics. Documentation for the management of incidental findings. 2012 Retrieved from http://neuroethics.med.ubc.ca/res/new-documentation-for-the-management-of-incidental-findings.
- National Research Ethics Service. Information sheets and consent forms. Guidance for researchers and reviewers, 2011. UK: National Patient Safety Agency; 2011. Retrieved from http://www.wales.nhs.uk/sitesplus/documents/866/Guidelines%20NRES%20on%20Information%20Sheet%20%26%20Consent%20Form.pdf. [Google Scholar]
- The Nuremberg Code. Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10. Vol. 2. Washington, DC: U.S. Government Printing Office; 1949. pp. 181–182. [Google Scholar]
- Office for Human Research Protections. Federal policy for the protection of human subjects (‘Common Rule’) 2016 Mar 18; Retrieved from http://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html.
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JP, Cole C, Gluck JP, Shoemaker JM, Petree LE, Helitzer DL, Holdsworth MT. Stakeholder opinions and ethical perspectives support complete disclosure of incidental findings in MRI research. Ethics & Behavior. 2015;25(4):332–350. doi: 10.1080/10508422.2014.938338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancher CE, Shoemaker JM, Petree LE, Holdsworth M, Phillips JP, Helitzer DL. Disclosing neuroimaging incidental findings: A qualitative thematic analysis of health literacy challenges. BMC Medical Ethics. 2016;17(1):58. doi: 10.1186/s12910-016-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Wong EC. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43(11):2311. doi: 10.1212/WNL.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):812–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Royal JM, Peterson BS. The risks and benefits of searching for incidental findings in MRI research scans. The Journal of Law, Medicine & Ethics. 2008;36(2):305–314. doi: 10.1111/j.1748-720X.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, Uchiyama H, Fukushi T, Sakura O, Tatsuya K, Japan Children’s Study Group Incidental findings of brain magnetic resonance imaging study in a pediatric cohort in Japan and recommendation for a model management protocol. Journal of Epidemiology. 2010;20(Suppl. 2):S498–S504. doi: 10.2188/jea.JE20090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JM, Holdsworth MT, Aine C, Calhoun VD, De La Garza R, Ewing SF, Sanjuan P. A practical approach to incidental findings in neuroimaging research. Neurology. 2011;77(24):2123–2127. doi: 10.1212/WNL.0b013e31823d7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer S, Booth TC, Widdershoven G, Jansen O, Fesl G, Von Kummer R, Reiter-Theil S. fMRI. Berlin, Germany: Springer; 2013. Incidental findings in neuroimaging research: Ethical considerations; pp. 311–318. [Google Scholar]
- Underwood E. When a brain scan bears bad news. Science. 2012;338(6106):455. doi: 10.1126/science.338.6106.455. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Van Der Lugt A. Incidental findings on brain MRI in the general population. New England Journal of Medicine. 2007;357(18):1821. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Davies H, Booth TC, Laurie G, Compston A, Freeman C, Crabbe F. Acting on incidental findings in research imaging. BMJ. 2015;351 doi: 10.1136/bmj.h5190. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Illes J. Managing incidental findings in human subjects research: Analysis and recommendations. The Journal of Law, Medicine & Ethics. 2008;36(2):219–248. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association. 2013;310(20):2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]


