ABSTRACT
Severe burn causes systemic inflammation and hypercatabolism, resulting in damage to multiple organs distant to the burn site, including the musculoskeletal system. Bone mass and muscle loss have been reported. However, tendon that connects bone and muscle has not been studied in comparable detail. Here we aimed to characterize the molecular and functional changes in Achilles tendon triggered by severe burn. Forty male Sprague–Dawley rats received 40% total body surface area scald burn. Achilles tendons were collected up to 14 days postburn. Sham-treated animals served as a control group. We analyzed tendons for changes in expression of IL-6, IL-1β, TNF, MMP9, MMP13, TGFβ1, Collagens I and III, and for morphological and biomechanical changes. Gene expression of IL-6 and IL-1β as well as MMP9 and MMP13 increased in rat tendon 3 days after burn. Col3a1 increased at day 3 and col1a1 at day 7. At day 14, TGFβ1 increased, whereas the protein ratio for collagens I/III decreased, indicating tendon remodeling. Histological analysis with H&E and Picrosirius red staining further revealed a decrease in organized collagen fibers 14 days after burn. Biomechanical analysis showed a decrease in stiffness and ultimate force of tendons in burn rats.
We conclude that tendinopathy was observed in Achilles tendon 14 days after severe burn, via the induction of inflammation and remodeling. The present study provides a model of tendinopathy that may be used for the development of therapeutic approaches after burn.
Keywords: Biomechanical property, collagen alignment, tendinopathy, thermal injury
INTRODUCTION
Severe burn is defined as a full-thickness burn of greater than 30% of total body surface area (TBSA). It occurs at a rate of approximately 5/100,000 persons per year globally and may affect multiple organs, even those distantly located from the burn site (1). In the musculoskeletal system, severe burn induces hypercatabolism in muscle and bone due to the activation of systemic inflammation, and disuse from long immobilization periods (2–6). Muscle loss and atrophy postburn have been associated with increased levels of TNF-α (7), whereas bone mass loss has been associated with circulating TNF-α, IL-1β, and IL-6, which are increased shortly after burn (8–10). Despite the findings of mass loss in both skeletal muscle and bone, there are limited studies on the molecular and structural effects of burn injury on tendon.
Tendon injuries are common events occurring in sports and other activities (11–13). Tendons are mostly composed of type I collagen fibrils aggregated together into fibers to provide tensile strength (14). There are also type III collagen fibrils intercalated into the collagen I fibers (14). When tendon experiences remodeling or injury, collagen III content increases, resulting in a decrease in tensile strength (15). On the contrary, metalloproteinases such as the collagenase MMP13 can cleave the collagen triple helix, creating fragments that are further degraded by gelatinases such as MMP9 (16). In addition, modulators of musculoskeletal growth and differentiation, such as TGFβ1, have been reported to coincide with scar formation and healing in tendon (17).
In this study, we hypothesize that the systemic inflammation caused by severe burn will induce molecular and structural changes in Achilles tendon that ultimately debilitate this tissue. By understanding the local changes produced in tendon, it is possible to adopt additional preventive measures to improve healing and to aid with physical therapies that target muscle and bone.
MATERIALS AND METHODS
Animals
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center at Dallas in accordance with the Institutional and Association for Assessment and Accreditation of Laboratory Animal Care guidelines and in accordance with NIH guidelines. All animal procedures were carried out at the UT Southwestern animal facility. A total of 40 adult male Sprague–Dawley rats (Charles Rivers), 270–300 g, were acclimated in an animal facility a week before the experiment. Animals were fed a pellet diet (Harlan Teklad #2018) ad libitum and housed in a reversed 12-h light/dark cycle with maintained room temperature.
Burn procedure
Animals were randomly divided into five groups: control (n = 11), and 1 day (n = 6), 3 days (n = 6), 7 days (n = 6), and 14 days (n = 11) postburn. Under 2% to 4% isoflurane inhalation anesthesia, animal hair was removed from the dorsal and lateral surfaces and the animals were secured in a specifically constructed template device with opening size of 10 cm length and 7 cm wide (curved). Dorsal skin amounting to 40% of TBSA was exposed through the device aperture and immersed in 95°C to 100°C water for 10 s. The burned animals then received intraperitoneally 4 mL/kg body weight/TBSA% of lactated Ringer's solution for resuscitation immediately after injury. Animals were given analgesia intraperitoneally (buprenorphine HCl 0.05 mg/kg) immediately and 12 h postburn. Control animals received sham treatment with the procedure of hair removal, anesthesia, and submersion in room temperature water. Control animals were not subjected to resuscitation procedure because injecting this amount of liquid intraperitoneally will cause cardiac failure and/or abdominal compartmentalization syndrome that would be fatal.
Tissue harvesting
Animals were euthanized with anesthetic overdose. Achilles tendons were isolated from surrounding tissue and dissected from both sides of hind limbs. The proximal ends of the Achilles tendons were disconnected at the end of the gastrocnemius, plantaris, and soleus, and the distal ends at the calcaneus. Left leg tendons of eight control rats and six burn rats for each time point (1, 3, 7, and 14 days) were stored in RNAlater (Qiagen), whereas right leg tendons were snap frozen for protein extraction. Samples were stored in −80°C for further analysis. Tendon RNA was obtained with RNAeasy Universal kit (Qiagen). cDNA conversion was done with iScript kit (Bio-Rad), and qPCR with SsoFast Eva Green kit (Bio-Rad). Primers for IL-6, TNF, IL-1β, col1a1, col3a1, MMP9, MMP13, and TGFβ1 were purchased from QuantiTect Primers (Qiagen). Gene expression was calculated using the ΔΔCt method with 18 s as housekeeping.
For protein extraction, tendon tissue was homogenized in RIPA buffer (Invitrogen) with proteinase inhibitor cocktail (Sigma). Protein quantification was performed with BCA assay (Pierce); 10 μg of total protein was used for Western blot. Antibodies included anti-collagen I (Abcam), anti-collagen III (Abcam), goat anti-mouse HRP (Pierce), and goat anti-rabbit HRP (Pierce), which were prepared in 2% BSA. Blots were developed with ECL (Pierce) using a CCD camera system.
Histology
Right leg Achilles tendons were dissected from three control rats and five burn rats (14 days). Parts of the calcaneus bone and gastrocnemius muscle were included. Tendons were fixed in 10% neutralized buffer formalin and decalcified with 10% formic acid. Paraffin sections of 5 μm were processed for hematoxylin and eosin (H&E) staining, as well as Picrosirius red staining. Samples were visualized in a Nikon Eclipse Ti microscope or in an Olympus BH2-RFCA using polarized light. The image analysis for Picrosirius red was performed using ImageJ (National Institutes of Health (18)). Several regions of 320 × 320 pixels were selected from the central area of each tendon. After converting the image into 8-bit and 256 color, the measurements of red, green, and blue colors were postprocessed in Excel. For each piece, the (green + blue)/red values were calculated and grouped as control vs. 14 days postburn, where green + blue represent more organized fibers compared with red.
Biomechanics
Three tendons from the control group and five tendons from the burn rats at 14 days postburn were dissected, leaving part of muscle and foot, and wrapped with cold PBS-soaked gauze to prevent dehydration. Sandpaper was fixed to both tendon extremities. Specimens were then clamped and tested using an Instron 5565 universal testing system (Instron Corp., Norwood, MA) equipped with a 5-kN load cell. After precondition using a cyclic load oscillating from 0% to 2.5% strain for 20 cycles, samples were finally pulled until failure at a crosshead speed of 6 mm/min collecting force and deformation data throughout the test. Tendon material properties, ultimate tensile force, and stiffness were calculated from the force-deformation curve.
Statistics
Data are presented as mean ± error propagation for gene expression and mean ± standard deviation elsewhere. Data were analyzed in GraphPad Prism 7 with one-way ANOVA and Fisher LSD posthoc test, or by unpaired Student t test when comparing two variables (P < 0.05 being significant).
RESULTS
Changes in gene expression of cytokines, MMPs, and TGFβ1 in Achilles tendon after burn
The gene expression of the proinflammatory cytokines in tendon tissue IL-1β and IL-6 was affected by severe burn. Both increased and reached a peak level at 3 days postburn, being significantly higher compared with control group, P < 0.05. The expression of TNF was also higher than control but did not reach a significant level. After 7 days, all three genes decreased back to control levels (Fig. 1A).
Fig. 1.
Changes in expression of proinflammatory cytokines, metalloproteases, and collagens in Achilles tendon after severe burn.
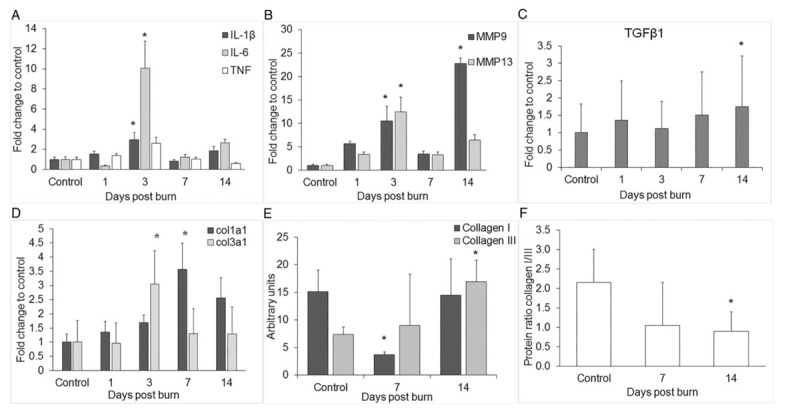
(A) Gene expression of cytokines IL-1β, IL-6, and TNF (B) metalloproteinases MMP9 and MMP13, (C) TGFβ1 and (D) collagens col1a1 and col3a1 were examined with qPCR. (E) Protein levels of collagen I and collagen III obtained by Western blot and normalized to total protein (F). Collagen I/III protein ratio was calculated from the Western blot results. Data are shown as mean with error propagation in (A) and (B). Data are shown as mean ± SD in (C) and (D). ∗P < 0.05 vs. control.
We next analyzed whether the expression of the matrix metalloproteinases commonly affected in tendinopathy were altered by burn. The results showed that the expression of both MMP9 and MMP13 continually increased after the injury and reached significantly higher levels at 3 days postburn compared with the control group, pP < 0.05 (Fig. 1B). MMP9 showed a second significant increase at 14 days postburn by more than 20-fold compared with control, P < 0.05 (Fig. 1B).
TGFβ1, which has been linked to tendon repair and healing, was also analyzed. We found a significant upregulation at day 14 postburn (P < 0.05) (Fig. 1C).
Changes in collagen expression and collagen I/III ratio
The gene expression of col3a1 significantly increased at day 3 postburn, whereas col1a1 did it at day 7 postburn (Fig. 1D). Protein levels of collagen I were significantly lower at day 7 postburn, P < 0.05 (Fig. 1E) and were restored back to control levels by day 14. Collagen III protein, on the contrary, increased significantly at day 14 postburn, P < 0.05 (Fig. 1E). The protein ratio of collagens I/III showed a significant decrease at day 14 postburn (Fig. 1F).
Histological and biomechanical evidence of tendon remodeling
We analyzed the control and 14-day postburn tendons stained with H&E to further investigate signs of remodeling. Tenocytes and extracellular matrix fibers were found aligned with the longitudinal axis in the control group. Most of the nuclei of tenocytes showed an elongated shape. In contrast, tendons from animals at 14 days postburn showed less parallel organization of cells and fibers, as well as hypercellularity with cell aggregation and round nuclei (Fig. 2A). To observe collagen organization in more detail, tendon sections were stained with Picrosirius red and analyzed under polarized light. A representative image of a control rat showed that collagen fibers were more organized and well aligned compared with 14 days postburn, where tendon fibers showed less organization (Fig. 2B). Our method of quantification of green, blue, and red colors showed that the (green + blue)/red ratio significantly decreased 14 days postburn, P < 0.05 (Fig. 2C).
Fig. 2.
Histological evidence of changes in Achilles tendon after severe burn.
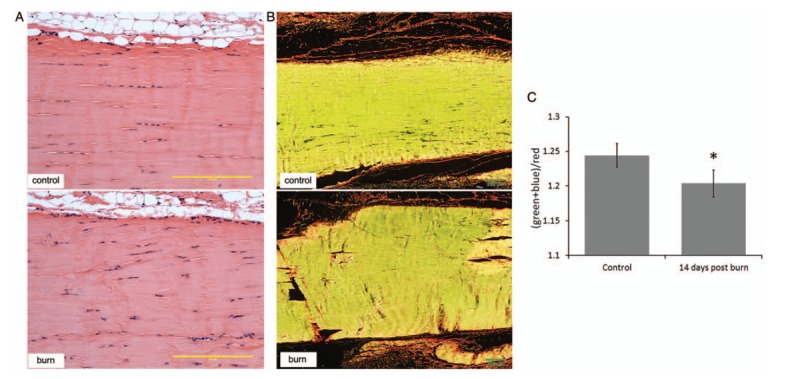
(A) Tendons from control rats (upper panel) and 14-day postburn rats (lower panel) were stained with hematoxylin and eosin to show cell morphology and gross fiber organization. (B) Slides from the same tendons were used for Picrosirius red staining and analyzed with polarized light to show fiber organization of control (upper panel) vs. 14-day postburn rats (lower panel). (C) Quantification of (green + blue)/red of the Picrosirius red images under polarized light was used to determine organization of fibers. Scale bars = 200 μm. ∗P < 0.05 vs. control.
To assess tendon function in parallel with histological changes, we measured the ultimate force of the left tendons of the same control and 14-day postburn rats utilized for histology (Fig. 3A). Data obtained from three control rats and five burn rats showed that control tendons had a higher ultimate tensile force or maximum force before failure (Fig. 3B). The average ultimate force for the control group was 43.7 ± 3.0 N, whereas tendons of the 14-day postburn group showed an average ultimate force of less than half that of the controls at 19.9 ± 9.7 N, P < 0.05.
Fig. 3.
Biomechanical changes in Achilles tendon after severe burn.
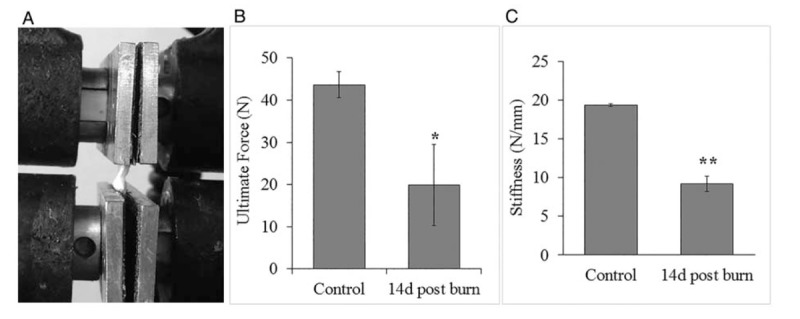
(A) Example of tendon set-up for biomechanical testing. Tendons are pulled until failure. (B) Maximum load values obtained from the force-deformation curve of three control rats and five injured rats 14 days postburn. (C) Stiffness values calculated from force-deformation curves comparing no-burn vs. 14-day postburn tendons. ∗P < 0.05, ∗∗P < 0.001 vs. control.
We also calculated tendon stiffness, which is the amount of measured deformation from an applied force. Control tendons had an average stiffness of 19.38 ± 0.16 N/mm that is significantly different from 14-day postburn rats with an average stiffness of 9.18 ± 0.99 N/mm, P < 0.001 (Fig. 3C).
DISCUSSION
In accordance with an induction of acute inflammation, we observed a significant increase in gene expression of the proinflammatory cytokines IL-1β and IL-6 as well as the matrix metalloproteinases involved in remodeling, MMP9 and MMP13. We observed a decrease in collagen I/III protein expression, upregulation of TGFβ1, histological signs of remodeling, and a decrease in ultimate tensile strength of tendon 14 days postburn. Taken together, these results demonstrate, for the first time, the presence of inflammation and remodeling in tendon distant from the burn site after severe burn. This is consistent with similar findings in the literature for both skeletal muscle and bone (1–4, 7).
Serum levels of IL6 have been previously reported to increase rapidly after burn and stay higher than nonburn for several days (10, 19). The local increase in IL-1β and IL-6 expression that we observed in tendons indicates an inflammatory response in this tissue. IL-6 has been suggested to also act as a growth factor by stimulating the synthesis of collagen in response to mechanical loading (20). Therefore, the rise of both IL-1β and IL-6 levels points toward the initiation of acute inflammation in the tendon with a concomitant induction of collagen synthesis.
Previous reports have shown the involvement of IL-1β in the upregulation of MMPs and tissue remodeling in tendon (21, 22). Even though MMPs are necessary for normal tissue homeostasis, an imbalance in the activity of these metalloproteases has been shown to be detrimental to tissue healing after injury, as an improvement of Achilles tendon repair can be obtained by the use of the nonselective MMP inhibitor, doxycycline (23). More investigation is needed to assess whether the increase in proinflammatory cytokines is mediated by tenocytes or by macrophage infiltration into the tissue after generation of the systemic response and to evaluate chronic inflammation at later time points.
Our results revealed a significant decrease in the collagen I protein level at 7 days postburn, with a subsequent increase akin to the control level by day 14. We propose that the initial decrease in collagen I can be explained by the upregulation of MMP13 and MMP9, generated by IL-1β. Along with this, an analysis of gene expression of collagens showed that collagen III mRNA was upregulated at day 3, whereas its protein was detected elevated at day 14 postburn. Collagen I showed significantly lower protein level at day 7, together with an upregulation of its mRNA on the same time point and followed by restored protein levels similar to control by day 14 postburn. The protein ratio collagen I/III was significantly reduced by day 14. A similar shift in collagen expression at early time points has been observed for tendon-to-bone healing in rotator cuff injury (17). Analysis of gene expression of TGFβ1 showed significant upregulation at day 14. This is an important factor involved in musculoskeletal tissue differentiation that has been reported to correlate with formation of scar tissue and less organized fibers in healing tendon (17). The synthesis of collagen III protein, upregulation of TGFβ1, and the decrease in the collagen I/III protein ratio suggest an induction of scar tissue or remodeling (15). Another proinflammatory cytokine that is upregulated in rheumatic diseases and present in tendon enthesis is IL-17a (24). This is produced by entheseal CD3+ lymphocytes, peripheral blood CD4+ T cells, and entheseal γ/δ T cells. We analyzed its gene expression throughout the time course, but did not find statistical significance (data not shown). This could be explained due to the chronic nature of rheumatic diseases compared with this more acute response.
Histological observations of collagen alignment and tenocyte distributions showed less tissue organization 14 days postburn compared with control. H&E staining showed less fiber alignment, cell aggregations, and even round nuclear morphology on some cells. This, together with the observation of changes in collagen composition, further indicates cellular events for tissue remodeling and possible weakening of the tendon. Collagen distribution and alignment are crucial for the transmission of forces in the tendon (14). In mature tissues, fibrils can be found parallel to the axial direction of tissue and also interwoven with the presence of a crimp pattern that provides its tensile properties (14, 15, 25). This anisotropy will derive into nonuniform strains on the cells, which can alter the cell morphology and response to differential mechanical forces. To address whether molecular changes in tissue structure will also affect its functional mechanics, the tensile force and deformation were measured. Overall, our findings showed that ultimate force and stiffness were reduced at 14 days postburn compared with controls. These biomechanical results correlate with histological findings of less organized fibers. It is possible that the initial loss in collagen I followed by an induction of synthesis of both collagens I and III results in fiber disorganizationbecause collagen III has been previously reported as accumulated at the rupture site in tendon (15). Although previous reports have shown that Achilles tendon could react to an inflammatory event produced in surrounding tissue (26), this study is the first to report that remote systemic inflammation is capable of inducing a local inflammatory response and remodeling in the tendon. Other models of trauma that generate systemic inflammation, such as fracture hematoma, have reported a similar pattern of circulating cytokines (27–29). Fracture hematoma with additional chest trauma has shown that systemic inflammation is capable of interfering with the normal inflammatory process on fracture healing (30). This evidence is supportive to the hypothesis that a trauma-generated systemic inflammation is potentially detrimental for soft tissues like tendon.
Severe burn patients suffer skeletal muscle and bone mass loss that can be attenuated by the introduction of physical activity. Considering the tendon as the connector and force transmitter between skeletal muscle and bone, it is essential to understand tendon's behavior in response to severe injury. Future investigation regarding the modulation of the inflammatory cascade and MMP inhibition accompanied by regulated physical therapy that allows proper tissue loading to prevent disuse, but also prevent overuse (31–35) may be promising for the improvement of recovery after severe burn and other traumatic injuries.
Acknowledgments
The authors thank the Hofmann Fund for resident research from the Department of Orthopaedic Surgery and The Golden Charity Guild Charles R. Baxter, MD, Chair Department of Surgery fund. The authors would also like to thank The Molecular Pathology Core of UT Southwestern for processing the samples for histology and Dave Primm for his help in editing this manuscript.
Footnotes
Authors’ contributions: PH, JS, and SW designed the experiments and project. PH, DB, TM, JW, RH, DC, and JS performed the experiments. PH, HL, JW, KM, TW, and JS analyzed the data. All authors were involved in drafting and revising the manuscript.
Financial support: Department of Defense, grant number W81XWH-13-1-0462.
The authors report no conflicts of interest.
REFERENCES
- 1.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol 19 9:777–783, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg 232 4:455–465, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein GL. Burn-induced bone loss: importance, mechanisms, and management. J Burns Wounds 5:e5, 2006. [PMC free article] [PubMed] [Google Scholar]
- 4.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery 128 2:312–319, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Analan PD, Leblebici B, Adam M, Sariturk C. Bone loss during the acute stage following burn injury: is it local or systemic? West Indian Med J 19 9:777–783, 2016. [Google Scholar]
- 6.Klein GL, Wolf SE, Goodman WG, Phillips WA, Herndon DN. The management of acute bone loss in severe catabolism due to burn injury. Horm Res 48 Suppl. 5:83–87, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Song J, Saeman MR, De Libero J, Wolf SE. Skeletal muscle loss is associated with TNF mediated insufficient skeletal myogenic activation after burn. Shock 44 5:479–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamgadge S, Shetty A, Tamgadge A, Bhalerao S, Periera T, Gotmare S. Study of polarization colors in the connective tissue wall of odontogenic cysts using picrosirius red stain. J Orofac Sci 7 2:119, 2015. [Google Scholar]
- 9.O’Halloran E, Kular J, Xu J, Wood F, Fear M. Non-severe burn injury leads to depletion of bone volume that can be ameliorated by inhibiting TNF-alpha. Burns 41 3:558–564, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 30 5:503–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 39 12:1338–1344, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Doral MN, Bozkurt M, Turhan E, Donmez G, Demirel M, Kaya D, Atesok K, Atay OA, Maffulli N. Achilles tendon rupture: physiotherapy and endoscopy-assisted surgical treatment of a common sports injury. Open Access J Sports Med 1:233–240, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley G. Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol 4 2:82–89, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports 10:312–320, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res 20:1352–1357, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol 21 2:185–195, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res 24 3:541–550, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82 (7–8):518–529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock 26 1:13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Andersen MB, Pingel J, Kjaer M, Langberg H. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol (1985) 110 6:1549–1554, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1β induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res 20 1:36–39, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res 21 2:256–264, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kessler MW, Barr J, Greenwald R, Lane LB, Dines JS, Dines DM, Drakos MC, Grande DA, Chahine NO. Enhancement of Achilles tendon repair mediated by matrix metalloproteinase inhibition via systemic administration of doxycycline. J Orthop Res 32 4:500–506, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdorfer L, Korn T, Weiss S, Forster R, Prinz I. Interleukin-23-dependent gamma/delta T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 68 10:2476–2486, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Provenzano PP, Vanderby R., Jr Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol 25 2:71–84, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Vieira CP, Guerra Fda R, de Oliveira LP, de Almeida Mdos S, Pimentel ER. Alterations in the Achilles tendon after inflammation in surrounding tissue. Acta Ortop Bras 20 5:266–269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claes L, Ignatius A, Lechner R, Gebhard F, Kraus M, Baumgartel S, Recknagel S, Krischak GD. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop 82 2:223–227, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8 3:133–143, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Recknagel S, Bindl R, Brochhausen C, Gockelmann M, Wehner T, Schoengraf P, Huber-Lang M, Claes L, Ignatius A. Systemic inflammation induced by a thoracic trauma alters the cellular composition of the early fracture callus. J Trauma Acute Care Surg 74 2:531–537, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Ignatius A, Ehrnthaller C, Brenner RE, Kreja L, Schoengraf P, Lisson P, Blakytny R, Recknagel S, Claes L, Gebhard F, et al. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. J Trauma 71 4:952–960, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enwemeka CS. Inflammation, cellularity, and fibrillogenesis in regenerating tendon: implications for tendon rehabilitation. Phys Ther 69 10:816–825, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Hardee JP, Porter C, Sidossis LS, Borsheim E, Carson JA, Herndon DN, Suman OE. Early rehabilitative exercise training in the recovery from pediatric burn. Med Sci Sports Exerc 46 9:1710–1716, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godleski M, Oeffling A, Bruflat AK, Craig E, Weitzenkamp D, Lindberg G. Treating burn-associated joint contracture: results of an inpatient rehabilitation stretching protocol. J Burn Care Res 34 4:420–426, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Paratz JD, Stockton K, Plaza A, Muller M, Boots RJ. Intensive exercise after thermal injury improves physical, functional, and psychological outcomes. J Trauma Acute Care Surg 73 1:186–194, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Saeman MR, Baer LA, Cai AR, Wade CE, Wolf SE. Exercise altered the skeletal muscle microRNAs and gene expression profiles in burn rats with Hindlimb unloading. J Burn Care Res 38 1:11–19, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


