With the increasing incidence of pathogenic fungi and drug-resistant fungi in clinic, it has become very important to develop the novel rate-limiting enzyme 14α-demethylase (CYP51) as an antifungal inhibitor.
With the increasing incidence of pathogenic fungi and drug-resistant fungi in clinic, it has become very important to develop the novel rate-limiting enzyme 14α-demethylase (CYP51) as an antifungal inhibitor.
Abstract
With the increasing incidence of pathogenic fungi and drug-resistant fungi in clinic, it has become very important to develop the novel rate-limiting enzyme 14α-demethylase (CYP51) as an antifungal inhibitor. In this study, a method involving structure-based virtual screening was employed. First, a publicly available database was obtained from the Dow Chemical Company, and the database was screened by the designed pharmacophore model of CYP51 inhibitors. Then, the pharmacophore search hits were docked into the CYP51 crystal structure. Finally, sixteen compounds were selected for in vitro antifungal inhibition assay, and most of the compounds showed a certain degree of antifungal activity. In particular, compounds 3, 4, and 9 exhibited significant antifungal and anti-drug resistance activities by blocking the synthesis of ergosterol. The molecular docking and ADME/T properties of the compounds 3, 4, and 9 were further predicted, and the results indicated that they can form hydrophobic and coordination interactions with the active sites of CYP51. At the same time, compounds 4 and 9 showed promising drug-like properties. This study reveals that the compounds can be further optimized and developed as lead compounds.
1. Introduction
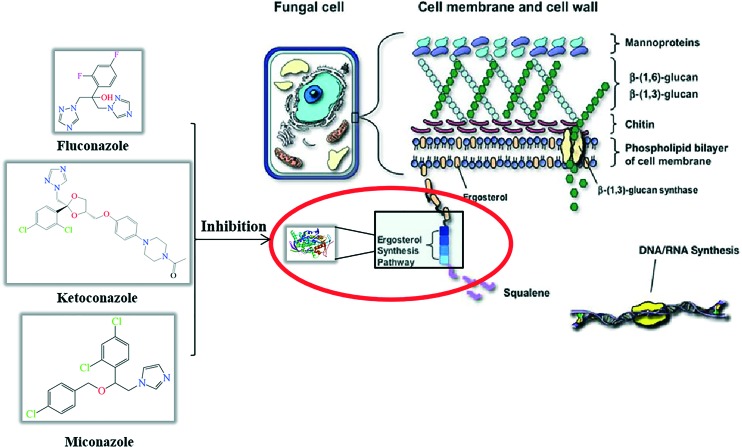
With the abuse of broad spectrum antibiotics and anticancer and immunosuppressive agents in clinic, deep fungal infection and resistant fungal infections have been increasing dramatically; these infections are associated with high mortality, and they are difficult to cure, especially in immunocompromised patients.1,2 Some fungal infections present a serious threat to human health. Therefore, it is becoming increasingly important to find novel and efficient antifungal inhibitors.3 Clinically, the design and development of antifungal inhibitors are mainly aimed at influencing the biosynthesis pathways of fungal glucan and ergosterol.4 The inhibitors of glucan synthase are mainly some antibiotics and although they exhibit good curative effect, there are some disadvantages: they cannot be taken orally, they are difficult to synthesize, and they are expensive.5 In contrast, most of the ergosterol synthase inhibitors do not have these associated problems and thus, they are used to achieve the goal of treating fungal diseases by using their effects on the ergosterol synthesis.6 The mechanism of ergosterol synthesis reveals that the rate-limiting enzyme 14α-demethylase (CYP51) plays an important role in the synthesis of ergosterol; if its activity is inhibited, the synthesis of ergosterol will be blocked such that the upstream components gather in the cell, resulting in the death of fungal cells.7,8 Among these CYP51 inhibitors, azole compounds have been developed for many years, and they include fluconazole, ketoconazole, and miconazole (Fig. 1). These compounds are widely used in front-line antifungal therapy, which can block ergosterol synthesis by inhibiting the activity of CYP51.9–11 However, these inhibitors also have some disadvantages: they are susceptible to drug resistance and toxicity, especially drug resistance. Once the pathogenic fungi are produced, it is extremely difficult to eliminate them.12 Therefore, drug developers strive to discover novel structures of CYP51 inhibitors to solve the problem of fungal resistance with less toxicity.
Fig. 1. Composition of fungal cell membrane and cell wall.
With the goal of finding a novel skeleton for CYP51 inhibitors, we performed structure-based virtual screening using the designed pharmacophore model of CYP51 inhibitors.13 Sixteen compounds were selected, and their antifungal activities were detected. Additionally, the binding modes of the compounds with higher activities and ADME/T (absorption, distribution, metabolism, excretion, and toxicity) values were also predicted. This study provides some new compound skeletons that can be further developed as more potent CYP51 inhibitors.
2. Results and discussion
2.1. Structure-based virtual screening
In recent years, the method of structure-based virtual screening has played an important role in the process of drug discovery. And the method gives more attention to the drug-like factors according to the combination mode of the fragments binding with its target protein.14,15 Thus, we hope to discover novel skeleton CYP51 inhibitors and obtain their binding modes through cascaded pharmacophore matching and molecular docking.
A proper pharmacophore model can help us to understand the structural features of the ligands and guide the discovery of lead compounds. Thus, the selection of a suitable pharmacophore model is important for the screening of CYP51 inhibitors. In a previous study, the pharmacophore model of CYP51 inhibitors was constructed by clustering the common chemical features from five representative CYP51 inhibitors with diverse scaffolds, and it displayed good recognition characteristics through the validation of the test set.13 In addition, the crystal structure of CYP51 with a potent ligand (VT1161) (PDB code: ; 5TZ1) has been resolved;16 it exhibits structural information, and it also contains chemical features of ligand binding in the active sites.
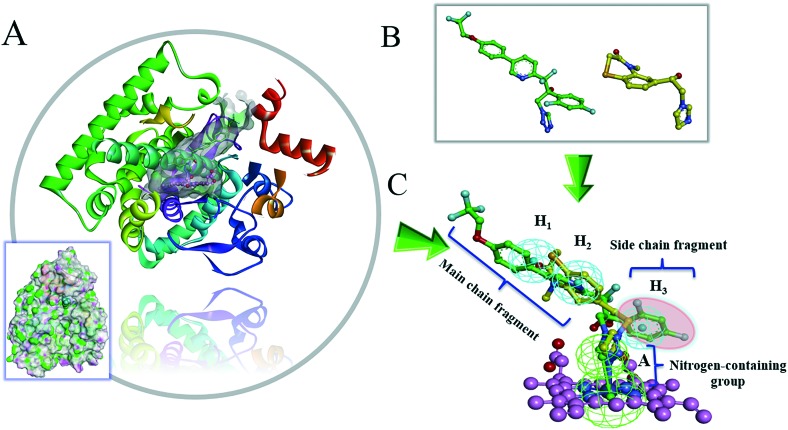
As shown in Fig. 2, the active site of CYP51 was analyzed, and it was found that the crystal ligand VT1161 binds to the active sites of CYP51 by hydrophobic interaction and coordination bond interaction. The ligand VT1161 contains three structural parts: the main chain fragment, the side chain fragment, and the nitrogen-containing group. To further understand the structural characteristics of CYP51 inhibitors, VT1161 was superimposed with the novel CYP51 inhibitor (benzothiazine compound).17 From this result, it was seen that the novel CYP51 inhibitor molecule contained two essential structural parts as the core area (the main chain fragment and the nitrogen-containing group). The main chain fragment matched with the hydrophobic groups of the pharmacophore, which indicated the part of the structure that consisted hydrophobic aromatic groups. The nitrogen-containing group matched with the hydrogen bond acceptor group of the pharmacophore, and it formed a coordination bond with the heme group in the bottom of the active cavity. When the side chain fragment was discarded, the novel benzothiazine compound maintained the inhibitory activity of CYP51,18 which suggested that the side chain fragment was not the essential fragment of the CYP51 inhibitor.
Fig. 2. Pharmacophoric description of the ligand in the CYP51 active site. (A) Crystal structure of CYP51 bound to VT1161. The compound is shown using cyan sticks. An electrostatic potential surface was added to the active site around the compound. The characteristic partial Hinger and G-loop structures are shown as secondary structures. (B) The structure of the ligand (VT1161) and the novel CYP51 inhibitor (benzothiazine compound). The compounds are shown using sticks. (C) The superposition diagram of the pharmacophore and the binding molecules (VT1161 and benzothiazine compound).
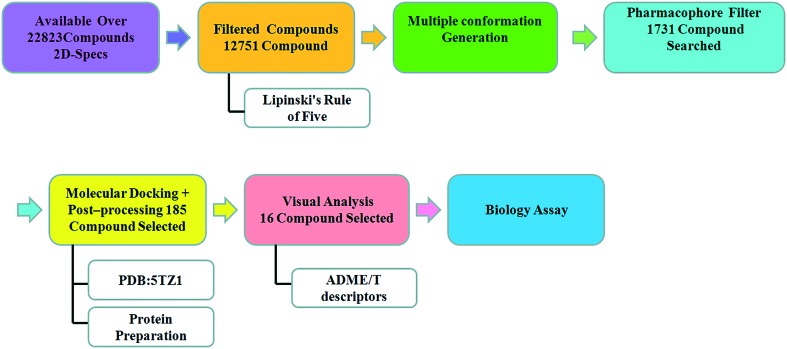
The workflow of the virtual screening is shown in Fig. 3. The publicly available database (ligands) SPECS obtained from the Dow Chemical Company was chosen for virtual screening. First, the two-dimensional structures of the molecules in the Specs database were converted to the corresponding three-dimensional structures. The Lipinski's rule of five was used to filter out the compounds with inappropriate physical and chemical properties, and the flexible multi-conformation searching for the remaining compounds was performed to increase the number of structures. Then, the database of multiple conformations was further matched with the common feature pharmacophore model, and 1731 hit compounds containing pharmacophore features were screened. To reduce the number of “non-hits”, it is effective to apply the strategy of molecular docking. The compounds obtained from the pharmacophore screening were docked into the CYP51 crystal structure and scored by the softwares Lib-DOCK and CDOCKER. According to the score values of Lib-DOCK, the top 10% compounds were selected, and these compounds were further validated by CDOCKER. The compounds with appropriate binding energy values (≤6) were selected.19,20 Finally, sixteen compounds were chosen and purchased for in vitro bioassay testing (Table 1).
Fig. 3. Workflow of the virtual screening protocol.
Table 1. Selection of hit compounds from virtual screening.

|

|
|

|
||||

|

|
|

|

|

|

|

|
| Compd 1 | Compd 2 | Compd 3 | Compd 4 | ||||
|

|

|

|
||||

|
|

|

|

|

|

|

|
| Compd 5 | Compd 6 | Compd 7 | Compd 8 | ||||

|

|

|

|
||||
|

|

|

|

|

|

|

|
| Compd 9 | Compd 10 | Compd 11 | Compd 12 | ||||

|

|

|

|
||||
|

|

|

|

|

|

|

|
| Compd 13 | Compd 14 | Compd 15 | Compd 16 | ||||
2.2. Bioassay validation
To evaluate the antifungal activities of the hit compounds obtained from virtual screening, in vitro antifungal activity experiments were performed according to the protocols from the NCCLS. Fluconazole (FLC) was selected as the reference drug. The broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) of the 16 hit compounds in 96-well plates.
The in vitro antifungal activities of the compounds are listed in Table 2. It can be seen that most of the compounds show antifungal activity, which suggests the rationality of the virtual screening. The MIC value of the compounds with nitrogen-containing groups (pyridine, imidazole) (3, 4, 6, 7, 9–15) is 2–32 μg ml–1 against pathogenic Candida fungi (C. albicans, C. glabrata, C. krusei, and C. tropicalis). Most of the compounds show weaker antifungal activity than the positive control drug (FLC); this is observed as FLC, due to its structural characteristics, has higher binding ability than the test compounds in the active cavity.
Table 2. In vitro antifungal activities of the selected compounds (MIC, μg ml–1).
| (Compound) | MIC (μg ml–1) |
||||
| C. alb. | C. gla. | C. kru, | C. tro. | A. fum | |
| FLC | 4 | 2 | 2 | 2 | 32 |
| 1 | >64 | 32 | 32 | >64 | 32 |
| 2 | 32 | >64 | 32 | 16 | >64 |
| 3 | 8 | 8 | 4 | 2 | 16 |
| 4 | 4 | 2 | 4 | 4 | 8 |
| 5 | 32 | >64 | 32 | >64 | 32 |
| 6 | 16 | 16 | 32 | 8 | >64 |
| 7 | 16 | 8 | 16 | 32 | 32 |
| 8 | 32 | >64 | 32 | 32 | >64 |
| 9 | 8 | 4 | 8 | 16 | 16 |
| 10 | 32 | 16 | 32 | 32 | 32 |
| 11 | 32 | 32 | >64 | 32 | 32 |
| 12 | 16 | 32 | >64 | 32 | 32 |
| 13 | 16 | 8 | 16 | 4 | 32 |
| 14 | 32 | 8 | 16 | 32 | >64 |
| 15 | 32 | 16 | 32 | 16 | 32 |
| 16 | 32 | >64 | 32 | >64 | >64 |
In particular, compounds 3, 4, 9, and 13 exhibit higher antifungal activities than the compounds containing carboxyl groups (1 and 5), suggesting that the nitrogen-containing groups are more likely to form coordination bonds with the porphyrin group of CYP51 than carboxyl groups, which can enhance the antifungal activity of CYP51, and this can result in the inhibition of fungi. In addition, it is noteworthy that some compounds (3, 4 and 9) also exhibit appropriate inhibitory activities against Aspergillus fumigatus (A. fumigatus), whereas compound 13 (32 μg ml–1) displays weak activity against Aspergillus fumigatus. Therefore, compounds 3, 4 and 9 exhibit broad spectrum antifungal effects with moderate to excellent antifungal activities and thus, they deserve our further study.
2.3. Antifungal resistance activity testing
At present, the problem of fungal drug resistance is becoming more and more serious. The widespread use of fluconazole has led to the emergence of resistant fungi associated with prolonged treatment periods, which has become a major clinical problem in fungal infection therapy. Therefore, it is very important to find new types of inhibitors that can be effective against fluconazole-resistant strains of C. albicans. The effective compounds 3, 4 and 9 were further evaluated for their ability to inhibit fluconazole-resistant strains of C. alb (C. alb. Strain100 and C. alb. Strain103). Compounds 3, 4 and 9 displayed antifungal activities, which inhibited strains 100 and 103, and the MIC values were in the range from 4 to 16 μg ml–1 (Table 3). In particular, compound 4 showed clear anti-resistant fungal activity.
Table 3. In vitro antifungal activities of the target compounds (MIC, μg ml–1).
| (Compound) | C. alb. Strain100 | C. alb. Strain103 |
| FLC | >64 | >64 |
| 3 | 8 | 16 |
| 4 | 8 | 4 |
| 9 | 8 | 16 |
2.4. Dose-dependent effect on sterol composition in Candida albicans (ATCC SC5314)
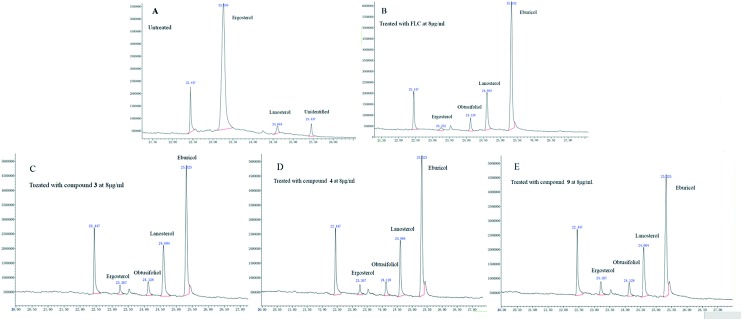
The biosynthetic pathway of ergosterol has been successfully used in studying the mechanism of action of antifungal agents.21–23 The antifungal mechanisms of compounds 3, 4 and 9 were studied by analyzing the composition of ergosterol after the treatment of C. albicans SC5314 cells using GC-MS; the untreated group and the fluconazole-treated group were used as positive controls, and cholesterol was added as an internal standard. The GC-MS analysis results are shown in Fig. 4 and Table 4. In the untreated control, the ergosterol content reached 90.3% of the total sterol fraction, and the lanosterol content was 3.9%. However, other 14-methylated sterols (obtusifoliol and eburicol) were not observed.
Fig. 4. Analysis of sterol composition in untreated group (A) or FLC-treated group (B) or compound 3 (C), 4 (D), 9 (E)-treated group of C. albicans by GC-MS.
Table 4. Analysis of sterol composition in C. albicans by GC-MS.
| Sterol | % of total sterols (C. alb.
a
) |
||||
| Control b | FLC c | 3 d | 4 d | 9 d | |
| Lanosterol | 3.9 | 19.9 | 20.3 | 22.4 | 23.1 |
| Eburicol | — | 67.2 | 63.4 | 59.0 | 54.7 |
| Obtusifoliol | — | 6.3 | 7.4 | 7.1 | 8.2 |
| Ergosterol | 90.3 | 2.5 | 4.6 | 5.8 | 9.1 |
| Unidentified | 5.8 | 4.1 | 4.3 | 5.7 | 4.9 |
aAbbreviations: C. alb. Candida albicans (ATCC SC10231).
bControl (no drug).
cTreated with FLC at 8 μg ml–1.
dTreated with compound 3, 4, 9 at 8 μg ml–1.
The CYP51 inhibitor can cause changes in sterol composition, which has been confirmed in earlier reports.24,25 In this study, when C. albicans was treated with 8 μg ml–1 FLC for 48 h, the content of ergosterol reduced to 2.5% from 90.3% of the total amount of sterol fraction, whereas the lanosterol and eburicol contents increased to 19.9% and 67.2%, respectively. Interestingly, treatments with compounds 3, 4 and 9 also resulted in noticeable accumulation of eburicol; their contents increased to 63.4%, 59.0% and 54.7%, respectively. In addition, it is worth noting that ergosterol levels showed significant decline and for compounds 3, 4 and 9, the ergosterol contents reduced to 4.6%, 5.8% and 9.1%, respectively. This result suggested that compounds 3, 4, and 9 have the same mechanism of action as fluconazole, and they can block ergosterol synthesis by inhibiting CYP51.
2.5. Interactions between the compounds and CYP51
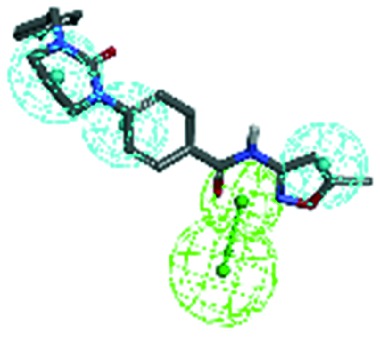
In this study, molecular docking was performed to investigate the binding modes of compounds 3, 4 and 9 with CYP51 using Discovery Studio Visualizer 4.0, which can help rationalize the available SAR and evaluate the regional influence on biological activity. The co-crystallized molecule VT1161 was used as the reference molecule, and compounds 3, 4 and 9 exhibited similar binding modes with VT1161, which has been shown in Table 5.
Table 5. Binding patterns of the three potential compounds with CYP51.
| Reference molecule VT1620 |

|

|

|
| Compound 3 |

|

|

|
| Compound 4 |

|

|

|
| Compound 9 |

|

|

|
From this result, it can be seen that the selected compounds 3, 4, and 9 and the reference molecule VT1161 contain two essential fragments (the main chain fragment and the nitrogen-containing groups) and one non-essential fragment (the side chain fragments), and these fragments and groups are well superimposed with those of the reference molecule (VT1161). The main chain fragments of compounds 3, 4 and 9 are composed of aromatic groups, which can form π–π and π–alkylation interactions with the hydrophobic amino acid residues (Leu121, Tyr132, Pro230, Gly303, and Leu 376), polar amino acid residues (Tyr118), and basic amino acid residues (His377). The nitrogen-containing group is composed of pyridine or azole group, which can compete to block the synthesis of ergosterol by the formation of coordination bonds with porphyrin. The side chain fragments are the non-essential fragments of CYP51 inhibitors, the side chain fragments of compounds 2 and 3 are abandoned, and compound 4 with the side chain fragment can form hydrophobic interactions with the amino acid residues (Gly303).
Based on the above-mentioned elucidations, it is evident that the main chain fragments and the nitrogen-containing groups of the compounds can form relatively strong interactions with the active target CYP51. The binding modes for these compounds and CYP51 are crucial in determining the active site regions and further optimizing the potent CYP51 inhibitors as templates.
2.6. Theoretical evaluation of ADME/T properties
To further study the medicinal properties of these compounds, ADME/T properties of compounds 3, 4, 9 and fluconazole were calculated.
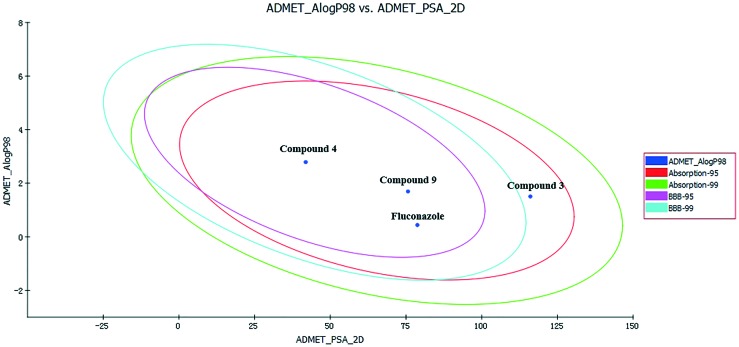
The two analogous 95% and 99% confidence ellipses for the blood–brain barrier (BBB) penetration and human intestinal absorption (HIA) models are shown in the biplot Fig. 5. All three screened compounds and the control drug (fluconazole) were distributed in the 99% confidence region for BBB penetration and HIA, which indicated that the prediction of ADMET values exhibited appropriate reliability. The detailed results for the pharmacokinetic parameters and toxicity analyses are shown in Table 6.
Fig. 5. Plot of PSA versus AlogP for the candidate compounds. Abbreviations: ADME/T, absorption, distribution, metabolism, excretion and toxicity; AlogP, the logarithm of the partition coefficient between octanol and water; PSA, polar surface area; 2D, two-dimensional; BBB, blood brain barrier.
Table 6. In silico ADME/T prediction of the three potential compounds compared with fluconazole.
| ADME/T parameters | 3 | 4 | 9 | Fluconazole |
| AlogP98 | 1.764 | 2.986 | 1.942 | 0.75 |
| PSA | 113.836 | 39.706 | 73.448 | 76.556 |
| BBB penetration | — | 0.14 | –0.716 | –1.134 |
| Aqueous solubility | –3.964 | –3.699 | –3.001 | –1.976 |
| Hepatotoxicity | 0.986 | 0.264 | 0.774 | 0.847 |
| CYP2D6 | 0.673 | 0.356 | 0.326 | 0.217 |
AlogP98 can determine the compounds' hydrophilicity, and an appropriate value of AlogP98 directly affects the compounds' absorption and penetration. When the value is less than 5, it indicates that the compounds' hydrophilicity is within a reasonable range. The range of compounds' AlogP98 is 0.75–2.986, and these values are located in the allowed range. Likewise, the PSA parameter is also directly related to drug bioavailability; the PSA values of the compounds are all less than 140, indicating that the compounds have high oral bioavailability. Thus, all of the compounds can be efficiently absorbed in the human intestine, and they can strongly bind to the plasma protein. In addition, it is noteworthy that compounds 4 and 9 exhibit low BBB penetration, and they can inhibit cytochrome P450; these compounds can slow down the rapid metabolism of drugs by inhibiting CYP2D6 and increase the time of drug action. However, all of these compounds have some hepatotoxicity; compound 4 exhibits lower hepatotoxicity than fluconazole. Computational pharmacokinetic and toxicology studies on the active compounds 3, 4 and 9 compared with those on fluconazole suggest that these compounds can be used as good starting points for further developing and designing new derivatives.
3. Conclusions
At present, it is observed that most antifungal inhibitors have significant side effects. Thus, it is very important to find new and effective antifungal inhibitors due to the increase in endogenous pathogenic fungal infections and the emergence of fungal resistance. CYP51, as a key antifungal target enzyme, can effectively guide the design of antifungal inhibitors. In this study, by the method of virtual screening, we tried to find novel skeletal CYP51 inhibitors as lead compounds. We screened the compound database of Dow Chemical Company through the early construction of pharmacophore model and molecular docking. Finally, 16 compounds were identified, and most of the screened compounds showed significant antifungal activities; among these compounds, compounds 3, 4, 9 and 13 exhibited higher antifungal activities than the other screened compounds by blocking the synthesis of ergosterol. At the same time, compounds 3, 4 and 9 also showed a broader antibacterial spectrum and some inhibitory effects on drug resistant Candida albicans strains. We further conducted the molecular docking and theoretical evaluation of ADME/T properties of compounds 3, 4 and 9. Compounds 3, 4 and 9 contained two essential fragments (the main chain fragments and the nitrogen-containing groups), and the main chain fragments of the compounds could form hydrophobic interactions with the amino acid residues. The nitrogenous group (pyridine) of the compound could form coordination bonds with heme in the active cavity of CYP51. To further study the medicinal properties of these compounds, the theoretical evaluations of ADME/T properties of compounds 3, 4 and 9 were conducted. Through the prediction of BBB, AlogP98, and PSA, compounds 4 and 9 were considered to be good lead compounds in a reasonable range, which suggested that these compounds could be further optimized for the discovery of novel antifungal agents.
Thus, it can be seen that the nitrogenous group of the compounds can be substituted by pyridine, which can reduce the toxicity and side effects. The side chain fragments are not the essential groups, and they have only small effect on the activity of the compounds. The main chain fragments are made up of hydrophobic groups, which have great influence on the activity of the compounds. These results suggested that these compounds could be further optimized for the discovery of novel antifungal agents.
4. Experimental section
4.1. Structure-based virtual screening
All the work of screening and docking was processed on the Dell PowerEdge R900 workstation. Ligand preparation and pharmacophore screening were performed using Discovery Studio 3.0 (DS 3.0). Part of the molecular docking process was performed using Lib-DOCK and CDOCKER modules in DS 3.0, and the affinity of protein–ligand binding was calculated. The interactions between the inhibitors and the receptor were displayed via Discovery Studio Visualizer 4.0. In addition, the ADMET value was predicted by the module of ADMET descriptors.
4.2. Preparation of ligands
The publicly available database (ligands) SPECS from the Dow Chemical Company was chosen for virtual screening. Discovery Studio 3.5 was used to convert the two-dimensional structures of the molecules in the Specs database to the corresponding three-dimensional structures. The structure of each compound was hydrogenated, and energy optimization was performed. The database was initially filtered using the Lipinski's rule of five and the Filter Molecules program in DS 3.0 to remove the molecules with unwanted physical and chemical properties. After filtering, the remaining molecules in the database were utilized to generate multiple conformations using the conformation search and minimization program in DS 3.0. The following parameters were set: maximum conformations = 255, energy threshold = 20 kcal mol–1 and root-mean-square distance (RMSD) = 0.8 Å.
4.3. Preparation of receptor and molecular docking
In this docking experiment, the co-crystallized protein receptor CYP51 of C. albicans (PDB code: ; 5TZ1) was selected as the docking receptor, and it was processed using the protein preparation wizard in CDOCK. First, the water molecules around the protein were removed, and the loop segments, missing residues, and polar hydrogen atoms were added. Second, the co-crystallized protein ; 5TZ1 was chosen as the CYP51 receptor using the module “Define Selected Molecule as Receptor” in the tool panel of “defining and editing the binding site”. Third, the position of the CYP51's active cavity was determined through the co-crystallized ligand VT-1062 (X: 70.61, Y: 66.284, and Z: 4.177), and the radius was set to 12. Then, the ligand and the active site coordinates were introduced in the docking parameter browsers. The other docking parameters were set as the default values.
4.4. Pharmacophore screening
The pharmacophore model of the CYP51 inhibitors was constructed in the previous report, and the pharmacophore was used to search the multiple conformation database of the available compounds using the ligand profiler protocol in DS 3.0. In the screening process, the corresponding parameter settings were opened in the parameter browser. The “Input Type” was “Ligands”; the “Input Ligands” was “DC03-221007.sd: All”. The appropriate pharmacophore model was selected in the settings bar: “Input File Pharmacophores”. The parameter group was expanded, and the “Save Aligned Ligands” was saved as “True”. The other parameters were adopted by default values.
4.5. ADME/T prediction
The properties of ADMET include absorption, distribution, metabolism, excretion and toxicity of drugs in the human body. It is very necessary for improving the chances of success of drug research and reducing the waste of funds. In this study, ADME/T prediction is used to guide the selection and optimization of leading compounds in the early stages of drug development. The specific operation process is as follows: the “ADMET descriptors” module is selected, and the parameter browser is opened. The small molecule compound files are imported. In the parameter settings, aqueous solubility, blood brain barrier penetration, CYP2D6 binding, hepatotoxicity, intestinal absorption and plasma protein binding are chosen as research objects.
4.6. Antifungal activity test
The compounds were tested for antifungal and antifungal resistance activity. The in vitro minimum inhibitory concentrations (MIC) were determined using the standard guidelines described in the National Committee for Clinical Laboratory Standards (NCCLS). The bacteria were derived from the Chinese medical fungus Preservation Center and Shenyang Pharmaceutical University. In the experiment, FLC was selected as the positive control drug. All of the compounds were dissolved in DMSO and serially diluted with the growth medium. The concentrations of all the compounds were 64, 32, 16, 8, 4, 2, 1, 0.5, and 0.25 (μg mL–1). The daily growth of fungi was observed under 35 °C culture conditions.
4.7. GC-MS analysis of sterol composition
In this process, Candida albicans (ATCC SC5314) was selected as the test strain, and FLC was purchased as the positive control drug. In the experiment, 1640 culture medium was used as the nutrient solution, and the concentration of drug was set to 8 μg ml–1. After 16 hours of cultivation, the sterol component was extracted using petroleum ether, and the extracted samples were used for GC/MS detection. The chromatographic conditions were as follows: the inlet temperature was 250 °C, the starting temperature was 100 °C, which was maintained for 1 min; then, the temperature was raised to 300 °C and maintained for 10 min. The carrier gas was He, and the flow rate was set to 1 ml min–1.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81703357), the Shandong Provincial Natural Science Foundation (Grant No. ZR2017BH102), Shandong Antibody Pharmaceutical Collaborative Innovation Fund (Grant No. CIC-AD1837) and the Tai-Shan Scholar Research Fund of Shandong Province of China. We also acknowledge the Shandong Collaborative Innovation Center for Antibody Drugs and Engineering Research Center for Nanomedicine and Drug Delivery systems for this research.
References
- George J. A. Infect. Dis. Clin. North Am. 2011;25:201–225. doi: 10.1016/j.idc.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez Santiago T. M., Pritt B., Gibson L. E., Comfere N. I. J. Am. Acad. Dermatol. 2014;71:293–301. doi: 10.1016/j.jaad.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Enoch D. A., Ludlam H. A., Brown N. M. J. Med. Microbiol. 2006;55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- Turel O. Expert Rev. Anti-infect. Ther. 2011;9:325–338. doi: 10.1586/eri.10.163. [DOI] [PubMed] [Google Scholar]
- Guitard J., Tabone M. D., Senghor Y., Cros C., Moissenet D., Markowicz K., Valin N., Leverger G., Hennequin C. J. Infect. 2016;73:607–615. doi: 10.1016/j.jinf.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Pooja C., Prasher P., Singh P., Pawar K., Vikramdeo K. S., Mondal N., Komath S. S. Eur. J. Med. Chem. 2014;80:325–339. doi: 10.1016/j.ejmech.2014.04.063. [DOI] [PubMed] [Google Scholar]
- Hlavica P. Biochim. Biophys. Acta. 2013;1834:205–220. doi: 10.1016/j.bbapap.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. J., Garvey E. P., Moore W. R., Rafferty S. W., Yates C. M., Schotzinger R. J. Bioorg. Med. Chem. Lett. 2014;24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Wang L., Yang W., Wang K., Hu Y. Bioorg. Med. Chem. Lett. 2012;22:4887–4890. doi: 10.1016/j.bmcl.2012.05.070. [DOI] [PubMed] [Google Scholar]
- Cao X., Xu Y., Cao Y., Wang R., Zhou R., Chu W., Yang Y. Eur. J. Med. Chem. 2015;102:471–476. doi: 10.1016/j.ejmech.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Zhao S., Zhao L., Zhang X., Liu C., Hao C., Xie H., Sun B., Zhao D., Cheng M. Eur. J. Med. Chem. 2016;123:514–522. doi: 10.1016/j.ejmech.2016.07.067. [DOI] [PubMed] [Google Scholar]
- Van Acker H., Van Dijck P., Coenye T. Trends Microbiol. 2014;22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Sun B., Huang W., Liu M. J. Mol. Graphics Modell. 2017;73:157–165. doi: 10.1016/j.jmgm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Jorgensen W. L. Science. 2004;303:1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- Bleicher K. H., Böhm H. J., Müller K., Alanine A. I. Nat. Rev. Drug Discovery. 2003;2:369–378. doi: 10.1038/nrd1086. [DOI] [PubMed] [Google Scholar]
- Hargrove T. Y., Friggeri L., Wawrzak Z., Qi A., Hoekstra W. J., Schotzinger R. J., York J. D., Guengerich F. P., Lepesheva G. I. J. Biol. Chem. 2017;292:6728–6743. doi: 10.1074/jbc.M117.778308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffella F., Macchiarulo A., Milanese L., Vecchiarelli A., Costantino G., Pietrella D., Fringuelli R. J. Med. Chem. 2005;48:7658–7666. doi: 10.1021/jm050685j. [DOI] [PubMed] [Google Scholar]
- Sun B., Huang W., Liu M., Lei K. J. Mol. Graphics Modell. 2017;77:1–8. doi: 10.1016/j.jmgm.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Li R. J., Wang J., Xu Z., Huang W. X., Li J., Jin S. F., Zhao D. M., Cheng M. S. ChemMedChem. 2014;9:1012–1022. doi: 10.1002/cmdc.201400016. [DOI] [PubMed] [Google Scholar]
- Li R. J., Su X. L., Chen Z., Huang W. X., Wang Y. L., Wang K. B., Lin B., Wang J., Cheng M. S. RSC Adv. 2015;5:23202–23209. [Google Scholar]
- Warrilow A. G., Hull C. M., Parker J. E., Garvey E. P., Hoekstra W. J., Moore W. R., Schotzinger R. J., Kelly D. E., Kelly S. L. Antimicrob. Agents Chemother. 2014;58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R. M., Cao Y. B., Fan K. H., Xu Y., Gao P. H., Zhou Y. J., Dai B. D., Tan Y. H., Wang S. H., Tang H., Liu H. T., Jiang Y. Y. Acta Pharmacol. Sin. 2009;30:1709–1716. doi: 10.1038/aps.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. J. Invest. Dermatol. 1981;76:438–441. doi: 10.1111/1523-1747.ep12521036. [DOI] [PubMed] [Google Scholar]
- Barker K. S., Crisp S., Wiederhold N., Lewis R. E., Bareither B., Eckstein J., Barbuch R., Bard M., Rogers P. D. J. Antimicrob. Chemother. 2004;54:376–385. doi: 10.1093/jac/dkh336. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Wang Y., Liu W., Liu N., Zhang Y. Q., Dong G. Q., Liu Y., Li Z. G., He X. M., Miao Z. Y., Yao J. Z., Li J., Zhang W. N., Sheng C. Q. ACS Med. Chem. Lett. 2014;5:506–511. doi: 10.1021/ml400492t. [DOI] [PMC free article] [PubMed] [Google Scholar]