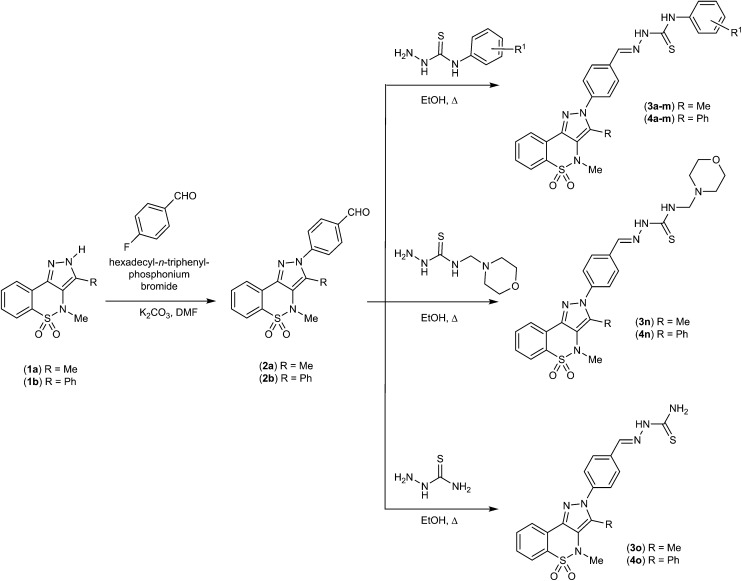
Binding mode of potent inhibitor (green) & cognate ligand (pink) in the active site of MAO-B.
Binding mode of potent inhibitor (green) & cognate ligand (pink) in the active site of MAO-B.
Abstract
Two new series of pyrazolobenzothiazine-based carbothioamides (3a–o and 4a–o) were synthesized using saccharin as the starting material. The synthesized derivatives were investigated for their ability to inhibit monoamine oxidases (MAO). Compound 3b was found to be a very potent MAO-A inhibitor with an IC50 value of 0.003 ± 0.0007 μM, while compound 4d was the most effective inhibitor of MAO-B having an IC50 value of 0.02 ± 0.001 μM. Molecular docking studies were performed to identify the probable binding modes in the active site of the monoamine oxidase enzymes. The synthetic and computational investigations in the current work suggested that these newly identified inhibitors may serve as a powerful starting point for the exploration and optimization of potential therapeutic agents targeting Parkinson's disease.
Introduction
Parkinson's disease (PD), a progressive neurodegenerative disorder, is considered as the second most prevalent disease after Alzheimer's disease affecting up to 10 million people throughout the world.1 These neurodegenerative disorders are mainly characterized by motor and non-motor symptoms such as depression, anxiety, etc.2 Although a significant volume of research has generated numerous chemical entities for the treatment of motor-related symptoms of PD, a decline in the dopamine level in neuronal synapses still subsists. Therefore, dopaminergic agonists are thought to play a crucial role in the therapy of PD.1,2
Monoamine oxidase (MAO; EC 1.4.3.4) is a flavoenzyme responsible for the metabolism and regulation of neurotransmitters such as 5-hydroxytryptamine, norepinephrine and dopamine in the central nervous system.3 MAO is located in the outer mitochondrial membranes of neuronal, glial cells4 particularly abundant in the liver and brain.5 The two main classifications (MAO-A and MAO-B) of these isozymes having differentiated substrate specificity, different amino acid sequences and differing sensitivity to inhibitors have been recognized. MAO-A preferentially oxidizes biogenic amines such as norepinephrine and serotonin, whereas MAO-B plays its critical role in the deamination of β-phenylethylamine. A range of structurally diverse heterocyclic compounds including pyrrolyl-oxazolidinones, triazolothiadiazoles, triazolothiadiazines, thiazolylhydrazones, piperidyl-thienyl chalcones and their 2-pyrazoline derivatives have been reported to inhibit monoamine oxidases.2,6–9 The adverse side effects posed by these MAO-inhibitors in clinics mean that there is an urgent need to develop potent bioactive inhibitors having fewer side effects and better activity.
Thiosemicarbazones of α-(N)-heterocyclic aldehydes or ketones have been demonstrated as potent biologically active compounds. In the past few years, various derivatives of this family have been reported to possess antibacterial,10 antiviral,11 antimalarial12,13 and anticancer14 activities. The structure–activity relationship (SAR) data revealed that the substitution at N-(4) of the thiosemicarbazone fragment as well as at the parent aldehyde or ketone is of key importance to the extent and type of biological activity.15
In the current study to explore new structural fragments for the inhibition of monoamine oxidases, we focused on the pyrazolobenzothiazine core. This heterocyclic motif is an intriguing scaffold for the discovery of new chemical entities (NCEs) for pharmacotherapy, evidenced by examples with significant potential as potent anti-inflammatories, analgesics,16 and anti-depressants17 and as selective focal adhesion kinase (FAK) inhibitors18 in addition to HCV replication inhibitors.19 Thiophene-based pyrazoline derivatives having a carbothioamide tail were found to offer potential as antidepressants.20 Recently, we have reported new pyrazolobenzothiazine derivatives showing potential as anti-HIV agents21 and as antioxidants.22–24 These literature precedents led us to target new analogues having a carbothioamide side chain. We speculated that the extended side chain could potentially modulate the inhibitory activity by helping the target compound to reside in the active pocket of the enzyme. With this aim, and in continuation of our previous reports on the identification of MAO inhibitors,7,9 the synthetic derivatives were screened for their monoamine oxidase inhibition. The detailed SAR studies with the help of molecular docking have also been evaluated and are described here.
Results and discussion
Chemistry
The synthetic approach to new carbothioamides 3a–o and 4a–o is depicted in Scheme 1. The key precursor compounds 1a and 1b were prepared from sodium saccharin according to our previously reported methods.21,22,24,25 Specifically, N-alkylation of a commercial saccharin sodium salt with suitable alkyl halides in dimethylformamide (DMF),22 ring expansion to benzothiazine ring systems, N-methylation and finally construction of a pyrazole ring on benzothiazine by cyclization with hydrazine hydrate provide the respective pyrazolo[4,3-c][1,2]benzothiazine 5,5-dioxide scaffolds (1a and 1b). We have already reported the characterization of 1a and 1b by spectroscopic methods as well as by X-ray crystallography.26,27
Scheme 1. Synthetic route to pyrazolobenzothiazine-based carbothioamides 3a–o and 4a–o.
The N-arylated key intermediates (2a and 2b) were delivered as single regioisomers by treating compounds 1a and 1b, respectively, with 4-fluorobenzaldehyde under mild base phase transfer conditions. The absorption peaks at 1727 and 1737 cm–1 in the IR spectra of compounds 2a and 2b, respectively, and singlets at the chemical shift values of 10.03 and 10.23 ppm, respectively, in the 1H NMR spectra confirmed the introduction of the formyl moiety.
Finally, the title carbothioamides 3a–o and 4a–o were prepared by the condensation reaction of compounds 2a and 2b with a series of diverse thiosemicarbazides in alcoholic media in the presence of 0.3 mL of glacial acetic acid (Scheme 1). The reactions proceeded to completion in 30–45 min affording a wide range of derivatives 3a–o and 4a–o in 80–90% yields (Table 1).
Table 1. Synthesis of pyrazolobenzothiazine-based carbothioamides 3a–o and 4a–o.
| ||||
| Entry | Products | R1 | Reaction time (min) | Yield (%) |
| 1 | 3a | 2-Methylphenyl | 36 | 87 |
| 2 | 3b | 4-Methylphenyl | 35 | 89 |
| 3 | 3c | Phenyl | 30 | 84 |
| 4 | 3d | 4-Ethylphenyl | 32 | 85 |
| 5 | 3e | 2,4-Dimethylphenyl | 37 | 82 |
| 6 | 3f | 2,6-Dimethylphenyl | 40 | 90 |
| 7 | 3g | 2-Chlorophenyl | 31 | 85 |
| 8 | 3h | 3-Chlorophenyl | 34 | 83 |
| 9 | 3i | 4-Chlorophenyl | 31 | 89 |
| 10 | 3j | 2-Fluorophenyl | 32 | 87 |
| 11 | 3k | 3-Fluorophenyl | 33 | 88 |
| 12 | 3l | 4-Fluorophenyl | 34 | 82 |
| 13 | 3m | Benzyl | 41 | 83 |
| 14 | 3n | Methylmorpholine | 45 | 80 |
| 15 | 3o | H | 31 | 86 |
| 16 | 4a | 2-Methylphenyl | 40 | 87 |
| 17 | 4b | 4-Methylphenyl | 41 | 84 |
| 18 | 4c | Phenyl | 40 | 81 |
| 19 | 4d | 4-Ethylphenyl | 38 | 85 |
| 20 | 4e | 2,4-Dimethylphenyl | 39 | 81 |
| 21 | 4f | 2,6-Dimethylphenyl | 41 | 87 |
| 22 | 4g | 2-Chlorophenyl | 42 | 83 |
| 23 | 4h | 3-Chlorophenyl | 40 | 84 |
| 24 | 4i | 4-Chlorophenyl | 41 | 85 |
| 25 | 4j | 2-Fluorophenyl | 37 | 83 |
| 26 | 4k | 3-Fluorophenyl | 38 | 84 |
| 27 | 4l | 4-Fluorophenyl | 40 | 82 |
| 28 | 4m | Benzyl | 38 | 84 |
| 29 | 4n | Methylmorpholine | 44 | 81 |
| 30 | 4o | H | 38 | 89 |
All the final compounds were fully characterized by spectroscopic techniques. The IR spectra of compounds 3a–o and 4a–o showed characteristic CH N absorbance in the regions of 1593–1603 and 1597–1627 cm–1, respectively, and C S absorption in the 1150–1169 and 1157–1182 cm–1 regions, and N–H stretches were observed at 3270–3349 and 3310–3350 cm–1, respectively. The 1H NMR spectra showed singlet peaks in the range of 10.2–10.3 and 11.9–12.1 ppm for the protons of N–NH and NH groups of the thiosemicarbazone moiety. For compounds 3n and 4n, NH peaks were observed as a triplet at 9.27 and 8.55 ppm, respectively, and the C–H proton was observed at 8.22–8.25 ppm. The 13C NMR spectra also fully supported the assignments of carbons of the C S group observed at 175.9–177.7 ppm.
Monoamine oxidase inhibition
The in vitro monoamine oxidase (A and B) inhibitory activity of 2-(4-(3,4-dimethyl/4-methyl-3-phenyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-phenyl-hydrazine carbothioamides 3a–o and 4a–o was assayed using standard methods.7,28 After the initial screening of these compounds, IC50 values were calculated for derivatives which showed >50% inhibition (Table 2). Clorgyline and deprenyl were used as standard inhibitors with IC50 values of 0.0045 ± 0.0003 and 0.0196 ± 0.001 μM, respectively.
Table 2. Monoamine oxidase inhibition of pyrazolobenzothiazine-based carbothioamides 3a–o and 4a–o.
| Compound | MAO-A | MAO-B |
| IC50 ± SEM (μM) |
||
| 3a | 0.01 ± 0.0005 | 3.13 ± 0.14 |
| 3b | 0.003 ± 0.0007 | 29.5 ± 4.68 |
| 3c | 3.69 ± 0.04 | 4.15 ± 0.57 |
| 3d | 1.77 ± 0.03 | 2.91 ± 0.04 |
| 3e | 1.13 ± 0.001 | 0.11 ± 0.005 |
| 3f | 0.01 ± 0.0005 | 6.11 ± 0.07 |
| 3g | 4.06 ± 0.07 | 6.21 ± 0.09 |
| 3h | 1.11 ± 0.003 | 2.89 ± 0.07 |
| 3i | 3.49 ± 0.05 | 0.43 ± 0.006 |
| 3j | 1.42 ± 0.004 | 3.83 ± 0.03 |
| 3k | 0.47 ± 0.007 | 1.68 ± 0.005 |
| 3l | 30.2 ± 5.02 | 41% a |
| 3m | 76.2 ± 7.31 | 4.72 ± 0.38 |
| 3n | 64.8 ± 5.28 | 4.34 ± 0.06 |
| 3o | 31.2 ± 4.07 | 36% a |
| 4a | ND b | ND b |
| 4b | ND b | ND b |
| 4c | 27.2 ± 3.62 | 2.49 ± 0.03 |
| 4d | 8.18 ± 1.94 | 0.02 ± 0.001 |
| 4e | 9.15 ± 2.04 | 0.75 ± 0.004 |
| 4f | 65.7 ± 7.91 | 0.77 ± 0.005 |
| 4g | 33.8 ± 4.62 | 0.68 ± 0.003 |
| 4h | 7.73 ± 0.16 | 20.9 ± 2.74 |
| 4i | 2.06 ± 0.01 | 3.27 ± 0.04 |
| 4j | 0.54 ± 0.006 | 19% a |
| 4k | 9.02 ± 0.08 | 1.76 ± 0.008 |
| 4l | 2.33 ± 0.03 | 6.99 ± 0.05 |
| 4m | 10.6 ± 1.01 | 0.64 ± 0.007 |
| 4n | 3.07 ± 0.19 | 2.99 ± 0.06 |
| 4o | 0.97 ± 0.07 | 2.39 ± 0.05 |
| Clorgyline | 0.0045 ± 0.0003 | 61.35 ± 1.13 |
| Deprenyl | 67.25 ± 1.02 | 0.0196 ± 0.001 |
a% inhibition at 100 μM end concentration.
bND: not determined.
In the 3a–o series, compounds 3a, 3b and 3f exhibited selective inhibition of MAO-A as compared to MAO-B. For the series 4a–o, approximately half of the tested compounds showed better selectivity for MAO-B with five compounds showing sub-micromolar IC50 values. Compound 3b, containing 4-methylphenyl as R1, was the most potent inhibitor of MAO-A depicting an IC50 value of 0.003 ± 0.0007 μM which indicates ∼1.5-fold higher inhibition compared to that of the standard inhibitor, clorgyline (IC50 = 0.0045 ± 0.0003 μM). Compounds 3a and 3f also exhibited good inhibition with IC50 values of 0.01 ± 0.0005 μM. Notably, 3a has a 2-methylphenyl R1 unit; whereas, 3f has a 2,6-dimethylphenyl substituent. Compound 3e, having R1 = 2,4-dimethylphenyl, also retained a significant IC50 value (1.13 ± 0.001 μM). These data indicate that methylphenyl and dimethylphenyl derivatives may be more potent and selective inhibitors towards MAO-A, all showing 2–4 orders of magnitude selectivity for MAO-A over MAO-B inhibition. When the weakly electron-donating methyl group was replaced with an electron-withdrawing halogen substituent such as chlorine and fluorine in the 3a–o series, somewhat different results were observed. For 3-chloro/fluoro substitutions (3h and 3k), high inhibitory values were noticed compared to the other halogenated analogues.
Among the 4a–o series, compound 4j having 2-fluorophenyl and 4o having thiosemicarbazide groups were the only sub-micromolar inhibitors of MAO-A with IC50 values of 0.54 ± 0.006 and 0.97 ± 0.07 μM, respectively. Notably, for the 4a–o series, a number of compounds (i.e.4d–g and 4m) showed 1–2 orders of magnitude selectivity for MAO-B. The most active compound in the series was 4d, having an IC50 value of 0.02 ± 0.001 μM against MAO-B, which is comparable to the reference deprenyl (IC50 = 0.0196 ± 0.001 μM). Other compounds in the series which showed potent inhibitory activity were 4e–g and 4m, all with IC50 values in the sub-micromolar range.
Molecular docking
The AutoDock 4.2 program was used for automated molecular docking. Docking validation was carried out by re-docking the ligand extracted from the experimentally determined enzyme–ligand adduct. To rationalize the obtained in vitro biological results, molecular docking studies were performed against human MAO-A (PDB ID: ; 2Z5Y) and MAO-B (PDB ID: ; 2V5Z). The binding pattern of the synthesized inhibitors was established by molecular docking studies. The docking method was able to reproduce the experimentally observed bound conformation of the co-crystallized ligand with rmsd <2 Å. By re-docking the crystallographic ligand harmine (HRM) in the active site of MAO-A, similar interactions were seen with the residue Tyr407 to those shown by HRM in the crystal structure of MAO-A. Similarly, safinamide was re-docked in the active site of MAO-B and interactions were found with residues Tyr435 and Tyr398. The original co-crystallized carboline ligand (7-methoxy-1-methyl-9H-beta-carboline, HRM) with MAO-A (PDB ID: ; 2Z5Y) was also π-stacked against Tyr407. A FAD coenzyme was oriented towards either side by Tyr407 and Tyr444 residues in MAO-A. For inhibition of MAO-A, this π-interaction with one or both of the tyrosine residues (Tyr407 and Tyr444) appeared to be important.29 Important van der Waals interactions were detected for the reference ligand HRM with residues Tyr69, Ile180, Phe208, Val210, Ile335, Phe352 and Tyr444. Polar interactions were observed with the coenzyme FAD and residues Tyr407, Cys323 and Gln215.
The docking study of the most potent compound 3b against MAO-A was carried out to gain further structural insights into the binding mode and possible interactions with the active site of the enzyme. The 4-methylphenyl moiety of 3b was oriented deep towards FAD and residues Tyr407 and Tyr444 forming π-stacked interactions, whereas the benzothiazine ring was oriented towards the hydrophobic pocket of the active site. Fig. 1 shows an overlay of the binding mode of this potent compound as well as the cognate ligand within the active site of MAO-A. Van der Waals interactions were observed with residues Ile325, Ile335, Phe352, Tyr69 and Tyr197. Polar interactions were observed with the coenzyme FAD and residues Tyr407, Gln215, Val210, Phe208, Ile180 and Cys323.
Fig. 1. Putative binding mode of inhibitor 3b (carbon atoms colored green) and the crystallographic inhibitor harmine (carbon atoms colored pink) in the active site of MAO-A.
In the case of MAO-B, in vitro results evidenced that 4d was the most suitable compound for docking studies. Before docking the potent inhibitor, in the active site of the MAO-B enzyme, molecular docking studies of the co-crystallized ligand safinamide (S)-(+)-2-[4-(fluorobenzyloxy-benzylamino)propionamide] were performed. The ligand was reproduced successfully with an RMSD value of 2.0 Å. For MAO-B docking analysis, the phenylbenzothiazine ring of 4d was found to be oriented towards the FAD molecule in the substrate cavity of the enzyme and π-stacked against Tyr398 and Tyr435 residues. These interactions were found to be important for MAO-B inhibition.30 Similar interactions were observed for the co-crystallized ligand safinamide (SAG). Fig. 2 shows an overlay of the binding mode of the potent compound as well as safinamide in the active site of MAO-B. Van der Waals interactions were observed for SAG with residues Gln206, Cys172, Ile198, Tyr326 and Ile199. The coenzyme FAD and residue Phe168 showed polar interactions. The fluorobenzyl moiety of safinamide was noted to be oriented more towards the entrance cavity, and similarly, the ethyphenyl side chain of 4d was oriented towards the entrance cavity. Moreover, 4d showed important van der Waals interactions with residues Phe103, Leu164, Phe168, Ile199, Tyr326 and Phe343. Apart from π–π interactions with Tyr398 and Tyr435, FAD and residues Gln206 and Ile316 showed polar interactions with 4d. The molecular docking studies gave rise to the hypothesis that there is a crucial correlation between the inhibitory activity and the aryl substituents attached and their ring position.
Fig. 2. Putative binding mode of inhibitor 4d (carbon atoms colored green) and the crystallographic inhibitor safinamide (carbon atoms colored pink) in the active site of MAO-B.
Conclusion
This work reports the synthesis of two series of novel pyrazolobenzothiazine-based carbothioamides and their evaluation as inhibitors of monoamine oxidases A and B to identify new potent leads against both MAO-A and MAO-B. Compound 3b was found to be a very potent selective MAO-A inhibitor with an IC50 value of 0.003 ± 0.0007 μM; whereas, compound 4d was the most effective selective inhibitor against MAO-B having an IC50 value of 0.02 ± 0.001 μM. The data suggested that addition of various methylphenyl groups as a substituent of the pyrazolobenzothiazine-based carbothioamide may lead to enhanced inhibition for MAO-A. In addition to enzymatic evaluation, the computational analysis of the enzyme–inhibitor interaction energy of each derivative revealed plausible putative binding modes of the extended structure of 3b and 4d along with the active sites of MAO-A and MAO-B. Our observations may set a platform for future investigations in the design of better and selective MAO inhibitors.
Experimental
All the chemicals were purchased from E. Merck, Sigma/Aldrich or Fluka and used without purification. General melting points were obtained on a Gallenkamp melting point apparatus and were uncorrected. IR spectra were recorded as KBr pellets on a Perkin Elmer infrared spectrophotometer. 1H NMR spectra were recorded in DMSO-d6 on a Bruker/XWIN NMR (300 and 400 MHz) and TMS was used as an internal standard (chemical shifts, δ in ppm). Mass spectra were recorded on a Jeol MS Route instrument. Ultrasound-mediated reactions were carried out in a Clifton ultrasonic bath (29 T2A, 300 W, DU-4) made by Nickel Electro Ltd.
General procedure for the synthesis of 4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2a) and 4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2b)
3,4-Dimethyl-2,4-dihydropyrazolo[4,3-c][1,2]benzothiazine 5,5-dioxide (1a) (12.50 g; 50.0 mmol) or 4-methyl-3-phenyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (1b) (15.55 g; 50.0 mmol), 4-fluorobenzaldehyde (7.44 g; 60.0 mmol) and anhydrous K2CO3 (8.30 g; 60.0 mmol) were dissolved in anhydrous DMF (25 mL) followed by the addition of hexadecyltriphenylphosphonium bromide (2.54 g; 5.0 mmol). The reaction mixture was refluxed under a nitrogen atmosphere for 40 min. Ice-cold water was added and the resulting pale-yellow precipitates were filtered, washed with excess water and recrystallized from ethanol.
4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2a)
Pale off-yellow solid; yield: 75%. m.p.: 231 °C; FT-IR (KBr) cm–1: 3097, 2994, 2927, 2836, 2771, 1727, 1598, 1445, 1359, 1244, 1161, 821, 725; 1H NMR (DMSO, 300 MHz) δ: 2.49 (3H, s, CH3), 3.09 (3H, s, N–CH3), 7.52 (1H, t, J = 7.5 Hz, Ar–H), 7.64 (2H, d, J = 6.3 Hz, Ar–H), 7.67 (2H, d, J = 6.4 Hz, Ar–H), 7.91 (1H, d, J = 6.6 Hz, Ar–H), 7.95 (1H, d, J = 8.4 Hz, Ar–H), 8.04 (1H, d, J = 7.2 Hz, Ar–H), 10.03 (1H, s, CH); 13C NMR (DMSO-d6, 75 MHz) δ: 11.0 (C–CH3), 40.0 (N–CH3), 124.3 (Ar–C), 124.8 (Ar–C), 124.9 (2C) (Ar–C), 125.5 (Ar–C), 127.8 (Ar–C), 129.3 (2C) (Ar–C), 130.9 (Ar–C), 132.6 (Ar–C), 133.0 (Ar–C), 133.6 (Ar–C), 135.5 (Ar–C), 139.7 (Ar–C), 144.0 (N C, Ar–C), 190.9 (–HC O); MS m/z: (ES–) 353 (M+), (ES+) 354.1 (M + H+); HR-MS (ES+): calcd for C18H16N3O3S (M + H+), 354.0912; found, 354.0907.
4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2b)
Pale off-yellow solid; yield: 70%; FT-IR (KBr) cm–1: 3079, 2987, 2847, 2779, 1737, 1701, 1598, 1456, 1362, 1204, 1162, 824, 723; 1H NMR (DMSO-d6, 300 MHz) δ: 3.23 (3H, s, N–CH3), 7.43–7.57 (2H, m, Ar–H), 7.67 (1H, t, J = 7.5 Hz, Ar–H), 7.75–7.83 (4H, dd, J1 = 9.0 Hz, J2 = 15.4 Hz, Ar–H), 7.91 (1H, d, J = 7.8 Hz, Ar–H), 7.99 (2H, d, J = 7.2 Hz, Ar–H), 8.01 (2H, d, J = 7.2 Hz, Ar–H), 8.13 (1H, d, J = 7.2 Hz, Ar–H), 10.23 (1H, s, CH); 13C NMR: (DMSO-d6, 75 MHz) δ: 38.7 (N–CH3), 124.3 (2C) (Ar–C), 124.7 (Ar–C), 125.1 (Ar–C), 125.2 (Ar–C), 126.9 (Ar–C), 127.1 (Ar–C), 127.8 (Ar–C), 128.3 (Ar–C), 129.2 (Ar–C), 129.4 (Ar–C), 129.9 (2C) (Ar–C), 130.5 (Ar–C), 131.0 (Ar–C), 133.3 (Ar–C), 135.6 (Ar–C), 137.9 (Ar–C), 139.1 (Ar–C), 140.2 (Ar–C), 143.0 (N C, Ar–C), 191.0 (–HC O); MS m/z: 416.1 (ES+, M + H+); HR-MS (ES+): calcd for C23H18N3O3S (M + H+), 416.1069; found, 416.1064.
Synthesis of pyrazolobenzothiazine-based carbothioamides (3a–o and 4a–o)
4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2a) or 4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (2b) and various thiosemicarbazides were added in equimolar quantities to ethanol followed by the addition of 0.3–0.5 mL of glacial acetic acid. Then, the reaction mixture was allowed to reflux for 1 to 3 h depending on the reactivity of 4-phenylthiosemicarbazides. The resulting precipitates were filtered, washed with hot methanol and dried.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(o-tolyl)hydrazine carbothioamide (3a)
Off-white powder; m.p.: 197 °C; FT-IR (KBr) cm–1: 3272, 3021, 2978, 2923, 1601, 1469, 1377, 1326, 1150, 1069, 970, 850, 737; 1H NMR (DMSO-d6, 400 MHz) δ: 2.26 (3H, s, C–CH3), 2.46 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.22 (2H, dd, J1 = 3.6 Hz, J2 = 6.8 Hz, Ar–H), 7.26–7.30 (3H, m, Ar–H), 7.71 (2H, d, J = 8.8 Hz, Ar–H), 7.84 (1H, dt, J1 = 12 Hz, J2 = 7.6 Hz, J3 = 15.6 Hz, Ar–H), 7.95 (1H, d, J = 7.2 Hz, Ar–H), 8.07 (1H, d, J = 8.0 Hz, Ar–H), 8.14 (2H, d, J = 8.4 Hz, Ar–H), 8.23 (1H, s, CH), 10.13 (1H, s, NH), 11.94 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 17.8 (C–CH3), 39.2 (N–CH3), 115.1 (Ar–C), 115.4 (Ar–C), 123.8 (Ar–C), 124.0 (Ar–C), 124.5 (Ar–C), 124.7 (Ar–C), 125.9 (Ar–C), 126.8 (Ar–C), 127.3 (Ar–C), 128.4 (Ar–C), 128.8 (Ar–C), 129.6 (Ar–C), 130.0 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 134.0 (Ar–C), 135.5 (Ar–C), 136.7 (Ar–C), 138.1 (Ar–C), 139.6 (–N C), 140.9 (C N), 141.0 (C N), 176.9 (C S); MS m/z (ES–): 515; HR-MS (ES–): calcd for C26H23N6O2S2 (M – H+), 515.1324; found, 515.1323.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(p-tolyl)hydrazine carbothioamide (3b)
Off-white powder; m.p.: 200 °C; FT-IR (KBr) cm–1: 3292, 3164, 2985, 2933, 1597, 1516, 1472, 1372, 1335, 1258, 1166, 1062, 967, 826, 769; 1H NMR (DMSO-d6, 300 MHz) δ: 2.31 (3H, s, C–CH3), 2.46 (3H, s, C–CH3), 3.04 (3H, s, N–CH3), 7.17 (2H, d, J = 8.4 Hz, Ar–H), 7.41 (2H, d, J = 8.4 Hz, Ar–H), 7.71 (3H, d, J = 8.4 Hz, Ar–H), 7.83 (1H, t, J = 15 Hz, Ar–H), 7.93 (1H, d, J = 7.8 Hz, Ar–H), 8.06 (1H, d, J = 7.8 Hz, Ar–H), 8.12 (2H, d, J = 8.4 Hz, Ar–H), 8.22 (1H, s, CH), 10.15 (1H, s, NH), 11.88 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 20.6 (C–CH3), 39.6 (N–CH3), 123.8 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.6 (2Ar–C), 125.9 (2Ar–C), 127.4 (Ar–C), 128.5 (4Ar–C), 129.6 (Ar–C), 131.9 (Ar–C), 133.5 (Ar–C), 134.0 (Ar–C), 134.6 (Ar–C), 135.7 (Ar–C), 136.5 (Ar–C), 138.1 (–N C), 139.7 (C N), 141.4 (C N), 176.2 (C S); MS m/z (ES–): 515; HR-MS (ES–): calcd for C26H24N6O2S2 (M – H+), 515.1324; found, 515.1318.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-phenylhydrazine carbothioamide (3c)
Light-brown powder; m.p.: 184 °C; FT-IR (KBr) cm–1: 3292, 3160, 2985, 2933, 1603, 1517, 1478, 1377, 1335, 1261, 1166, 1062, 967, 821, 767; 1H NMR (CDCl3, 400 MHz) δ: 2.45 (3H, s, C–CH3), 3.10 (3H, s, N–CH3), 7.27 (1H, d, J = 5.4 Hz, Ar–H), 7.42 (2H, t, J = 6.0 Hz, Ar–H), 7.57 (3H, t, J = 6.3 Hz, Ar–H), 7.67 (3H, t, J = 6.0 Hz, Ar–H), 7.82 (2H, d, J = 6.3 Hz, Ar–H), 7.95 (1H, d, J = 5.7 Hz, Ar–H), 8.00 (1H, s, CH), 8.08 (1H, d, J = 5.7 Hz, Ar–H), 9.20 (1H, s, NH), 10.21 (1H, s, NH); 13C NMR (CDCl3, 100 MHz) δ: 10.8 (C–CH3), 39.9 (N–CH3), 124.1 (Ar–C), 124.6 (Ar–C), 124.9 (Ar–C), 125.0 (2Ar–C), 126.5 (Ar–C), 127.9 (Ar–C), 128.3 (2Ar–C), 128.9 (4Ar–C), 129.1 (Ar–C), 132.5 (Ar–C), 132.8 (Ar–C), 132.9 (Ar–C), 133.4 (Ar–C), 137.7 (Ar–C), 139.3 (–N C), 140.8 (C N), 141.4 (C N–N), 175.9 (C S); MS m/z (ES–): 501; HR-MS (ES–): calcd for C25H22N6O2S2 (M – H+), 501.1167; found, 501.1162.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(4-ethylphenyl)hydrazine carbothioamide (3d)
Off-white powder; m.p.: 195 °C; FT-IR (KBr) cm–1: 3267, 3000, 2918, 2863, 1600, 1489, 1374, 1318, 1231, 1163, 1041, 962, 815, 740; 1H NMR (DMSO-d6, 300 MHz) δ: 1.20 (3H, t, J = 7.8 Hz, C–CH3), 2.26 (3H, s, C–CH3), 2.62 (2H, q, J = 7.8 Hz, C–CH2), 3.05 (3H, s, N–CH3), 7.22 (2H, d, J = 8.1 Hz, Ar–H), 7.45 (2H, d, J = 7.8 Hz, Ar–H), 7.72 (3H, d, J = 8.1 Hz, Ar–H), 7.84 (1H, t, J = 7.5 Hz, Ar–H), 7.95 (1H, d, J = 7.5 Hz, Ar–H), 8.10 (1H, d, J = 7.5 Hz, Ar–H), 8.14 (2H, d, J = 8.1 Hz, Ar–H), 8.24 (1H, s, CH), 10.18 (1H, s, NH), 11.92 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 15.7 (C–CH3), 27.7 (C–CH2), 39.2 (N–CH3), 123.1 (Ar–C), 123.8 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.9 (Ar–C), 126.5 (Ar–C), 127.3 (Ar–C), 127.5 (Ar–C), 128.4 (Ar–C), 128.6 (Ar–C), 129.6 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 134.0 (Ar–C), 135.5 (Ar–C), 136.7 (Ar–C), 137.6 (Ar–C), 138.10, 139.7 (–N C), 140.9 (C N), 141.4 (C N–N), 176.2 (C S); MS m/z (ES–): 529.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(2,4-dimethylphenyl)hydrazine carbothioamide (3e)
Off-white powder; m.p.: 303 °C; FT-IR (KBr) cm–1: 3211, 3121, 2980, 1603, 1505, 1436, 1360, 1261, 1159, 1064, 967, 842, 743; 1H NMR (DMSO-d6, 300 MHz) δ: 2.20 (3H, s, C–CH3), 2.30 (3H, s, C–CH3), 2.47 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.03 (1H, d, J = 8.1 Hz, Ar–H), 7.12 (1H, d, J = 7.2 Hz, Ar–H), 7.15 (1H, s, Ar–H), 7.71 (3H, d, J = 8.1 Hz, Ar–H), 7.84 (1H, t, J = 7.8 Hz, Ar–H), 7.94 (1H, d, J = 8.1 Hz, Ar–H), 8.07 (1H, d, J = 7.8 Hz, Ar–H), 8.13 (2H, d, J = 8.4 Hz, Ar–H), 8.22 (1H, s, CH), 10.13 (1H, s, NH), 11.94 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 17.7 (C–CH3), 20.6 (C–CH3), 39.9 (N–CH3), 113.1 (Ar–C), 116.9 (Ar–C), 123.8 (Ar–C), 124.1 (Ar–C), 124.5 (Ar–C), 124.8 (Ar–C), 126.4 (Ar–C), 127.4 (Ar–C), 128.4 (Ar–C), 128.5 (Ar–C), 129.6 (Ar–C), 130.6 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 134.0 (Ar–C), 134.2 (Ar–C), 135.2 (Ar–C), 135.4 (Ar–C), 135.8 (Ar–C), 138.1 (–N C), 139.6 (C N), 141.1 (C N–N), 177.1 (C S); MS m/z (ES–): 529; HR-MS (ES–): calcd for C27H25N6O2S2 (M – H+), 529.1480; found, 529.1485.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(2,6-dimethylphenyl)hydrazine carbothioamide (3f)
Off-white powder; m.p.: 178 °C; FT-IR (KBr) cm–1: 3315, 2966, 2922, 2856, 1603, 1504, 1437, 1374, 1233, 1169, 1064, 968, 841, 746; 1H NMR (DMSO-d6, 400 MHz) δ: 2.21 (6H, s, C–CH3), 2.47 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.13 (2H, d, J = 8.4 Hz, Ar–H), 7.71 (4H, t, J = 8.0 Hz, Ar–H), 7.84 (1H, t, J = 8.0 Hz, Ar–H), 7.95 (1H, d, J = 8.0 Hz, Ar–H), 8.07 (1H, d, J = 8.0 Hz, Ar–H), 8.14 (2H, d, J = 8.0 Hz, Ar–H), 8.22 (1H, s, CH), 10.02 (1H, s, NH), 11.89 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 18.0 (C–CH3), 20.6 (C–CH3), 40.1 (N–CH3), 113.1 (Ar–C), 117.6 (Ar–C), 123.8 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.7 (Ar–C), 126.9 (Ar–C), 127.5 (Ar–C), 128.4 (Ar–C), 128.6 (Ar–C), 129.7 (Ar–C), 130.9 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 134.0 (Ar–C), 134.1 (Ar–C), 135.2 (Ar–C), 135.5 (Ar–C), 136.4 (Ar–C), 138.2 (–N C), 139.7 (C N), 141.0 (C N–N), 177.2 (C S); MS m/z (ES–): 529; HR-MS (ES–): calcd for C27H26N6O2S2 (M – H+), 529.1480; found, 529.1485.
N-(2-Chlorophenyl)-2-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (3g)
Off-white powder; m.p.: 220 °C; FT-IR (KBr) cm–1: 3271, 2924, 1593, 1507, 1439, 1374, 1261, 1169, 1063, 967, 839, 745; 1H NMR (DMSO-d6, 300 MHz) δ: 2.46 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.31–7.43 (4H, m, Ar–H), 7.57 (1H, d, J = 7.8 Hz, Ar–H), 7.69 (2H, dd, J1 = 8.4 Hz, J2 = 19.8 Hz, Ar–H), 7.84 (1H, t, J = 7.8 Hz, Ar–H), 7.95 (2H, d, J = 7.8 Hz, Ar–H), 8.10 (2H, dd, J1 = 8.4 Hz, J2 = 13.5 Hz, Ar–H), 8.25 (1H, s, CH), 10.22 (1H, s, NH), 12.12 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 39.7 (N–CH3), 115.1 (Ar–C), 115.8 (Ar–C), 123.8 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.7 (Ar–C), 127.1 (Ar–C), 127.3 (Ar–C), 127.9 (Ar–C), 128.4 (Ar–C), 129.3 (Ar–C), 129.6 (Ar–C), 130.1 (Ar–C), 130.9 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 136.6 (Ar–C), 138.1 (–N C), 139.8 (C N), 141.6 (C N–N), 176.9 (C S); MS m/z (ES–): 535; HR-MS (ES–): calcd for C25H20ClN6O2S2 (M – H+), 535.0778; found, 535.0780.
N-(3-Chlorophenyl)-2-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (3h)
Creamy white powder; m.p.: 221 °C; FT-IR (KBr) cm–1: 3304, 2979, 2924, 1596, 1509, 1436, 1374, 1252, 1169, 1064, 966, 841, 745; 1H NMR (DMSO-d6, 400 MHz) δ: 2.48 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.37 (1H, d, J = 7.8 Hz, Ar–H), 7.49 (1H, t, J = 7.8 Hz, Ar–H), 7.68 (2H, d, J = 7.8 Hz, Ar–H), 7.70–7.85 (2H, m, Ar–H), 7.90 (2H, t, J = 7.8 Hz, Ar–H), 8.05 (2H, d, J = 7.8 Hz, Ar–H), 8.12 (2H, d, J = 7.8 Hz, Ar–H), 8.19 (2H, d, J = 7.8 Hz, Ar–H), 8.32 (1H, s, CH), 10.42 (1H, s, NH), 12.12 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 39.7 (N–CH3), 115.2 (Ar–C), 115.9 (Ar–C), 124.1 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 127.3 (Ar–C), 128.6 (Ar–C), 129.5 (Ar–C), 129.7 (Ar–C), 130.1 (Ar–C), 130.9 (Ar–C), 131.8 (Ar–C), 132.1 (Ar–C), 133.5 (Ar–C), 133.8 (Ar–C), 134.0 (Ar–C), 136.6 (Ar–C), 138.1 (–N C), 139.8 (C N), 140.5 (C N–N), 176.9 (C S); MS m/z (ES–): 535; HR-MS (ES–): calcd for C25H20ClN6O2S2 (M – H+), 535.0778; found, 535.0777.
N-(4-Chlorophenyl)-2-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (3i)
Light-yellow crystalline solid; m.p.: 225 °C; FT-IR (KBr) cm–1: 3280, 3129, 2974, 2926, 1589, 1506, 1437, 1371, 1258, 1157, 1064, 965, 827, 744; 1H NMR (DMSO-d6, 400 MHz) δ: 2.26 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.45 (2H, d, J = 8.4 Hz, Ar–H), 7.62 (2H, d, J = 8.8 Hz, Ar–H), 7.73 (3H, t, J = 7.6 Hz, Ar–H), 7.85 (1H, t, J = 7.6 Hz, Ar–H), 7.95 (1H, d, J = 7.6 Hz, Ar–H), 8.07 (1H, d, J = 8.0 Hz, Ar–H), 8.15 (2H, d, J = 8.4 Hz, Ar–H), 8.26 (1H, s, CH), 10.22 (1H, s, NH), 12.12 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 39.6 (N–CH3), 115.2 (Ar–C), 115.8 (Ar–C), 123.8 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 127.6 (Ar–C), 128.5 (Ar–C), 129.6 (Ar–C), 129.6 (Ar–C), 130.0 (Ar–C), 130.9 (Ar–C), 131.8 (Ar–C), 132.1 (Ar–C), 133.5 (Ar–C), 133.8 (Ar–C), 134.0 (Ar–C), 136.5 (Ar–C), 138.1 (N C), 139.8 (C N), 141.8 (C N–N), 176.9 (C S); MS m/z (ES–): 535; HR-MS (ES–): calcd for C25H20ClN6O2S2 (M – H+), 535.0778; found, 535.0783.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(2-fluorophenyl)hydrazine carbothioamide (3j)
Light yellow powder; m.p.: 308 °C; FT-IR (KBr) cm–1: 3325, 3102, 2958, 1598, 1508, 1433, 1374, 1344, 1272, 1163, 1065, 968, 846, 745; 1H NMR (DMSO-d6, 300 MHz) δ: 3.05 (3H, s, C–CH3), 3.34 (3H, s, N–CH3), 7.21–7.34 (4H, m, Ar–H), 7.51 (1H, t, J = 7.5 Hz, Ar–H), 7.73 (3H, d, J = 8.7 Hz, Ar–H), 7.84 (1H, t, J = 7.8 Hz, Ar–H), 7.95 (1H, d, J = 8.8 Hz, Ar–H), 8.10 (2H, dd, J1 = 7.8 Hz, J2 = 16.2 Hz, Ar–H), 8.25 (1H, s, CH), 10.11 (1H, s, NH), 12.10 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 38.7 (N–CH3), 115.6 (Ar–C), 115.8 (Ar–C), 123.8 (Ar–C), 123.9 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.7 (Ar–C), 127.0 (Ar–C), 127.2 (Ar–C), 127.3 (Ar–C), 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 129.6 (Ar–C), 130.3 (Ar–C), 131.9 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 138.1 (–N C), 139.8 (C N), 141.7 (C N–N), 177.5 (C S); MS m/z (ES–): 519; HR-MS (ES–): calcd for C25H20FN6O2S2 (M – H+), 519.1073; found, 519.1078.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(3-fluorophenyl)hydrazine carbothioamide (3k)
Light yellow powder; m.p.: 312 °C; FT-IR (KBr) cm–1: 3340, 2966, 1595, 1513, 1433, 1374, 1345, 1271, 1167, 1069, 967, 843, 744; 1H NMR (DMSO-d6, 300 MHz) δ: 2.47 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.06 (1H, t, J = 7.8 Hz, Ar–H), 7.38–7.48 (2H, m, Ar–H), 7.64 (1H, t, J = 11.1 Hz, Ar–H), 7.73 (3H, t, J = 7.8 Hz, Ar–H), 7.84 (1H, t, J = 7.8 Hz, Ar–H), 7.92 (1H, d, J = 7.8 Hz, Ar–H), 8.11 (3H, dd, J1 = 8.4 Hz, J2 = 16.8 Hz, Ar–H), 8.27 (1H, s, CH), 10.31 (1H, s, NH), 12.11 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 38.7 (N–CH3), 111.7 (Ar–C), 112.0 (Ar–C), 112.2 (Ar–C), 112.5 (Ar–C), 121.4 (Ar–C), 123.8 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 127.4 (Ar–C), 128.3 (Ar–C), 128.6 (Ar–C), 129.4 (Ar–C), 129.5 (Ar–C), 129.6 (Ar–C), 131.8 (Ar–C), 133.4 (Ar–C), 133.7 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 138.1 (–N C), 139.8 (C N), 142.0 (C N–N), 175.9 (C S); MS m/z (ES–): 519; HR-MS (ES–): calcd for C25H20FN6O2S2 (M – H+), 519.1073; found, 519.1072.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(4-fluorophenyl)hydrazine carbothioamide (3l)
Light yellow powder; m.p.: 310 °C; FT-IR (KBr) cm–1: 3338, 3126, 2997, 2963, 1601, 1507, 1435, 1374, 1345, 1267, 1156, 1066, 964, 830, 740; 1H NMR (DMSO-d6, 400 MHz) δ: 2.47 (3H, s, C–CH3), 3.06 (3H, s, N–CH3), 7.23 (2H, t, J = 8.8 Hz, Ar–H), 7.55 (3H, dd, J1 = 4.8 Hz, J2 = 8.8 Hz, Ar–H), 7.72 (2H, dd, J1 = 4.0 Hz, J2 = 8.4 Hz, Ar–H), 7.85 (1H, t, J = 8.4 Hz, Ar–H), 7.95 (1H, d, J = 8.0 Hz, Ar–H), 8.11 (3H, dd, J1 = 8.4 Hz, J2 = 16.4 Hz, Ar–H), 8.25 (1H, s, CH), 10.26 (1H, s, NH), 12.00 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 38.8 (N–CH3), 114.6 (Ar–C), 114.8 (Ar–C), 123.8 (Ar–C), 123.9 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 127.0 (Ar–C), 127.2 (Ar–C), 127.4, 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 129.6 (Ar–C), 130.3 (Ar–C), 131.8 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 138.1 (–N C), 139.7 (C N), 141.6 (C N–N), 176.5 (C S); MS m/z (ES–): 519; HR-MS (ES–): calcd for C25H20FN6O2S2 (M – H+), 519.1073; found, 519.1083.
N-Benzyl-2-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (3m)
Off-white powder; m.p.: 247 °C; FT-IR (KBr) cm–1: 3322, 3150, 3059, 2971, 2938, 1601, 1541, 1504, 1438, 1374, 1331, 1259, 1165, 1064, 963, 841, 743; 1H NMR (DMSO-d6, 400 MHz) δ: 2.50 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 4.87 (2H, d, J = 6.0 Hz, N–CH2), 7.25 (2H, t, J = 7.2 Hz, Ar–H), 7.35 (3H, q, J = 8.0 Hz, Ar–H), 7.71 (2H, dd, J1 = 4.0 Hz, J2 = 8.4 Hz, Ar–H), 7.84 (1H, t, J = 6.4 Hz, Ar–H), 7.95 (1H, d, J = 8.0 Hz, Ar–H), 8.05 (3H, dd, J1 = 3.6 Hz, J2 = 8.8 Hz, Ar–H), 8.18 (1H, s, CH), 9.25 (1H, t, J = 6.4 Hz, NH), 11.76 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.3 (C–CH3), 38.8 (N–CH3), 46.6 (C–CH2), 123.8 (Ar–C), 123.9 (Ar–C), 124.0 (Ar–C), 124.4 (Ar–C), 124.7 (Ar–C), 126.7 (Ar–C), 127.1 (Ar–C), 127.3 (Ar–C), 128.1 (Ar–C), 128.5 (Ar–C), 129.6 (Ar–C), 130.3 (Ar–C), 131.8 (Ar–C), 132.5 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 134.1 (Ar–C), 138.0 (Ar–C), 139.4 (–N C), 139.6 (C N), 141.0 (C N–N), 177.6 (C S); MS m/z (ES–): 515; HR-MS (ES–): calcd for C26H23N6O2S2 (M – H+), 515.1324; found, 515.1325.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(morpholinomethyl)hydrazine carbothioamide (3n)
Off-white powder; m.p.: 250 °C; FT-IR (KBr) cm–1: 3325, 3130, 2987, 2936, 1610, 1561, 1501, 1438, 1376, 1336, 1259, 1165, 1064, 965, 844, 744; 1H NMR (DMSO-d6, 400 MHz) δ: 2.45 (4H, t, J = 2.4 Hz, C–CH2), 3.05 (3H, s, C–CH3), 3.35 (3H, s, N–CH3), 3.58 (4H, t, J = 4.8 Hz, O–CH2), 4.89 (2H, d, J = 5.2 Hz, N–CH2), 7.43 (2H, dd, J1 = 4.4 Hz, J2 = 8.4 Hz, Ar–H), 7.50 (2H, dd, J1 = 4.0 Hz, J2 = 8.4 Hz, Ar–H), 7.72 (2H, dd, J1 = 4.4 Hz, J2 = 8.4 Hz, Ar–H), 7.84 (1H, dt, J1 = 1.2 Hz, J2 = 7.6 Hz, J3 = 16.0 Hz, Ar–H), 8.05 (1H, dd, J1 = 3.6 Hz, J2 = 8.8 Hz, Ar–H), 8.19 (1H, s, CH), 9.45 (1H, t, J = 6.0 Hz, NH), 11.89 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 10.2 (C–CH3), 38.8 (N–CH3), 57.4 (2C, N–CH2), 69.9 (2C, O–CH2), 81.2 (N–CH2–N), 123.9 (Ar–C), 124.0 (Ar–C), 124.4 (Ar–C), 127.4 (Ar–C), 128.5 (Ar–C), 129.7 (Ar–C), 130.8 (Ar–C), 132.6 (Ar–C), 133.5 (Ar–C), 134.1 (Ar–C), 138.0 (Ar–C), 139.4 (Ar–C), 139.7 (–N C), 140.8 (C N), 143.1 (C N–N), 177.7 (C S); MS m/z (ES–): 524.
2-(4-(3,4-Dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (3o)
Light yellow powder; m.p.: 271 °C; FT-IR (KBr) cm–1: 3463, 3343, 3253, 2986, 2949, 1585, 1499, 1431, 1371, 1333, 1261, 1157, 1061, 962, 842, 768; 1H NMR (DMSO-d6, 300 MHz) δ: 2.46 (3H, s, C–CH3), 3.05 (3H, s, N–CH3), 7.70 (3H, d, J = 8.8 Hz, Ar–H), 7.84 (1H, t, J = 7.5 Hz, Ar–H), 7.95 (1H, d, J = 7.8 Hz, Ar–H), 8.08 (3H, t, J = 9.0 Hz, Ar–H), 8.14 (2H, s, NH2), 8.29 (1H, s, CH), 11.56 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 10.3 (C–CH3), 38.7 (N–CH3), 123.8 (Ar–C), 124.1 (Ar–C), 124.4 (Ar–C), 124.7 (Ar–C), 127.4 (Ar–C), 128.2 (Ar–C), 128.8 (Ar–C), 129.6 (Ar–C), 130.8 (Ar–C), 131.8 (Ar–C), 133.4 (Ar–C), 133.9 (Ar–C), 134.1 (Ar–C), 138.0 (–N C), 139.6 (C N), 140.9 (C N–N), 178.2 (C S); MS m/z (ES–): 425; HR-MS (ES–): calcd for C19H17N6O2S2 (M – H+), 425.0854; found, 425.0851.
2-(4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(o-tolyl)hydrazine carbothioamide (4a)
Off-white powder; m.p.: 206 °C; FT-IR (KBr) cm–1: 3349, 3211, 3063, 1604, 1511, 1461, 1348, 1265, 1182, 1061, 970, 838, 771; 1H NMR (DMSO-d6, 400 MHz) δ: 2.31 (3H, s, C–CH3), 2.90 (3H, s, N–CH3), 7.14 (2H, dd, J1 = 2.4 Hz, J2 = 6.4 Hz, Ar–H), 7.21–7.31 (2H, m, Ar–H), 7.49 (1H, t, J = 7.2 Hz, Ar–H), 7.59 (2H, t, J = 8.0 Hz, Ar–H), 7.65 (2H, d, J = 8.8 Hz, Ar–H), 7.73 (3H, m, Ar–H), 8.04 (3H, t, J = 7.2 Hz, Ar–H), 8.17 (2H, d, J = 8.8 Hz, Ar–H), 8.26 (1H, s, CH), 10.15 (1H, s, NH), 11.96 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 17.8 (C–CH3), 39.7 (N–CH3), 123.8 (Ar–C), 123.9 (Ar–C), 124.2 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 125.9 (Ar–C), 126.0 (Ar–C), 126.8 (Ar–C), 127.3 (Ar–C), 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 128.8 (Ar–C), 129.0 (Ar–C), 129.2 (Ar–C), 129.7 (Ar–C), 130.0 (Ar–C), 130.0 (Ar–C), 130.3 (Ar–C), 131.8 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.1 (Ar–C), 138.0 (–N C), 139.7 (C N), 140.9 (C N–N), 177.0 (C S); MS m/z (ES–): 577; HR-MS (ES–): calcd for C31H25N6O2S2 (M – H+), 577.1480; found, 577.1483.
2-(4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(p-tolyl)hydrazine carbothioamide (4b)
Off-white powder; m.p.: 205 °C; FT-IR (KBr) cm–1: 3315, 3065, 2921, 1622, 1501, 1463, 1347, 1261, 1182, 1057, 968, 835, 767; 1H NMR (DMSO-d6, 400 MHz) δ: 2.29 (3H, s, C–CH3), 2.90 (3H, s, N–CH3), 7.13–7.20 (2H, dd, J1 = 8.4 Hz, J2 = 20.4 Hz, Ar–H), 7.42 (2H, d, J = 8.4 Hz, Ar–H), 7.49 (2H, t, J = 7.6 Hz, Ar–H), 7.59 (2H, t, J = 7.6 Hz, Ar–H), 7.66 (2H, d, J = 8.8 Hz, Ar–H), 7.72 (4H, 2× dd, J1 = 7.6 Hz, J2 = 14.0 Hz, Ar–H), 8.05 (2H, dd, J1 = 2.4 Hz, J2 = 7.2 Hz, Ar–H), 8.17 (1H, d, J = 8.4 Hz, Ar–H), 8.26 (1H, s, CH), 10.20 (1H, s, NH), 11.94 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 20.6 (C–CH3), 38.5 (N–CH3), 123.8 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.2 (Ar–C), 125.9 (Ar–C), 126.0 (Ar–C), 126.5 (Ar–C), 127.2 (Ar–C), 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 128.9 (Ar–C), 129.0 (Ar–C), 129.2 (Ar–C), 129.7 (Ar–C), 130.0 (Ar–C), 130.1 (Ar–C), 130.5 (Ar–C), 131.8 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 138.0 (–N C), 139.7 (C N), 140.9 (C N–N), 176.2 (C S); MS m/z (ES–): 577; HR-MS (ES–): calcd for C31H25N6O2S2 (M – H+), 577.1480; found, 577.1485.
2-(4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-phenylhydrazine carbothioamide (4c)
Off-white powder; m.p.: 191 °C; FT-IR (KBr) cm–1: 3310, 3058, 2922, 1602, 1508, 1463, 1347, 1263, 1181, 1059, 968, 835, 769; 1H NMR (DMSO-d6, 400 MHz) δ: 2.90 (3H, s, N–CH3), 7.14 (2H, dd, J1 = 2.8 Hz, J2 = 7.2 Hz, Ar–H), 7.24–7.26 (2H, m, Ar–H), 7.33 (2H, t, J = 7.2 Hz, Ar–H), 7.47 (1H, t, J = 7.6 Hz, Ar–H), 7.57 (2H, t, J = 8.0 Hz, Ar–H), 7.65 (2H, d, J = 8.8 Hz, Ar–H), 7.69–7.76 (3H, m, Ar–H), 8.04 (2H, t, J = 7.6 Hz, Ar–H), 8.17 (2H, d, J = 8.8 Hz, Ar–H), 8.24 (1H, s, CH), 10.15 (1H, s, NH), 11.95 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 38.5 (N–CH3), 123.5 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.2 (Ar–C), 125.9 (Ar–C), 126.0 (Ar–C), 126.3 (Ar–C), 127.2 (Ar–C), 128.2 (Ar–C), 128.2 (Ar–C), 128.5 (Ar–C), 128.9 (Ar–C), 129.0 (Ar–C), 129.1 (Ar–C), 129.7 (Ar–C), 129.9 (Ar–C), 130.3 (Ar–C), 130.5 (Ar–C), 131.5 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.0 (Ar–C), 137.9 (–N C), 139.7 (C N), 140.9 (C N–N), 176.0 (C S); MS m/z (ES–): 563; HR-MS (ES–): calcd for C30H23N6O2S2 (M – H+), 563.1324; found, 563.1326.
N-(4-Ethylphenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4d)
Off-white powder; m.p.: 215 °C; FT-IR (KBr) cm–1: 3310, 2958, 2922, 2853, 1597, 1517, 1462, 1346, 1260, 1180, 1061, 968, 835, 771; 1H NMR (DMSO-d6, 400 MHz) δ: 1.20 (3H, t, J = 8.4 Hz, C–CH3), 2.60 (2H, q, J = 8.0 Hz, C–CH2), 2.90 (3H, s, N–CH3), 7.14 (1H, d, J = 8.8 Hz, Ar–H), 7.21 (2H, t, J = 8.4 Hz, Ar–H), 7.41–7.77 (4H, m, Ar–H), 7.59 (2H, t, J = 7.6 Hz, Ar–H), 7.66 (1H, d, J = 8.8 Hz, Ar–H), 7.70–7.77 (3H, m, Ar–H), 7.90 (1H, t, J = 8.0 Hz, Ar–H), 8.02 (2H, d, J = 7.2 Hz, Ar–H), 8.17 (1H, d, J = 9.6 Hz, Ar–H), 8.27 (1H, s, CH), 10.20 (1H, s, NH), 11.95 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 15.7 (C–CH3), 27.7 (C–CH2), 39.7 (N–CH3), 123.8 (Ar–C), 123.9 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.9 (Ar–C), 126.3 (Ar–C), 127.3 (Ar–C), 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 129.3 (Ar–C), 129.6 (Ar–C), 129.9 (Ar–C), 130.0 (Ar–C), 130.5 (Ar–C), 131.7 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 138.0 (–N C), 139.6 (C N), 141.0 (C N–N), 176.2 (C S); MS m/z (ES–): 591; HR-MS (ES–): calcd for C27H25N6O2S2 (M – H+), 591.1637; found, 591.1639.
N-(2,4-Dimethylphenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4e)
Off-white powder; m.p.: 249 °C; FT-IR (KBr) cm–1: 3310, 3058, 2922, 1602, 1508, 1463, 1347, 1263, 1181, 1059, 968, 835, 769; 1H NMR (DMSO-d6, 300 MHz) δ: 2.20 (3H, s, C–CH3), 2.30 (3H, s, C–CH3), 2.90 (3H, s, N–CH3), 7.05 (2H, d, J = 7.8 Hz, Ar–H), 7.11 (1H, dd, J1 = 6.3 Hz, J2 = 10.8 Hz, Ar–H), 7.50 (1H, d, J = 6.6 Hz, Ar–H), 7.57 (2H, d, J = 7.5 Hz, Ar–H), 7.63 (1H, t, J = 8.1 Hz, Ar–H), 7.72 (2H, t, J = 7.5 Hz, Ar–H), 8.05 (4H, dd, J1 = 2.8 Hz, J2 = 9.2 Hz, Ar–H), 8.17 (3H, d, J = 9.8 Hz, Ar–H), 8.24 (1H, s, CH), 10.06 (1H, s, NH), 11.91 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 17.7 (C–CH3), 20.6 (C–CH3), 38.5 (N–CH3), 123.6 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 125.9 (Ar–C), 126.0 (Ar–C), 126.1 (Ar–C), 126.4 (Ar–C), 127.3 (Ar–C), 128.2 (Ar–C), 128.3 (Ar–C), 128.5 (Ar–C), 128.8 (Ar–C), 129.2 (Ar–C), 129.7 (Ar–C), 130.3 (Ar–C), 130.6 (Ar–C), 131.8 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 135.2 (Ar–C), 135.4 (Ar–C), 138.9 (–N C), 139.7 (C N), 140.9 (C N–N), 177.2 (C S); MS m/z (ES–): 591; HR-MS (ES–): calcd for C32H27N6O2S2 (M – H+), 591.1637; found, 591.1614.
N-(2,6-Dimethylphenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4f)
Light yellow powder; m.p.: 256 °C; FT-IR (KBr) cm–1: 3248, 3128, 2978, 2913, 2853, 1601, 1503, 1452, 1356, 1260, 1157, 1059, 968, 841, 779; 1H NMR (DMSO-d6, 300 MHz) δ: 2.21 (6H, s, C–CH3), 2.91 (3H, s, N–CH3), 7.02 (2H, d, J = 8.1 Hz, Ar–H), 7.07–7.16 (3H, m, Ar–H), 7.49 (1H, t, J = 6.6 Hz, Ar–H), 7.56 (2H, d, J = 7.8 Hz, Ar–H), 7.61 (1H, t, J = 8.4 Hz, Ar–H), 7.72 (1H, d, J = 7.5 Hz, Ar–H), 8.07 (4H, dd, J1 = 2.8 Hz, J2 = 9.8 Hz, Ar–H), 8.17 (2H, d, J = 9.2 Hz, Ar–H), 8.25 (1H, s, CH), 10.07 (1H, s, NH), 11.96 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 17.7 (C–CH3), 20.7 (C–CH3), 38.6 (N–CH3), 123.5 (Ar–C), 123.5 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 125.9 (Ar–C), 126.0 (Ar–C), 126.2 (Ar–C), 126.3 (Ar–C), 127.3 (Ar–C), 128.3 (Ar–C), 128.6 (Ar–C), 128.9 (Ar–C), 129.2 (Ar–C), 129.7 (Ar–C), 130.0 (Ar–C), 130.5 (Ar–C), 130.7 (Ar–C), 131.8 (Ar–C), 133.1 (Ar–C), 133.6 (Ar–C), 133.9 (Ar–C), 135.2 (Ar–C), 135.4 (Ar–C), 138.9 (–N C), 139.8 (C N), 140.8 (C N–N), 177.1 (C S); MS m/z (ES–): 591; HR-MS (ES–): calcd for C32H27N6O2S2 (M – H+), 591.1637; found, 591.1634.
N-(2-Chlorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4g)
Off-white powder; m.p.: 225 °C; FT-IR (KBr) cm–1: 3318, 3157, 1612, 1508, 1464, 1347, 1261, 1182, 1059, 969, 835, 767; 1H NMR (DMSO-d6, 400 MHz) δ: 2.91 (3H, s, N–CH3), 7.15 (1H, d, J = 7.2 Hz, Ar–H), 7.34 (1H, t, J = 7.6 Hz, Ar–H), 7.40 (1H, t, J = 7.6 Hz, Ar–H), 7.50 (2H, d, J = 7.2 Hz, Ar–H), 7.59 (2H, t, J = 12.0 Hz, Ar–H), 7.68 (3H, t, J = 7.2 Hz, Ar–H), 8.05 (4H, dd, J1 = 2.8 Hz, J2 = 7.2 Hz, Ar–H), 8.15 (3H, d, J = 8.0 Hz, Ar–H), 8.28 (1H, s, CH), 10.25 (1H, s, NH), 12.15 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 38.8 (N–CH3), 115.5 (Ar–C), 115.7 (Ar–C), 123.5 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 125.9 (Ar–C), 126.1 (Ar–C), 127.1 (Ar–C), 128.1 (Ar–C), 128.2 (Ar–C), 128.8 (Ar–C), 129.1 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 130.0 (Ar–C), 130.5 (Ar–C), 131.8 (Ar–C), 133.1 (Ar–C), 133.5 (Ar–C), 133.9 (Ar–C), 134.6 (Ar–C), 139.6 (Ar–C), 139.9 (Ar–C), 140.0 (–N C), 140.9 (C N), 143.7 (C N–N), 176.6 (C S); MS m/z (ES–): 597; HR-MS (ES–): calcd for C30H22ClN6O2S2 (M – H+), 597.0934; found, 597.0935.
N-(3-Chlorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4h)
Off-white powder; m.p.: 208 °C; FT-IR (KBr) cm–1: 3310, 3158, 1606, 1548, 1461, 1346, 1265, 1182, 1059, 963, 832, 767; 1H NMR (DMSO-d6, 300 MHz) δ: 2.90 (3H, s, N–CH3), 7.16 (1H, d, J = 7.2 Hz, Ar–H), 7.36 (1H, t, J = 7.2 Hz, Ar–H), 7.40 (1H, t, J = 7.5 Hz, Ar–H), 7.51 (2H, d, J = 7.8 Hz, Ar–H), 7.60 (2H, t, J = 8.4 Hz, Ar–H), 7.73 (2H, dd, J1 = 2.4 Hz, J2 = 7.1 Hz, Ar–H), 7.85 (2H, t, J = 7.1 Hz, Ar–H), 8.06 (4H, dd, J1 = 3.6 Hz, J2 = 8.1 Hz, Ar–H), 8.17 (2H, d, J = 8.4 Hz, Ar–H), 8.29 (1H, s, CH), 10.27 (1H, s, NH), 12.18 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 39.0 (N–CH3), 114.6 (Ar–C), 115.0 (Ar–C), 123.5 (Ar–C), 124.3 (Ar–C), 124.4 (Ar–C), 125.0 (Ar–C), 125.3 (Ar–C), 126.1 (Ar–C), 127.1 (Ar–C), 128.3 (Ar–C), 129.1 (Ar–C), 129.2 (Ar–C), 129.3 (Ar–C), 129.5 (Ar–C), 129.6 (Ar–C), 129.8 (Ar–C), 130.4 (Ar–C), 130.4 (Ar–C), 132.1 (Ar–C), 133.1 (Ar–C), 133.7 (Ar–C), 134.7 (Ar–C), 138.6 (Ar–C), 139.9 (Ar–C), 140.0 (Ar–C), 140.5 (–N C), 143.7 (C N), 149.0 (C N–N), 177.6 (C S); MS m/z (ES–): 597; HR-MS (ES–): calcd for C30H22ClN6O2S2 (M – H+), 597.0934; found, 597.0911.
N-(4-Chlorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4i)
Off-yellow powder; m.p.: 222 °C; FT-IR (KBr) cm–1: 3310, 3138, 2922, 1602, 1508, 1463, 1347, 1263, 1181, 1059, 968, 835, 769; 1H NMR (DMSO-d6, 300 MHz) δ: 2.90 (3H, s, N–CH3), 7.13 (1H, d, J = 6.9 Hz, Ar–H), 7.47 (4H, dd, J1 = 8.7 Hz, J2 = 19.2 Hz, Ar–H), 7.59 (5H, t, J = 8.7 Hz, Ar–H), 7.67 (2H, d, J = 8.1 Hz, Ar–H), 8.05 (3H, d, J = 7.1 Hz, Ar–H), 8.18 (2H, d, J = 8.4 Hz, Ar–H), 8.28 (1H, s, CH), 10.30 (1H, s, NH), 12.07 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 38.7 (N–CH3), 120.5 (Ar–C), 121.8 (Ar–C), 123.4 (Ar–C), 123.6 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.2 (Ar–C), 126.1 (Ar–C), 127.0 (Ar–C), 127.6 (Ar–C), 127.9 (Ar–C), 128.9 (Ar–C), 129.1 (Ar–C), 129.2 (Ar–C), 129.4 (Ar–C), 129.7 (Ar–C), 129.8 (Ar–C), 130.0 (Ar–C), 130.3 (Ar–C), 130.4 (Ar–C), 130.5 (Ar–C), 133.1 (Ar–C), 134.8 (Ar–C), 138.6 (Ar–C), 139.6 (Ar–C), 141.6 (–N C), 143.7 (C N), 158.8 (C N–N), 177.5 (C S); MS m/z (ES–): 597; HR-MS (ES–): calcd for C30H22ClN6O2S2 (M – H+), 597.0934; found, 597.0930.
N-(2-Fluorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4j)
Light yellow powder; m.p.: 243 °C; FT-IR (KBr) cm–1: 3370, 3168, 2972, 1600, 1558, 1467, 1347, 1261, 1178, 1059, 968, 835, 769; 1H NMR (DMSO-d6, 300 MHz) δ: 2.90 (3H, s, N–CH3), 7.13 (1H, d, J = 6.9 Hz, Ar–H), 7.46 (4H, dd, J1 = 8.7 Hz, J2 = 16.2 Hz, Ar–H), 7.46–7.51 (2H, m, Ar–H), 7.58 (2H, t, J = 7.5 Hz, Ar–H), 7.67 (2H, d, J = 8.1 Hz, Ar–H), 7.72 (1H, t, J = 3.9 Hz, Ar–H), 8.05 (3H, d, J = 8.4 Hz, Ar–H), 8.16 (2H, d, J = 8.1 Hz, Ar–H), 8.27 (1H, s, CH), 10.14 (1H, s, NH), 12.14 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 38.7 (N–CH3), 115.5 (Ar–C), 115.8 (Ar–C), 123.5 (Ar–C), 124.0 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 126.1 (Ar–C), 127.0 (Ar–C), 128.9 (Ar–C), 129.1 (Ar–C), 129.1 (Ar–C), 129.7 (Ar–C), 129.8 (Ar–C), 130.0 (Ar–C), 130.3 (Ar–C), 130.4 (Ar–C), 130.6 (Ar–C), 131.1 (Ar–C), 131.9 (Ar–C), 133.1 (Ar–C), 133.9 (Ar–C), 134.8 (Ar–C), 138.1 (Ar–C), 139.6 (Ar–C), 141.6 (–N C), 143.7 (C N), 159.0 (C N–N), 177.5 (C S); MS m/z (ES–): 581; HR-MS (ES–): calcd for C30H22FN6O2S2 (M – H+), 581.1230; found, 581.1238.
N-(3-Fluorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4k)
Off-white powder; m.p.: 242 °C; FT-IR (KBr) cm–1: 3377, 3158, 2971, 1601, 1552, 1461, 1343, 1262, 1178, 1059, 965, 831, 769; 1H NMR (DMSO-d6, 400 MHz) δ: 2.90 (3H, s, N–CH3), 7.14 (1H, d, J = 7.6 Hz, Ar–H), 7.54 (4H, dd, J1 = 8.0 Hz, J2 = 16.4 Hz, Ar–H), 7.58 (2H, t, J = 6.8 Hz, Ar–H), 7.67–7.75 (3H, m, Ar–H), 7.89 (2H, t, J = 7.6 Hz, Ar–H), 7.99 (1H, d, J = 8.1 Hz, Ar–H), 8.06 (2H, d, J = 7.6 Hz, Ar–H), 8.18 (2H, d, J = 7.6 Hz, Ar–H), 8.30 (1H, s, CH), 10.23 (1H, s, NH), 12.13 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 38.9 (N–CH3), 111.7 (Ar–C), 111.9 (Ar–C), 112.3 (Ar–C), 112.6 (Ar–C), 121.5 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.2 (Ar–C), 126.1 (Ar–C), 127.1 (Ar–C), 128.3 (Ar–C), 128.8 (Ar–C), 129.1 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 129.9 (Ar–C), 130.5 (Ar–C), 130.7 (Ar–C), 131.2 (Ar–C), 31.9 (Ar–C), 133.6 (Ar–C), 133.9 (Ar–C), 138.1 (Ar–C), 139.6, 141.8 (–N C), 141.9 (C N), 147.0 (C N–N), 177.6 (C S); MS m/z (ES–): 581; HR-MS (ES–): calcd for C30H22FN6O2S2 (M – H+), 581.1230; found, 581.1235.
N-(4-Fluorophenyl)-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4l)
Off-white powder; m.p.: 247 °C; FT-IR (KBr) cm–1: 3373, 3168, 2973, 1604, 1544, 1465, 1348, 1261, 1177, 1057, 966, 837, 769; 1H NMR (DMSO-d6, 400 MHz) δ: 2.90 (3H, s, N–CH3), 7.14 (1H, d, J = 8.0 Hz, Ar–H), 7.23 (2H, t, J = 8.4 Hz, Ar–H), 7.49 (2H, t, J = 7.6 Hz, Ar–H), 7.53–7.61 (4H, m, Ar–H), 7.69 (4H, dd, J1 = 6.4 Hz, J2 = 8.4 Hz, Ar–H), 8.05 (2H, d, J = 8.0 Hz, Ar–H), 8.18 (2H, d, J = 8.4 Hz, Ar–H), 8.27 (1H, s, CH), 10.27 (1H, s, NH), 12.03 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 38.8 (N–CH3), 114.6 (Ar–C), 114.8 (Ar–C), 120.9 (Ar–C), 121.5 (Ar–C), 123.9 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 125.9 (Ar–C), 126.1 (Ar–C), 127.1 (Ar–C), 128.3 (Ar–C), 128.4 (Ar–C), 128.9 (Ar–C), 129.1 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 129.9 (Ar–C), 130.8 (Ar–C), 131.1 (Ar–C), 131.9 (Ar–C), 133.6 (Ar–C), 133.9 (Ar–C), 138.1 (Ar–C), 139.7 (Ar–C), 141.7 (–N C), 141.9 (C N), 149.1 (C N–N), 176.5 (C S); MS m/z (ES–): 581; HR-MS (ES–): calcd for C30H22FN6O2S2 (M – H+), 581.1230; found, 581.1229.
N-Benzyl-2-(4-(4-methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4m)
Off-white powder; m.p.: 182 °C; FT-IR (KBr) cm–1: 3370, 3068, 2972, 1628, 1517, 1463, 1347, 1251, 1171, 1054, 965, 831, 767; 1H NMR (DMSO-d6, 300 MHz) δ: 2.90 (3H, s, N–CH3), 4.87 (2H, d, J = 6.0 Hz, N–CH2), 7.12 (1H, d, J = 6.9 Hz, Ar–H), 7.35 (5H, d, J = 6.6 Hz, Ar–H), 7.50 (1H, d, J = 6.9 Hz, Ar–H), 7.58 (2H, t, J = 7.5 Hz, Ar–H), 7.64 (2H, d, J = 8.4 Hz, Ar–H), 7.69–7.73 (3H, m, Ar–H), 8.03–8.09 (4H, m, Ar–H), 8.21 (1H, s, CH), 9.27 (1H, t, J = 6.3 Hz, NH), 11.78 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 38.7 (N–CH3), 46.6 (N–CH2), 115.5 (Ar–C), 115.8 (Ar–C), 118.9 (Ar–C), 123.5 (Ar–C), 124.3 (Ar–C), 124.6 (Ar–C), 125.3 (Ar–C), 126.1 (Ar–C), 126.7 (Ar–C), 127.2 (Ar–C), 128.1 (Ar–C), 128.6 (Ar–C), 129.1 (Ar–C), 129.2 (Ar–C), 129.3 (Ar–C), 129.7 (Ar–C), 129.9 (Ar–C), 130.3 (Ar–C), 130.4 (Ar–C), 130.5 (Ar–C), 131.9 (Ar–C), 133.1 (Ar–C), 133.9 (Ar–C), 135.0 (Ar–C), 139.3 (Ar–C), 139.7 (–N C), 140.8 (C N), 143.7 (C N–N), 177.7 (C S); MS m/z (ES–): 577; HR-MS (ES–): calcd for C31H25N6O2S2 (M – H+), 577.1480; found, 577.1485.
2-(4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)-N-(morpholinomethyl)hydrazine carbothioamide (4n)
Off-yellow powder; m.p.: 220 °C; FT-IR (KBr) cm–1: 3377, 3067, 2972, 2957, 1613, 1567, 1453, 1346, 1250, 1171, 1057, 961, 837, 765; 1H NMR (DMSO-d6, 400 MHz) δ: 2.46 (4H, t, J = 2.4 Hz, C–CH2), 3.01 (3H, s, C–CH3), 3.55 (4H, t, J = 4.8 Hz, O–CH2), 4.87 (2H, d, J = 5.2 Hz, N–CH2), 7.45 (2H, dd, J1 = 4.4 Hz, J2 = 8.4 Hz, Ar–H), 7.68 (2H, t, J = 8.0 Hz, Ar–H), 7.75 (2H, q, J = 7.2 Hz, Ar–H), 7.84–7.91 (2H, m, Ar–H), 7.98 (2H, d, J = 8.0 Hz, Ar–H), 8.04 (1H, t, J = 9.2 Hz, Ar–H), 8.17 (2H, d, J = 7.6 Hz, Ar–H), 8.17 (1H, s, CH), 8.55 (1H, t, J = 6.7 Hz, NH), 11.64 (1H, s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 39.7 (N–CH3), 53.2 (2C, N–CH2), 56.6 (2C, O–CH2), 66.2 (N–CH2–N), 115.5 (Ar–C), 115.8 (Ar–C), 123.5 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 127.7 (Ar–C), 128.1 (Ar–C), 128.6 (Ar–C), 129.2 (Ar–C), 129.3 (Ar–C), 129.6 (Ar–C), 129.9 (Ar–C), 130.3 (Ar–C), 130.5 (Ar–C), 131.9 (Ar–C), 133.1 (Ar–C), 133.9 (Ar–C), 135.0 (Ar–C), 139.3 (Ar–C), 139.7 (–N C), 140.3 (C N), 143.7 (C N–N), 177.7 (C S); MS m/z (ES–): 586.
2-(4-(4-Methyl-5,5-dioxido-3-phenylbenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzylidene)hydrazine carbothioamide (4o)
Off-white powder; m.p.: 245 °C; FT-IR (KBr) cm–1: 3471, 3363, 3257, 2989, 2933, 1582, 1499, 1451, 1373, 1331, 1264, 1159, 1061, 962, 842, 769; 1H NMR (DMSO-d6, 300 MHz) δ: 2.90 (3H, s, N–CH3), 4.87 (2H, d, J = 6.0 Hz, N–CH2), 7.12 (1H, d, J = 6.9 Hz, Ar–H), 7.35 (2H, d, J = 6.6 Hz, Ar–H), 7.50 (1H, d, J = 6.9 Hz, Ar–H), 7.58 (2H, t, J = 7.5 Hz, Ar–H), 7.64 (1H, d, J = 8.4 Hz, Ar–H), 7.69–7.73 (2H, m, Ar–H), 8.03–8.09 (2H, m, Ar–H), 8.21 (1H, s, CH), 9.27 (2H, t, J = 6.3 Hz, NH2), 11.78 (1H, s, NH); 13C NMR (DMSO-d6, 75 MHz) δ: 38.7 (N–CH3), 121.5 (Ar–C), 123.8 (Ar–C), 124.4 (Ar–C), 124.6 (Ar–C), 125.2 (Ar–C), 126.1 (Ar–C), 127.1 (Ar–C), 127.9 (Ar–C), 128.6 (Ar–C), 129.1 (Ar–C), 129.1 (Ar–C), 129.3 (Ar–C), 129.6 (Ar–C), 129.9 (Ar–C), 130.4 (Ar–C), 131.9 (Ar–C), 133.6 (Ar–C), 133.9 (Ar–C), 134.1 (Ar–C), 139.6 (–N C), 140.7 (C N), 143.7 (C N–N), 178.2 (C S); MS m/z (ES–): 487; HR-MS (ES–): calcd for C24H19N6O2S2 (M – H+), 487.1011; found, 487.1018.
In vitro monoamine oxidase inhibition assay
The inhibitory activity of the synthesized compounds (3a–o and 4a–o) against monoamine oxidases A and B was determined by a previously used method7,28 with slight modifications. The assay was performed in a polystyrene 96-well plate having a flat bottom containing 200 μL of the total reaction mixture. For each assay, the mixture was composed of 136 μL of 50 mM phosphate buffer (pH 7.4), 2 μL of test compound (0.1 mM) and 45 μL of enzyme pre-incubated at 37 °C for 10 min. The enzymatic reaction was initiated by the addition of 5 μL of the substrate (p-tyramine, 3 mM) and 12 μL of Amplex Red reagent and the mixture was further incubated at 37 °C for 10 min. Consequently, the production of H2O2 and resorufin was measured using a microplate fluorescence reader (FLx 800, Bio-Tek Instruments, Inc., Winooski, USA) based on the fluorescence produced at the excitation and emission wavelength of 544 nm and 590 nm, respectively. For all the test compounds, the assay was performed in triplicate. IC50 values of the compounds showing activity more than 50% were determined by further diluting the mixture to eight different concentrations. Deprenyl and clorgyline were used as standard inhibitors. The IC50 values were determined using Graph PRISM software version 5.0 (San Diego, CA, USA) as the mean ± S.E.M. of three assays.
Molecular docking protocols
Structure selection
Molecular docking studies were conducted to investigate putative interactions of the compounds in the active site of the monoamine oxidases. In order to perform efficient docking studies, the crystallographic structures of human MAO (A & B) were downloaded from the Protein Data Bank (ID: 2Z5Y at 2.17 Å and ; 2V5Z at 1.7 Å).29,30 MAO-A exists as a monomer while MAO-B as a homo-dimer. Before the docking studies, chain B of MAO-B was removed and only chain A was retained. The co-enzyme, flavin adenine dinucleotide (FAD), was kept in the oxidized state as described previously.31 The enzyme structure was protonated with the Protonate3D32 algorithm implemented in the molecular modeling tool MOE.33 The structure was energy-minimized using the Amber99 force field including all crystallographic solvent molecules. The backbone atoms were restrained with a small force in order to avoid collapse of the binding pockets during energy minimization calculations. After minimization, the water molecules and co-crystallized bound compounds were removed.
Compound preparation
The 3D structural coordinates of all the compounds were generated using MOE followed by assignment of protonation and ionization states in the physiological pH range by using the “wash” module. Afterwards, the structures of the compounds were energy-minimized with the MMFF94x force field for docking studies.33
Docking studies
The docking studies of all the compounds were carried out using AutoDock 4.2.34 Method validation was carried out by re-docking the extracted ligand that had co-crystallized with the enzyme. The docking method was able to reproduce the experimentally observed conformation for ligands in the enzyme with an rmsd of less than 2 Å. AutoDock uses AutoGrid to calculate affinity maps, in which the dimensions of the grid box were 80 × 80 × 80, that were centered on the nitrogen atom N5 of FAD as described earlier.30 The Lamarckian genetic algorithm (LGA) was used for docking, and the number of GA runs was set to 100. All other docking parameters were used as defaults. For each docking result, the conformation with the lowest binding free energy was accepted as the most probable model of interaction. The conformations and ligand–enzyme interactions were analyzed by the Discovery studio visualizer v4.35
Supplementary Material
Acknowledgments
J. Iqbal is thankful to the Organization for the Prohibition of Chemical Weapons (OPCW), The Hague, The Netherlands and the Higher Education Commission of Pakistan for the financial support through Project No. 20-3733/NRPU/R&D/14/520.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00570e
References
- Reis J., Cagide F., Chavarria D., Silva T., Fernandes C., Gaspar A., Uriarte E., Remião F., Alcaro S., Ortuso F., Borges F. J. Med. Chem. 2016;59:5879–5893. doi: 10.1021/acs.jmedchem.6b00527. [DOI] [PubMed] [Google Scholar]
- Zaib S., Rizvi S. U. F., Aslam S., Ahmad M., Al-Rashida M., Iqbal J. Med. Chem. 2015;11:497–505. doi: 10.2174/1573406410666141229101130. [DOI] [PubMed] [Google Scholar]
- Mathew B., Suresh J., Anbazhagan S., Dev S. Biomed. Aging Pathol. 2014;4:297–301. [Google Scholar]
- Westlund K. N., Denney R. M., Rose R. M., Abell C. W. Neuroscience. 1988;25:439–456. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S., Dalvie D. K., Castagnoli N., Taylor T. J. Chem. Res. Toxicol. 2001;14:1139–1162. doi: 10.1021/tx010073b. [DOI] [PubMed] [Google Scholar]
- Mai A., Artico M., Esposito M., Sbardella G., Massa S., Befani O., Turini P., Giovannini V., Mondovì B. J. Med. Chem. 2002;45:1180–1183. doi: 10.1021/jm015578d. [DOI] [PubMed] [Google Scholar]
- Khan I., Bakht S. M., Ibrar A., Abbas S., Hameed S., White J. M., Rana U. A., Khan S. U.-D., Zaib S., Shahid M., Iqbal J. RSC Adv. 2015;5:21249–21267. [Google Scholar]
- Chimenti F., Maccioni E., Secci D., Bolasco A., Chimenti P., Granese A., Befani O., Turini P., Alcaro S., Ortuso F., Cardia M. C., Distinto S. J. Med. Chem. 2007;50:707–712. doi: 10.1021/jm060869d. [DOI] [PubMed] [Google Scholar]
- Zaib S., Rizvi S. U. F., Aslam S., Ahmad M., Abid S. M. A., Al-Rashida M., Iqbal J. Med. Chem. 2015;11:580–589. doi: 10.2174/1573406410666141226131252. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Kumar P., Joshi R., Iqbal P. F., Saleem K. Eur. J. Med. Chem. 2008;43:2029–2034. doi: 10.1016/j.ejmech.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Field H. J., Reading M. J. Antiviral Res. 1987;7:245–256. doi: 10.1016/0166-3542(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Divatia S. M., Rajani D. P., Rajani S. D., Patel H. D. Arabian J. Chem. 2014 doi: 10.1016/j.arabjc.2014.09.007. [DOI] [Google Scholar]
- Biot C., Pradines B., Sergeant M. H., Gut J., Rosenthal P. J., Chibale K. Bioorg. Med. Chem. Lett. 2007;17:6434–6438. doi: 10.1016/j.bmcl.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Xie W., Xie S., Zhou Y., Tang X., Liu J. Eur. J. Med. Chem. 2014;81:22–27. doi: 10.1016/j.ejmech.2014.05.001. [DOI] [PubMed] [Google Scholar]
- West D. X., Padhye S. B., Sonawane P. B. Struct. Bonding. 1991;76:1. [Google Scholar]
- West D. X., Liberta A. E., Padhye S. B., Chikate R. C., Sonawane P. B., Kumbhar A. S., Yerande R. G. Coord. Chem. Rev. 1993;123:49. [Google Scholar]
- Sharma P. K., Sawhney S. Bull. Chem. Soc. Jpn. 1993;66:3843–3846. [Google Scholar]
- Shavel, Jr J.., Mendham N. J. and Zinnes H., US Pat., 3408347, 1968.
- Tomita N., Hayashi Y., Suzuki S., Oomori Y., Aramaki Y., Matsushita Y., Iwatani M., Iwata H., Okabe A., Awazu Y., Isono O., Skene R. J., Hosfield D. J., Miki H., Kawamoto T., Hori A., Baba A. Bioorg. Med. Chem. Lett. 2013;23:1779–1785. doi: 10.1016/j.bmcl.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Barreca M. L., Manfroni G., Leyssen P., Winquist J., Kaushik-Basu N., Paeshuyse J., Krishnan R., Iraci N., Sabatini S., Tabarrini O., Basu A., Danielson U. H., Neyts J., Cecchetti V. Eur. J. Med. Chem. 2013;56:2270–2282. doi: 10.1021/jm301643a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B., Suresh J., Anbazhagan S. EXCLI J. 2014;13:437–445. [PMC free article] [PubMed] [Google Scholar]
- Aslam S., Ahmad M., Zia-ur-Rehman M., Montero C., Detorio M., Parvez M., Schinazi R. F. Arch. Pharmacal Res. 2014;37:1380–1393. doi: 10.1007/s12272-013-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Siddiqui H. L., Gardiner J. M., Parvez M., Aslam S. Med. Chem. Res. 2013;22:794–805. [Google Scholar]
- Ahmad M., Siddiqui H. L., Zia-ur-Rehman M., Parvez M. Eur. J. Med. Chem. 2010;45:698–704. doi: 10.1016/j.ejmech.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Siddiqui H. L., Ahmad S., Parvez M., Tizzard G. J. J. Chem. Crystallogr. 2010;40:1188–1194. [Google Scholar]
- Ahmad M., Siddiqui H. L., Azam M., Bukhari I. H., Parvez M. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2010;66:o616. doi: 10.1107/S1600536810005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Siddiqui H. L., Rizvi U. F., Ahmad S., Parvez M. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2010;66:o862. doi: 10.1107/S1600536810009359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stössel A., Schlenk M., Hinz S., Küppers P., Heer J., Gütschow M., Müller C. E. J. Med. Chem. 2013;56:4580–4596. doi: 10.1021/jm400336x. [DOI] [PubMed] [Google Scholar]
- Son S. Y., Ma J., Kondou Y., Yoshimura M., Yamashita E., Tsukihara T. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C., Wang J., Pisani L., Caccia C., Carotti A., Salvati P., Edmondson D. E., Mattevi A. J. Med. Chem. 2007;50:5848–5852. doi: 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- Toprakçí M., Yelekçi K. Bioorg. Med. Chem. Lett. 2005;15:4438–4446. doi: 10.1016/j.bmcl.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Labute P., Protonate 3D, Chemical Computing Group, 2007, http://www.chemcomp.com/journal/proton.htm.
- MOE, version 2014.0901; Chemical Computing Group (CCG), Montreal, Canada, http://www.chemcomp.com/MOEMolecular_Operating_ Environment.htm.
- Morris G. M., Huey R., Lindstrom W., Sanner M. F., Belew R. K., Goodsell D. S., Olson A. J. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accelrys Software Inc., Discovery Studio Modeling Environment, Release 4.0, Accelrys Software Inc., San Diego, CA, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.