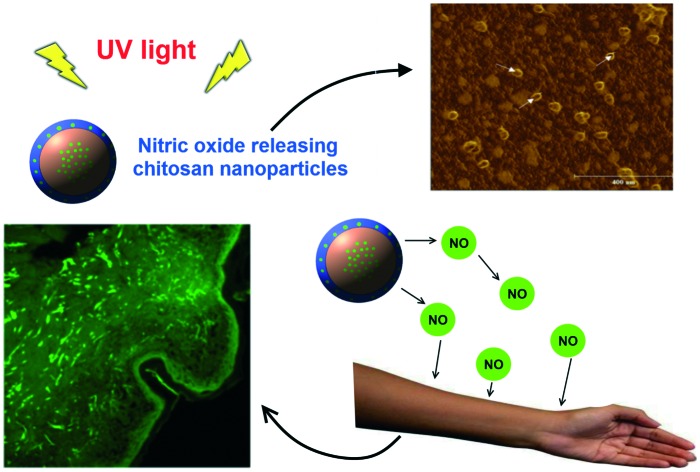
 The use of nanoparticle-based transdermal delivery systems is a promising approach to efficiently carry and deliver therapeutic agents for dermal and systemic administration.
The use of nanoparticle-based transdermal delivery systems is a promising approach to efficiently carry and deliver therapeutic agents for dermal and systemic administration.
Abstract
The use of nanoparticle-based transdermal delivery systems is a promising approach to efficiently carry and deliver therapeutic agents for dermal and systemic administration. Nitric oxide (NO) is a key molecule that plays important roles in human skin such as the control of skin homeostasis, skin defense, control of dermal blood flow, and wound healing. In addition, human skin contains stores of NO derivatives that can be mobilized and release free NO upon UV irradiation with beneficial cardiovascular effects, for instance the control of blood pressure. In this work, the NO donor precursor glutathione (GSH) was encapsulated (encapsulation efficiency of 99.60%) into ultra-small chitosan nanoparticles (CS NPs) (hydrodynamic size of 30.65 ± 11.90 nm). GSH-CS NPs have a core-shell structure, as revealed by atomic force microscopy and X-ray photoelectron spectroscopy, in which GSH is protected in the nanoparticle core. Nitrosation of GSH by nitrous acid led to the formation of the NO donor S-nitrosogluthathione (GSNO) into CS NPs. The GSNO release from the CS NPs followed a Fickian diffusion described by the Higuchi mathematical model. Topical application of GSNO-CS NPs in intact human skin significantly increased the levels of NO and its derivatives in the epidermis, as assayed by confocal microscopy, and this effect was further enhanced by skin irradiation with UV light. Therefore, NO-releasing CS NPs are suitable materials for transdermal NO delivery to local and/or systemic therapies.
Introduction
The free radical nitric oxide (NO) is an important signalling molecule involved in several physiologic and pathophysiologic processes in mammals including the dilation of blood vessels, the immune response, antioxidant and anticancer activities, cell communication, and the inhibition of platelet adhesion and aggregation, among others.1–6 NO is an important molecule synthesized by several skin cells, and due to its chemical nature (small size, lack of charge and lipophilicity) it easily diffuses among cells and tissues playing important roles in the bioregulation of several processes in human skin, such as the promotion of wound healing, the control of dermal blood flow, erythema formation, skin defense and pigmentation.7,8 NO is endogenously synthesized by a family of three nitric oxide synthase (NOS) enzymes: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS).9–11 Both eNOS and nNOS express constitutive NO for homeostatic activities, such as the control of blood flow, skin pigmentation and the delay of wrinkle onset.12,13 In contrast, iNOS produces higher amounts of NO in response to cell-specific stimuli.6 The three NOS isoforms are expressed in human skin cells.
Topical applications of NO-releasing biomaterials are able to increase dermal blood flow in human healthy volunteers14,15 and diabetic rats16 to promote and accelerate tissue repair of acute,17,18 and ischaemic wounds,19 to combat cutaneous infections caused by bacteria,20 protozoa,21 and fungi,22 and to have toxic effects against skin cancer.23 In addition, several papers reported that the human skin contains stores of NO derivatives, present as oxidized forms of NO, predominantly nitrate (NO3–), nitrite (NO2–), and S-nitrosothiols (RSNOs), which can be mobilized to release free NO to the systemic circulation upon UV irradiation producing beneficial effects on cardiovascular health by controlling blood pressure and obesity.24–29 In addition, Mowbray et al. demonstrated that skin exposure of healthy subjects to physiological quantities of UVA irradiation increased arterial vasodilation and decreased blood pressure.30 Although sunlight exposure is a risk factor for skin cancer, it also increases NO bioavailability leading to cardiovascular benefits.
Due to the importance of NO in biological systems, there is increasing interest in developing vehicles able to carry and to deliver therapeutic amounts of NO in human skin, where NO may have local and/or systemic effects.31–33 In this work, the low molecular weight NO donor S-nitrosoglutathione (GSNO) was incorporated into chitosan nanoparticles (CS NPs) for topical NO delivery in human skin in order to enhance the cutaneous NO derivative stores. Chitosan is a biopolymer obtained through the deacetylation of chitin and employed for several biomedical applications including gene and drug delivery and treatment of wound infections, due to its unique properties such as biocompatibility, biodegradability, antimicrobial, antioxidant, and mucoadhesion characters.34–37 Recently, engineered nanoparticles have been successfully applied in topical delivery systems.38–40 In particular, ultra-small polymeric nanoparticles (smaller than 40 nm) show superior penetration into skin strata, carrying and delivering active molecules to the desired site of application.41
In this context, this work describes the synthesis and characterization of ultra-small GSNO-containing CS NPs and the kinetic profiles and mechanisms of GSNO release from the nanoparticles under physiological conditions. Moreover, GSNO–CS NPs were topically applied on ex vivo human skin sections and the intracutaneous distribution and enrichment of NO through the epidermis was quantified. To the best of our knowledge, this is the first work to report on the effects of UV irradiation on skin sections pre-treated with NO-releasing nanoparticles. GSNO–CS NPs have chemical, structural, and morphological properties suitable for efficient transdermal delivery of NO in human skin. The results highlight the potential therapeutic effects of combined administration of GSNO–CS NPs and UV irradiation to efficiently load NO in human skin for local and/or systemic (cardiovascular) applications.
Results and discussion
Synthesis and characterization of GSH–CS NPs
The combination of low molecular weight NO donors, such as GSNO, with nanoparticles has been extensively explored to allow the delivery of the unstable NO molecules directly to the therapeutic site in a sustained and safe manner.31–33 Biocompatible and biodegradable polymeric nanoparticles are promising platforms for drug delivery.33 The polysaccharide chitosan is widely used in topical drug delivery systems due to its mucoadhesion, antioxidant and antimicrobial activities.34,36 In the work we describe here, CS NPs were obtained based on the cross-linking between positively charged chitosan chains and the negatively charged polyanion TPP.42 The tripeptide GSH, precursor of the NO donor GSNO, was encapsulated into CS NPs via electrostatic interactions. DLS measurements revealed that the hydrodynamic size, polydispersity index (PDI), and zeta potential of GSH-CS NPs were 30.65 ± 11.90 nm, 0.258 ± 0.008, and +26.1 ± 0.5 mV, respectively. These results confirm the formation of GSH–CS NPs in the nanoscale with a monodal size distribution due to the electrostatic interactions between chitosan, TPP and GSH. Fig. S1 in the ESI‡ shows a representative size distribution curve of the GSH–CS NPs obtained by DLS measurements, and the corresponding autocorrelation curve, showing a monodal size distribution. The obtained PDI value suggested a minor particle polydispersity. The positive zeta potential value is attributed to the positive charge excess of protonated amino and thiol groups from GSH and amino groups from chitosan at low pH (2.75), which is in agreement with the charge balance of the system. It should be noted that positively charged nanoparticle surfaces might interact better with biomolecules/biological targets with negative charges, such as DNA or tumor surfaces, highlighting the importance of CS NPs in the biological medium.43
The chemical surface composition of GSH–CS NPS was acquired by XPS and then compared to the total composition of the system. The elements carbon, oxygen, nitrogen, sulfur and phosphorus are all present at the nanoparticles surface, as shown in Table S1 in the ESI.‡ However, the molar ratio of GSH to chitosan, calculated from the values shown in Table S1,‡ varies considerably, being 17.5 : 1 for the total composition and 2.8 : 1 for the nanoparticle surface. These results indicate that the outer surface layer of GSH-CS NPs is richer in chitosan and depleted in GSH, compared to the core.
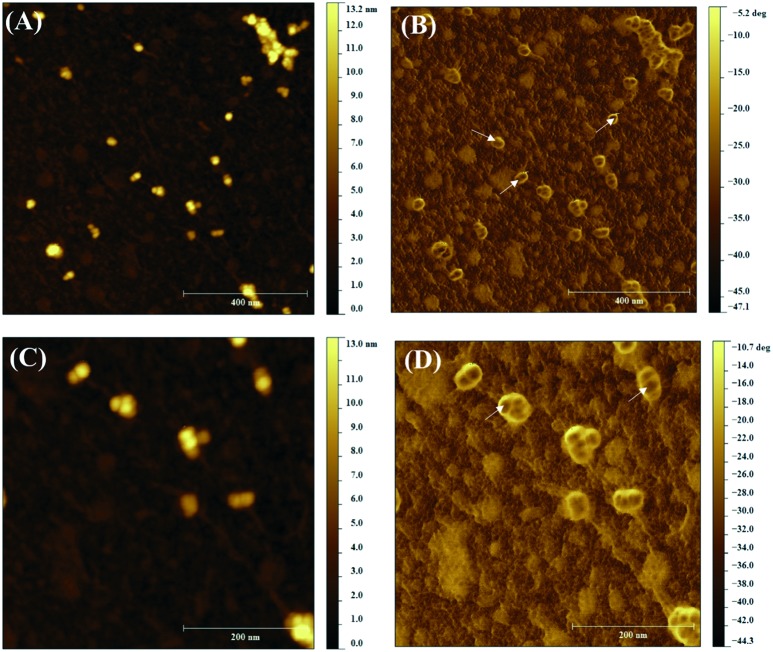
AFM was used to further understand the surface structure and the average size of GSH–CS NPs at the solid state. Topography and phase contrast images from different areas were simultaneously acquired and some representative micrographs are displayed in Fig. 1.
Fig. 1. AFM topographic (A, C) and phase contrast (B, D) images of GSH–CS NPs (GSH concentration of 100 mmol L–1).
As can be observed in Fig. 1A and C, the nanoparticles are approximately uniform spheres, with an average diameter of 21.7 ± 3.8 nm. These results are in agreement with the hydrodynamic size measured by DLS (30.65 ± 11.90 nm), since the DLS value is expected to be higher than the size obtained by AFM due to the hydration of the NP surface.44 For topical applications, the skin penetration of ultra-small nanoparticles, with size smaller than 40 nm, is enhanced into the skin strata via hair follicle stem cells, allowing a superior dermatological application.41
Another important aspect shown by AFM is that the inner part of the nanoparticles is darker than the shell as shown by the white arrows in the phase contrast image (Fig. 1B and D), reinforcing that the chemical compositions of the core and shell are distinct, as detected by XPS analysis. The encapsulation efficiency of GSH in CS NPs was found to be 99.60 ± 0.01%. Such a high value reveals the strong electrostatic interaction between GSH and the NP components, indicating the success of nanoparticle preparation.
All these observations can be used to propose a nanostructure model for GSH–CS NPS, as shown in Fig. S2 in the ESI:‡ they have a core–shell structure with GSH encapsulated (with a high encapsulation efficiency) within the CS NP core.
The encapsulation of GSH would protect the molecule from degradation while promoting a sustained release of the active drug. The core–shell structured nanoparticles are very suitable carrier systems for drug delivery and have advantages over simple nanoparticles, such as, increased dispersibility, biocompatibility and chemical stability.45 Important therapeutic agents, such as plasmid DNA,46 and acyclovir,47 were successfully incorporated into CS NPs. The incorporation of therapeutic molecules into CS NPs would increase drug thermal and photochemical stabilities, with enhanced drug permeation through the skin.41 CS NPs have been employed to deliver plasmid DNA and antisense oligonucleotides for topical skin deliver applications.41
GSNO release profiles in vitro from free and encapsulated GSNO
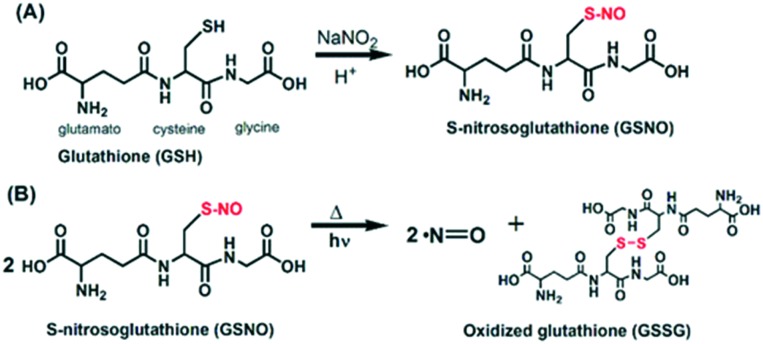
The thiol group of free or encapsulated GSH was nitrosated by reacting with an equimolar amount of NaNO2 in an acidified solution leading to the formation of free or GSNO–CS NPs. GSNO acts as a spontaneous NO donor due to the homolytic bond cleavage with free NO release and the formation of oxidized glutathione (GS–SG) (Fig. 2). This reaction occurs thermally and can be catalyzed by irradiation with UV or visible light.48,49
Fig. 2. Nitrosation of glutathione (GSH) by sodium nitrite (NaNO2) yielding S-nitrosoglutathione (GSNO) (A), which thermally or photochemically releases free NO (B).
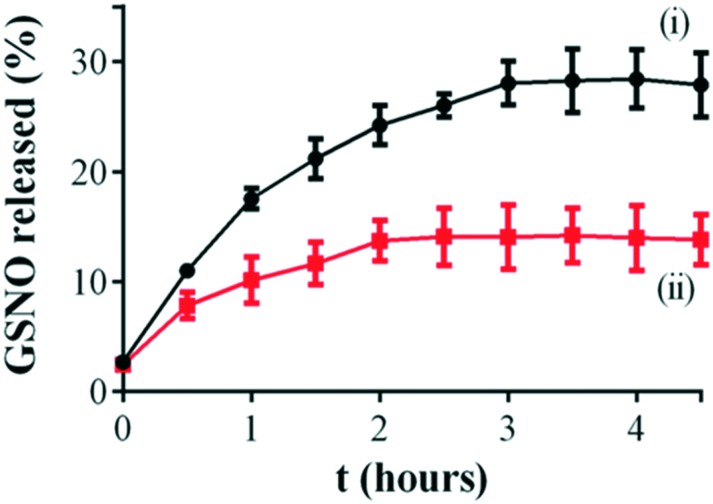
Once encapsulated in CS NPs, GSNO is expected to diffuse from the polymeric matrix to the exterior solution, where the intact GSNO releases free NO by S–N cleavage. Therefore, the release of an intact GSNO molecule from the CS NPs was monitored by using a Franz diffusion cell. Fig. 3 shows the kinetics of GSNO release from CS NPs, compared to the diffusion of free GSNO. As can be observed, the GSNO release profiles comprise two sequential phases. Firstly, GSNO is rapidly released from the donor compartment to the receptor compartment through the membrane, in the first 2.5 h of monitoring. Secondly, the rates of GSNO release from the donor compartment of the Franz diffusion cell to the receptor compartment significantly decreased, for both free and encapsulated GSNO, creating a steady state, which continues for at least 4.5 h. This profile is in accordance with kinetics studies for encapsulated GSNO in chitosan/alginate nanoparticles reported by Wu et al.50
Fig. 3. In vitro percentage of cumulative GSNO release from: (i) free GSNO, and (ii) GSNO–CS NPs, using a vertical Franz diffusion cell with a polysulfone membrane disc filter. Initial GSNO concentration of 10 mmol L–1. The error bars represent the mean of three independent experiments ± standard error.
In order to elucidate the main release mechanism of GSNO from CS NPs, the Higuchi mathematical model was applied on the kinetic curves of Fig. 3. Correlation coefficients (r2) of 0.991 and 0.995 for free GSNO and for GSNO–CS NPs, respectively, were obtained. This result indicates a Fickian diffusion of the GSNO (free or encapsulated into CS NPs), which corroborates with a previous study reported by our group.51
The release constants (KH) of GSNO were 15.650 ± 0.456 and 7.751 ± 1.228% h–0.5 for free GSNO for GSNO–CS NPs, respectively. It should be noted that the KH value of the free GSNO is 2-fold higher than the value obtained for GSNO encapsulated into CS NPs. Furthermore, the total GSNO released from CS NPs was found to be ca. 12% of the initial amount of encapsulated NO donor after 4 h 30 min of monitoring. In contrast, GSNO released through the membrane reached ca. 30% of the initial amount of GSNO after the same period of monitoring. These results indicate that the encapsulation of GSNO into CS NPs promoted a sustained GSNO diffusion.
NO release from GSNO–CS NPs is expected to occur following the processes: (i) intact GSNO is released from the nanoparticle core to the exterior medium, where GSNO would release free NO by its thermal or photochemical decomposition,52 and/or intact GSNO may react with cysteine residues of important proteins in trans-nitrosation reactions,53 outside of the nanoparticles; (ii) GSNO is decomposed within the polymeric nanoparticle yielding free NO, which diffuses through the polymeric layer of the nanoparticle to the exterior medium. In both cases, a sustained GSNO/NO release is obtained upon incorporation of the NO donor into CS NPs. In a similar manner, Duong et al. reported that the encapsulation of GSNO into polymeric nanoparticles significantly improved NO stability.54
Topical application of GSNO–CS NPs: NO loading in human skin
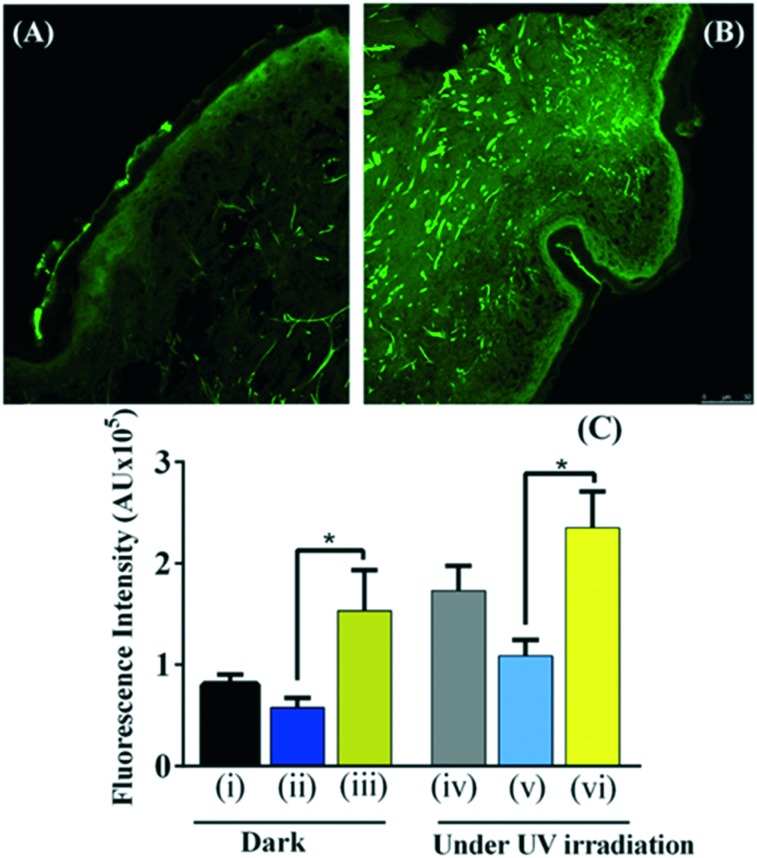
GSNO–CS NPs (GSNO concentration of 100 mmol L–1) were topically applied on ex vivo human skin sections to investigate NO permeation through the skin and the NO loading in the epidermis upon skin treatment with the nanoparticles, in the dark and under UV light irradiation. We selected to irradiate skin sections with UV light of 290 nm for 20 min since previous investigation from this group revealed that these are the optimal irradiation conditions for increasing NO pool in human skin (data not shown). Fig. 4A and B show the representative confocal florescence microscopy images of human skin sections: untreated (A) and treated with GSNO–CS NPs for 6 h, in the dark. All the skin sections were pre-incubated with the NO fluorochrome. Topical application of GSNO–CS NPs increased cutaneous fluorescence intensity (Fig. 4B) most markedly in the epidermis and stratum corneum, compared to untreated skin (control group) (Fig. 4A). Fig. 4C shows the fluorescence intensities of human skin sections: (i) untreated (control group); (ii) pre-treated with GSH–CS NPs, (iii) pre-treated with GSNO–CS NPs; in the dark or followed by UV irradiation (λ = 290 nm) for 20 min. It can be observed that topical application of GSNO–CS NPs significantly increased fluorescence intensities, in the dark and followed UV irradiation for 20 min, compared with the other groups. Indeed, dermal application of NO-releasing nanoparticles increased in ca. 2-fold the NO pool in comparison with topically applied GSH–CS NPs. Moreover, irradiation of skin sections with UV light increased the fluorescence intensities in all groups, compared to the non-irradiated skin sections (dark conditions). The combination of skin pre-treated with GSNO–CS NPs followed by UV irradiation led to the highest increase in the NO fluorescence signal intensity in the epidermis (Fig. 4C). In fact, a 2.3-fold increase in the fluorescence intensity was found for skin sections treated with NO-releasing nanoparticles, compared with GSH–CS NPs, for irradiated groups.
Fig. 4. NO permeation through ex vivo human skin sections upon treatment with GSNO–CS NPs (GSNO concentration of 100 mmol L–1) for 6 h, followed by 20 min of UV irradiation, compared with those in the dark. Confocal florescence microscopy images of human skin were acquired through incubation of the skin sections with the NO fluorochrome 4,5-diaminofluorescein diacetate (DAF-2DA) for 24 h prior to the addition of: GSNO–CS NPs, GSH–CS NPs, and no treatment (untreated skin sections). Representative images of human skin sections: (A) untreated skin in the dark; and (B) skin treated with GSNO–CS NPs in the dark. (C) Means of fluorescence intensities due to the presence of NO in the epidermis of skin sections (n = 6) of: (i) untreated skin in the dark; (ii) pre-treated skin with GSH–CS NPs in the dark; (iii) pre-treated skin with GSNO–CS NPs in the dark; (iv) untreated skin under UV irradiation for 20 min; (v) pre-treated skin with GSH–CS NPs followed by UV-irradiation for 20 min and (vi) pre-treated skin with GSNO–CS NPs followed by UV-irradiation for 20 min. UV irradiation with 290 nm. *P < 0.05 and AU, arbitrary units.
Taken together, these results demonstrated that topical application of GSNO–CS NPs in the dark and under UV light irradiation significantly increased NO pool in human skin. Both treatments led to the highest NO detection in the epidermis.
NO is a key signalling and modulator molecule in many tissues and organs, including the human skin. NO is continuously synthesized in the human skin by several cells.12,13 Important papers demonstrated that topical applications of NO promotes and accelerates wound healing,17–19 controls skin homeostasis,55 increases dermal blood flow,14,15 and has protective functions in skin defense against pathogens and cancer conditions.12,13 As a small lipophilic free radical, NO has a diffusion coefficient of ca. 3300 μm2 s–1 at 37 °C,56 which is comparable to similar small and neutral diatomic molecules.57 Therefore, NO is expected to have a diffusion distance of 500 μm in tissues, allowing its penetration to the dermis and epidermis, upon topical application.58 Following a cutaneous application, NO has the ability to rapidly penetrate the lipid-rich structure of the stratum corneum barrier due to its chemical nature.59 Hence, following topical application, NO might exert local effects such as the increase of dermal blood flow,14,15 regulation of apoptosis following UV radiation, wound healing and regulation of keratinocyte homeostasis.30
Nanoparticles have been extensively employed in several biomedical applications, including transdermal and dermal delivery of therapeutic agents and antimicrobial applications.40,60–62 The chemical composition, size, surface components, and charge of the nanoparticles dictate their performance in dermatological drug delivery systems.40,41 In this work, the NO delivery though human skin was performed by applying GSNO–CS NPs. Our results demonstrated that CS NPs have a small size and positive zeta potential, making them suitable for NO delivery through the skin. The positive charges of CS NPs facilitate the affinity with negatively charged phospholipids, altering the skin membrane permeability and thus reducing the skin barrier properties.63,64 Interestingly, the CS NPs synthesized in this work have a small size (less than 40 nm as assayed by DLS and AFM), which facilitates the nanoparticle penetration through human skin via trans-appendageal routes. Several studies demonstrated that nanoparticles smaller than 40 nm can reach hair follicles after topical application to animal and human skin.39,41,65,66
Penetration of nanoparticles through the skin may occur via lipid channels and/or the follicular route. Due to the particular properties of GSNO–CS NPs, the intact NO-releasing nanoparticles are able to penetrate into the superficial layers of the stratum corneum, and then release the encapsulated GSNO and/NO locally into the deeper skin layers. Alternatively, the nanoparticles may remain in the outer layers of the stratum corneum and epidermis, where GSNO and/or NO is released and permeated through the skin. In both manners, topical application of GSNO–CS NPs significantly increased the NO pool in the epidermis, suggesting that this material might find important applications for transdermal NO delivery therapies, including the treatment of disturbed dermal circulation, wound infections and skin ulceration.28,29
Fig. 4 shows that UV light irradiation increases NO levels in the skin, and a significant enhancement of cutaneous NO stores was achieved by the combination of skin pre-treated with GSNO–CS NPs followed by UV light irradiation. Previous studies demonstrated that the human skin contains large stores of NO derivatives (nitrite, nitrate, S-nitrosothiols, NO–heme species), which release free NO upon UV irradiation.24–29 Fig. 4 indicates that there are NO stores within the human skin that are able to release NO upon UV irradiation, and topical administration of NO-releasing nanoparticles has the ability to replenish the endogenous NO stores in human skin. Moreover, UV light irradiation efficiently catalyzes the decomposition of skin permeated GSNO in the skin layers leading to the formation of bioactive NO.28,29 It has been demonstrated that NO and its derivatives, generated in the skin under UV irradiation, have the ability to influence the cardiovascular system controlling several disorders such as hypertension, thrombosis, injury and atherosclerosis.59,67,68
UV light administration to healthy subjects decreased blood pressure, and thus the risk of stroke and coronary heart diseases.27 UV light induced beneficial cardiovascular effects that require cutaneous bioactive NO rather than NO in bloodstream.69 It should be noted that the deleterious effects of sunlight are well known, such as skin cancer and premature aging.70 Topical application of GSNO–CS NPs enhanced NO levels in the epidermis, which may have a protective effect against the negative effects of UV irradiation due to the increase of Bcl-2 expression and concomitant inhibition of UVA-induced overexpression of the Bax protein.28,29,71 It should be noted that depletion of skin NO stores may occur following prolonged UV irradiation with reduced clinical benefits. Topical application of NO-releasing CS NP might revert this depletion by efficiently loading NO to the skin.
Conclusions
This paper describes the synthesis and characterization of ultra-small GSNO–CS NPs and their topical application to intact human skin. The nanoparticles consist of a core–shell structure; this configuration allows the protection of the encapsulated molecule promoting a sustained release of the active drug. Topical application of GSNO–CS NPs on intact human skin significantly increased the levels of NO in the epidermis, indicating the transdermal efficacy of NO-releasing CS NPs. The combination of cutaneous administration of GSNO–CS NPs followed by skin UV irradiation further enhanced the NO and its derivatives loaded in human skin. To the best of our knowledge, this is the first report on the demonstration of the efficacy of NO-releasing CS NPs in cutaneous application.
Supplementary Material
Acknowledgments
This work was supported by FAPESP (Procs. 2015/00393-8, 2016/10347-6), the Brazilian Network on Nanotoxicology (Grant number: 552120/2011-1) (MCTI/CNPq), the Laboratory of Nanostructure Synthesis and Biosystem Interactions-NANOBIOSS (MCTI) (Grant number: 402280-2013), and Newton Advanced Fellowship (The Royal Society NA140046). The authors thank the Brazilian Nanotechnology National Laboratory/Center for Research in Energy and Materials (LNNano/CNPEM) for technical support during XPS and AFM analyses.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00502k
References
- Bonavida B., Garban H. Redox Biol. 2015;6:486–494. doi: 10.1016/j.redox.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl U., Fraccarollo D., Widder J. D., Micka J., Neuser J., Bauersachs J., Schäfer A. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0123621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Kim J., Lee Y. M., Im S., Park H., Kim W. J. J. Controlled Release. 2015;220:624–630. doi: 10.1016/j.jconrel.2015.08.057. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Nitric Oxide Biology and Pathobiology, 2nd edn, Academic Press, San Diego, 2000. [Google Scholar]
- Sagi Y., Heiman M., Peterson J. D., Musatov S., Scarduzio M., Logan S. M., Kaplitt M. G., Surmeier D. J., Heintz N., Greengard P. Proc. Natl. Acad. Sci. U. S. A. 2014;111:17636–17641. doi: 10.1073/pnas.1420162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller B. J. Invest. Dermatol. 2009;129:834–842. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- Cals-Grierson M. M., Ormerod A. D. Nitric Oxide. 2004;10:179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Weller R. Clin. Exp. Dermatol. 1999;24:388–391. doi: 10.1046/j.1365-2230.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Costa E. D., Rezende B. A., Cortes S. F., Lemos V. S. Front. Physiol. 2016;7:206. doi: 10.3389/fphys.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Q., Song W., Li L., Fan X. Mol. Brain. 2016;30:1–8. doi: 10.1186/s13041-016-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch-Gerharz D., Ruzicka T., Kolb-Bachofen V. Arch. Dermatol. Res. 1998;290:643–651. doi: 10.1007/s004030050367. [DOI] [PubMed] [Google Scholar]
- Bruch-Gerharz D., Ruzicka T., Kolb-Bachofen V. J. Invest. Dermatol. 1998;110:1–7. doi: 10.1046/j.1523-1747.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- Seabra A. B., Fitzpatrick A., Paul J., Oliveira M. G., Weller R. Br. J. Dermatol. 2004;151:977–983. doi: 10.1111/j.1365-2133.2004.06213.x. [DOI] [PubMed] [Google Scholar]
- Vercelino R., Cunha T. M., Ferreira E. S., Cunha F. Q., Ferreira S. H., de Oliveira M. G. J. Mater. Sci.: Mater. Med. 2013;24:2157–2169. doi: 10.1007/s10856-013-4973-7. [DOI] [PubMed] [Google Scholar]
- Seabra A. B., Pankotai E., Fecher M., Somlai A., Kiss L., Biro L., Szabo C., Kollai M., de Oliveira M. G., Lacza Z. Br. J. Dermatol. 2007;156:814–818. doi: 10.1111/j.1365-2133.2006.07718.x. [DOI] [PubMed] [Google Scholar]
- Amadeu T. P., Seabra A. B., de Oliveira M. G., Costa A. M. A. J. Eur. Acad. Dermatol. Venereol. 2007;21:629–637. doi: 10.1111/j.1468-3083.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- Amadeu T. P., Seabra A. B., de Oliveira M. G., Costa A. M. A. J. Surg. Res. 2008;149:84–93. doi: 10.1016/j.jss.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Georgii J. L., Amadeu T. P., Seabra A. B., de Oliveira M. G., Costa A. M. A. J. Tissue Eng. Regener. Med. 2011;5:612–619. doi: 10.1002/term.353. [DOI] [PubMed] [Google Scholar]
- Neidrauer M., Ercan U. K., Bhattacharyya A., Samuels J., Sedlak J., Trikha R., Barbee K. A., Weingarten M. S., Joshi S. G. J. Med. Microbiol. 2014;63:203–209. doi: 10.1099/jmm.0.067322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez V., Seabra A. B., Reguera R. M., Khandare J., Calderon M. Chem. Soc. Rev. 2016;45:152–168. doi: 10.1039/c5cs00674k. [DOI] [PubMed] [Google Scholar]
- Regev-Shoshani G., Crowe A., Miller C. C. J. Appl. Microbiol. 2013;114:536–544. doi: 10.1111/jam.12047. [DOI] [PubMed] [Google Scholar]
- Chaudhary S. C., Singh T., Kapur P., Weng Z. P., Arumugam A., Elmets C. A., Kopelovich L., Athar M. Toxicol. Appl. Pharmacol. 2013;268:249–255. doi: 10.1016/j.taap.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Kolb-Bachofen V., Liu D., Lundberg J. O., Revelo L. P., Suschek C. V., Weller R. B. Eur. Heart J. 2010;31:1041–1045. doi: 10.1093/eurheartj/ehq069. [DOI] [PubMed] [Google Scholar]
- Geldenhuys S., Hart P. H., Endersby R., Jacoby P., Feelisch M., Weller R. B., Matthews V., Gorman S. Diabetes. 2014;63:1–11. doi: 10.2337/db13-1675. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Titze J., Weller R. Curr. Opin. Nephrol. Hypertens. 2016;25:11–15. doi: 10.1097/MNH.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Fernandez B. O., Hamilton A., Lang N. N., Gallagher J. M. C., Newby D. E., Feelisch M., Weller R. B. J. Invest. Dermatol. 2014;134:1839–1846. doi: 10.1038/jid.2014.27. [DOI] [PubMed] [Google Scholar]
- Opländer C., Deck A., Volkmar C. M., Kirsch M., Liebmann J., Born M., van Abeelen F., van Faassen E. E., Kröncke K. D., Windolf J., Suschek C. V. Free Radical Biol. Med. 2013;65:1363–1377. doi: 10.1016/j.freeradbiomed.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Opländer C., Müller T., Baschin M., Bozkurt A., Grieb G., Windolf J., Pallua N., Suschek C. V. Nitric Oxide. 2013;28:24–32. doi: 10.1016/j.niox.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Mowbray M., McLintock S., Weerakoon R., Lomatschinsky N., Jones S., Rossi A. G., Weller R. B. J. Invest. Dermatol. 2009;129:834–842. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- Seabra A. B., Durán N. J. Mater. Chem. 2010;20:1624–1637. [Google Scholar]
- Seabra A. B., Durán N. Curr. Nanosci. 2012;8:520–525. [Google Scholar]
- Seabra A. B., Justo G. Z., Haddad P. S. Biotechnol. Adv. 2015;33:1370–1379. doi: 10.1016/j.biotechadv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Choi C., Nam J. P., Nah J. W. J. Ind. Eng. Chem. 2016;33:1–10. [Google Scholar]
- Dey A., Koli U., Dandekar P., Jain R. Polymer. 2016;93:44–52. [Google Scholar]
- Elgadir M. A., Uddin M. S., Ferdosh S., Adam A., Chowdhury A. J. K., Sarker M. Z. I. J. Food Drug Anal. 2015;23:619–629. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva R., Ragelle H., Rieux A., Duhem N., Jérôme C., Préat V. Adv. Polym. Sci. 2011;244:19–44. [Google Scholar]
- Ahmed T. A., El-Say K. M. Life Sci. 2014;110:35–43. doi: 10.1016/j.lfs.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Caon T., Porto L. C., Granada A., Tagliari M. P., Silva M. A. S., Simões C. M. O., Borsali R., Soldi V. Eur. J. Pharm. Sci. 2014;52:165–172. doi: 10.1016/j.ejps.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Ozcan I., Azizoglu E., Senyigit T., Ozyazici M., Ozer O. Int. J. Nanomed. 2013;8:461–475. doi: 10.2147/IJN.S40519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Tsai P.-C., Ramezanli T., Michniak-Kohn B. B. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2013;5:205–218. doi: 10.1002/wnan.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo V. F., Lancheros C. A. C., Narciso A. M., Valereto E. C. S., Kobayashi R. K. T., Seabra A. B., Nakazato G. Int. J. Pharm. 2014;473:20–29. doi: 10.1016/j.ijpharm.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Zou P., Yang X., Wang J., Li Y., Yu H., Zhang Y., Liu G. Food Chem. 2016;190:1174–1181. doi: 10.1016/j.foodchem.2015.06.076. [DOI] [PubMed] [Google Scholar]
- Liu C., Guo J., Wang W., Hu J., Wang C., Hu S. J. Mater. Chem. 2009;19:4764–4770. [Google Scholar]
- Chatterjee K., Sarkar S., Jagajjanani K. R., Paria S. Adv. Colloid Interface Sci. 2014;209:8–39. doi: 10.1016/j.cis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Wu W., Perrin-Sarrado C., Ming H., Lartaud I., Maincent P., Hu X.-M., Sapin-Minet A., Gaucher C. Nanomedicine. 2016;12:1795–1803. doi: 10.1016/j.nano.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Hasanovic A., Zehl M., Reznicek G., Valenta C. J. Pharm. Pharmacol. 2009;61:1609–1616. doi: 10.1211/jpp/61.12.0004. [DOI] [PubMed] [Google Scholar]
- Seabra A. B., de Oliveira M. G. Biomaterials. 2004;25:3773–3782. doi: 10.1016/j.biomaterials.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Shishido S. M., Seabra A. B., Loh W., de Oliveira M. G. Biomaterials. 2003;24:3543–3553. doi: 10.1016/s0142-9612(03)00153-4. [DOI] [PubMed] [Google Scholar]
- Wu W., Perrin-Sarrado C., Ming H., Lartaud I., Maincent P., Hu X.-M., Sapin-Minet A., Gaucher C. Nanomedicine. 2016;12:1795–1803. doi: 10.1016/j.nano.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Marcato P. D., Adami L. F., de M. Barbosa R., Melo P. S., Ferreira I. R., de Paula L., Duran N., Seabra A. B. Curr. Nanosci. 2013;9:1–7. [Google Scholar]
- Gao S., Fan L., Yuan Z., Bond P. L. Appl. Microbiol. Biotechnol. 2015;99:2305–2312. doi: 10.1007/s00253-014-6211-8. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Lipton S. A. Trends Pharmacol. Sci. 2016;37:73–84. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong H. T. T., Kamarudin Z. M., Erlich R. B., Li Y., Jones M. W., Kavallaris M., Boyer C., Davis T. P. Chem. Commun. 2013;49:4190–4192. doi: 10.1039/c2cc37181b. [DOI] [PubMed] [Google Scholar]
- Weller R. Clin. Exp. Dermatol. 2003;28:511–514. doi: 10.1046/j.1365-2230.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- Malinski T., Taha Z., Grunfeld S., Patton S., Kapturczak M., Tomboulian P. Biochem. Biophys. Res. Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- Lancaster Jr. J. R. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschek C. V., Schewe T., Sies H., Kroncke K. D. Biol. Chem. 2006;387:499–506. doi: 10.1515/BC.2006.065. [DOI] [PubMed] [Google Scholar]
- Opländer C., Römer A., Paunel-Görgülü A., Fritsch T., van Faassen E. E., Mürtz M., Bozkurt A., Grieb G., Fuchs P., Pallua N., Suschek C. V. Clin. Pharmacol. Ther. 2012;91:1074–1082. doi: 10.1038/clpt.2011.366. [DOI] [PubMed] [Google Scholar]
- Duong H. T. T., Adnan N. N. M., Barraud N., Basuki J. S., Kutty S. K., Jung K., Kumar N., Davis T. P., Boyer C. J. Mater. Chem. B. 2014;2:5003–5011. doi: 10.1039/c4tb00632a. [DOI] [PubMed] [Google Scholar]
- Nguyen T. K., Selvanayagam R., Ho K. K. K., Chen R., Kutty S. K., Rice S. A., Kumar N., Barraud N., Duong H. T. T., Boyer C. Chem. Sci. 2016;7:1016–1027. doi: 10.1039/c5sc02769a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong H. T. T., Ho A., Davis T. P., Boyer C. J. Polym. Sci., Part A: Polym. Chem. 2014;52:2099–2103. [Google Scholar]
- Chang H. W., Lin Y. S., Tsai Y. D., Tsai M. L. J. Appl. Polym. Sci. 2013;127:169–176. [Google Scholar]
- Zhou X., You G., Liu D., Yau K. Acta Polym. Sin. 2009;8:781–785. [Google Scholar]
- Matos B. N., Reis T. A., Gratieri T., Gelfuso G. M. Int. J. Biol. Macromol. 2015;75:225–229. doi: 10.1016/j.ijbiomac.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Raber A. S., Mittal A., Schafer J., Bakowsky U., Reichrath J., Vogt T., Schaefer U. F., Hansen S., Lehr C. M. J. Controlled Release. 2014;179:25–32. doi: 10.1016/j.jconrel.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Carnicer R., Crabtree M. J., Sivakumaran V., Casadei B., Kass D. A. Antioxid. Redox Signaling. 2013;18:1078–1099. doi: 10.1089/ars.2012.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Peng H., Ning F., Yao L., Luo M., Zhao Q., Zhu X., Xiong H. Food Chem. 2014;152:307–315. doi: 10.1016/j.foodchem.2013.11.147. [DOI] [PubMed] [Google Scholar]
- Weller R. Blood Purif. 2016;41:130–134. doi: 10.1159/000441266. [DOI] [PubMed] [Google Scholar]
- Wright F., Weller R. B. Maturitas. 2015;81:425–431. doi: 10.1016/j.maturitas.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Paunel A. N., Dejam A., Thelen S., Kirsch M., Horstjann M., Gharini P., Mqrtz M., Kelm M., Groot H., Kolb-Bachofen V., Suschek C. V. Free Radical Biol. Med. 2015;38:606–615. doi: 10.1016/j.freeradbiomed.2004.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.