Abstract
Substantial evidence indicates that long-term exposure to fine particulate matter from multiple combustion sources contributes to cardiovascular disease. An integrated exposure-response approach uses evidence from exposures to air pollution, second hand smoke, and active cigarette smoking to explore mortality exposure-risk relationships. Although there are limitations, this approach provides a useful framework to evaluate consistency and coherency of the evidence and to estimate burden of disease from air pollution.
Keywords: air pollution, cigarette smoke, cardiovascular disease, particulate matter
Why an IER Approach?
Humans are exposed to combustion-related fine particulate matter (particles ≤ 2.5 µm in aerodynamic diameter), or PM2.5, from multiple sources, including ambient air pollution, household air pollution (HAP), second hand cigarette smoke (SHS), and active smoking. There is substantial evidence that breathing combustion-derived PM2.5 from these sources contributes to cardiovascular disease (CVD).1 Recently, estimators of PM2.5 relative risk (RR), termed integrated exposure-response functions (IER) have been developed that integrate CVD mortality risk estimates for air pollution, SHS, and active smoking. They describe the shape of the PM2.5-CVD mortality relationship over a broad range of exposure to PM2.5.2–4
The development of IER was motivated by two research needs. First, the need to address the plausibility of reported CVD mortality risk estimates for air pollution and SHS relative to risk from active smoking. Exposures to PM2.5 from air pollution and SHS are extremely small compared to that from active smoking. However, moderately elevated long-term exposures to ambient PM2.5 (20 µg/m3) and comparable exposures to SHS are associated with an approximately 25–30% increased risk of CVD mortality1,5,6–estimates much larger than expected based on proportional or linear extrapolations of the effects of active smoking. Second, there was a need to develop risk functions to estimate burden of disease attributable to ambient PM2.5 across a wide range of exposures, including areas with extremely high concentrations of air pollution, but where no direct epidemiologic evidence was available.4,7
In response to these research needs, the quantitative relationship between CVD mortality and exposure to PM2.5 from ambient air pollution, SHS and tobacco smoking was explored,2,3 observing a non-linear relationship. Building on that work, an IER estimator was developed to estimate the global burden of disease attributable to ambient PM2.5.4 Using the IER estimator, recent estimates of the contribution of PM2.5 air pollution exposures to global burden of disease from CVD are substantial.7 Recent reports regarding CVD mortality risks from light cigarette smoking,8 air pollution,9,10,11 SHS6 and HAP12 provide new evidence bearing on the validity of the IER approach. This viewpoint incorporates this new evidence, focuses on the broader implications of the IER approach to estimating the PM2.5-CVD risk relationship, and emphasizes that the IER approach does not provide an established “true” estimator but is an evolving conceptual and pragmatic framework with its validity depending on integrating ongoing empirical evidence.
Stylized Illustration of IER Approach
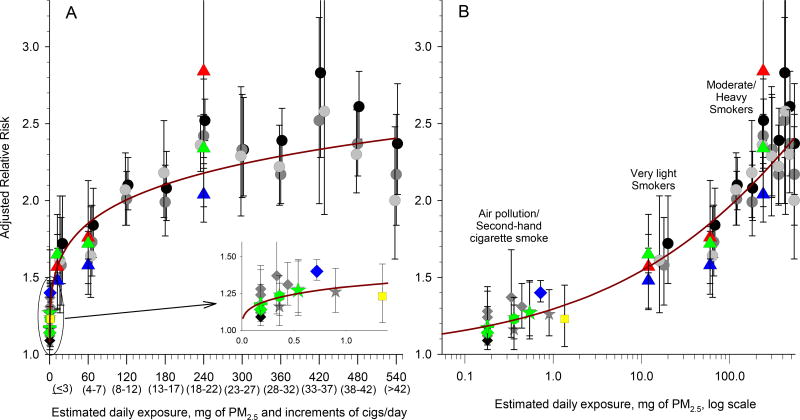
The Figure illustrates an IER approach using air pollution, SHS and active smoking evidence to evaluate the PM2.5-CVD mortality exposure-response relationship. Adjusted relative risks (RRs) (95% CIs) are plotted over estimated daily exposure of PM2.5 and increments of cigarette smoking. Associations at low exposures are further illustrated as an inset with a magnified scale (Panel A) and are also presented on the log scale (Panel B). Gray and black circles represent smoking-related RR estimates for ischemic heart, cardiovascular, and cardiopulmonary disease mortality and gray diamonds and stars represent estimates from studies of air pollution and second hand cigarette smoke as previously documented.2,3
RR estimates of the PM2.5-CVD mortality exposure-response relationship from several important new studies are superimposed as colored symbols (red, blue, and green indicate females, males, and both sexes, respectively). Triangles represent estimates for coronary heart disease mortality associated with smoking 1, 5, and 20 cigarettes/day.8 The green star represents the estimate for ischemic heart disease mortality associated with second-hand cigarette smoke.6 RR estimates for CVD mortality based on two recent analyses US cohorts9,11 are represented by a green diamond and plus sign. A meta-analysis of cohort studies5 of ambient air pollution provides the RR estimate for CVD mortality associated with 20 µg/m3 of PM2.5 (mean exposures across the cohorts ranged from 4 to 28 µg/m3) represented by the green hex. The estimate for CVD mortality associated with 40 µg/m3 of PM2.5 (mean exposures, 43.7 µg/m3) based on a large national cohort of Chinese men10 is represented by the blue diamond. A recent RR estimate for CVD mortality from exposure to HAP from solid fuel use in rural China12 is represented by the yellow square.
The maroon curve illustrates an IER function, with the mathematical form used in global burden of disease estimates as documented elsewhere,4,7 specifically:
Lessons learned
The integrated evidence regarding the CVD-PM2.5 mortality risk relationship from air pollution, SHS, and active smoking is remarkably coherent (Figure). Breathing combustion-related PM2.5 appears to contribute to CVD mortality risk in a way that is largely exposure-dependent, but nonlinear. The exposure-response is relatively steep at low exposures and levels off at higher exposures. Understanding this empirical evidence is critical for making informed estimates of burden of disease from air pollution, especially when extrapolating to highly polluted areas with inadequate exposure-response estimates. The IER approach illustrates why reducing CVD mortality risk from cigarette smoking is more effectively accomplished by complete smoking cessation, even among light smokers, rather than smoking reduction.
Figure.
Illustration of the IER approach to evaluate the PM2.5-CVD mortality exposure-response relationship. Relative risks (RRs) (95% CIs) of CVD-related mortality plotted over estimated daily exposure of PM2.5 and increments of cigarette smoking. An IER function is fit through the data. Details provided in “Stylized Illustration of IER Approach” subsection of text.
The IER approach is instructive in exploring plausible biological mechanisms. Evidence indicates that PM2.5 exposures from cigarette smoke or air pollution affect multiple physiological pathways1 and is suggestive of a saturation phenomenon, where relatively low exposures to PM2.5, either from very light smoking or even from SHS and air pollution, appear sufficient to induce adverse biological responses and increase the risk of CVD. Increasing exposure further elevates risk, but at a decreasing marginal rate.
The IER approach provides a framework to evaluate the consistency and coherency of evidence regarding the contribution of PM2.5 exposure to CVD risk. For example, the recent meta-analytic RR estimates of smoking 1, 5, and 20 cigarettes8 are consistent with earlier reported results2,3 and provide additional corroboration of the overall shape of the exposure-response relationship. The estimated effect of smoking a single cigarette, narrows the evidence gap between active smoking and ambient air pollution—and appears consistent with the IER function. The recent China cohort study10 also helps narrow the evidence gap, but does not fit the IER as well, suggesting that the IER may be underestimating effects at the high concentrations in China. The recent HAP study in rural China12 is also informative. Given the uncertainty in estimates of RR and average differential PM2.5 exposures specific to solid fuel use versus clean fuel (tentatively ~55 µg/m3), the estimates are reasonably consistent with the IER.
Limitations
Despite its utility as both a conceptual framework and a practical approach for risk estimates for PM2.5 from diverse combustion sources, neither specific IER estimators nor the IER approach more generally provides a unified PM2.5 mass risk model that fully and accurately defines risk relationships between CVD and PM2.5 from all sources and under all circumstances. This approach has been constantly evolving and specific estimated IER functions change with new empirical evidence.
One limitation of the IER approach is that it requires uncertain assumptions regarding scaling exposures to PM2.5 from different sources. In the Figure, for active smoking, daily inhaled exposure is 12 mg PM2.5 per cigarette. For air pollution and SHS, daily average inhaled exposure is estimated as PM2.5 concentrations (mg/m3) multiplied by average daily inhalation rates (18 m3/day) as discussed elsewhere.2,3 However, changes in cigarette design and compensatory smoking behavior make estimates of PM2.5 exposure per cigarette smoked uncertain. Scaling of exposure estimates requires assumptions regarding ventilation rates, yields of PM2.5 per cigarette, and estimates of average concentrations from air pollution and SHS. Nevertheless, the nonlinear exposure-response relationship is observed even for alternative exposure scaling approaches. For example, simply using number of cigarettes smoked per day (with no additional scaling) demonstrates a nonlinear or non-proportional exposure-response relationship with the excess risk of smoking a single cigarette being approximately 40 percent of the excess risk of smoking 20 cigarettes per day.
Another limitation is the assumption that health impacts are dependent on PM2.5 mass exposure without allowance for variations in toxicity that depend on source, chemical composition, or other characteristics. Even when excluding air pollution evidence, however, and only using evidence from exposure to cigarette smoke, the non-linear exposure-response function illustrated in the Figure is nearly identical. The range of exposures associated with active smoking alone results in an exposure-response function that is not linear passing through the origin (Figure 1 Panel A). Assuming no excess risk at zero exposure, a monotonic exposure-response function based only on the evidence of excess risk due to active smoking would require a non-linear function that is steeper at low exposures and flattens out at high exposures. The non-proportional excess risk associated with SHS provides further evidence of an exposure-response curve that is relatively steep at low exposures.
The assumption of equivalent toxicity across sources is more problematic when exposures to air pollution from multiple sources are also included. Differential toxicity of PM2.5 from different combustion sources remains poorly understood. Recent observations have led some to hypothesize that particles from fossil fuel combustion (especially coal combustion) are relatively more toxic than particles from other sources such as biomass burning.13 If true, the IER approach might underestimate PM2.5 health effects in areas with extensive burning of coal and other fossil fuels—one possible explanation for the discrepancy between IER predictions and the results of the recent cohort study from China noted above.10 However, there is currently insufficient evidence to clearly differentiate toxicity by physical, chemical, or source characteristics.4,14 There is a need for further related research. Furthermore, as illustrated in the Figure, similar exposures to particles from SHS and ambient air pollution are associated with approximately comparable elevated risks of CVD mortality.
Another limitation of the IER illustrated in the Figure is that it does not allow for a sigmoidal shaped function. When applying IER function to estimate global burden of disease, the excess risk has been estimated in contrast to a small, but non-zero, counterfactual level of pollution exposure—implicitly assuming no effects below the counterfactual level.7 A more elegant, and possibly more accurate approach would be to use flexible risk-response functions that allow for sigmoidal shapes. As the number of cohort studies of long-term exposure to air pollution grow, meta-analytic methods that more flexibly estimate the shape of the exposure-response function using only studies of ambient air pollution is becoming possible.
Finally, the IER approach assumes that excess risk from one source of PM2.5 exposure is not dependent upon another source. The evidence however, suggests that smokers are also affected by exposure to air pollution, indicating interaction between smoking and air pollution not accounted for by this approach.15
Conclusions
Despite its limitations, the IER approach has been useful in estimating the size and shape of the PM2.5-CVD mortality risk-exposure relationship. Evidence provided by an IER approach is remarkably coherent and provides intriguing evidence that breathing combustion-related fine particulate matter contributes to CVD risk as a function of estimated daily dose of PM2.5, whether the exposure is from active smoking, second hand smoking, or ambient air pollution. The PM2.5-CVD mortality exposure-response relationship is not linear, with large marginal effects at low exposures and declining marginal effects at higher exposures. These findings are consistent with evidence of significant public health effects of PM2.5 air pollution even across relatively less polluted communities. These results are also consistent with findings that SHS contributes to CVD risk and that policies that reduce SHS exposures can reduce risk of CVD events.
The IER approach has been useful in making informed estimates of burden of disease from ambient air pollution and in exploring biological mechanisms. A feature of the IER development is that it has been ongoing with emerging evidence being integrated into the estimates of the exposure-response functions. Alternative meta-analytic methods that estimate the shape and uncertainty in the exposure response relationship using evidence from the growing number of air pollution studies may be used in future estimates of global burden of disease, especially when additional studies from some of the most highly polluted areas of the world are made available. The IER approach to evaluate integrated evidence of CVD effect of PM2.5 from air pollution, SHS, active smoking, and other sources, however, will remain useful to provide context, plausibility, and to evaluate the consistency and coherency of the overall evidence.
Acknowledgments
Sources of Funding
Funding support included grants from the National Institutes of Environmental Health Sciences (NIH ES019217), US Environmental Protection Agency Center for Air, Climate, and Energy Solutions (CACES) (EPA Grant Number R835873), and the Mary Lou Fulton Professorship at Brigham Young University.
Footnotes
Disclosures
None
References
- 1.Brook RD, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the Scientific Statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, III, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle E, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett RT, Pope CA, III, Ezzati M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122:397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. doi: 10.1186/s12889-015-2489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j3984. doi: 10.1136/bmj.j3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope CA, III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 10.Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, Liang R, Wang W, Qi J, Wang L, Zhou M. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125(11):117002. doi: 10.1289/EHP1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker JD, Kravets N, Vaidyanathan A. Particulate matter air pollution exposure and heart disease mortality risk by race and ethnicity in the United States. Circulation. 2018;137:1688–1697. doi: 10.1161/CIRCULATIONAHA.117.029376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu K, Qiu G, Chan KH, et al. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. JAMA. 2018;319:1351–1361. doi: 10.1001/jama.2018.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, Ito K, Jerrett M, Gapstur SM, Diver WR, Pope CA. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air Pollution. Environ Health Perspect. 2016;124:785–94. doi: 10.1289/ehp.1509777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanek LW, Sacks JD, Dutton SJ, Dubois JJB. Attributing health effects to apportioned components and sources of particulate matter: An evaluation of collective results. Atmos Environ. 2011;45:5655–5663. [Google Scholar]
- 15.Turner MC, Cohen A, Burnett RT, Jerrett M, Diver WR, Gapstur SM, Krewski D, Samet JM, Pope CA., III Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ. Res. 2017;154:304–310. doi: 10.1016/j.envres.2017.01.024. [DOI] [PubMed] [Google Scholar]