 A series of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k) were synthesized and evaluated for their α-glucosidase inhibitory activity.
A series of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k) were synthesized and evaluated for their α-glucosidase inhibitory activity.
Abstract
A series of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k) were synthesized and evaluated for their α-glucosidase inhibitory activity. The majority of compounds exhibited potent inhibitory activity in the range of 5.44 ± 0.13 to 50.45 ± 0.39 μM, when compared to the standard drug acarbose (IC50 = 817.38 ± 6.27 μM). Among the compounds in the series, compounds 5k, 6a, 6b, 6e, 6h and 6k showed potent inhibitory potential with IC50 values of 20.95 ± 0.21, 16.11 ± 0.19, 7.72 ± 0.16, 7.91 ± 0.17, 6.59 ± 0.15 and 5.44 ± 0.13 μM, respectively. Compound 6k (IC50 = 5.44 ± 0.13 μM), containing chloro and rhodanine groups at the 2- and 4-positions of the phenyl ring respectively, was found to be the most active compound that inhibits α-glucosidase activity. Furthermore, molecular docking studies were performed to understand the binding interactions between the molecule and enzyme.
Introduction
Diabetes mellitus (DM) is a growing public health problem in both developed and developing countries and the number of people with diabetes is expected to reach 366 million by 2030.1 It is a group of metabolic diseases characterized by hyperglycemia.2 The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs including the eyes, kidneys, nerves, heart, and blood vessels.3,4 α-Glucosidase is the key enzyme catalyzing the final step in the digestive process of carbohydrates. Inhibition of α-glucosidase is a useful treatment to treat type II diabetes mellitus by delaying the absorption of glucose after meals.5 Currently, some glucosidase inhibitors, such as acarbose, miglitol, and voglibose, are being clinically used for the treatment of type II diabetes mellitus.6 In addition, α-glucosidase inhibitors are also used as anti-hepatitis, anti-cancer and anti-HIV agents.7–10 Therefore, the design and development of new α-glucosidase inhibitors is an important objective in medicinal chemistry.
Thiazolidine-2,4-dione is an important class of heterocyclic compounds due to their broad range of biological activities, including antidiabetic,11 antioxidative,12 anticancer,13 anti-inflammatory14 and antimicrobial.11 Several thiazolidine-2,4-dione derivatives, like pioglitazone and rosiglitazone, have been approved by the Food and Drug Administration (FDA) for treating type 2 diabetes.15 Recently, Chinthala et al. have reported that a novel series of thiazolidinedione derivatives with triazole ring act as a new class of α-glucosidase inhibitors (Fig. 1).16 Like thiazolidine-2,4-diones, rhodanine (2-thio-2,4-thiazolidinedione) and its derivatives have attracted wide attention in medicinal chemistry.17 Rhodanine derivatives have been reported to exhibit a variety of biological activities such as antimalarial,18 antioxidative,19 antimicrobial,20 anticancer,21 and antidiabetic activities.22 In particular, epalrestat is a rhodanine-3-acetic acid analogue and acts as a potent aldose reductase inhibitor, which has been approved for the treatment of diabetic complications, such as neuropathy, nephropathy and cataract (Fig. 1).23
Fig. 1. The structures of some commercial drugs and pharmacologically active compounds.
On the other hand, phthalimide derivatives have been widely reported to possess various biological activities like, anticancer,24 anti-HIV,25 antimicrobial,26 antioxidative26 and antiinflammatory27 activities. It is an interesting point that numerous phthalimide derivatives have been reported to show potent inhibitory activity against α-glucosidase.28–31 For example, compound series I,28II,29 and III (ref. 30) displayed potent α-glucosidase inhibitory activity (Fig. 1). Accordingly, phthalimide could be used as a pharmacophore to design new more active compounds for α-glucosidase.
In continuation of our interest in the design and development of novel α-glucosidase inhibitors,32–36 we report herein the design and synthesis of a hybrid scaffold by incorporating phthalimide with thiazolidine-2,4-dione or rhodanine into a single molecule. The synthesized compounds were tested for their in vitro α-glucosidase inhibitory activity. Molecular docking was performed to investigate the interaction of the synthesized compounds with the active site of α-glucosidase.
Results and discussion
Chemistry
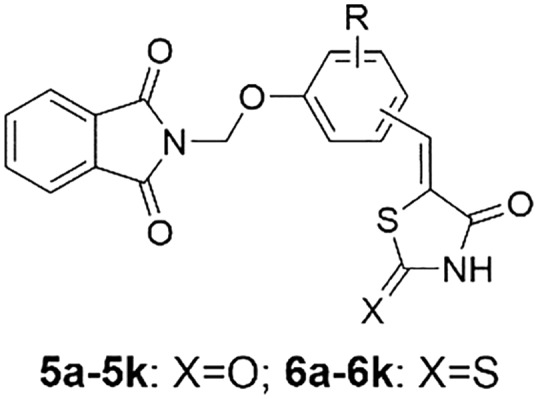
A series of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k) were synthesized as shown in Scheme 1. Phthalimide 1 was reacted with formaldehyde to form 2-(hydroxymethyl)isoindoline-1,3-dione 2, which reacted with thionyl chloride to provide 2-(chloromethyl)isoindoline-1,3-dione 3. Treatment of 3 with different commercially available hydroxyl-substituted aromatic aldehydes in the presence of anhydrous K2CO3 in dry acetone afforded the intermediates 4a–4k. Knoevenagel condensation of 4a–4k with rhodanine or thiazolidine-2,4-dione in the presence of β-alanine in refluxing acetic acid afforded the target compounds (5a–5k, 6a–6k). Based on the literature, the isomeric ratio was presumed to be of mainly the Z-stereoisomer.37,38 All the title compounds are new and have not been reported in the literature to date.
Scheme 1. Reagents and conditions: (a) 38% formaldehyde–H2O, reflux, 3 h; (b) SOCl2, reflux, 5 h; (c) hydroxy-substituted benzaldehyde, K2CO3, KI, acetone, reflux, 6 h; (d) β-alanine, AcOH, reflux, 6 h.
The structures of all the title compounds (5a–5k, 6a–6k) were elucidated from spectral data. For instance, the 1H NMR spectrum of 5a showed a singlet at δ 3.75 ppm due to the methoxyl protons of the phenyl ring. The methylene protons of –OCH2N– appeared as a singlet at δ 5.60 ppm. The doublet of doublet peak of C6–H of the phenyl ring was observed at δ 7.14 ppm. Two doublet signals at δ 7.23 and δ 7.31 ppm were attributed to C2–H and C5–H of the phenyl ring, respectively. The proton of –CH C appeared at δ 7.76 as a singlet signal. The four protons of phthalimide appeared as a multiplet in the regions 7.90–7.93 ppm and 7.96–7.98 ppm. The single peak of the NH proton of thiazolidine-2,4-dione was observed at δ 12.56 ppm. All these data are in agreement with the structure of compound 5a. Moreover, in their 13C NMR spectra, the number of signals equals the number of different carbons in the molecule (ESI†).
α-Glucosidase inhibition assay
All synthetic compounds were evaluated for their in vitro α-glucosidase inhibitory activities. The results showed that the majority of compounds exhibited potent inhibitory activity in the range 5.44 ± 0.13 to 50.45 ± 0.39 μM, when compared to the standard drug acarbose (IC50 = 817.38 ± 6.27 μM (ref. 39 and 40)). Among the compounds in the series, compounds 5k, 6a, 6b, 6e, 6h and 6k showed potent inhibitory potential with IC50 values of 20.95 ± 0.21, 16.11 ± 0.19, 7.72 ± 0.16, 7.91 ± 0.17, 6.59 ± 0.15 and 5.44 ± 0.13 μM, respectively. Compound 6k (IC50 = 5.44 ± 0.13 μM), containing chloro and rhodanine groups at the 2- and 4-positions of the phenyl ring respectively, was found to be the most active compound that inhibits α-glucosidase activity. Compounds 5a, 5b, 5e, 5h, 5j, 6c, 6g, 6i and 6j also showed good inhibition with IC50 values of 35.14 ± 0.28, 29.26 ± 0.24, 25.60 ± 0.22, 22.48 ± 0.23, 50.45 ± 0.39, 33.98 ± 0.26, 39.81 ± 0.36, 34.82 ± 0.29 and 35.79 ± 0.25 μM respectively. The other compounds displayed low α-glucosidase inhibitory activity (Table 1).
Table 1. α-Glucosidase inhibitory activity of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k).
| |||
| Compound | Substituted position | R | IC50 a (μM) |
| 5a | Para | 2-MeO | 35.14 ± 0.28 |
| 5b | Para | 2-EtO | 29.26 ± 0.24 |
| 5c | Meta | H | >100 |
| 5d | Ortho | H | >100 |
| 5e | Para | H | 25.60 ± 0.22 |
| 5f | Ortho | 6-OMe | >100 |
| 5g | Para | 2,6-diMeO | >100 |
| 5h | Para | 2-NO2 | 22.48 ± 0.23 |
| 5i | Ortho | 4-Cl | >100 |
| 5j | Ortho | 4-NO2 | 50.45 ± 0.39 |
| 5k | Para | 2-Cl | 20.95 ± 0.21 |
| 6a | Para | 2-MeO | 16.11 ± 0.19 |
| 6b | Para | 2-EtO | 7.72 ± 0.16 |
| 6c | Meta | H | 33.98 ± 0.26 |
| 6d | Ortho | H | >100 |
| 6e | Para | H | 7.91 ± 0.17 |
| 6f | Ortho | 6-OMe | >100 |
| 6g | Para | 2,6-diMeO | 39.81 ± 0.36 |
| 6h | Para | 2-NO2 | 6.59 ± 0.15 |
| 6i | Ortho | 4-Cl | 34.82 ± 0.29 |
| 6j | Ortho | 4-NO2 | 35.79 ± 0.25 |
| 6k | Para | 2-Cl | 5.44 ± 0.13 |
| Acarbose | 817.38 ± 6.27 | ||
aAcarbose is the standard for α-glucosidase inhibition activity.
The structure–activity relationship of these compounds has been established. The results suggested that the thiazolidine-2,4-dione or rhodanine group located at the 4-position of the phenyl ring resulted in the best activity (5c–5e, 6c–6e). Shifting the thiazolidine-2,4-dione or rhodanine group to the 2- or 3-position decreased the activity drastically. A comparison of the thiazolidine-2,4-dione derivatives (5a–5k) with their rhodanine counterparts (6a–6k) demonstrated that the replacement of O with S significantly increased the activity. Introduction of an electron-withdrawing group such as Cl (5k, 6k) and NO2 (5h, 6h) into the 2-position of the phenyl ring results in an increase in the inhibitory activity. Comparing the inhibitory activity of 5a (IC50 = 35.14 ± 0.28 μM) and 5g (IC50 > 100 μM), the result showed that the introduction of methoxyl into the phenyl ring results in a decrease in the inhibitory activity. In particular, compound 6k (IC50 = 5.44 ± 0.13 μM) with a chloro substitution at the 2-position of the right phenyl ring and a rhodanine group at the 4-position of the right phenyl ring was found to be the most active compound. Compounds 6b, 6e, and 6h also displayed good inhibition with IC50 values of 7.72 ± 0.16, 7.91 ± 0.17 and 6.59 ± 0.15 μM, respectively. In summary, the obtained structure–activity relationship information is useful in future structural optimization. The binding interactions of the most active molecules with the active site of α-glucosidase were confirmed through molecular docking studies.
Homology model
The crystallographic structure of Saccharomyces cerevisiae's α-glucosidase enzyme has not been reported. To understand the ligand–enzyme interactions, the 3D structure of α-glucosidase was built by means of MODELLER 9.15 homology modeling software (; http://salilab.org/modeller/). The sequence in a FASTA format of α-glucosidase was retrieved from UniProt (access code P53341). The crystallographic structure of Saccharomyces cerevisiae isomaltase (PDB ID: ; 3AJ7, resolution 1.30 Å) with a 72.4% sequence identity with the target was selected as the template for homology modeling.41 The quality of the homology model was verified by PROCHECK (; http://services.mbi.ucla.edu/PROCHECK/). The result showed that the model could be used to study the interactions between this class of compounds and the active site of α-glucosidase.36
Molecular docking
The theoretical binding mode between 5k and Saccharomyces cerevisiae α-glucosidase is shown in Fig. 2. Compound 5k adopted an “L-shaped” conformation in the pocket of α-glucosidase. The phthalimide group of 5k stretched into the hydrophobic pocket that consisted of Phe-157, Phe-177 and Leu-218, forming stable hydrophobic bonds. Detailed analysis showed that the 2-chlorophenyl group of 5k formed CH–π interactions with the residues of Phe-157, Phe-177 and Phe-300. It was shown that the residues of Thr-215 (bond length: 3.4 Å), Asp-214 (length: 3.4 and 2.3 Å) and His-248 (length: 3.2 Å) formed four hydrogen bonds with 5k, which were the main interactions between 5k and α-glucosidase.
Fig. 2. Compound 5k was docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase.
To explain the activity order of 5k and 6k against α-glucosidase, 6k was then docked into the binding site of α-glucosidase, and the theoretical binding mode between 6k and α-glucosidase is shown in Fig. 3A. The interactions between 6k and α-glucosidase were nearly the same as those of the precursor 5k (Fig. 3B). The only difference was that the lengths of the hydrogen bonds between 6k and α-glucosidase were all shorter than those of 5k, which made 6k more active than 5k against α-glucosidase (Fig. 3B). In addition, the estimated binding energies were –8.7 kcal mol–1 for 5k and –9.4 kcal mol–1 for 6k, respectively, which was consistent with the results of the in vitro α-glucosidase inhibitory assay.
Fig. 3. (A) Compound 6k was docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase. (B) Compounds 5k and 6k were docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase (overlapped).
To explain the activity order of 6e and 6k against α-glucosidase, 6e was then docked into the binding site of α-glucosidase, and the theoretical binding mode between 6e and α-glucosidase is shown in Fig. 4A. Compound 6e adopted an “L-shaped” conformation in the pocket of α-glucosidase. The phthalimide group of 6e was located at the hydrophobic pocket, surrounded by the residues of Phe-157, Phe-177 and Leu-218, forming stable hydrophobic bonds. Detailed analysis showed that the phenyl group in the middle of 6e formed CH–π interactions with the residues of Phe-157, Phe-177 and Phe-300. It was shown that the residues of Thr-215 (bond length: 3.3 Å), Asp-214 (length: 3.4 and 2.5 Å) and His-248 (length: 3.2 Å) formed four hydrogen bonds with 6e, which were the main interactions between 6e and α-glucosidase. The interactions between 6k and α-glucosidase were almost the same as those of 6e (Fig. 4B). The only difference was that the lengths of the three hydrogen bonds between 6k and α-glucosidase were shorter than those of 6e, which made 6k more active than 6e against α-glucosidase (Fig. 4B). In addition, the estimated binding energies were –8.9 kcal mol–1 for 6e and –9.4 kcal mol–1 for 6k, respectively, which was consistent with the results of the in vitro anti-α-glucosidase assay.
Fig. 4. (A) Compound 6e was docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase. (B) Compounds 6e and 6k were docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase (overlapped).
In order to further explain the activity order of 6e and 6c against α-glucosidase, 6c was then docked into the binding site of α-glucosidase, and the result is displayed in Fig. 5A. The interactions between 6c and α-glucosidase were nearly the same as those of 6e (Fig. 5B). The main difference was that the oxygen atom of 6e formed an extra hydrogen bond with the residue of Thr-215 (length: 3.3 Å), which made 6e more active than 6c against α-glucosidase (Fig. 5B). In addition, the estimated binding energies were –8.3 kcal mol–1 for 6c and –8.9 kcal mol–1 for 6e, respectively, which was consistent with the results of the in vitro anti-α-glucosidase assay. In summary, the above molecular simulations give us a rational explanation of the interactions between 5k, 6c, 6e, 6k and α-glucosidase, which provided valuable information for further development of α-glucosidase inhibitors.
Fig. 5. (A) Compound 6c was docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase. (B) Compounds 6c and 6e were docked to the binding pocket of Saccharomyces cerevisiae α-glucosidase (overlapped).
Experimental
Equipment and materials
All starting materials and reagents were purchased from commercial suppliers. TLC was performed on 0.20 mm silica gel 60 F254 plates (Qingdao Ocean Chemical Factory, Shandong, China). Nuclear magnetic resonance spectra (NMR) were recorded on a Bruker spectrometer (400 MHz) with TMS as an external reference and are reported in parts per million.
General procedures for the synthesis of 5a–5k and 6a–6k
A mixture of 4 (1.0 mmol), β-alanine (1.5 mmol), rhodanine or thiazolidine-2,4-dione (3 mmol) in AcOH (10 mL) was stirred at 120 °C for 6 h. After the completion of the reaction, the precipitates that formed were collected by filtration and washed with boiling water (3 × 10 mL), ethanol (3 × 10 mL), and ether (3 × 10 mL). The wet product was dried in a vacuum at 60 °C for 24 h.
(Z)-5-(4-((1,3-Dioxoisoindolin-2-yl)methoxy)-3-methoxybenzylidene)thiazolidine-2,4-dione (5a)
Yellow solid; yield 78.5%; m. p. 225–228 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.75 (s, 3H, OCH3), 5.60 (s, 2H, OCH2N), 7.14 (dd, 1H, J = 8.4 Hz, 2.0 Hz, ArH), 7.23 (d, 1H, J = 2.0 Hz, ArH), 7.31 (d, 1H, J = 8.4 Hz, ArH), 7.76 (s, 1H, CH), 7.90–7.93 (m, 2H, phthalimide), 7.96–7.98 (m, 2H, phthalimide), 12.56 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.1, 66.8, 114.7, 117.9, 122.3, 123.5, 124.2, 128.7, 131.7, 132.2, 135.6, 147.8, 150.7, 167.3, 167.7, 168.3; HRMS (ESI) calcd for [M – H]– C20H13N2O6S: 409.0500, found: 409.0511.
(Z)-5-(4-((1,3-Dioxoisoindolin-2-yl)methoxy)-3-ethoxybenzylidene)thiazolidine-2,4-dione (5b)
Yellow solid; yield 81.3%; m. p. 230–233 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 1.27 (t, 3H, J = 6.8 Hz, OCH2C[combining low line]H[combining low line]3[combining low line]), 3.99 (q, 2H, J = 6.8 Hz, OC[combining low line]H[combining low line]2[combining low line]CH3), 5.60 (s, 2H, OCH2N), 7.12 (dd, 1H, J = 8.4 Hz, 2.0 Hz, ArH), 7.21 (d, 1H, J = 2.0 Hz, ArH), 7.30 (d, 1H, J = 8.4 Hz, ArH), 7.75 (s, 1H, CH), 7.90–7.93 (m, 2H, phthalimide), 7.95–7.97 (m, 2H, phthalimide), 12.56 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 14.8, 64.5, 67.2, 115.7, 119.2, 122.4, 123.6, 124.2, 129.0, 131.7, 132.2, 135.6, 148.0, 150.3, 167.3, 167.7, 168.3; HRMS (ESI) calcd for [M – H]– C21H15N2O6S: 423.0656, found: 423.0665.
(Z)-5-(3-((1,3-Dioxoisoindolin-2-yl)methoxy)benzylidene)thiazolidine-2,4-dione (5c)
Light yellow solid; yield 82.6%; m. p. 229–231 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.63 (s, 2H, OCH2N), 7.23 (dd, 2H, J = 8.0 Hz, 2.0 Hz, ArH), 7.32 (t, 1H, J = 2.0 Hz, ArH), 7.49 (t, 1H, J = 8.0 Hz, ArH), 7.75 (s, 1H, CH), 7.90–7.93 (m, 2H, phthalimide), 7.95–7.99 (m, 2H, phthalimide), 12.64 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 66.0, 118.2, 118.5, 123.7, 124.2, 124.8, 131.0, 131.7, 131.8, 135.0, 135.6, 157.2, 167.4, 167.7, 168.2; HRMS (ESI) calcd for [M – H]– C19H11N2O5S: 379.0394, found: 379.0399.
(Z)-5-(2-((1,3-Dioxoisoindolin-2-yl)methoxy)benzylidene)thiazolidine-2,4-dione (5d)
Light yellow solid; yield 69.6%; m. p. 228–230 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.67 (s, 2H, OCH2N), 7.21 (t, 1H, J = 8.0 Hz, ArH), 7.39 (d, 1H, J = 8.0 Hz, ArH), 7.47–7.55 (m, 2H, ArH), 7.79 (s, 1H, CH), 7.88–7.91 (m, 2H, phthalimide), 7.93–7.95 (m, 2H, phthalimide), 12.47 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.5, 68.2, 115.5, 119.5, 123.9, 126.3, 126.5, 127.1, 129.0, 131.5, 135.3, 144.8, 153.1, 166.7, 167.1, 168.2; HRMS (ESI) calcd for [M – H]– C19H11N2O5S: 379.0394, found: 379.0403.
(Z)-5-(4-((1,3-Dioxoisoindolin-2-yl)methoxy)benzylidene)thiazolidine-2,4-dione (5e)
Light yellow solid; yield 68.7%; m. p. 240–242 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.66 (s, 2H, OCH2N), 7.26 (d, 2H, J = 8.8 Hz, ArH), 7.58 (d, 2H, J = 8.8 Hz, ArH), 7.76 (s, 1H, CH), 7.91–7.93 (m, 2H, phthalimide), 7.97–7.99 (m, 2H, phthalimide), 12.54 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 65.7, 116.9, 121.9, 124.2, 127.4, 131.7, 131.8, 132.4, 135.6, 153.8, 167.4, 168.0, 168.4; HRMS (ESI) calcd for [M – H]– C19H11N2O5S: 379.0394, found: 379.0405.
(Z)-5-(2-((1,3-Dioxoisoindolin-2-yl)methoxy)-3-methoxybenzylidene)thiazolidine-2,4-dione (5f)
Light yellow solid; yield 85.3%; m. p. 230–232 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.87 (s, 3H, OCH3), 5.49 (s, 2H, OCH2N), 7.83–7.85 (m, 1H, ArH), 7.23–7.25 (m, 2H, ArH), 7.59 (s, 1H, CH), 7.78–7.83 (m, 4H, phthalimide), 12.26 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.5, 68.2, 115.5, 119.5, 123.9, 126.3, 126.5, 127.1, 129.0, 131.5, 135.3, 144.8, 153.1, 166.7, 167.1, 168.2; HRMS (ESI) calcd for [M – H]– C20H13N2O6S: 409.0500, found: 409.0511.
(Z)-5-(4-((1,3-Dioxoisoindolin-2-yl)methoxy)-3,5-dimethoxybenzylidene)thiazolidine-2,4-dione (5g)
Yellow solid; yield 72.7%; m. p. 231–233 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.58 (s, 6H, OCH3), 5.44 (s, 2H, OCH2N), 6.82 (s, 2H, ArH), 7.72 (s, 1H, CH), 7.88–7.94 (m, 4H, phthalimide), 12.60 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.4, 68.0, 107.6, 123.5, 123.9, 130.4, 131.7, 132.3, 135.4, 136.4, 154.0, 167.4, 167.6, 168.2; HRMS (ESI) calcd for [M – H]– C21H15N2O7S: 439.0605, found: 439.0610.
(Z)-5-(4-((1,3-Dioxoisoindolin-2-yl)methoxy)-3-nitrobenzylidene)thiazolidine-2,4-dione (5h)
Yellow solid; yield 80.6%; m. p. 227–229 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.82 (s, 2H, OCH2N), 7.82 (s, 1H, CH), 7.85 (d, 1H, J = 8.8 Hz, ArH), 7.89 (d, 1H, J = 2.0 Hz, ArH), 7.91–7.95 (m, 2H, phthalimide), 7.96–8.00 (m, 2H, phthalimide), 8.12 (d, 1H, J = 2.0 Hz, ArH), 12.70 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.5, 119.2, 124.3, 125.1, 126.8, 128.1, 129.6, 131.6, 135.2, 135.7, 141.3, 150.3, 167.1, 167.6, 167.8; HRMS (ESI) calcd for [M – H]– C19H10N3O7S: 424.0245, found: 424.0256.
(Z)-5-(5-Chloro-2-((1,3-dioxoisoindolin-2-yl)methoxy)benzylidene)thiazolidine-2,4-dione (5i)
Yellow solid; yield 72.9%; m. p. 237–239 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.67 (s, 2H, OCH2N), 7.34 (d, 1H, J = 2.4 Hz, ArH), 7.53 (d, 1H, J = 8.8 Hz, ArH), 7.58 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 7.67 (s, 1H, CH), 7.88–7.90 (m, 2H, phthalimide), 7.91–7.94 (m, 2H, phthalimide), 12.55 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.6, 119.2, 124.2, 125.3, 126.3, 126.9, 127.5, 128.1, 131.6, 132.0, 135.6, 154.5, 167.1, 167.2, 167.8; HRMS (ESI) calcd for [M – H]– C19H10ClN2O5S: 413.0004, found: 413.0010.
(Z)-5-(2-((1,3-Dioxoisoindolin-2-yl)methoxy)-5-nitrobenzylidene)thiazolidine-2,4-dione (5j)
Yellow solid; yield 64.1%; m. p. 253–254 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.86 (s, 2H, OCH2N), 7.73 (d, 1H, J = 8.8 Hz, ArH), 7.75 (s, 1H, CH), 7.91–7.93 (m, 2H, phthalimide), 7.97–7.99 (m, 2H, phthalimide), 8.23 (d, 1H, J = 2.4 Hz, ArH), 8.38 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 12.62 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 66.9, 115.7, 123.8, 124.1, 124.3, 124.3, 127.6, 128.0, 131.6, 135.7, 142.3, 160.0, 167.2, 167.3, 167.6; HRMS (ESI) calcd for [M – H]– C19H10N3O7S: 424.0245, found: 424.0253.
(Z)-5-(3-Chloro-4-((1,3-dioxoisoindolin-2-yl)methoxy)benzylidene)thiazolidine-2,4-dione (5k)
Light yellow solid; yield 68.2%; m. p. 238–239 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.73 (s, 2H, OCH2N), 7.58 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 7.62 (d, 1H, J = 8.8 Hz, ArH), 7.71 (d, 1H, J = 2.4 Hz, ArH), 7.75 (s, 1H, CH), 7.92–7.94 (m, 2H, phthalimide), 7.98–8.00 (m, 2H, phthalimide), 12.63 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.0, 117.6, 123.7, 123.9, 124.3, 128.9, 130.2, 130.5, 131.6, 132.3, 135.7, 153.7, 167.2, 167.6, 168.0; HRMS (ESI) calcd for [M – H]– C19H10ClN2O5S: 413.0004, found: 413.0001.
(Z)-2-((2-Methoxy-4-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6a)
Yellow solid; yield 75.4%; m. p. 243–246 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.77 (s, 3H, OCH3), 5.61 (s, 2H, OCH2N), 7.16 (dd, 1H, J = 8.4 Hz, 2.0 Hz, ArH), 7.22 (d, 1H, J = 2.0 Hz, ArH), 7.34 (d, 1H, J = 8.4 Hz, ArH), 7.62 (s, 1H, CH), 7.91–7.93 (m, 2H, phthalimide), 7.96–7.98 (m, 2H, phthalimide), 13.81 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.2, 66.8, 114.8, 117.7, 124.2, 124.3, 128.5, 131.7, 132.2, 135.6, 148.2, 150.7, 167.3, 169.8, 195.9; HRMS (ESI) calcd for [M – H]– C20H13N2O5S2: 425.0271, found: 425.0275.
(Z)-2-((2-Ethoxy-4-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6b)
Yellow solid; yield 83.6%; m. p. 264–267 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 1.25 (t, 3H, J = 6.8 Hz, OCH2C[combining low line]H[combining low line]3[combining low line]), 4.00 (q, 2H, J = 6.8 Hz, OC[combining low line]H[combining low line]2[combining low line]CH3), 5.61 (s, 2H, OCH2N), 7.14 (dd, 1H, J = 8.4 Hz, 2.0 Hz, ArH), 7.20 (d, 1H, J = 2.0 Hz, ArH), 7.32 (d, 1H, J = 8.4 Hz, ArH), 7.61 (s, 1H, CH), 7.90–7.93 (m, 2H, phthalimide), 7.94–7.97 (m, 2H, phthalimide), 13.81 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 14.8, 64.6, 67.2, 115.9, 119.1, 124.2, 124.3, 128.9, 131.7, 132.2, 135.6, 148.4, 150.3, 167.3, 169.8, 195.9; HRMS (ESI) calcd for [M – H]– C21H15N2O5S2: 439.0428, found: 439.0439.
(Z)-2-((3-((4-Oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6c)
Yellow solid; yield 65.9%; m. p. 258–259 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.64 (s, 2H, OCH2N), 7.24–7.28 (m, 2H, ArH), 7.33 (s, 1H, ArH), 7.50 (t, 1H, J = 8.0 Hz, ArH), 7.61 (s, 1H, CH), 7.90–7.93 (m, 2H, phthalimide), 7.95–7.99 (m, 2H, phthalimide), 13.88 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 66.1, 118.4, 118.9, 124.2, 126.8, 131.2, 131.6, 131.7, 135.0, 135.6, 157.3, 167.4, 169.7, 196.1; HRMS (ESI) calcd for [M – H]– C19H11N2O4S2: 395.0166, found: 395.0177.
(Z)-2-((2-((4-Oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6d)
Yellow solid; yield 74.5%; m. p. 248–250 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.69 (s, 2H, OCH2N), 7.22 (t, 1H, J = 7.2 Hz, ArH), 7.36–7.40 (m, 1H, ArH), 7.48 (d, 1H, J = 7.2 Hz, ArH), 7.55 (dt, 1H, J = 7.2 Hz, 1.6 Hz), 7.63 (s, 1H, CH), 7.88–7.91 (m, 2H, phthalimide), 7.93–7.95 (m, 2H, phthalimide), 13.69 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.2, 116.9, 123.8, 124.1, 124.2, 126.6, 126.9, 129.8, 131.6, 133.2, 135.6, 155.9, 167.3, 169.4, 196.3; HRMS (ESI) calcd for [M – H]– C19H11N2O4S2: 395.0166, found: 395.0177.
(Z)-2-((4-((4-Oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6e)
Yellow solid; yield 66.1%; m. p. 251–254 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.67 (s, 2H, OCH2N), 7.27 (d, 2H, J = 8.8 Hz, ArH), 7.59 (d, 2H, J = 8.8 Hz, ArH), 7.63 (s, 1H, CH), 7.91–7.94 (m, 2H, phthalimide), 7.96–7.99 (m, 2H, phthalimide), 13.78 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 65.7, 117.0, 123.7, 124.2, 127.3, 131.7, 131.9, 133.0, 135.6, 158.6, 167.3, 169.9, 196.0; HRMS (ESI) calcd for [M – H]– C19H11N2O4S2: 395.0166, found: 395.0175.
(Z)-2-((2-Methoxy-6-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6f)
Yellow solid; yield 77.4%; m. p. 233–235 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.88 (s, 3H, OCH3), 5.50 (s, 2H, OCH2N), 6.80–6.82 (m, 1H, ArH), 7.25–7.26 (m, 2H, ArH), 7.42 (s, 1H, CH), 7.78–7.79 (m, 4H, phthalimide), 13.48 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.6, 68.2, 115.9, 119.8, 123.8, 126.7, 127.1, 128.2, 129.0, 131.3, 135.4, 144.9, 153.2, 167.1, 168.5, 196.3; HRMS (ESI) calcd for [M – H]– C20H13N2O5S2: 425.0271, found: 425.0281.
(Z)-2-((2,6-Dimethoxy-4-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6g)
Yellow solid; yield 80.4%; m. p. 241–243 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 3.59 (s, 6H, OCH3), 5.45 (s, 2H, OCH2N), 6.82 (s, 2H, ArH), 7.58 (s, 1H, CH), 7.89–7.94 (m, 4H, phthalimide), 13.85 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 56.4, 68.1, 108.0, 124.0, 125.5, 130.3, 131.7, 132.2, 135.4, 136.8, 154.1, 167.4, 169.7, 195.9; HRMS (ESI) calcd for [M – H]– C21H15N2O6S2: 455.0377, found: 455.0386.
(Z)-2-((2-Nitro-4-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6h)
Yellow solid; yield 58.1%; m. p. 237–239 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.83 (s, 2H, OCH2N), 7.67 (s, 1H, CH), 7.85 (d, 1H, J = 8.8 Hz, ArH), 7.89 (d, 1H, J = 2.4 Hz, ArH), 7.91–7.94 (m, 2H, phthalimide), 7.98–8.00 (m, 2H, phthalimide), 8.12 (d, 1H, J = 2.4 Hz, ArH), 13.89 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.5, 119.3, 124.3, 127.2, 128.0, 129.3, 131.6, 135.7, 135.7, 141.4, 150.5, 167.1, 169.8, 190.9, 195.7; HRMS (ESI) calcd for [M – H]– C19H10N3O6S2: 440.0017, found: 440.0006.
(Z)-2-((4-Chloro-2-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6i)
Yellow solid; yield 62.9%; m. p. 243–245 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.68 (s, 2H, OCH2N), 7.35 (d, 1H, J = 2.4 Hz, ArH), 7.50 (s, 1H, CH), 7.53 (d, 1H, J = 8.8 Hz, ArH), 7.59 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 7.87–7.91 (m, 2H, phthalimide), 7.91–7.94 (m, 2H, phthalimide), 13.74 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 67.5, 119.1, 124.2, 125.2, 126.1, 127.6, 128.9, 129.0, 131.5, 132.4, 135.6, 154.6, 167.2, 169.3, 195.9; HRMS (ESI) calcd for [M – H]– C19H10ClN2O4S2: 428.9776, found: 428.9788.
(Z)-2-((4-Nitro-2-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6j)
Yellow solid; yield 53.5%; m. p. 250–251 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.88 (s, 2H, OCH2N), 7.60 (s, 1H, CH), 7.73 (d, 1H, J = 8.8 Hz, ArH), 7.91–7.95 (m, 2H, phthalimide), 7.95–7.99 (m, 2H, phthalimide), 8.22 (d, 1H, J = 2.4 Hz, ArH), 8.39 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 13.86 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 66.9, 115.8, 123.9, 124.2, 1243, 125.2, 127.9, 129.8, 131.6, 135.7, 142.4, 159.9, 167.2, 169.4, 195.5; HRMS (ESI) calcd for [M – H]– C19H10N3O6S2: 440.0017, found: 440.0027.
(Z)-2-((2-Chloro-4-((4-oxo-2-thioxothiazolidin-5-ylidene)methyl)phenoxy)methyl)isoindoline-1,3-dione (6k)
Yellow solid; yield 83.4%; m. p. 248–251 °C; 1H NMR (d6-DMSO, 400 MHz) δ: 5.74 (s, 2H, OCH2N), 7.56 (dd, 1H, J = 8.8 Hz, 2.4 Hz, ArH), 7.61 (s, 1H, CH),7.64 (d, 1H, J = 8.8 Hz, ArH), 7.73 (d, 1H, J = 2.4 Hz, ArH), 7.92–7.95 (m, 2H, phthalimide), 7.97–8.00 (m, 2H, phthalimide), 13.86 (s, 1H, NH); 13C NMR (d6-DMSO, 100 MHz) δ: 66.9, 117.6, 124.0, 124.3, 128.8, 130.2, 130.7, 131.6, 132.8, 135.7, 153.9, 167.2, 170.0, 195.8; HRMS (ESI) calcd for [M – H]– C19H10ClN2O4S2: 428.9776, found: 428.9769.
In vitro assay of α-glucosidase inhibitory activity
α-Glucosidase inhibitory activity was assayed by using 0.1 M phosphate buffer (pH 6.8) at 37 °C. The enzyme (0.1 U/mL) in phosphate buffer saline was incubated with various concentrations of the test compounds at 37 °C for 15 min. Then 1.25 mM p-nitrophenyl α-d-glucopyranoside was added to the mixture as a substrate. After further incubation at 37 °C for 30 min, the absorbance was measured spectrophotometrically at 405 nm. The sample solution was replaced by DMSO as a control. Acarbose was used as a positive control. All experiments were carried out in triplicate. The % inhibition has been obtained using the formula: Inhibition (%) = (1 – ΔAsample/ΔAcontrol) × 100%. The IC50 value is defined as the concentration of sample inhibiting 50% of α-glucosidase activity under the stated assay conditions.
Molecular docking
Molecular docking studies were performed to investigate the binding mode between the compounds 5k, 6c, 6e and 6k and α-glucosidase using AutoDock Vina 1.1.2. The 3D structures of 5k 6c, 6e and 6k were obtained using ChemBioDraw Ultra 14.0 and ChemBio3D Ultra 14.0 software programs. The AutoDockTools 1.5.6 package was employed to generate the docking input files. The search grid of α-glucosidase was identified as center_x: –19.676, center_y: –7.243, and center_z: –21.469 with dimensions size_x: 15, size_y: 15, and size_z: 15. The value of exhaustiveness was set to 20. For Vina docking, the default parameters were used if not mentioned. The best-scoring poses as judged by the Vina docking score were chosen and visually analyzed using PyMOL 1.7.6 software (; http://www.pymol.org/).
Conclusions
In conclusion, a series of novel thiazolidine-2,4-dione or rhodanine derivatives (5a–5k, 6a–6k) were synthesized and evaluated for their α-glucosidase inhibitory activity. Compound 6k (IC50 = 5.44 ± 0.13 μM), containing chloro and rhodanine groups at the 2- and 4-positions of the phenyl ring respectively, was found to be the most active compound that inhibits α-glucosidase activity, when compared to the standard drug acarbose (IC50 = 817.38 ± 6.27 μM). The binding interaction of these active compounds with α-glucosidase was confirmed through molecular docking.
Conflict of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81660574).
Footnotes
†Electronic supplementary information (ESI) available: NMR spectra of all target compounds 5a–5k, 6a–6k. See DOI: 10.1039/c7md00173h
References
- Dinparast L., Valizadeh H., Bahadori M. B., Soltani S., Asghari B., Rashidi M.-R. J. Mol. Struct. 2016;1114:84–94. [Google Scholar]
- DeFronzo R. A. Med. Clin. North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Lopez-Candales A. J. Med. 2001;32:283–300. [PubMed] [Google Scholar]
- Deshpande A. D., Harris-Hayes M., Schootman M. Phys. Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. A., Gulve E. A., Wang M. H. Chem. Rev. 2004;104:1255–1282. doi: 10.1021/cr0204653. [DOI] [PubMed] [Google Scholar]
- Joshi S. R., Standl E., Tong N., Shah P., Kalra S., Rathod R. Expert Opin. Pharmacother. 2015;16:1959–1981. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- Pili R., Chang J., Partis R. A., Mueller R. A., Chrest F. J., Passaniti A. Cancer Res. 1995;55:2920–2926. [PubMed] [Google Scholar]
- Mehta A., Zitzmann N., Rudd P. M., Block T. M., Dwek R. A. FEBS Lett. 1998;430:17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- Rawlings A. J., Lomas H., Pilling A. W., Lee M. J. R., Alonzi D. S., Rountree J. S. S., Jenkinson S. F., Fleet G. W. J., Dwek R. A., Jones J. H., Butters T. D. ChemBioChem. 2009;10:1101–1105. doi: 10.1002/cbic.200900025. [DOI] [PubMed] [Google Scholar]
- Zitzmann N., Mehta A. S., Carrouée S., Butters T. D., Platt F. M., McCauley J., Blumberg B. S., Dwek R. A., Block T. M. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Figueroa S., Ramirez-Espinosa J. J., Estrada-Soto S., Almanza-Perez J. C., Roman-Ramos R., Alarcon-Aguilar F. J., Hernandez-Rosado J. V., Moreno-Diaz H., Diaz-Coutino D., Navarrete-Vazquez G. Chem. Biol. Drug Des. 2013;81:474–483. doi: 10.1111/cbdd.12102. [DOI] [PubMed] [Google Scholar]
- Kruk I., Bozdag-Dundar O., Ceylan-Unlusoy M., Ertan R., Aboul-Enein H. Y., Michalska T. Luminescence. 2009;24:230–235. doi: 10.1002/bio.1105. [DOI] [PubMed] [Google Scholar]
- Li Q. B., Wu J. D., Zheng H., Liu K., Guo T. L., Liu Y. Y., Eblen S. T., Grant S., Zhang S. J. Bioorg. Med. Chem. Lett. 2010;20:4526–4530. doi: 10.1016/j.bmcl.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Wang G., Deng C., Xie C., Ma L., Yang J., Qiu N., Xu Q., Chen T., Peng F., Chen J., Qiu J., Peng A., Wei Y., Chen L. Eur. J. Med. Chem. 2011;46:5941–5948. doi: 10.1016/j.ejmech.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Camp H. S. Curr. Opin. Investig. Drugs. 2003;4:406–411. [PubMed] [Google Scholar]
- Chinthala Y., Kumar Domatti A., Sarfaraz A., Singh S. P., Kumar Arigari N., Gupta N., Satya S. K. V. N., Kotesh Kumar J., Khan F., Tiwari A. K., Paramjit G. Eur. J. Med. Chem. 2013;70:308–314. doi: 10.1016/j.ejmech.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Tomasic T., Masic L. P. Expert Opin. Drug Discovery. 2012;7:549–560. doi: 10.1517/17460441.2012.688743. [DOI] [PubMed] [Google Scholar]
- Chauhan K., Sharma M., Saxena J., Singh S. V., Trivedi P., Srivastava K., Puri S. K., Saxena J. K., Chaturvedi V., Chauhan P. M. S. Eur. J. Med. Chem. 2013;62:693–704. doi: 10.1016/j.ejmech.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Ungoren S. H., Albayrak S., Gunay A., Yurtseven L., Yurttas N. Tetrahedron. 2015;71:4312–4323. [Google Scholar]
- Guo M., Zheng C. J., Song M. X., Wu Y., Sun L. P., Li Y. J., Liu Y., Piao H. R. Bioorg. Med. Chem. Lett. 2013;23:4358–4361. doi: 10.1016/j.bmcl.2013.05.082. [DOI] [PubMed] [Google Scholar]
- Fu H. S., Hou X. B., Wang L., Dun Y. Y., Yang X. Y., Fang H. Bioorg. Med. Chem. Lett. 2015;25:5265–5269. doi: 10.1016/j.bmcl.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Murugan R., Anbazhagan S., Narayanan S. S. Eur. J. Med. Chem. 2009;44:3272–3279. doi: 10.1016/j.ejmech.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Ramirez M. A., Borja N. L. Pharmacotherapy. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- Zahran M. A. H., Abdin Y. G., Osman A. M. A., Gamal-Eldeen A. M., Talaat R. M., Pedersen E. B. Arch. Pharm. 2014;347:642–649. doi: 10.1002/ardp.201400073. [DOI] [PubMed] [Google Scholar]
- Magalhaes U. D. O., Souza A. M. T. D., Albuquerque M. G., Brito M. A. D., Bello M. L., Cabral L. M., Rodrigues C. R. Drug Des., Dev. Ther. 2013;7:953–961. doi: 10.2147/DDDT.S47057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamie P. F., Phillopes J. N., El-Gendy A. O., Rarova L., Gruz J. Molecules. 2015;20:16620–16642. doi: 10.3390/molecules200916620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A. M., El-Azab A. S., Al-Suwaidan I. A., ElTahir K. E. H., Asiri Y. A., Abdel-Aziz N. I., Abdel-Aziz A. A. M. Eur. J. Med. Chem. 2015;92:115–123. doi: 10.1016/j.ejmech.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Bian X., Wang Q., Ke C., Zhao G., Li Y. Bioorg. Med. Chem. Lett. 2013;23:2022–2026. doi: 10.1016/j.bmcl.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Singh J., Raju R., Maiti B. C., Maity T. K. Asian J. Chem. 2011;23:1581–1584. [Google Scholar]
- Pascale R., Carocci A., Catalano A., Lentini G., Spagnoletta A., Cavalluzzi M. M., De Santis F., De Palma A., Scalera V., Franchini C. Bioorg. Med. Chem. 2010;18:5903–5914. doi: 10.1016/j.bmc.2010.06.088. [DOI] [PubMed] [Google Scholar]
- Pluempanupat W., Adisakwattana S., Yibchok-Anun S., Chavasiri W. Arch. Pharmacal Res. 2007;30:1501–1506. doi: 10.1007/BF02977317. [DOI] [PubMed] [Google Scholar]
- Wang G., Wang J., He D., Li X., Li J., Peng Z. Bioorg. Med. Chem. Lett. 2016;26:2806–2809. doi: 10.1016/j.bmcl.2016.04.071. [DOI] [PubMed] [Google Scholar]
- Wang G., Wang J., He D., Li X., Li J., Peng Z. Heterocycles. 2016;92:1430–1439. [Google Scholar]
- Wang G., Peng Z., Wang J., Li J., Li X. Bioorg. Med. Chem. Lett. 2016;26:5719–5723. doi: 10.1016/j.bmcl.2016.10.057. [DOI] [PubMed] [Google Scholar]
- Wang G., Peng Z., Wang J., Li J., Li X. Bioorg. Med. Chem. 2016;24:5374–5379. doi: 10.1016/j.bmc.2016.08.061. [DOI] [PubMed] [Google Scholar]
- Wang G., Peng Z., Wang J., Li X., Li J. Eur. J. Med. Chem. 2017;125:423–429. doi: 10.1016/j.ejmech.2016.09.067. [DOI] [PubMed] [Google Scholar]
- Sing W. T., Lee C. L., Yeo S. L., Lim S. P., Sim M. M. Bioorg. Med. Chem. Lett. 2001;11:91–94. doi: 10.1016/s0960-894x(00)00610-7. [DOI] [PubMed] [Google Scholar]
- Fresneau P., Cussac M., Morand J. M., Szymonski B., Tranqui D., Leclerc G. J. Med. Chem. 1998;41:4706–4715. doi: 10.1021/jm9801399. [DOI] [PubMed] [Google Scholar]
- Rahim F., Malik F., Ullah H., Wadood A., Khan F., Javid M. T., Taha M., Rehman W., Rehman A. U., Khan K. M. Bioorg. Chem. 2015;60:42–48. doi: 10.1016/j.bioorg.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Niaz H., Kashtoh H., Khan J. A. J., Khan A., Atia tul W., Alam M. T., Khan K. M., Perveen S., Choudhary M. I. Eur. J. Med. Chem. 2015;95:199–209. doi: 10.1016/j.ejmech.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Miyake H., Kusunoki M., Osaki S. FEBS J. 2010;277:4205–4214. doi: 10.1111/j.1742-4658.2010.07810.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








