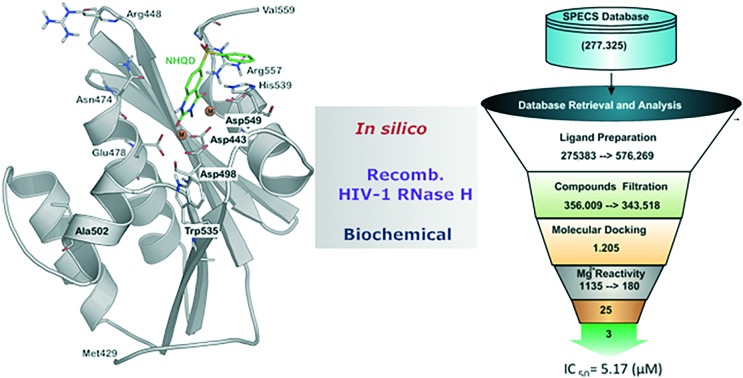
 In silico methods identified a new class of inhibitors for HIV-1 RT RNase H and magnesium complexation study reveals the binding mode of these compounds.
In silico methods identified a new class of inhibitors for HIV-1 RT RNase H and magnesium complexation study reveals the binding mode of these compounds.
Abstract
Persistent HIV infection requires lifelong treatment and among the 2.1 million new HIV infections that occur every year there is an increased rate of transmitted drug-resistant mutations. This fact requires a constant and timely effort in order to identify and develop new HIV inhibitors with innovative mechanisms. The HIV-1 reverse transcriptase (RT) associated ribonuclease H (RNase H) is the only viral encoded enzyme that still lacks an efficient inhibitor despite the fact that it is a well-validated target whose functional abrogation compromises viral infectivity. Identification of new drugs is a long and expensive process that can be speeded up by in silico methods. In the present study, a structure-guided screening is coupled with a similarity-based search on the Specs database to identify a new class of HIV-1 RNase H inhibitors. Out of the 45 compounds selected for experimental testing, 15 inhibited the RNase H function below 100 μM with three hits exhibiting IC50 values <10 μM. The most active compound, AA, inhibits HIV-1 RNase H with an IC50 of 5.1 μM and exhibits a Mg-independent mode of inhibition. Site-directed mutagenesis studies provide valuable insight into the binding mode of newly identified compounds; for instance, compound AA involves extensive interactions with a lipophilic pocket formed by Ala502, Lys503, and Trp (406, 426 and 535) and polar interactions with Arg557 and the highly conserved RNase H primer-grip residue Asn474. The structural insights obtained from this work provide the bases for further lead optimization.
Introduction
The increasing resistance to current therapeutic agents used for HIV drug regimens remains a major issue for effective acquired immune deficiency syndrome (AIDS) therapy.1,2 It has recently been estimated (2016) that more than 36.7 million people are living with HIV, and among these only 18.2 million are on antiretroviral therapy and nearly 1.1 million people died in 2015.1,3,4 Furthermore, 2.1 million new HIV infections occur every year. Although AIDS related mortality has been significantly reduced since the introduction of highly active antiretroviral therapy (HAART) (35% since 2005), most of the currently marketed drugs that are being used today for HIV therapy are prone to viral resistance.5,6 The number of newly infected people per year is constant and among these people there is an overall increase of transmitted drug resistant mutations (TDRMs) detected in antiretroviral treatment-naive patients.2,7 This resistance, which often results from lack of compliance, can relate to multiple types of drugs and can dramatically affect the outcome of HAART;8,9 therefore, there is a strong need to search for non-traditional chemo-targets for anti-HIV drug developments.10–12 Inhibition of the HIV-1 reverse transcriptase associated ribonuclease H (RNase H) function provides a novel target for anti-HIV chemotherapy, since it is the only viral encoded enzymatic function for which no inhibitor has gone into clinical development so far.13–15 Nevertheless, it has been shown that inactivation of the RNase H function by amino acidic substitution lead to non-infectious virions16 and its selective inhibition completely blocks viral replication.17,18
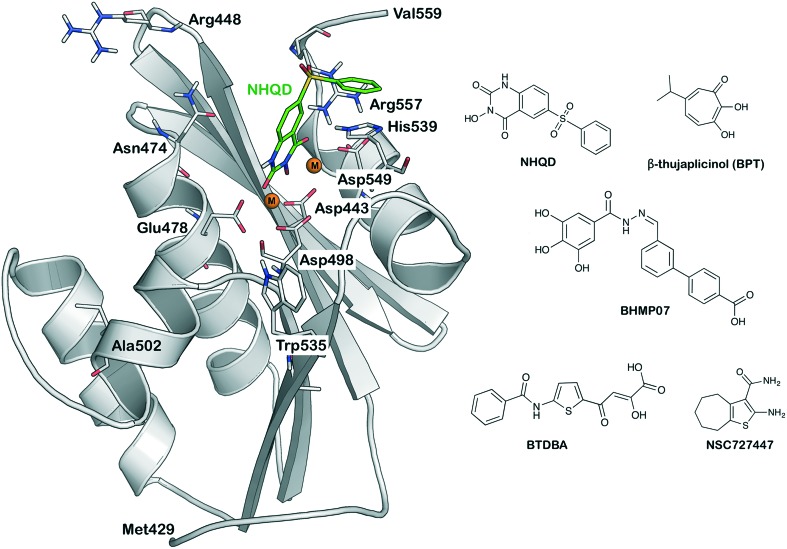
RNase H is one of the two catalytically active domains of the p66 subunit of HIV-1 reverse transcriptase, which is essential for viral genome replication by performing the hydrolysis of the viral RNA strand in the RNA/DNA hybrid during the retro-transcription process.19–22 In order to carry out the hydrolytic process, water molecules (to act as nucleophiles) and magnesium ions (Mg2+) (to initiate the deprotonation of water) coordinating with the highly conserved DDDE motif (Asp443, Asp498, Asp549 and Glu478) residues of RNase H are essential.23 The topology of the HIV-1 RNase H domain is well characterized by different studies.22,24,25 The active site is small and slightly hydrophobic and consists of a highly electronegative region, which is primarily due to the DDDE motif coordinated with the two catalytically active magnesium ions (Fig. 1).22,26
Fig. 1. The HIV-RT associated RNase H structural topology is shown with a bound ligand NHQD (green, ball and stick model). The catalytically important residues and magnesium ions (orange spheres) have been highlighted. The 2D structures of representative molecules belong to the active site and allosteric RNase H inhibitors.
In the past years, a large number of RNase H inhibitors have been reported but none of these inhibitors have yet reached the market.14,15,18,20,27–31 RNase H inhibitors can be classified into two categories based on the mode of action: (i) compounds that block the availability of magnesium ions for the hydrolytic process (the so called active-site inhibitors), such as diketo,17,32 N-hydroximide (e.g. NHQD, cf.Fig. 1),33 tropolone (e.g. β-thujaplicinol, cf.Fig. 1),34 pyrido-pyrimidinone,30N-hydroxy-naphthyridinone,35 thiocarbamates,27 and 5-nitro-furan-2-carboxylic acid36 and (ii) allosteric inhibitors that impair the efficiency of the RNase H activity by binding outside the catalytic domain, inducing conformational changes that affect proper substrate binding at the RNase H active site. This second class includes vinylogous ureas (e.g. NSC727447, cf.Fig. 1),37 acylhydrazones (e.g. BHMP07, cf.Fig. 1),29,38,39 propenones,25 isatines,40 and anthraquinones.41 Both classes of compounds present critical issues that hamper the process of drug development. On the one hand, Mg2+ coordination provides active site inhibitors with a strong and stable interaction with the catalytic domain, as shown for the last approved innovation for HIV-1 treatment, the integrase (IN) strand transfer inhibitors,42 and has been proved for the RNase H active site inhibitor RDS1759.17 However, both cases show that the Mg binding mechanism itself lacks specificity towards other polynucleotidyl transferases (and indeed it is deeply investigated in order to look for IN and RNase H dual inhibitors43–45) and that the metal-binding moiety needs to be flanked by additional fragments able to specifically interact with the molecular environment in order to achieve sufficient potency and specificity.46 On the other hand, it has been proved that compounds that bind to allosteric non-conserved regions, such as the non-nucleoside RT-inhibitors, are more prone to selection of resistant strains.2 Therefore, in order to identify effective inhibitors with a lower possibility of toxicity and drug resistance, there is a need to also target the most conserved peripheral regions of the HIV-1 RNase H domain, adjoining allosteric pockets that are reported to differ in location in humans and viruses.47,48
Recently, we proved that the lateral chains of prototype DKA inhibitors designed and synthesized by Costi et al.43,44 arrange within the active site binding the highly conserved residues Asn474, Gln475 and Tyr501 of the HIV-1 RNase H.17
These amino acids are part of the RNase H primer grip, so called because it extensively interacts with the DNA strain of the RNA/DNA heteroduplex, allowing the RNA to be in the right orientation to be cleaved by the active site. Mutations in the primer grip strongly impair viral replication49 by compromising RNase H specificity50 and efficiency.17 Compound BHMP07 (cf.Fig. 1), which binds to an allosteric pocket centered on Gln507 of p66 and composed of Leu425, Leu422, and Asn404 residues, was also reported to interact with Asn474 (H-bond), Gln500 (H-bond), Tyr501 (π–π stack), Asp498 (H-bond), Gly444 (H-bond) and Asp443 (H-bond) residues.47 This observation was further supported by a virtual enrichment study.48 Therefore, highly conserved residues within the RNase H domain represent a promising target for the development of efficient RNase H inhibitors, and this effort can be optimized using computer-aided drug design techniques.
With the availability of a large number of high-resolution 3D crystal structures of drug target proteins and of a large number of ligand data deposited in public databases, both ligand and structure-guided in silico approaches are extensively utilized to identify new ligands with affinity for a wide range of protein targets.51–53 Recently, a ligand-based virtual screening (VS) strategy was used on HIV-1 RT for the identification of dual inhibitors (polymerase and RNase H activity),29 leading to the identification of four compounds that inhibited the RNase H function in the low micromolar range, and the binding mode was further confirmed by a site-directed mutagenesis approach, thus confirming the effectiveness of this method for the identification of novel RNase H inhibitors. Along this line, we have developed various ligand- and structure-based virtual screening models such as machine learning methods, docking, shape-based evaluation, pharmacophore modeling and QM-based calculations.54,55 From a structure-based docking approach, we found about 80–90% accuracy in terms of “enrichment” compared to other methods. As a continuation, in the present study, we used virtual screening models from docking followed by shape-based methods to screen the Specs database (; www.specs.net) to identify new chemotypes able to bind within the RNase H domain and inhibit the RNase H function. The best-ranked compounds from in silico screening have been selected for in vitro testing. Among the 45 compounds screened for RNase H inhibition, three compounds showed IC50 values below 10 μM. In addition, the binding mode of these compounds has been investigated by site-directed mutagenesis and “induced-fit” molecular modeling to obtain insights for further lead optimization.
Results and discussion
1. Structure-based virtual screening
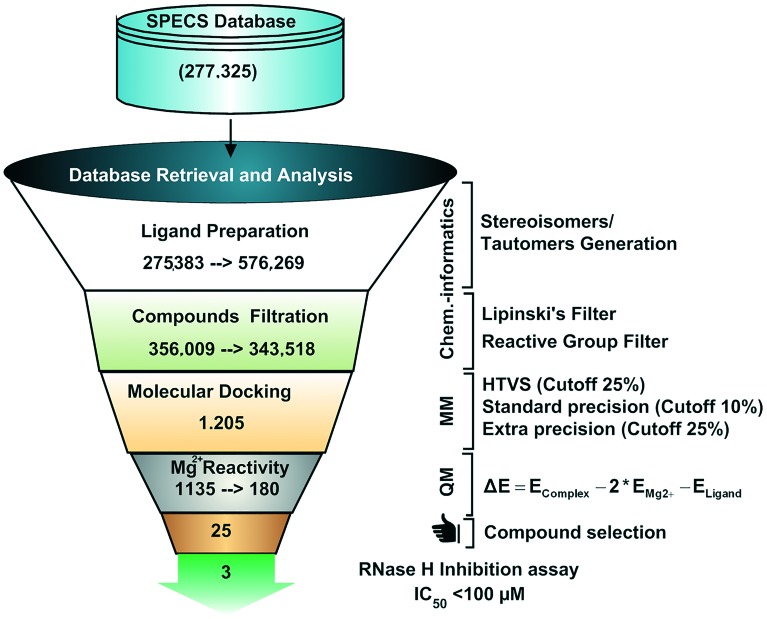
We have previously shown that effective virtual screening of RNase H inhibitors from large chemical databases could be achieved using a combination of docking and QM-based refinement calculations.54,55 In order to identify a novel chemo-type for RNase H inhibition and to validate previously developed computational methods, the best models were used to screen the Specs database (containing 277 325 drug-like compounds for purchase) for HIV-1 RNase H inhibition screening (Fig. 2). After application of general filters for the ADMET property score and reactive groups, 343 518 molecules were docked within a computational model of the HIV-1 RT associated RNase H domain.22 A set of 1205 compounds was obtained at the end of the docking-based virtual screening, and these compounds were subsequently used for QM-based refinement calculation based on density functional theory (DFT) calculations as previously described55 (ESI† S1). The best-ranked 180 compounds from the screening were sorted for further inspection. To select a diverse set of structures for the biochemical assays, these compounds were clustered according to structural similarity using the Canvas software from the Schrodinger suite.56 In total, 50 individual clusters were defined containing between 1 and 15 compounds and each cluster accounts for at least 75% structural similarity. In the next step, each individual cluster was visually inspected and the previously known compounds were removed from the hit list, which includes NSC727447, tropolone, pyrido-pyrimidinone and tropolone scaffolds (the list of compounds used for clustering is provided in ESI† S2). At the end, 25 structurally diverse compounds with the best scores (structures are provided in the ESI† S3) were chosen and purchased from the chemical vendor ; www.specs.net to be tested against the HIV RT-associated RNase H function in enzymatic assays.
Fig. 2. Overall workflow of structure-based virtual screening strategies applied.
2. Inhibition of HIV-1 RT associated RNase H function
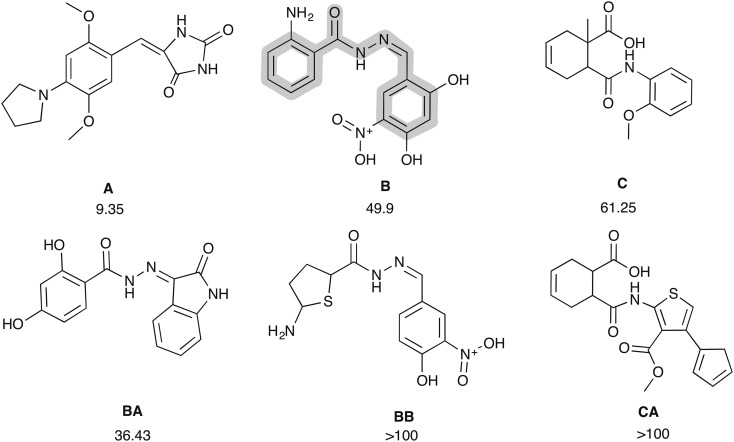
The selected compounds were tested against the HIV-1 RT associated RNase H function (Table 1). Out of 25 compounds tested (A–Y), 3 compounds inhibited the RNase H activity below an IC50 value of 100 μM and compound A showed an IC50 value of 9.35 μM. Notably, none of these compounds has previously been reported as an inhibitor for RNase H; however, compound A shares structural similarities with hydantoin molecules described as HIV RNase H inhibitors in the patent in 2005 (US 20100063118),57 and compound B shares very similar structural patterns with BHMP07 (cf.Fig. 3). These interesting similarities confirm the robustness of the VS methods in identifying potential RNase H inhibitors. Interestingly, among the active compounds, compound C, which exhibited a modest IC50 value (61.25 μM), presents a scaffold that has never been reported before in relation to the RNase H modulator (Fig. 3). Thus, further investigation on this scaffold could be interesting. To find more similar compounds which could potentially be used to understand the structure–activity relationship (SAR) for lead optimization, all three compounds A, B and C were used as query molecules for a further similarity-based search.
Table 1. In silico prediction scores and biological effects of VS hits on HIV-1 RT-associated RNase H activity.
| ID | Vendor code | Cluster | Chelation a | XP a | Mol wt (Da) | IC50 b (μM) |
| A d | AN-648/41665045 | 36 | –21.17 | –7.68 | 317.34 | 9.35 ± 0.007 |
| B d | AG-205/10367010 | 34 | –51.14 | –9.91 | 316.27 | 49.90 ± 9.7 |
| C d | AL-281/41935302 | 21 | –42.49 | –6.86 | 289.33 | 61.25 ± 2.7 |
| D d | AK-968/41922459 | 1 | –11.03 | –8.38 | 295.77 | >100 (73.40) c |
| E d | AK-968/12713114 | 3 | –34.61 | –8.01 | 241.33 | >100 (76.28) c |
| F d | AQ-099/42181915 | 4 | –59.34 | –8.57 | 286.10 | >100 (84.45) c |
| G d | AP-853/43445377 | 8 | –13.66 | –9.67 | 275.29 | >100 (82.70) c |
| H d | AF-399/07629003 | 10 | –22.42 | –9.18 | 228.21 | >100 (89.07) c |
| I d | AE-848/08809049 | 11 | –23.03 | –7.36 | 369.17 | >100 (69.14) c |
| J d | AN-648/41701113 | 11 | –13.91 | –6.86 | 348.38 | >100 (77.90) c |
| K d | AQ-149/42447764 | 13 | –87.78 | –7.27 | 299.35 | >100 (81.80) c |
| L d | AQ-149/43372378 | 13 | –25.62 | –7.19 | 319.40 | >100 (77.55) c |
| M d | AK-968/41922490 | 27 | –22.30 | –8.13 | 190.24 | >100 (83.52) c |
| N d | AI-204/31719028 | 28 | –37.80 | –8.45 | 237.28 | >100 (76.50) c |
| O d | AJ-292/41685670 | 28 | –35.68 | –9.83 | 309.34 | >100 (74.50) c |
| P d | AN-329/43248835 | 29 | –54.79 | –8.92 | 304.34 | >100 (83.70) c |
| Q d | AO-080/42837941 | 30 | –42.83 | –9.65 | 305.37 | >100 (86.00) c |
| R d | AK-968/41923774 | 42 | –33.40 | –9.04 | 322.34 | >100 (80.00) c |
| S d | AO-433/42007915 | 49 | –27.79 | –7.54 | 371.43 | >100 (80.41) c |
| T d | AF-399/41368457 | 45 | –34.22 | –9.34 | 335.36 | >100 (80.00) c |
| U d | AG-205/32708023 | 31 | –12.43 | –7.79 | 359.34 | >100 (59.43) c |
| V d | AG-690/33090008 | 39 | –35.05 | –7.49 | 313.35 | >100 (87.24) c |
| W d | AK-968/41922494 | 43 | –15.19 | –9.05 | 340.14 | >100 (94.56) c |
| X d | AK-968/40355672 | 26 | –18.26 | –8.15 | 236.31 | >100 (70.44) c |
| Y d | AO-080/43441633 | 30 | –35.71 | –8.50 | 374.44 | >100 (80.25) c |
| AA e | AG-690/36550050 | 36 | — | — | 424.30 | 5.17 ± 0.56 |
| AB e | AG-690/36165052 | 36 | — | — | 402.45 | 12.82 ± 0.68 |
| AC e | AH-487/15021157 | 36 | — | — | 456.33 | 8.73 ± 1.84 |
| AD e | AN-698/40677529 | 36 | — | — | 315.37 | 12.30 ± 1.92 |
| AE e | AN-698/40677530 | 36 | — | — | 331.83 | 16.06 ± 0.68 |
| AF e | AH-487/40935633 | 36 | — | — | 591.09 | 17.78 ± 4.13 |
| AG e | AH-487/41035472 | 36 | — | — | 331.37 | 19.31 ± 1.05 |
| AH e | AH-487/41457445 | 36 | — | — | 367.36 | 28.11 ± 4.66 |
| AI e | AH-487/40935863 | 36 | — | — | 260.29 | 52.80 ± 3.43 |
| AJ e | AN-988/14610013 | 36 | — | — | 266.68 | 84.20 ± 15.50 |
| AK e | AH-487/41449196 | 36 | — | — | 276.25 | 91.57 ± 11.92 |
| AL e | AN-698/40704420 | 36 | — | — | 294.29 | >100 (65.5) c |
| AM e | AG-690/40144774 | 36 | — | — | 265.29 | >100 (60.0) c |
| AN e | AH-487/40935550 | 36 | — | — | 388.11 | >100 (67.5) c |
| AO e | AH-487/41449786 | 36 | — | — | 296.71 | >100 (70.0) c |
| AP e | AG-690/40124767 | 36 | — | — | 274.13 | >100 (80.5) c |
| AQ e | AK-968/41022607 | 36 | — | — | 279.25 | >100 (89.5) c |
| BA e | AN-329/40081786 | 34 | — | — | 297.27 | 36.43 ± 5.83 |
| BB e | AF-399/15030247 | 34 | — | — | 321.32 | >100 (55.0) c |
| CA e | AK-968/15608063 | 21 | — | — | 391.47 | >100 (93.0) c |
aChelation and docking scores are in kcal mol–1.
bCompound concentration required to reduce the HIV-1-RT associated RNase H activity by 50%; the table reports the average and standard deviation of three independent experiments.
cPercentage of control activity in the presence of 100 μM concentration of compound.
dFirst screening.
eSecond screening (shape-based search).
Fig. 3. Initial hit molecules (A–C) from the first screening and compound “B” is highlighted in regions where it shares a common structural pattern with BHMP07. Compounds AQ and AR and analogs of B and C as found by the shape-based screening (see text for details).
Similarity-based search and structure activity relationships (SAR)
Similarity searches assume that molecules with similar physicochemical properties also have similar biological activity. This approach not only finds similar molecules or analogs but is also able to identify completely new chemo-types having similar pharmacophoric points.58 In this study, a combination of shape or pharmacophore-based (vROCS)59 and electrostatic-based similarity (EON)60 screening tools was used on three active compounds (A–C) in the Specs database, identifying the 20 best-scored compounds to be tested against the HIV RT-associated RNase H function in enzymatic assays. Among these, 17 molecules were analogs of compound A with structures varying greatly in terms of length and also functional groups (Fig. 4). Two compounds, BA and BB, were found as analogs of B, and only one CA was found as an analogue of compound C (cf.Fig. 3).
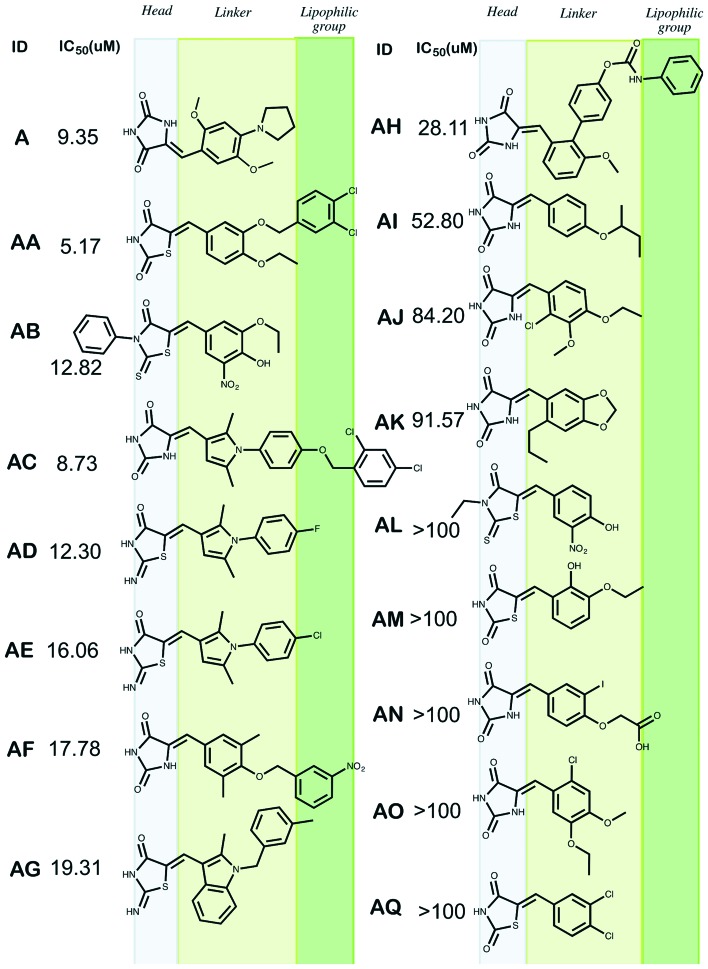
Fig. 4. Comparison of the structure and activity of A analogs.
Out of the 20 hits, 12 compounds inhibited the RNase H function with IC50 values ranging from 5 to 91 μM (cf.Table 1, compounds AA-AP). Interestingly, the better inhibitors were found among A analogs, with compound AA showing an IC50 value of 5.17 μM. The A analogs showed indeed a great variety of structures in terms of length and also functional groups; however, there is a clear pattern in the scaffold among the tested compounds, as hydantoin-like molecules that all belong to benzylidene hydantoins, a group less explored.57 Indeed, the structure of the compounds in the A series can be fragmented into three parts (moieties): a head (part a), a linker (part b) and a lipophilic group (part c). Part a is either an imidazolidinedione or a thiazolidinedione moiety. In some cases, one of the two carbonyl groups of the azolidinedione ring can be replaced by a thiocarbonyl or an imino moiety (Fig. 4).
Part b consists of a more or less complex aromatic linker: aromatic or heteroaromatic ring substituted with hydroxyl, alkyloxy or amino groups, halogens or again aryl rings. If the linker is composed of the biaryl fragments, such as the case of compounds AB, AC, AD, AG, the compound exhibits stronger inhibition compared to shorter compounds AH–AO (the sole exception is AA, discussed below).
Part c significantly modulates the RNase H function and is mostly composed of groups such as arylhalides or nitroaryl or carbamoylphenyl groups. The approximate length of parts a–c is found to be 7.5 Å (benzylideneimidazolidinedione), 3.5 Å (linker), and 4.5 Å (active region), respectively. Overall, the observation suggests that parts a and b are essential for the activity, while part c greatly modulates the activity against the RNase H function, depending on the nature of the substituent: the more lipophilic the substituent the more active is the inhibitor (compare derivatives AA, AB, and AE with the less potent AG derivative).
Among the B analogues, the results obtained from the shape-based screening, e.g., BA (IC50 = 36.43 μM) and BB (IC50 ≥ 100 μM) (Table 1, cf.Fig. 2), reveal that small changes in the structure lead to a large difference in inhibition, e.g., compounds B (IC50 = 49.9 μM) and BB. The major structural difference between these compounds lies in the presence of a hydrogen bond donor, e.g., hydroxyl or amino groups at the phenyl ring. Indeed, it has previously been shown that hydroxyl groups interact directly with negatively charged residues at the active site.39 Moreover, the decrease in molecular density of the molecule (molecular density is molecular weight divided by vdw_vol (amu Å–3)) results in an increasing inhibitory activity, e.g. BHMP07 (0.795) > BA (0.817) > B (0.837) > BB (0.861) (the molecular density of each molecule is provided in the parentheses). This observation is particularly interesting in relation to the design of new compounds in this series. Finally, only one analog, CA, of compound C was unable to inhibit the RNase H function up to a concentration of 100 μM.
Binding mode of inhibitors
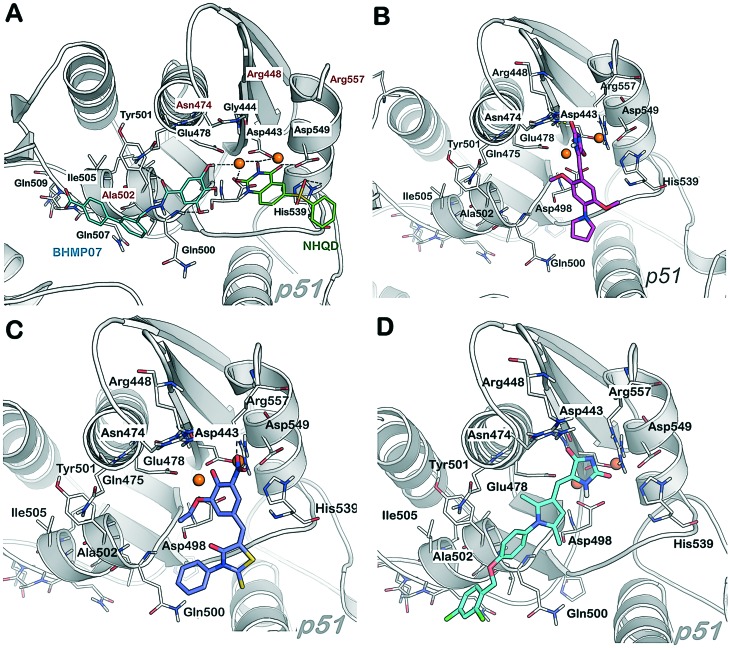
In order to acquire more information about the binding mode of the compounds, a further investigation was performed with a particular focus on the four “hits” identified, A, AB, and AC, comparing their binding mode with that of BHMP07 (an allosteric inhibitor) and N-hydroxy quinazolinedinone (a prototype inhibitor actively binding the active site) (NHQD, PDB ID: ; 3QIO).22 Since there is no crystal structure for BHMP07 bound to the RNase H domain, we compared the docking pose of BHMP07 from our docking methodology as previously reported.39
In the case of NHQD, for instance, His539 and Mg2+ interact with one of the three oxygen atoms of NHQD and the neighboring two oxygen atoms interact with the other Mg2+ (1) ion and bound water molecules (Fig. 5, panel A). Differently, the binding pose of BHMP07 matched the previously determined binding pose,39 having hydrogen bonds with Asn474, Gln500, Asp498, Gly444, Asp433 and π–π stacking with Tyr501 (Fig. 5, panel A).
Fig. 5. Comparison of the binding mode of known RNase H inhibitors in an X-ray crystal; important residues are highlighted. Panel A, N-hydroxy quinazolinedione inhibitor (NHQD, active site binder, green) and BHMP07 (allosteric site binder, cyan). Panel B, compound A (magenta). Panel C, compound AA (blue). Panel D, compound AB (cyan).
For the imidazolidinedione scaffold, the head portion of all the inhibitors lies in close vicinity to negatively charged residues (Asp433 or Asp498) and interacts with Asn474. Moreover, a negatively charged nitrogen atom in the imidazolidinedione ring (e.g. compound A, panel B) is partially exposed to the magnesium ions and Arg557, as we have observed for the active site inhibitor NHQD. Overall, the head portion of the inhibitors is exposed or interacts with Arg557 (ionic or π interaction); the polar interactions are found either with Gly444, Asn474, Asp498, Gln500 or His539 residues. The linker of the “hits” is primarily exposed to hydrophobic residues such as Ala502, Lys503, Trp (406, 426, 535) and Tyr501, thereby confirming that a linker composed as described above with one or two aromatic portions properly substituted can provide additional interactions contributing to a stronger inhibition compared to the compounds that have substitution with halo-groups or ends in this region.
The most important part of these hits is an “active region” fragment (preferably with arylhalides). Residues that lie in this region are more hydrophobic in nature, e.g., Phe346, Trp406, Tyr406, Tyr405, Ala502 and Ile505. In addition, residues such as Gln507 and Pro421 also significantly interact with most active compounds. Some of these residues are located between the polymerase domain and the RNase H domain of reverse transcriptase.
Compounds in the same scaffold such as A and AA showed a slightly different binding mode compared to the rest of the compounds (Fig. 5 panels B and C) since the negatively charged nitrophenol moiety of AA has high affinity towards the magnesium ions, and this ionic interaction pulls the whole compound towards the active site. Since these ligands are small in size, the ionic interactions dominate the overall binding pose. Moreover, the hydrophobic residue Trp535 strongly interacts with the phenyl substituent at position 3 of the thioxothiazolidine of AA and with the pyrrolidine ring of A, respectively. A similar binding mode was observed for hydrazone derivatives AQ and B (Fig. S3†), in which favorable interactions of the inhibitors with Arg557 (ionic/π interaction), Gly444 (H-bonding) and Ala446 (H-bonding) were found.
3. Investigation of magnesium complexation
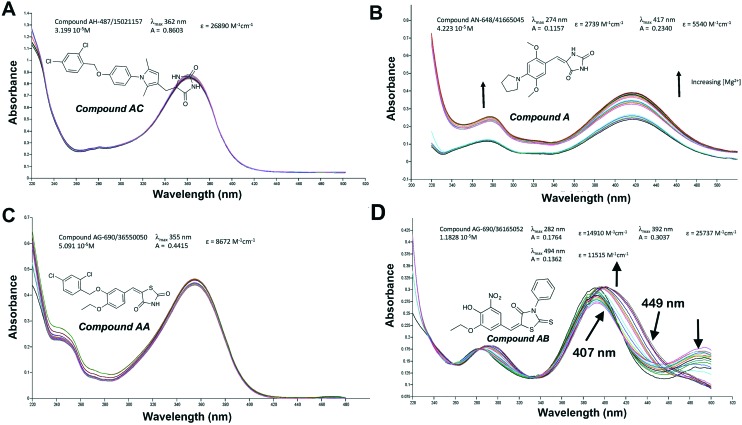
In order to investigate the potential importance of the interaction between the active compounds and Mg2+ ions, spectrophotometric complexation studies were carried out on selected compounds A, AA, AB, AC, AD, AE, AF and AG. Titration with MgCl2 did not induce significant changes in the UV-vis spectra of compounds AA, AC, AD, AE, AF and AG, thereby indicating a lack of interaction of these molecules with Mg2+ (Fig. 6 panels A and C and ESI† S4 to S8).
Fig. 6. Titration graphs of inhibitors with Mg2+; panel A: compound AC, panel B: compound A, panel C: compound AA and panel D: compound AB.
For compound A, we observed an overall increase in absorbance across the analyzed UV-vis spectrum which could reasonably be attributed to an increase in the solubility due to the presence of magnesium ions rather than to a real complexation (Fig. 6B).
Differently, titration of compound AB with MgCl2 produced an increase in the absorbance at 282 and 392 nm (hyperchromic effect) with a slight shift towards higher wavelengths (hypsochromic effect); furthermore, disappearance of the 494 nm maxima was observed. The titration spectra present two isosbestic points: the first at 407 nm appears at lower magnesium concentrations, the second at 449 nm appears at higher magnesium concentrations. This type of behavior suggests the presence of two subsequent complexation equilibria between AB and magnesium ions (Fig. 5D), supporting the hypothesis of the involvement of the interaction with the cofactor Mg2+ in the binding to the protein, and in agreement with the docking model.
This finding is very interesting since it is the first time that the inhibition of metalloenzymes is reported for phenol with flanking nitro and ethoxy group fragments. Compounds containing the nitrophenol fragment have been previously investigated as inhibitors of HIV-1 integrase (IN),61 showing that the nitrophenol moiety is not sufficient to achieve inhibition of IN. Indeed, this is in agreement with the much lower activity of compounds B (IC50 = 49 μM) and BB (IC50 = >100 μM) which contain a similar nitrophenol moiety but lack the ethoxy group, and are therefore not able to coordinate the Mg as compound AB does. The phenol with flanking nitro and ethoxy group fragments is structurally closely related to the more investigated 5-nitrovanillin (CHEBI ID: 48385), that, on its side, has never been investigated for such property. Therefore, this finding could represent a new insight for the development of the AB scaffold.
4. Site-directed mutagenesis
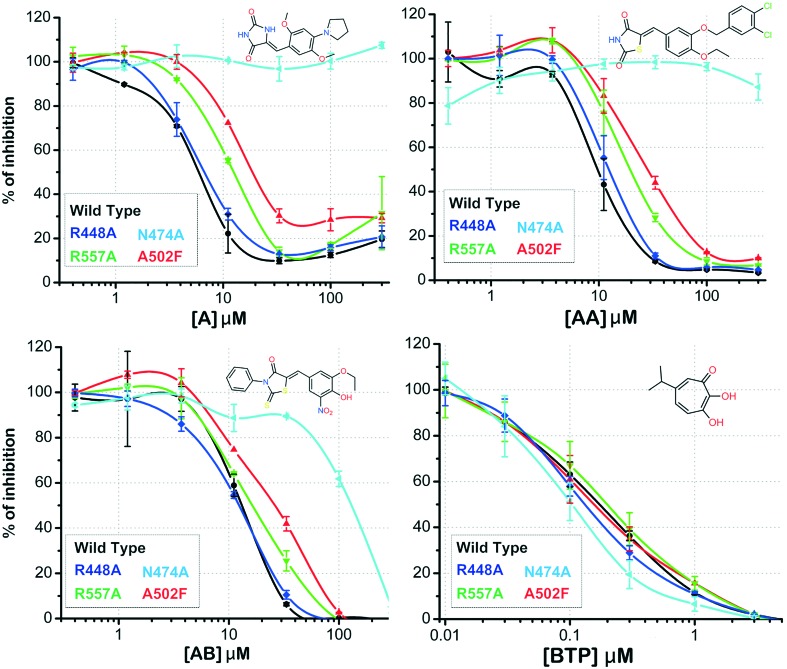
In order to experimentally verify the binding model suggested by the computational studies, the most active compounds A, AA and AB were chosen to perform site-directed mutagenesis, determining the independent impact of several amino acid substitutions on the potency of the compounds to inhibit the RNase H function. First, we assessed the impact of the substitution of Asn474, a primer grip residue, with Ala. Then we attempted to reduce the space available for the binding of the “active region” fragment by introducing a phenylalanine instead of Ala502. Moreover, we attempted to disrupt the interactions engaged by the head portion by mutating Arg557 into Ala. A further substitute, R448A, which was previously identified specifically affecting the activity of DKA active site inhibitors,17 was included as a negative control. The active-site inhibitor β-thujaplicinol (BTP)62 was used as a control (Fig. 7 and Table 2). To understand the binding mode of the compounds used in the site-directed mutagenesis studies, molecular docking based on the “induced-fit” approach was utilized in mutated RT RNase H structure models.
Fig. 7. A comparison of the binding modes of compounds A, AA, AB and BPT in the wild type and various clinically relevant mutants.
Table 2. Effect of selected compounds on the HIV-1 RT associated RNase H function of mutated RTs.
|
A
|
AA
|
AB
|
BTP |
|||||
| IC50 a (μM) | Fold b | IC50 a (μM) | Fold b | IC50 a (μM) | Fold b | IC50 a (μM) | Fold b | |
| Wild | 9.35 ± 0.73 | — | 5.1 ± 1.89 | — | 12.82 ± 1.05 | — | 0.20± 0.02 | — |
| A502F | 32.52 ± 2.83 | 3.5*** | 26.72 ± 3.14 | 5.3*** | 26.35 ± 1.01 | 2.1**** | 0.17 ± 0.03 | 0.85 |
| R557A | 20.54 ± 1.26 | 2.2*** | 12.53 ± 1.36 | 2.5** | 18.61 ± 0.42 | 1.5** | 0.21 ± 0.05 | 1.05 |
| R448A | 10.96 ± 1.12 | 1.20 | 7.01 ± 0.78 | 1.40 | 11.28 ± 0.64 | 0.9 | 0.14 ± 0.02 | 0.70 |
| N474A | >300 | >32**** | >300 | >59**** | 146 ± 12 | 11.4**** | 0.10 ± 0.01 | 0.50 |
aConcentration required to inhibit HIV-1 RT-associated RNase H activity by 50% obtained by three independent experiments (reported as average ± standard deviation). p value < 0.05 (*); p value < 0.01 (**); p value < 0.001 (***); p value < 0.0001(****).
bFold of increase with respect to WT RT.
Initially, the binding mode of BPT was investigated in four mutants (i.e. A502F, R557A, R4448A, N474A) and wild-type (WT). As shown in Table 2, there is no significant change in the IC50 of BPT in the mutants as compared to the WT. The “induced-fit” analysis also reveals that BPT exhibits a similar binding mode in all mutants compared to WT (cf. ESI† S9). BPT has been shown to inhibit the RNase H function through metal chelation. It is evident from the various binding poses that two of the three hydroxyl groups strongly interact with one of the magnesium ions in the active site. We observed a similar metal interaction pattern for compound AB (cf.Fig. 4C) which was indeed the only one to show the isosbestic point and the shift appropriate for actual Mg coordination. When assessed against the mutated enzymes, AB showed a lower impact of N474A substitution on its potency of inhibition compared to the other compounds, a slight increase in IC50 when assessed against A502F and no significant effect of R557A substitution, proving that AB inhibits RNase H through metal chelation.
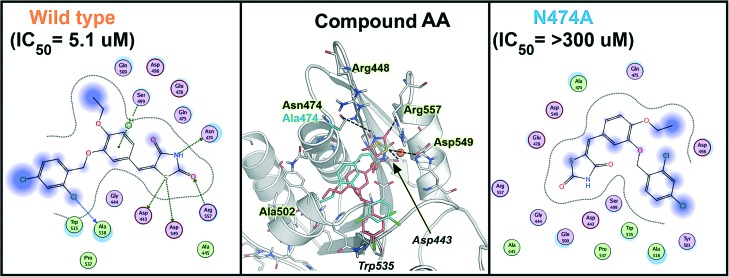
The other two compounds, AA and A, showed a similar behavior when tested against the mutated enzymes, with more pronounced variations in IC50 in the case of compound AA. Compound AA totally lost its potency of inhibition when assessed against N474A (>59-fold as compared to the WT) in accordance with the “induced-fit” calculation which clearly shows the different binding interaction of AA in N474A-substituted and WT RT (Fig. 8). Although the binding mode looks quite similar in both cases, the binding pose in N474A is slightly shifted, leading to a significant weakening of its key interactions with residues such as Ala538, Arg557, Asp549, Asp443, Asn474 and Ser499. Compound AA also showed a significant loss in potency in the case of A502F (5-fold) and R557A (2.5-fold), proving that it had multiple and strong interactions within the RNase H domain, and, as expected, no change was seen for R448A. The overall results show that compound AA has a binding mode that differs significantly from the one reported for NHQD,22 BHMP07,35 and DKAs,30 providing interesting perspectives for the hit optimization.
Fig. 8. A comparison of the various binding modes of compound AA in the RNase H active site; wild type is shown in orange and N474A mutant shown in cyan. Important residues are highlighted.
Furthermore, in order to confirm if the scaffolds are capable of improvements without becoming toxic, and to check if they are able to inhibit HIV-1 replication in cell-based assays, compounds A, AA, and AB were tested for cytotoxicity and inhibition of HIV-1 replication (data not shown). The results showed that compounds A, AA, and AB are not toxic up to 40 μM. Unfortunately, at the highest concentration tested, they were not able to inhibit viral replication; however, the structural insights gained from the present work will drive the scaffold improvements in order to achieve antiviral activity.
Conclusions
In the effort to search for novel inhibitors of the HIV-1 RT associated RNase H function, we have identified 4 scaffolds, A, AA, AB and AC, which inhibited the RNase H function in the low micromolar range. Binding affinity studies, computational investigation of the binding modes, magnesium complexation studies and site-directed mutagenesis revealed that the most active compound AA inhibits HIV-1 RNase H with an IC50 of 5.1 μM and showed a Mg-independent mode of action that involves multiple interactions within the RNase H domain, including one with the highly conserved residue Asn474. The molecular determinants identified in this study of the binding mode will serve as a good starting point to further ongoing lead optimization.
Materials and methods
In silico screening protocol
Specs compounds (www.specs.net) from the ZINC database63 were retrieved due to the fact that these compounds are commercially available for experimental screening. The purity of the final compounds (hits) used in the biochemical screening was verified by the chemical vendor using LC-MS and/or 1H NMR and showed at least 95% purity. Out of 277 324 compounds, 1943 compounds were removed due to missing stereo chemical information using the KNIME workflow.64 The remaining 275 381 compounds were imported into the OMEGA (a 3D conformation generating tool) tool from the OpenEye software.65 By default, OMEGA reports multiple conformers for all compounds using the MMFF94S force field; however, in the present study only a single, i.e., the lowest, energy conformer was used.
Compound preprocessing
3D coordinates of all the compounds were imported into the Maestro module66 available in the Schrödinger suite for further structural filtration and virtual screening process. The virtual screening (VS) workflow implemented in the Schrödinger suite was used. This includes ligand pre- and post-preparations66–69 (e.g. generation of tautomers, minimization etc.), Lipinski filters, reactive group filters and the docking workflow. In each step of the VS workflow, a significant reduction in the number of compounds was achieved.67
Homology modeling and structure-based screening
A computational model of the HIV-1 RT associated RNase H domain was built from an X-ray crystal structure (resolution of 1.4 Å) downloaded from the Protein Data Bank (PDB ID: 3QIO).22 Missing residues were added using Swiss-Model.70 Subsequently, the atomic coordinate of the protein was imported into the Maestro module66 implemented in the Schrödinger suite and the protein was further optimized (e.g., adding hydrogen atoms, assigning correct bond orders, building di-sulfide bonds and replacing crystal bound Mn2+ ions with catalytically active Mg2+ ions) using the Protein Preparation Wizard.69 The docking experiments were performed using the Glide module67 of the Schrödinger suite as described in the previous section. Before the compound screening, the protein structure and the docking protocol were assessed by reproducing the bound conformation of ligands (3-hydroxy-6-(phenylsulfonyl)quinazoline-2,4(1H,3H)-dione) by docking.54 In this study, water molecules at the active site beyond 3 Å from the bound ligand were deleted.
Shape-based similarity screening
The rapid overlay of chemical structures (ROCS)59 and electrostatic (EON)60 screening tools from OpenEye71 were used for shape and electrostatic based structural similarity searches. In both methods, the specs database is screened against the shape and electrostatic potential of a query molecule against a reference molecule. A detailed account of the theory behind these methods can be found elsewhere.53 Once the overlap is optimized, the shape or electrostatic similarity is computed using Tanimoto Combo (ranges from 0–2, a high score indicates a higher shape and pharmacophore similarity).
Structural clustering
For compound clustering, a set of 166 MACCS structural keys was computed; subsequently, the hierarchical clustering was employed using the Tanimoto similarity (TS) as the method of metric (TSAB = c/a + b – c, a = bits set to 1 in molecule A, b = bits set to 1 in molecule B, c = number of 1 bits common to molecules A and B).
Binding affinity prediction
Prediction of binding affinities for ligands in lead identification and optimization is a major challenge in computer-aided drug design. In this study, MM-GBSA was used to validate the binding mode of a series of ligands that bind to the protein. A detailed account of the theory behind this method can be found elsewhere.72,73 To capture the “induced-fit” effect, protein–ligand complexes from docking experiments were further energy optimized using Prime MM-GBSA with an implicit solvation model. During the optimization, both the protein and ligands were treated as flexible; for instance the 5 Å region of the protein around the ligand was treated as flexible.
HIV-DNA polymerase-independent RNase H activity determination
The HIV RT-associated RNase H activity was measured as described,32 briefly in a 100 μL reaction volume containing 50 mM Tris-HCl buffer pH 7.8, 6 mM MgCl2, 1 mM dithiothreitol (DTT), 80 mM KCl, 0.25 μM hybrid RNA/DNA 5′-GAUCUGAGCCUGGGAGCU-fluorescin-3′ (HPLC, dry, QC: Mass Check) (available from Metabion), 5′-dabcyl-AGCTCCCAGGCTCAGATC-3′ (HPLC, dry, QC: Mass Check), increasing concentrations of the inhibitor, whose dilution was made using water, and different amounts of enzymes according to a linear range of the dose–response curve: 20 ng of WT RT, 37.5 ng R448A, 62.5 ng N474A, 100 ng A502F and 75 ng R557A. The reaction mixture was incubated for 1 h at 37 °C, stopped by addition of EDTA, and the products were measured using a multi-label counter plate reader, Victor 3 (Perkin Elmer model 1420-051), equipped with filters for 490/528 nm (excitation/emission wavelength).
Site-directed mutagenesis
Amino acid substitutions were introduced into the p66 HIV-1 RT subunit of a HIV-1 RT group M subtype B coded in a p6HRT-prot plasmid using a QuikChange mutagenesis kit, following the manufacturer's instructions (Agilent Technologies Inc., Santa Clara, CA). Heterodimeric RT was expressed essentially and purified as described.74
Magnesium complexation study
Complexation studies were carried out on the following compounds: AN-648/41665045 (A); AG-690/36550050 (AA); AG-690/36165052 (AB); AH-487/15021157 (AC); AN-698/40677529 (AD); AN-698/40677530 (AE); AH-487/40935633 (AF); AH-487/41035472 (AG). The effects of the magnesium ions were evaluated by a spectrophotometric method, using a Perkin Elmer lambda 40 UV-vis spectrophotometer and a Hellma quartz cuvette with a 1 cm optical path. Magnesium chloride (1 M solution) was purchased from Sigma-Aldrich (Milano, Italy) and was diluted with absolute ethanol to obtain 13 stock solutions ranging from 4 10–5 to 0.2 M. Each studied compound was dissolved in 50–100 mL of absolute ethanol and the final concentration ranges between 3 × 10–5 and 8 × 10–5 M; the obtained solutions were maintained for 5 minutes in an ultrasonic bath. Each solution (3 mL) was placed in a cuvette and the UV-vis spectrum was recorded between 220 and 500 or 600 nm using ethanol as the reference. Thereafter, small volumes (10–15 μL) of the appropriate MgCl2 ethanolic stock solution were added both in the sample and in the reference cuvettes and the UV-vis spectra were recorded; the Mg2+ concentration in the solutions was increased from 0 to 100–200-fold with respect to the studied compound, in about twenty consecutive increments. Each experiment was conducted in triplicate.
Due to the scarce solubility of compound AH-487/40935633 (AF) in absolute ethanol, it was not possible to prepare a solution of known concentration; for this reason, only three spectra were recorded, one in the absence and two in the presence of a great excess of Mg2+. The titration graphs for the selected compounds are reported in the ESI.†
Author contributions
VP, JK and ET conceived and designed the experiments; VP and CS performed computational modeling, AC, NG, and FE performed RNase H inhibition experiments, LS, RC and FP performed chelation experiments. VP, AC, RDS, ET, and JK analyzed the data; VP, ET, JK, and RDS contributed reagents/materials/analysis tools. The manuscript was written through contributions of all the authors. All the authors have given approval to the final version of the manuscript.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This work has been supported by the Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, Denmark. The authors are thankful to ChemAxon and OpenEye scientific software for providing a free academic license. The authors thank the Italian MIUR for financial support (PRIN 2010, 2010W2KM5L_003).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00600d
References
- Fauci A. S. Nat. Med. 2003;9:839–843. doi: 10.1038/nm0703-839. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Gregson J., Parkin N., Haile-Selassie H., Tanuri A., Andrade Forero L., Kaleebu P., Watera C., Aghokeng A., Mutenda N., Dzangare J., Hone S., Hang Z. Z., Garcia J., Garcia Z., Marchorro P., Beteta E., Giron A., Hamers R., Inzaule S., Frenkel L. M., Chung M. H., de Oliveira T., Pillay D., Naidoo K., Kharsany A., Kugathasan R., Cutino T., Hunt G., Avila Rios S., Doherty M., Jordan M. R., Bertagnolio S. Lancet Infect. Dis. 2017 doi: 10.1016/S1473-3099(17)30702-8. [DOI] [Google Scholar]
- De Clercq E. Curr. Med. Chem. 2001;8:1543–1572. doi: 10.2174/0929867013371842. [DOI] [PubMed] [Google Scholar]
- UNAIDS report, on the global AIDS epidemic 2012: Join UN program on HIV/AIDS, 2012.
- De Clercq E. Biochim. Biophys. Acta, Mol. Basis Dis. 2002;1587:258–275. doi: 10.1016/s0925-4439(02)00089-3. [DOI] [PubMed] [Google Scholar]
- Zdanowicz M. M. Am. J. Pharm. Educ. 2006;70:1–9. doi: 10.5688/aj7005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margot N. A., Wong P., Kulkarni R., White K., Porter D., Abram M. E., Callebaut C., Miller M. D. J. Infect. Dis. 2017;215:920–927. doi: 10.1093/infdis/jix015. [DOI] [PubMed] [Google Scholar]
- Schneider A., Corona A., Sporing I., Jordan M., Buchholz B., Maccioni E., Di Santo R., Bodem J., Tramontano E., Wohrl B. M. Nucleic Acids Res. 2016;44:2310–2322. doi: 10.1093/nar/gkw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyshire J. Drugs. 1995;49:1–3. doi: 10.2165/00003495-199500491-00003. [DOI] [PubMed] [Google Scholar]
- FDA, Antiretroviral drugs used in the treatment of HIV infection, http://www.fda.gov, (accessed December, 2014).
- Flexner C. Nat. Rev. Drug Discovery. 2007;6:959–966. doi: 10.1038/nrd2336. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Debyser Z., Ikeda Y., Freed E. O., Stephens E., Yonemoto W., Buckheit R. W., Este J. A., Cihlar T. Antiviral Res. 2008;80:251–265. doi: 10.1016/j.antiviral.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Corona A., Esposito F., Tramontano E. Future Virol. 2014;9:445–448. [Google Scholar]
- Ilina T., Labarge K., Sarafianos S. G., Ishima R., Parniak M. A. Biology. 2012;1:521–541. doi: 10.3390/biology1030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano E., Di Santo R. Curr. Med. Chem. 2010;17:2837–2853. doi: 10.2174/092986710792065045. [DOI] [PubMed] [Google Scholar]
- Schatz O., Cromme F., Naas T. and Lindemann D., Gene Regulation and AIDS, 1990, portfolio, pp. 293–404. [Google Scholar]
- Corona A., Di Leva F. S., Thierry S., Pescatori L., Crucitti G. C., Subra F., Delelis O., Esposito F., Rigogliuso G., Costi R., Cosconati S., Novellino E., Di Santo R., Tramontano E. Antimicrob. Agents Chemother. 2014;58:6101–6110. doi: 10.1128/AAC.03605-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano E., Esposito F., Badas R., Di Santo R., Costi R., La Colla P. Antiviral Res. 2005;65:117–124. doi: 10.1016/j.antiviral.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Davies 2nd J. F., Hostomska Z., Hostomsky Z., Jordan S. R., Matthews D. A. Science. 1991;252:88–95. doi: 10.1126/science.1707186. [DOI] [PubMed] [Google Scholar]
- Himmel D. M., Maegley K. A., Pauly T. A., Bauman J. D., Das K., Dharia C., Clark A. D., Ryan K., Hickey M. J., Love R. A., Hughes S. H., Bergqvist S., Arnold E. Structure. 2009;17:1625–1635. doi: 10.1016/j.str.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel D. M., Sarafianos S. G., Dharmasena S., Hossain M. M., McCoy-Simandle K., Ilina T., Clark A. D., Knight J. L., Julias J. G., Clark P. K., Krogh-Jespersen K., Levy R. M., Hughes S. H., Parniak M. A., Arnold E. ACS Chem. Biol. 2006;1:702–712. doi: 10.1021/cb600303y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdon E. B., Liu Q., Leavitt S. A., Balakrishnan M., Perry J. K., Lancaster-Moyer C., Kutty N., Liu X. H., Squires N. H., Watkins W. J., Kirschberg T. A. Antimicrob. Agents Chemother. 2011;55:2905–2915. doi: 10.1128/AAC.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz O., Cromme F. V., Naas T., Lindemann D., Mous J., Legrice S. F. J. Adv. Appl. Biotechnol. 1990;7:293–303. [Google Scholar]
- Christen M. T., Menon L., Myshakina N. S., Ahn J., Parniak M. A., Ishima R. Chem. Biol. Drug Des. 2012;80:706–716. doi: 10.1111/cbdd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleddu R., Cannas V., Distinto S., Sarais G., Del Vecchio C., Esposito F., Bianco G., Corona A., Cottiglia F., Alcaro S., Parolin C., Artese A., Scalise D., Fresta M., Arridu A., Ortuso F., Maccioni E., Tramontano E. ChemMedChem. 2014;9:1869–1879. doi: 10.1002/cmdc.201402015. [DOI] [PubMed] [Google Scholar]
- Poongavanam V., Olsen J. M. H., Kongsted J. Integr. Biol. 2014;6:1010–1022. doi: 10.1039/c4ib00111g. [DOI] [PubMed] [Google Scholar]
- Di Grandi M., Olson M., Prashad A. S., Bebernitz G., Luckay A., Mullen S., Hu Y. B., Krishnamurthy G., Pitts K., O'Connell J. Bioorg. Med. Chem. Lett. 2010;20:398–402. doi: 10.1016/j.bmcl.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Didierjean J., Isel C., Querre F., Mouscadet J. F., Aubertin A. M., Valnot J. Y., Piettre S. R., Marquet R. Antimicrob. Agents Chemother. 2005;49:4884–4894. doi: 10.1128/AAC.49.12.4884-4894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distinto S., Esposito F., Kirchmair J., Cardia M. C., Gaspari M., Maccioni E., Alcaro S., Markt P., Wolber G., Zinzula L., Tramontano E. Eur. J. Med. Chem. 2012;50:216–229. doi: 10.1016/j.ejmech.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Kirschberg T. A., Balakrishnan M., Squires N. H., Barnes T., Brendza K. M., Chen X., Eisenberg E. J., Jin W., Kutty N., Leavitt S., Liclican A., Liu Q., Liu X., Mak J., Perry J. K., Wang M., Watkins W. J., Lansdon E. B. J. Med. Chem. 2009;52:5781–5784. doi: 10.1021/jm900597q. [DOI] [PubMed] [Google Scholar]
- Nowotny M., Gaidamakov S. A., Ghirlando R., Cerritelli S. M., Crouch R. J., Yang W. Mol. Cell. 2007;28:513–513. doi: 10.1016/j.molcel.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Costi R., Metifiot M., Chung S., Crucitti G. C., Maddali K., Pescatori L., Messore A., Madia V. N., Pupo G., Scipione L., Tortorella S., Di Leva F. S., Cosconati S., Marinelli L., Novellino E., Le Grice S. F. J., Corona A., Pommier Y., Marchand C., Di Santo R. J. Med. Chem. 2014;57:3223–3234. doi: 10.1021/jm5001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp K., Hang J. Q., Rajendran S., Yang Y. L., Derosier A., In P. W. K., Overton H., Parkes K. E. B., Cammack N., Martin J. A. Nucleic Acids Res. 2003;31:6852–6859. doi: 10.1093/nar/gkg881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budihas S. R., Gorshkova I., Gaidamakov S., Wamiru A., Bona M. K., Parniak M. A., Crouch R. J., McMahon J. B., Beutler J. A., Le Grice S. F. J. Nucleic Acids Res. 2005;33:1249–1256. doi: 10.1093/nar/gki268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. P., Yan Y. W., Prasad G. S., Smith R. F., Daniels C. L., Abeywickrema P. D., Reid J. C., Loughran H. M., Kornienko M., Sharma S., Grobler J. A., Xu B., Sardana V., Allison T. J., Williams P. D., Darke P. L., Hazuda D. J., Munshi S. J. Virol. 2010;84:7625–7633. doi: 10.1128/JVI.00353-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji H., Urano E., Futahashi Y., Hamatake M., Tatsumi J., Hoshino T., Morikawa Y., Yamamoto N., Komano J. J. Med. Chem. 2009;52:1380–1387. doi: 10.1021/jm801071m. [DOI] [PubMed] [Google Scholar]
- Chung S. M., Wendeler M., Rausch J. W., Beilhartz G., Gotte M., O'Keefe B. R., Bermingham A., Beutler J. A., Liu S. X., Zhuang X. W., Le Grice S. F. J. Antimicrob. Agents Chemother. 2010;54:3913–3921. doi: 10.1128/AAC.00434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K. A., Marchand B., Ong Y. T., Ndongwe T. P., Hachiya A., Michailidis E., Leslie M. D., Sietsema D. V., Fetterly T. L., Dorst C. A., Singh K., Wang Z., Parniak M. A., Sarafianos S. G. Antimicrob. Agents Chemother. 2012;56:2048–2061. doi: 10.1128/AAC.06000-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q. G., Menon L., Ilina T., Miller L. G., Ahn J., Parniak M. A., Ishima R. Chem. Biol. Drug Des. 2011;77:39–47. doi: 10.1111/j.1747-0285.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. M., Miller J. T., Johnson B. C., Hughes S. H., Le Grice S. F. J. J. Biol. Chem. 2012;287:4066–4075. doi: 10.1074/jbc.M111.314781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Kharlamova T., Distinto S., Zinzula L., Cheng Y. C., Dutschman G., Floris G., Markt P., Corona A., Tramontano E. FEBS J. 2011;278:1444–1457. doi: 10.1111/j.1742-4658.2011.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Tramontano E. Antiviral Chem. Chemother. 2014;23:129–144. doi: 10.3851/IMP2690. [DOI] [PubMed] [Google Scholar]
- Costi R., Metifiot M., Esposito F., Cuzzucoli Crucitti G., Pescatori L., Messore A., Scipione L., Tortorella S., Zinzula L., Novellino E., Pommier Y., Tramontano E., Marchand C., Di Santo R. J. Med. Chem. 2013;56:8588–8598. doi: 10.1021/jm401040b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzucoli Crucitti G., Metifiot M., Pescatori L., Messore A., Madia V. N., Pupo G., Saccoliti F., Scipione L., Tortorella S., Esposito F., Corona A., Cadeddu M., Marchand C., Pommier Y., Tramontano E., Costi R., Di Santo R. J. Med. Chem. 2015;58:1915–1928. doi: 10.1021/jm501799k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona A., di Leva F. S., Rigogliuso G., Pescatori L., Madia V. N., Subra F., Delelis O., Esposito F., Cadeddu M., Costi R., Cosconati S., Novellino E., di Santo R., Tramontano E. Antiviral Res. 2016;134:236–243. doi: 10.1016/j.antiviral.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Summa V., Petrocchi A., Bonelli F., Crescenzi B., Donghi M., Ferrara M., Fiore F., Gardelli C., Gonzalez Paz O., Hazuda D. J., Jones P., Kinzel O., Laufer R., Monteagudo E., Muraglia E., Nizi E., Orvieto F., Pace P., Pescatore G., Scarpelli R., Stillmock K., Witmer M. V., Rowley M. J. Med. Chem. 2008;51:5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- Corona A., Masaoka T., Tocco G., Tramontano E., Le Grice S. F. J. Future Med. Chem. 2013;5:2127–2139. doi: 10.4155/fmc.13.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts A. K., LaBarge K., Bauman J. D., Patel D. V., Himmel D. M., Arnold E., Parniak M. A., Levy R. M. J. Chem. Inf. Model. 2011;51:1986–1998. doi: 10.1021/ci200194w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julias J. G., McWilliams M. J., Sarafianos S. G., Arnold E., Hughes S. H. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9515–9520. doi: 10.1073/pnas.142123199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J. W., Lener D., Miller J. T., Julias J. G., Hughes S. H., Le Grice S. F. Biochemistry. 2002;41:4856–4865. doi: 10.1021/bi015970t. [DOI] [PubMed] [Google Scholar]
- Poongavanam V., Namasivayam V., Vanangamudi M., Al Shamaileh H., Veedu R. N., Kihlberg J., Murugan N. A. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2017;8:e1328. [Google Scholar]
- Schneider G., Bohm H. J. Drug Discovery Today. 2002;7:64–70. doi: 10.1016/s1359-6446(01)02091-8. [DOI] [PubMed] [Google Scholar]
- Vasanthanathan P., Lastdrager J., Oostenbrink C., Commandeur J. N. M., Vermeulen N. P. E., Jorgensen F. S., Olsen L. MedChemComm. 2011;2:853–859. [Google Scholar]
- Poongavanam V., Kongsted J. PLoS One. 2013;8:e73478. doi: 10.1371/journal.pone.0073478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poongavanam V., Steinmann C., Kongsted J. PLoS One. 2014;9:e98659. doi: 10.1371/journal.pone.0098659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvas, Schrödinger, LLC, New York, NY, 2014.
- Olson M. W., Di G. M. and Prashad A., US Pat., WO2005090316A1, 2005.
- Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., Mizote A., Rosen D., Thomas J. M., Izumi M., Ganesan A., Galione A., Churchill G. C. Nat. Chem. Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. A., Gallardo M. A., Pickup B. T. J. Comput. Chem. 1996;17:1653–1666. [Google Scholar]
- Muchmore S. W., Souers A. J., Akritopoulou-Zanze I. Chem. Biol. Drug Des. 2006;67:174–176. doi: 10.1111/j.1747-0285.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- Costi R., Santo R. D., Artico M., Massa S., Ragno R., Loddo R., La Colla M., Tramontano E., La Colla P., Pani A. Bioorg. Med. Chem. 2004;12:199–215. doi: 10.1016/j.bmc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Chung S. M., Himmel D. M., Jiang J. K., Wojtak K., Bauman J. D., Rausch J. W., Wilson J. A., Beutler J. A., Thomas C. J., Arnold E., Le Grice S. F. J. J. Med. Chem. 2011;54:4462–4473. doi: 10.1021/jm2000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin J. J., Sterling T., Mysinger M. M., Bolstad E. S., Coleman R. G. J. Chem. Inf. Model. 2012;52:1757–1768. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R. B., Nicolas C., Fabian D., Thomas R. G., Tobias K., Thorsten M., Peter O., Kilian T. and Bernd W., SIGKDD Explor. Newsl., 2009, vol. 11, pp. 26–31. [Google Scholar]
- Hawkins P. C. D., Skillman A. G., Warren G. L., Ellingson B. A., Stahl M. T. J. Chem. Inf. Model. 2010;50:572–584. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro, Schrödinger, LLC, New York, NY, 2014.
- Friesner R. A., Banks J. L., Murphy R. B., Halgren T. A., Klicic J. J., Mainz D. T., Repasky M. P., Knoll E. H., Shelley M., Perry J. K., Shaw D. E., Francis P., Shenkin P. S. J. Med. Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- LigPrep, Schrödinger, LLC, New York, NY, 2014.
- Sastry G. M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. J. Comput.-Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J., Schwede T. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- OpenEye, OpenEye Scientifc Software, CR Sanda Fe, NM 87508, USA (http://www.eyesopen.com), 2014.
- Lyne P. D., Lamb M. L., Saeh J. C. J. Med. Chem. 2006;49:4805–4808. doi: 10.1021/jm060522a. [DOI] [PubMed] [Google Scholar]
- Du J., Sun H. J., Xi L. L., Li J. Z., Yang Y., Liu H. X., Yao X. J. J. Comput. Chem. 2011;32:2800–2809. doi: 10.1002/jcc.21859. [DOI] [PubMed] [Google Scholar]
- Corona A., Meleddu R., Esposito F., Distinto S., Bianco G., Masaoka T., Maccioni E., Menendez-Arias L., Alcaro S., Le Grice S. F. J., Tramontano E. PLoS One. 2016;11:e0147225. doi: 10.1371/journal.pone.0147225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.