Abstract
Brain-derived neurotrophic factor (BDNF) is known as one of the members of the neurotropin family. BDNF-induced activation of its receptor tyrosine kinase B (TrkB) is associated with anoikis tolerance, tumor progression and poor prognosis in many types of malignancy. However, to the best of our knowledge, there are no reports describing the contribution of the BDNF/TrkB axis to cervical cancer. BDNF and TrKB expression in cervical cancer (CC) tissues and adjacent normal tissues from 87 patients were analyzed by immunohistochemistry, western blot analysis and quantitative PCR assays and the results showed that they were significantly higher in cancer tissues than that in normal adjacent tissues, respectively. Higher expression rates of BDNF and TrKB were observed in stage IIB or higher and BDNF expression was positively associated with lymph node metastasis. Notably, a high expression of TrKB may be contributed to poor survival time, which confirmed by Kaplan-Meier analysis. Compared to the corresponding CC cell lines, HeLa, SiHa, CASKI, C4-1 and C-33a, BDNF and TrKB expression was enhanced in anoikis-like apoptotic tolerance (AAT), a cell model established from cervical cancer cell lines. AAT cells showed a higher proliferation activity compared with CC cell lines, which was confirmed by a shorter G0/G1 phase, elevated cyclin A, cyclin D1 and c-myc, decreased caspase-3 and Bax, and increased Bcl-2. By contrast, the knockdown of TrKB expression reversed these changes in AAT cells, induced G0/G1 arrest and suppressed proliferation activity. The results of the present study show that PI3K/Akt signaling is involved in the BDNF/TrKB-induced proliferation of AAT cells in cervical cancer. These findings indicate that BDNF/TrKB pathway is a potential target for the treatment of cervical cancer.
Keywords: BDNF, TrkB, anoikis, PI3K/Akt, cervical cancer
Introduction
Statistics from the World Health Organization show that cervical cancer is the second most common malignancy globally in women after breast cancer (1). Cervical cancer is becoming increasingly more prevalent in younger women (2). For the histopathological type, cervical squamous cell carcinoma accounted for more than 80% of cases. In addition, the incidence of cervical and adenosquamous carcinoma has been on the increase (3). Early detection and good treatment can improve patient survival and prognosis (4). Tumor metastasis, a multi-stage cascade process, is an important part of the early stage of tumor metastasis, which allows tumor cells to migrate, and to break through the basement membrane into the blood and/or lymphatic vessels (5). Epithelial-mesenchymal transformation (EMT) refers to the transformation of epithelial cells into mesenchymal cells under specific conditions (6). When EMT occurs, the polarity of the epithelial cells is lost, and their contact with the surrounding cells and extracellular matrix is reduced, accompanied by enhanced cell migration and exercise capacity, as well as gradual loss of cell epithelial phenotype (6,7). These processes are closely associated with tumor metastasis and growth. Normal epithelial cells are adhesion-dependent, and their survival depends on the signal transduction between the cells and extracellular matrix, known as anchorage-dependent growth (8). Once these cells are separated from the extracellular matrix and lose the link between them, a programmed cell death commences, known as anoikis (9). Anoikis is a physiological barrier to metastases, and the ability of cells to obtain anoikis-like apoptotic cell death resistance is a prerequisite for tumor proliferation, metastasis and chemotherapy resistance (9,10).
Receptor tyrosine kinase B (TrkB) is a transmembrane protein that is a specific receptor for brain-derived neurotrophic factor (BDNF) and belongs to the neurotrophic factor receptor Trk family, which consists of extracellular glycosylated polypeptides, transmembrane region and cytoplasmic tyrosine kinase domain (11,12). TrkB can be activated by BNDF or neurotrophic factor (NT) −4/5 (13). BDNF, a member of the neurotrophin family, is associated with the development and regeneration of neurons and can also bind to its major receptor TrkB, resulting in the activation of downstream signal pathways (14). Activated TrKB plays an important role in the development and maturation of the nervous system (15). However, there is mounting evidence that TrKB plays an important role in promoting tumor formation and metastasis in some malignancies (15). Our previous findings showed that the upregulation of the BDNF/TrKB pathway promotes the epithelial-mesenchymal transition, as well as the migration and invasion of cervical cancer (16). Other findings have shown that the overexpression of TrKB can promote the proliferation and migration of cells by inhibiting anoikis, promoting growth and metastasis in several types of cancer cells (17,18). TrkB can bind to its ligand BDNF to induce multiple signal cascades, including the PI3K/AKT pathways (19). Moreover, the activated PI3K/AKT pathway by TrkB/BDNF can block the activation of caspase-3, thereby inducing anoikis tolerance (20). In addition, normal epithelial cells did not express or lowly expressed BDNF and TrkB, while the expression of BDNF and TrkB in various cancer cells was significantly enhanced (21,22). However, whether the TrkB/BDNF signaling pathway activates, as well as how to activate the downstream pathway and mediate cervical cancer cell anoikis tolerance remains to be clarified. Therefore, examinig the TrkB/BDNF pathway can be useful in determining the molecular mechanism of cervical cancer metastasis to develop the corresponding drugs and improve the survival rate of patients.
Materials and methods
Patients
Cervical cancer specimens of 87 patients were collected from the Affiliated Hospital of Southwest Medical University between March 2009 and December 2016. The present study was approved by the hospital institutional review board of the Affiliated Hospital of Southwest Medical University and informed consent was obtained from all the patients. There were 68 cases of squamous cell carcinoma, and 19 cases of adenocarcinoma. All the patients underwent total hysterectomy or radical mastectomy without preoperative radiotherapy and chemotherapy. Survival was defined as the interval between the date of surgery and the date of death or the last follow-up. Cervical cancer was staged according to FIGO staging (2009) (23): 67 cases were less than or equal to IIA, and 20 cases were more than IIB. Lymph node metastasis was identified in 21 cases, but no lymph node metastasis was found in 66 cases.
Immunohistochemical (IHC) staining
The samples from cancer and adjacent tissues were collected and fixed in 10% formalin. Formalin-fixed samples of tumors were routinely trimmed, processed, paraffin-embedded, sectioned and stained with hematoxylin and eosin. Sections were deparaffinized and rehydrated according to routine protocol. Sections were pre-incubated with 3% normal horse serum in PBS for 1 h at room temperature, and then incubated with primary antibodies (BDNF monoclonal antibody, 1:100 dilution, cat. no. ab108319; TrKB polyclonal antibody, 1:100 dilution, cat. no. ab18987; Abcam, Cambridge, UK) at 4°C overnight. The sections were incubated at room temperature for 30 min with goat anti-Rat IgG H&L (HRP, polyclonal antibody; cat. no. A10211; GenScript Biotech Corp., Piscataway, NJ, USA). Sections for staining scores were evaluated by combination of staining intensity and the percentage of positive staining. Scores of 0, 1+, and 2+ were considered a negative expression of BDNF and TrKB, while scores of 3+ were considered positive for the expression of BDNF and TrKB.
Cell culture and establishment of model of anoikis-like apoptotic tolerance (AAT)
The human cervical cancer cell lines HeLa, SiHa, CASKI, C4-1 and C-33a and the human papillomavirus immortalized ectocervical (Ect1/E6E7) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). They were grown in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics at 37°C under a humidified incubator with 5% CO2 and 95% air. For the establishment of the model of anoikis-like apoptotic tolerance, the logarithmic growth phase cells were digested and centrifuged, and then resuspended with fresh medium. The cell suspension was transferred to 6-well ULC plates at 5×105 cells per well, and the culture medium was replaced every 3 days. After suspension culture for 7 days, the cells were collected in a centrifuge tube and centrifuged at 500 × g for 5 min. The supernatant was discarded, and trypsin was added to digest cells into a single cell suspension. The cells were then resuspended with fresh culture medium, and transferred into 6-well routine plates to continue culture. After the cells were expanded, they were transferred to 6-well ULC plates again and the culture was continued as described above for 7 days, and then transferred to a 6-well routine plate to continue culture. Finally, surviving cells were used for the following determination and experiments.
Silencing TrKB by siRNA
Exponentially growing cells collected from normal and model cells of anoikis-like apoptotic tolerance were seeded in 6-well plates at 1×105 per well and cultured at 37°C for 24 h. Recombinant adenoviruses encoding negative control siRNA and TrkB siRNA were designed and constructed by Shanghai Jike Gene Chemical Technology Co., Ltd. (Shanghai, China). After cells were grown to 70% confluence, the model cells were divided into three groups, and treated with PBS (model group without treatment), adenoviruses encoding negative control siRNA (siNC group) and TrkB siRNA (siTrKB). Normal cells were treated with PBS and used as a control group. After transfection for 48 h, transfection efficiency was assessed using western blot and RT-PCR assays.
Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to determine cell proliferation. Cell viability was measured according to the manufacturer's protocol. In brief, the cells from control, model, siTrKB and negative siRNA were grown in a 96-well plate for 24 h. Then, 1 mg/ml of MTT (0.5 mg/l) per well was added into the cells and incubated at 37°C for 4 h. Absorbance at 490 nm was measured using a Bio-Rad microplate reader (Bio-Rad Laboratories, Richmond, CA, USA). Cell viability was expressed as a percentage of the average OD value compared to the control.
Cell apoptosis assay
Cells collected from control, model, siTrKB and negative siRNA were washed with PBS and harvested after centrifugation at 1,000 × g for 5 min. After being washed with PBS, the cells were added into diluted buffer (500 µl). FITC-labeled Annexin V and 5 µl PI (Biodesign International, Kennebunk, ME, USA) then were added, respectively. After incubation for 10 min at room temperature, apoptosis was determined by a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA).
Cell cycle determination by flow cytometry
Cells collected from control, model, siTrKB and negative siRNA were washed with PBS and harvested after centrifugation at 1,000 × g for 5 min. The cells were then adjusted at 1×106, resuspended, and fixed with 70% ethanol. The cells were then washed with PBS and incubated with 100 µl RNase A (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C. Subsequently, cell solution was stained using 400 µl PI (Sigma-Aldrich) for 30 min at 4°C. DNA content was determined on a flow cytometer.
Western blot analysis
Cells harvested from control, model, siTrKB and negative siRNA were washed with ice-cold PBS and incubated on ice in lysis buffer (100 µM Tris, 150 mM NaCl, 1% Triton X-100) for 30 min. The protein expression levels were determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Jiangsu, China) according to the manufacturer's protocol. Proteins in cells were separated by sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) (Pharmacia, Piscataway, NJ, USA) electrophoresis, and then transferred to polyvinylidene difluoride membranes ((Bio-Rad). After blocking with PBS containing 5% non-fat dry milk, the membranes were incubated with primary antibodies (BDNF monoclonal antibody, 1:1,000 dilution; cat. no. ab108319; TrKB polyclonal antibody, 1:1,000 dilution; cat. no. ab18987; cyclin A1 polyclonal antibody, 1:1,000 dilution; cat. no. ab53699; cyclin D1 monoclonal antibody, 1:5,000 dilution; cat. no. ab134175; c-Myc, monoclonal antibody, 1:5,000 dilution; cat. no. ab32072; caspase-3, polyclonal antibody, 1:500 dilution; cat. no. ab13847; Bax, monoclonal antibody, 1:1,000 dilution; cat. no. ab32503; Bcl-2, monoclonal antibody, 1:1,000 dilution; cat. no. ab32124; PI3K, monoclonal antibody, 1:1,000 dilution; cat. no. ab40776; p-PI3K, monoclonal antibody, 1:1,000 dilution; cat. no. ab125633; Akt, polyclonal antibody; 1:1,000 dilution, cat. no. ab8805; p-Akt, polyclonal antibody, 1:5,000 dilution; cat no. ab38449; all from Abcam) at 4°C overnight. After being washed with PBS, the membranes were incubated with HRP-conjugated polyclonal secondary antibodies (cat. no. P6782; Sigma-Aldrich) for 1 h. The detection of chemiluminescence was conducted with an enhanced chemiluminescence (ECL) western blotting detection system (Amersham Biosciences, Piscataway, NJ, USA).
Real-time RT-PCR analysis
Total RNA was isolated from the cells using a Qiagen RNeasy Mini kit according to the manufacturer's protocols. Total RNA (100 µg) from all the groups was reverse-transcribed into cDNA using TaqMan Reverse Transcription Reagent kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed using TaqMan Gene Expression Assays and the TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's protocol. Amplification and detection of mRNA were performed using a 7500 fast Real-Time PCR System (Applied Biosystems). Data were calculated using the 2−∆∆Cq method and normalized to control. PCR primers used in the study were: TrKB, 5′-CTGGCCTGGAATTGACGATG-3′ (forward) and 5′-ACCACAGCATAGACCGAGAG-3′ (reverse); BDNF, 5′-TGCGGGAGGAATTTCTGAGT-3′ (forward) and 5′-GCACTTAAAGCACGAGGTCC-3′ (reverse); cyclin A, 5′-ACAGAGGTTGGGAGTGGAAG-3′ (forward) and 5′-TCCATTCTGAGAACCCTGGG-3′ (reverse); cyclin D1, 5′-TTTGTTGTGTGTGCAGGGAG-3′ (forward) and 5′-TTTCTTCTTGACTGGCACGC-3′ (reverse); c-myc, 5′-ATTCTCTGCTCTCCTCGACG-3′ (forward) and 5′-CTGTGAGGAGGTTTGCTGTG-3′ (reverse). Caspase-3, 5′-AAAATACCAGTGGAGGCCGA-3′ (forward) and 5′-ATTCTGTTGCCACCTTTCGG-3′ (reverse). Bax, 5′-AAGAAGCTGAGCGAGTGTCT-3′ (forward) and 5′-GTTCTGATCAGTTCCGGCAC-3′ (reverse). GAPDH, 5′-GAGTAAGACCCCTGGACCAC-3′ (forward) and 5′-AACTGGTTGAGCACAGGGTA-3′ (reverse).
Statistical analysis
Data were expressed as means ± standard deviation according to at least three independent experiments. Independent Student's t-test and one-way ANOVA were used to evaluate the statistical comparison of two and multiple groups, respectively. Tukey's post hoc test was used after one-way ANOVA. Statistical analysis between cancer tissues and corresponding normal tissues was performed by McNemar test. The Kaplan-Meier analysis was used to determine overall survival time of patients between groups. Statistically significant differences were defined as P<0.05. Data statistics were performed using SPSS software (SPSS version 18; SPSS, Inc., Chicago, IL, USA).
Results
Correlation between clinicopathological parameters and expression of BNDF or TrKB
To examine the role of BNDF and TRKB in cervical cancer tissues, an immunohistochemistry assay was used to evaluate the expression levels of BNDF and TrKB in surgical specimens of human cervical cancer tissues. The representative stains are shown in Fig. 1 and the relationship between clinicopathological parameters and the expression of BDNF and TrKB in cancer tissues, are listed in Table I.
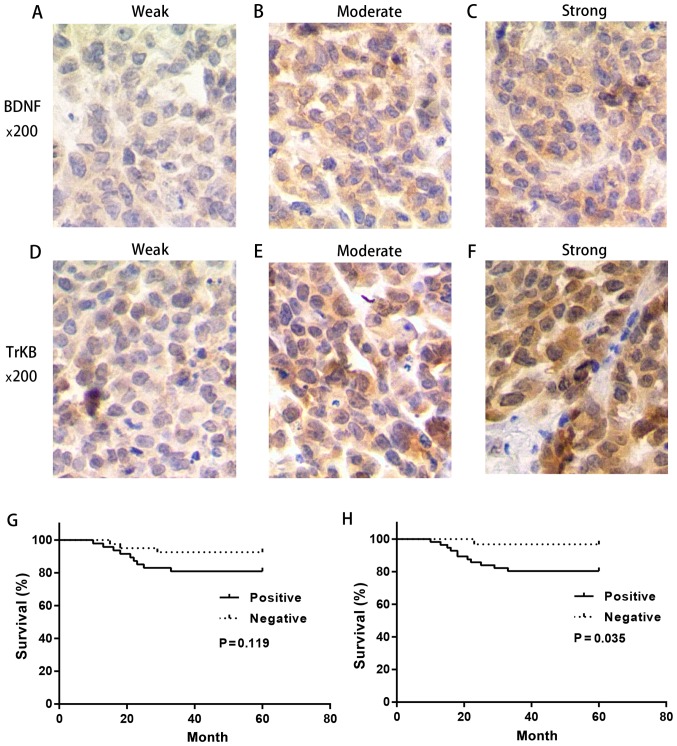
Figure 1.
Expression levels of BNDF and TrKB protein in cervical cancer tissues were detected by immunohistochemistry and negatively correlated with survival time. (A-C) Representative of weak, moderate and strong BNDF expression, respectively. (D-F) Representative of weak, moderate and strong TrKB expression, respectively. Overall survival time was prolonged in patients with a negative expression of (G) BDNF and (H) TrKB.
Table I.
Relationship between clinicopathological parameters and the expression of BDNF and TrKB.
| TrKB | BDNF | ||||
|---|---|---|---|---|---|
| Characteristics | No. | Positive (%) | P-value | Positive (%) | P-value |
| Age | |||||
| <50 | 56 | 35 (62.5) | 0.625 | 30 (53.6) | 0.910 |
| ≥50 | 31 | 21 (67.7) | 17 (54.8) | ||
| Histologic subtype | |||||
| Squamous cell carcinoma | 65 | 40 (61.5) | 0.344 | 32 (49.2) | 0.123 |
| Adenocarcinoma | 22 | 16 (72.7) | 15 (68.2) | ||
| FIGO stage | |||||
| ≤IIA | 67 | 39 (58.2) | 0.028 | 32 (47.8) | 0.032 |
| ≥IIB | 20 | 17 (85.0) | 15 (75) | ||
| Grade of differentiation | |||||
| Well | 35 | 20 (57.1) | 0.127 | 14 (40.0) | 0.065 |
| Moderate | 37 | 23 (62.2) | 22 (59.5) | ||
| Poor | 15 | 13 (86.7) | 11 (73.3) | ||
| LNM | |||||
| Positive | 21 | 18 (80.9) | 0.048 | 14 (66.7) | 0.182 |
| Negative | 66 | 38 (59.1) | 33 (50.0) | ||
No significant association between TrKB expression and age, histologic subtype and grade of differentiation was observed. However, there was a significant difference between the expression of TrKB and FIGO stage or lymph node metastasis (LNM). The expression level of TrKB was higher in patients with stage IIB or higher stage than that in patients with stage IIA or lower stage (P=0.028). In addition, a significantly higher rate of positive expression of TrKB was observed in positive LNM compared to that in negative LNM (P=0.048). These results suggested that TrKB was closely associated with poor clinicopathological parameters. There were no significant differences in positive BDNF expression among the subgroups of age, histologic subtype, grade of differentiation and LNM. However, a higher positive BDNF expression was observed in patients with stage IIB or higher stage and poor differentiation compared to patients with stage IIA or lower stage (P=0.032) and well differentiated (P=0.065).
High survival time and prolonged survival time were observed in the negative expression of BDNF and TrKB
We further determined the correlation between 5-year survival rate and clinicopathological parameters or the expression of BDNF and TrKB (Table II). The survival rate is closely associated with histologic subtype, grade of differentiation, LNM and TrKB expression. Patients with a positive BDNF expression had a survival rate of 80.8%, which was lower than that of patients with a negative BDNF expression, albeit this difference was not significant (P=0.116). We found that the survival rate for patients with a positive TrKB expression was significant lower than that for patients with a negative expression of TrKB (P=0.033).
Table II.
Relationship between clinicopathological parameters and the 5-year survival rate.
| Characteristics | No. | Survival no. (%) | P-value |
|---|---|---|---|
| Age | |||
| <50 | 56 | 48 (85.7) | 0.858 |
| ≥50 | 31 | 27 (87.1) | |
| Histologic subtype | |||
| Squamous cell carcinoma | 65 | 62 (95.4) | <0.001 |
| Adenocarcinoma | 22 | 13 (59.1) | |
| FIGO stage | |||
| ≤IIa | 67 | 60 (89.6) | 0.136 |
| ≥IIb | 20 | 15 (75.0) | |
| Grade of differentiation | |||
| Well | 35 | 34 (97.1) | 0.001 |
| Moderate | 37 | 33 (89.2) | |
| Poor | 15 | 8 (53.5) | |
| LNM | |||
| Positive | 21 | 11 (52.4) | <0.001 |
| Negative | 66 | 64 (96.9) | |
| BDNF | |||
| Positive | 47 | 38 (80.8) | 0.116 |
| Negative | 40 | 37 (92.5) | |
| TrKB | |||
| Positive | 56 | 45 (80.4) | 0.033 |
| Negative | 31 | 30 (96.8) | |
Then we performed the Kaplan-Meier analysis in different expression levels of BDNF and TrKB. It was evident that the survival time in patients with a negative BDNF and TrKB expression was prolonged compared with that in patients with a positive BDNF and TrKB expression, respectively (BDNF, P=0.119; TrKB, P=0.035) (Fig. 1). These results suggested that BDNF and TrKB were poor prognostic factors in cervical cancer.
Expression levels of BNDF and TrKB in cervical cancer tissues and cell lines
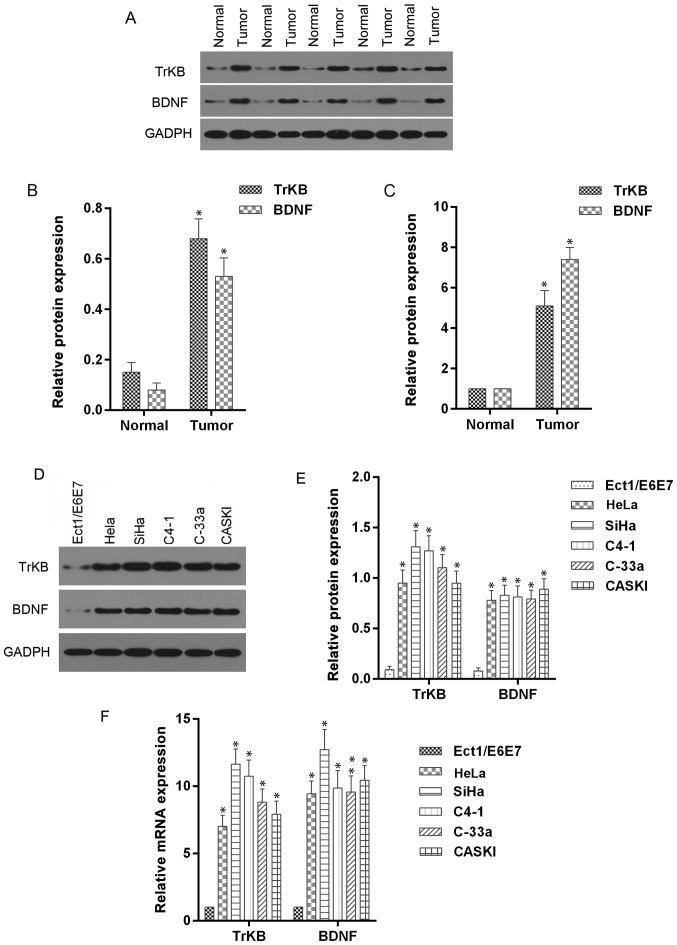
To further determine the roles of BDNF and TrKB in cervical cancer tissues and corresponding adjacent normal tissues, we determined the expression levels of BDNF and TrKB protein and mRNA. The McNemar test showed that the positive expression of BDNF and TrKB was more frequently observed in the cancer tissues compared to the normal tissues (Table III). Moreover, the protein and mRNA expression levels of BDNF and TrKB, analyzed by western blot and RT-qPCR assays, were higher in cancer tissues than those in normal tissues (Fig. 2). In addition, we determined the expression levels of BDNF and TrKB protein and mRNA in cervical cancer cell lines. As shown in Fig. 2, higher expression levels of BDNF and TrKB were observed in HeLa, SiHa, C4-1, C-33a and CASKI cells compared to those in normal Ect1/E6E7 cells. These results indicated that BDNF and TrKB expression were higher in cancer tissues and cells.
Table III.
Difference of BDNF and TrKB expression between cancer tissues and normal tissues.
| Bdnf expression | Trkb expression | ||||||
|---|---|---|---|---|---|---|---|
| Type of tissue | Case | Negative | Positive no. (%) | P-value | Negative | Positive no. (%) | P-value |
| Cancer | 87 | 40 | 47 (54.0) | <0.001 | 31 | 56 (64.4) | <0.001 |
| Normal | 87 | 75 | 12 (13.8) | 82 | 5 (5.7) | ||
Figure 2.
Overexpression of BDNF and TrKB was observed in both translational and transcriptional levels in cervical cancer tissues and cell lines compared with normal tissues and Ect1/E6E7 cells, respectively. Western blot and RT-qPCR assays were performed in five paired cancer tissues and normal tissue. (A and B) Western blot assay showed that BDNF and TrKB protein was higher in cervical cancer tissues than that in corresponding adjacent normal tissues. (C) Relative mRNA levels of BDNF and TrKB were higher than those in adjacent normal tissues. (D and E) The protein levels of BDNF and TrKB were evidently higher in cervical cancer cell lines than those in Ect1/E6E7 cells. (F) Relative mRNA levels of BDNF and TrKB were significantly higher in cervical cancer cell lines compared with those in Ect1/E6E7 cells. *P<0.05, cancer tissue vs. normal tissue or cancer cell lines vs. Ect1/E6E7 cells.
TrKB and BDNF expression is enhanced in model cells of anoikis-like apoptotic tolerance and decreased by TrKB siRNA treatment
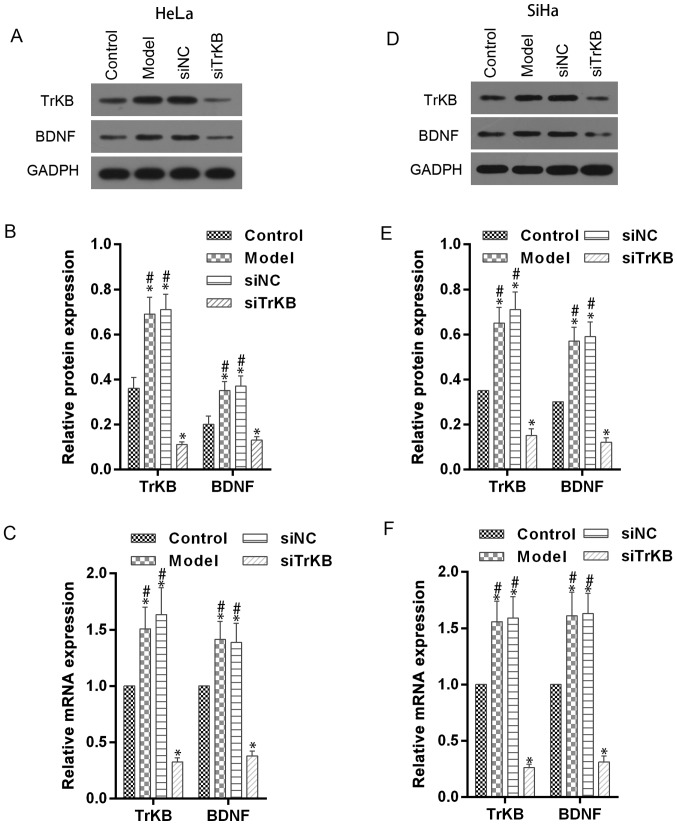
To evaluate the role of TrKB and BDNF in anoikis-like apoptotic tolerance (AAT), we established model cells of AAT from cancer cell lines HeLa and SiHa. We determined the expression of TrKB and BDNF using western blot and real-time RT-PCR assays in model cells HeLa and SiHa (Fig. 3). Compared with control cells, TrKB and BDNF expression was slightly increased in both HeLa and SiHa cells from the model group. We further silenced the expression of TrKB with siRNA. The expression of TrKB was reduced at the translational and transcriptional levels in TrKB siRNA-treated model cells HeLa and SiHa compared to cells from the model, siNC and control groups. In addition, the protein and mRNA expression level of BDNF was reduced after TrKB was silenced by TrKB siRNA.
Figure 3.
Western blot and RT-qPCR assays showed the downregulatory effect of TrKB siRNA on expression of BDNF and TrKB in AAT cells. Western blot and RT-qPCR assays were performed in HeLa and SiHa cells (control), AAT cells (model), AAT cells with negative TrKB treatment, and AAT cells with TrKB siRNA treatment. (A and B) For AAT cells derived from HeLa, protein levels of BDNF and TrKB were enhanced compared with corresponding HeLa cells, and attenuated in cells treated TrkB siRNA. (C) Relative mRNA levels of BDNF and TrKB were increased in AAT cells from model compared with corresponding HeLa cells, and decreased in AAT cells with TrKB siRNA treatment compared with AAT cells from model group. (D and E) Similarly, for AAT cells derived from SiHa, the protein levels of BDNF and TrKB were enhanced compared with corresponding SiHa cells, and attenuated in cells treated with TrkB siRNA. (F) Relative mRNA levels of BDNF and TrKB were increased in AAT cells (model group) compared with corresponding SiHa cells (control), and decreased in AAT cells with TrKB siRNA treatment compared with AAT cells (model group). *P<0.05, vs. control; #P<0.05, vs. siTrKB.
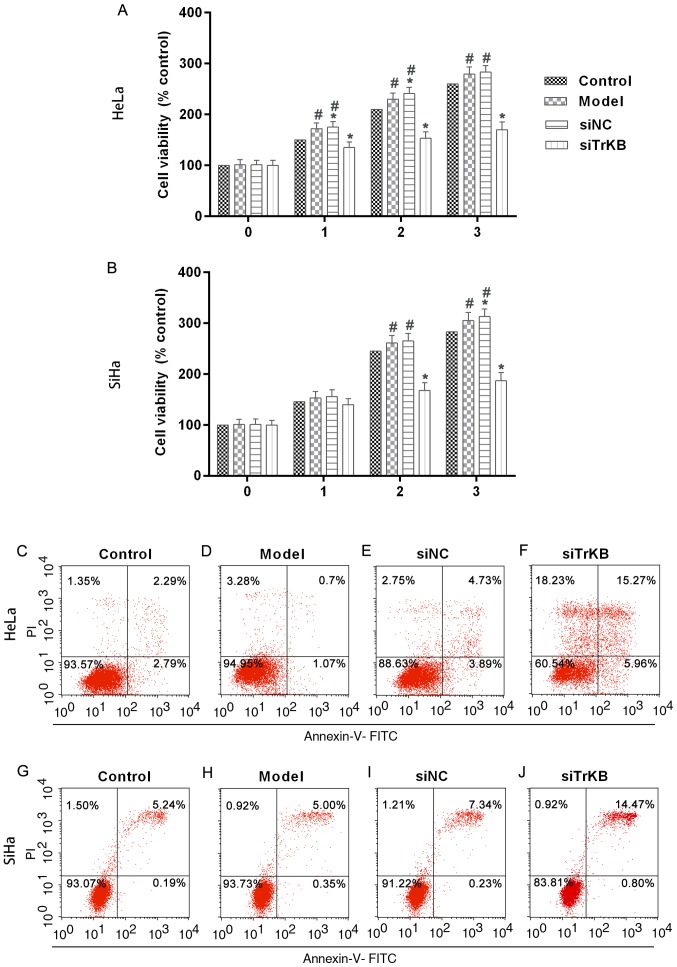
Proliferation is significantly inhibited and apoptosis is induced after treatment of cells with TrKB siRNA
To evaluate the function of TrKB in the proliferation of AAT cells, we determined cell viability by MTT assay after TrKB knockdown of cells (Fig. 4A and B). The results showed that cell proliferation was suppressed in cells treated with TrKB siRNA compared to that in cells of the control, model and siNC groups. After cells culture for 3 days, the cell proliferation was slightly increased for HeLa and SiHa cells in the model group compared to the control. However, silencing TrKB revealed an obvious inhibitory effect on the proliferation of AAT cervical cancer cell lines HeLa and SiHa compared to cells from the model group at day 3. These results suggested that AAT cells have a higher proliferation ability than HeLa and SiHa cells with no treatment. Moreover, TrKB plays an important role in promoting cell proliferation in HeLa and SiHa cervical cancer cell lines. We then determined the apoptosis of cells after silencing TrKB. The apoptosis of cells treated with TrKB siRNA was markedly increased compared to the control, model and siNC groups (Fig. 4C-J).
Figure 4.
Cell proliferation was suppressed and apoptosis was induced in cells treated with TrKB siRNA. After cells were cultured for 3 days, the cell viability was slightly higher for (A) HeLa and (B) SiHa cells from the model group than that in the controls. However, knockdown of TrKB showed obvious suppression on the proliferation of (A) HeLa and (B) SiHa cells from the siTrKB group compared to cells from the model group at day 3. Cell apoptosis was analyzed by flow cytometry. The apoptotic rate was increased in cells treated with siTrKB compared to the control, model and siNC groups in both (C-F) HeLa and (G-J) SiHa cells. *P<0.05, vs. control; #P<0.05, vs. siTrKB.
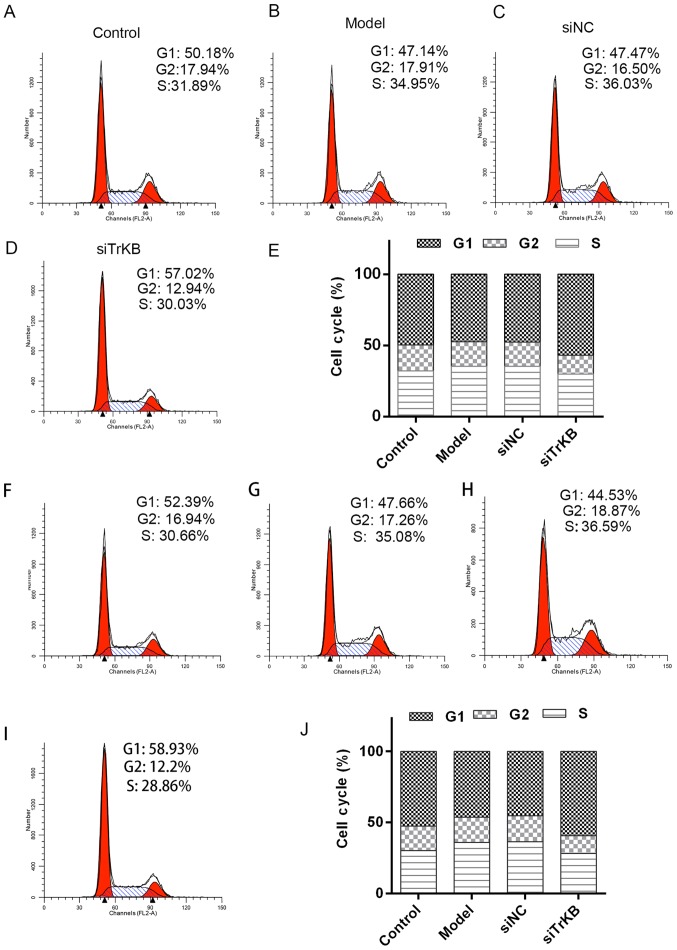
Knockdown of TrKB induced G0/G1 cell cycle arrest
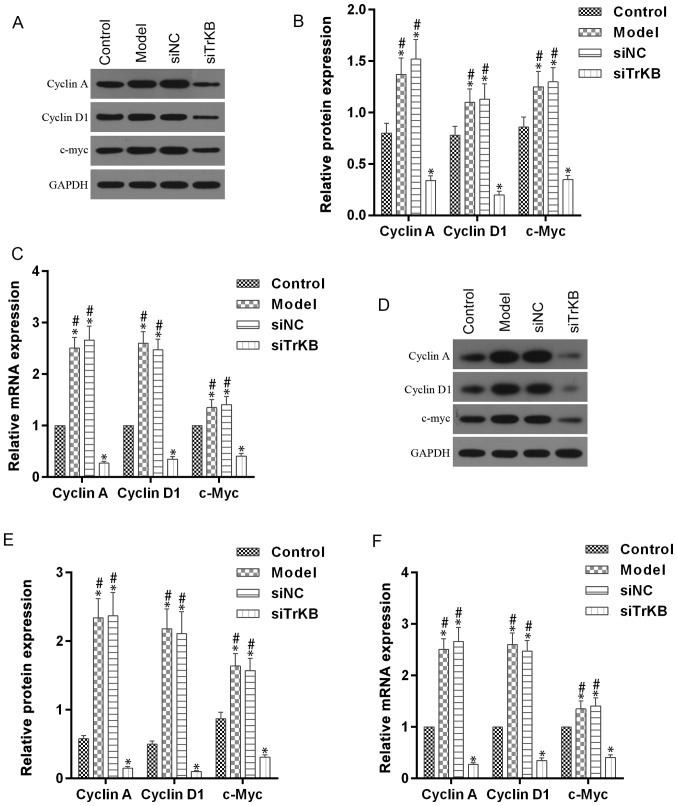
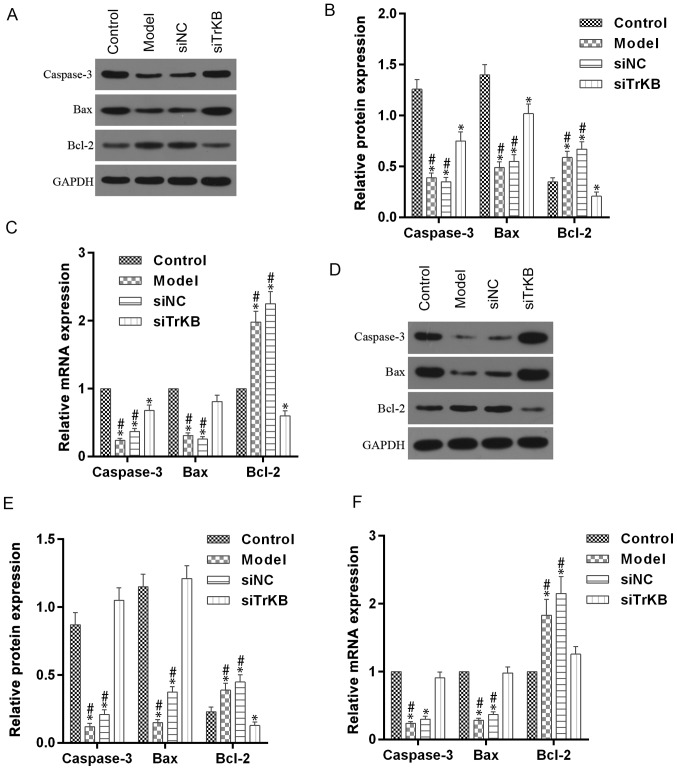
We tested the effects of TrKB on cell cycle by flow cytometry (Fig. 5). The time of G0/G1 phase was shortened for both HeLa and SiHa cells in the model group compared to the control group. However, G0/G1 phase of cells in the siTrKB group was lengthened compared to that in control. We further determined the expression of cell cycle-associated proteins, such as cyclin A, cyclin D1 and c-myc, and apoptosis-associated proteins, including caspase-3, Bax and Bcl-2 (Figs. 6 and 7). For HeLa cells, compared to the control group, the expression of cyclin A, cyclin D1 and c-myc were slightly increased for both protein and mRNA levels in HeLa cells. However, the protein and mRNA expression levels of cyclin A, cyclin D1 and c-myc were significantly decreased in siTrKB group after knockdown of siTrKB. For SiHa cells, the expression of cyclin A, cyclin D1 and c-myc were evidently increased, particularly for cyclin A and cyclin D1 in the model group, compared to control. Moreover, the expression of cyclin A, cyclin D1 and c-myc were significantly downregulated in the siTrKB group compared to the model and siNC groups. Furthermore, the attenuated expression of caspase-3 and Bax in HeLa and SiHa cells were observed in the model group compared to the control group. By contrast, the expression of caspase-3 and Bax were clearly enhanced in the siTrKB group compared to the model group. These results suggested that AAT cells in the model group have a high expression of cyclin A, cyclin D1, c-myc and Bcl-2, indicating high proliferation activity. These findings also revealed that TrKB plays an important role in promoting cell proliferation by regulating the expression of cyclin A, cyclin D1, c-my, caspase-3, Bax and Bcl-2.
Figure 5.
Silencing TrKB induced G0/G1 arrest in AAT cells derived for both HeLa and SiHa cells. (A-D) Cell cycle was determined by flow cytometry assay in different groups including the control, model, siNC and siTrKB in HeLa cells. (E) Comparison of the cell cycle between different groups showed that the time of G0/G1 phase was shortened and lengthened for AAT cells in the model and siTrKB groups compared to corresponding HeLa cells (control), respectively. (F-I) Cell cycle was determined by flow cytometry assay in different groups including the control, model, siNC and siTrKB in SiHa. (J) Comparison of the cell cycle between different groups showed that the time of G0/G1 phase was shortened and lengthened for AAT cells in the model and siTrKB groups compared to corresponding SiHa cell (control), respectively.
Figure 6.
Upregulated cell cycle-associated proteins, including cyclin A, cyclin D1 and c-Myc in AAT cells were decreased after silencing TrkB. (A-C) For HeLa cells, the expression levels of both protein and mRNA of cyclin A, cyclin D1 and c-Myc in cells from model group, analyzed by western blot analysis and RT-qPCR assays, respectively, were upregulated compared with those in cells from the control, while they were downregulated in cells from the siTrKB group. (D-F) For SiHa cells, the expression levels of both protein and mRNA of cyclin A, cyclin D1 and c-Myc in cells from the model group, determined by western blot analysis and RT-qPCR assays, respectively, were significantly upregulated compared with those in cells from the control, whereas they were clearly decreased in cells from siTrKB group. *P<0.05, vs. control; #P<0.05, vs. siTrKB.
Figure 7.
Enhanced expression of caspase-3 and Bax, and attenated Bcl-2 in ATT cells were reversed after knockdown of TrKB. (A and B) For HeLa cells, western blot assay showed that a significant decrease of caspase-3 and Bax protein and an obvious increase of Bcl-2 were observed in cells from the model group compared to those in cells from control, whereas these changes were reversed in cells from the siTrKB group. (C) In addition, RT-qPCR detection showed that the profile of mRNA expression was similar to protein expression. (D and E) For SiHa cells, the expression levels of caspase-3 and Bax protein were significantly increased, and Bcl-2 expression was slightly increased, compared with those in cells from control. However, those changes were significantly reversed in cells treated with TrKB. (F) RT-qPCR detection showed that a similar profile of mRNA expression was observed compared to protein expression. *P<0.05, vs. control; #P<0.05, vs. siTrKB.
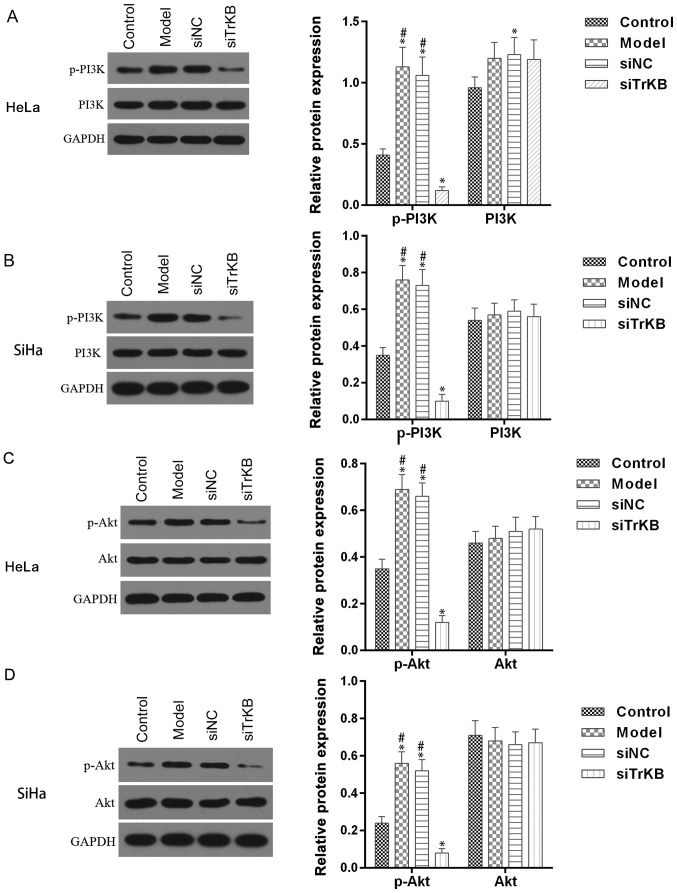
PI3K/Akt pathway is activated in AAT cells
Activation of PI3K/Akt signaling pathway is associated with many forms of cancer, including cervical cancer. We examined whether the PI3K/Akt signaling pathway is involved in BDNF/TrKB pathway-induced proliferation. As shown in Fig. 8, the phosphorylation of PI3K/Akt was enhanced in both HeLa and SiHa cells from the model group compared to the control. However, the enhanced phosphorylation of PI3K/Akt observed in the model group was significantly decreased in cells from the siTrKB group. These results indicated that PI3K/Akt pathway is stimulated in ATT cells and regulated by the BDNF/TrKB pathway.
Figure 8.
Enhanced phosphorylation of PI3K and Akt was reduced after cells with knockdown of TrKB expression. For both (A) HeLa and (B) SiHa cells, the phosphorylation of PI3K was obviously increased in cells from the model group compared to that in cells from the control group, whereas it was significantly decreased in cells from the siTrKB group compared with that in cells from the model group. However, no significant changes of PI3K protein were observed in the groups. For both (C) HeLa and (D) SiHa cells, the phosphorylation of Akt was clearly increased in cells from the model group compared to that in cells from the control group, whereas it was evidently decreased in cells from the siTrKB group compared with that in cells from the model group. However, no significant changes of Akt protein were observed in the groups. *P<0.05, vs. control; #P<0.05, vs. siTrKB.
Discussion
Recently, the BDNF/TrKB pathway was reported as a new signaling pathway promoting cancer cell survival and inhibiting apoptosis (24–26). We determined the role of BDNF/TrKB in cervical cancer. Our findings revealed that the overexpression of BDNF/TrKB was observed in cervical cancer tissues and cell lines compared to adjacent normal tissues and normal cell lines, respectively. Moreover, the enhanced expression of BDNF/TrKB was observed in the model cells of anoikis-like apoptotic tolerance (AAT) established in this study, compared to the control (cancer cells without treatment) and higher proliferation activity was observed in AAT cells than in common cancer cell lines, such as HeLa and SiHa cells. However, when the BDNF/TrKB pathway was blocked by TrKB siRNA, a high growth activity of AAT cells was significantly attenuated. In addition, we found that the enhanced activation of PI3K/Akt observed in AAT cells was evidently suppressed after silencing the expression of TrKB.
In addition to the survival of central neurons, differentiation, growth and development, BDNF plays an important role in maintaining physiological function (27). When BDNF binds to its receptor tyrosine kinase receptor B (tyrosine kinase receptor B, TrkB), phosphorylation of TrKB is induced and the intracellular tyrosine kinase signaling pathway is activated; these are closely associated with tumor cell proliferation, anti-anoikis ability, as well as invasion and metastasis (24–26,28). Although there are many studies on BDNF and TrkB, especially in the study of tumor progression (22,29), the role they play in cervical cancer tissues is unclear. The overexpression of BDNF/TrKB has been found in gastric cancer (30), lung cancer (31), breast cancer (32), nasopharyngeal carcinoma (33), hepatic carcinoma (26), and a low expression in the corresponding normal adjacent tissues (14). Moreover, the expression of BDNF and TrkB is associated with tumor malignancy (31,34). Our results showed that a high expression of BDNF and TrKB were found in cervical cancer tissues and cell lines compared with normal cervical tissues. Moreover, the survival rate for patients with positive BDNF or TrkB expression was significantly lower than that for patients with a negative expression of TrKB. These results suggested that BDNF/TrKB axis is closely associated with poor prognosis in various carcinomas and plays a major role in cervical cancer.
The BDNF/TrKB pathway plays an important role in anoikis-like apoptotic tolerance (AAT) in several forms of cancer, and is involved in resistance to anoikis, allowing for the survival of cancer cells during systemic circulation (28,35). In the present study, we established a cell model of AAT that expressed higher levels of BDNF and TrKB than cancer cell lines, HeLa and SiHa. These results demonstrated that AAT cells have higher proliferation activity and can accelerate the formation of tumors, which are consistent with other reports that the formation of AAT cells is closely linked to tumor metastasis, and invasion and anti-apoptotic ability of cancer cells (14,24–26,28). Since the roles of BDNF and TrKB were considered poor prognostic factors (31,34), we hypothesized that an enhanced expression of BDNF/TrKB in AAT cells is an important event associated with high proliferation activity of AAT cells. In agreement with our hypothesis, the proliferation activity of AAT cells were significantly suppressed when TrKB expression was downregulated.
Activated TrKB by BDNF can induce the activation of several downstream signaling pathways, including PI3K/AKT, JAK/STAT, PLC/PKC, AMPK/ACC and RAS/ERK pathways (36). We furthermore tested the PI3K/Akt signaling pathway, which is involved in the regulation of tumor growth, metastasis, and invasion, such as thyroid cancer and lung cancer (37–40). The phosphorylation of both PI3K and Akt in AAT cells was significantly elevated in comparison with the corresponding HeLa and SiHa cells. However, after downregulation of TrKB expression, phosphorylation of both PI3K and Akt in AAT cells was clearly inhibited in AAT cells. These findings are in agreement with observations in other investigations (20,34,39–41). Therefore, we suggest that the PI3K/Akt signaling pathway is an important pathway mediating the BDNF/TrKB-induced proliferation of AAT cells in cervical cancer. Notably, we also found that the protein and mRNA expression level of BDNF was reduced after TrKB was silenced by siTrKB, which indicates regulation of BDNF. Cheng et al reported that cAMP/PKA pathway is also involved in BDNF-induced secretion of BDNF in an autocrine manner (42). However, further investigations should be conducted to elucidate this phenomenon.
In summary, we found that BDNF and TrKB are overexpressed in both cancer tissues and cell lines. High expression of BDNF and TrKB is closely and positively correlated with high FIGO stage, lymph node metastasis and with poor prognosis. In addition, in AAT cells developed from HeLa and SiHa cells, BDNF and TrKB expression was enhanced and is essential for high proliferation activity. Moreover, we demonstrated that the PI3K and Akt signaling pathways are involved in BDNF/TrKB-induced proliferation of AAT cells in cervical cancer. Thus, we suggest that the BDNF/TrKB pathway is a potential target for the treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used in this study are included in this published article.
Authors' contributions
YY wrote the manuscript. YY and HQY performed the experiments including immunohistochemical staining, cell culture, cell transfection, and cell apoptosis assay. YY and QCR participated in cell cycle assay, western blot assay and real-time RT-PCR assay. YY, HQY and QCR conducted the statistical analysis. YY and HQY revised the manuscript. All authors read and approved the final the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jeebun N, Agnihotri S, Manraj SS, Purwar B. Study of cervical cancers in mauritius over a twelve years period (1989–2000) and role of cervical screening. Internet J Oncol. 2005;3:2. [Google Scholar]
- 2.Morris BJ, Nightingale B, inventors. A method of detection of carcinogenic human papillomavirus. US Patent: 6,218,104 B1. 2001 Filed December 30, 1997; issued April 17.
- 3.Yasuda S, Kojima A, Maeno Y, Oki N, Miyahara Y, Sudo T, Takekida S, Yamaguchi S, Nishimura R. Poor prognosis of patients with stage Ib1 adenosquamous cell carcinoma of the uterine cervix with pelvic lymphnode metastasis. Kobe J Med Sci. 2006;52:9–15. [PubMed] [Google Scholar]
- 4.Dankert-Roelse JE, te Meerman GJ. Long term prognosis of patients with cystic fibrosis in relation to early detection by neonatal screening and treatment in a cystic fibrosis centre. Thorax. 1995;50:712–718. doi: 10.1136/thx.50.7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SQ, Yu H, Zhang LL. Clinical implications of increased lymph vessel density in the lymphatic metastasis of early-stage invasive cervical carcinoma: A clinical immunohistochemical method study. BMC Cancer. 2009;9:64. doi: 10.1186/1471-2407-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (emt)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2014;33:1649–1657. doi: 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McConkey DJ, Bondar V. Regulation and function of detachment-induced cell death (Anoikis) in cancer progression and metastasis. In: Gewirtz DA, Holt SE, Grant S, editors. Cancer Drug Discovery and Development: Apoptosis, Senescence, and Cancer. Humana Press, Inc.; Totowa, NJ: 2007. pp. 109–122. [DOI] [Google Scholar]
- 9.Venetsanakos E, Mirza A, Fanton C, Romanov SR, Tlsty T, Mcmahon M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp Cell Res. 2002;273:21–33. doi: 10.1006/excr.2001.5424. [DOI] [PubMed] [Google Scholar]
- 10.Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. Anoikis resistance: An essential prerequisite for tumor metastasis. Int J Cell Biol. 2012;2012:306879. doi: 10.1155/2012/306879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: Evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/MCB.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider R, Schweiger M. A novel modular mosaic of cell adhesion motifs in the extracellular domains of the neurogenic trk and trkB tyrosine kinase receptors. Oncogene. 1991;6:1807–1811. [PubMed] [Google Scholar]
- 13.Strohmaier C, Carter BD, Urfer R, Barde YA, Dechant G. A splice variant of the neurotrophin receptor trkB with increased specificity for brain-derived neurotrophic factor. EMBO J. 1996;15:3332–3337. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Meng X, Huang Y, Lv Z, Liu J, Wang G, Meng W, Xue S, Zhang Q, Zhang P, Chen G. MicroRNA-497 inhibits thyroid cancer tumor growth and invasion by suppressing BDNF. Oncotarget. 2017;8:2825–2834. doi: 10.18632/oncotarget.13747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Yu Y, Zhang S, Wang X, Yang Z, Ou G. Overexpression of TrkB promotes the progression of colon cancer. APMIS. 2010;118:188–195. doi: 10.1111/j.1600-0463.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Ye HQ, Ren QC. Upregulation of the BDNF/TrKB pathway promotes epithelial-mesenchymal transition, as well as the migration and invasion of cervical cancer. Int J Oncol. 2018;52:461–472. doi: 10.3892/ijo.2017.4230. [DOI] [PubMed] [Google Scholar]
- 17.Xing ZS, Bai ZM, Liu ZX, Chong Z. AB038. High tropomyosin related kinase (TrkB) expression induces epithelial-mesenchymal transition, anoikis resistance and metastasis in prostatic cancer cells. Transl Androl Urol. 2016;5(Suppl 1):AB038. doi: 10.21037/tau.2016.s038. [DOI] [Google Scholar]
- 18.Bao W, Qiu H, Yang T, Luo X, Zhang H, Wan X. Upregulation of trkb promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma. PLoS One. 2013;8:e70616. doi: 10.1371/journal.pone.0070616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi D, Ouyang C, Wang Y, Zhang S, Ma X, Song Y, Yu H, Tang J, Fu W, Sheng L, et al. HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt signaling pathway in stroke. Brain Res. 2014;1577:69–76. doi: 10.1016/j.brainres.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 21.Germanà A, Sánchez-Ramos C, Guerrera MC, Calavia MG, Navarro M, Zichichi R, García-Suárez O, Pérez-Piñera P, Vega JA. Expression and cell localization of brain-derived neurotrophic factor and TrkB during zebrafish retinal development. J Anat. 2010;217:214–222. doi: 10.1111/j.1469-7580.2010.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odate S, Nakamura K, Onishi H, Kojima M, Uchiyama A, Nakano K, Kato M, Tanaka M, Katano M. TrkB/BDNF signaling pathway is a potential therapeutic target for pulmonary large cell neuroendocrine carcinoma. Lung Cancer. 2013;79:205–214. doi: 10.1016/j.lungcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Liang Y, He Z, An Y, Zhao W, Wu J. Autocrine activity of BDNF induced by the STAT3 signaling pathway causes prolonged TrkB activation and promotes human non-small-cell lung cancer proliferation. Sci Rep. 2016;6:30404. doi: 10.1038/srep30404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au CW, Siu MX, Liao X, Wong ES, Ngan HY, Tam KF, Chan DC, Chan QK, Cheung AN. Tyrosine kinase B receptor and BDNF expression in ovarian cancers-Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009;281:151–161. doi: 10.1016/j.canlet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Guo D, Hou X, Zhang H, Sun W, Zhu L, Liang J, Jiang X. More expressions of BDNF and TrkB in multiple hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis, supressed invasion of HepG2 and HCCLM3 cells. J Exp Clin Cancer Res. 2011;30:97. doi: 10.1186/1756-9966-30-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minichiello L, Casagranda F, Tatche RS, Stucky CL, Postigo A, Lewin GR, Davies AM, Klein R. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron. 1998;21:335–345. doi: 10.1016/S0896-6273(00)80543-7. [DOI] [PubMed] [Google Scholar]
- 28.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 29.Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT, Poon RT, Fan ST. Platelet activation during tumor development, the potential role of BDNF-TrkB autocrine loop. Biochem Biophys Res Commun. 2006;346:981–985. doi: 10.1016/j.bbrc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Okugawa Y, Tanaka K, Inoue Y, Kawamura M, Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108:121–130. doi: 10.1038/bjc.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura K, Harada T, Wang S, Ijichi K, Furuyama K, Koga T, Okamoto T, Takayama K, Yano T, Nakanishi Y. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer. 2012;78:100–106. doi: 10.1016/j.lungcan.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res. 2011;17:1741–1752. doi: 10.1158/1078-0432.CCR-10-1890. [DOI] [PubMed] [Google Scholar]
- 33.Ng YK, Wong EY, Lau CP, Chan JP, Wong SC, Chan AS, Kwan MP, Tsao SW, Tsang CM, Lai PB, et al. K252a induces anoikis-sensitization with suppression of cellular migration in Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma cells. Invest New Drugs. 2012;30:48–58. doi: 10.1007/s10637-010-9513-4. [DOI] [PubMed] [Google Scholar]
- 34.Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429–4436. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- 35.Geiger TR, Peeper DS. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 2007;67:6221–6229. doi: 10.1158/0008-5472.CAN-07-0121. [DOI] [PubMed] [Google Scholar]
- 36.Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, Nanjappa V, Narayana J, Somani BL, Mukherjee KK, et al. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal. 2013;7:301–307. doi: 10.1007/s12079-013-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol Res. 2009;39:177–186. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 38.Nakashio A, Fujita N, Tsuruo T. Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell migration via downregulation of the PI3K-Akt signaling pathway. Int J Cancer. 2002;98:36–41. doi: 10.1002/ijc.10166. [DOI] [PubMed] [Google Scholar]
- 39.Nozhat Z, Hedayati M. PI3K/AKT pathway and its mediators in thyroid carcinomas. Mol Diagn Ther. 2016;20:13–26. doi: 10.1007/s40291-015-0175-y. [DOI] [PubMed] [Google Scholar]
- 40.Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol. 2016;49:1325–1333. doi: 10.3892/ijo.2016.3628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008;10:R80. doi: 10.1186/bcr2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci USA. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are included in this published article.