Abstract
Radiosensitivity of prostate cancer (PCa) cells promotes the curative treatment for PCa. The present study was designed to investigate the synergistic effect of genistein and AG1024 on the radiosensitivity of PCa cells. The optimal X-irradiation dose (4 Gy) and genistein concentration (30 µM) were selected by using the CCK-8 assay. Before X-irradiation (4 Gy), PC3 and DU145 cells were treated with genistein (30 µM), AG1024 (10 µM) and their combination. All treatments significantly reduced cell proliferation and enhanced cell apoptosis. Using flow cytometric analysis, we found that genistein arrested the cell cycle at S phase and AG1024 arrested the cell cycle at G2/M phase. Genistein treatment suppressed the homologous recombination (HRR) and the non-homologous end joining (NHEJ) pathways by inhibiting the expression of Rad51 and Ku70, and AG1024 treatment only inhibited the NHEJ pathway via the inactivation of Ku70 as detected by western blot analysis. Moreover, the combination treatment with genistein and AG1024 more effectively radiosensitized PCa cells than single treatments by suppressing cell proliferation, enhancing cell apoptosis and inactivating the HRR and NHEJ pathways. In vivo experiments demonstrated that animals receiving the combination treatment with genistein and AG1024 displayed obviously decreased tumor volume compared with animals treated with single treatment with either genistein or AG1024. We conclude that the combination of genistein (30 µM) and AG1024 (10 µM) exhibited a synergistic effect on the radiosensitivity of PCa cells by suppressing the HRR and NHEJ pathways.
Keywords: radiosensitivity, prostate cancer, genistein, AG1024, DNA repair
Introduction
Radiotherapy is an important curative treatment option for patients with metastatic tumors such as prostate cancer (PCa) (1,2). High tumor recurrence and therapy resistance rate in patients with PCa (>30%) suggest that cellular radioresistance is a major obstacle and challenge to effective radiotherapy for PCa (3,4). Improved radiotherapy modalities and treatments for PCa are warranted and being assessed in pre-clinical applications.
Radiotherapy usually induces severe damage in genomic DNA, including DNA single-strand breaks (SSBs) and double-strand breaks (DSBs) (5). In higher eukaryotes, DNA-DSBs are repaired by two major DNA repair pathways, the homologous recombination (HRR) and the non-homologous end joining (NHEJ) pathways (9). Cell cycle arrest, apoptosis and autophagy are typical impairments induced by radiotherapy (6–8). In response to DNA damage, cell cycles are mainly interfered by genotoxic stress at two checkpoints: G1/S and G2/M transitions (9). DNA damage could be monitored by characterizations of inhibited DNA replication at G1/S checkpoint and damaged chromosome segregation at G2/M checkpoint (9,10). Irradiation-mediated genotoxic stress is caused by sustained DNA damage that overloads DNA repair capacity by the HRR and NHEJ pathways (5). This DNA repair failure finally results in increased cell apoptosis, cell cycle arrest and autophagy, thus, inducing radiosensitivity.
Radiosensitivity of tumor cells could be achieved by targeting signalings, gene transcriptions and non-coding RNAs (microRNAs and lncRNAs) which ultimately regulate cell cycle progression, apoptosis and induce cell cycle checkpoint defects (4,6,11–14). Two important tyrosine kinase receptors, insulin-like growth factor 1 receptor (IGF1R) and epidermal growth factor receptor (EGFR), play essential roles in cancer development and cell cycle progression, invasion and radiosensitivity via the activation of downstream pathways (5,15). IGF1R gene silencing was found to enhance the sensitivity of human PCa cells to DNA-damaging agents (16).
P13K/Akt/mTOR signaling is responsive to various stresses and plays a critical role in cell cycle, apoptosis, autophagy, invasion, metastasis, tumorigenesis and radiosensitivity of tumor cells (5,17–19). Genistein is a tyrosine-specific protein kinases inhibitor, topoisomerase II poison, which inactivates EGFR, IGF1R and Akt-mediated signalling (20–23). It was reported that genistein inhibited DSB repair through the inactivation of DNA-dependent protein kinases (DNA-PKs) and led to NHEJ and HRR incompleteness (24). Tyrosine kinase inhibitor (tyrphostin) AG1024 is a specific IGF1R inhibitor, which has been reported to radiosensitize PCa cells and breast cancer cells (5,18). However, less information is known about the radiosensitivity of PCa cells to the combination treatment with AG1024 and genistein.
In the present study, we investigated the synergistic effect of combination treatment with AG1024 and genistein on the radiosensitivity of PCa cells. Before X-irradiation, PC3 and DU145 PCa cells were treated with genistein, AG1024 and their combination. The radiosensitivity of PCa cells was evaluated by changes in DNA damage, cell proliferation, apoptosis, cell cycle distribution and the inactivation of the NHEJ and HRR pathways. The synergistic effect of AG1024 and genistein on the sensitivity of PCa cells to radiotherapy is discussed. This study provides new insights into the compensation/synergic effect on NHEJ and HRR pathways for DNA damage repair by AG1024 and genistein.
Materials and methods
Cell lines, culture conditions and treatments
The human PCa cell lines PC3 and DU145 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1640 medium (Gibco-BRL; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco-BRL; Thermo Fisher Scientific) at 37°C under 5% CO2 in a humidified incubator. For cell treatment, PC3 and DU145 cells were cultured in RPMI-1640 medium supplemented with genistein (purity >98%, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and AG1024 (Sigma-Aldrich; Merck KGaA) at series concentrations. Genistein (0, 10, 20, 30, 50 and 100 µM) and AG1024 (10 µM) (5) were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). X-ray irradiation was delivered using an X-6 MV photon linear accelerator (Varian Associates Inc., Palo Alto, CA, USA) at room temperature with a dose rate of 2 Gy/min (25). Cells treated with DMSO were considered as negative control for irradiation or drug treatment.
Cell viability and in vitro cytotoxicity assay
The optimal concentration of genistein and optimal dose of X-ray irradiation were selected using a Cell Counting Kit-8 (CCK-8) assay kit (Sigma-Aldrich; Merck KGaA). The cytotoxicity of genistein and X-irradiation to human PCa cells was assessed using cell viability analysis. Cells were placed into 96-well plates, pretreated with DMSO, genistein, AG1024 and combination of genistein and AG1024 for 1 h, followed by X-irradiation. Then, cells were incubated in RPMI-1640 medium for another 24 h in conditions as above. Ten microliters of CCK-8 solution per well was added into the cell culture for 2 h, and then the cell culture was subjected to a microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) for determination of the optical density (OD450nm).
Soft agar colony formation analysis
Monolayer clonogenic assay was performed to investigate the effect of genistein, AG1024 and the combination on colony formation ability. Cells were placed into 6-well plates and grown to log phase, followed by transferring to medium containing DMSO, genistein, AG1024 and genistein plus AG1024. After a 1-h incubation, the cells were irradiated with 4 Gy of X-irradiation and incubated in RPMI-1640 medium for 24 h. Then, the cells were treated with trypsin (Gibco-BRL; Thermo Fisher Scientific, Inc.), transferred to a 6-cm diameter tissue culture dish with fresh RPMI-1640 medium and incubated for 12 days. Medium was replaced every third day. For colony detection, dishes were washed with phosphate-buffered saline (PBS) for two times, fixed with methanol at 4°C for 15 min, and stained with Giemsa (Sigma-Aldrich; Merck KGaA) for 30 min. Colony counting was performed on clearly visible colonies (colony with >50 cells or diameter >50 µm) using a light microscope (BX51; Olympus Optical Co., Ltd., Tokyo, Japan). Each experiment was performed in triplicate.
Flow cytometric analysis
The effects of the combination treatment of genistein and AG1024 and single treatments on cell cycle distribution and apoptosis were detected using a flow cytometer (BD Biosciences, San Jose, CA, USA). For cell cycle analysis, cells under different treatments for 24 h were harvested (trypsin; Gibco-BRL; Thermo Fisher Scientific, Inc.), fixed (70% ethanol), stained with 50 µg/ml propidium iodide (PI) solution, and were subjected to flow cytometry. Cell cycle distribution at G0/G1, S and G2/M phase was analyzed. For cell apoptosis analysis, fixed cells were incubated with PI and Annexin V-FITC for 10 min in the dark, and then subjected to a BD FACSCalibur flow cytometer (BD Biosciences). Cells undergoing early apoptosis were considered as apoptotic cells (Annexin V-positive and PI-negative, Annexin V+/PI−). Each experiment was conducted in triplicate.
Immunofluorescence
Immunofluorescence analysis was performed to detect the γH2AX foci formation as described by Wang et al (5). In brief, 1×106 cells/ml of PC3 and DU145 cells were seeded into 6-well plates with coverslips and were treated with different treatments combined with X-irradiation for 24 h. The cells were then fixed with 4% paraformaldehyde for 20 min, incubated with 0.2% Triton X-100 in PBS for 5 min, and coverslips were blocked with 5% bovine serum albumin (BSA; Gibco-BRL; Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Then slips with fixed cells were incubated with specific primary antibody against phospho-histone γH2AX (1:500; cat. no. 2595; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight, followed by incubation with Cy3-labelled goat anti-rabbit fluorescent secondary antibody (1:2,000; cat. no. 111-165-003; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature and 1 µg/ml DAPI (Invitrogen; Thermo Fisher Scientific, Inc.) for additional 10 min in the dark. Images were captured using an Olympus laser scanning confocal microscopy (LEXT 3100; Olympus Corp., Tokyo, Japan).
Western blot analysis
Cells were placed into 6-well plates and incubated using the different treatments as above. Cells were harvested at 24 h post X-irradiation. Cellular and nuclear protein was isolated using RIPA buffer (Pierce Inc., Beijing, China). Proteins were prepared as described by Liu et al (26). Western blot analysis was performed according to the standard methods. Specific primary antibodies of anti-phospho (p)-IGF1R (Tyr1135), -IGF1R, -ATM, -ATM(Ser1981), -Bax, -Bcl2, -cleaved caspase-3, -Ku70, -Rad51, -DNA-PKcs and -GAPDH were purchased from Cell Signaling Technology, Inc. Primary antibody p-DNA-PKcs (Thr2609) was purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA, USA; cat. no. sc-101664).
In vivo tumor radiation protocol
The in vivo subcutaneous mouse tumors were produced by subcutaneously injecting 5×106 DU145 cells, mixed with BD Matrigel (BD Biosciences), into the flank of male nude mice (6–7 weeks old, 18–20 g, n=60) provided by the Experimental Animal Center of the Fourth Military Medical University (5). Animals were maintained with ad libitum access to food and water for 5 days at 25±1°C in environmental chambers, with 40–50% humidity and 12 h light: 12 h dark cycle. A digital Vernier caliper was used for measuring tumor volume [V = 0.5 × tumor length (mm) × tumor width2 (mm2)]. Twenty days later, mice were randomly divided into four groups (n=15 in each group): the DMSO + IR (control) group received X-irradiation every three days for 5 times (15-day treatment course), with orally intubated with 200 mg/kg/day DMSO; the genistein + IR group received 100 mg/kg/day genistein, 100 mg/kg/day DMSO and X-irradiation for 5 times; the AG1024 + IR group received 100 mg/kg/day AG1024, 100 mg/kg/day DMSO and X-irradiation for 5 times; the Combination (genistein + AG1024) + IR group received 100 mg/kg/day genistein, 100 mg/kg/day AG1024, plus with X-irradiation for 5 times. The in vivo therapeutic efficacy of the different treatments on in vivo tumors was evaluated using changes in tumor volume and proliferation index (PI, PI=Vtreatment/Vcontrol) (5). Body weight (g) of experimental animals were recorded. Multiple nodes in one mouse were circled into one circle and the accumulated volume was calculated as above. All mice were sacrificed by anesthesia and the tumors were removed on day 15 after the 1st administration of genistein, AG1024 and the combination treatment. The animal experiment protocols were approved by the Ethics Committee of the Fourth Military Medical University (Xi'an, China).
Statistical analyses
Each cellular experiment was performed in triplicate. All quantitative data and continuous variables are expressed as mean ± standard deviation (SD). Statistical analysis was performed using the unpaired two-tailed Student's t-test in SPSS software 17.0 (SPSS, Inc., Chicago, IL, USA). GraphPad Prism 6 (Graphpad Software, Inc., San Diego, CA, USA) was used for plotting. Statistical significant P-value is given as P≤0.05.
Results
Cytotoxicity of irradiation and genistein in PCa cells
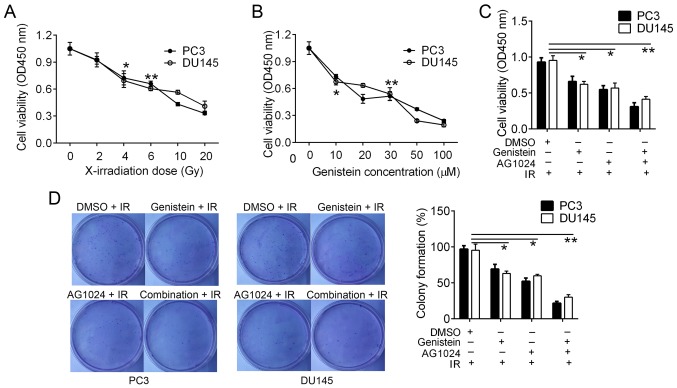
The frequently used concentration of AG1024 is 10 µM for cancer cells (5,18). In order to select suitable doses of X-irradiation and genistein, we treated PC3 and DU145 cells with different doses of X-irradiation (0, 2, 4, 6, 10 and 20 Gy) and genistein (0, 10, 20, 30, 50 and 100 µM). Cell cytotoxicity was detected using the CCK-8 assay. In comparison analysis, we found that cell viability was significantly inhibited by ≥4-Gy X-irradiation (P<0.05; Fig. 1A) and ≥10 µM genistein (P<0.05; Fig. 1B). Thus, we selected 4-Gy X-irradiation and 30 µM genistein as the optimal conditions for cell cytotoxicity in the PCa cells.
Figure 1.
Effect of X-irradiation, genistein and the combination of genistein and AG1024 on PCa cell proliferation. (A and B) Cell viability in response to different doses of X-irradiation and genistein. The selected optimal condition was 4-Gy X-irradiation and 30 µM genistein. (C) Cell viability analysis with the single and combined treatment of genistein and AG1024. (D) Colony formation analysis. Combination treatment with genistein and AG1024 and single treatments revealed an inhibitory effect on cell proliferation. *P<0.05 vs. control (A and B, dose 0; C and D, DMSO). **P<0.01 vs. control (A and B, dose 0; C and D, DMSO). PCa, prostate cancer; IR, X-irradiation.
Combination of genistein and AG1024 enhances X-irradiation-reduced PC3 and DU145 cell proliferation
We pretreated PC3 and DU145 cells with genistein (30 µM), AG1024 (10 µM) and the combination before X-irradiation (4 Gy). Twenty-four hours later, we confirmed that the combination of genistein and AG1024 significantly decreased cell proliferation (P<0.01), followed by genistein (30 µM, P<0.05) and AG1024 (10 µM, P<0.05) as compared with that of the control cells (Fig. 1C). Using colony formation assay, we detected that the colony numbers of PC3 and DU145 cells were reduced by treatments of genistein (30 µM), AG1024 (10 µM) and the combination treatment (P<0.05; Fig. 1D). PC3 and DU145 cells pretreated with the combination of genistein (30 µM) and AG1024 (10 µM) before X-irradiation (4 Gy) showed the lowest frequency of colony formation (P<0.01), followed by cells pretreated with either genistein (30 µM) or AG1024 (10 µM) before X-irradiation (4 Gy, P<0.05; Fig. 1D). These results showed that the combination treatment of genistein and AG1024 and single treatments enhanced PCa cell cytotoxicity by X-irradiation. In addition, the combination of genistein and AG1024 indicated higher efficacy in the inhibition of cell proliferation than either genistein or AG1024 single treatment, suggesting a synergistic inhibition effect on PCa cell proliferation.
Combination treatment of genistein and AG1024 and single treatments induce cell cycle arrest
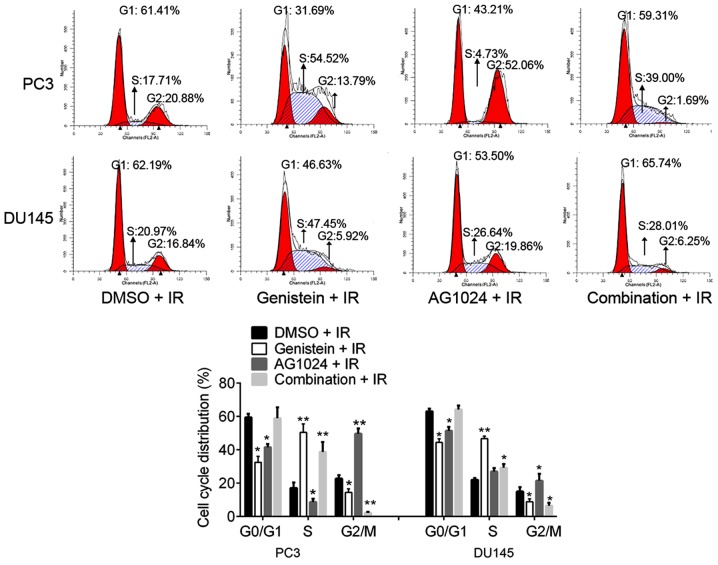
Cell cycle distribution analysis showed the percentages of cells at G0/G1, S and G2/M phases were significantly disturbed by genistein (30 µM), AG1024 (10 µM) and the combination treatment. As compared with the control (DMSO + irradiation), genistein (30 µM) treatment significantly decreased the percentages of cells at G0/G1 and G2/M phases (P<0.05), and increased the percentages of cells at S phases (P<0.01), revealing that genistein induced S cell cycle arrest (Fig. 2). In addition, we observed that AG1024 treatment induced G2/M cell cycle arrest in PC3 and DU145 cells. AG1024 treatment significantly increased the percentages of cells at the G2/M phases (P<0.01 for PC3 cells; P<0.05 for DU145 cells) and reduced the percentages of cells at G0/G1 and/or S phases (P<0.05).
Figure 2.
Cell cycle distribution by flow cytometric analysis. PC3 and DU145 cells were pretreated with genistein (30 µM), AG1024 (10 µM) and their combination before X-irradiation (4 Gy). After X-irradiation, cells were incubated for 24 h. Cell cycle arrest at S phase and G2/M phase was observed in genistein (30 µM) and AG1024 (10 µM) pretreated cells, respectively. The combination of genistein and AG1024 arrested cell cycle at the G2/M phase. *P<0.05 vs. control (DMSO + IR). **P<0.01 vs. control (DMSO + IR). IR, X-irradiation.
Notably, the combination treatment with genistein (30 µM) and AG1024 (10 µM) significantly increased the percentages of cells at the S phase (P<0.01 for PC3 cells; P<0.05 for DU145 cells) and decreased the percentages of cells at G2/M phases (P<0.01 for PC3 cells; P<0.05 for DU145 cells). The percentages of cells at G0/G1 phases were concomitant with those of the control cells (DMSO + IR; P>0.05). Taken together, we found that genistein (30 µM) induced S cell cycle arrest, AG1024 (10 µM) induced G2/M cell cycle arrest, and the combination of genistein (30 µM) and AG1024 (10 µM) induced S cell cycle arrest. In addition, the combination treatment with genistein and AG1024 showed a synergistic inhibitory effect on chromosome segregation at the G2/M phase, but not DNA replication at the G0/G1 phase in PCa cells.
Combination treatment with genistein and AG1024 enhances X-irradiation-induced apoptosis in PC3 and DU145 cells
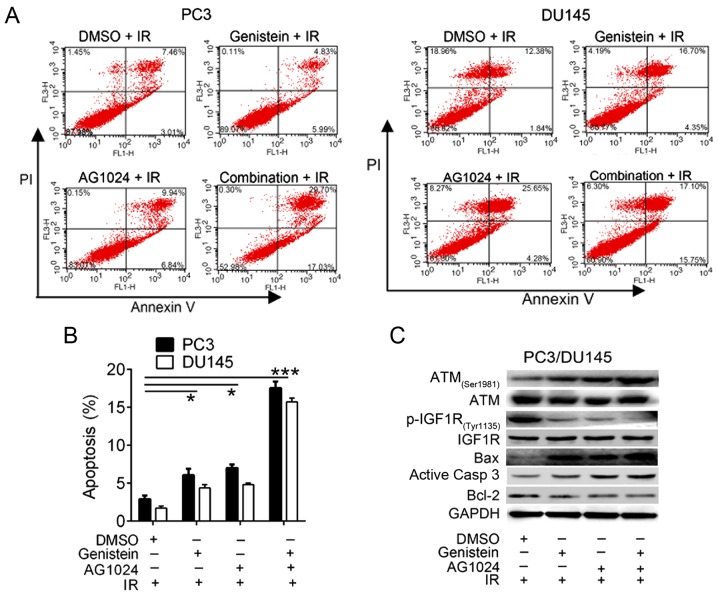
We then confirmed that the treatment of genistein (30 µM), AG1024 (10 µM) and their combination promote cell apoptosis using flow cytometric analysis. Significant enhancement of cell apoptosis was observed in cells treated with genistein (30 µM, P<0.05), AG1024 (10 µM, P<0.05), and both of them (P<0.01) combined with X-irradiation (Fig. 3A and B). These data demonstrated that genistein (30 µM) and/or AG1024 (10 µM) treatment enhanced X-irradiation-induced PCa cell apoptosis, and the combination treatment with genistein and AG1024 synergistically promoted cell radiosensitivity.
Figure 3.
Analysis of cell apoptosis and expression of cell apoptosis-related proteins. (A and B) Cell apoptosis analysis was analyzed using Annexin V/PI flow cytometric analysis. (C) The expression of cell apoptosis-related proteins was detected using western blot analysis. *P<0.05 vs. control (DMSO + IR). ***P<0.001 vs. control (DMSO + IR). PI, propidium iodide; R, X-irradiation.
Using western blot analysis, we detected the expression of cell apoptosis-related proteins and pathway. In comparison with the control cells (DMSO + irradiation), the expression of ATM (Ser1981), Bax and active caspase-3 were increased, whereas the expression of p-IGF1R (Tyr1135) and Bcl-2 were decreased in PC3 and DU145 cells treated with genistein (30 µM) and/or AG1024 (10 µM) plus X-irradiation (Fig. 3C). Thus, we suggested that both genistein and AG1024 induced PCa cell apoptosis via the activation of apoptosis-related pathways, which may be associated with the inactivation of IGF1R. In addition, combination of genistein and AG1024 synergistically modulated the expression of these proteins, showing synergistic effect on irradiation-induced cell apoptosis of PCa cells.
Genistein and/or AG1024 enhances irradiation-induced DSB in PCa cells
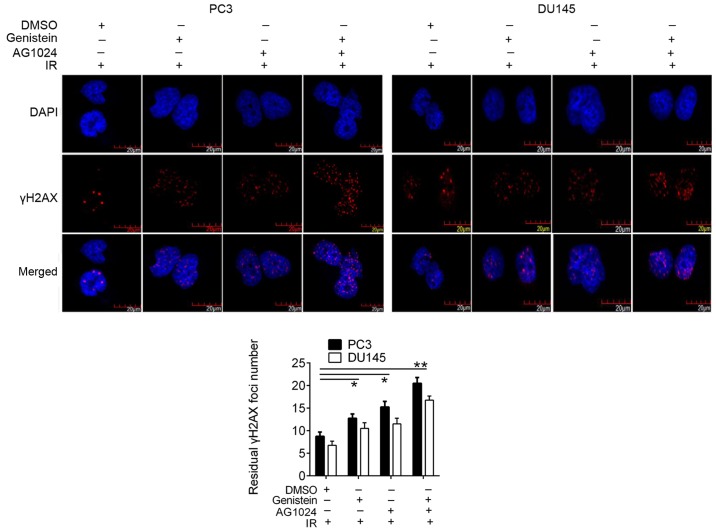
We then revealed the increased γH2AX foci formation, a DSB marker, in genistein (30 µM) and/or AG1024 (10 µM) treated PCa cells (Fig. 4), suggesting the elevation of DNA damage by pretreatment with genistein, AG1024, and combination before X-irradiation. In PC3 and DU145 cells pretreated with different treatments before X-irradiation, the numbers of residual γH2AX foci were significantly elevated as compared with that in the control cells (P<0.05). Moreover, PCa cells treated with the combined treatment with genistein and AG1024 demonstrated a higher number of γH2AX foci than cells treated with single treatments of either genistein or AG1024, showing synergistic enhancement of γH2AX foci formation by genistein and AG1024.
Figure 4.
Analysis of γH2AX foci formation in treated PCa cells. γH2AX foci were indicated by purple foci in cells. More purple-colored foci were formed in cells pretreated with genistein (30 µM) and/or AG1024 (10 µM) before X-irradiation (4 Gy). *P<0.05 vs. control (DMSO + IR). **P<0.01 vs. control (DMSO + IR). PCa, prostate cancer; IR, X-irradiation.
Combination of genistein and AG1024 enhances radiosensitivity of PCa cells via the inactivation of HRR and NHEJ pathways
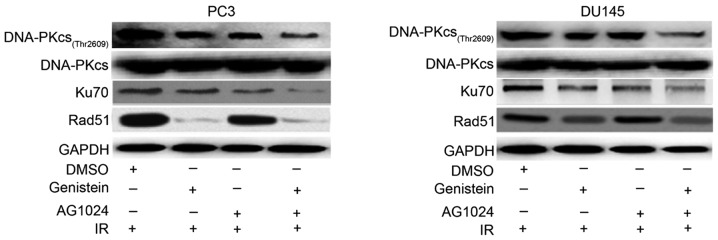
Using western blot analysis, we detected the decreased expression of NHEJ-related DNA repair proteins DNA-PKcs (Thr2609) and Ku70 in PCa cells pretreated with genistein (30 µM), AG1024 (10 µM) and their combination as compared with the control (Fig. 5). PCa cells pretreated with the combination of genistein and AG1024 before X-irradiation showed the lowest expression levels of nuclear DNA-PKcs and Ku70, followed by PCa cells pretreated with either genistein (30 µM) or AG1024 (10 µM). However, the expression of nuclear Rad51, an enzymatic component of HRR, was decreased by genistein alone and in combination with AG1024, but not AG1024 alone. These results revealed that genistein and the combination of genistein and AG1024 enhanced the radiosensitivity of PCa cells via the inhibition of both HRR and NHEJ pathways, while AG1024 promoted the radiosensitivity of PCa cells only by inactivating the NHEJ pathway.
Figure 5.
Expression of DNA repair-associated proteins. Expression of NHEJ-related DNA repair proteins DNA-PKcs (Thr2609), Ku70 and HRR-related protein Rad51 in PCa cells. Three proteins were nuclear proteins. DNA-PKcs (Thr2609) and Ku70 were inhibited by treatment of genistein (30 µM), AG1024 (10 µM), and their combination, whereas Rad51 was only influenced by genistein (30 µM). PCa, prostate cancer; IR, X-irradiation.
Genistein and/or AG1024 enhance cancer radiotherapy in a preclinical model of PCa tumors
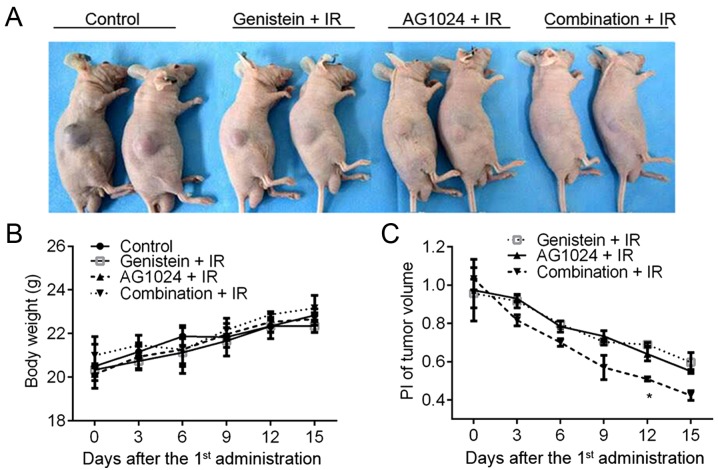
The in vitro cellular experiments demonstrated the single treatment with either genistein (30 µM) or AG1024 (10 µM) and the combination treatment radio-sensitized PCa cells to X-irradiation. To investigate the in vivo effect of genistein and AG1024 on cancer radiotherapy, we constructed the in vivo subcutaneous tumor model using DU145 cells. Before X-irradiation, mice were orally intubated with DMSO, genistein (100 mg/kg/day), AG1024 (100 mg/kg/day), and the combination of genistein and AG1024. The body weight of the mouse model increased from 18–20 g at the onset (6–7 weeks of age) to 20–24 g at the end of this study (11–12 weeks of age). At the day 15 post the 1st administration of genistein and/or AG1024, the tumor volume ranged from 1,697.20 mm3 in control mice to 252.01 mm3 in mice treated with the combination of genistein and AG1024 (Fig. 6A). No difference was observed in body weight between the groups (Fig. 6B). As compared with the control mice (DMSO + IR), significantly decreased PI of tumor volume was observed in mice treated with genistein, AG1024, and the combination (P<0.05; Fig. 6C). Moreover, at day 12 post the 1st administration of the agent, the PI of the tumor volume in mice treated with the combination of genistein and AG1024 was significantly lower than those in the mice treated with either genistein or AG1024 (P<0.05). The in vivo experiments showed that the treatment with either genistein or AG1024 radiosensitized PCa tumors, and the combination of them showed a synergistic effect on inhibiting tumor growth and sensitizing PCa to radiotherapy in vivo.
Figure 6.
In vivo effect of genistein and AG1024 on PCa cells. Animals received 100 mg/kg/day genistein, 100 mg/kg/day AG1024, or their combination thm (genistein + AG1024) before X-irradiation for 15 days. (A) The image of xenograft tumor-bearing mice at day 15 post the 1st time of medicine treatment. (B) Body weight of the experimental animals. (C) Tumor volume (V, mm3) change during treatment time was indicated by the proliferation index (PI, PI=Vtreatment/Vcontrol). Significant decrease in PI was observed in mice treated with the combination treatment at day 12 post treatment, compared with those in the other groups. *P<0.05 vs. genistein or AG1024. PCa, prostate cancer; IR, X-irradiation.
Discussion
The present study investigated whether the combination of genistein and AG1024 could enhance the radiosensitivity of PCa cells to X-irradiation. The data confirmed that the single and combination of genistein and AG1024 radiosensitized PCa cells to X-irradiation by arresting the cell cycle, promoting cell apoptosis, and inhibiting DNA repair via the inactivation of the NHEJ pathway and/or HRR pathway.
Radiotherapy to cancer cells induces cell damage by causing genomic DNA damage overloading DNA repair capacity, which finally results into mitotic catastrophe in tumor cells (5). Cell cycle arrest, apoptosis and autophagy are typical impairments induced by radiotherapy (6–8). Both genistein and AG1024 regulate DNA damage and enhance the radiosensitivity of tumor cells (5,17,28). Genistein has been proven to induce DNA damage, apoptosis, and cell cycle arrest at the G2/M phase via the ATM-p53-dependent pathway (Fig. 7), p38 MAPK activation, NF-κB and other pathways (12,27–29). In addition, genistein inhibited IGF-1 signaling pathway and inactivated Akt signaling pathways (30). In addition, genistein has been reported to arrest cell cycle at the G0/G1 phase in MCF-7, HB4a and BG-1 cells (31,32). Tyrphostin AG1024 is an inhibitor of IGF1R. The inhibition of IGF1R enhances tumor cell radiosensitivity (13). In this study, we confirmed that the pretreatment of genistein and AG1024 before X-irradiation promoted DNA damage and cell apoptosis in PC3 and DU145 cells, thus, enhancing the radiosensitivity of PCa cells to X-irradiation.
Figure 7.
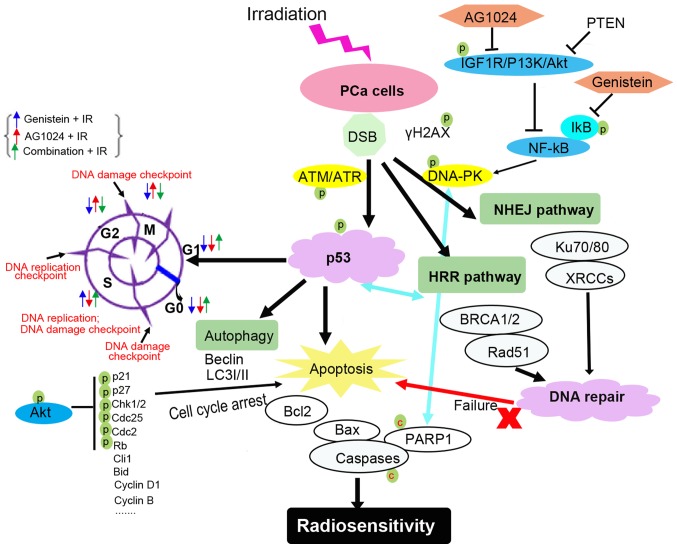
Schematic depiction of genistein and AG1024-mediated DNA-repair pathways related to radiosensitivity of PCa cells. The IGF1R/P13K/Akt and NF-κB pathways are associated with the AG1024 and genistein-mediated inhibition of DNA repair pathways, respectively. AG1024 and genistein promote cell apoptosis via ATM/ATR/p53-dependent downstream pathways or factors. AG1024 and genistein arrests cell cycle arrest at G2/M phase and S phase, respectively. The DNA repair capacity is controlled by HRR and NHEJ pathways in PCa cells. The failures of DNA repair and cell cycle arrest result in enhancement of cell apoptosis, thus, enhancing the radiosensitivity of tumor cells. PCa, prostate cancer; HRR, homologous recombination; NHEJ, non-homologous end joining.
The cell cycle is regulated by check point proteins, including G0/G1 phase checkpoints, S phase checkpoints and G2/M phase checkpoints. These checkpoints are responsible for the genomic instability of tumor cells and are responsive to irradiation-induced DNA damage (14,33). These checkpoints control cell cycle arrest and the radiosensitivity of tumor cells (Fig. 7). Under irradiation-mediated genotoxic stress, increased DNA-DSB challenges the stability of genomic DNA and the DNA repair capacity determines the cell survival and the radiosensitivity of cancer cells (5). Under irradiation, DNA damage is sensed by DNA-PK/ATM and ATM-related kinase (ATR)-mediated signaling. The activation of this signaling promotes p53 phosphorylation and activates p53-mediated responses (34,35). The DNA damage checkpoints, including G2/M phase, S phase and G0-S transition checkpoints, are sensitive to and responsible for irradiation-mediated DNA damage (36). The p53-dependent p21 phosphorylation promoted cell cycle arrest at the G1 phase by inhibiting cyclin D1, cyclin E and CDK2 (32,37), while G2/M cell cycle arrest was induced by decreased cyclin B and Chk2/cell division cycle 25C (Cdc25C)/Cdc2, which was negatively regulated by p53-dependent GADD45 expression (8,9,27,8). Genistein and AG1024-induced G2/M cell cycle arrest has been reported in human colon cancer cells and breast cancer cells (28,29,40), whereas genistein and AG1024-induced G1 cell cycle arrest has also been confirmed in BG-1 ovarian cancer and DU145 PCa cells (18,32). These results demonstrated that genistein or AG1024-mediated cell arrest is dependent on cell types or other unknown conditions. Our current data demonstrated that genistein induced PCa cell cycle arrest at the S phase, and AG1024 induced PCa cell cycle arrest at the G2/M phase. In addition, the combination of genistein and AG1024 arrested the cell cycle at the S phase, showing a synergistic effect on cell cycle distribution with a genistein tendency.
In tumor cells, the NHEJ and HRR pathways enhance DNA repair capacity and modulate cell sensitivity and resistance to radiotherapy (5). The inhibition of IGF1R enhances radiosensitivity and delays DSB repair by inactivating NHEJ and HRR pathways (13). Previous reports have shown that the expression of the Ku70/80 gene and DNA-PKcs activity did not correlate with DSB repair capacity and cellular radiosensitivity in normal human fibroblasts (41). In tumor cells, the NHEJ pathway is mediated by DNA-PK-dependent phosphorylation of Ku70/80 and the HRR pathway is mediated by BRCA1/Rad51 expression (4,41). In comparison with normal tumor cells, increased NHEJ and HRR pathways were detected in radio-resistant PCa cells (4). On contrast, Chang et al indicated that the combination of P13K/mTOR inhibitor and 6-Gy radiation inhibited both NHEJ and HRR pathways, thus, radiosensitizing PCa cells (4). It was reported that genistein radiosensitized breast cancer cells via inactivating the HRR repair pathway by inhibiting Rad51 expression (28). IGF1R inhibitor AZ12253801 radiosensitized PCa cells through inactivating NHEJ repair pathway (13). Our data showed that genistein radiosensitized PCa cells via inhibiting both NHEJ and HRR pathways, and AG1024 enhanced the radiosensitivity of PCa cells to X-irradiation via the inhibition of the NHEJ pathway. Additionally, the combination of AG1024 and genistein showed a synergistic effect on the inhibition of NHEJ and HRR pathways and the radiosensitivity of PCa cells to X-irradiation, showing the therapeutic potential of the combination treatment with AG1024 and genistein for PCa.
In summary, we confirmed that both AG1024 and genistein radiosensitized PCa cells by arresting the cell cycle at the G2/M and S phase, respectively. In addition, the genistein-induced radiosensitivity of PCa cells was mediated by the inhibition of both HRR and NHEJ repair pathways, while AG1024 induced radiosensitivity of PCa cells only through the inhibition of the NHEJ pathway. Both in vitro and in vivo experiments indicated that the combination of AG1024 and genistein synergistically enhanced the radiosensitivity of PCa cells. Even so, we are still not entirely clear concerning the crosstalk between AG1024 and genistein. The mechanisms and differences in cell cycle arrest and DNA repair pathways between AG1024 and genistein were not explored in the present study. We hypothesize that the investigation of cyclin expression may be helpful for uncovering the differences in genistein and AG1024-mediated cell cycle arrest and the radiosensitivity of PCa cells. More experiments should be performed before the clinical therapy of PCa by using the combination of AG1024 and genistein.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from the National Natural Science Foundation of China (grant no. 81672535) and the National Natural Science Foundation of Shaanxi Province (grant no. 2016JM8145).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
QT and JM conceived and designed the study. QT, JM, JS, LY, FY, WZ, RL and LW performed the experiments. QT and JM analysed the data. QT and JM wrote the manuscript. YW and HW reviewed and checked the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The animal experiment protocols were approved by the Ethics Committee of the Fourth Military Medical University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cojoc M, Peitzsch C, Kurth I, Trautmann F, Kunz-Schughart LA, Telegeev GD, Stakhovsky EA, Walker JR, Simin K, Lyle S, et al. Aldehyde dehydrogenase is regulated by β-catenin/TCF and promotes radioresistance in prostate cancer progenitor cells. Cancer Res. 2015;75:1482–1494. doi: 10.1158/0008-5472.CAN-14-1924. [DOI] [PubMed] [Google Scholar]
- 2.Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol. 2014;90:615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimura T, Noma N, Sano Y, Ochiai Y, Oikawa T, Fukumoto M, Kunugita N. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol. 2014;112:302–307. doi: 10.1016/j.radonc.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Yuan JL, Zhang YT, Ma JJ, Xu P, Shi CH, Zhang W, Li YM, Fu Q, Zhu GF, et al. Inhibition of both EGFR and IGF1R sensitized prostate cancer cells to radiation by synergistic suppression of DNA homologous recombination repair. PLoS One. 2013;8:e68784. doi: 10.1371/journal.pone.0068784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang KB, Zhu C, Yong SK, Gao Q, Wong MC. Enhanced sensitivity of celecoxib in human glioblastoma cells: Induction of DNA damage leading to p53-dependent G1 cell cycle arrest and autophagy. Mol Cancer. 2009;8:66. doi: 10.1186/1476-4598-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–1410. [PubMed] [Google Scholar]
- 8.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Wu LJ, Tashino S, Onodera S, Ikejima T. Protein tyrosine kinase pathway-derived ROS/NO productions contribute to G2/M cell cycle arrest in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res. 2010;44:792–802. doi: 10.3109/10715762.2010.481302. [DOI] [PubMed] [Google Scholar]
- 10.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 11.Su H, Jin X, Shen L, Fang Y, Fei Z, Zhang X, Xie C, Chen X. Inhibition of cyclin D1 enhances sensitivity to radiotherapy and reverses epithelial to mesenchymal transition for esophageal cancer cells. Tumor Biol. 2016;37:5355–5363. doi: 10.1007/s13277-015-4393-z. [DOI] [PubMed] [Google Scholar]
- 12.Cui S, Wienhoefer N, Bilitewski U. Genistein induces morphology change and G2/M cell cycle arrest by inducing p38 MAPK activation in macrophages. Int Immunopharmacol. 2014;18:142–150. doi: 10.1016/j.intimp.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Chitnis MM, Lodhia KA, Aleksic T, Gao S, Protheroe AS, Macaulay VM. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene. 2014;33:5262–5273. doi: 10.1038/onc.2013.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992;71:543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- 15.Stegeman H, Kaanders JH, van der Kogel AJ, Iida M, Wheeler DL, Span PN, Bussink J. Predictive value of hypoxia, proliferation and tyrosine kinase receptors for EGFR-inhibition and radiotherapy sensitivity in head and neck cancer models. Radiother Oncol. 2013;106:383–389. doi: 10.1016/j.radonc.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochester MA, Riedemann J, Hellawell GO, Brewster SF, Macaulay VM. Silencing of the IGF1R gene enhances sensitivity to DNA-damaging agents in both PTEN wild-type and mutant human prostate cancer. Cancer Gene Ther. 2005;12:90–100. doi: 10.1038/sj.cgt.7700775. [DOI] [PubMed] [Google Scholar]
- 17.Wen B, Deutsch E, Marangoni E, Frascona V, Maggiorella L, Abdulkarim B, Chavaudra N, Bourhis J. Tyrphostin AG 1024 modulates radiosensitivity in human breast cancer cells. Br J Cancer. 2001;85:2017–2021. doi: 10.1054/bjoc.2001.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisielewska J, Ligeza J, Klein A. The effect of tyrosine kinase inhibitors, tyrphostins: AG1024 and SU1498, on autocrine growth of prostate cancer cells (DU145) Folia Histochem Cytobiol. 2008;46:185–191. doi: 10.2478/v10042-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 19.Shukla V, Chandra V, Sankhwar P, Popli P, Kaushal JB, Sirohi VK, Dwivedi A. Phytoestrogen genistein inhibits EGFR/PI3K/NF-κB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Advances. 2015;5:56075–56085. doi: 10.1039/C5RA06167A. [DOI] [Google Scholar]
- 20.Hari S, Vasudevan V, Kasibhotla S, Reddy D, Venkatappa M, Devaiah D. Anti-inflammatory dietary supplements in the chemoprevention of oral cancer. Cancer Res Front. 2016;2:380–395. doi: 10.17980/2016.380. [DOI] [Google Scholar]
- 21.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 22.Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-W. [DOI] [PubMed] [Google Scholar]
- 23.Markovits J, Linassier C, Fossé P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 24.Liu XX, Sun C, Jin XD, Li P, Zheng XG, Zhao T, Li Q. Genistein sensitizes sarcoma cells in vitro and in vivo by enhancing apoptosis and by inhibiting DSB repair pathways. J Radiat Res. 2016;57:227–237. doi: 10.1093/jrr/rrv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marampon F, Gravina G, Ju X, Vetuschi A, Sferra R, Casimiro M, Pompili S, Festuccia C, Colapietro A, Gaudio E, et al. Cyclin D1 silencing suppresses tumorigenicity, impairs DNA double strand break repair and thus radiosensitizes androgen-independent prostate cancer cells to DNA damage. Oncotarget. 2016;7:5383–5400. doi: 10.18632/oncotarget.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Majumder S, McCall W, Sartor CI, Mohler JL, Gregory CW, Earp HS, Whang YE. Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res. 2005;65:3404–3409. doi: 10.1158/0008-5472.CAN-04-4292. [DOI] [PubMed] [Google Scholar]
- 27.Fang Y, Zhang Q, Wang X, Yang X, Wang X, Huang Z, Jiao Y, Wang J. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int J Oncol. 2016;48:1016–1028. doi: 10.3892/ijo.2016.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Sun C, Jin X, Li P, Ye F, Zhao T, Gong L, Li Q. Genistein enhances the radiosensitivity of breast cancer cells via G2/M cell cycle arrest and apoptosis. Molecules. 2013;18:13200–13217. doi: 10.3390/molecules181113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC, Du W, Yuan CS. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289–296. doi: 10.3892/ijo.2013.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Ju J, Park S, Hong SJ, Yoon S. Inhibition of IGF-1 signaling by genistein: Modulation of E-cadherin expression and downregulation of β-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr Cancer. 2012;64:153–162. doi: 10.1080/01635581.2012.630161. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboy MS, Marcarini JC, de Souza AO, de Paula NA, Dorta DJ, Mantovani MS, Ribeiro LR. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J Med Food. 2014;17:218–225. doi: 10.1089/jmf.2013.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang KA, Kang NH, Yi BR, Lee HR, Park MA, Choi KC. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17β-estradiol or bisphenol A via the inhibition of cell cycle progression. Int J Oncol. 2013;42:733–740. doi: 10.3892/ijo.2012.1719. [DOI] [PubMed] [Google Scholar]
- 33.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5:a012716. doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, Tokhunts R, Koldobskiy MA, Fu C, Barrow R, et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol Cell. 2014;54:119–132. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elledge SJ. Cell cycle checkpoints: Preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 37.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JJ, Lee JH, Gu MJ, Han JH, Cho WK, Ma JY. Agastache rugosa Kuntze extract, containing the active component rosmarinic acid, prevents atherosclerosis through up-regulation of the cyclin-dependent kinase inhibitors p21 WAF1/CIP1 and p27 KIP1. J Functional Food. 2017;30:30–38. doi: 10.1016/j.jff.2016.12.025. [DOI] [Google Scholar]
- 39.Oki T, Sowa Y, Hirose T, Takagaki N, Horinaka M, Nakanishi R, Yasuda C, Yoshida T, Kanazawa M, Satomi Y, et al. Genistein induces Gadd45 gene and G2/M cell cycle arrest in the DU145 human prostate cancer cell line. FEBS Lett. 2004;577:55–59. doi: 10.1016/j.febslet.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Veldwijk MR, Zhang Q, Li ZB, Xu WC, Fu S. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer. 2013;13:297. doi: 10.1186/1471-2407-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasten U, Plottner N, Johansen J, Overgaard J, Dikomey E. Ku70/80 gene expression and DNA-dependent protein kinase (DNA-PK) activity do not correlate with double-strand break (dsb) repair capacity and cellular radiosensitivity in normal human fibroblasts. Br J Cancer. 1999;79:1037–1041. doi: 10.1038/sj.bjc.6690166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.