 Cisplatin as a chief chemotherapy has nephro-toxicity and so we have tried to develop a novel antitumor drug based on a combination of cobalt metal ion with an organic moiety.
Cisplatin as a chief chemotherapy has nephro-toxicity and so we have tried to develop a novel antitumor drug based on a combination of cobalt metal ion with an organic moiety.
Abstract
Cisplatin as a chief chemotherapy has nephro-toxicity and so we have tried to develop a novel antitumor drug based on a combination of cobalt metal ion with an organic moiety. The antitumor activity of the complex was tested in vitro and in vivo against murine Ehrlich ascites carcinoma (EAC). Antioxidant capacity and nucleic acids content were determined. Cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex significantly diminished tumor load. It decreased the tumor proliferation rate and obviously increased the life span of EAC-bearing mice. It reversed the haematological parameters back towards normal, reduced liver enzymes and urea, and increased albumin and total protein. Antioxidant parameters levels were reversed towards normal. An assessment was conducted by comparing these results with those obtained using the standard drug, cisplatin. The results suggest that the cobalt complex can be considered as a potent anticancer agent as it showed appreciable antitumor activity in EAC-bearing mice that was almost analogous to that of the reference standard, cisplatin.
Introduction
Since the discovery and clinical approval of cisplatin, efforts have been made to develop novel antitumor drugs that can overcome cisplatin’s systemic and organ-specific toxicity and to improve its chemotherapy clinical effectiveness.1 Among the non-platinum compounds with antitumor potential, transition-metal complexes have been investigated on the assumption that endogenous metals may be less toxic.3,4
There is an increasing attention towards oxime–hydrazone complexes owing to their biological action.4 Several successful antitumor medications are molecules of natural origin or are constructed from their synthetic analogs. Metal-based compounds have exceptional criteria increasing their activity as anticancer agents.5,6
Cobalt is an essential trace mineral in humans, displaying a lot of useful biological functions.7 From some investigations, it was found that some complexes of cobalt(ii) with different ligands decreased drastically the viability and proliferation of cultured tumor cells and stimulated DNA damage.8 Many Schiff base ligands derived from pyridine derivatives and their cobalt(ii) complexes have also been found to inhibit the growth of tumor cells.9
Reactive oxygen species (ROS) formed through biochemical pathways in the body, such as the superoxide anion and hydrogen peroxide, are very reactive and strongly damaging short-lived chemical species.10 Antioxidants are able to decrease oxidative stress-encouraged carcinogenesis via scavenging of ROS directly and/or via hindering cell proliferation secondary to protein phosphorylation. Both enzymatic and non-enzymatic antioxidants exist in the intracellular and extracellular environment to detoxify ROS.11
The present study aimed to design a complex based on the combination of cobalt(ii) metal and the diacetyl monoxime-2-hydrazinopyridine ligand, and to validate its anticancer activity in comparison with cisplatin.
Experimental
Chemicals, drugs and kits
Freshly prepared cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex was used as a potential anticancer drug and cisplatin was used as the reference standard anticancer drug. Cisplatin (Myllal, France) was obtained from a local pharmacy. Solvents redistilled by standard techniques were used. Cobalt acetate anhydrous was purchased from Sigma (St. Louis, MO, USA) and diacetyl monoxime-2-hydrazinopyridine ligand (L) was kindly supplied by Prof. Mohamed M. Hassanien (Professor of analytical chemistry – Industrial Education Collage, Beni-Suef University, Egypt). Other chemicals and reagents with the highest available pure grade were used. Malondialdehyde (MDA), catalase (CAT) and total antioxidant capacity (TAC) kits were purchased from BIODIAGNOSTIC, Cairo, Egypt. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) kits were obtained from Diamond Company (Cairo, Egypt). The kit for albumin was purchased from STANBIO Company (USA).
Synthesis, characterization, and in solution preparation of cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex
Equimolar amounts (1 × 10–3 M) of L and cobalt acetate anhydrous were mixed in absolute ethanol then heated under reflux for 24 hours. The obtained red solid complex had a melting point of >300 °C. Its elemental analysis gave C 48.2%, H 5.1%, N 27.8%, and Co 11.6%, suggesting the chemical formula [Co(L)2OAc]. The IR spectrum of L showed three strong bands at 1575, 930 and 3340 cm–1, assigned to γ(C N) imine, γ(N–O) vibration and γ(OH) oxime, respectively. The IR spectrum of [Co(L)2OAc] showed that L behaves as a mononegative bidentate ligand coordinating via (C N) imine and (OH) oxime groups with the displacement of the H atom from the latter group in the complex. This was supported by the shift of γ(C N) imine to lower wave number and the disappearance of the signal attributed to the H proton of (OH) from the oxime group of the complex. The electronic spectrum of the diamagnetic [Co(L)2OAc] complex suggested an octahedral structure supported by the appearance of the characteristic magnetic moment band at 20 000 cm–1.
Cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex was prepared in solution after application of Job's method (continual variation method) to specify the stoichiometric composition of the chelate.12 Bands in the UV region (200–340 nm) are characteristic of L and the new distinctive band of the complex was detected at 489 nm by the addition of cobalt acetate anhydrous solution. The absorbance plot at 489 nm against the mole fraction of L had maximum absorbance at X = 0.66, verifying that the stoichiometric ratio for the complexation of Co(ii) and L is 1 : 2.
The cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex, [CoII(L)2]2+, was freshly prepared in solution by mixing two volumes of diacetyl monoxime-2-hydrazinopyridine solution (0.01 M in absolute ethanol, freshly prepared) with one volume of freshly prepared cobalt acetate anhydrous solution (0.01 M in bidistilled water). The stability constant of the complex was examined spectrophotometrically at 489 nm and it was found to be 3.5 × 109.
Tumor cell transplantation
The parent Ehrlich ascites carcinoma (EAC) cell line was kindly provided by the National Cancer Institute, Cairo University, Egypt, and it was conserved in mice through serial intraperitoneal transplantations of 1 × 106 viable tumor cells in 0.2 ml of saline. EAC cells were collected 7 days after intraperitoneal implantation. The harvested cells were diluted with saline to obtain a concentration of 5 × 106 viable EAC cells per ml. A volume of 0.2 ml saline (1 × 106 EAC cells) was implanted intraperitoneally into normal mice.4
Animals
Female mice were used in this study because they are known to be more susceptible to Ehrlich ascites carcinoma than male mice. Adult female Swiss albino mice obtained from National Cancer Institute, Cairo, Egypt with an average body weight of 20 to 25 g were used. The animals were housed according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health.13
They were housed in steel mesh cages (10 mice per cage) and maintained for two weeks prior to experimental use, under constant conditions of 12 : 12 hours light/dark cycle, temperature of 23 ± 2 °C and controlled humidity conditions, and they were fed with the standard mice pellet diet and water ad libitum.
Determination of median lethal dose (LD50)
The LD50 value of cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex was determined according to the method of Probit.14 The fresh complex solution was injected intraperitoneally to 7 groups of mice (each containing n = 2) at different doses equivalent to 2.5, 5, 7.5, 10, 15 and 20 mg cobalt(ii) diacetyl monoxime-2-hydrazinopyridine per kg.
Anti-haemolytic effect of complex
To test the anti-haemolytic effects of the cobalt(ii) complex, normal human erythrocytes in phosphate buffered saline (PBS) solution containing 200 μM of m-chloroperbenzoic acid (m-CPBA), the acid concentration which gave the maximum haemolytic effect, were subjected to UV light for 30 min. After incubation, the contents were centrifuged and the absorbance of the supernatants as an indication of the photo-haemolytic effect was measured at 546 nm.15
Superoxide dismutase (SOD)-like activity of complex
SOD-like activity of the cobalt(ii) complex was determined by the method of Dechatelet et al. (1974).16 0.1 ml of the complex (0.01 M) was added to a mixture of nitro blue tetrazolium salt (NBT) and NADH in a pyrophosphate buffer (pH = 8.3). The changes in the optical density were recorded per minute for five minutes at 560 nm after the addition of phenazine methosulfate. The percent inhibition of color development was calculated based on that of a control tube.
In vitro cytotoxicity assay
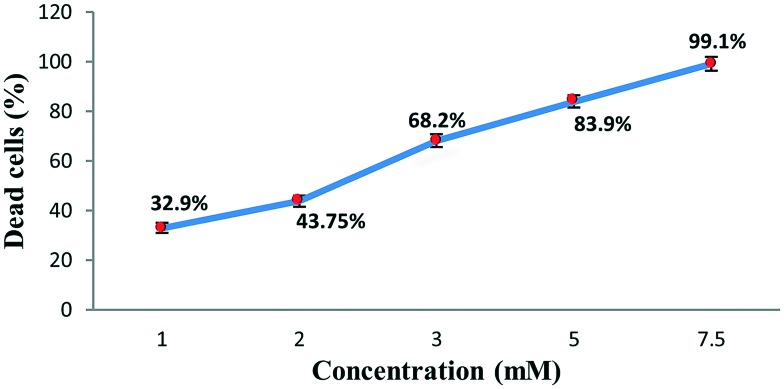
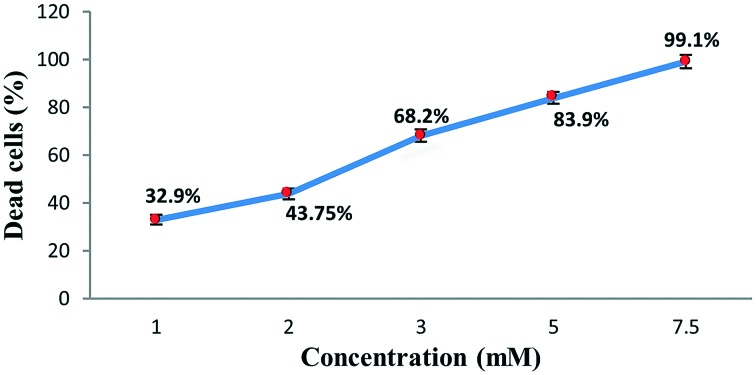
In vitro short-term cytotoxic activity of the complex was investigated via the trypan blue exclusion method of MacLimans et al.17 against EAC cells. A volume of 0.2 ml of 0.32% trypan blue was mixed with 0.2 ml of EAC cells [previously mixed with different concentrations of the cobalt complex (1, 2, 3, 5 and 7.5 mM) and incubated for 10 minutes at 37 °C], then the number of viable tumor cells (unstained) was counted within 5 minutes after incubation using a haemocytometer at a microscopic magnification of ×100. The % of viable tumor cells per ml was then calculated using the formula: viable tumor cells % = (UC/TC) × 100, where UC is the unstained cell count (viable cells) and TC is the total cell count (stained and unstained).
In vivo anticancer assay
Experimental design
To study the anticancer activity of the cobalt(ii) complex on EAC-bearing mice, mice were randomly divided into seven groups of ten mice each. Group I served as the normal control group and did not receive any treatment. Group II served as EAC control; EAC bearing-mice received the respective vehicles. After 24 hours of tumor inoculation, the mice of group III were injected intraperitoneally with 3.5 mg kg–1 of cisplatin once and the mice of groups IV, V, VI and VII were injected intraperitoneally with the freshly prepared cobalt(ii) complex for consecutive nine days at doses of 0.34, 0.46, 0.69 and 1.37 mg kg–1 per day, respectively.
To study the effects of the cobalt(ii) complex on the normal mice, two additional animal groups were included: a vehicle mice group that received the respective vehicles only and a normal healthy mice group that were injected intraperitoneally with the freshly prepared cobalt(ii) complex with a dose of 1.37 mg kg–1 per day (1/10 of the LD50) for nine consecutive days.
Collection of samples
One day after the last dose, mice were fasted for 18 hours then six mice from each group were sacrificed for estimation of tumor proliferation, haematological, hepatic anti-oxidative and serum biochemical parameters, and liver tissue DNA and RNA contents. The remaining mice in each group were kept alive for measurement of survival parameters. The ascitic fluids containing EAC cells were collected and their volumes were measured. Blood samples were collected, left to clot and the sera were separated by cooling centrifugation (4 °C) at 3000 rpm for 10 min and stored at –20 °C until used. The liver of each mouse was removed, rapidly rinsed with ice-cold saline and dried on filter paper. Weighed dried liver was homogenized in 0.95% NaCl solution for 15 min and the resultant homogenate (10%, w/v) was then centrifuged at 4 °C and the resultant supernatant was used for determination of hepatic anti-oxidative parameters.
SOD and catalase (CAT) activities in liver tissue homogenate
SOD activity in liver homogenate was assayed as in Dechatelet et al.16 CAT activity was determined according to the kit instructions.
Glutathione reduced form (GSH), total antioxidant capacity (TAC) and MDA levels in liver tissue homogenate
GSH was determined in liver tissues using the method of Moron et al.18 MDA and TAC were determined using the kit instructions.
Liver and kidney function tests and nucleic acids contents
AST and ALT activities and albumin level were determined according to the kits instructions. Total protein, urea, DNA and RNA contents were assayed according to the methods of Lowry et al.,19 Fawcett et al.,20 Dische and Schwartez21 and Mejbaum,22 respectively.
Haematological parameters
Red blood cells (RBCs), white blood cells (WBCs) and platelets (PLT) counts and haemoglobin (Hb) level were estimated using the methods of D'Armour et al.,23 Dacie and Lewis,15 Brecher and Cronkite,24 and Drabkin and Austin,25 respectively.
Survival parameters
Cancer anti-proliferation effects of the complex were assessed by observation of changes with respect to body weight, tumor volume, viable tumor cells count, median survival time (MST) and percentage increase in life span (% ILS). MST was monitored by recording the mortality daily for 6 weeks and % ILS was calculated using the following equations: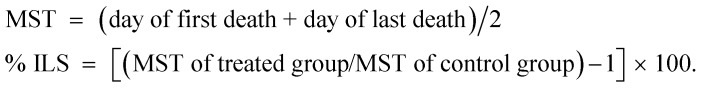
Weight variation parameter
Animals were weighed every 3 days from day zero to calculate the differences in weight gain between treated groups and the control EAC group.
All experimental procedures were approved by the Animal House of Biochemistry, Chemistry Department, Faculty of Science, Damietta University, Egypt.
In vitro tests were done at least in triplicate while in vivo tests were done in duplicate and when variation was more than 25% the sample was tested again.
Statistical analysis
Data were expressed as the mean ± standard deviation (mean ± S.D.). Student's t-test was used to perform the statistical analysis between two groups. A value of p < 0.05 was considered significant for all analyses. All statistics were obtained according to the statistical software system, instat version 3.10 (Graph pad, USA).
Results
Anti-haemolytic effects of complex
In vitro study revealed that the maximum cyto-protective effect of the cobalt complex for human erythrocytes from photo-irradiative damage was 91.10% at a concentration of 0.01 M, as shown in Table 1.
Table 1. In vitro anti-haemolytic effect and SOD-like activity of cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex (0.01 M).
| Complex (0.01 M) | Anti-haemolytic effect (%) | SOD-like activity (% inhibition) |
| Cobalt(ii) diacetyl monoxime-2-hydrazinopyridine | 91.10 ± 2.57 | 78.155 ± 2.283 |
SOD-like activity of complex
The cobalt(ii) complex showed good SOD-like activity of about 78.155% at a concentration of 0.01 M (Table 1).
In vitro cytotoxicity assay
The complex was tested using short-term in vitro cytotoxicity towards EAC cells as a preliminary screening technique with the trypan blue exclusion method (cell viability test) for its cytotoxic potential. Results are shown in Fig. 1.
Fig. 1. In vitro effect of cobalt complex on cell viability. Results are expressed as mean ± S.D.
In vivo anticancer evaluation
In vivo cytotoxic assay
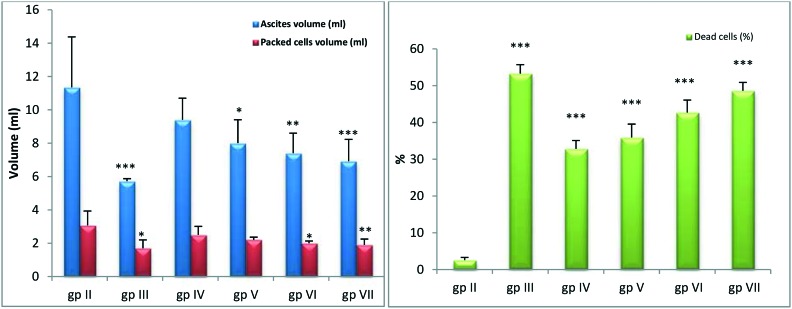
Fig. 2 illustrates that treated groups with four doses of the complex showed significant decreases (p < 0.05–p < 0.001) in ascites and packed cell volumes with significant increases (p < 0.001) in dead cell percentages compared to the EAC control.
Fig. 2. In vivo ascites volume, packed cells volume and dead cells percentage in tumorized and treated mice. *, **, *** Significant at p < 0.05, p < 0.01 and p < 0.001, respectively compared to EAC-bearing group. gp II: EAC-bearing mice; gp III: EAC-bearing mice treated with cisplatin; gp IV: EAC-bearing mice treated with (0.34 mg kg–1) complex; gp V: EAC-bearing mice treated with (0.46 mg kg–1) complex; gp VI: EAC-bearing mice treated with (0.69 mg kg–1) complex; gp VII: EAC-bearing mice treated with (1.37 mg kg–1) complex.
Body weights
The tumor inoculated control animals showed continuous weight gain until day 9. Cisplatin or complex administration reduced the weight gain at the end of the treatment course as compared to the control (Table 2).
Table 2. Effects of complex doses on body weights (g) of tumorized mice groups.
| Days |
|||||
| Groups | 0 | 3 | 6 | 9 | % decrease in body weight by treatment |
| Normal control | 18.97 ± 2.82 | 21.5 ± 0.87 | 22.4 ± 3.87 | 24.07 ± 4.3 | — |
| EAC control | 22.8 ± 2.74 | 25.1 ± 2 | 27.3 ± 2.3 | 29.31 ± 1.07 | — |
| EAC + cisplatin | 22.22 ± 2.85 | 25.6 ± 2 | 27.7 ± 1.53 | 25.47 ± 0.96 | 8.05 |
| EAC + complex (dose: 0.34 mg kg–1) | 21.8 ± 1.25 | 22.1 ± 2.53 | 24.16 ± 1.25 | 23.8 ± 2.03 | 1.49 |
| EAC + complex (dose: 0.46 mg kg–1) | 22.7 ± 1.16 | 23.5 ± 1.32 | 25.03 ± 0.35 | 24.3 ± 0.57 | 2.91 |
| EAC + complex (dose: 0.69 mg kg–1) | 22.16 ± 1.04 | 22.2 ± 1.31 | 24.03 ± 1.98 | 23.1 ± 2.33 | 3.78 |
| EAC + complex (dose: 1.37 mg kg–1) | 25.1 ± 0.76 | 26.1 ± 1.55 | 26.9 ± 1.47 | 25.2 ± 1.28 | 6.32 |
| Healthy + solvent | 20.2 ± 1.12 | 21.96 ± 0.96 | 24.2 ± 1.17 | 24.26 ± 1.02 | — |
| Healthy + complex | 20.33 ± 1.04 | 20.6 ± 0.97 | 21.6 ± 0.68 | 21 ± 0.75 | — |
Survival parameters
The effects of the complex on survival of tumor-bearing mice are shown in Table 3. Cisplatin prolonged the MST with respect to its control. It showed an increase in the lifespan of animals by >30%. Similarly, all the complex doses prolonged the median survival times in a dose-dependent manner. The influence of the complex on % ILS was 23.07%, as represented by the highest dose.
Table 3. Effect of complex on survival time against EAC induced animals.
| Groups | Median survival time (MST) (days) | Percentage increase of life span (% ILS) |
| Normal control | >33 | >153.85 |
| EAC control | 13 | 0 |
| EAC + cisplatin | 18 | 38.5 |
| EAC + complex (dose: 0.34 mg kg–1) | 14.5 | 11.53 |
| EAC + complex (dose: 0.46 mg kg–1) | 15 | 15.38 |
| EAC + complex (dose: 0.69 mg kg–1) | 15.5 | 19.23 |
| EAC + complex (dose: 1.37 mg kg–1) | 16 | 23.07 |
| Healthy + solvent | 28 | 115.38 |
| Healthy + complex | >33.5 | >157.69 |
Haematological parameters
Tumor-bearing animals treated with the complex or with cisplatin showed significant increases (p < 0.05–p < 0.001) in RBCs count and Hb levels but they showed decreases in WBCs count (p < 0.001) and in PLT count (Table 4).
Table 4. Effects of complex on the haematological parameters of EAC-bearing mice.
| Groups | RBCs (1 × 106 mm–3) | WBCs (1 × 103 mm–3) | PLT (1 × 103 mm–3) | Hb (g dl–1) |
| Normal control | 7.43 ± 0.95 | 16.6 ± 0.58 | 918.17 ± 128 | 10.95 ± 0.69 |
| EAC control | 5.44 ± 0.5## | 41.62 ± 0.47### | 1352.5 ± 277### | 7.5 ± 0.46### |
| EAC + cisplatin | 7 ± 0.42*** | 19.55 ± 1.04*** | 1047.5 ± 187* | 10.48 ± 0.66*** |
| EAC + complex (dose: 0.34 mg kg–1) | 6.44 ± 0.49#,* | 24.32 ± 0.64###,*** | 1269.8 ± 85# | 8.82 ± 0.59###,** |
| EAC + complex (dose: 0.46 mg kg–1) | 6.64 ± 0.44** | 22.84 ± 0.77###,*** | 1141.5 ± 128 | 9.53 ± 0.53##,*** |
| EAC + complex (dose: 0.69 mg kg–1) | 6.8 ± 0.48** | 21.95 ± 1.5###,*** | 1135 ± 123 | 9.73 ± 0.55#,*** |
| EAC + complex (dose: 1.37 mg kg–1) | 6.89 ± 0.42*** | 20.29 ± 0.71###,*** | 1095.5 ± 138 | 10 ± 0.69*** |
| Healthy + solvent | 8.09 ± 0.4 | 14.58 ± 2.1 | 891.6 ± 201.7 | 10.42 ± 0.33 |
| Healthy + complex | 8 ± 0.16 | 10.61 ± 0.98### | 1223.5 ± 66# | 11 ± 0.51 |
Lipid peroxidation and antioxidants
As evident from Table 5, treatment by the complex significantly reduced MDA levels, p < 0.001. On the other hand, it significantly elevated the mean GSH contents (p < 0.001), SOD activities (p < 0.001), TAC levels (p < 0.01–p < 0.001), and CAT activities (p < 0.05–p < 0.001) in liver tissues of the treated tumorized mice compared to the non-treated tumorized mice.
Table 5. Mean activities of SOD and catalase (CAT) in liver tissues and mean levels of hepatic reduced glutathione (GSH), total antioxidant capacity (TAC) and malondialdehyde (MDA).
| Groups | MDA (μmole g–1 tissue × 10–4) | SOD (%) | CAT (U g–1 tissue) | GSH (mmoles g–1 tissue × 10–3) | TAC (mM) |
| Normal control | 4.21 ± 0.12 | 39.15 ± 0.47 | 2.77 ± 0.4 | 16.042 ± 1.13 | 23.59 ± 0.32 |
| EAC control | 8.36 ± 0.16### | 23.5 ± 0.54### | 0.94 ± 0.19### | 9.09 ± 0.86### | 10.01 ± 0.52### |
| EAC + cisplatin | 5.68 ± 0.08*** | 37.07 ± 0.8** | 2.24 ± 0.35*** | 14.79 ± 0.65*** | 21.7 ± 0.6*** |
| EAC + complex (dose: 0.34 mg kg–1) | 6.73 ± 0.55###,*** | 31.69 ± 0.54###,*** | 1.42 ± 0.13### | 12.74 ± 0.56###,*** | 15.96 ± 0.67###,*** |
| EAC + Co-complex (dose: 0.46 mg kg–1) | 6.52 ± 0.56###,*** | 32.68 ± 0.6###,*** | 1.56 ± 0.26###,* | 12.81 ± 0.37###,*** | 16.16 ± 0.99##,** |
| EAC + complex (dose: 0.69 mg kg–1) | 5.78 ± 0.47###,*** | 33.68 ± 0.45###,*** | 1.76 ± 0.15###,* | 13.15 ± 0.92###,*** | 16.77 ± 0.58###,*** |
| EAC + complex (dose: 1.37 mg kg–1) | 5.24 ± 0.13##,*** | 34.89 ± 0.59###,*** | 2.06 ± 0.055###,*** | 13.55 ± 0.53###,*** | 18.67 ± 0.59 ###,*** |
| Healthy + solvent | 4.51 ± 0.79 | 34.28 ± 0.68### | 2.88 ± 0.53 | 16.34 ± 0.86 | 22.36 ± 1.15 |
| Healthy + complex | 3.58 ± 0.64 | 34.68 ± 0.35### | 2.91 ± 0.47 | 12.1 ± 0.78### | 21.52 ± 0.51### |
Nucleic acids contents
For nucleic acids contents, treated groups with complex doses showed significant decreases in DNA (p < 0.001) and in RNA (p < 0.05–p < 0.001) compared to the EAC-bearing group (Table 6).
Table 6. Mean serum activities of ALT and AST, mean serum levels of albumin (ALB) and urea, and mean levels of hepatic total protein, DNA and RNA.
| Groups | ALT (U ml–1) | AST (U ml–1) | ALB (g%) | Total protein (g%) | Urea (mg%) | DNA (mg ml–1) | RNA (mg ml–1) |
| Normal control | 42.05 ± 1.42 | 55.28 ± 0.65 | 5.7 ± 0.4 | 6.37 ± 0.066 | 44.8 ± 0.51 | 0.1 ± 0.01 | 0.76 ± 0.06 |
| EAC control | 76.2 ± 0.75### | 87 ± 0.82### | 1.4 ± 0.47### | 3.37 ± 0.015### | 64.9 ± 0.38### | 0.22 ± 0.012### | 1.01 ± 0.1### |
| EAC + cisplatin | 44.7 ± 0.66*** | 60.2 ± 0.96*** | 3.6 ± 0.55*** | 5.13 ± 0.073*** | 47.91 ± 0.49*** | 0.111 ± 0.02*** | 0.81 ± 0.056*** |
| EAC + complex (dose: 0.34 mg kg–1) | 52.6 ± 0.56###,*** | 68.5 ± 0.55###,*** | 2.6 ± 0.46###,*** | 4.11 ± 0.33###,* | 51.68 ± 0.52###,*** | 0.158 ± 0.01###,*** | 0.88 ± 0.067* |
| EAC + Co-complex (dose: 0.46 mg kg–1) | 51.05 ± 0.69###,*** | 67 ± 0.89###,*** | 2.8 ± 0.37###,*** | 4.33 ± 0.052###,*** | 49.96 ± 0.43###,*** | 0.138 ± 0.04*** | 0.87 ± 0.063* |
| EAC + complex (dose: 0.69 mg kg–1) | 49.88 ± 0.41###,*** | 63 ± 1.8###,*** | 3.1 ± 0.2###,*** | 4.6 ± 0.36###,*** | 48.64 ± 0.59###,*** | 0.135 ± 0.01*** | 0.84 ± 0.05** |
| EAC + complex (dose: 1.37 mg kg–1) | 47.7 ± 0.54###,*** | 61.8 ± 1.47###,*** | 3.3 ± 0.12###,*** | 4.82 ± 0.46###,*** | 48.12 ± 0.66###,*** | 0.115 ± 0.017*** | 0.82 ± 0.027*** |
| Healthy + solvent | 39.8 ± 0.4### | 52 ± 0.89# | 3.8 ± 0.52### | 5.32 ± 0.73# | 48.24 ± 0.8### | 0.114 ± 0.021 | 0.74 ± 0.08 |
| Healthy + complex | 38.8 ± 0.4### | 65.3 ± 3.55### | 4.1 ± 0.85### | 5.67 ± 0.4### | 48.87 ± 0.4### | 0.047 ± 0.002## | 0.69 ± 0.083 |
Biochemical parameters
Table 6 illustrates that treated tumor-bearing groups with complex doses showed significant decreases (p < 0.001) in AST and ALT serum activities and in serum urea levels compared to tumor-bearing non-treated group. On the other hand, these groups showed significant increases in albumin levels (p < 0.001) compared to those of non-treated tumor group.
Treated tumorized mice with the complex showed also significant increases in total protein contents (p < 0.05 – p < 0.001) as compared to those of non-treated tumorized mice.
Discussion
Transition metals, including cobalt, display different oxidation states and can react with a number of negatively charged molecules. This property has led to the current development of metal-based drugs, which are considered to be prospective candidates for pharmacological and therapeutic applications.2 The advancement of metal-based antitumor medicines has been induced by the clinical success of cisplatin in tumor treatment; however its bad side effects obligated researchers to attempt trials of new anticancer drugs that can be less toxic. This was a strong motive for us to design the current complex, which is based on the combination of cobalt, an essential trace metal in humans, and the diacetyl monoxime-2-hydrazinopyridine ligand to validate its anticancer activity in comparison with cisplatin, the chief chemotherapeutic drug, on the hypothesis that compatible metals may be safer.
In the current investigation, a rapid increase in ascitic tumor volume was observed in EAC-bearing mice and treatment with the cobalt complex reduced the intraperitoneal tumor burden by decreasing the tumor volume, tumor weight and viable tumor cells count, along with a life span increase. Therefore, it may be hypothesized that the increase in life span may be due to the decrease in nutritional ascitic volume and a delay in cell proliferation.26 This confirms the anticancer activity of the complex against EAC cells because prolongation of life span is a dependable principle for judging the anticancer efficacy of any compound.27 These results also indicate that the complex has a direct connection with tumor cells at higher doses as they directly absorb the anticancer drug in the peritoneal cavity, resulting in cell lysis through a direct and cytotoxic mechanism.
In this work, a considerable increase in body weight of the animals was observed in EAC control mice due to the fast and progressive accumulation of ascites tumor cells. Treatment with the complex caused a marked reduction in the body weight, indicating the feature of inhibition of tumor cells progression.
In the present in vitro studies, it was found that the complex was able to protect the erythrocytes from photo-haemolysis via quenching the produced free radicals. This is supported by the earlier evidence that verified that ROS are responsible for photo-haemolysis of erythrocytes induced by m-CPBA.28 This haemolytic state may be due to direct cellular membrane injury caused by the formed lipid peroxides. In addition, the complex showed a high percentage (about 80%) of SOD-like activity. This confirmed the ability of the complex to scavenge O2–˙ produced during photosensitization of erythrocytes. The ability of this complex to prevent RBCs damage confirms its capacity to act as a free radical scavenger that may be used in photodynamic therapy after further experimental validation.
Increase in biochemical marker enzymes activities has a good association with the transformed cells count in cancer cases.29 It was hypothesized that free amino acid utilization in protein construction of speedily dividing cancer cells may cause enzyme activity disturbances in hepatocytes.30 Borentain et al.31 stated that liver carcinoma enhances both AST and ALT activities and its surgical elimination caused their reduction to near normal. In the current investigation, AST and ALT activities increase following EAC implantation may be attributed to cell membrane transport disorder and enzyme outflow. This is suggestive of the beginning of liver cell destruction due to hepatic malfunction and disturbance of AST and ALT biosynthesis with the hepatocyte membrane permeability changes. Complex administration significantly decreased serum AST and ALT activities, indicating conservation of hepatocyte cell membrane functional integrity.
The results of the present study showed that the mean levels of total proteins in liver tissues of tumorized mice were reduced, verifying the incidence of a catabolic status accompanying tumor growth.32 This was confirmed by their re-elevation together with hepatic DNA and RNA contents decrease following tumor killing by the complex. The latter results confirm the ability of the studied complex not only to treat the tumor cells but also to prevent their metastasis to the liver.
Moreover, the observed significant elevation in blood urea content of tumor-bearing mice might be attributed to the tumor catabolic effect and high urea production rate. This was confirmed by the previous similar findings of Hussein and Azab.33
Recently, Shreaz et al. attributed the fungicidal effectiveness of a Co(ii) complex of the type [Co(C40H40N4)Cl2] to the loss of membrane integrity.34 Besides, investigations of the anticancer effectiveness of some Co(ii) complexes using various human cancer cells in vitro reflected that Co(ii) complexes have apoptotic properties.2 Previously, Pizarro and Sadler35 reported that DNA is supposed to be the chief target for many metal-based drugs. These drugs can form specific damage to DNA that stimulates its apoptosis. As one of these transition metal complexes, Co(ii) complexes have the ability to act as DNA-binding agents.2 Therefore, it can be hypothesized that the present complex may follow a similar mechanism to that of Pizarro and Sadler during killing of EAC cells. This conclusion is based on the drop in the mean levels of nucleic acids after treatment of the tumorized mice with the complex. However, this hypothesis still needs further investigation for more validation.
Key enzymes in antioxidant defence mechanisms are SOD and CAT. These antioxidant enzymes are exposed to alteration during carcinogenesis and after tumor formation.36–38 Disequilibrium in the antioxidant defence mechanism makes cells more sensitive to free radicals.39 Low levels of antioxidant enzymes result in accumulation of free oxygen radicals with consequent destruction of DNA, RNA, lipids and proteins and may ultimately affect cancer cells.40,41 Lipid peroxidation is a spontaneous catalytic free radical chain propagating reaction that is known to be associated with cell pathological conditions.42 Its high level can result in degeneration of tissues. Lipid peroxides can be transferred from the initial site via the blood and induce damage by propagating the lipid peroxidation process.43 Experimental animal or human tumors affect several functions of the vital organs, particularly the liver, although the location of the tumor does not interfere directly with organ function.44 MDA, the end product of lipid peroxidation, is found to be higher in tumorized tissues than in healthy tissues.45
Both GSH and SOD are abundant in the liver and they participate in cell protection against ROS and against cancer development.43,46 SOD catalyzes the breakdown of the superoxide anion into oxygen and hydrogen peroxide and GSH detoxifies hydrogen peroxide in presence of GSH peroxidase.47 Sun et al.48 observed an inhibition in SOD activity in EAC-bearing animals that may be attributed to loss of mitochondria and loss of Mn SOD activity in EAC cells, resulting in a lowering in total hepatic SOD activity. In addition, a significant reduction in plasma CAT activity in tumor-bearing mice is in agreement with analogous findings reported by Bozzi et al.,49 which showed a very low CAT activity observed in tumor cells.
In the present study, decreases in hepatic antioxidants concomitant with elevated MDA of the tumorized animals suggest existence of an oxidative stress state and subsequent cellular and tissue damage, and possibly EAC cells and hepatocytes damage as well. This is actually the case because treatment with the complex inhibited hepatic lipid peroxidation, as indicated by the reduction of MDA towards normal levels and the re-elevation in the activities of both SOD and CAT enzymes. These enzymes can defend liver tissues against oxidative stress through scavenging superoxide radicals and the formed H2O2, the substrates of SOD and CAT enzymes, respectively. This emphasizes the reduction in free radicals and the subsequent decrease in cell membrane damage and MDA production in the treated tumor-bearing mice.
The chief complications that have emerged in cancer chemotherapy are myeloid-suppressor and anaemia because of lowering in RBCs or Hb content.50 In the current work, tumorized mice showed reduced Hb content and RBCs count, which may be due to the suppressive action of EAC on bone marrow erythropoiesis.51 This is possibly attributed to iron deficiency of haemolytic or myelopathic state.52 Treatment with the complex resulted in appreciable improvements in Hb content and RBCs count towards normal. These improvements clearly indicate that the complex possesses a protective action on the haemopoietic system. Induction of Hb following treatment with the complex may be due to enhancement of iron levels, which may improve the haemopoietic function.
One of the most important criteria for evaluating clinically efficient anticancer drugs is that they can reduce the WBCs count of tumorized animals.53 In the present study, complex administration returned RBCs, WBCs and PLT counts and Hb content towards normal levels. This indicated that the complex exhibits haematopoietic protecting activity without myelotoxicity, the most common side effect of cancer chemotherapy.
Conclusions
Finally, cobalt(ii) diacetyl monoxime-2-hydrazinopyridine complex can be considered as a potent anticancer agent with minimal side effects as it exhibited a significant antitumor activity in EAC-bearing mice that is almost comparable to that of the reference standard, cisplatin.
Footnotes
†The authors declare no competing interests.
References
- Wani W. A., Baig U., Shreaz S., Shiekh R. A., Iqbal P. F., Jameel E., Ahmad A., Mohd-Setapar S. H., Mushtaqueh Md., Hun L. T. New J. Chem. 2016;40:1063–1090. doi: 10.1039/C5NJ01449B. [DOI] [Google Scholar]
- Selvaganapathy M., Raman N. J. Chem. Biol. Ther. 2016;1(2):108–124. [Google Scholar]
- Saad E. A., Hassanien M. M., El-lban F. W. Biochem. Biophys. Res. Commun. 2017;484:579–585. doi: 10.1016/j.bbrc.2017.01.137. [DOI] [PubMed] [Google Scholar]
- Saad E. A., Hassanien M. M., Elneely E. A. Appl. Organomet. Chem. 2016 doi: 10.1002/aoc.3684. [DOI] [Google Scholar]
- Dorr M., Meggers E. Curr. Opin. Chem. Biol. 2014;19(1):76–81. doi: 10.1016/j.cbpa.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Li G. Y., Guan R. L., Ji L. N., Chao H. Coord. Chem. Rev. 2014;281:100–113. [Google Scholar]
- Prashanth L., Kattapagari K. K., Chitturi R. T., Baddam V. R., Prasad L. K. J. Dr. NTR Univ. Health Sci. 2015;4:75–85. [Google Scholar]
- Du Y. H., Huang J., Weng X. C., Zhou X. Curr. Med. Chem. 2010;17:173–189. doi: 10.2174/092986710790112648. [DOI] [PubMed] [Google Scholar]
- Raman N., Pothiraj K., Baskaran T. J. Mol. Struct. 2011;1000:135–144. [Google Scholar]
- Habib S. A., Saad E. A., Elsharkawy A. A., Attia Z. R. Adv. Med. Sci. 2015;60(2):179–185. doi: 10.1016/j.advms.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Ozkan A., Fiskin K. THOD, Turk Hematol.-Onkol. Derg. 2004;14:52–60. [Google Scholar]
- Harris D. C., Freeman W. H. and Company, Quantitative Chemical Analysis, New York, 4th edn, 1995, pp. 529–530. [Google Scholar]
- NIH [National Institutes of Health], Guide for the Care and Use of Laboratory Animals, National Academy Press, DC, Washington, 7th edn, 1996. [Google Scholar]
- Finney D. J., Probit Analysis, Cambridge University Press, London, 3rd edn, 1971. [Google Scholar]
- Dacie J. V. and Lewis S. M., Practical Hematology, Churchill-Livingstone, New York, 1984, pp. 152–178. [Google Scholar]
- Dechatelet L. R., McCall C. E., McPhail L. C., Johnston R. B. J. Clin. Invest. 1974;53:1197–1201. doi: 10.1172/JCI107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLimans W. F., Davis E. V., Glover F. L., Rake G. W. J. Immunol. 1957;79:428–436. [PubMed] [Google Scholar]
- Moron M. S., Depierre J. W., Mannervik B. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Lowry O. M., Rosenbrough N. J., Farr A. L., Randall R. J. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- Fawcett J. K., Soctt J. E. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische Z., Schwartez K. Microchim. Acta. 1937;2:13–19. [Google Scholar]
- Mejbaum W. Z. Physiol. Chem. 1939;258:117–120. [Google Scholar]
- D’Amour F. F., Blood F. R. and Belden D. A., The manual for Laboratory Work in mammalian Physiology, The University of Chicago Press, Chicago, 1965, pp. 148–150. [Google Scholar]
- Brecher G., Cronkite E. P. J. Appl. Physiol. 1950;3:365. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- Drabkin D. L., Austin J. H. J. Biol. Chem. 1932;98:719–733. [Google Scholar]
- Sur P., Bag S. P., Khanam J. A. Neoplasma. 1997;44:197–201. [PubMed] [Google Scholar]
- Gupta M., Mazumder U. K., Sambath kumar R., Sivakumar T., Vamsi M. L. M. J. Pharmacol. Sci. 2004;94:177–184. doi: 10.1254/jphs.94.177. [DOI] [PubMed] [Google Scholar]
- Kurata M., Suzuki M., Agar N. S. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol. 1993;106(3):477–487. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- Zimmer R., Thomas P. Cancer Res. 2001;61:2822–2826. [PubMed] [Google Scholar]
- Abu-Sinna G., Esmat A. M., Al-Zahaby N. A., Soliman T. M. I. Toxicon. 2003;37:1509–1524. [Google Scholar]
- Borentain P., Gerolami R., Dodero F., Chrestian M. A., Quillichini F., Rdissone J., Perrimond H., Chamlian A., Gerolami A. Gastroenterol. Clin. Biol. 2006;30:304–306. doi: 10.1016/s0399-8320(06)73170-0. [DOI] [PubMed] [Google Scholar]
- Korekane H., Nishikawa A., Imamura K. Arch. Biochem. Biophys. 2003;412(2):216–222. doi: 10.1016/s0003-9861(03)00041-9. [DOI] [PubMed] [Google Scholar]
- Hussein S. A., Azab M. E. Egypt. J. Biochem. 1997;15:6180. [Google Scholar]
- Shreaz S., Shiekh R. A., Raja V., Wani W. A., Behbehani J. M. Chem.-Biol. Interact. 2016;247:64–74. doi: 10.1016/j.cbi.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Pizarro A. M., Sadler P. J. Biochimie. 2009;91(10):1198–1211. doi: 10.1016/j.biochi.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad E. A., Habib S. A. Free Radicals Antioxid. 2013;139:265–272. [Google Scholar]
- Saad E. A. Natural Science. 2013;5(1):1–6. doi: 10.4236/ns.2013.51001. [DOI] [Google Scholar]
- Fridovich I. Adv. Enzymol. Relat. Areas Mol. Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Saad E. A., Hassanien M. M., El-Hagrasy M. A., Radwan K. H. Int. J. Pharm. Pharm. Sci. 2015;7(7):397–402. [Google Scholar]
- Yariktas M., Doner F., Dogru H., Aynali G., Yonden Z. and Delibas N., Malondialdehyde levels and antioxidant enzyme activities in head and neck malign tumors (in Turkish), Süleyman Demirel University School of Medicine Journal, 2003, vol. 10, pp. 65–67. [Google Scholar]
- Abdel Malak C. A., Toson E. A., El-sharabasy M. M., El-sherbiny M., Saad E. A. Journal of the Medical Research Institute. 2003;24(2):1–13. [Google Scholar]
- Saad E. A., Toson E. A., Ahmed G. M. Int. J. Pharm. Pharm. Sci. 2015;7(6):72–76. [Google Scholar]
- Sinclair A. J., Barnett A. H., Lunie J. Br. J. Hosp. Med. 1990;43:334–344. [PubMed] [Google Scholar]
- DeWys W. D. Cancer Res. 1982;42:721s–726s. [PubMed] [Google Scholar]
- Yagi K. Chem. Phys. Lipids. 1991;45:337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Singh K., Ahluwalia P. Indian J. Clin. Biochem. 2002;17:29–33. doi: 10.1007/BF02867938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad E. A. Biochem. Biophys. Res. Commun. 2012;423:147–151. doi: 10.1016/j.bbrc.2012.05.102. [DOI] [PubMed] [Google Scholar]
- Sun Y., Oberley L. W., Elwell J. H., Sierra Rivera E. Int. J. Cancer. 1989;44:1028–1033. doi: 10.1002/ijc.2910440615. [DOI] [PubMed] [Google Scholar]
- Bozzi A., Mavelli J., Finazzi Agro A., Strom R., Wolf A. M., Mondovi B., Rotilio G. Mol. Cell. Biochem. 1976;10:11–16. doi: 10.1007/BF01731676. [DOI] [PubMed] [Google Scholar]
- Opare Kennedy D., Kojima A., Hasuma T. Chem.-Biol. Interact. 2001;134:113–133. doi: 10.1016/s0009-2797(00)00251-9. [DOI] [PubMed] [Google Scholar]
- De Gowin R. L., Gibson D. P. Exp. Hematol. 1978;6:568–575. [PubMed] [Google Scholar]
- Barger A., Medicinal Chemistry, John Wiley & Sons, London, 3rd edn, 1981, pp. 602–653. [Google Scholar]
- KunduSen S., Bala A., Kar B., Bhattacharya S., Mazumder K. U., Gupta M., Haldar K. P. Alternative Medicine Studies. 2012;2:48–51. [Google Scholar]