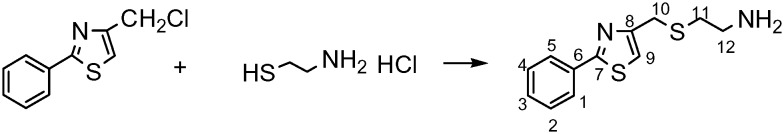 Synthesis and evaluation of antitumoral, antiinflammatory or inflammation-targeted properties of Pd(ii), Cu(ii), Re(i), and 99mTc(i) thiazole-based complexes is presented.
Synthesis and evaluation of antitumoral, antiinflammatory or inflammation-targeted properties of Pd(ii), Cu(ii), Re(i), and 99mTc(i) thiazole-based complexes is presented.
Abstract
A new thiazole-containing multidentate ligand 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, L, was synthesized and used to prepare new complexes of the formula PdIILCl2 (Pd–L), CuIIL2Cl2 (Cu–L) and fac-[Re/99mTcI(CO)3(L)]+ (Re/99mTc–L). The ligand L and the metal complexes were characterized spectroscopically. Furthermore, the structures of Re–L and Cu–L were elucidated by X-ray crystallography. Ligand L acts as a bidentate (Nth, S) chelator in Pd–L, as a bidentate (N, S) chelator in Cu–L and as a tridentate (Nth, S, N) chelator in Re–L. The radiotracer 99mTc–L was synthesized in high yield and characterised by HPLC comparison with the Re–L analog. The synthesized compounds were evaluated for their anti-inflammatory and cytotoxic properties. The compounds exhibited low anti-inflammatory activity with Pd–L showing the highest activity among them. The cytotoxic activity of the ligand and the complexes against several human cancer cell lines (cervical adenocarcinoma HeLa, colorectal adenocarcinoma LS-174T, lung adenocarcinoma A549, breast adenocarcinoma MDA-MB-231 and normal human lung fibroblast cell line MRC-5) was examined using the MTT assay. The complex Cu–L exhibited the highest cytotoxicity and the complex Pd–L showed the best tumor selectivity. The changes in the cell cycle phase distribution were determined by flow cytometry and it was found that ligand L shows the highest apoptotic activity. The biodistribution studies of 99mTc–L in mice showed fast tissue clearance. Of all the thiazole-containing compounds, the palladium complex appears to be more promising for future efforts.
1. Introduction
Metals have been used in medicine to treat various conditions since ancient times. Their use in the development of modern therapeutics increased tremendously after the discovery of the anticancer drug cisplatin. The advantages of metal complexes as synthons of drug molecules include the great chemical diversity of transition metals that, in combination with a variety of ligands available, can create a palette of compounds with a plethora of properties and offers the possibility to discover novel drugs with new mechanisms of action. Furthermore, due to the physical properties that a number of metals possess, their coordination compounds find application as imaging agents (radiodiagnostic) as well as contrast agents for magnetic resonance imaging.1
The search for new antitumor coordination compounds with increased tumor selectivity, broad spectrum of action and limited side-effects compared to the cisplatin prototype remains a challenging area of research and many efforts have focused on the development of either new improved derivatives of cisplatin or other non-platinum metal complexes as well as on the development of combinations of active organic drugs with metals.2,3 In this context, palladium analogs have been developed, generating new compounds with antitumor properties, since palladium has many chemical similarities with platinum. However, initial studies showed that the palladium compounds exchange ligands faster than the platinum analogs,4 thus limiting their access to the biological target. In the field of non-platinum compounds showing antitumor potential, copper-based complexes have been investigated on the assumption that endogenous metals may be less toxic.5 Organometallic complexes are attracting interest due to their kinetic stability and flexibility as building blocks for pharmaceutical applications. In particular, organometallic rhenium(i) tricarbonyl compounds have been shown to exhibit antitumor properties.6 In addition, rhenium radionuclides 186Re/188Re are both beta-emitters and are used for cancer radiotherapy.7 Furthermore, the radioactive surrogate metal of rhenium, technetium-99m, is a gamma-emitter and a protagonist in nuclear medicine imaging. Currently, research involving technetium-99m focuses on the development of tumor-targeted agents.8
Thiazole-containing compounds not only exist naturally as part of biologically important molecules, such as thiamine (vitamin B1) and bleomycin, but also include clinically useful drugs, such as abafungin, cefotaxime,9 meloxicam,10 thiabendazole,11 nizatidine, ritonavir,12 niridazole13etc. Thiazole derivatives exhibit a variety of pharmacological activities, such as antibacterial,14,15 fungicidal,16,17 antioxidant,18 anti-inflammatory,19,20 anticancer,21–24 anti-HIV,25,26 antibiotic,27 local anaesthetic,28 analgesic and antipyretic,29 against schizophrenia30 among others. For the above reasons, thiazole-containing pharmacophores are promising and are actively used in the development of new therapeutics.31
As a part of our continuing research on the coordination chemistry of multidentate ligands, this work focuses on the development of a new thiazole-containing metal chelator, 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, for the construction of new palladium(ii), copper(ii) and rhenium(i) tricarbonyl complexes. Given the fact that this ligand contains three different donor atoms, a thiazole-N (Nth), a thioether-S and an amine-N in combination with the different nature of first row transition metal copper, second row palladium and third row rhenium, three different coordination compounds were obtained. This diverse series of metal complexes was evaluated for cytotoxicity in a number of human adenocarcinoma cell lines as well as in a non-tumorous cell line. Furthermore, the anti-inflammatory properties of these compounds were evaluated. Finally, the technetium-99m radiotracer analog was also prepared and its biodistribution was studied in vivo (Fig. 1).
Fig. 1. Structures of the rhenium(i) tricarbonyl, copper(ii) and palladium(ii) complexes synthesized with the new ligand L.
2. Experimental
2.1. General
All the chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). [NEt4]2[ReBr3(CO)3] was prepared from Re2CO10 according to the literature32 which was then converted to [Re(H2O)3(CO)3]+.33 Precursor fac-[99mTc(CO)3(H2O)3]+ was prepared by a standard method where 1 mL of [99mTcO4–] 99Mo/99mTc generator eluate was added to a kit containing 5.5 mg NaBH4, 4 mg Na2CO3 and 15 mg Na–K tartrate, purged with CO gas, and the mixture was heated in boiling water for 30 min.34 The synthesis of 2-phenyl-4-chloromethyl thiazole was performed with a method similar to the one described in the literature.35 ESI-MS data were obtained on an MSQ Plus™ LC/MS (Thermo Scientific, Waltham, MA, USA). IR spectra were recorded as KBr pellets on a Perkin-Elmer FT-IR Spectrum BX spectrophotometer in the region 500–4000 cm–1. 1H and 13C NMR spectra were recorded on an Agilent DD2 500 MHz spectrometer and a Varian “Gemini 2000” (200 MHz) spectrometer. HPLC analysis was performed on an Agilent HP 1100 series pump, connected to a Gabi gamma detector (Raytest) and an HP 1100 multiple wavelength detector. Separations were achieved on an Agilent Eclipse XDB-C18 column (25 cm × 4.6 mm, 5 μm) eluted with a binary gradient system of solvent A: 0.1% TFA in water and solvent B: methanol at 1 mL min–1 flow rate. Initial composition consisted of 100% A–0% B which linearly converted to 25% A–75% B over 15 min and to 5% A–95% B from 15 to 20 min. The composition remained constant from 20 to 25 min at 95% B.
2.2. Chemical synthesis
2.2.1. Synthesis of 2-((2-phenylthiazol-4-yl)methylthio)ethanamine (L)
4-Chloromethyl-2-phenylthiazole (1 mmol, 209 mg) and 2-aminoethanethiol hydrochloride (1.5 mmol, 170.4 mg) were dissolved in a mixture of ethanol (8 ml)–water (5 ml), and then a solution of NaOH (2 M, 1.5 mL) was added dropwise. The reaction mixture was stirred at room temperature under N2 for 18 h (Scheme 1). Then, the solvent was evaporated in vacuo to dryness. The residue was redissolved in ethanol and the solid formed was filtered. The filtrate was condensed to dryness and the product was purified by silica gel column chromatography with dichloromethane–methanol–ammonium hydroxide (9 : 0.85 : 0.15) as the eluent. Yield: 210 mg (84%); tR = 15.29 min. IR (KBr): νmax/cm–1 3346.3, 3047.1, 2918.9, 1640.9, 1555.4, 1517.0, 1504.1, 1478.5, 1470.0, 1431.5, 1388.7, 1341.7, 1247.4, 1239.1, 1222.1, 1175.0, 1076.7, 1034.0, 1008.3, 996.9, 871.6, 824.6, 764.7, 696.3, 649.3, 585.2, 572.4, 400.1, 314.5. 1H NMR δ (500 MHz; CDCl3) 7.99–7.84 (m, 2H, Ph), 7.45–7.35 (m, 3H, Ph), 7.09 (s, 1H, H-9), 3.86 (s, 1H, H-10), 2.87 (t, J = 6.3 Hz, 2H, H-12), 2.66 (t, J = 6.3 Hz, 2H, H-11). 13C NMR δ (126 MHz; CDCl3) 168.32 (C-7), 154.78 (C-8), 133.47 (C-6), 128.87 (C-2/C-4), 126.5 (C-1/C-5), 115.15 (C-9), 40.88 (C-12), 36.10 (C-10), 31.35 (C-11). ESI(+)-MS (m/z): (C12H14N2S2) 250.06 calc. for [M + H]+, 251.12 found.
Scheme 1. Synthesis of 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, L.
2.2.2. Synthesis of the rhenium complex, fac-[Re(CO)3(L)] (Re–L)
A solution of [Re(H2O)3(CO)3]+ (0.15 mmol) in methanol (2 mL) was added a solution of ligand L (39.01 mg, 0.15 mmol) in methanol (5 mL) and the mixture was heated under reflux for 4 h. The solvent was evaporated to dryness, and the residue was crystallized by slow evaporation from methanol/water. Yield: 46 mg (51.1%); tR = 17.48 min. IR (KBr): νmax/cm–1 3431.2, 3215.0, 3112.9, 3068.5, 2965.3, 2024.2 (CO), 1915.7 (CO), 1602.6, 1383.3, 1243.1, 1171.9, 1047.5, 992.9, 926.6, 745.0, 696.2, 638.0, 528.6, 494.0. 1H NMR δ (500 MHz; CD3OD) 7.94 (s, 1H, H-9), 7.70–7.57 (m, 5H, Ph), 5.53 (br, 1H, NH), 4.78 (d, J = 16.9 Hz, 1H, H-10), 4.28 (d, J = 16.9 Hz, 1H, H-10), 3.91 (br, 1H, NH), 3.11 (ddd, J = 12.9, 5.9, 4.7 Hz, 1H, H-12), 2.84 (tdd, J = 10.0, 9.2, 5.4 Hz, 1H, H-11), 2.69 (tdd, J = 12.8, 8.3, 4.1 Hz, 1H, H-11), 2.55 (ddd, J = 13.1, 8.9, 4.1 Hz, 1H, H-12). 13C NMR δ (126 MHz; CD3OD) 175.15 (C-7), 154.86 (C-8), 133.16 (C-6), 131.50 (C-3), 129.28 (C-2/C-4), 129.02 (C-1/C-5), 118.73 (C-9), 48.08 (C-12), 36.76 (C-10), 35.55 (C-11). ESI(+)-MS (m/z): (C15H14N2S2O3Re) 521.00 calc. for [M]+, 521.11 found.
2.2.3. Synthesis of the copper(ii) complex, CuCl2L2 (Cu–L)
Copper(ii) chloride (13.4 mg, 0.1 mmol) was dissolved in methanol (5 mL) and a solution of ligand L (50.12 mg, 0.2 mmol) in methanol (10 mL) was added. The reaction mixture was stirred for 5 h. The greenish solid obtained was isolated by filtration, washed with water to remove any free copper and dried at room temperature. Single crystals of Cu–L suitable for X-ray measurements were obtained by slow recrystallization from a mixture of ether/methanol by evaporation. A deep green solid powder was obtained. Yield: 34.5 mg (54.33%); tR = 15.97 min. IR (KBr): νmax/cm–1 3448.61, 3280.67, 3233.89, 3113.87, 2921.14, 2651.08, 2177.65, 1623.54, 1576.25, 1514.44, 1498.85, 1459.71, 1438.01, 1409.54, 1304.01, 1235.56, 1113.64, 1096.68, 1050.10, 1001.87, 976.43, 926.86, 764.56, 727.60, 708.57, 691.18, 631.95, 599.41.
2.2.4. Synthesis of the palladium(ii) complex, PdCl2L (Pd–L)
An aqueous solution of K2[PdCl4] (25.01 mg, 0.1 mmol) was mixed with a methanolic solution of L (32.64 mg, 0.1 mmol) and the mixture reacted for 2 h at room temperature. A pale yellow precipitate of the complex Pd–L was obtained, filtered off, washed with cold water to remove any free palladium, and air-dried. Yield: 31.5 mg (80.36%); tR = 14.55 min. IR (KBr): νmax/cm–1 3444.54, 3190.00, 3109.44, 3090.44, 2978.04, 2933.41, 1618.76, 1577.52, 1495.83, 1459.64, 1404.80, 1322.99, 1236.67, 1136.19, 1074.33, 1001.96, 929.78, 843.47, 766.82, 712.83, 690.62. 1H NMR δ (200 MHz; DMSO-d6) 8.02–7.93 (m, 2H, Ph), 7.89 (s, 1H, H-9), 7.57–7.46 (m, 3H, Ph), 5.16 (s, 2H, NH2), 4.62 (d, J = 13.8 Hz, 1H, H-10), 4.34 (d, J = 13.9 Hz, 1H, H-10), 2.96–2.89 (m, 1H, H-11), 2.85–2.74 (m, 2H, H-12), 2.71–2.60 (m, 1H, H-11). 13C NMR δ (50 MHz, DMSO-d6) 168.09 (C-7), 149.59 (C-8), 132.80 (C-6), 130.67 (C-3), 129.40 (C-2/C-4), 126.40 (C-1/C-5), 120.24 (C-9), 47.70 (C-12), 37.22 (C-10), 35.83 (C-11). ESI(+)-MS (m/z): (C12H14N2S2Cl2Pd) 390.91 calc. for [M – Cl]+, 391.05 found.
2.3. X-Ray crystal structure determination
Crystals of both compounds were taken from the mother liquor and mounted at room temperature on a Bruker Kappa APEX 2 diffractometer equipped with a Triumph monochromator using Mo Kα radiation. Calculation of cell dimensions and crystal system determination were performed using at least 182 high θ reflections with I > 10σ(I). Data collection (φ and ω scans) and processing (cell refinement, data reduction and numerical absorption correction based on dimensions) were performed using the SAINT and SADABS programs.36,37 The structure was solved by means of the SUPERFLIP package.38 The CRYSTALS version 14.40 program package was used for structure refinement by full-matrix least-squares methods on F2 and the rest of all subsequent calculations.39 Molecular illustrations with 50% ellipsoid probability were drawn using CAMERON incorporated into crystals.40 All non-disordered non-hydrogen atoms have been anisotropically refined. All hydrogen atoms were found at their expected positions and were refined using appropriate riding constraints to the pivot atoms. Crystallographic details for both compounds are summarized in Table 1. Further details on the crystallographic studies as well as atomic displacement parameters are given as ESI† in the form of cif files.
Table 1. Crystallographic data for Re–L and Cu–L.
| Crystal data | ||
| CCDC deposition number | CCDC 1821097 | CCDC 1821098 |
| Chemical formula | C15H14N3O6ReS2 | C24H28Cl2CuN4S4 |
| M r | 582.62 | 635.23 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| Temperature (K) | 295 | 295 |
| a (Å) | 26.3056(13) | 19.911(11) |
| b (Å) | 9.4798(4) | 8.771(4) |
| c (Å) | 16.1346(6) | 8.088(4) |
| β (°) | 106.786(2) | 93.416(15) |
| V (Å3) | 3852.1(3) | 1409.9(12) |
| Z | 8 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm–1) | 6.56 | 1.28 |
| Crystal size (mm) | 0.43 × 0.19 × 0.16 | 0.17 × 0.16 × 0.06 |
| Absorption correction | Numerical | Numerical |
| T min, Tmax | 0.29, 0.35 | 0.81, 0.93 |
| Reflections | ||
| Measured | 38 228 | 23 119 |
| Independent | 9952 | 2636 |
| Observed [I > 2.0σ(I)] | 6648 | 1881 |
| R int | 0.021 | 0.034 |
| (sin θ/λ)max (Å–1) | 0.682 | 0.613 |
| Refinement | ||
| R[F2 > 2σ(F2)] | 0.036 | 0.047 |
| wR(F2) | 0.054 | 0.101 |
| S | 1.00 | 1.00 |
| No. of reflections | 6648 | 1881 |
| No. of parameters | 487 | 160 |
| H-Atom treatment | H-Atom parameters constrained | H-Atom parameters constrained |
| Δρmax, Δρmin (e Å–3) | 1.68, –2.74 | 0.65, –0.57 |
2.4. Synthesis and stability of 99mTc–L
The precursor [99mTc(CO)3(H2O)3]+ (5 mCi/185 MBq, 0.5 mL) (pH 6) was added via a syringe to a crimped vial with L (50 μL, 0.01 M) and the mixture was heated at 95 °C for 30 min. Complex formation was verified by HPLC. The HPLC-purified 99mTc complex (50 μL, approx. 10 MBq) was incubated with 0.5 mL of 0.1 M pH 7.4 PBS at 37 °C for 18 h. The mixtures were analyzed by HPLC at 1 and 18 h.
2.5. In vitro cell studies
2.5.1. In vitro cytotoxicity
The examined compounds were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. Human cancer cell lines, cervical adenocarcinoma HeLa, colorectal adenocarcinoma LS-174T, lung adenocarcinoma A549 and breast adenocarcinoma MDA-MB-231, and a normal cell line, embryonic lung fibroblast MRC-5, were grown in RPMI-1640 medium and seeded into 96-well microtiter plates (cell densities: 2000, 7000, 5000, 5000 and 5000 cells per well for each cell line, respectively). 24 hours later, five concentrations of complexes (compounds) (concentrations ranged from 12.5 μM to 200 μM for L and Pd–L and from 3.1 μM to 50 μM for Cu–L) were added to the cells, except the control cells, according to the standardized procedure.41 Control cells were grown only in nutrient medium. After 72 hours of treatment with the compounds, cytotoxic effects on cells were determined by the MTT assay, according to the method of Mosmann, Ohno and Abe.42,43 Cisplatin was used as a positive control.
To calculate the cell survival (%), the absorbance (A) of cells treated with various concentrations of the complexes was divided by the absorbance of the control and multiplied by 100. The calculated IC50 was the concentration of the compound that inhibited cell survival by 50% compared with the control. The results were obtained from three independent experiments and presented with standard deviations.
The conditions for cell culture growth were described earlier.41 The cell lines were received from the American Type Culture Collection (Manassas, VA, USA) and the reagents were products of Sigma-Aldrich, St. Louis, MO.
2.5.2. Cell cycle analysis
200 000 HeLa cells per well were seeded into 6-well plates overnight and then treated with IC50 and 2 × IC50 concentrations of the examined compounds for 24 hours. Collected cells were fixed in 70% ethanol and stored at –20 °C for one week. The cells were washed, treated with RNAse A (100 μg mL–1) at 37 °C for 30 min and stained with propidium iodide (PI) (40 μg mL–1).41 The results were obtained using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and CELLQuest software (BD Biosciences), for 10 000 cells per sample.
2.6. Animal studies
The animal studies were approved by the Aristotle University Committee for animal experimentation and were performed according to the EU guidelines. The animals were housed under standard conditions and received a diet of commercial food pellets and water ad libitum prior to experimentation.
2.6.1. Inhibition of the carrageenin-induced edema
Edema was induced in the right hind paw of mice (AKR) by the intradermal injection of 0.05 mL of 2% carrageenin in water. Both sexes were used, but pregnant females were excluded. Each group was composed of 6–10 animals. The tested compounds (0.01 mmol per kg body weight) were suspended in water with a few drops of Tween-80 and ground in a mortar before use and were given intraperitoneally simultaneously with the carrageenin injection. The mice were euthanized 3.5 h after the carrageenin injection. The difference between the weight of the injected and uninjected paws was calculated for each animal. It was compared with that in control animals (treated with water) and expressed as percent inhibition of the edema (CPE% values). Each experiment was performed in duplicate, and the standard deviation was less than 10%.
2.6.2. Biodistribution studies of 99mTc–L
Inflammation edema was induced in ten-week-old BALB/c mice of approximately 25 g weight by intramuscular injection of 2% carrageenin (0.1 mL) into their right hind limb 2 h prior to the experiment. Each animal was then injected intravenously with 370 kBq of the purified 99mTc–L in 0.1 mL saline. The animals were sacrificed at 5 min and 1 h post-injection (p.i.) by cervical dislocation followed by blood withdrawal and cardiectomy. Organs and tissues of interest were excised rapidly and weighed, and their radioactivity was determined using a γ-counter. The activity of the tissue samples was decay-corrected and calibrated by comparing the counts in the tissue with the counts of a standard solution corresponding to 1% of the injected dose. Counts of the sample and calibration aliquots were measured in the γ-counter at the same time. The amount of activity in the selected tissues and organs is expressed as a percent of the injected dose per gram tissue (% ID per g). Values are quoted as the mean % ID ± standard deviation (SD) of four mice per group. Blood volume and muscle mass were estimated at 7 and 43% of body weight, respectively.
3. Results and discussion
3.1. Synthesis and characterization
Ligand L, 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, was prepared in high yield (Scheme 1) and was used to prepare the new Re(i), Cu(ii), Pd(ii) and Tc(i) complexes. Ligand L is soluble in water, methanol, chloroform, dichloromethane and dimethyl sulfoxide. The reaction of [Re(H2O)3(CO)3]+ with an equimolar amount of ligand L in methanol led to the formation of complex fac-[Re(L)(CO)3], Re–L (Fig. 1). Re–L was crystallized by slow evaporation from methanol/water. A new copper(ii) complex with ligand L, having the formula CuCl2L2 (Fig. 1), was synthesized by the reaction of copper(ii) chloride and L in methanol in a Cu : L molar ratio of 1 : 2. Single crystals of Cu–L were obtained by slow recrystallization from a mixture of ether/methanol by evaporation. The complex Pd–L (Fig. 1) was obtained by mixing an aqueous solution of K2[PdCl4] and an equimolar amount of L in methanol.
The infrared spectra of the complexes were compared with that of the free ligand in order to determine the involvement of the coordination sites in chelation. The IR spectrum of the free ligand shows two bands at 1640.9 and 1555.4 cm–1 that correspond to the stretching vibrations of the thiazole ring, ν(–C N) and ν(–C C).44 A shift in the stretching frequency of these bands to a lower wavenumber was observed in the IR spectra of Re–L and Pd–L, which indicates the involvement of the thiazole nitrogen atom in the coordination, while the thiazole is not involved in the coordination in Cu–L.45 Strong bands in the region 2024–1915 cm–1 in the IR spectrum of the complex Re–L indicate the presence of three carbonyl ligands in a facial arrangement.46–48 The C–S–C band of the free ligand, which appears at 764.7 cm–1, showed a very little effect by complexation indicating that the sulfur atom is not involved in the chelation.49–51 Asymmetric ν(–CH2) stretching vibrations of moderate intensities were recorded around 2900 cm–1. Another band observed at 1455–1476 cm–1 was assigned to νasym(C6H5), while the band at 1220 cm–1 was assigned to ν(C–N) stretching. The coordination of nitrogen to the metal is further supported by the appearance of a new band of medium intensity in the region 440–490 cm–1 due to v(M–N) vibration.52
The 1H NMR spectrum of L was recorded in CDCl3, that of complex Re–L in CD3OD and that of complex Pd–L in DMSO-d6. The 1H NMR spectra of the ligand and the complexes showed signals in the aromatic region at δ 7.10–7.95 ppm that correspond to protons H-1 to H-9. The downfield shift of H-9 in the Re and Pd complexes signifies the coordination of the thiazole ring with the metals. In addition, the signals that correspond to the aliphatic protons H-10, H-11 and H-12 were shifted indicating the coordination mode of L with fac-[Re(CO)3]+ and PdCl2, respectively. In particular, for the Re–L complex where L has coordinated as a tridentate (Nth, S, N) chelator, each methylene proton exhibits different shifts due to the different chemical environments upon coordination with the metal, at 4.78 and 4.28 ppm for H-10, 2.84 and 2.69 ppm for H-11, and 3.11 and 2.55 ppm for H-12 and, in addition, two visible signals of the two NH2 protons at 5.53 and 3.91 ppm, respectively, indicating the coordination of the terminal nitrogen. In the palladium complex, the H-10 signals appear at 4.62 and 4.34 ppm and the H-11 signals are observed at 2.96–2.89 (m) and 2.71–2.60 (m) ppm, respectively, while the H-12 and NH2 protons show only one signal at 2.85–2.74 (m) ppm and 5.16 ppm, respectively, indicating that the terminal nitrogen has not coordinated with the metal. Therefore, from the NMR study, it was concluded that in Pd–L, L has coordinated as a bidentate (Nth, S) chelator. The 13C NMR spectrum of the ligand showed the carbon signals of the thiazole ring at 115, 155 and 168 ppm (ref. 53) as well as the signals due to the phenyl ring in the range of 126.0–133.43 ppm. Analogous signals were observed for the Re and Pd complexes as well.
The radio-tracer fac-[99mTc(CO)3L]+, 99mTc–L, was synthesized in high radiochemical yield (>95%) and was characterized by radio-HPLC comparison of its elution time (99mTc–L tR = 17.24 min) with that of its analog Re–L (tR = 17.47 min) (Fig. 2). The same elution time of the two compounds indicates that they share the same structure.46,47
Fig. 2. HPLC chromatograms of 99mTc–L (blue, front trace) and its analog Re–L (red, back trace).
3.2. Description of the structures
The monoclinic unit cell of the compound Re–L comprises eight independent monocationic complexes and eight nitrate counter ions. Each asymmetric unit contains two complexes with very slight differences between them. The ligand 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, L, acts as a tridentate chelating agent as it is coordinated to the Re(i) cation through the thiazole nitrogen atom, the methylthio sulfur atom and the terminal ethanamine nitrogen atom. This coordination mode forms two stable five-membered chelate rings as shown in Fig. 3. Three carbon atoms from three carbonyl molecules are also connected to the Re(i) cation fulfilling the final coordination number of six. The geometry around the metal is distorted octahedral. As the most axial vector passes through C14 and S1, these atoms lie on the octahedral axial positions while the equatorial plane is formed from N1, N2, C13 and C15 atoms (Fig. 2). The interatomic distances and angles of the compound are similar to those of analogous complexes32–34,46–48 found in the literature and are given in Table 2. Slight to moderate hydrogen bonding interactions arise between the nitrate oxygen atoms and the protons of the primary amine group.
Fig. 3. Molecular structure of one of the two Re–L complexes present in the asymmetric unit together with the nitrate counter ion.
Table 2. Selected bond lengths [Å] and angles [°] for Re–L.
| Distances | |||
| Re1–N1 | 2.204(5) | C1–C6 | 1.390(9) |
| Re1–N2 | 2.206(5) | C2–C3 | 1.386(9) |
| Re1–S1 | 2.4627(16) | C3–C4 | 1.361(11) |
| Re1–C13 | 1.889(6) | C4–C5 | 1.407(11) |
| Re1–C14 | 1.940(7) | C5–C6 | 1.369(8) |
| Re1–C15 | 1.874(6) | C6–C7 | 1.468(8) |
| N1–C12 | 1.488(7) | C8–C9 | 1.410(9) |
| N2–C7 | 1.300(8) | C8–C10 | 1.445(8) |
| N2–C8 | 1.370(7) | C11–C12 | 1.517(8) |
| S1–C10 | 1.778(6) | C13–O1 | 1.171(7) |
| S1–C11 | 1.830(6) | C14–O2 | 1.135(7) |
| S2–C7 | 1.737(6) | C15–O3 | 1.170(7) |
| S2–C9 | 1.654(8) | ||
| Angles | |||
| N1–Re1–N2 | 84.33(18) | S1–Re1–C14 | 177.13(19) |
| N1–Re1–S1 | 80.56(13) | C13–Re1–C14 | 89.5(3) |
| N2–Re1–S1 | 79.57(13) | N1–Re1–C15 | 91.6(2) |
| N1–Re1–C13 | 173.8(2) | N2–Re1–C15 | 173.6(2) |
| N2–Re1–C13 | 93.4(2) | S1–Re1–C15 | 94.9(2) |
| S1–Re1–C13 | 93.40(19) | C13–Re1–C15 | 90.1(3) |
| N1–Re1–C14 | 96.6(2) | C14–Re1–C15 | 85.4(3) |
| N2–Re1–C14 | 99.9(2) | C14–Re1–C15 | 85.4(3) |
In the monoclinic unit cell of Cu–L, two independent neutral centrosymmetric complexes are present. Each complex comprises one Cu(ii) cation placed on the center of symmetry and symmetrically coordinated to two ligand molecules and two chlorine anions. Each ligand 2-((2-phenylthiazol-4-yl)methylthio)ethanamine, L, acts in this case as a bidentate chelating agent. The Cu(ii) cation is coordinated to the methylthio sulfur atoms and the terminal ethanamine nitrogen atoms forming stable five-membered rings. The final distorted octahedral geometry around copper and the coordination number of six are fulfilled by the two chlorine anions (Fig. 4). The selected geometrical parameters of the complex are given in Table 3 and are in accordance with similar complexes found in the literature.54–56
Fig. 4. Molecular structure of the Cu–L complex.
Table 3. Selected bond lengths [Å] and angles [°] for Cu–L.
| Distances | |||
| Cu1–Cl1 | 2.4010(16) | C6–C7 | 1.454(7) |
| Cu1–N1 | 2.004(4) | C7–S2 | 1.701(5) |
| Cu1–S1 | 2.7163(16) | C8–C9 | 1.292(7) |
| N1–C12 | 1.455(6) | C8–C10 | 1.470(7) |
| N2–C7 | 1.316(6) | C9–S2 | 1.722(5) |
| N2–C8 | 1.305(6) | C10–S1 | 1.817(5) |
| C1–C2 | 1.397(7) | C11–C12 | 1.506(7) |
| C1–C6 | 1.392(7) | C11–S1 | 1.762(5) |
| C2–C3 | 1.391(8) | ||
| Angles | |||
| S1i–Cu1–Cl1i | 84.27(6) | N1i–Cu1–N1 | 180 |
| S1i–Cu1–N1i | 82.83(12) | Cl1–Cu1–N1 | 88.71(12) |
| Cl1i–Cu1–N1i | 88.71(12) | S1i–Cu1–S1 | 180 |
| S1i–Cu1–Cl1 | 95.73(6) | Cl1i–Cu1–S1 | 95.73(4) |
| Cl1i–Cu1–Cl1 | 180 | N1i–Cu1–S1 | 97.17(13) |
| N1i–Cu1–Cl1 | 91.29(13) | Cl1–Cu1–S1 | 84.27(4) |
| S1i–Cu1–N1 | 97.17(12) | N1–Cu1–S1 | 82.83(13) |
| Cl1i–Cu1–N1 | 91.29(12) | ||
3.3. Anti-inflammatory activity
The compounds were evaluated for their anti-inflammatory activity in vivo. The results are shown in Fig. 5. As can be seen from these data, our compounds showed low to moderate anti-inflammatory activity. The best activity was observed for Pd–L (40.9% CPE), where the indomethacin standard exhibited 47% CPE, followed by Cu–L (35.1% CPE), L (30.1% CPE) and Re–L (21.4% CPE) which showed the lowest anti-inflammatory activity. The study of the structure–activity relationship revealed that the activity of these complexes depends on the metal. Thus, introduction of Cu to the complex increases the anti-inflammatory activity of the initial ligand. The replacement of Cu with Pd furnished the most active compound, while introduction of Re diminished the anti-inflammatory activity compared to the initial ligand. Since none of the compounds tested exhibited significant activity in this in vivo test, they were not studied further (e.g. to determine anti-COX properties that would allow us to explain their mode of action).
Fig. 5. % Inhibition of carrageenan paw edema in mice after injection of 0.01 mmol of the ligand L or the M–L complexes, where M = Re, Cu or Pd.
3.4. In vitro cytotoxicity
The viability of a series of adenocarcinoma cell lines as well as a normal cell line was evaluated by the MTT assay after treatment of the cells for 72 h with all the compounds. Our compounds showed good to moderate and dose-dependent cytotoxic effects against various adenocarcinoma cell lines (Table 4). Ligand L and palladium complex Pd–L exhibited moderate to low cytotoxicity, with L showing higher cytotoxicity against the HeLa, LS-174T and A549 cancer cell lines, while the cytotoxicity had an inverse trend in the case of the MDA-MB-231 cancer cell line, against which Pd–L showed higher cytotoxicity compared to L. The complex Pd–L shows the highest activity against HeLa cells, among the tested cell lines, and furthermore it exhibited the lowest toxicity, two to three times lower, in normal MRC-5 cells compared to the cancer cells. In general, Pd–L shows good selectivity. Therefore, Pd–L shows better selectivity compared to L with selectivity indexes of 3.04 for Pd–L and 2.79 for L, respectively, for HeLa cells. The complex Cu–L exhibited the highest cytotoxicity among the other investigated compounds. In particular, it was found to be more cytotoxic against the A549 and MDA-MB-231 cell lines compared to cisplatin. Nevertheless, it was found to be more cytotoxic against the normal MRC-5 cell line, which suggests poor selectivity.
Table 4. Concentrations of compounds that induced 50% decrease in target cell survival.
| Compound | HeLa | LS-174T | IC50 (μM) | ||
| A549 | MDA-MB-231 | MRC-5 | |||
| L | 38.67 ± 0.72 | 46.29 ± 3.2 | 103.46 ± 3.95 | 131.16 ± 2.22 | 108.14 ± 1.12 |
| Pd–L | 47.52 ± 1.53 | 60.28 ± 2.82 | 114.75 ± 2.19 | 76.92 ± 2.17 | 144.68 ± 2.67 |
| Cu–L | 6.13 ± 0.03 | 7.28 ± 0.8 | 7.38 ± 0.2 | 3.76 ± 0.16 | 4.42 ± 0.22 |
| Cisplatin | 2.24 ± 0.17 | 5.38 ± 1.34 | 10.12 ± 0.58 | 11.62 ± 1.73 | 39.85 ± 0.64 |
3.5. Cell cycle analysis
Cell cycle analysis in the HeLa cell line (which was the most sensitive to L and Pd–L among the tested cell lines) was performed in order to obtain an early estimate of whether there is any apoptotic response of the cells after treatment with the compounds for 24 h, at two different concentrations (IC50 and 2 × IC50). Results from our experiments showed a dose-dependent increase in the percentages of HeLa cells in the subG1 phase 24 hours after exposure (Fig. 6). The cell cycle studies indicate that Pd–L as well as ligand L induced an appreciable increase in the subG1 phase (cells entering apoptosis). The highest increase in the percentage of HeLa cells in the subG1 phase was observed after treatment with ligand L (12.64% at IC50 and 18.14% at 2 × IC50 for control = 5.53%), followed by Pd–L (9.37% at IC50 and 14.96% at 2 × IC50). The significant increase in the percentages of treated cells in the subG1 phase after exposure to the tested compounds indicates the ability of the investigated compounds to induce apoptosis. For complex Cu–L, no accumulation of apoptotic cells was measured and therefore no conclusion as to its potential mechanism of cytotoxicity can be drawn at this point. It should be noted that the most sensitive cell lines to Cu–L were not HeLa but MDA-MB-231 and MRC-5. However, because it exhibited no tumor selectivity, it was not evaluated any further.
Fig. 6. Changes in the cell cycle phase distribution of HeLa cells after treatment for 24 h.
3.6. Biodistribution in mice
The biodistribution studies of 99mTc–L (Table 5) showed a fast blood clearance of 0.46 ± 0.06% ID per g at 1 h p.i. Its liver and kidney uptake was 14.50 ± 0.76% ID per g and 9.75 ± 1.10% ID per g at 1 h p.i., respectively. In 1 h, 23.38 ± 2.3% of the injected dose was found in the intestine and 22.51 ± 3.63% ID in the urine. No accumulation of the radiotracer was observed in the edema compared to normal muscle at 1 h p.i. with values of 0.47 ± 0.08 and 0.46 ± 0.04% ID per g, respectively. The heart uptake was 2.18 ± 0.18% ID per g at 5 min p.i. and 1.52 ± 0.14% ID per g at 1 h, which is attributed to the cationic nature of 99mTc–L.57
Table 5. Biodistribution of 99mTc–L in mice (mean ± SD).
| % ID per organ |
% ID per g |
|||
| Organ | 5 min | 1 h | 5 min | 1 h |
| Blood | 2.83 ± 0.22 | 0.87 ± 0.12 | 1.49 ± 0.18 | 0.46 ± 0.06 |
| Heart | 0.25 ± 0.00 | 0.20 ± 0.03 | 2.18 ± 0.18 | 1.53 ± 0.14 |
| Liver | 25.69 ± 2.12 | 22.22 ± 1.50 | 17.18 ± 1.18 | 14.50 ± 0.76 |
| Lungs | 0.25 ± 0.01 | 0.15 ± 0.03 | 1.87 ± 0.42 | 1.03 ± 0.13 |
| Muscle | 7.38 ± 0.04 | 5.25 ± 0.10 | 0.63 ± 0.03 | 0.46 ± 0.04 |
| Kidneys | 32.62 ± 2.27 | 3.55 ± 0.41 | 93.81 ± 7.66 | 9.75 ± 1.10 |
| Spleen | 0.09 ± 0.01 | 0.05 ± 0.00 | 1.43 ± 0.05 | 0.73 ± 0.08 |
| Intestine | 16.60 ± 0.95 | 23.38 ± 2.30 | 10.37 ± 0.94 | 15.56 ± 1.83 |
| Stomach | 0.54 ± 0.08 | 1.13 ± 0.61 | 2.27 ± 0.17 | 4.91 ± 3.72 |
| Urine | 6.80 ± 1.15 | 22.51 ± 3.63 | — | — |
| Edema | — | — | 0.82 ± 0.11 | 0.47 ± 0.08 |
In this work, we synthesized and evaluated the cytotoxicity as well as the anti-inflammatory properties of a new thiazole-containing ligand L and its respective metal complexes M–L (M = Pd, Cu, Re). It is interesting as a starting point that the ligand itself exhibits moderate cytotoxicity and anti-inflammatory activity. Thiazoles have been shown in the literature to exhibit a variety of biological properties, and anti-inflammatory and antitumor properties are among them. The respective complexes M–L exhibit biological properties that are mostly related to the nature of the metal. The palladium complex Pd–L has structural similarities to cisplatin; it is square planar, it has a neutral bidentate ligand and two cis chlorine atoms that are exchangeable in aqueous solution. It is known that palladium complexes are about 105 times more reactive than their Pt(ii) analogs (Pd being a second row metal vs. Pt being a third row one) leading to rapid hydrolysis of the leaving group. The hydrolysed species [Pd(L)(H2O)2]2+, being cationic, does not diffuse through the cellular membranes and therefore it cannot interact with its targets. This would explain its lower toxicity in the cell lines in comparison to cisplatin. However, its good selectivity along with its moderate anti-inflammatory activity makes it the most promising compound in this work. In future work, a bidentate labile ligand could be used instead of the two chlorines to improve the complex's pharmacological profile.
The copper complex is octahedral and has two axial chlorines that could be exchanged in water, but since they are not in cis conformation as in the palladium complex, their structures cannot be directly compared. On the other hand, copper(ii) is very reactive and transchelation could occur in vivo, in either axial or equatorial positions. Its cytotoxicity was found to be very high, comparable to that of cisplatin against all the cancer cell lines, however it was more toxic against normal cells which renders it unsuitable as an anticancer agent. Its anti-inflammatory properties were comparable to those of L and Pd–L.
It should be noted that initially the rhenium complex Re–L was included in the cytotoxicity study, however in preliminary studies it was found to be inactive, hence it was not studied further (data not included). As far as its anti-inflammatory properties are concerned, Re–L was found to be the least active. Rhenium(i) tricarbonyl complexes with tridentate ligands are inert and this could explain the absence of biological activity observed in Re–L. Its in vivo inertness can be perceived by extrapolation of the biodistribution results of its radioactive analog 99mTc–L, where fast clearance from circulation was observed via renal and hepatobiliary excretion.
4. Conclusions
The new tridentate thiazole-containing ligand coordinated in different modes with each of the three metals used, Cu(ii), Pd(ii) and Re(i). With copper(ii), it formed an octahedral homodimeric (S, N) complex, with palladium(ii) a bidentate (Nth, S) chelate and with the Re tricarbonyl core a tridentate (Nth, S, N) cationic complex. The Re–L compound did not exhibit significant anti-inflammatory properties, while the analogous 99mTc–L radiotracer did not exhibit in vivo accumulation in carrageenan-induced edema in the right hind limb. Compounds L and Pd–L exhibited similarly moderate antitumor activity against the tested adenocarcinoma cell lines where the tumor selectivity of Pd–L showed the most promise. L and Pd–L both induced apoptosis in HeLa cells. Cu–L was the most cytotoxic and exhibited poor selectivity. In terms of the anti-inflammatory properties of these compounds, an increase in their activity was observed in Cu–L and Pd–L versus the ligand L, while Pd–L exhibited the most significant anti-inflammatory properties among the tested compounds. Overall, Pd–L exhibited the most promising biological properties in terms of both antitumor and anti-inflammatory activities and future studies could focus on the development of palladium complexes with an improved pharmacological profile.
Conflicts of interest
The authors declare no competing interest.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Ministry of Education, Science and Technological Development of the Republic of Serbia (project no. 172016 and 175011).
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1821097 and 1821098. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8md00067k
References
- Guo Z., Sadler P. J. Angew. Chem., Int. Ed. 1999;38:1513–1531. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghani N. T., Mansour A. M. Eur. J. Med. Chem. 2012;47:399. doi: 10.1016/j.ejmech.2011.11.008. [DOI] [PubMed] [Google Scholar]
- (a) Roy S., Westmaas J. A., Buda F., Reedijk J. J. Inorg. Biochem. 2009;103:1278. doi: 10.1016/j.jinorgbio.2009.07.004. [DOI] [PubMed] [Google Scholar]; (b) Montagner D., Gandin V., Marzano C., Longato B. J. Inorg. Biochem. 2011;105:919. doi: 10.1016/j.jinorgbio.2011.03.009. [DOI] [PubMed] [Google Scholar]
- (a) Akdi K., Vilaplana R. A., Kamah S., Navarro J. A. R., Salas J. M., González-Vílcheza F. J. Inorg. Biochem. 2002;90:51. doi: 10.1016/s0162-0134(02)00370-7. [DOI] [PubMed] [Google Scholar]; (b) Matilla A., Tercero J. M., Dung N. H., Voissat B., Pérez J. M., Alonso C., Martín-Ramos D., Niclós-Gutiérrez J. J. Inorg. Biochem. 1994;55:235. doi: 10.1016/0162-0134(94)85008-9. [DOI] [PubMed] [Google Scholar]
- Marzano C., Pellei M., Tisato F., Santini C. Anti-Cancer Agents Med. Chem. 2009;9:185–211. doi: 10.2174/187152009787313837. [DOI] [PubMed] [Google Scholar]
- Leonidova A., Gasser G. ACS Chem. Biol. 2014;9(10):2180–2193. doi: 10.1021/cb500528c. [DOI] [PubMed] [Google Scholar]
- Donnelly P. S. Dalton Trans. 2011;40:999–1010. doi: 10.1039/c0dt01075h. [DOI] [PubMed] [Google Scholar]
- Papagiannopoulou D. J. Labelled Compd. Radiopharm. 2017;60:502–520. doi: 10.1002/jlcr.3531. [DOI] [PubMed] [Google Scholar]
- Chhabria T. M., Patel S., Modi P., Brahmksatriya P. S. Curr. Top. Med. Chem. 2016;16:2841–2862. doi: 10.2174/1568026616666160506130731. [DOI] [PubMed] [Google Scholar]
- Engelhardt G., Homma D., Schlegel K., Utzmann R., Schnitzler C. Inflammation Res. 1995;44(10):423–433. doi: 10.1007/BF01757699. [DOI] [PubMed] [Google Scholar]
- Van Arman G. G., Campbell W. C. Tex. Rep. Biol. Med. 1975;33(2):303–311. [PubMed] [Google Scholar]
- De Souza M. V. N., De Almeida M. V. Quim. Nova. 2003;26:366–373. [Google Scholar]
- Kilpatrick M. E., El Masry N. A., Bassily S., Farid Z. Am. J. Trop. Med. Hyg. 1982;31:1164–1167. doi: 10.4269/ajtmh.1982.31.1164. [DOI] [PubMed] [Google Scholar]
- (a) Holla B. S., Malini K. V., Rao B. S., Sarojini B. K., Kumari N. S. Eur. J. Med. Chem. 2003;38:313. doi: 10.1016/s0223-5234(02)01447-2. [DOI] [PubMed] [Google Scholar]; (b) Verma A., Saraf S. K. Eur. J. Med. Chem. 2008;43:897. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]; (c) Aridoss G., Amirthaganesan S., Kim M. S., Kim J. T., Jeong Y. T. Eur. J. Med. Chem. 2009;44:4199. doi: 10.1016/j.ejmech.2009.05.015. [DOI] [PubMed] [Google Scholar]; (d) Abdel-Wahab B. F., Abdel-Aziz H. A., Ahmed E. M. Eur. J. Med. Chem. 2009;44:2632–2635. doi: 10.1016/j.ejmech.2008.09.029. [DOI] [PubMed] [Google Scholar]
- (a) Kouatly O., Geronikaki A., Zoumpoulakis P., Camoutsis Ch., Sokovic M., Ciric A., Glamoclija J. Bioorg. Med. Chem. 2010;18(1):426–443. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]; (b) Zablotskaya A., Segal I., Geronikaki A., Eremkina T., Belyakov S., Petrova M., Shestakova I., Zvejniece L., Vizma Nikolajeva V. Eur. J. Med. Chem. 2013;70:846–856. doi: 10.1016/j.ejmech.2013.10.008. [DOI] [PubMed] [Google Scholar]; (c) Haroun M., Tratrat C., Tsolaki E., Geronikaki A. Comb. Chem. High Throughput Screening. 2016;19(1):51–57. doi: 10.2174/1386207319666151203002348. [DOI] [PubMed] [Google Scholar]
- (a) Cukurovali A., Yilmaz I., Gur S., Kazaz C. Eur. J. Med. Chem. 2006;41:201. doi: 10.1016/j.ejmech.2005.01.013. [DOI] [PubMed] [Google Scholar]; (b) Bharti S. K., Nath G., Tilak R., Singh S. K. Eur. J. Med. Chem. 2010;45:651–660. doi: 10.1016/j.ejmech.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Mishra L., Singh V. K. Agric. Biol. Chem. 1991;55:1883. [Google Scholar]
- (a) De S., Adhikari S., Tilak-Jain J., Menon V. P., Devasagayam T. P. A. Chem.-Biol. Interact. 2008;173:215. doi: 10.1016/j.cbi.2008.03.011. [DOI] [PubMed] [Google Scholar]; (b) Geronikaki A. A., Pitta E. P., Liaras K. S. Curr. Med. Chem. 2013;20(36):4460–4480. doi: 10.2174/09298673113209990143. [DOI] [PubMed] [Google Scholar]; (c) Gouda M. A., Berghot M. A., Baz E. A., Hamama W. S. Med. Chem. Res. 2012;21(7):1062–1070. [Google Scholar]
- Franklin P. X., Pillai A. D., Rathod P. D., Yerande S., Nivsarkar M., Padh H., Vasu K. K., Sudarsanam V. Eur. J. Med. Chem. 2008;43:129. doi: 10.1016/j.ejmech.2007.02.008. [DOI] [PubMed] [Google Scholar]
- (a) Kouatly O., Geronikaki A., Kamoutsis Ch., Hadjipavlou-Litina D., Eleftheriou Ph. Eur. J. Med. Chem. 2009;44:1198–1204. doi: 10.1016/j.ejmech.2008.05.029. [DOI] [PubMed] [Google Scholar]; (b) Lagunin A. A., Geronikaki A., Eleftheriou P. T., Hadjipavlou-Litina D. I., Filimonov D. A., Poroikov V. V. J. Med. Chem. 2008;51:1601–1609. doi: 10.1021/jm701496h. [DOI] [PubMed] [Google Scholar]
- Liu Z. Y., Wang Y. M., Li Z. R., Jiang J. D., Boykin D. W. Bioorg. Med. Chem. Lett. 2009;19(19):5661–5664. doi: 10.1016/j.bmcl.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Luzina E. L., Popov A. V. Eur. J. Med. Chem. 2009;44:4944–4953. doi: 10.1016/j.ejmech.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Turan-Zitouni G., Altıntop M. D., Özdemir A., Kaplancıklı Z. A., Çiftçi G. A., Temel H. E. Eur. J. Med. Chem. 2016;107:288–294. doi: 10.1016/j.ejmech.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Gomha S. M., Kheder N. A., Abdelaziz M. R., Mabkhot Y. N., Alhajoj A. Chem. Cent. J. 2017;11:25. doi: 10.1186/s13065-017-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Pitta E., Crespan E., Geronikaki A., Maga G., Samuele A. Lett. Drug Des. Discovery. 2010;7(4):228–234. [Google Scholar]; (b) Pitta E., Geronikaki A., Surmava S., Eleftheriou Ph., Mehta V., Van der Eycken E. J. Enzyme Inhib. Med. Chem. 2013;28(1):113–122. doi: 10.3109/14756366.2011.636362. [DOI] [PubMed] [Google Scholar]
- Madni M., Hameed S., Ahmed M. N., Tahir M. N., Al-Masoudi N. A., Pannecouque C. Med. Chem. Res. 2017;26:2653–2665. [Google Scholar]
- Mostafa M. S., El-Salam N. M. A. Der Pharma Chemica. 2013;5:1–7. [Google Scholar]
- Geronikaki A., Vicini P., Theophilidis G., Lagunin A., Poroikov V., Dabarakis N., Modarresi H., Dearden J. C. Eur. J. Med. Chem. 2009;44(2):473–481. doi: 10.1016/j.ejmech.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Saravanan G., Alagarsamy V., Prakash C. R., Kumar P. D., Selvam T. P. Asian J. Res. Pharm. Sci. 2011;1(4):134–138. [Google Scholar]
- Gupta V. Sci. Int. 2013;1:253–260. [Google Scholar]
- Yenilmez H. Y., Sevim A. M., Bayır Z. A. Synth. Met. 2013;176:11–17. [Google Scholar]
- Alberto R., Egli A., Abram U., Hegetschweiler K., Gramlich V., Schubiger P. A. J. Chem. Soc., Dalton Trans. 1994:2815–2820. [Google Scholar]
- Kydonaki T. E., Tsoukas E., Mendes F., Hatzidimitriou A. G., Paulo A., Papadopoulou L. C. J. Inorg. Biochem. 2016;160:94–105. doi: 10.1016/j.jinorgbio.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Schibli R., La Bella R., Alberto R., Garcia-Garayoa E., Ortner K., Abram U. Bioconjugate Chem. 2000;11:345–351. doi: 10.1021/bc990127h. [DOI] [PubMed] [Google Scholar]
- Sprague J. M., Land A. H., Ziegler C. J. Am. Chem. Soc. 1946;68:2155–2159. doi: 10.1021/ja01215a010. [DOI] [PubMed] [Google Scholar]
- Bruker Analytical X-ray Systems, Inc. Apex2, Version 2 User Manual, M86-E01078, Madison, WI, 2006.
- Siemens Industrial Automation, Inc., SADABS: Area-Detector Absorption Correction, Madison, WI, 1996.
- Betteridge P. W., Carruthers J. R., Cooper R. I., Prout K., Watkin D. J. J. Appl. Crystallogr. 2003;36:1487. [Google Scholar]
- Palatinus L., Chapuis G. J. Appl. Crystallogr. 2007;40:786–790. [Google Scholar]
- Watkin D. J., Prout C. K. and Pearce L. J., CAMERON, Chemical Crystallography Laboratory, Oxford, UK, 1996.
- Matić I. Z., Aljančić I., žiŽak ž., Vajs V., Jadranin M., Milosavljević S., Juranić Z. BMC Complementary Altern. Med. 2013;13:36. doi: 10.1186/1472-6882-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ohno M., Abe T. J. Immunol. Methods. 1991;145:199–203. doi: 10.1016/0022-1759(91)90327-c. [DOI] [PubMed] [Google Scholar]
- Venkatraman R., Hossain H. A., Fronczek F. R. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2010;66:541–542. doi: 10.1107/S1600536810013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos C. A., Fanourgakis P. V., Christidis P. C., Nikolov G. Polyhedron. 1999;18:1661–1668. [Google Scholar]
- (a) He H., Lipowska M., Xu X., Taylor A. T., Carlone M., Marzilli L. G. Inorg. Chem. 2005;44:5437–5446. doi: 10.1021/ic0501869. [DOI] [PubMed] [Google Scholar]; (b) Karagiorgou O., Patsis G., Pelecanou M., Raptopoulou C. P., Terzis A., Siatra-Papastaikoudi T., Alberto R., Pirmettis I., Papadopoulos M. Inorg. Chem. 2005;44:4118–4120. doi: 10.1021/ic050254r. [DOI] [PubMed] [Google Scholar]; (c) He H., Lipowska M., Xu X., Taylor A. T., Marzilli L. G. Inorg. Chem. 2007;46:3385–3394. doi: 10.1021/ic0619299. [DOI] [PubMed] [Google Scholar]
- (a) Papagiannopoulou D., Makris G., Tsoukalas C., Raptopoulou C. P., Terzis A., Pelecanou M., Pirmettis I., Papadopoulos M. S. Polyhedron. 2010;29:876–880. [Google Scholar]; (b) Karagiorgou O., Papagiannopoulou D., Kyprianidou P., Patsis G., Panagiotopoulou A., Tsoukalas C., Raptopoulou C. P., Pelecanou M., Pirmettis I., Papadopoulos M. Polyhedron. 2009;28:3317–3321. [Google Scholar]; (c) He H., Morley J. E., Twamley B., Groeneman R. H., Bucar D.-K., MacGillivray L. R., Benny P. D. Inorg. Chem. 2009;48:10625–10634. doi: 10.1021/ic901159r. [DOI] [PubMed] [Google Scholar]; (d) Papagiannopoulou D., Tsoukalas C., Makris G., Raptopoulou C. P., Psycharis V., Leondiadis L., Gdniazdowska E., Koźminski P., Fuks L., Pelecanou M., Pirmettis I., Papadopoulos M. S. Inorg. Chim. Acta. 2011;378:333–337. [Google Scholar]
- (a) Alves S., Paulo A., Correia J. D. G., Domingos Â., Santos I. J. Chem. Soc., Dalton Trans. 2002:4714–4719. [Google Scholar]; (b) Vitor R. F., Alves S., Correia J. D. G., Paulo A., Santos I. J. Organomet. Chem. 2004;689:4764–4774. [Google Scholar]
- Nakamoto K., Infrared and raman spectra of inorganic and coordination compounds, Part A & B, John Wiley & Sons, NewYork, 5th edn, 1998. [Google Scholar]
- Neelakantan M. A., Marriappan S. S., Dharmaraja J., Jeyakumar T., Muthukumaran K. Spectrochim. Acta, Part A. 2008;71:628–635. doi: 10.1016/j.saa.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Omar M. M., Mohamed G. G. Spectrochim. Acta, Part A. 2005;61:929–936. doi: 10.1016/j.saa.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Kumari A., Tandon J. P., Singh R. V. Appl. Organomet. Chem. 1993;7:655. [Google Scholar]
- Abd-Elzaher M. M. J. Chin. Chem. Soc. 2004;51:499–504. [Google Scholar]
- Hossain M. E., Alam M. N., Begum J., Akbar Ali M., Nazimuddin M., Smith F. E., Hynes R. C. Inorg. Chim. Acta. 1996;249:207–213. [Google Scholar]
- Addison A. W., Rao T. N., Reedijk J., van Rijn J., Verschoor G. C. J. Chem. Soc., Dalton Trans. 1984:1349–1356. [Google Scholar]
- González-Álvarez M., Alzuet G., Borrás J., del Castillo Agudo L., Montejo-Bernardo J. M., García-Granda S. J. Biol. Inorg. Chem. 2003;8:112–120. doi: 10.1007/s00775-002-0394-7. [DOI] [PubMed] [Google Scholar]
- (a) Platts E. A., North T. L., Pickett R. D., Kelly J. D. J. Nucl. Cardiol. 1995;2:317–326. doi: 10.1016/s1071-3581(05)80076-5. [DOI] [PubMed] [Google Scholar]; (b) Carvalho P., Chiu M. L., Kronauge J. F., Kawamura M., Jones A. G., Holman B. L., Piwnica-Worms D. J. Nucl. Med. 1992;33:1516–1522. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.