 The benzisoxazole analogs represent one of the privileged structures in medicinal chemistry and there has been an increasing number of studies on benzisoxazole-containing compounds.
The benzisoxazole analogs represent one of the privileged structures in medicinal chemistry and there has been an increasing number of studies on benzisoxazole-containing compounds.
Abstract
The benzisoxazole analogs represent one of the privileged structures in medicinal chemistry and there has been an increasing number of studies on benzisoxazole-containing compounds. The unique benzisoxazole scaffold also exhibits an impressive potential as antimicrobial, anticancer, anti-inflammatory, anti-glycation agents and so on. This review examines the state of the art in medicinal chemistry as it relates to the comprehensive and general summary of the different benzisoxazole analogs, their use as starting building blocks of multifarious architectures on scales sufficient to drive human drug trials. The number of reports describing benzisoxazole-containing highly active compounds leads to the expectation that this scaffold will further emerge as a potential candidate in the field of drug discovery.
1. Introduction
Heterocyclic compounds are an exceedingly important class of compounds and they have attracted more attention for diverse biological studies. All available natural and synthetic heterocyclic compounds can participate in biochemical reactions in the human body.1 The engineering and rationale behind drug design are closely related to the strategic incorporation of heterocyclic-like fragments with specific physicochemical properties. The potency and selectivity through bioisosteric replacements, lipophilicity, polarity, and aqueous solubility can ultimately be fine-tuned to the point of altering and conditioning the possible mechanisms of action of pharmaceutical drugs in an attempt to obtain molecularly targeted agents.2
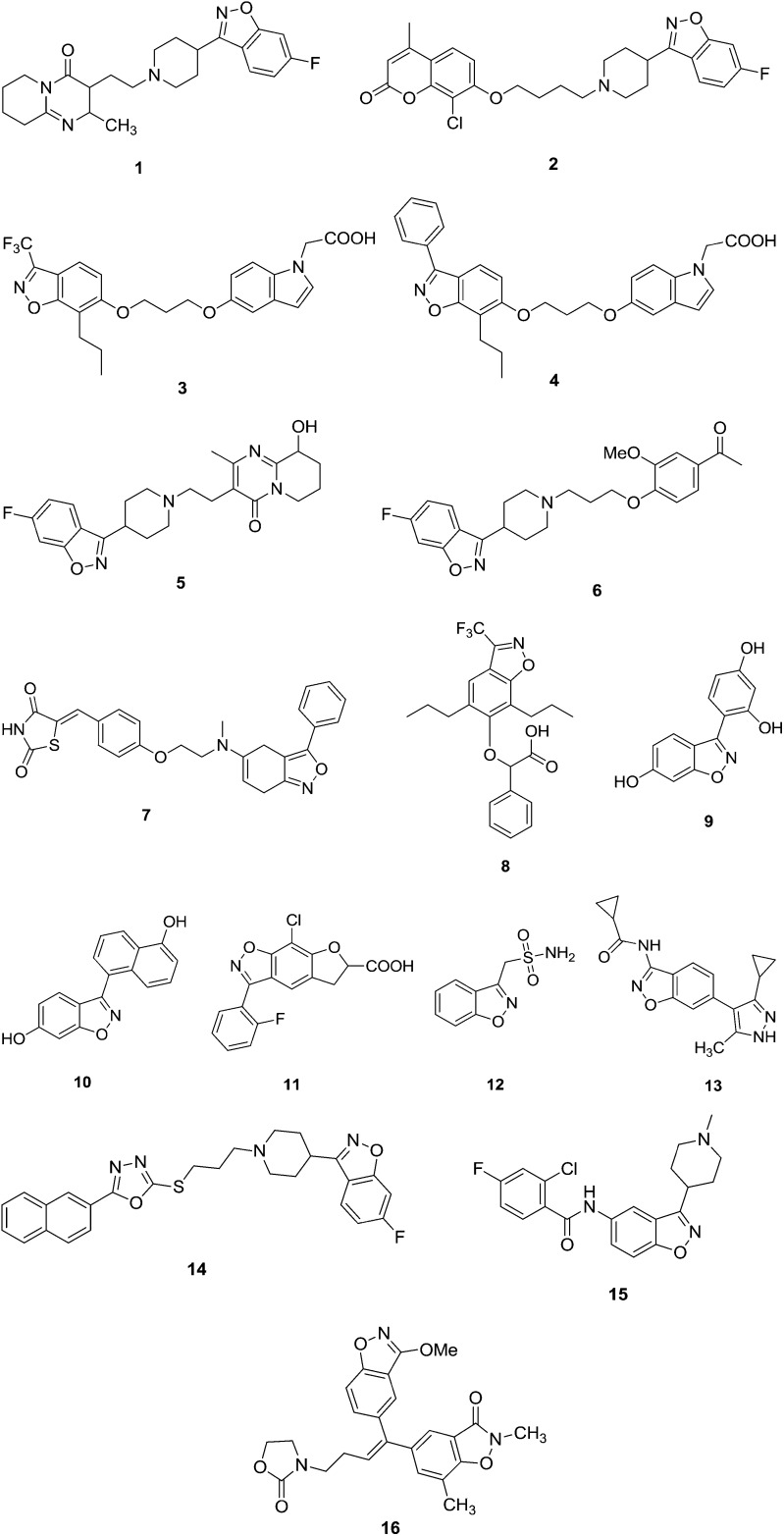
Numerous benzisoxazole moieties have been found to possess a broad spectrum of pharmacological activities, which encouraged research activities in these areas. The presence of an amide bond, benzisoxazoles, chromans and fluorine atom substituents can alter the chemical properties, disposition, and biological activities of drugs.3 Many fluorinated compounds, 1,2-benzisoxazole derivatives and various amides are currently used in the treatment of diseases.4 These include antidepressants, anti-inflammatory agents, antimalarial drugs, antipsychotics, antiviral agents, steroids, and anesthetics. The 1,2-benzisoxazole ring containing drugs zonisamide and risperidone have been used as anticonvulsants.5 Fluorine substitution can also have a profound effect on drug disposition, in terms of distribution, drug clearance, route(s), and extent of drug metabolism.6 Some representatives of these benzisoxazole-containing drugs are reported in Fig. 1(1–16).
Fig. 1. Some of the representative benzisoxazole analogues with biological potential.
Benzisoxazoles are currently the most important building blocks in drug discovery, with a high number of positive hits encountered in biological screens of this heterocycle and its derivatives. The benzisoxazole template forms the molecular backbone, possesses versatile binding properties with a frequently occurring binding motif, and provides potent and selective ligands for a range of different biological targets in medicinal chemistry. The benzisoxazole scaffold and its analogues are important pharmacophores that can be found in biologically active compounds across a number of different therapeutic areas as anti-HIV,7,8 antimicrobial,9,10 antipsychotic,11–14 anti-inflammatory,15–17 analgesic,18,19 dopamine and serotonin receptor,20 anticonvulsant,21–24 acetylcholine esterase,25 anticancer,26–29 antioxidation,30 antidiabetic31–35 agents and so on.
Benzisoxazole compounds are also used in the preparation of various functional materials for synthetic chemistry and also present in various drug molecules. This review is an attempt to expand the huge potentiality and is focused on the various biological activities of benzisoxazoles and their structure–activity relationship and docking studies.
2. Importance of medicinal chemistry in drug designing
Medicinal chemistry is a scientific discipline, which has progressed rapidly over the past few decades. Facilitated by technological advancements, the early understanding of medicinal chemistry as “synthesizing bioactive molecules” has become one of the connecting bridges of a variety of related scientific disciplines.36 Medicinal chemistry is an interdependent mature science that encompasses the discovery, development, identification, and interpretation of the mode of action of biologically active compounds at the molecular level. It may be viewed as the melting pot of synthetic chemistry and molecular pharmacology that emphasizes the study of the structure–activity relationship (SAR) of drug molecules.37
2.1. Antimicrobial activities
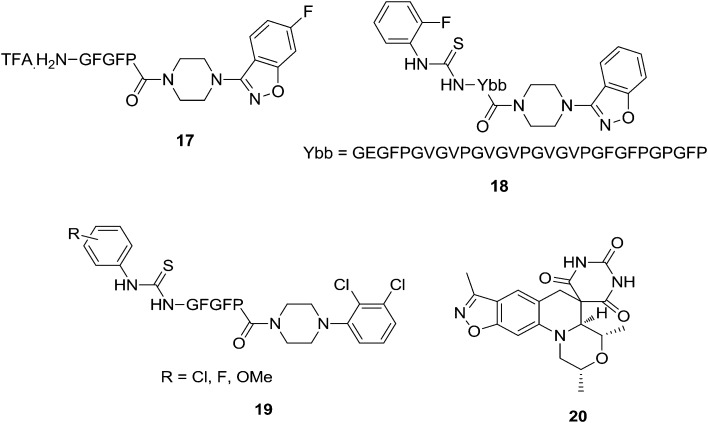
Antibacterial and antifungal microbial infections imply a considerable risk to human health and life. The development of novel drugs is still a challenge to enlarge the panel of resources of available antibacterial and antimicrobial drugs.38 Suhas et al.39 synthesized several amino acid/elastin-based peptides conjugated to a benzisoxazole derivative and tested them for their in vitro antimicrobial activities. The results revealed that conjugation plays a dominant role in inhibiting the growth of microorganisms tested. All the benzisoxazole–amino acid/peptide conjugates have shown improved antimicrobial activity (6–19 μg mL–1). Compound 17 showed excellent antimicrobial activity compared to the standard antibiotics. Further, conjugates of tricosamers have revealed extraordinary activity (3–5 μg mL–1) against all the fungal species tested which were found to be almost five times more potent than the reference drug used. Suhas et al.40 extended their previous work to urea and thiourea benzisoxazole derivatives conjugated to amino acids/peptides. In their work, the authors conjugated piperazine heterocycles to amino acids/peptides and evaluated their inhibitory activities on the growth of pathogenic bacteria and fungi. Compounds 18 and 19 exhibited highly promising activity. To study the structure–activity relationship, the introduction of S in place of O in the urea has led to enhanced results. The presence of electron withdrawing substituents, particularly F, is found to be essential for potent activity compared to electron donating groups. Among the analogs of the two heterocyclic conjugates, benzisoxazole nucleus bearing compounds have shown better activity than the piperazine moiety containing derivatives. The most salient feature of this study is that the F and Cl-containing urea/thiourea derivatives of tricosamer conjugates 18 (1.25 mg mL–1) and 19 (1.75 mg mL–1) have nearly 20–25 (Fig. 2) fold greater activity than the standards. Thus, their findings provide a new opportunity for the development of novel antimicrobials to overcome the ever-increasing problem of drug resistance. The persistence and escalation of bacterial resistance to current drug regimens continue to prompt efforts toward identifying novel antibacterial agents. A compound with a spirocyclic architecture fused to a benzisoxazole ring represents a new class of antibacterial agents that operate by inhibition of DNA gyrase as corroborated in an enzyme assay and by the inhibition of insertion of the precursor thymidine into DNA during cell growth. Highly active compounds against Staphylococcus aureus had a sufficiently high solubility, high plasma protein free fraction, and favorable pharmacokinetics, suggesting that in vivo efficacy could be demonstrated, which was realized with compound 20 (Fig. 2) in S. aureus mouse infection models. A high drug exposure NOEL on oral dosing in rats suggested that a high therapeutic margin could be achieved. Importantly, compound 20 was not cross-resistant with other DNA gyrase inhibitors such as fluoroquinolone and aminocoumarin antibacterials. Hence, this type of analog compound showed considerable promise for the treatment of infections caused by multi-drug resistant bacteria, including S. aureus.41
Fig. 2. Compounds with antimicrobial activity: compounds 17–20.
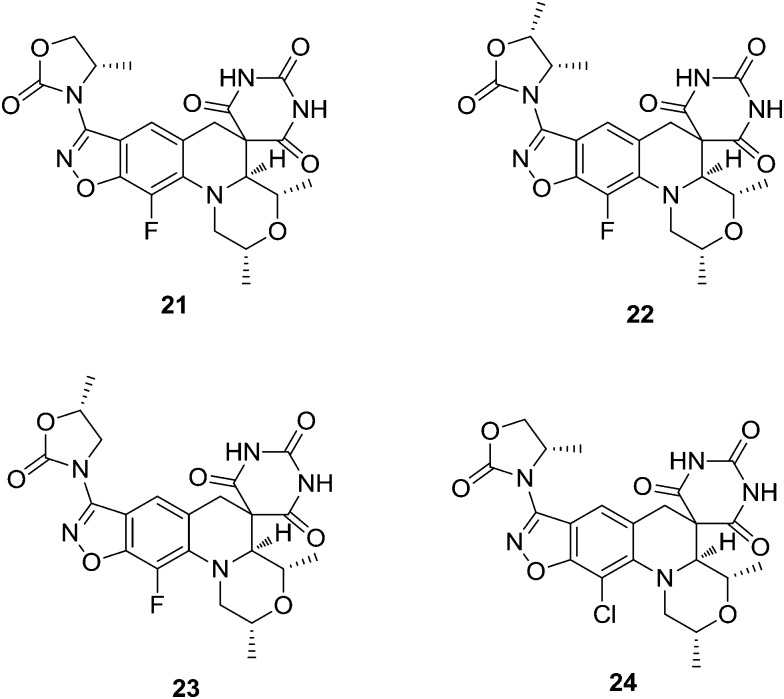
A novel class of bacterial type-II topoisomerase inhibitor displaying a spiropyrimidine-trione architecture fused to a benzisoxazole scaffold shows potent activity against Gram-positive and fastidious Gram-negative bacteria. Basarab et al.42 synthesized a new series of benzisoxazoles with N-linked oxazolidinone substituents, which improved the antibacterial activity of the initially described compounds of the class. These compounds showed favorable PK properties and demonstrated efficacy in an in vivo Staphylococcus aureus infection model. Inhibition of the topoisomerases DNA gyrase and topoisomerase IV from both Gram-positive and Gram-negative organisms was demonstrated. The compounds showed a clean in vitro toxicity profile, including no genotoxicity and no bone marrow toxicity at the highest evaluated concentrations or other issues that have been problematic for some fluoroquinolones. Compound 21 was identified for advancement into human clinical trials for the treatment of uncomplicated gonorrhea based on a variety of beneficial attributes including its potent activity and favorable safety profile. Compounds 21–24 (Fig. 3) showed excellent activity (MIC values <10 μM) versus E. coli, although the values were not sufficiently low to anticipate efficacy against the pathogen at reasonable doses in an in vivo situation.
Fig. 3. Potential antimicrobial agents (21–24).
Ravi et al.43 synthesized a series of novel methylene bridged benzisoxazolyl imidazothiadiazole derivatives and screened them for their antibacterial and antifungal activities using an agar well diffusion method. Some of the synthesized compounds showed excellent antimicrobial activities. Mainly compounds 25–28 (Fig. 4) have shown very good activity against Bacillus subtilis-ATCC 6633 and Escherichia coli-ATCC 35218. Compound 28 has exhibited very good activity against all the bacterial strains; however, compounds 25 and 26 were highly active against Escherichia coli-ATCC 35218 when compared to the standard drug ampicillin. In the study of the structure–activity relationships, the presence of electron withdrawing chloro and bromo functional groups showed excellent antimicrobial activity compared to electron donating groups.
Fig. 4. Potential antimicrobial agents (25–28).
Chaker et al.44 synthesized a new series of 3-substituted-2,1-benzisoxazole (anthranils) analogs and evaluated them for their in vitro antibacterial activity against representative Gram-positive (Bacillus subtilis and Staphylococcus aureus) and Gram-negative bacteria (Pseudomonas aeruginosa and Cronobacter sakazakii) and against two fungal (Geotrichum candidum and Candida albicans) strains. The antimicrobial activities of all the synthesized compounds were determined, and among them, compounds 29–31 (Fig. 5) showed potential antimicrobial activity against the tested pathogens (29, MIC = 44.8 μM against G. candidum, 30 and 31, MIC = 839.5 and 584.2 μM, respectively).
Fig. 5. Potential antimicrobial agents (29–31).
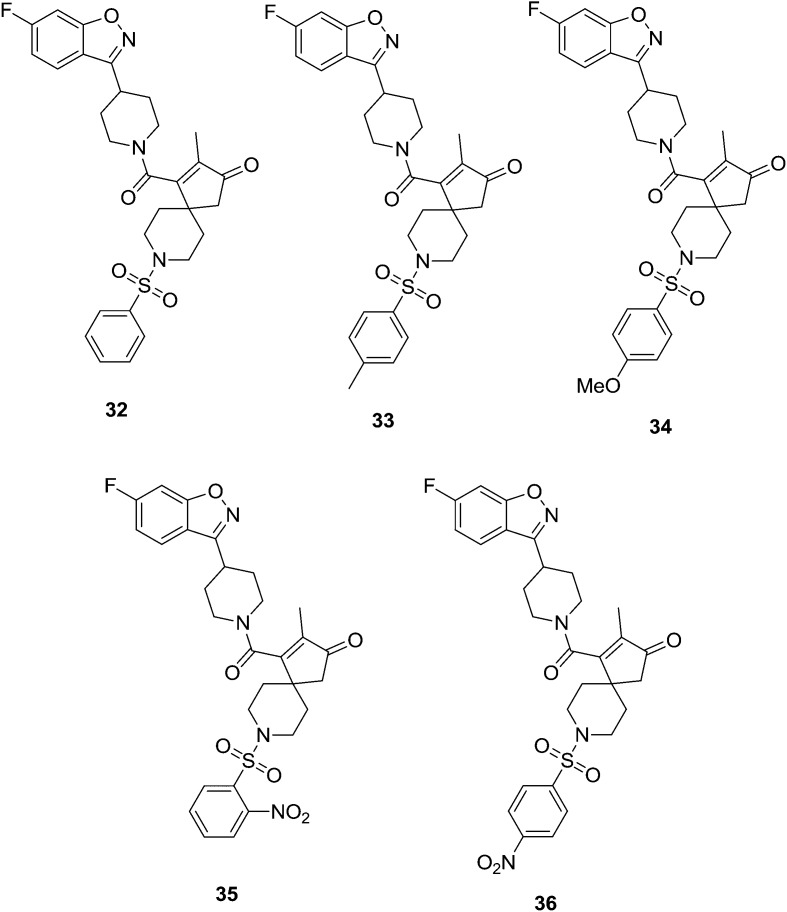
Shivaprasad et al.45 synthesized a series of novel benzisoxazole derivatives and tested them for their antibacterial, antioxidant and anti-inflammatory activities. Compound 32, a benzisoxazole derivative without substitution on the phenyl ring, showed good antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Salmonella typhi and Bacillus subtilis. Benzisoxazoles 33 and 34 bearing methyl and methoxy substituents, respectively, exhibited prominent antioxidant activity and 35 and 36, bearing the electron withdrawing nitro group, showed good anti-inflammatory activity. Thus, a new class of benzisoxazole derivatives can be incorporated into the family of bioactive heterocyclic compounds. It should also be noted that in general compounds bearing activating groups on the phenyl ring, such as 33 and 34 (Fig. 6), showed good antioxidant activities, and those with deactivating groups exhibited anti-inflammatory activities.
Fig. 6. Benzisoxazole analogues with potential biological activities.
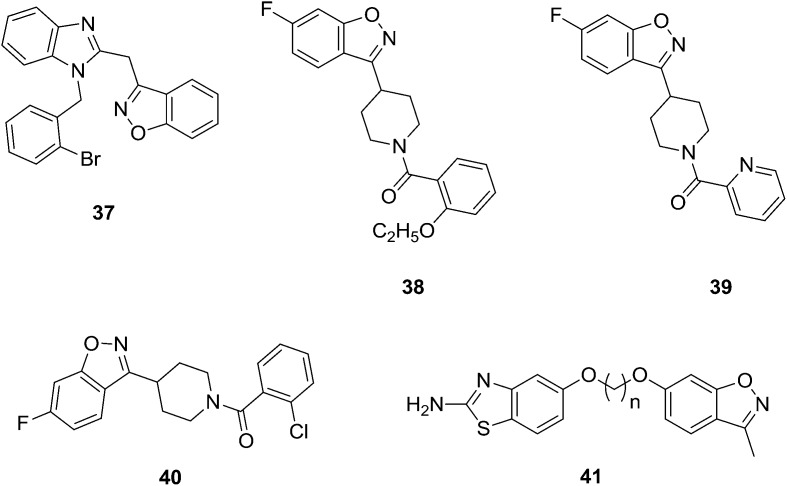
Vaidya et al.46 synthesized a series of new benzisoxazole derived analogs and screened them for their in vitro antibacterial activity. Some of the compounds were found to have good anti-bacterial activity against S. typhimurium; however, they were found to have less activity against S. aureus. Compound 37 was found to have high activity even at 1 μg mL–1 compared to cephalexin against S. aureus. These compounds were also tested against PDE-IV for a potential anti-asthmatic effect and against DP-IV and PTP-1B for potential anti-diabetic effects. Unfortunately, the results were disappointing. Priya et al.47 synthesized a series of benzisoxazole derivatives and screened them for their in vitro antimicrobial activity against different bacterial and fungal pathogens, such as Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens, Xanthomonas campestris pvs, X. oryzae, Aspergillus niger, A. flavus, Fusarium oxysporum, Trichoderma spp., F. monaliforme, and Penicillum spp., by using disc diffusion and microdilution methods. Compounds 38–40 (Fig. 7) (MIC value was lower than the standard) showed excellent inhibitory activity compared to standard antibiotics. Among these compounds, 39 showed potent inhibitory activity against all the strains and was found to be non-strain dependent. Kumbhare and coworkers48 have designed and prepared benzisoxazole derivatives of benzothiazole 41 and reported their antimicrobial activity.
Fig. 7. Synthesized compounds (37–41) with potential antimicrobial activity.
2.2. Antiglycation and urease activities
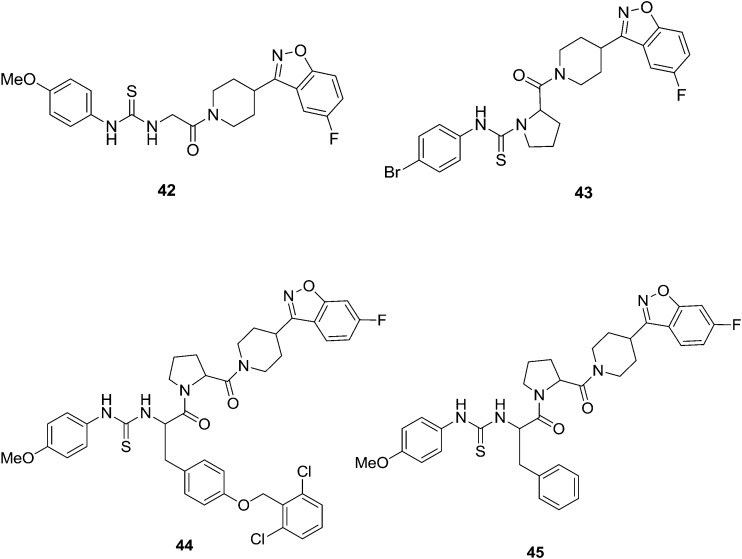
Diabetes mellitus (DM) is a one of the metabolic disorders observed when the body is inefficiently able to oxidize carbohydrates due to disruption in the insulin function.49 DM causes a long-term damage in various body functions which may also cause failure of various organ functions.50 The present estimation says that about 150 million people worldwide are suffering from diabetes and this number will increase to 220 million by 2010 and 300 million by 2050.51 90% of the global population is affected by type II diabetes.52 Shantharam et al.53 reported a new series of urea/thiourea derivatives of Gly/Pro conjugated benzisoxazole derivatives and these were screened for their in vitro antiglycation activity. Compounds 42 and 43 (Fig. 8) showed promising activity with IC50 < 5 mM compared to the standard rutin (IC50 = 41.9 mM). Further, it was found that compounds containing methoxy and bromine substituents have exerted highly potent activity. Moreover, the previously-mentioned authors continued their research on the novel development of anti-glycating agents in medicinal chemistry. The authors designed and synthesized four dipeptides with varying hydrophobicities at the N-terminus, and these were conjugated to the benzisoxazole heterocycle and their conversion to the corresponding urea/thiourea derivatives was monitored.54 All the synthesized compounds were screened for their in vitro anti-glycation and urease activities. Preliminary studies revealed that compounds with methoxy at the para position and bromo at the ortho position of the aryl moiety along with the thiourea system has significantly enhanced antiglycation and anti-urease activities when compared to their bioisosteric counterpart, the methoxy/bromo substituted aryl moiety along with the urea system. The best antiglycation activity was found for the benzisoxazole conjugated Tyr–Pro dipeptide (44) (IC50 value is 4.3 μM) analogue with a methoxy group at the para position and the best anti-urease activity was found for Phe–Pro (45) (IC50 value is 4.9 μM) for urease with a methoxy group at the para position (Fig. 8). The results clearly indicate the importance of peptides towards the potency of protein glycation and urease inhibition. Further, the trend showed that the increase in the hydrophobicity of the molecule increases the anti-glycation and urease inhibition activities. In conformation to earlier findings,53 the compounds with thiourea are more potent than their corresponding urea analogs. The order of activity is found to be KP < VP < FP < YP, which is analogous to the order of their hydrophobicities.55–57
Fig. 8. Synthesized compounds (42–45) with potential antiglycation activity.
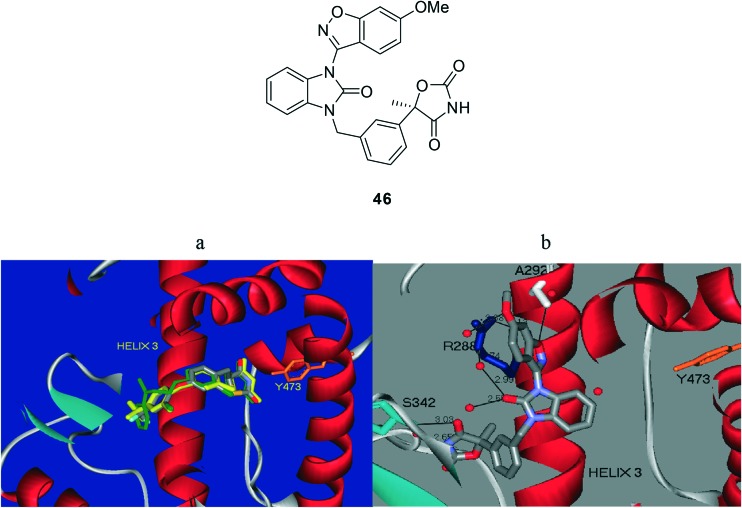
Diabetes is a metabolic disorder escalating in the world population at an alarming rate.58 Liu et al.59 developed a new series of benzimidazolone carboxylic acids and oxazolidinedione derivatives in search for selective PPARγ modulators (SPPARγMs) as potential therapeutic agents for the treatment of type II diabetes mellitus (T2DM) with improved safety profiles relative to rosiglitazone and pioglitazone, the currently marketed PPARγ full agonist drugs. The lead compound, 46, displayed robust glucose and insulin lowering activities similar or superior to that of rosiglitazone in rodent models of T2DM. These unique biological properties displayed by SPPARγM 46 make it an attractive candidate for further evaluation as a potential therapeutic agent for the treatment of T2DM. Compound 46 was cocrystallized with the human PPARγ ligand binding domain (LDB), and its structure was compared with those of rosiglitazone and pioglitazone, two prototypical thiazolidinedione-based PPARγ full agonists. As shown in Fig. 9, the two full agonists bind across helix 3 with the acidic thiazolidinedione moiety interacting through hydrogen binding with Tyr473 in helix 12, thereby stabilizing this activated receptor–ligand complex.60
Fig. 9. PPARγ ligand binding domain showing ligand–protein interactions of (a) full PPARγ agonists rosiglitazone (green) and pioglitazone (yellow) (PDB access code 2PRG) and (b) PPARγ selective modulator, compound 46 (gray) (PDB access code ; 3TY0). The helix 3 region is marked, and the tyrosine 473 residue is shown in orange.
2.3. Anticonvulsant activity
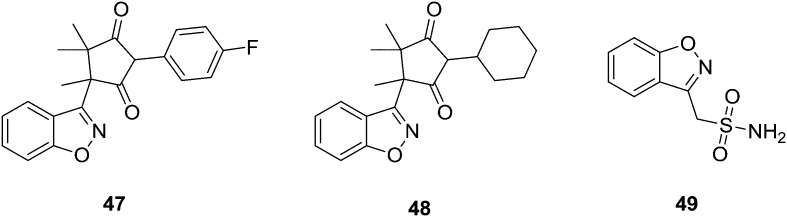
Malik et al.61 synthesized a series of benzisoxazole analogs and screened them for their in vitro anticonvulsant activities. Preliminary anticonvulsant activity screening was performed using maximal electroshock (MES) and subcutaneous pentylenetetrazole (scPTZ) tests after intraperitoneal (IP) injection into mice, which are the most widely-employed models for early identification of anticonvulsant candidates. Their acute neurological toxicity (NT) was determined to apply the rotorod test. The quantitative evaluation after oral administration in rats showed that the most active compound was 47 with an ED50 value of 14.90 mg kg–1. Similarly, the most potent in the scPTZ test was compound 48 with an ED50 value of 42.30 mg kg–1. These molecules were more potent and less neurotoxic than phenytoin and ethosuximide, which were used as the reference antiepileptic drugs. Their structural modification can play an essential role in the search for new anticonvulsants. Uno and associates62 have synthesized several 3-(sulfamoylmethyl)-1,2-benzisoxazole derivatives from 3-(bromomethyl)-1,2-benzisoxazole by its reaction with sodium bisulfite, followed by chlorination and amination. Some of the derivatives displayed marked anticonvulsant activity in mice. The introduction of a halogen atom at the 5-position of the benzisoxazole ring caused an increase in activity and neurotoxicity; the substitution of a sulfamoyl group caused a decrease in the activity. The activity of monoalkylated compounds might be the result of biotransformation. Compound 49 was thought to be the most promising anticonvulsant agent (Fig. 10).
Fig. 10. Most active synthesized anticonvulsant agents.
2.4. Antitubercular activity
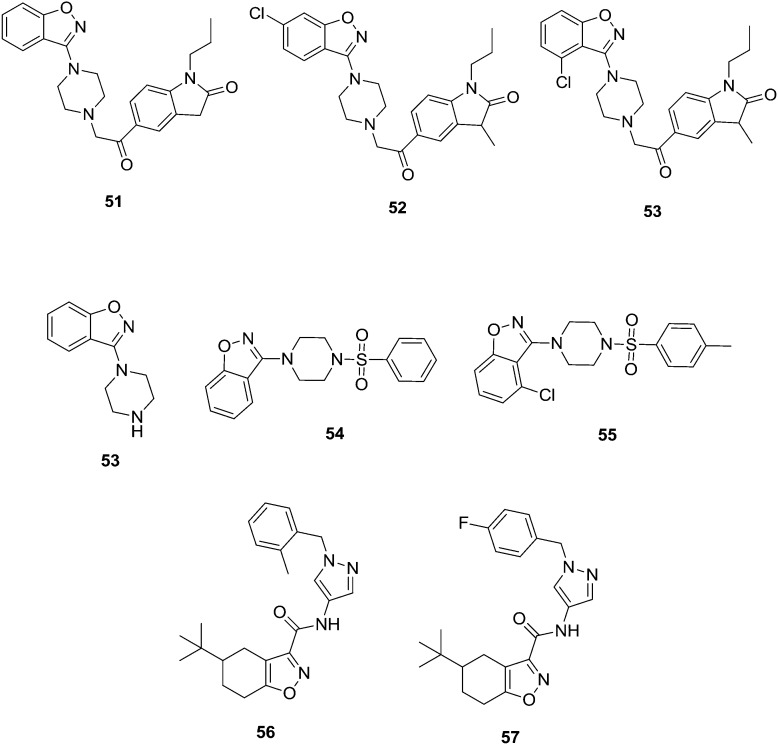
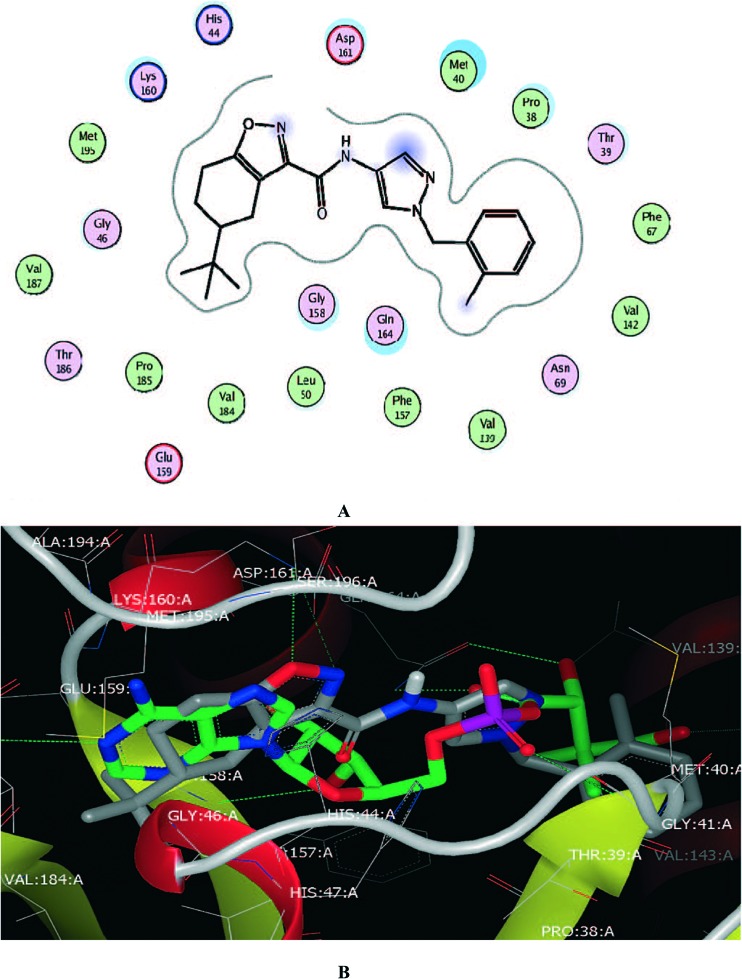
Naidu et al.63 synthesized a series of benzisoxazole analogs and screened them for their in vitro anti-tubercular activity against the Mycobacterium tuberculosis H37Rv strain. Compounds 50–52 (MIC value is 3.12 μg mL–1) showed good anti-tubercular activity. The results of benzo[d]isoxazoles showed essential pharmacophoric features that might lead to them as promising candidates in developing anti-tubercular agents. Moreover, the authors continued their research on the development of novel tuberculosis agents. Later, the same authors developed and synthesized a series of twenty-four novel benzisoxazole analogs and tested them for their in vitro anti-tubercular activity against the Mycobacterium tuberculosis (MTB) H37Rv strain. The synthesized compounds exhibited minimum inhibitory concentrations (MIC) between 3.125 and >50 μg mL–1. Among the tested compounds, 53 and 54 exhibited good activity (MIC = 6.25 μg mL–1 and 3.25 μg mL–1, respectively) and 55 (MIC = 6.25 μg mL–1) exhibited very good antitubercular activity. In addition, the analogues were subjected to toxicity studies against mouse macrophage (RAW 264.7) cell lines to analyze the selectivity profile of the newly synthesized compounds and the selectivity index of the most active compound was found to be >130, indicating suitability of the compound for further drug development.64 Pantothenate synthetase (PS) is one of the potential new antimicrobial targets that may also be useful for the treatment of the nonreplicating persistent forms of Mycobacterium tuberculosis. Subash et al.65 synthesized a new series of benzisoxazole derived analogs and screened them for their potential as in vitro Mycobacterium tuberculosis PS inhibitors. Compounds 56 (IC50 120 nM) and 57 (IC50 nM) showed potent anti-tuberculosis activity (Fig. 11). In the molecular docking studies of ligand 56, the binding of the tert-butyl group in the hydrophobic pockets of the binding site is consistent with its importance for the inhibitory activity of the ligands (Fig. 12).
Fig. 11. Most active synthesized anti-tuberculosis agents (51–57).
Fig. 12. Protein–ligand interactions between (R)-56 and PS (PDB: ; 2A88): (green) hydrophobic, (light-purple) polar, (blue ring) basic, (red ring) acidic. The tetrahydrobenzoisoxazole ring of 1a occupies the position of the adenine ring (A and B) of the reaction intermediate cocrystallized with PS in PDB1N2H.
2.5. Antipsychotic activities
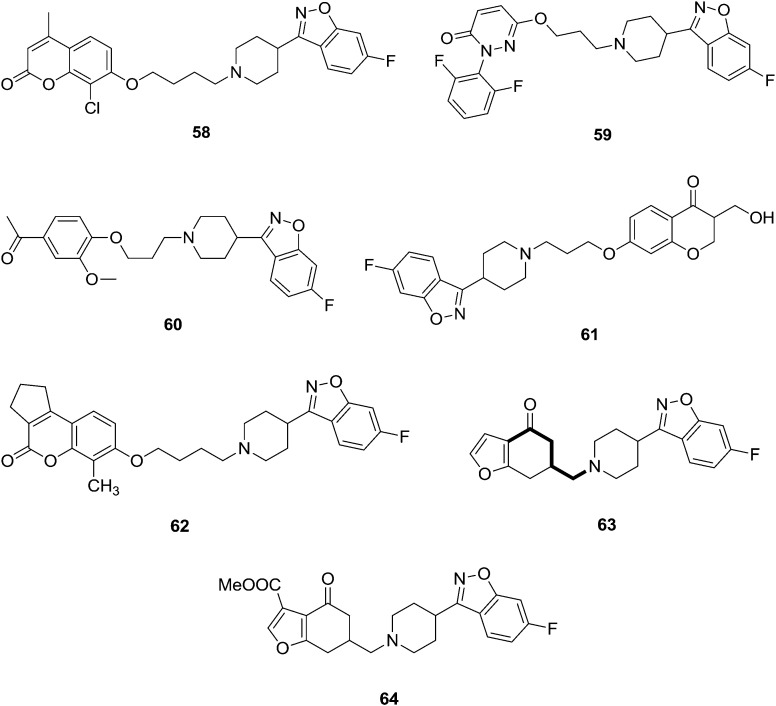
Chen et al.66 reported and synthesized a novel series of coumarin-based benzothiazole derivatives and these were screened for their antipsychotic activities. In this work, the authors mainly focused on potent dopamine D2, D3, and serotonin 5-HT1A and 5-HT2A receptor properties. The most-promising derivative was 58 (Fig. 13). This derivative possesses unique pharmacological features, including high affinity for dopamine D2 and D3 and serotonin 5-HT1A and 5-HT2A receptors. Moreover, it possesses low affinity for 5-HT2C and H1 receptors (to reduce the risk of obesity associated with chronic treatment) and hERG channels (to reduce the incidence of torsade des points). In animal models, compound 58 inhibited the apomorphine-induced climbing behavior, MK-801-induced hyperactivity, and the conditioned avoidance response without observable catalepsy at the highest dose tested. Further, fewer preclinical adverse events were noted with 58 compared with risperidone in assays that measured prolactin secretion and weight gain. Acceptable pharmacokinetic properties were also noted with 58. Taken together, 58 may constitute a novel class of drugs for the treatment of schizophrenia. Nowadays, multi-targeting ligands have attracted great interest as possible new atypical antipsychotics. Combinations of dopamine and serotonin receptor ligands within single molecules might afford new therapeutic opportunities. X. Cao et al.67 synthesized a novel series of 6-hydroxypyridazinone benzisoxazoles and their binding behaviors to different receptors in terms of atypical antipsychotic behaviors were noted. The most potent compound (59) exhibited excellent affinities for certain receptors (D2, Ki = 0.5 ± 0.07 nM; 5-HT1A, Ki = 5.9 ± 0.8 nM; 5-HT2A, Ki = 0.3 ± 0.01 nM; 5-HT6, Ki = 0.5 ± 0.04 nM) and combined with low affinities for the H1, 5-HT2C, and adrenergic a1 receptors. In contrast to risperidone, compound 59 exhibited a high cataleptic threshold; this may be useful in the development of a novel class of drugs treating schizophrenia. Albers and associates studied iloperidone 60 (Fig. 13), a new-generation atypical antipsychotic agent, acting as a serotonin/dopamine (5-HTzA/Dz) antagonist. Chemically, iloperidone is a benzisoxazole, like risperidone, and shows a multiple receptor binding profile, sharing this feature with the other atypical antipsychotic agents. They have reported its pharmacokinetics and pharmacodynamics, together with an evaluation of its clinical safety and efficacy results.68
Fig. 13. Benzisoxazole analogues with potent antipsychotic activities (58–64).
Bolos et al.69 synthesized a series of novel benzisoxazole analogs and they tested their potential as antipsychotics in several in vitro and in vivo assays. Some of the synthesized compounds possessed a good affinity for D2 receptors, together with a greater affinity for 5-HT2 receptors, a profile which has been proposed as a model for atypical antipsychotics. Several agents also displayed a high potency in the climbing mice assay on oral administration, suggesting a potent antipsychotic effect compared to reference standards. Compound 61 (Fig. 13) showed good binding affinities for the D2 receptor (IC50 17.0 nM) and 5-HT2A receptor (IC50 6.2 nM). According to these results, compound 61 (abaperidone, FI-8602) has been selected for clinical development.
Chen et al.70 synthesized a series of new coumarin-based benzisoxazole analogs and these were screened as potential multitarget antipsychotics. The catalepsy test is a common and widely used preclinical screening test for the propensity of an antipsychotic drug to induce EPS in humans.71 Compound 62 (Fig. 13) showed high affinity for dopamine D2 (12.7 nM) and D3, serotonin 5-HT1A (7.8 nM), 5-HT2A (2.2 nM) receptors, with a low affinity for the H1 receptor. Compound 62 affinity observations indicated that introducing substituents at the 8-position is useful to increase affinity for the D2, 5-HT1A, and 5-HT2A receptors. In vivo animal models showed that compound 62 had high potential for treating symptoms of schizophrenia without causing catalepsy. Compound 62 had a higher threshold for catalepsy induction compared with the two currently marketed atypical antipsychotics, risperidone and clozapine. Compound 62 might be useful for developing a novel class of drugs for the treatment of schizophrenia. The complex etiology of schizophrenia has prompted researchers to develop clozapine-related multitarget strategies to combat its symptoms. Aranda et al.72 synthesized a new series of 6-aminomethylbenzofuranones in an effort to find new chemical structures with balanced affinities for 5-HT2 and dopamine receptors. In addition, compound 63 (QF1004B) was used as a tool to elucidate the role of 5-HT2C receptors in mediating antipsychotic effects and metabolic adverse events. Compound 64 (QF1018B) showed moderate to high affinities for D2 and 5-HT2A receptors, and its 5-HT2A/D2 ratio was predictive of an atypical antipsychotic profile.
2.6. Anticancer activities
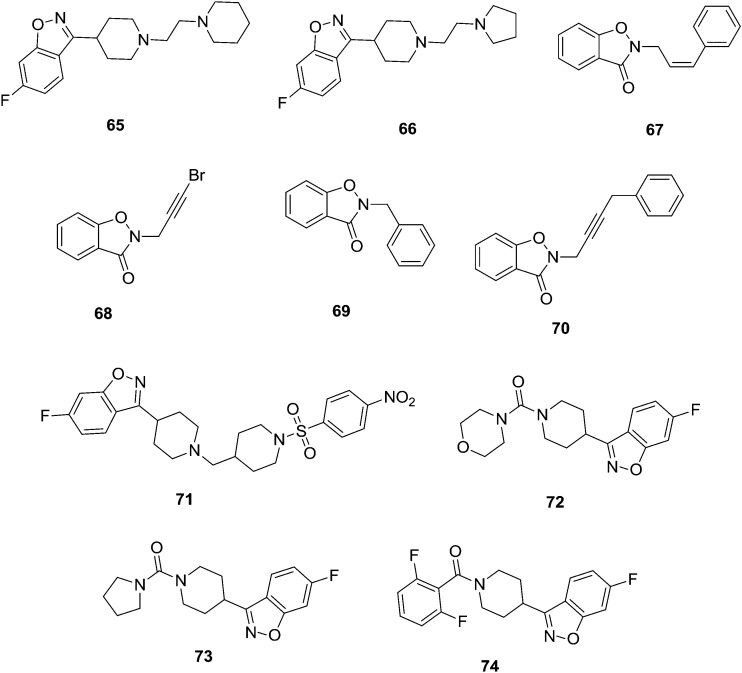
Development of new anticancer therapeutic agents is one of the key challenges in medicinal chemistry. Cancer, a universal name for a group of diseases, is found all over the world.73 Cancer is the second leading cause of death worldwide. There is always a huge demand for novel anticancer drugs and diverse new natural or synthetic compounds are developed continuously by scientists. Presently, large numbers of drugs in clinical practice have shown a pervasive side effect and multidrug resistance. K. S. Rangappa and Basappa74 synthesized a series of new benzisoxazole analogs and these were screened for their in vitro inhibitory activity of acetylcholinesterase (AChE) against targets from different species, such as pure electric eel AChE, human serum AChE, and rat brain AChE. Compounds 65 (IC50 = 1.5, 2.22, 1.29 μM) and 66 (IC50 = 2.51, 3.29, 5.3 μM) showed strong inhibition against AChE from different sources: electric eel, human serum and rat brain homogenate, respectively. Structure–activity relationships were studied by comparing the basicities of the different substituted heterocyclic ring systems at the C-3 position of the 1,2-benzisoxazoles derivatives. Anand et al.75 synthesized a novel series of benzisoxazole-substituted-allyl derivatives and their in vitro antioxidant and anticancer activities were evaluated. Compounds 67–70 (Fig. 14) were identified as the best hit against HT-29 human colon cancer cells.
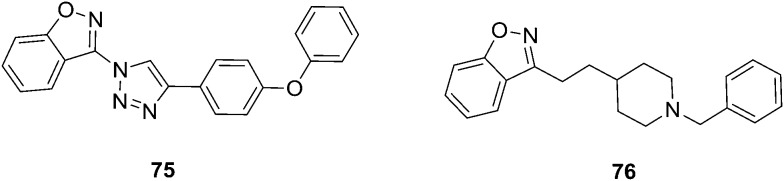
Fig. 14. Benzisoxazole analogues that are potential anticancer agents (65–74).
Chen et al.76 synthesized a series of new amide derivatives of benzisoxazole analogs and these were evaluated as potential multi-target antipsychotic agents. Compound 71 showed high affinity for dopaminergic and serotonergic (5-HT1A and 5-HT2A) receptors and low affinity for H1 receptors and hERG channels. Furthermore, animal models showed that compound 71 had a high potential for treating symptoms of schizophrenia without causing catalepsy. Compound 71 (Fig. 14) showed potential as an antipsychotic lead candidate. Biological results demonstrate that the novel derivative may serve as a promising antipsychotic lead and hence pave the way for further investigation around this chemical space. Benaka Prasad et al.77 synthesized a series of novel benzisoxazole derivatives and these were evaluated for their in vitro antiproliferative activity against viable human skin fibroblast cells and carcinoma cells, namely, HeLa cells, HT-29 cells, MCF-7 cells, HepG-2 by using the MTT assay. Compounds 72–74 (Fig. 14) have shown potential and the remaining compounds showed less cell growth inhibition. Among the amide substituted compounds, the minimum 37.75% inhibitory activity was shown by 72 against HepG-2 cells. Compound 73 exhibited 39.19% antiproliferation against HepG-2 cells. Similarly, compound 74 showed moderate 42.06% antiproliferation against HepG-2 cells. From the SAR studies, it is revealed that the inhibition by compounds 72 and 73 could be attributed to the basicity of the morpholine and pyrrolidine ring, respectively. The inhibition by compound 74 may be due to the presence of the electron withdrawing difluorophenyl ring attached to the parent compound.
The innovation of the HDAC family of proteins, and avenues of how to modulate them have provided a new dimension to the design of therapeutic agents against a broad range of cancers via epigenetic approaches. Ashwini et al.78 developed a series of novel 1,2,3-triazoles derived from benzisoxazole analogs and these were screened for their in vitro antiproliferative effect on human acute myeloid leukemia (AML) cells. Compound 75 was found to be the most potent antiproliferative agent with an IC50 of 2 μM against MV4-11 cells using the MTT assay. 75 was identified as the most potent cytotoxic agent against various AML cell lines (MOLM13, MOLM14, and MV4-11 cells) while showing selectivity over bone marrow cells. Ligand-based as well as structure-based analyses of the interaction partners suggested HDACs as a molecular mode-of-action of 75. The lead compound 75 hence significantly decreased cell proliferation, induced mitochondrial-mediated apoptosis, and disrupted the cell cycle of MOLM13, MOLM14, and MV4-11 cells. Computational analysis suggested HDAC as the target of 75, which has been validated by increased acetylation of histone H3 in a dose-dependent manner, as well as induction of p21 and tubulin acetylation. Villalobos et al.,79 have developed a series of N-benzylpiperidine benzisoxazoles (76, Fig. 15) as potent and selective inhibitors of the enzyme acetylcholinesterase (AChE). The benzisoxazole heterocycle was found to be an appropriate bioisosteric replacement for the benzoyl functionality present in the N-benzylpiperidine class of inhibitors. They have concluded that the N-benzylpiperidine benzisoxazoles may be suitable compounds for the palliative treatment of Alzheimer's disease.
Fig. 15. Benzisoxazole analogues that are potential anticancer agents.
2.7. Other biological activities
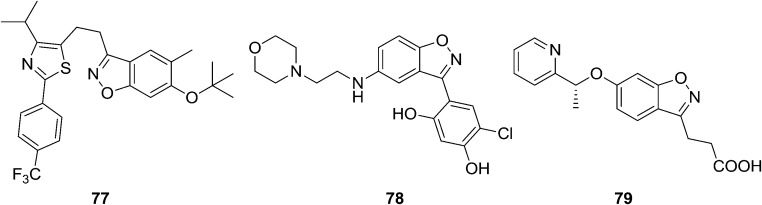
Privileged heterocyclic structures present a new concept of understanding bioactive molecular diversity, and these can also be used for the identification of new structural units that contribute as pharmacophores for different pharmacologic activities.80 It can bind to multiple targets with high affinity, thus aiding the discovery of novel bioactive agents. Numerous encouraging investigations demonstrated that these privileged structures should be extensively exploited for their therapeutic benefits.81,82 The basic trick of exploiting privileged structures for scaffold is re-evolution or refining. The influence of this approach is exemplified in the discovery of other new potential drugs or therapeutic applications by refining privileged structures in pharmacological agents.83 Sakuma et al.84 synthesized a novel series of benzisoxazole derived analogs and these were screened for their peroxisome proliferator-activated receptor d agonist activity. Compound 77 exhibited potent human PPARδ transactivation activity. The EC50 of compound 77 is 0.011 μM. Furthermore, it stimulated the differentiation of oligodendrocyte precursor cells in vitro. This indicates that this potential drug may be effective for the treatment of demyelinating disorders such as multiple sclerosis. Heat shock protein 90 (Hsp90) is a molecular chaperone that is responsible for activating many signaling proteins and is a promising target in tumor biology. Gopalsamy et al.85 identified small-molecule benzisoxazole derivatives of 78 as an Hsp90 inhibitors. Crystallographic studies showed that these compounds bind in the ATP binding pocket interacting with the Asp93. Structure-based optimization led to the identification of potent analog 78, with good biochemical profiles. Acute pancreatitis (AP) is an inflammatory disease of the pancreas that is usually triggered by gallstones or excessive alcohol consumption. Walker et al.86 developed and optimized a series of KMO inhibitors using novel Pf KMO crystal structures and rationalized some unexpected SAR on the human KMO enzyme. The compound 28 showed potent cellular activity, excellent aqueous solubility, and a DMPK profile suitable for dosing. The effect on plasma concentrations of kynurenine pathway metabolites after administration of 79 recapitulates the previously observed effect using tool KMO inhibitor compounds and Kmo knockout mice.87 Compound 79 showed protection against extrapancreatic tissue injury to the kidneys and lungs during experimental AP in rats. Compound 79 will now be progressed toward clinical evaluation (Fig. 16).
Fig. 16. Benzisoxazole analogues that are potential biological agents.
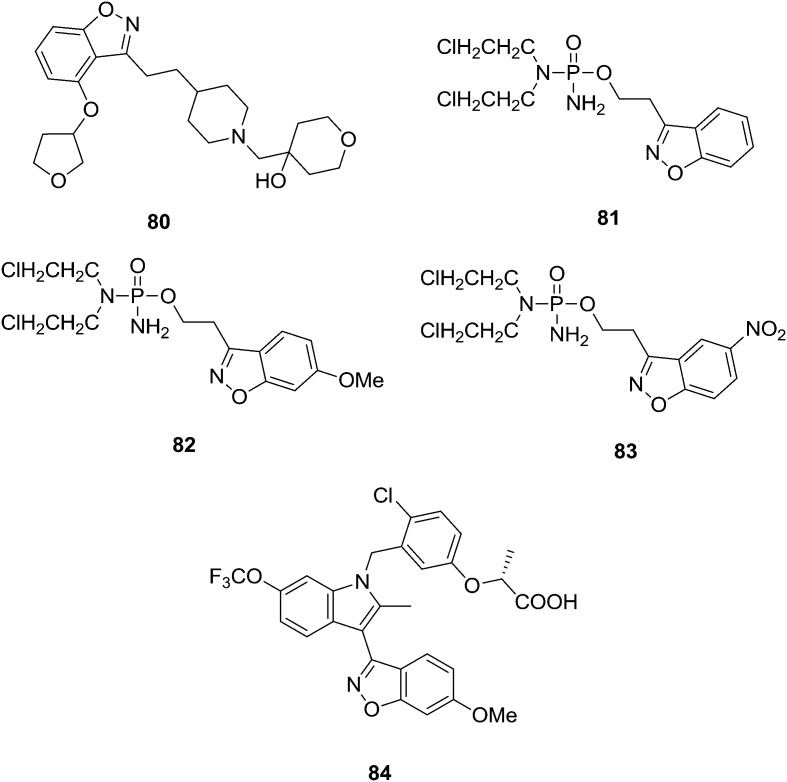
The cognitive impairments observed in Alzheimer's disease (AD) are in part a consequence of reduced acetylcholine (ACh) levels resulting from a loss of cholinergic neurons. Preclinically, serotonin 4 receptor (5-HT4) agonists are reported to modulate the cholinergic function and therefore may provide a new mechanistic approach for treating cognitive deficits associated with AD. Brodney et al.88 designed and synthesized a new series of potent, selective, and brain penetrant 5-HT4 agonists. The overall goal of the medicinal chemistry strategy was an identification of structurally diverse clinical candidates with varying intrinsic activities. The exposure–response relationships between binding affinity, intrinsic activity, receptor occupancy, drug exposure, and pharmacodynamic activity in relevant preclinical models of AD were utilized as key selection criteria for advancing compounds. On the basis of their excellent balance of pharmacokinetic attributes and safety, one lead 5-HT4 partial agonist candidate 80 was chosen for further clinical development. Jain and Kwon89 synthesized benzisoxazole phosphorodiamidate analogs as prodrugs of phosphoramide mustard requiring bioreductive activation. As expected, the proposed prodrugs 81–83 showed 3–5-fold more potent cytotoxins than the controls, which are lacking in the phosphoramide mustard group (Fig. 17). Among the phosphorodiamidate prodrug, 82 was the most potent cytotoxic, with an IC50 value of 241 μM, while 4 and 12 showed slightly weaker cytotoxicity with IC50 values of 411 and 311 μM, respectively. Liu et al.90 synthesized a series of new metabolically robust benzisoxazole selective PPARc modulators (compound 84). In vitro studies of 84 show that these are partial agonists and they exhibit reduced adipogenesis in human adipocytes. In vivo studies with SPPARcMs result in potent glucose lowering in DB/DB mice and attenuate increases in heart weight and brown adipose tissue that is typically observed in rats upon treatment with PPARc full agonists. Compound 84 was also studied in male Sprague–Dawley rats. After once-daily dosing for two weeks, 12 (AUC = 1030 μM h, ∼1.6–10× efficacy exposure) resulted in less CH and BAT increases compared to that of rosiglitazone (AUC = 2700 μM h, ∼4–10× efficacy exposure), which was included as an internal positive control.
Fig. 17. Benzisoxazole analogues that are potential biological agents.
2.8. Molecular binding targets
Glycogen synthase kinase-3 (GSK-3) as a target
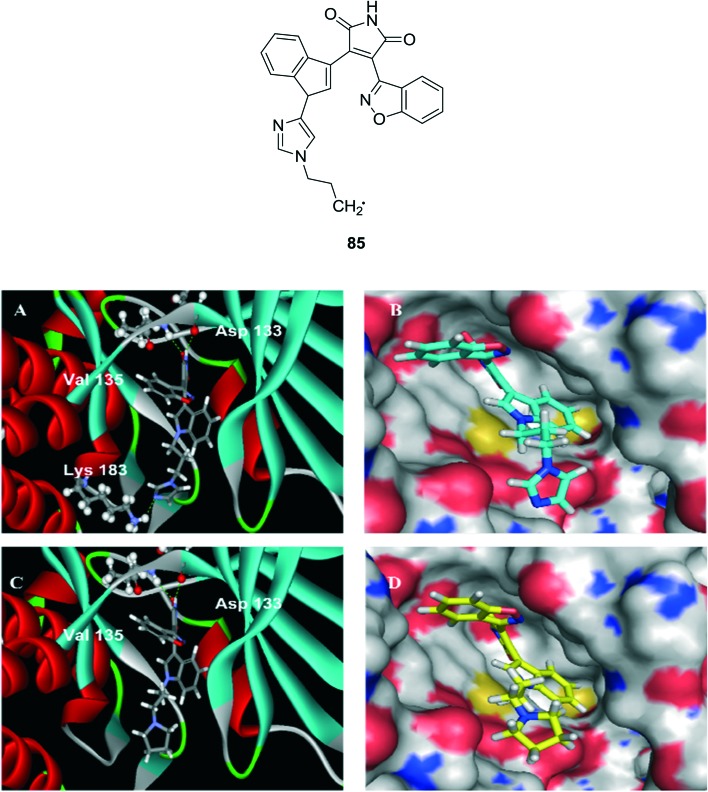
GSK3 has attracted some considerable interest because it has the unconventional characteristics for a kinase of being constitutively active, its substrates usually need to be pre-phosphorylated by another kinase, and it is inhibited, rather than activated, in response to stimulation of the two main signaling pathways known to impinge on GSK3, the insulin and Wnt pathways. One of these developments was the discovery that GSK3 is a key kinase contributing to abnormal phosphorylation of the microtubule-binding protein tau in the process thought to cause neurofibrillary tangles in Alzheimer's disease.91,92 In conjunction with these discoveries and the ensuing enormous growth in recognizing new actions of GSK3, many additional inhibitors of GSK3 were developed as GSK3 began to be seriously considered as a therapeutic target.93 Potential therapeutic applications include many prevalent conditions, such as cancer, cardiovascular diseases, diabetes, inflammatory conditions, neurodegenerative diseases, and psychiatric diseases, as well as other less prevalent conditions. Such an expansive influence on cellular signaling and association with multiple disease processes has stimulated skeptics to inquire how GSK3 can manage all of its tasks in an organized fashion, and how such a pervasive kinase can reasonably be thought of as a feasible therapeutic target. The mammalian GSK-3 has two isoforms – GSK-3α and GSK-3β – sharing homology (84% overall) at the catalytic domain (98%) but they significantly differ in their N-terminal. The GSK-3β plays a critical role in glycogen metabolism, and it has evolved to be a target to decrease the occurrence of diseases as type 2 diabetes, stroke, cancer, chronic inflammatory processes, bipolar disorders and Alzheimer's disease. Many a potent drug have been developed from various approaches but they are not truly fit to target the decrease in occurrence of pathological conditions.94 A series of synthetic compounds have been analyzed against GSK-3β. Among them, compound 85 showed the highest inhibition in an enzymatic assay against GSK-3β (IC50 (nM) 0.73 ± 0.02) compared to staurosporine (IC50 (nM) 72.2 ± 3.6). And its molecular docking study was performed for the published X-ray crystal structure of GSK-3β (PDB ID: ; 1Q3D) and the study results could explain the fact that 85 showed significantly improved potency compared to other derivatives and staurosporine (Fig. 18).
Fig. 18. Docking of compound 85 to the GSK-3β crystal structure. (A) Ribbon presentation of compound 85 bound to GSK-3β; (B) surface presentation of compound 85 docking into GSK-3β; (C) ribbon presentation of compound 85 bound to GSK-3β; and (D) surface presentation of compound 85 docking into GSK-3β.
As an anti-tubercular agent
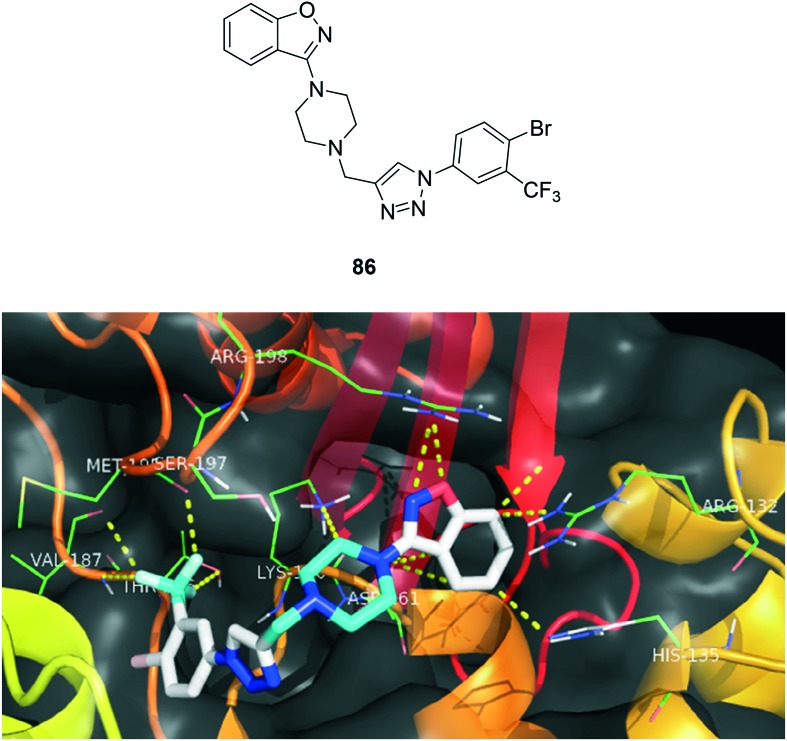
Tuberculosis (TB) is a multisystemic infectious disease caused by Mycobacterium tuberculosis (or TB), a rod-shaped bacterium. TB may stand for the disease or the bacteria that cause the disease and is the most common cause of infectious disease-related mortality worldwide (about 10 million people worldwide were sick with TB in 2015, and about 1.8 million people died from TB worldwide in 2015 according to the World Health Organization (WHO)). The most common site (about 85%) for TB to develop is in the pulmonary tract. Humans are the only known hosts for Mycobacterium tuberculosis (although animals can get infected). However, the CDC estimates one-third of the world's population is infected with TB with about 1.8 million deaths per year. About 60% of all TB-infected people are located in India, Indonesia, China, Nigeria, Pakistan, and South Africa. These bacteria are slow growing, aerobic, and can grow within body cells (an intracellular parasitic bacterium). Its unique cell wall helps protect it from the body's defenses and gives mycobacteria the ability to retain certain dyes like fuchsin (a reddish dye) after an acid rinse that rarely happens with other bacterial, fungal, or parasitic genera. Mycobacteria that escape destruction by body defenses may be spread by blood or lymphatic pathways to most organs, with preference to those that oxygenate well. Many a failure of synthetic drugs lead to resistance to the bacteria, causing them to become multidrug resistant TB (MDR-TB), extensively drug resistant TB (XDR-TB) and totally drug resistant TB (TDR-TB) or extremely drug resistant TB (XXDR-TB).95 Since the past few decades, bedaquiline and delamanid are used to healing pulmonary MDR-TB patients in life-threatening conditions. In these aspects, no novel anti-TB drugs have surfaced out till now in both efficacy and safety aspects.96,97 Naidu et al.98 synthesized a series of compounds and tested them to understand the role of the compounds and their efficacy as an anti-tuberculosis agent. To analyse the interaction profile of the most active anti-TB compounds, we performed docking studies on the mycobacterial pantothenate synthetase (PS) enzyme (; 4MQ6.pdb) to understand ligand–receptor binding interactions. The unique multiple interactions observed for compound 86 may be responsible for its potency. From this study, we wish to propose that interactions with Lys 160, Met 195, Ser 197, and Arg 132 play a major role in the design of an inhibitor for the PS enzyme and the binding pattern of the most active compound 86 is depicted in Fig. 19. The future is waiting for a new class of drug-like candidates for TB in the forms like benzo[d]isoxazoles to produce vital pharmacophoric features that might lead to promising candidates for further drug development towards treating TB.
Fig. 19. Interaction pattern of the compound 86 with mycobacterial pantothenate synthase enzyme.
3. Conclusion
It is evident from the above discussion that benzisoxazole and its derivatives have immense potential as various biological agents. In the discovery of potential drugs, benzisoxazole is an important pharmacophore, and several research laboratories worldwide are focused on the synthesis of different benzisoxazole derivatives for the development of novel and more potent drugs. This review article focused on the biological activities of synthetic benzisoxazole derivatives against various biological drug candidates.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Grant No. 21772150) and Wuhan University of Technology for financial support.
References
- Bioactive Heterocycles II, Topics in Heterocyclic Chemistry, Eguchi S., 2007, vol. 8, XII, p. 249. [Google Scholar]
- Gomtsyan A. Chem. Heterocycl. Compd. 2012;48:7–10. [Google Scholar]
- Kirk K. L. and Filler R., In Biomedical Frontiers of Fluorine Chemistry, Symposium Series, American Chemical Society, Washington, DC, 1996, vol. 639, pp. 1–24. [Google Scholar]
- Gelders Y. G., Heylen S. L. E., Vander B. G., Reyntjens A. J. M., Janssen P. A. J. Pharmacopsychiatry. 1990;23:206–211. doi: 10.1055/s-2007-1014509. [DOI] [PubMed] [Google Scholar]
- Dollery C., Therapeutic drugs, Churchill Livingstone, Edinburgh, UK, 1999. [Google Scholar]
- Park B. K., Kitteringham N. R. Drug Metab. Rev. 1994;26:605–643. doi: 10.3109/03602539408998319. [DOI] [PubMed] [Google Scholar]
- Deng B. L., Cullen M. D., Zhou Z., Hartman T. L., Buckheit R. W., Pannecouque C., Clercq E. D., Fanwick P. E., Cushmana M. Bioorg. Med. Chem. 2006;14:2366–2374. doi: 10.1016/j.bmc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Liang D. B., Yujie Z., Tracy L. H., Karen W., Robert W. B., Christophe P., Erik D. C., Mark C. Eur. J. Med. Chem. 2009;44:1210–1214. [Google Scholar]
- Wierenga W., Evans B. R., Zurenko G. E. J. Med. Chem. 1984;27:1212–1215. doi: 10.1021/jm00375a022. [DOI] [PubMed] [Google Scholar]
- Kendall H. D. J. Med. Chem. 1994;59:7008. [Google Scholar]
- Hrib N. J., Jurcak J. G., Burgher K. L., Conway P. G., Hartman H. B., Kerman L. L., Roehr J. E., Woods A. T. J. Med. Chem. 2003;37:2308–2314. doi: 10.1021/jm00041a009. [DOI] [PubMed] [Google Scholar]
- Davis L., Effland R. C., Klein J. T., Dunn R. W., Geyer H. M., Petko W. M. Drug Des. Discovery. 1992;8:225–240. [PubMed] [Google Scholar]
- Strupczewski J. T., Allen R. C., Gardner B. A. J. Med. Chem. 1985;28:761–769. doi: 10.1021/jm00383a012. [DOI] [PubMed] [Google Scholar]
- Jamssen P. A. J., Niemegeer C. J. E. J. Pharmacol. Exp. Ther. 1988;244:685–691. [PubMed] [Google Scholar]
- Walsh D. A., Anti-inflammatory derivatives of 3-aryl-2,1-benzisoxazole, European Patent 0260924, 1987.
- Rajeev J., Agarwal D. D., Damodharan M. J. Indian Chem. Soc. 1995;72:825. [Google Scholar]
- Saunders J. C., Williamson W. R. J. Med. Chem. 1999;22:1554–1558. doi: 10.1021/jm00198a026. [DOI] [PubMed] [Google Scholar]
- Khedekar P. B., Bahekar R. H., Chopade R. S., Umathe S. N., Rao A. R., Bhusari K. P. Drug Res. 2003;53:640. doi: 10.1055/s-0031-1299806. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Curr. Med. Res. Opin. 2004;20:577–586. doi: 10.1185/030079904125003313. [DOI] [PubMed] [Google Scholar]
- Nuhrich A., Varache-Lembege M., Vercauteren J., Dokhan R., Renard P., Devaux G. Eur. J. Med. Chem. 1996;31:957–964. [Google Scholar]
- Matsushitha N. Y., Ono N. and Ytakechi, Jpn Kokai Tokkyo JP, 04173780, 1992; CA, 117212485, 1992.
- Aiello S., Wells G., Stone E. L., Kadri H., Bazzi R., Bell D. R., Stevens M. F. G., Matthews C. S., Bradshaw T. D., Westwell A. D. J. Med. Chem. 2008;51:5135–5139. doi: 10.1021/jm800418z. [DOI] [PubMed] [Google Scholar]
- Arakawa K., Inamasu M., Matsumoto M., Okumura K., Yasuda K. Chem. Pharm. Bull. 1997;45:1984–1993. doi: 10.1248/cpb.45.1984. [DOI] [PubMed] [Google Scholar]
- Stiff D. D., Robicheau J. T., Zemaitis M. A. Xenobiotica. 1992;22:1–11. doi: 10.3109/00498259209053097. [DOI] [PubMed] [Google Scholar]
- Clive B. P., Scott E. S., Phillip S. S., Michael R. K. Nucl. Med. Biol. 1999;26:99–103. [Google Scholar]
- Sharath Chandra S. P., Mahadimane Vishwaprakash P. Int. J. Pharma Sci. Res. 2015;6:3606–3611. [Google Scholar]
- Vedi G. M., Kulkarni A. A., Geetha K. M., Manisha S., Vishal S. Med. Chem. 2013;1:48–53. [Google Scholar]
- Naoki T. and Takashi I., Preparation of indazole and benzisoxazole derivatives as Raf inhibitors for treatment of cancer, WO 2009028655 A1 20090305, 2009.
- Shinichi K., Hata M. K., Masuo S. K., Masamitsu H., Yasushi M., Shigeru T. and Minoru S., Phenyl-2,1-benzisoxazoles for treatment of cancer, EP 63383 A1 19821027, 1982.
- Rajashekar R. C. B., Reddy R., Revathy S. T., Krish S., Sabiah S. Res. J. Pharm., Biol. Chem. Sci. 2014;5:873–880. [Google Scholar]
- Farr R. A. and Peet N. P., Novel glucohydrolase inhibitors useful as antidiabetic agents, WO Patent 011867, 1992.
- Ziyang C., Preparation of benzisoxazole derivatives as anti-diabetic agents, CN 105037290 A 20151111, 2015.
- Purohit S. S., Veerapur V. P. Inter. J. Pharma Bio Sci. 2012;3:142–149. [Google Scholar]
- Shi Q. G., Dropinski J. F., McKeever B. M., Shihua X. J. Med. Chem. 2005;13:4457–4468. doi: 10.1021/jm0502135. [DOI] [PubMed] [Google Scholar]
- Kun L., Libo X. and Brian J. A., Preparation of benzisoxazolyloxyacetic acids for treatment of diabetes and lipid disorders, US 20020173663 A1 20021121, 2002.
- Holbrook S. Y. L., Garneau-Tsodikova S. Med. Chem. Commun. 2017;8:1739–1741. doi: 10.1039/c7md90030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman H. Compr. Med. Chem II. 2007;8:7–15. [Google Scholar]
- Kathiravan M. K., Salake A. B., Chothe A. S., Dudhe P. B., Watode R. P., Mukta M. S., Gadhwe S. Bioorg. Med. Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Suhas R., Chandrashekar S., Gowda D. C. Eur. J. Med. Chem. 2011;46:704–711. doi: 10.1016/j.ejmech.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Suhas R., Chandrashekar S., Gowda D. C. Eur. J. Med. Chem. 2012;48:179–191. doi: 10.1016/j.ejmech.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Basarab G. S., Brassil P., Doig P., Galullo V. J. Med. Chem. 2014;57:9078–9095. doi: 10.1021/jm501174m. [DOI] [PubMed] [Google Scholar]
- Basarab G. S., Doig P., Galullo V., Kern G. J. Med. Chem. 2015;58:6264–6282. doi: 10.1021/acs.jmedchem.5b00863. [DOI] [PubMed] [Google Scholar]
- Ravi S. L., Nitinkumar S. S., Kamble R. R., Khazi I. A. M. Eur. J. Med. Chem. 2009;44:2828–2833. doi: 10.1016/j.ejmech.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Chaker A., Najahi E., Chatriant O., Valentin A., Tene N., Treilhou M., Chabchoub F., Nepveu F. Arabian J. Chem. 2017;10:S2464–S2470. [Google Scholar]
- Shivaprasad C. M., Jagadish S., Swaroop T. R., Mohan C. D., Roopashree R., Sharath Kumar K. S., Rangappa K. S. Eur. J. Chem. 2014;5:91–95. [Google Scholar]
- Vaidya S. D., Siva Kumara B. V., Kumar R. V., Bhisea U. N., Mashelkar U. C. J. Heterocycl. Chem. 2007;44:685–691. [Google Scholar]
- Priya B. S., Basappa, Nanjunda Swamy S., Rangappa K. S. Bioorg. Med. Chem. 2005;13:2623–2628. doi: 10.1016/j.bmc.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Kumbhare R. M., Ingle V. N. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2009;48:996–1000. [Google Scholar]
- Abesundara K. J., Matsui T., Matsumoto K. J. Agric. Food Chem. 2004;52:2541–2545. doi: 10.1021/jf035330s. [DOI] [PubMed] [Google Scholar]
- Funke I., Melzig M. F. Braz. J. Pharmacog. 2006;16:1–5. [Google Scholar]
- Li Y., Wen S., Kota B. P., Peng G., Li G. Q., Yamahara J., Roufogalis B. D. J. Ethnopharmacol. 2005;99:239–244. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Youn J. Y., Park H. Y., Cho K. H. Diabetes Res. Clin. Pract. 2004;66S:S149–S155. doi: 10.1016/j.diabres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Shantharam C. S., Suyoga Vardhan D. M., Suhas R., Sridhara M. B., Gowda D. C. Eur. J. Med. Chem. 2013;60:325–332. doi: 10.1016/j.ejmech.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Shantharam C. S., Suyoga Vardhan D. M., Channe Gowda D. Int. J. Chem. Pharm. Sci. 2013;4:102–112. [Google Scholar]
- Urry D. W., Gowda D. C., Parker T. M., Luan C. H., Reid M. C., Harris C. M., Pattanaik A., Harris R. D. Biopolymers. 1992;32:1243–1250. doi: 10.1002/bip.360320913. [DOI] [PubMed] [Google Scholar]
- Kempe Gowda B. K., Prasad H. S., Rangappa K. S., Gowda D. C. Int. J. Chem. Kinet. 2002;34:39–48. [Google Scholar]
- Prasad K. U., Iqbal M. A., Urry D. W. Int. J. Pept. Protein Res. 1985;25:408–413. doi: 10.1111/j.1399-3011.1985.tb02193.x. [DOI] [PubMed] [Google Scholar]
- Albrecht S. S., Kuklina E. V., Bansil P., Jamieson D. J., Whiteman M. K., Kourtis A. P., Posner S. F., Callaghan W. J. Med. Chem. 2011;54:8541–8554. [Google Scholar]
- Weiguo L., Fiona L., Liu K., Wood H. B. J. Med. Chem. 2011;54:8541–8554. doi: 10.1021/jm201061j. [DOI] [PubMed] [Google Scholar]
- Gani O. A. B. S., Sylte I. Chem. Biol. Drug Des. 2008;72:50–57. doi: 10.1111/j.1747-0285.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Malik S., Ahuja P., Sahu K., Khan S. A. Eur. J. Med. Chem. 2014;84:42–50. doi: 10.1016/j.ejmech.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Uno H., Kurokawa M., Masuda Y., Nishimura H. J. Med. Chem. 1979;22:80–183. doi: 10.1021/jm00188a011. [DOI] [PubMed] [Google Scholar]
- Naidu K. M., Gajanan R. N., Chandra Sekhar K. V. G. Arabian J. Chem. 2015 doi: 10.1016/j.arabjc.2015.02.025. [DOI] [Google Scholar]
- Naidu K. M., Suresh A., Subbalakshmi J., Sriram D., Yogeeswari P., Raghavaiah P., Chandra Sekhar K. V. G. Eur. J. Med. Chem. 2014;87:71–78. doi: 10.1016/j.ejmech.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Subash V., Brunsteiner M., Uddin R., Wan B., Franzblau S. G., Petukhov P. A. J. Med. Chem. 2008;51:1999–2002. doi: 10.1021/jm701372r. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang S., Xu X., Liu X. J. Med. Chem. 2013;56:4671–4690. doi: 10.1021/jm400408r. [DOI] [PubMed] [Google Scholar]
- Cao X., Chen Y., Zhang Y., Qiu Y., Yu M., Xu X., Liu X., Liu B. F., Zhang G. Eur. J. Med. Chem. 2016;124:713–728. doi: 10.1016/j.ejmech.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Albers L. J., Musenga A., Raggi M. A. Expert Opin. Invest.Expert Opin. Invest. DrugsDrugs. 2008;17:61–75. doi: 10.1517/13543784.17.1.61. [DOI] [PubMed] [Google Scholar]
- Bolos J., Anglada L., Gubert S., Planas J. M., Agut J., Prıincep M., Fuente A., Sacristan A., Ortiz J. J. Med. Chem. 1998;41:5402–5409. doi: 10.1021/jm9810396. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lan Y., Wang S., Zhang H., Xu X., Liu X., Yu M., Liu B. F., Zhang G. Eur. J. Med. Chem. 2014;74:427–439. doi: 10.1016/j.ejmech.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Xiberas X., Martinot J. L., Mallet L., Artiges E., Loch C., Maziere B., Martinot M. L. P. Br. J. Psychiatry. 2001;179:503–508. doi: 10.1192/bjp.179.6.503. [DOI] [PubMed] [Google Scholar]
- Aranda R., Villalba K., Ravina E., Masaguer C. F. J. Med. Chem. 2008;51:6085–6094. doi: 10.1021/jm800602w. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Kaneko Y., Suwa K., Ebara S., Nakazawa K., Yasuno K. Bioorg. Med. Chem. 2005;13:2509–2522. doi: 10.1016/j.bmc.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Rangappa K. S., Basappa J. Phys. Org. Chem. 2005;18:773–778. [Google Scholar]
- Anand M., Selvaraj V., Alagar M. Korean J. Chem. Eng. 2014;31:659–663. [Google Scholar]
- Chen Y., Lan Y., Cao X., Xu X., Zhang J., Yu M., Liu X., Liua B. F., Zhang G. Med. Chem. Commun. 2015;6:831–838. [Google Scholar]
- Benaka Prasad S. B., Vinaya K., Ananda Kumar C. S., Swarup S., Rangappa K. S. Invest. New Drugs. 2009;27:534–542. doi: 10.1007/s10637-008-9205-5. [DOI] [PubMed] [Google Scholar]
- Ashwini N., Garg M., Mohan C. D. Bioorg. Med. Chem. 2015;23:6157–6165. doi: 10.1016/j.bmc.2015.07.069. [DOI] [PubMed] [Google Scholar]
- Villalobos A., Blake J. F., Biggers C. K., Butler T. W., Chapin D. S., Chen Y. L. J. Med. Chem. 1994;37:721–2734. doi: 10.1021/jm00043a012. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang P., Liu X. Mini-Rev. Med. Chem. 2011;11:1130–1142. doi: 10.2174/138955711797655407. [DOI] [PubMed] [Google Scholar]
- Song Y., Zhan P., Liu X. Curr. Pharm. Des. 2013;19:7141–7154. doi: 10.2174/13816128113199990505. [DOI] [PubMed] [Google Scholar]
- Song Y., Zhan P., Zhang Q., Liu X. Curr. Pharm. Des. 2013;19:1528–1548. [PubMed] [Google Scholar]
- Song Y., Chen W., Kang D., Zhang Q., Zhang P., Liu X. Comb. Chem. High Throughput Screening. 2014;17:536–553. doi: 10.2174/1386207317666140122101631. [DOI] [PubMed] [Google Scholar]
- Sakuma S., Endo T., Kanda T., Nakamura H., Yamasaki S., Yamakawa T. Bioorg. Med. Chem. 2011;19:3255–3264. doi: 10.1016/j.bmc.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Gopalsamy A., Shi M., Golas J., Vogan E., Jacob J. J. Med. Chem. 2008;51:373–375. doi: 10.1021/jm701385c. [DOI] [PubMed] [Google Scholar]
- Walker L. A., Ancellin N., Beaufils B., Bergeal M. J. Med. Chem. 2017;60:3383–3404. doi: 10.1021/acs.jmedchem.7b00055. [DOI] [PubMed] [Google Scholar]
- Mole D. J., Webster S. P., Uings I., Zheng X. Nat. Med. 2016;22:202–209. doi: 10.1038/nm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodney M. A., Johnson D. E., Sawant-Basak A., Coffman K. J. J. Med. Chem. 2012;55:9240–9254. doi: 10.1021/jm300953p. [DOI] [PubMed] [Google Scholar]
- Jain M., Kwon C. H. J. Med. Chem. 2003;46:5428–5436. doi: 10.1021/jm020581y. [DOI] [PubMed] [Google Scholar]
- Liu K., Black R. M., Acton J. J. Bioorg. Med. Chem. Lett. 2005;15:2437–2440. doi: 10.1016/j.bmcl.2005.03.092. [DOI] [PubMed] [Google Scholar]
- Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., Anderton B. H. Neurosci. Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R. FEBS Lett. 1992;314:315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H., Martinez A. Front. Mol. Neurosci. 2011;4:32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Li M., Zhou Y., Pang T., Xu L., Cao J., Han L., Li Y., Wang W., Gao J., Li J. Molecules. 2013;18:5498–5516. doi: 10.3390/molecules18055498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chakaya J., Centis R., D'Ambrosio L., Mwaba P., Bates M., Kapata N., Nyirenda T., Chanda D., Mfinanga S., Hoelscher M. Lancet Respir. Med. 2015;3:220–234. doi: 10.1016/S2213-2600(15)00063-6. [DOI] [PubMed] [Google Scholar]
- Velayati A., Farnia P., Masjedi M. R. Int. J. Clin. Exp. Med. 2013;6:307. [PMC free article] [PubMed] [Google Scholar]
- Agertt V. A., Marques L. L., Bonez P. C., Dalmolin T. V., de Oliveira G. N. M., de Campos M. M. A. Tuberculosis. 2013;93:318–321. doi: 10.1016/j.tube.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Naidu K. M., Srinivasarao S., Agnieszka N., Ewa A. K., Kumar M. M. K., Sekhar K. V. G. Bioorg. Med. Chem. Lett. 2016;26:2245–2250. doi: 10.1016/j.bmcl.2016.03.059. [DOI] [PubMed] [Google Scholar]