 An F-atom in the phenylalkyl side chain of GluN2B antagonists does not affect the affinity towards GluN2B receptors.
An F-atom in the phenylalkyl side chain of GluN2B antagonists does not affect the affinity towards GluN2B receptors.
Abstract
The influence of an F-atom in the side chain of benzo[7]annulen-7-amines on the affinity towards GluN2B subunit containing NMDA receptors and the selectivity over related receptors was investigated. The synthesis of 5a and 5b was performed by reductive amination of the ketone 6 with primary alkanamines 14a and 14b bearing an F-atom in β-position. The GluN2B affinities of non-fluorinated and fluorinated ligands 4 and 5 are almost identical. The low impact of the F-atom on GluN2B affinity was unexpected, as it influences several chemical and physicochemical properties of the ligands. However, introduction of the F-atom led to reduced selectivity over σ receptors. Whereas 5a and 5b display still a 2–3-fold preference for GluN2B over σ1 receptors, they show almost the same affinity to GluN2B and σ2 receptors.
1. Introduction
The amino acid (S)-glutamate is the most important excitatory neurotransmitter in the central nervous system. It interacts with G-protein coupled receptors (mGlu receptors), which are differentiated into 8 subtypes termed mGlu1-8, and ligand gated ion channels (iGlu receptors). Three subtypes of (S)-glutamate-gated ion channels are known, which are termed according to their prototypical ligands AMPA (2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid), kainate (according to the natural product kainic acid) and NMDA (N-methyl-d-aspartate).1,2
The NMDA receptor is composed of four subunits forming the heterotetrameric ion channel. Cloning has resulted in seven genes encoding these subunits, which are classified into three types: GluN1 (with 8 splice variants GluN1a–h), GluN2 (with four types GluN2A–D) and GluN3 (with two types GluN3A and GluN3B).3–5
We are particularly interested in the modulation of the activity of NMDA receptors containing the GluN2B subunit. The GluN2B subunit is found only in some regions of the central nervous system (e.g. cortex, hippocampus, striatum), other regions (e.g. cerebellum) do not express the GluN2B subunit. NMDA receptor antagonists inhibiting only GluN2B subunit containing receptors should lead to a better side effect profile, since the NMDA receptors are addressed in only some regions of the central nervous system.6 Moreover, the GluN2B subunit offers an additional binding site, the so-called ifenprodil binding site. Ligands interacting with this binding site in the periphery of the ion channel (N-terminal part of the receptor) lead to a mere modulation than complete inhibition of the ion channel, which also contributes to a more favorable side effect profile of such type of ligands.7–13
NMDA receptor antagonists blocking the ion channel by interaction with the ifenprodil binding site at the N-terminal domain of the GluN2B subunit could be used for the treatment of ischemia/stroke, neurodegenerative disorders (Parkinson's and Alzheimer's disease), neuropathic pain,9 migraine, and alcohol withdrawal symptoms.3,6,7
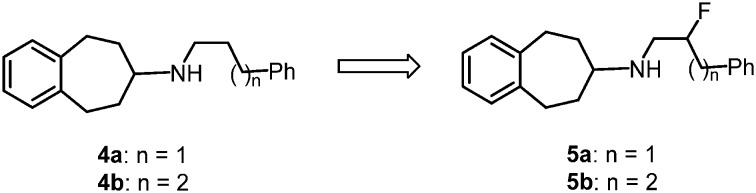
The aminopropanol Ro 25-6981 (1) represents a promising GluN2B selective NMDA receptor antagonist, which is able to protect cultured cortical neurons against glutamate-induced toxicity14 (Fig. 1). Very recently we have reported on novel benzo[7]annulen-7-amines15–17 resulting from rearrangement of the substructures of Ro 25-6981. Both the alcohol 2 (cis-isomer) and the unsubstituted benzo[7]annulenamine 3 showed low Ki values (GluN2B binding) of 16 nM (cis-isomer) and 11 nM, respectively. Moreover, the alcohol 2 (cis-isomer) was active as GluN2B antagonist in the low nanomolar range (IC50 = 12 nM).15 Removal of the 2-methoxy group of 3 led to the benzo[7]annulenamines 4 displaying unexpectedly high GluN2B affinity. 4a and 4b differing in the length of the phenylalkyl moiety bind with similar affinity towards GluN2B containing NMDA receptors (Ki = 16 and 17 nM).16
Fig. 1. Design of GluN2B ligands with a fluorine atom in the phenylalkyl side chain.
Starting from the phenylalkyl derivatives 4, it should be investigated whether the introduction of a fluorine atom in the side chain (5) will be tolerated by GluN2B subunit containing NMDA receptors. The results of this study should be used for the development of a fluorinated PET tracer labeling selectively NMDA receptors with the GluN2B subunit. The fluorine atom with its high electronegativity will reduce the basicity of an amino moiety in β-position by approx. two log units. Thus the formation of ionic and H-bond interactions of the basic amino moiety will be modulated by the fluorine atom. On the other hand, the fluorine atom in the side chain modulates the lipophilicity and is able to form H-bonds with H-bond donating functional groups.18,19 Moreover, a fluorine atom should influence the conformation of the alkyl side chain, as a gauche orientation of the fluorine atom and the basic amino moiety in β-position represents a preferred conformation.20,21
2. Synthesis
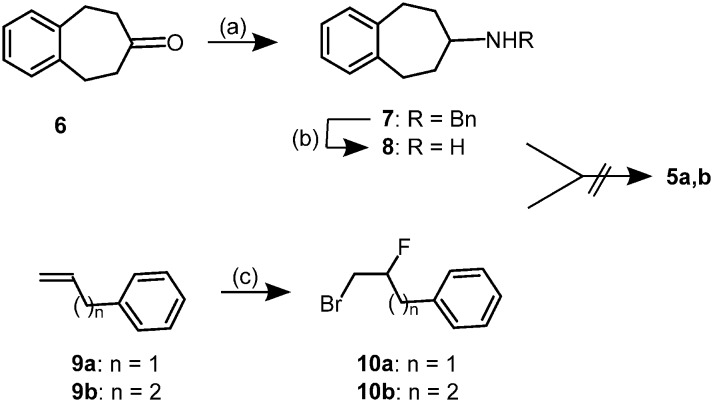
In the first approach the fluorinated benzo[7]annulen-7-amines 5 should be prepared by alkylation of amines 7 or 8 with fluorinated phenylalkyl bromides 10. Reductive amination of ketone 6 with benzylamine and NaBH(OAc)3 provided the benzylamine 7 (ref. 16) which was debenzylated with H2 and Pd/C to give the primary amine 8 (Scheme 1).
Scheme 1. Alkylation with β-fluoroalkyl bromides 10. Reagents and reaction conditions: (a) BnNH2, NaBH(OAc)3, CH2Cl2, rt, 16 h, 81%. (b) H2 (balloon), Pd/C, CH3OH, rt, 21 h, 82%. (c) NEt3·3 HF, NBS, CH2Cl2, rt, 3–23 h, 64% (10a), 63% (10b).
According to a literature protocol,22 allylbenzene 9a was reacted with NEt3·3 HF and N-bromosuccinimide (NBS) to form the bromofluoropropane 10a. (Scheme 1) As the regioselectivity of this addition was poor, an 80 : 20-mixture of 10a and its regioisomer was isolated in 64% yield, which was used for alkylation of amines 7 and 8. The same reagents (NEt3·3 HF, NBS) were employed to transform the homologous butenylbenzene 9b into the bromofluorobutane 10b. The reaction of 9b took place with high regioselectivity resulting only in the desired regioisomer 10b.
All attempts to react the bromofluoroalkanes 10a and 10b with the primary amine 8 or the secondary amine 7 failed to produce the fluoroalkylated amines 5a and 5b. Even heating of a solution of primary amine 8 and bromofluoroalkanes 10 in DMSO to reflux did not lead to any transformation. Therefore, a reductive amination of ketone 6 with fluorinated primary amines 14 was envisaged instead of alkylation of amine 8.
For the synthesis of the 2-fluoro-3-phenylpropyl derivative 5a, oxirane 11a23 was opened regioselectively with NaN3 in aqueous CH3OH to yield the azidoalcohol 12a24 (Scheme 2). Transformation of 12a into fluoroazide 13a was performed with DAST (diethylaminosulfur trifluoride) at –78 °C. Reduction of the azido moiety of 13a with H2 led to the fluorinated primary amine 14a. In contrast to the alkylation of amine 8 with alkyl bromides 10a, the reaction of the ketone 6 with the primary amine 14a in the presence of NaBH(OAc)3 (ref. 25) led to a clean transformation and produced the 2-fluoro-3-phenylpropyl derivative 5a in 50% yield.
Scheme 2. Synthesis of (2-fluoro-3-phenylpropyl)amine 5a. Reagents and reaction conditions: (a) NaN3, CH3OH, H2O, reflux, 1 h, 88%. (b) DAST, CH2Cl2, –78 °C to rt, 3 h, 30%. (c) H2 (balloon), Pd/C, CH3OH, rt, 4 h, 86%. (d) ketone 6, NaBH(OAc)3, CH2Cl2, rt, 16 h, 50%.
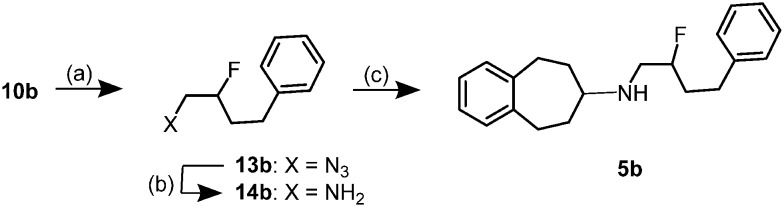
The bromofluorobutyl derivative 10b, which was obtained with high regioselectivity (see Scheme 1), served as starting material for the synthesis of the fluorobutyl derivative 5b (Scheme 3). Reaction of 10b with NaN3 afforded the fluoroazide 13b,26 which was reduced with H2 and Pd(OH)2/C to give the primary amine 14b. Reductive amination25 of ketone 6 with primary amine 14b provided the 2-fluorobutylamine 5b in 34% yield.
Scheme 3. Synthesis of (2-fluoro-4-phenylbutyl)amine 5b. Reagents and reaction conditions: (a) NaN3, DMSO, 65 °C, 2 h, 99%. (b) H2 (balloon), Pd(OH)2/C, CH3OH, HCl, rt, 7 h, 51%. (c) ketone 6, NaBH(OAc)3, CH2Cl2, rt, 24 h, 34%.
3. Receptor affinity
The affinity towards GluN2B subunit containing NMDA receptors was determined in receptor binding studies using the radioligand [3H]ifenprodil.27,28 The receptor material was obtained from L(tk–) cells stably transfected with a vector containing the genetic information of GluN1a and GluN2B subunits. The synthesis of NMDA receptor subunits was induced by addition of dexamethasone. Finally, membrane preparations were prepared and standardized.
Table 1 clearly indicates that the introduction of an F-atom is well tolerated by the GluN2B receptor: the phenylpropylamines 4a and 5a without and with an F-atom in β-position to the amino moiety show almost the same GluN2B affinity. The GluN2B affinity of the homologous phenylbutylamine 5b with an F-atom in the side chain is slightly reduced compared to its non-fluorinated analog 4b. It can be concluded that the GluN2B affinity of this type of ligands is only slightly changed by introduction of an F-atom in the phenylalkyl side chain.
Table 1. Receptor affinities of fluorinated benzo[7]annulenamines 5 compared with the affinities of lead compounds 4 without F atom and some reference compounds.

| ||||||
| Compd. | X | n |
K
i ± SEM [nM] (n = 3) |
|||
| GluN2B | PCP a | σ 1 | σ 2 | |||
| 4a 16 | H | 1 | 16 ± 4.0 | 0% | 150 | 27 ± 12 |
| 4b 16 | H | 2 | 17 ± 2.0 | 2% | 216 | 55 ± 7.0 |
| 5a | F | 1 | 17 ± 1.0 | 2% | 54 ± 6.0 | 14 ± 5.0 |
| 5b | F | 2 | 27 ± 10 b | 3% | 82 ± 24 b | 19 ± 2.0 b |
| Ifenprodil | 10 ± 0.7 | — | 125 ± 24 | 98 ± 34 | ||
| Eliprodil | 13 ± 2.5 | — | — | — | ||
| Dexoxadrol | — | 32 ± 7.4 | — | — | ||
| (+)-MK-801 | — | 3.4 ± 0.8 | — | — | ||
| Haloperidol | — | — | 6.3 ± 1.6 | 78.1 ± 2.3 | ||
| Di-o-tolylguanidine | — | — | 89 ± 29 | 57.5 ± 18 | ||
aDue to the very low affinity towards the PCP binding site of the NMDA receptor, only the inhibition of radioligand binding (%) at the high test compound concentration of 1 μM is given in the table.
bThe Ki values were determined four times (n = 4).
In addition to the interaction with the ifenprodil binding site of the NMDA receptor, the affinity of the fluorinated benzo[7]annulenamines 5 towards the PCP (phencyclidine) binding site within the channel pore of the NMDA receptor was also determined.29,30 As it can be seen from Table 1, the amines 4 and 5 did not interact considerably with the PCP binding site at a concentration of 1 μM indicating high selectivity over the PCP binding site.
Since the prototypical lead compound ifenprodil31,32 and the benzo[7]annulenamines 4 interact with σ1 and σ2 receptors as well (see Table 1), the affinity of the fluorinated compounds 5 towards both σ receptor subtypes was also recorded.33–35 Compared to ifenprodil and the lead compounds 4 the σ1 affinity of the fluorinated compounds 5 is increased. Both fluorinated compounds 5a and 5b show σ1 receptor affinity, which is approx. 3-fold lower than their GluN2B affinity. Overall, the fluorinated compounds 5 show still a preference for the GluN2B receptor, which is however reduced compared to the non-fluorinated ligands 4.
A similar observation was made in the σ2 field. Both fluorinated ligands 5 reveal increased σ2 affinity compared to ifenprodil and the non-fluorinated analogs 4. Whereas compounds 4 show a 2–3-fold preference for GluN2B receptors over σ2 receptors, the GluN2B and the σ2 affinity of the fluorinated ligands 5 are almost identical. Obviously, the selectivity or preference of GluN2B ligands with a benzo[7]annulenamine scaffold over σ2 receptors was lost upon introduction of a F-atom into the phenylalkyl side chain.
4. Conclusion
The effect of introduction of an F-atom into the phenylalkyl side chain of GluN2B ligands was investigated. Both fluorinated ligands 5 and non-fluorinated ligands 4 possess almost identical GluN2B affinity in the low nanomolar range and show high selectivity over the PCP binding site of the NMDA receptor. Introduction of an F-atom into the side chain resulted in reduced selectivity of 5a and 5b over both σ receptor subtypes. They still display a preference for GluN2B over σ1 receptors, but do not have any selectivity over the σ2 subtype. Although only two compounds have been synthesized and biologically evaluated, the study proves the principle of F-tolerance, which will be further exploited with additional examples.
5. Experimental
5.1. Chemistry, general
Unless otherwise noted, moisture sensitive reactions were conducted under dry nitrogen. Acetonitrile was dried over molecular sieves 3 Å. CH2Cl2 was distilled over CaH2. Thin layer chromatography (tlc): silica gel 60 F254 plates (Merck). Flash chromatography (fc): silica gel 60, 40–64 μm (Merck); parentheses include: diameter of the column (d), fraction size (v), eluent, Rf value. Melting point: melting point apparatus Mettler Toledo MP50 Melting Point System, uncorrected. MS: microOTOF-Q II (Bruker Daltonics); APCI, atmospheric pressure chemical ionization. IR: FT-IR spectrophotometer MIRacle 10 (Shimadzu) equipped with ATR technique. Nuclear magnetic resonance (NMR) spectra were recorded on Agilent 600-MR (600 MHz for 1H, 151 MHz for 13C) or Agilent 400-MR spectrometer (400 MHz for 1H, 101 MHz for 13C); δ in ppm related to tetramethylsilane and measured referring to CHCl3 (δ = 7.26 ppm (1H NMR) and δ = 77.2 ppm (13C NMR)) and CHD2OD (δ = 3.31 ppm (1H NMR) and δ = 49.0 ppm (13C NMR)); trichlorofluoromethane (CCl3F) was used as reference compound in 19F NMR spectroscopy; coupling constants are given with 0.5 Hz resolution; the assignments of 13C and 1H NMR signals were supported by 2-D NMR techniques where necessary. HPLC: Merck Hitachi equipment; UV detector: L-7400; autosampler: L-7200; pump: L-7100; degasser: L-7614; column: LiChrospher® 60 RP-select B (5 μm); LiChroCART® 250–4 mm cartridge; flow rate: 1.0 mL min–1; injection volume: 5.0 μL; detection at λ = 210 nm; solvents: A: water with 0.05% (v/v) trifluoroacetic acid; B: acetonitrile with 0.05% (v/v) trifluoroacetic acid: gradient elution: (A%): 0–4 min: 90%, 4–29 min: 90 → 0%, 29–31 min: 0%, 31–31.5 min: 0 → 90%, 31.5–40 min: 90%. The purity of all compounds was determined by this method. The purity of all test compounds is higher than 95%.
5.2. Synthetic procedures
5.2.1. 6,7,8,9-Tetrahydro-5H-benzo[7]annulen-7-amine·HOAc (8·HOAc)
A mixture of benzylamine 7 (126 mg, 0.50 mmol), HOAc (2 drops), Pd/C (10% w/w, 31.7 mg) and CH3OH (10 mL) was stirred under H2 (balloon) at rt for 21 h. The suspension was filtered over Celite® and the filtrate was concentrated in vacuo. The compound was used without further purification. Colorless solid, mp 170 °C, yield 91.2 mg (82%). C13H19NO2 (221.3). Purity (HPLC): 95%, tR = 12.8 min. Exact MS (APCI): m/z = calcd. for C11H16N [MH+] 162.1277, found 162.1281. FT-IR (neat): ν [cm–1] = 3013 (NH3+), 2936, 2847 (C–Halkyl), 1381 (COO–).
5.2.2. (3-Bromo-2-fluoropropyl)benzene (10a)
Et3N·3HF (2.7 mL, 17 mmol) was added to a solution of 9a (591 mg, 5.0 mmol) in CH2Cl2 (25 mL). The solution was cooled to 0 °C and subsequently NBS (980 mg, 5.5 mmol) was added. The mixture was stirred at 0 °C for 30 min and at rt for 16 h. Ice (ca. 50 mL) and 28% NH4OH (30 mL) were added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were washed with 1 M HCl (2 × 25 mL) and saturated NaHCO3 solution (50 mL). The organic layer was dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (4 cm, hexane, 30 mL, Rf = 0.28). Colorless oil, yield 699 mg (64%). Mixture of regioisomers 8 : 2 (NMR). The mixture was used without further purification. C9H10BrF (217.1). Purity (HPLC): 71%, tR = 22.1 min. Exact MS (APCI): m/z = calcd. for C9H1179BrF [MH+] 217.0023, found 216.9979. 1H NMR (CDCl3): δ (ppm) = 3.00 (dd, J = 24/6.1 Hz, 2H, CH2Br,), 3.31–3.46 (m, 2H, CH2Ph), 4.78 (dq, J = 47/6.2 Hz, 1H, CHF), 7.15–7.30 (m, 5H, arom.). 19F NMR (CDCl3): δ (ppm) = –175.2. FT-IR (neat): ν [cm–1] = 3063 (CH2–Br), 2962, 2905 (C–Halkyl), 1605, 1586, 1497 (C–Harom).
5.2.3. (4-Bromo-3-fluorobutyl)benzene (10b)
Et3N·3HF (9.3 mL, 56.8 mmol) was added to a solution of 9b (3000 mg, 22.7 mmol) in CH2Cl2 (25 mL). The solution was cooled to 0 °C and subsequently NBS (4450 mg, 25 mmol) was added. The mixture was stirred at 0 °C for 30 min and at rt for 16 h. Ice (ca. 100 mL) and 28% NH4OH (30 mL) were added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were washed with 1 M HCl (2 × 25 mL) and saturated NaHCO3 solution (50 mL). The organic layer was dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (6 cm, l = 16 cm, hexane, 50 mL, Rf = 0.15). Colorless oil, yield 3332 mg (63%). C10H12BrF (231.1). Purity (HPLC): 88%, tR = 23.2 min. Exact MS (APCI): m/z = calcd. for C10H1281BrF [M] 232.0086, found 232.0082. 1H NMR (CDCl3): δ (ppm) = 1.91–2.07 (m, 2H, CH2CH2Ph), 2.60–2.82 (m, 2H, CH2Ph), 3.41 (dd, J = 19.6/5.1 Hz, 2H, CH2Br), 4.64 (dquint, J = 49/5.2 Hz, 1H, CHF), 7.19–7.24 (m, 3H, 2-CHar, 4-CHar, 6-CHar), 7.29–7.33 (m, 2H, 3-CHar, 5-CHar). 13C NMR (CDCl3): δ (ppm) = 31.1 (d, 1C, J = 4.2 Hz, CH2Ph), 33.7 (d, 1C, J = 25.2 Hz, CH2Br), 35.3 (d, 1C, J = 20.8 Hz, CH2CH2Ph), 91.1 (d, 1C, J = 175.4 Hz, CHF), 126.4 (1C, C-4ar), 128.6 (2C, C-2ar, C-6ar), 128.7 (2C, C-3ar, C-5ar), 140.7 (1C, C-1ar). 19F NMR (CDCl3): δ (ppm) = –179.95. FT-IR (neat): ν [cm–1] = 3063 (CH2–Br), 2951, 2928, 2862 (C–Halkyl), 1601, 1586, 1497 (C–Harom).
5.2.4. 1-Azido-3-phenylpropan-2-ol (12a)24
A solution of oxirane 11a (670 mg, 5.6 mmol), NaN3 (975 mg, 15 mmol) and NH4Cl (401 mg, 7.5 mmol) in H2O (3 mL) and CH3OH (15 mL) was heated to reflux for 1 h. Ethyl acetate (50 mL) and H2O (50 mL) were added, the organic layer was separated and the aqueous layer was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with brine (50 mL), dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (4 cm, cyclohexane/ethyl acetate = 1 : 1, 20 mL, Rf = 0.25). Colorless oil, yield 782 mg (79%). C9H11N3O (177.2). Purity (HPLC): 87%, tR = 17.4 min. Exact MS (APCI): m/z = calcd. for C9H12NO [MH+ – N2] 150.0913, found 150.914. 1H NMR (CDCl3): δ (ppm) = 2.71–2.84 (m, 2H, CH2N3), 3.24 (dd, J = 12/6.8 Hz, 1H, CH2Ph), 3.32 (dd, J = 12/6.8 Hz, 1H, CH2Ph), 3.90–3.97 (m, 1H, CHOH), 7.14–7.21 (m, 3H, arom.), 7.23–7.28 (m, 2H, arom.). FT-IR (neat): ν [cm–1] = 3395 (O–H), 2099 (–N3), 1601, 1586, 1493 (C–Harom), 1080 (C–O).
5.2.5. (3-Azido-2-fluoropropyl)benzene (13a)
At –78 °C, a solution of DAST (806 mg, 5.0 mmol) in CH2Cl2 (5 mL) was added to a solution of 12a (709 mg, 4.0 mmol) in CH2Cl2 (5 mL). The cooling bath was removed and the mixture was stirred for 3 h. H2O (10 mL) was added and the mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (4 cm, hexane/ethyl acetate = 10 : 1, 20 mL, Rf = 0.18). Colorless oil, yield 213 mg (30%). C9H10FN3 (179.2). Purity (HPLC): 86%, tR = 21.7 min. Exact MS (APCI): m/z = calcd. for C9H11FN [MH – N2] 152.0876, found 152.0857. 1H NMR (CDCl3): δ (ppm) = 2.83–3.05 (m, 2H, CH2N3), 3.25–3.39 (m, 2H, CH2Ph), 4.67–4.86 (m, 1H, CHF), 7.13–7.27 (m, 5H, arom.), 19F NMR (CDCl3): δ (ppm) = –182.1. FT-IR (neat): ν [cm–1] = 3028 (Aryl-H), 2936 (C–Halkyl), 2099 (–N3), 1605, 1585, 1497 (C–Harom).
5.2.6. (4-Azido-3-fluorobutyl)benzene (13b)26
A solution of 10b (3265 mg, 14.1 mmol) and NaN3 (1378 mg, 21.2 mmol) in DMSO (20 mL) was heated to 65 °C for 2 h. Then, ethyl acetate (60 mL) was added, and the mixture was washed with H2O (2 × 40 mL) and brine (40 mL). The organic layer was dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (6 cm, l = 8 cm, hexane/ethyl acetate = 20 : 1, 60 mL, Rf = 0.25). Colorless oil, yield 2720 mg (99%). C10H12FN3 (193.2). Purity (HPLC): 99%, tR = 22.6 min. Exact MS (APCI): m/z = calcd. for C10H13FN [MH – N2] 166.1032, found 166.1035. 1H NMR (CDCl3): δ (ppm) = 1.77–2.15 (m, 1H, CH2CH2Ph), 2.68–2.88 (m, 1H, CH2CH2Ph), 3.31–3.47 (m, 2H, CH2N3), 4.54–4.72 (m, 1H, CHF), 7.19–7.24 (m, 3H, 2-CHar, 4-CHar, 6-CHar), 7.29–7.33 (m, 2H, 3-CHar, 5-CHar). 13C NMR (CDCl3): δ (ppm) = 31.1 (d, 1C, J = 4.5 Hz, CH2Ph), 34.0 (d, 1C, J = 20.5 Hz, CH2CH2Ph), 54.5 (d, 1C, J = 21.8 Hz, CH2N3), 91.9 (d, 1C, J = 173.5 Hz, CHF), 126.4 (1C, C-4ar), 128.6 (2C, C-2ar, C-6ar), 128.7 (2C, C-3ar, C-5ar), 140.7 (1C, C-1ar). 19F NMR (CDCl3): δ (ppm) = –186.1. FT-IR (neat): ν [cm–1] = 3028 (Aryl-H), 2928 (C–Halkyl), 2095 (–N3), 1601, 1586, 1497 (C–Harom).
5.2.7. 2-Fluoro-3-phenylpropan-2-amine (14a)
A mixture of 13a (89.6 mg, 0.50 mmol), Pd/C (10% w/w, 50 mg) and CH3OH (4 mL) was stirred under H2 (balloon) at rt for 4 h. The suspension was filtered over Celite® and the filtrate was concentrated in vacuo. Colorless solid, yield 71.5 mg (86%). C9H12FN (153.2). Since the purity of compound 14a was very high, it was used for the reductive amination of ketone 6 without further purification.
5.2.8. 2-Fluoro-4-phenylbutan-1-amine (14b)
A mixture of 13b (2640 mg, 13.7 mmol), Pd(OH)2/C (20% w/w, 270 mg), 1 M HCl (12 mL) and CH3OH (30 mL) was stirred under H2 (balloon) at rt for 7 h. The suspension was filtered over Celite® and the filtrate was concentrated in vacuo. The product was purified by fc (6 cm, l = 12 cm, CH2Cl2/CH3OH/Et3N = 93.5 : 5 : 1.5, 60 mL, Rf = 0.17). Colorless oil, yield 1165 mg (51%). C10H15FN (167.2). Purity (HPLC): 99%, tR = 13.1 min. Exact MS (APCI): m/z = calcd. for C10H15FN [MH+] 168.1183, found 168.1172. 1H NMR (CDCl3): δ (ppm) = 1.76–2.04 (m, 2H, CH2CH2Ph), 2.68–2.73 (m, 1H, CH2Ph), 2.80–2.90 (m, 3H, CH2Ph, CH2NH2), 4.39–4.51 (m, 1H, CHF), 7.18–7.22 (m, 3H, 2-CHar, 4-CHar, 6-CHar), 7.28–7.31 (m, 2H, 3-CHar, 5-CHar). 13C NMR (CDCl3): δ (ppm) = 31.4 (d, 1C, J = 4.4 Hz, CH2Ph), 34.3 (d, 1C, J = 20.7 Hz, CH2CH2Ph), 46.4 (d, 1C, J = 21.7 Hz, CH2NH2), 94.9 (d, 1C, J = 168.5 Hz, CHF), 126.2 (1C, C-4ar), 128.57 (2C, C-2ar, C-6ar), 128.61 (2C, C-3ar, C-5ar), 141.3 (1C, C-1ar). 19F NMR (CDCl3): δ (ppm) = –190.2. FT-IR (neat): ν [cm–1] = 3028 (Aryl-H), 3001 (NH3+), 2955, 2920 (C–Halkyl), 1597, 1512, 1497 (C–Harom).
5.2.9. N-(2-Fluoro-3-phenylpropyl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-7-amine (5a)
A mixture of ketone 6 (64.1 mg, 0.40 mmol), primary amine 14a (65.9 mg, 0.43 mmol), NaBH(OAc)3 (424 mg, 2.0 mmol) and CH2Cl2 (14 mL) was stirred at rt for 24 h. Saturated NH4Cl solution (10 mL) was added and the mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (2 cm, CH2Cl2/CH3OH/Et3N = 200 : 1 : 1, 7 mL, Rf = 0.22) and preparative tlc (CH2Cl2/CH3OH/Et3N = 50 : 1 : 1). Colorless solid, mp 52 °C, yield 44.3 mg (37%). C20H24FN (297.4). Purity (HPLC): 96%, tR = 18.9 min. Exact MS (APCI): m/z = calcd. for C20H25FN [MH+] 298.1966, found 298.1948. 1H NMR (CDCl3): δ (ppm) = 1.34 (q, J = 11 Hz, 2H, 6-CH2, 8-CH2), 2.07 (m, 2H, 6-CH2, 8-CH2), 2.66–2.86 (m, 5H, 5-CH2, 9-CH2, 7-CH), 2.85–2.91 (m, 2H, NCH2), 2.91–3.06 (m, 2H, CH2Ph), 4.81–4.94 (m, 1H, CHF), 7.10 (m, 4H, 1-CH, 2-CH, 3-CH, 4-CH), 7.22–7.27 (m, 3H, 1-CHaryl, 3-CHaryl, 5-CHaryl), 7.30–7.34 (m, 2H, 2-CHaryl, 4-CHaryl). 13C NMR (CDCl3): δ (ppm) = 32.2 (2C, C-5, C-9), 34.1, 34.2 (2C, C-6, C-8), 39.6 (d, 1C, J = 21.2 Hz, CH2Ar), 50.1 (d, 1C, J = 21.0 Hz, NHCH2), 61.2 (1C, C-7), 94.1 (d, 1C, J = 171.4 Hz, CHF), 126.37, 126.38 (2C, C-2, C-3), 126.8 (1C, C-4aryl), 128.7 (2C, C-3aryl, C-5aryl), 129.02, 129.03 (2C, C-1, C-4), 129.5 (2C, C-2aryl, C-6aryl), 136.83 (d, J = 5.5 Hz, 1C, C-1aryl), 142.45, 142.48 (2C, C-1a, C-4a). 19F NMR (CDCl3): δ (ppm) = –184.24. FT-IR (neat): ν [cm–1] = 3325 (NH), 3021 (Aryl-H), 2928, 2882 (C–Halkyl), 1601, 1510, 1493 (C–Harom).
5.2.10. N-(2-Fluoro-4-phenylbutyl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-7-amine (5b)
A mixture of ketone 6 (100 mg, 0.60 mmol), primary amine 14b·HCl (201 mg, 1.20 mmol), NaBH(OAc)3 (255 mg, 1.20 mmol) and CH2Cl2 (10 mL) was stirred at rt for 24 h. Saturated NH4Cl solution (10 mL) was added and the mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried (Na2SO4), filtered, concentrated in vacuo and the residue was purified by fc (2 cm, l = 31 cm, CH2Cl2/CH3OH/Et3N = 196 : 1 : 3, 7 mL, Rf = 0.18). Colorless oil, yield 64 mg (34%). C21H26FN (311.4). Purity (HPLC): 99%, tR = 20.0 min. Exact MS (APCI): m/z = calcd. for C21H27FN [MH+] 312.2122, found 312.2103. 1H NMR (CDCl3): δ (ppm) = 1.38 (m, 2H, 6-CH2, 8-CH2), 1.82–2.04 (m, 2H, CH2CH2Ph), 2.14 (m, 2H, 6-CH2, 8-CH2), 2.68–2.95 (m, 9H, 5-CH2, 9-CH2, CH2CH2Ph, NHCH2, 7-CH), 4.66–4.79 (m, 1H, CHF), 7.08–7.13 (m, 4H, 1-CH, 2-CH, 3-CH, 4-CH), 7.18–7.21 (m, 3H, 1-CHaryl, 3-CHaryl, 5-CHaryl), 7.27–7.30 (m, 2H, 2-CHaryl, 4-CHaryl). 13C NMR (CDCl3): δ (ppm) = 31.4 (d, 1C, J = 4.6 Hz, CH2CH2Ph), 32.2 (2C, C-5, C-9), 33.4, 33.8 (2C, C-6, C-8), 35.0 (d, 1C, J = 20.5 Hz, CH2CH2Ph), 50.4 (d, 1C, J = 20.8 Hz, NHCH2), 61.2 (1C, C-7), 92.9 (d, 1C, J = 167.8 Hz, CHF), 126.3 (1C, C-4aryl), 126.45 (2C, C-2, C-3), 128.58 (2C, C-2aryl, C-6aryl), 128.64 (2C, C-3aryl, C-5aryl), 129.05, 129.07 (2C, C-1, C-4), 141.2 (1C, C-1aryl), 142.29, 142.32 (2C, C-1a, C-4a). 19F NMR (CDCl3): δ (ppm) = –187.4. FT-IR (neat): ν [cm–1] = 3021 (Aryl-H), 2927, 2847 (C–Halkyl), 1601, 1586, 1493 (C–Harom).
5.3. Receptor binding studies
5.3.1. Materials
The recombinant L(tk–) cells stably expressing the GluN2B receptor were a generous donation of Prof. Steinhilber (Frankfurt, Germany). Cell incubator: Heracell 120 (Thermo Fisher Scientific, Langenselbold, Germany). Homogenizers: Elvehjem Potter (B. Braun Biotech International, Melsungen, Germany) and Soniprep 150, MSE, London, UK). Centrifuges: cooling centrifuge model Rotina 35R (Hettich, Tuttlingen, Germany) and high-speed cooling centrifuge model Sorvall RC-5C plus (Thermo Fisher Scientific, Langenselbold, Germany). Multiplates: standard 96-well multiplates (Diagonal, Muenster, Germany). Shaker: self-made device with adjustable temperature and tumbling speed (scientific workshop of the institute). Vortexer: Vortex Genie 2 (Thermo Fisher Scientific, Langenselbold, Germany). Harvester: MicroBeta FilterMate-96 Harvester. Filter: printed Filtermat Typ A and B. Scintillator: Meltilex (Typ A or B) solid state scintillator. Scintillation analyzer: MicroBeta Trilux (all Perkin Elmer LAS, Rodgau-Jügesheim, Germany). Chemicals and reagents were purchased from different commercial sources and of analytical grade.
5.3.2. Cell culture and preparation of membrane homogenates for the GluN2B assay27
Mouse L(tk–) cells stably transfected with the dexamethasone-inducible eukaryotic expression vectors pMSG NR1-1a, pMSG GluN2B (1 : 5 ratio) were grown in Modified Earl's Medium (MEM) containing 10% of standardized FCS (Biochrom AG, Berlin, Germany). The expression of the NMDA receptor at the cell surface was induced after the cell density of the adherent growing cells had reached approximately 90% of confluency. For the induction, the original growth medium was replaced by a growth medium containing 4 μM dexamethasone and 4 μM ketamine (final concentration). After 24 h the cells were harvested by trypsination and pelleted (10 min, 5000 × g).
For the binding assay, the cell pellet was resuspended in PBS buffer and the number of cells was determined using an improved Neubauer's counting chamber (VWR, Darmstadt, Germany). Subsequently, the cells were lysed by sonication (4 °C, 6 × 10 s cycles with breaks of 10 s). The resulting cell fragments were centrifuged with a high performance cool centrifuge (20 000 × g, 4 °C). The supernatant was discarded and the pellet resuspended in a defined volume of phosphate buffer saline (PBS) yielding cell fragments of approximately 500 000 cells per mL. The suspension of membrane homogenates was sonicated again (4 °C, 2 × 10 s cycles with breaks of 10 s) and stored at –80 °C.
5.3.3. Protein determination
The protein concentration was determined by the method of Bradford,36 modified by Stoscheck.37 The Bradford solution was prepared by dissolving 5 mg of Coomassie Brilliant Blue G 250 in 2.5 mL of EtOH (95%, v/v). 10 mL deionized H2O and 5 mL phosphoric acid (85%, m/v) were added to this solution, the mixture was stirred and filled to a total volume of 50.0 mL with deionized water. The calibration was carried out using bovine serum albumin as a standard in 9 concentrations (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0 and 4.0 mg mL–1). In a 96-well standard multiplate, 10 μL of the calibration solution or 10 μL of the membrane receptor preparation were mixed with 190 μL of the Bradford solution, respectively. After 5 min, the UV absorption of the protein–dye complex at λ = 595 nm was measured with a platereader (Tecan Genios, Tecan, Crailsheim, Germany).
5.3.4. General protocol for the binding assay27
The test compound solutions were prepared by dissolving approximately 10 μmol (usually 2–4 mg) of test compound in DMSO so that a 10 mM stock solution was obtained. To obtain the required test solutions for the assay, the DMSO stock solution was diluted with the respective assay buffer. The filtermats were presoaked in 0.5% aqueous polyethylenimine solution for 2 h at room temperature before use. All binding experiments were carried out in duplicates in 96-well multiplates. The concentrations given are the final concentrations in the assay. Generally, the assays were performed by addition of 50 μL of the respective assay buffer, 50 μL test compound solution in various concentrations (10–5, 10–6, 10–7, 10–8, 10–9 and 10–10 mol L–1), 50 μL of corresponding radioligand solution and 50 μL of the respective receptor preparation into each well of the multiplate (total volume 200 μL). The receptor preparation was always added last. During the incubation, the multiplates were shaken at a speed of 500–600 rpm at the specified temperature. Unless otherwise noted, the assays were terminated after 120 min by rapid filtration using the harvester. During the filtration each well was washed five times with 300 μL of water. Subsequently, the filtermats were dried at 95 °C. The solid scintillator was melted on the dried filtermats at a temperature of 95 °C for 5 min. After solidifying of the scintillator at room temperature, the trapped radioactivity in the filtermats was measured with the scintillation analyzer. Each position on the filtermat corresponding to one well of the multiplate was measured for 5 min with the [3H]-counting protocol. The overall counting efficiency was 20%. The IC50-values were calculated with the program GraphPad Prism® 3.0 (GraphPad Software, San Diego, CA, USA) by non-linear regression analysis. Subsequently, the IC50 values were transformed into Ki-values using the equation of Cheng and Prusoff.38 The Ki-values are given as mean value ± SEM from three independent experiments.
5.3.5. Performing of the GluN2B assay27
The competitive binding assay was performed with the radioligand [3H]-ifenprodil (60 Ci mmol–1; Perkin Elmer). The thawed cell membrane preparation from the transfected L(tk–) cells (about 20 μg protein) was incubated with various concentrations of test compounds, 5 nM [3H]-Ifenprodil, and TRIS/EDTA-buffer (5 mM/1 mM, pH 7.5) at 37 °C. The non-specific binding was determined with 10 μM unlabeled ifenprodil. The Kd value of ifenprodil is 7.6 nM.
5.3.6. Affinity toward σ1 and σ2 receptors and the PCP binding site of the NMDA receptor
The affinity toward the PCP binding site of the NMDA receptor29,30 and the affinity toward the σ1 and σ2 receptors33–35 were recorded as previously described.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) which is gratefully acknowledged.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00621c
References
- Bräumer-Osborne H., Egebjerg J., Nielsen E. O., Madsen U., Krogsgaard-Larsen P. J. Med. Chem. 2000;43:2609–2645. doi: 10.1021/jm000007r. [DOI] [PubMed] [Google Scholar]
- Kew J. N. C., Kemp J. A. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Paoletti P., Neyton J. Curr. Opin. Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Lodge D. Neuropharmacology. 2009;56:6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Singh S. K., Mancusco R., Gouaux E. Nature. 2006;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Mony L., Kew J. N. C., Gunthorpe M. J., Paoletti P. Br. J. Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Layton M. E., Kelly III M. J., Rodzinak K. J. Curr. Top. Med. Chem. 2006;6:697–709. doi: 10.2174/156802606776894447. [DOI] [PubMed] [Google Scholar]
- Wu L.-J., Zhou M. Neurotherapeutics. 2009;6:693–702. doi: 10.1016/j.nurt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Curr. Drug Targets. 2001;2:28–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Karakas E., Simorowski N., Furukawa H. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E., Furukawa H. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-H., Lü W., Michel J. C., Goehring A., Du J., Sond X., Gouaux E. Nature. 2014;511(7508):191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Mutel V., Trube G., Malherbe P., Kew J. N., Mohacsi E., Heitz M. P., Kemp J. A. J. Pharmacol. Exp. Ther. 1997;283(3):1285–1292. [PubMed] [Google Scholar]
- Benner A., Bonifazi A., Shirataki C., Temme L., Schepmann D., Quaglia W., Shoji O., Watanabe Y., Daniliuc C., Wünsch B. ChemMedChem. 2014;9:741–751. doi: 10.1002/cmdc.201300547. [DOI] [PubMed] [Google Scholar]
- Gawaskar S., Schepmann D., Bonifazi A., Wünsch B. Bioorg. Med. Chem. 2014;22:6638–6646. doi: 10.1016/j.bmc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Gawaskar S., Schepmann D., Bonifazi A., Robaa D., Sippl W., Wünsch B. Bioorg. Med. Chem. Lett. 2015;25:5748–5751. doi: 10.1016/j.bmcl.2015.10.076. [DOI] [PubMed] [Google Scholar]
- Smart B. S. J. Fluorine Chem. 2001;109:3–11. [Google Scholar]
- Hagmann W. K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- O'Hagan D. Chem. Soc. Rev. 2008;37(2):308–319. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- Hyla-Kryspin I., Grimme S., Hruschka S., Haufe G. Org. Biomol. Chem. 2008;6:4167–4175. doi: 10.1039/b810108f. [DOI] [PubMed] [Google Scholar]
- Alvernhe G., Laurent A., Haufe G. Synthesis. 1987:562–564. [Google Scholar]
- Taber D. F., Paquette C. M., Gu P., Tian W. J. Org. Chem. 2013;78:9772–9780. doi: 10.1021/jo4014996. [DOI] [PubMed] [Google Scholar]
- Cai G., Chau J. N., Dominguez C., Lu Y. and Rishton G. M., US6921762 B2, 2005.
- Abdel-Magid A. F., Mehrman S. J. Org. Process Res. Dev. 2006;10:971–1031. [Google Scholar]
- Silverman R. B. and Xue F., US8299100 B2, 2012.
- Schepmann D., Frehland B., Lehmkuhl K., Tewes B., Wünsch B. J. Pharm. Biomed. Anal. 2010;53:603–608. doi: 10.1016/j.jpba.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Tewes B., Frehland B., Schepmann D., Schmidtke K.-U., Winckler T., Wünsch B. Bioorg. Med. Chem. 2010;18:8005–8015. doi: 10.1016/j.bmc.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Schepmann D., Köhler J., Würthwein E.-U., Wünsch B. Bioorg. Med. Chem. 2010;18:7855–7867. doi: 10.1016/j.bmc.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Köhler J., Bergander K., Fabian J., Schepmann D., Wünsch B. J. Med. Chem. 2012;55:8953–8957. doi: 10.1021/jm301166m. [DOI] [PubMed] [Google Scholar]
- Williams K. Curr. Drug Targets. 2001;2:28–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Falck E., Begrow F., Verspohl E., Wünsch B. J. Pharm. Biomed. Anal. 2014;88:96–105. doi: 10.1016/j.jpba.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Maier C. A., Wünsch B. J. Med. Chem. 2002;45:438–448. doi: 10.1021/jm010992z. [DOI] [PubMed] [Google Scholar]
- Meyer C., Neue B., Schepmann D., Yanagisawa S., Yamaguchi J., Würthwein E.-U., Itami K., Wünsch B. Bioorg. Med. Chem. 2013;21:1844–1856. doi: 10.1016/j.bmc.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Bourgeois C., Werfel E., Galla F., Lehmkuhl K., Torres-Gomez H., Schepmann D., Kögel B., Christoph T., Straßburger W., Engelberger W., Soeberdt M., Hüwel S., Galla H.-J., Wünsch B. J. Med. Chem. 2014;57:6845–6860. doi: 10.1021/jm500940q. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M. Elsevier. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-C., Prusoff W. H. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.