 Arylated 5-hydroxy-pyrrol-2-ones were prepared in 2 synthetic steps from mucochloric acid and optimised as CCK2-selective ligands using a range of assays.
Arylated 5-hydroxy-pyrrol-2-ones were prepared in 2 synthetic steps from mucochloric acid and optimised as CCK2-selective ligands using a range of assays.
Abstract
Arylated 5-hydroxy-pyrrol-2-ones were prepared in 2 synthetic steps from mucochloric acid and optimised as CCK2-selective ligands using radiolabelled binding assays. CCK antagonism was confirmed for the ligands in isolated tissue preparations. DSS (dextran sulfate sodium)-induced inflammation was analysed for derivative 7 and PNB-001 with L-365,260 as a standard. The IC50 of PNB-001 was determined to be 10 nM. Subsequent in vivo evaluation confirmed anti-inflammatory activity with respect to IBD assays. The best molecule, PNB-001, showed analgesic activity in the formalin test and in the hotplate assay, in which the analgesic effect of 1.5 mg kg–1 PNB-001 was equivalent to 40 mg kg–1 tramadol. The CCK2-selective antagonist PNB-001 protected rats against indomethacin-induced ulceration at similar doses. The GI protection activity was found to be more potent than that of the 10 mg kg–1 dose of prednisolone, which served as a standard.
Introduction
Inflammatory bowel syndrome (IBS) is a complex symptom of unknown etiology, characterized by abdominal pain or discomfort associated with disturbed defecation and often bloating. Inflammatory bowel disease (IBD) encompasses at least two forms of intestinal inflammation expressed as ulcerative colitis and Crohn's disease. Although many other inflammatory disorders affect the gastrointestinal tract, most can be distinguished by a specific underlying process from IBD. Stress may exacerbate IBS symptoms and the CCK2 receptor is known to mediate anxiety1 and panic attacks. Though several drugs such as anti-cholinergic and anti-spasmodic agents and tricyclic anti-depressants for IBS and steroids for IBD are of limited use, inflammatory bowel disease is still classified as an unmet medical need and hundreds of studies are ongoing worldwide.
The aim of the drug discovery programme was to target the underlying mechanism of the disease, thus targeting cholecystokinin (CCK) pathways. CCK, a peptide hormone, which is extensively found in the gastrointestinal tract (GIT), is widely distributed throughout the nervous system.2 Cholecystokinin acts as a neuromodulator/gut hormone, and CCK-ligands, agonists and antagonists3 have been extensively investigated as potential drug targets.4 CCK-antagonists were studied as growth inhibitors in certain forms of cancer,5 as anxiolytics,6 in the treatment of schizophrenia,7 in satiety8 and as anti-panic agents.9 An agonist, the shortened CCK tetrapeptide, was found to induce panic in patients.10
Despite the progress of several CCK receptor antagonists11 to different phases of clinical trials, only proglumide was marketed as Milid for the treatment of gastric ulcer.
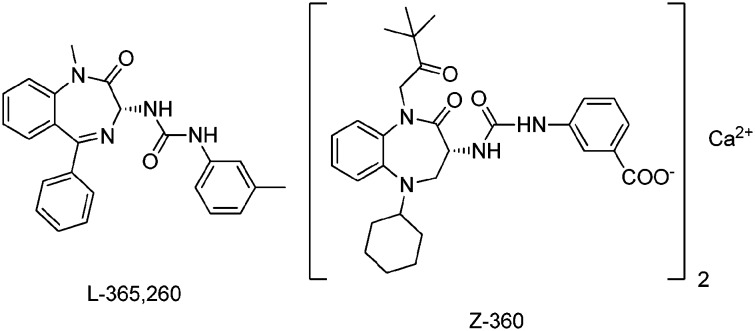
Merck's standard CCK2 antagonist12 is outlined in Fig. 1. Z-360 is the most recent derivative derived from the original lead structure, in which the N was moved to the 5-position. The N-1 was alkylated, the 5-phenyl group was replaced by a cyclohexyl group and the water solubility was enhanced by converting the Me group on the side chain into a carboxylic acid. All structural optimisations did not address the main underlying problem with respect to poor pharmacokinetic properties, such as low solubility and very low membrane penetration resulting from the large polar surface area of the molecule.
Fig. 1. CCK2/gastrin antagonists: Merck's standard and the most recent addition.
PNB Vesper Life Science systematically investigated a hydroxy-pyrrolone scaffold and an isobutyl derivative13 which served as lead structures in this programme towards novel anti-inflammatory analgesics targeting the underlying mechanism of inflammatory ulceration.
A full biological evaluation of lactam 14, now PNB-001, is reported here in this publication from CCK-5 pentagastrin antagonism to initial anti-inflammatory analgesic activity.
Results and discussion
Synthesis
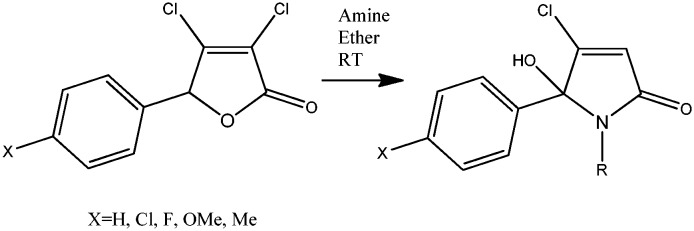
Mucochloric acid was reacted in the presence of a Lewis acid via SE reactions into a series of arylated dichlorinated furanones. In addition to halogenated (X = Cl, F) 5-arylated 2(5H)-furanones further molecules containing donor substituents were prepared. The chemical yields were generally lower and the binding affinity also decreased in the resulting final molecules. Arylated 2(5H)-furanones, containing nitro-groups or trifluoro-methyl groups, could not be prepared.
Subsequent reaction of the 5-arylated 3,4-dichloro-2(5H)-furanones with amines, in particular with aryl alkyl amines, furnished 15 arylated N-alkylated hydroxy-pyrrolones and the 2nd step of the synthetic sequence is outlined in Scheme 1.
Scheme 1. Synthesis of lactams 1–15 from 3,4-dichloro-5-arylated 2(5H)-furanones. For the synthesis of building blocks from mucochloric acid, see Lattmann et al., MedChemComm 2016.
The 5-arylated 2(5H)-furanones reacted selectively in the ester position and no reaction in the 4-position was observed. Previously, the substitution in the 4-position was described for pseudo-esters,14 and here, a ring-opening–ring-closure mechanism for the formation of hydroxy-pyrrolones is proposed in Scheme 2.
Scheme 2. Chemical mechanism for the formation of 5-hydroxy-5-aryl-pyrrol-2-ones with amines.
The first step in the reaction sequence of the dichlorinated 2(5H)-furanone is the ring opening and amide formation from the corresponding lactone. The keto form of the acyclic amide was then in situ converted into a lactam through the elimination of hydrogen chloride.
Analysis by chiral HPLC showed a 50 : 50 racemic mixture of the novel template in solution in methanol.
SAR optimisation
Initially, SARs were fully explored and optimised for the CCK2/gastrin receptor, using a radiolabelled binding assay with iodinated CCK8. The results, expressed in IC50, are outlined in Table 1.
Table 1. CCK binding affinity using radioligands with cortex and pancreatic membranes. IC50 is presented in micromolar; N = 3.
| Lactam | X | R | CCK-A [μM] | CCK-B [μM] |
| Lorglumide | — | — | 0.17 ± 0.01 | >10 |
| L-365,260 | — | — | 0.25 ± 0.01 | 0.003 ± 0.001 |
| PNB-028 | F | Isobutyl- | 0.012 ± 0.001 | 0.75 ± 0.21 |
| 1 | H | Cyclopropyl- | 7.5 ± 0.3 | >10 |
| 2 | Cl | Cyclopropyl- | 4.0 ± 0.3 | >10 |
| 3 | H | Cyclopentyl- | 0.36 ± 0.30 | 0.84 ± 0.40 |
| 4 | Cl | Cyclopentyl- | 2.50 ± 0.20 | >10 |
| 5 | H | Cyclohexyl- | >10 | >10 |
| 6 | H | Ph- | >10 | >10 |
| 7 | H | Bz- | 0.80 ± 0.04 | 0.022 ± 0.002 |
| 8 | Cl | Bz- | 0.51 ± 0.04 | 0.020 ± 0.004 |
| 9 | F | Bz- | 0.32 ± 0.01 | 0.017 ± 0.002 |
| 10 | MeO | Bz- | 0.21 ± 0.02 | 4.5 ± 0.4 |
| 11 | Me | Bz- | 1.5 ± 0.04 | 4.1 ± 0.3 |
| 12 | H | Methylbenzyl- | 0.60 ± 0.04 | 0.21 ± 0.01 |
| 13 | H | Methylbenzyl- | 0.42 ± 0.03 | 0.21 ± 0.15 |
| 14 | H | Phenylethyl- | >10 | 0.022 ± 0.002 |
| 15 | Cl | Phenylethyl- | >10 | 0.030 ± 0.001 |
Lorglumide served as the CCK1 standard and L-365,260 was used as the CCK2 standard. For a better comparison from the initial starting point, the CCK1 antagonist PNB-028 is also included in Table 1.
Cyclic substituents on the N-position, such as cyclopropyl, cyclopentyl and cyclohexyl, provided activity in the micromolar range (lactams 1–5). Anilines were inactive and the introduction of the benzyl group furnished a dual ligand in the nanomolar range (lactam 7). Halogen atoms on the 5-phenyl group marginally enhanced the binding affinity and the best derivative was the fluorinated N-benzyl lactam 9.
Donor substituents on the 5-phenyl group, such as para-methoxy and methyl groups, resulted in ligands with manifold lower activities (lactams 10 and 11). The introduction of a chiral amine, such as methyl benzyl amine provided diastereoisomers (12 and 13), which were separated by column chromatography, but both isomers exhibited a lower affinity than the parent benzyl derivative 7. Further optimisation on the N-benzyl group was performed, but the introduction of para F- and methoxy-groups, using p-fluorobenzyl amine and p-methoxybenzyl amine, did not enhance the binding affinity for the CCK receptor.
The introduction of a spacer, which is a single CH2 group, resulted in the formation of a phenyl-ethyl derivative 14, which represented a highly CCK2-selective ligand.
Lactam 14 is 450 times selective for the CCK2/gastrin receptor. Halogenation, the introduction of a para-chlorine atom into the phenyl position, producing lactam 15, did not enhance the binding affinity any further, possibly due to the drug receptor interaction of the phenyl group with a lipophilic cavity within the CCK receptor.
Molecular modelling – ligand docking
The docking of phenylethyl pyrrolone 14 into the CCK2 receptor is outlined in Fig. 2 for one final pose of minimal energy and some key drug receptor interactions are highlighted.
Fig. 2. Docking of CCK antagonist PNB-001 into the CCK2 receptor.
The 2-carbonyl group of the central pyrrolone template interacts via hydrogen bonding with the N group of Trp-114. The phenyl group of the N-phenylethyl side chain binds towards the aromatic indole system of Trp-114, and electron-withdrawing groups should enhance these aromatic interactions. The lipophilic pocket in the CCK2 receptor composed of Ile-184 and Leu-133 principally allows a wide range of substituents, but only phenyl and not cyclohexyl can be realised synthetically. The 5-phenyl group of the pyrrolone template binds via Ile-184 and Leu-133, based on van der Waals interactions and not aromatic interactions. Therefore, the introduction of electron-withdrawing groups into the 5-phenyl group, such as a chlorine atom, does not enhance the binding affinity and optimisation on this side has an overall limited effect. Interestingly, the metabolism of the 5-phenyl derivative 14 may result in a p-hydroxy-phenyl metabolite, which may interact additionally with His-122 via a strong hydrogen bond, and the role of metabolites is currently under investigation.
The molecular structure of lactam 14 (Table 1) which is now PNB-001 was designed from an iso-propyl lead structure via the benzyl derivative 7 (Fig. 3). PNB-001 contains an N-phenyl-ethyl substituent, which is required for high CCK2/gastrin selectivity.15
Fig. 3. Lead design from a CCK1 antagonist to a CCK2-selective molecule.
The mixed dual-acting N-benzylated pyrrolone 7 (Table 1) shows the expected pharmacological profile of a mixed CCK antagonist16 and its role in brain cancer is currently being elucidated.
The phenyl ethyl derivative PNB-001 completed preclinical development as an anti-inflammatory analgesic and progressed into phase 1 clinical trials for the treatment of IBD.
Isolated tissue preparations
CCK antagonism
The CCK2-selective ligand PNB-001 exhibited a potent binding affinity and the CCK antagonism was studied17 using pentagastrin (CCK-5)-induced contractions of the rat duodenum.
Initially, CCK4 was used,18 but CCK4 has a low solubility and low potency in the micro-molar range. The best CCK2-selective agonist is CCK-5 and it was used to analyse the agonist or antagonist properties of the ligand (Fig. 4).
Fig. 4. Responses to CCK-5 in the absence and presence of PNB-001: CCK-5, CCK-5 + 10 nM PNB-001, CCK-5 + 30 nM PNB-001, and CCK-5 + 100 nM PNB-001; N = 2 for each data point. Values are ±SEM.
The concentration response curve of pentagastrin, CCK-5, was recorded and shifted to the right by a nanomolar concentration of PNB-001, thus confirming the antagonistic properties19 of this ligand.
100 nM PNB-001 with 30 min incubation time fully blocked the CCK4- and CCK5-induced contractions. For the 5 min incubation cycle, 10 nM PNB-001 shifted the CCK-5 concentration response curve to the right, and from 30 nM onwards, the antagonist PNB-001 showed a reduced maximum response. At high nanomolar concentrations, PNB-001 acted as an insurmountable gastrin/cholecystokinin antagonist.
As the clinical trial outcome of selective CCK/gastrin antagonists was found to be questionable in the treatment of anxiety and depression,20 the aim of the programme was to focus on selectively designing CCK2 antagonists for the gastrointestinal system and to focus on inflammation thereof.
Inflammatory bowel disease, IBD, is classified as an unmet medical need by the FDA and allows fast track approval. The indomethacin-induced ulceration is a standard in vivo assay for IBD.21
In vitro inflammation
In vitro, spontaneous contractions correlate with inflammation and anti-inflammatory steroids such as dexamethasone reduced spontaneous contractions of the duodenum.22
DSS, dextran sulfonic acid sodium, is a standard agent to induce inflammation for IBD in vivo and in vitro and its effect is slower and milder than that of trinitrobenzene sulfonic acid, TNBSA.23
DSS was used here in vitro using isolated organ preparations and preparations of the duodenum worked best in this assay.24
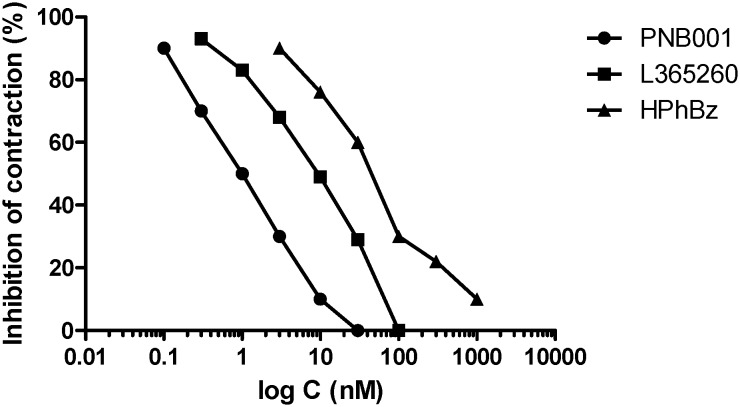
L-365,260 was applied as the CCK2 standard and the mixed CCK antagonist HPhBz, lactam 7, together with the highly CCK2-selective antagonist PNB-001, were all tested under the same conditions. Their concentration response curves, showing how these agents inhibit contractions at very low concentrations, are outlined in Fig. 5.
Fig. 5. The inhibitory concentration–response curves of PNB-001, L-365,260 and HPhBz (lactam 7) for DSS-stimulated contractions.
An IC50 of 1 nM was determined for CCK2 antagonist PNB-001. The Merck CCK2 standard had ten times lower IC50 and the benzylated derivative HPhBz, lactam 7, had the lowest anti-inflammatory effect. Considering the chemical similarity between lactam 7 and PNB-001, the addition of an extra CH2 group resulted in a remarkable effect. The CCK1 antagonism may oppose the anti-inflammatory effect, but the possible mechanistic pathway is outside the scope of this publication.
Based on the in vitro results with respect to anti-inflammatory activity, PNB-001 was selected for further in vivo assays. This programme was concerned with inflammation of the GI tract and the indomethacin-induced ulceration is the best model for IBD (inflammatory bowel disease) and ulcerative colitis, which is associated with the release of gastric acid. Here, the anti-inflammatory action of the molecule, supported by anti-gastric activity, was supposed to create a synergistic therapeutic effect.
In vivo evaluation
Inflammatory/ulceration protection
Gastrin triggers the release of gastric acid in the stomach and the content of the GI system moves from the duodenum, jejunum and ileum to the colon. The inflammation was assessed in this order by organ weight and inflammation scores. The measurement of weight as an inflammation parameter is reliable and the induction of inflammation/ulceration resulted in an increased organ weight (Fig. 6).
Fig. 6. Indomethacin-induced ulceration. Weights of the GI tract treated with PNB-001.
Indomethacin increased the weight in all observed organ parts and this increase of weight was reduced dose-dependently by PNB-001 for the duodenum, jejunum, and ileum. The effect of protection is observed for all parts of the GI system and PNB-001 performed better than the steroid standard prednisolone.
The second accessed parameter was scores of inflammation and the results are outlined in Fig. 7.
Fig. 7. Inflammation scores of the GI tract treated with PNB-001.
Treatment was presented for each individual organ and scores of inflammation were determined. Most interestingly, the gaps in the treatment groups with zero inflammation are present in the duodenum for both 5 and 20 mg kg–1 doses of PNB-001 via oral administration. Indomethacin induced an inflammation score of 5 in the duodenum, which was fully protected by both doses of the CCKB antagonist and reduced to 2 by prednisolone at 10 mg kg–1.
In Fig. 8, selected photos of the gastrointestinal system with ulceration are depicted. In the duodenum, the perforation is clearly visible and this is fully prevented by a low 5 mg kg–1 dose of PNB-001. A similar effect on indomethacin-induced ulcers is seen for the jejunum and this damage of the GI system was prevented by PNB-001.
Fig. 8. Top: Photos of the duodenum; bottom: photos of the jejunum. Selected parts of the GI system, indomethacin-induced ulcers and damage prevented by CCK2/gastrin antagonist PNB-001.
Analgesic evaluation
CCK antagonists potentiate the analgesia induced by opiates and usually, except for proglumide, have no analgesic effect on their own.25 For Z-360, an interesting weak analgesic effect in the formalin test was observed.26
Thus, a first evaluation of the analgesic properties of PNB-001 was performed using the formalin test (Fig. 9).
Fig. 9. Analgesic anti-inflammatory activity of PNB-001 for formalin-induced pain in rats.
Opiates, such as morphine, reduce the response in the form of licks and bites to formalin in the first phase of the assay. Anti-inflammatory agents are active in this assay in the second phase.
PNB-001 showed some opiate-like effects only at the 1.5 mg kg–1 dose (phase 1) and displayed also the best anti-inflammatory effect at this dose (phase 2). The 5 mg kg–1 dose by IP administration is equivalent to the 20 mg kg–1 dose by oral administration, but had a lower analgesic effect than the 1.5 mg kg–1 dose. However, the activity in the second phase of the formalin test reconfirmed the anti-inflammatory activity found in vitro and in vivo.
In order to evaluate the pain managing properties of the new agent, the partial opiate agonist tramadol was included in a last study, in which the hot plate assay was used to evaluate analgesic activity (Fig. 10).
Fig. 10. Hotplate test of PNB-001 in mice by IP administration.
Bar 1 is the baseline with a response time of about 12 s. The maximum analgesic effect in the hotplate is 26 s for 40 mg kg–1 tramadol, which is a more than 100% change from the control.
The analgesic efficacy of 0.5 mg kg–1 PNB-001 by IP administration as a single agent was found to be equivalent to that of 40 mg kg–1 tramadol (SC administration). Interestingly, there is no significant potentiation of tramadol analgesia with PNB-001. The selective CCK2/gastrin antagonist worked here in this assay as an analgesic on its own. This analgesic activity may be found useful in the treatment of IBD/IBS pain additionally.
Conclusions
A novel template, which originally exhibited CCK1 activity,27 was optimised as a CCK2/gastrin-selective antagonist.
The target molecule was synthesised in only 2 steps from readily available starting materials and will potentially deliver affordable therapeutic agents.
By designing a potent CCK2/gastrin antagonist for inflammatory diseases and not CNS disorders, a first-in-class CCK2 antagonist with analgesic anti-inflammatory activity was developed under the consideration of membrane penetration, half-life and bioavailability.
By focussing on unmet medical needs such as inflammatory bowel disease, IND was granted in India and the FDA approval is expected in late 2019.
The mild analgesic effect of PNB-001 may offer an alternative drug treatment to tramadol, which is closing the gap of pain management between NSAIDs and opiates.
Supplementary Material
Acknowledgments
This experimental work was partly supported by PNB Vesper Life Sciences Pvt, India.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00707d
References
- Hughes J., Boden P., Costallt B., Domeneyt A., Kellyt E., Horwell D., Hunter J., Pinnock R., Woodruff G. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6728. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz R. Med. Res. Rev. 2003;23:559. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- McDonald I. M. Expert Opin. Ther. Pat. 2001;11:445. [Google Scholar]
- Bock M. G., DiPardo R. M., Mellin E. C., Newto N. C. J. Med. Chem. 1994;37:722. doi: 10.1021/jm00032a003. [DOI] [PubMed] [Google Scholar]
- Lattmann E., Arayarat P. Khon Kaen Sci. J. 2003;31:178. [Google Scholar]
- Dourish C. T., Ravard S. Trends Pharmacol. Sci. 1990;11:271. doi: 10.1016/0165-6147(90)90004-r. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Czachura J. F., Stockton M. E., Howbert J. J. J. Pharmacol. Exp. Ther. 1993;264:480. [PubMed] [Google Scholar]
- Dourish C. T., Rycroft W., Iversen S. D. Science. 1989;245:1509. doi: 10.1126/science.2781294. [DOI] [PubMed] [Google Scholar]
- Trivedi B. K. Curr. Med. Chem. 1994;1:313. [Google Scholar]
- Bradwejn J., Koszycki D., Meterissian G. Can. J. Psychiatry. 1990;35:83. doi: 10.1177/070674379003500115. [DOI] [PubMed] [Google Scholar]
- Noble F., Wank S. A., Crawley J. N., Bradwejn J., Seroogy K. B., Hamon M., Roques B. P. Pharmacol. Rev. 1999;51:4. [PubMed] [Google Scholar]
- Woodruff G. N., Hughes J. Annu. Rev. Pharmacol. Toxicol. 1991;31:469. doi: 10.1146/annurev.pa.31.040191.002345. [DOI] [PubMed] [Google Scholar]
- Lattmann E., Russell S. T., Schwalbe C. H., Shortt A., Balaram P. N., Theochari E., Alharbi M., Narayanan R., Lattmann P. Med. Chem. Commun. 2016;7:1138. [Google Scholar]
- Lattmann E., Sattayasai N., Schwalbe C. H., Niamsanit S., Billington D. C., Lattmann P., Langley C. A., Singh H., Dunn S. Curr. Drug Discovery Technol. 2006;3:125. doi: 10.2174/157016306778108857. [DOI] [PubMed] [Google Scholar]
- Lattmann E., Sattayasai J., Billington D. C., Poyner D. R., Puapairoj P., Tiamkao S., Airarat W., Singh H., Offel M. J. Pharm. Pharmacol. 2002;54:827. doi: 10.1211/0022357021779005. [DOI] [PubMed] [Google Scholar]
- Lattmann E., Boonprakob Y., Sattayasai J. Drug Discoveries Ther. 2008;2:344. [PubMed] [Google Scholar]
- D'Amato M., Stamford I. F., Bennett A. Br. J. Pharmacol. 1991;102:391. doi: 10.1111/j.1476-5381.1991.tb12184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradwejn J., Koszycki D., Couetoux du Tertre A., van Megen H., den Boer J., Westenberg H. Arch. Gen. Psychiatry. 1994;51:486. doi: 10.1001/archpsyc.1994.03950060050005. [DOI] [PubMed] [Google Scholar]
- Kenakin T., Jenkinson S., Watson C. J. Pharmacol. Exp. Ther. 2006;319:710. doi: 10.1124/jpet.106.107375. [DOI] [PubMed] [Google Scholar]
- Sramek J. J., Kramer M. S., Reines S. A., Cutler N. R. Anxiety. 1994;1:141. doi: 10.1002/anxi.3070010308. [DOI] [PubMed] [Google Scholar]
- Yasuoka T., Sasaki M., Fukunaga T., Tsujikawa T., Fujiyama Y., Kushima R., Goodlad R. A. Int. J. Exp. Pathol. 2003;84:231. doi: 10.1111/j.1365-2613.2003.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss G., Diener M. J. Vet. Med., A. 1999;46:123. doi: 10.1046/j.1439-0442.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Hosseini J., Goldhill J., Bossone C., Piñeiro-Carrero V., Shea-Donohue T. Neurogastroenterol. Motil. 1999;11:347. doi: 10.1046/j.1365-2982.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- Barthó L., Holzer P., Lembeck F., Lippe I. T., Setnikar I. Br. J. Pharmacol. 1987;90:753. doi: 10.1111/j.1476-5381.1987.tb11229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattmann E., Sattayasai J., Schwalbe C. H., Boonprakob Y., Dunn S., Fajana F., Lattmann P. Arch. Pharm. 2016;349:456. doi: 10.1002/ardp.201600036. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Horii T., Hamano H., Eta R., Ozaki T., Orikawa Y., Yoshii K., Kawabata Y., Hori Y., Seto K., Takei M., Kuraishi Y. Biol. Pharm. Bull. 2010;33:244. doi: 10.1248/bpb.33.244. [DOI] [PubMed] [Google Scholar]
- Ponnusamy S., Lattmann E., Lattmann P., Thiyagarajan T., Padinjarethalakal B. N., Narayanan R. Oncol. Rep. 2016;35:2097. doi: 10.3892/or.2016.4588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.