ABSTRACT
Background:
Dextran-70 is a more potent plasma volume expander than albumin but use has been hampered because of its antithrombotic properties. However, also albumin has antithrombotic properties and little is known about relative effects of these two colloids on coagulation in vivo when controlling for differences in efficacy as plasma volume expanders.
Aim:
Compare effects of dextran-70 and albumin on coagulation at a dose resulting in equal plasma volume expansion.
Methods:
Guinea pigs were subjected to a 25 mL/kg hemorrhage during 20 min and randomized to resuscitation with either 6% dextran-70 at a dose of 15 mL/kg or 5% albumin at a dose of 25 mL/kg (n = 14 in each group) during 30 min starting 1 h of shock. Blood samples were collected at the completion of resuscitation and at 4 h. Plasma volume was measured using 125I-albumin and the effect on coagulation was evaluated using whole blood thrombelastography (TEG), measurement of plasma fibrinogen and von Willebrand factor (vWF) concentrations and vWF glycoprotein 1b (GP1b) A activity.
Results:
Plasma volumes after resuscitation were similar in the groups at both time points. Dextran-70 resulted in a transient prolongation of TEG clot amplification time (K) at the completion of resuscitation compared with albumin. TEG clot initiation (R) and strength (MA) did not differ between the treatments at any of the time points. Albumin reduced vWF concentrations to a larger extent than dextran at both time points, whereas no difference in vWF GP1bA activity or in plasma fibrinogen and could be detected.
Conclusion:
In equipotent doses with regard to plasma volume expansion, dextran-70 transiently prolongs clot amplification time more than albumin whereas dextran-70 reduces plasma vWF concentrations less than albumin.
Keywords: Fibrinogen, plasma volume, thrombelastography, von Willebrand factor, whole blood
INTRODUCTION
Fluid resuscitation in the critically ill patient is a controversial topic. Given that colloids are more efficacious plasma volume expanders than crystalloids, use of colloids may improve outcome by reducing harmful effects of fluid overload (1, 2). Studies showing that the use of albumin in subgroups of critically ill patients may improve outcome align with this hypothesis (3, 4). However, albumin is expensive and availability is limited meaning that the study of alternatives to albumin is of considerable interest.
After several reports of detrimental effects of hydroxylethyl starches in critically ill patients (5, 6) dextran-70 has emerged as a potential alternative to albumin. Dextrans are a group of naturally occurring polysaccharides produced by bacteria and may be found for example in human dental plaques (7). Dextran-70 has been used a plasma volume expander since the forties and is a more efficacious plasma volume expander than albumin (8–10). If administered after the injection of small sized (1 kD) dextran molecules, dextran-70 has an incidence of allergic reactions similar to that of albumin (11).
Dextran-70 has antithrombotic effects that may be attributed both to dilution of coagulation factors secondary to plasma volume expansion, and to specific effects on coagulation such as decreased activity of the von Willebrand factor (vWF) and factor VIII (12). Additionally, dextran is suggested to affect fibrin polymerization and stability (13–15). The antithrombotic effects have hampered use dextran-70 as plasma volume expander because of fear of bleeding. While some support for this concern may be inferred from two small retrospective studies in septic patients (16, 17), a recent larger study could not demonstrate an association between dextran-70 and clinically relevant bleeding in sepsis (18).
Resuscitation with albumin is also suggested to affect coagulation (19–23) and to our knowledge only one in-vivo study has compared the anticoagulant effect of albumin with dextran-70 (24). In the latter study it was shown dextran-70 impairs coagulation more than albumin if administered in the same volumes for resuscitation in a rabbit model of hemorrhage. However, given that dextran-70 is a more potent plasma volume expander than albumin, plasma volumes after resuscitation are likely to have differed. Therefore, it is unknown to what extent effects of albumin and dextran-70 can be referred to dilution of coagulation factors and to what extent they can be referred to intrinsic effects of respective colloid on coagulation.
Based on these considerations we hypothesized that the proposed larger effects of dextran-70 on coagulation, relative to those of albumin, are largely secondary to differences in different efficacy as plasma volume expanders. The objective of the present study was therefore to compare effects of dextran-70 and albumin when administered in equipotent doses with regard to plasma volume expansion. For this purpose guinea pigs were exposed to a 30% hemorrhage and were then resuscitated to equal plasma volume with either 5% albumin or dextran-70. Effects on coagulation were estimated with thrombelastography of whole blood, measurement of plasma fibrinogen and vWF concentrations and vWF activity.
MATERIALS AND METHODS
Animals
The Lund University Ethical Committee for Animal Research (M309-12) approved the study. Animals were treated in accordance with the guidelines of the National Institutes of Health for Care and Use of Laboratory animals. Sawdust was used as bedding and animals had unlimited access to standard rodent chow and water and were housed in 12 h light/dark cycles at a temperature of 24°C. Guinea pigs were used as experimental model because rats are allergic to dextran and because guinea pig platelet function is suggested to resemble that of humans (25, 26). A total of 35 adult male Dunkin Hartley guinea pigs (Taconic, Ejby, Denmark) weighing 378 ± 22 g were used in the study.
Anesthesia and preparation
The animals had free access to water and food until the start of the experiment. Anesthesia was induced by placing animals in a covered glass container with a continuous supply of 5% isoflurane (Isoflurane Baxter, Baxter AB, Sweden). After a tracheostomy, the animals were connected to a ventilator (Ugo Basile; Biological Research Apparatus, Comerio, Italy) and anesthesia was maintained with isoflurane at a dose of 1.5–1–8% in humidified room air. Tidal volumes were 10 mL/kg and a positive end-expiratory pressure of 3 to 4 cm H2O was applied to prevent atelectasis. The core temperature was kept at 37.1°C to 37.3°C using a heating pad. The left carotid artery was cannulated for the measurement of mean arterial blood pressure and for blood sampling. To prevent thrombosis the arterial line was filled with heparin in 0.9% sodium chloride at a concentration of 50E/mL. Blood gases, sodium, hematocrit were measured using a portable analyzer (I-STAT; Abbot Point of Care Inc, Abbot Park, Ill). The right and left jugular veins were cannulated to measure central venous pressure for injections and fluid administration. After the completion of the protocol, the animals were killed with an intravenous injection of potassium chloride.
Measurement of plasma volume
Plasma volume was determined by calculating the distribution volume of a known amount of human 125I-albumin. Briefly, this was accomplished by measuring the radioactivity in 100 μL of plasma taken 5 min after an intravenous 0.5 mL injection of 125I-albumin. Administered dose of albumin was calculated by subtracting the activity in the used syringe, and needle from the activity in the syringe prior to injection. The increase in radioactivity in plasma was calculated by subtracting the activity in a plasma sample taken just before the injection from that taken 5 min after the injection. Free iodine was measured continuously following precipitation with 10% trichloroacetic acid, and it was found to be 3.8 ± 2.7% in the prepared samples. Radioactivity was measured with a gamma counter (Wizard 1480; Wallac, Turku, Finland).
Thrombelastography
Blood coagulation was evaluated using thrombelastography (TEG) analysis of 450 uL of blood collected in a vial containing 50 uL of 3.2% buffered sodium citrate. After 30 min at room temperature, analysis was conducted in a computerized thrombelastograph (TEG, model 5000, Software Version 4.1.73, database version 1.0.16, Hemoscope Corporation [Niles, Ill]) according to the manufacturer's recommendations. The clot activator used was the RapidTEG assay that activates clot formation with kaolin (intrinsic activator) and recombinant mouse tissue factor (extrinsic activator). The variables presented here are reaction time (R) (min) reflecting rate of initial fibrin formation (clot initiation), clot amplification (K) the time from the end of R until clot amplitude reaches 20 mm, reflecting speed of clot formation, the maximal amplitude (MA) (mm) reflecting maximum clot strength, and clot lysis after 30 min (LY30) reflecting fibrinolysis. All the samples were run in TEG heparinase cups (blocking potential heparin effects from the arterial line, see above).
Fibrinogen and von Willebrand factor
Effects of dextran relative to those of albumin in equipotent doses on fibrinogen plasma concentration, vWF antigen concentration, and activity were analyzed in separate animals. Blood was collected in citrated tubes as described above and centrifuged for 20 min at 2,000 × g. Plasma was separated and immediately frozen at −70°C. This plasma was analyzed with commercial available tests adopted for human plasma (see below). Plasma concentration of fibrinogen was analyzed using an ELISA according to the instructions by the manufacturer (Zymutest Fibrinogen, Aniara Diagnostica, West Chester, Ohio). The vWF antigen concentration was analyzed with a latex immunoassay: vWF Ag (Siemens) on a BCS-XP (Siemens). Latex-particles are coated with antibodies against vWF. Upon binding, agglutination occurs, which is measured optically. The function of vWF was analyzed with latex vWF glycoprotein 1b (GP1b) activity test Innovance (Siemens). It relies on a monoclonal antibody that recognizes the functional GPIb-binding epitope on vWF and reflects vWF effects on platelet adhesion and aggregation (27).
Experimental protocol
An overview of the experimental protocol is presented in Figure 1. At 10 min after the completion of surgical preparation and baseline measurements were performed. Animals were then subjected to a hemorrhage of 25 mL/kg over 30 min via the cannulated femoral artery. This volume of hemorrhage corresponds to ≈ 30% of blood volume as calculated by dividing plasma volume at baseline by (1-Hct). At 1 h after the completion of the hemorrhage a blood sample for blood gas analysis was collected. Animals were then resuscitated with either 6% dextran-70 in 0.9% NaCl (Macrodex, Meda, Solna, Sweden) at dose of 15 mL/kg or 5% Albumin in 0.9% NaCl (Alburex, CSL Behring) at a dose of 25 mL/kg during 30 min. The lower dose of dextran-70 was chosen to give equal plasma volume expansion as 5% albumin and was chosen based on previous studies and pilot experiments (10, 28). Blood samples for measurement of plasma volume, arterial blood gases, TEG, fibrinogen, vWF Ag and vWF GP1b activity were collected immediately after completion of resuscitation and 4h after completion of resuscitation. For comparison a group of animals resuscitated with dextran-70 at a dose of 25 mL/kg was also included in the TEG analysis. After the completion of the protocol, the animals were killed with an intravenous injection of potassium chloride.
Fig. 1.
Schematic figure of experimental protocol.
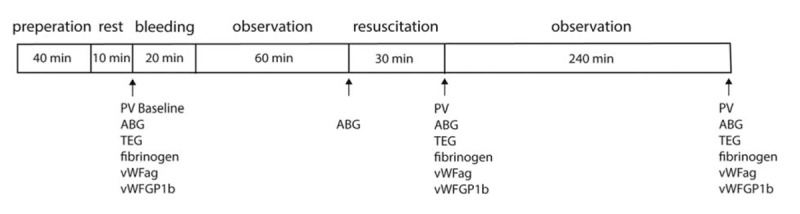
ABG indicates arterial blood gas; PV, plasma volume; TEG, thrombelastography; vWF Ag, von Willebrand factor antigen; vWF GP1b, vWF glycoprotein 1b. Note that TEG parameters on one hand and fibrinogen, vWF Ag and vWF GP1b activity on the other hand were analyzed in separate animals.
Statistical analysis
Based on our objective to compare effects albumin and dextran-70 on coagulation in animals receiving equipotent volumes of colloid in each group, we assumed that a difference in plasma volume expansion between the albumin treated group and the equipotent dextran-70 groups of less than 4 mL/kg was considered to be negligible. Based on a standard deviation of 3 mL/kg it could be calculated that a sample size of 8 was required for a power of 80% (29). Plasma volumes, hemodynamic, and laboratory data between groups at the different time points were analyzed using one-way ANOVA followed by Turkey multiple comparison test or Student t test as appropriate. A P value < 0.05 was considered statistically significant. Data are presented as mean ± SD. Prism software version 6.0d for Mac OS was used for the analysis (GraphPad Software, San Diego, Calif).
RESULTS
Laboratory and hemodynamic data
One animal died 1 h after resuscitation with dextran-70 at a dose of 15 mL/kg and this animal was not included in the analysis. Hemodynamic and laboratory data are presented in Table 1. No differences in laboratory and hemodynamic data could be detected prior to resuscitation. At the completion of resuscitation and at 240 min after the completion of resuscitation hematocrit was lower and mean arterial pressure higher in the group resuscitated with dextran-70 at a dose of 25 mL/kg than in the other groups.
Table 1.
Laboratory and hemodynamic data
| Dextran-70 15 mL/kg (n = 8) | Dextran-70 25 mL/kg (n = 6) | Albumin 25 mL/kg (n = 8) | |
| Baseline | |||
| MAP (mm Hg) | 34 ± 4 | 34 ± 5 | 34 ± 7 |
| Heart rate | 239 ± 22 | 228 ± 16 | 241 ± 20 |
| Hct | 39 ± 3 | 40 ± 2 | 39 ± 3 |
| pH | 7.4 ± 0.02 | 7.4 ± 0.03 | 7.4 ± 0.11 |
| pCO2 (kPa) | 4.7 ± 0.26 | 4.7 ± 0.3 | 4.9 ± 0.27 |
| pO2, (kPa) | 11 ± 1.9 | 10.7 ± 1.8 | 11.8 ± 1.3 |
| Na (mmol/L) | 135 ± 1 | 134 ± 1 | 135 ± 2 |
| K (mmol/L) | 4.4 ± 0.4 | 4.5 ± 0.8 | 4.5 ± 0.5 |
| Before resuscitation | |||
| MAP, mm Hg | 29 ± 4 | 28 ± 2 | 26 ± 4 |
| Heart rate | 241 ± 21 | 238 ± 20 | 245 ± 22 |
| Hct | 24 ± 3 | 26 ± 2 | 25 ± 3 |
| pH | 7.5 ± 0.07 | 7.5 ± 0.06 | 7.5 ± 0.05 |
| pCO2 (kPa) | 4.7 ± 0.5 | 4.6 ± 0.4 | 4.7 ± 0.29 |
| pO2, (kPa) | 11.9 ± 0.8 | 12 ± 1.4 | 12.1 ± 0.8 |
| Na (mmol/L) | 135 ± 2 | 135 ± 2 | 136 ± 2 |
| K (mmol/L) | 4.9 ± 0.6 | 5 ± 0.8 | 4.7 ± 0.2 |
| At completion of resuscitation | |||
| MAP (mm Hg) | 45 ± 8 | 53 ± 7 | 44 ± 6 |
| Heart rate | 261 ± 30 | 279 ± 22 | 255 ± 30 |
| Hct | 16 ± 1 | 14 ± 2 | 16 ± 2 |
| pH | 7.6 ± 0.05 | 7.5 ± 0.03 | 7.5 ± 0.05 |
| pCO2 (kPa) | 45 ± 0.38 | 4.7 ± 0.5 | 4.7 ± 0.49 |
| pO2, (kPa) | 11.3 ± 11 | 11.9 ± 1.2 | 11.7 ± 1.4 |
| Na (mmol/L) | 136 ± 1 | 138 ± 1 | 137 ± 2 |
| K (mmol/L) | 4.4 ± 0.2 | 4.4 ± 0.7 | 4.3 ± 0.4 |
| At 240 min after resuscitation | |||
| MAP (mm Hg) | 37 ± 4 | 40 ± 4 | 36 ± 4 |
| Heart rate | 261 ± 29 | 244 ± 24 | 252 ± 18 |
| Hct | 15 ± 1 | 14 ± 1 | 15 ± 1 |
| pH | 7.5 ± 0.05 | 7.5 ± 0.06 | 7.5 ± 0.06 |
| pCO2 (kPa) | 4.4 ± 0.4 | 4.0 ± 0.5 | 4.4 ± 0.4 |
| pO2, (kPa) | 10.5 ± 3.2 | 12.3 ± 1.9 | 9.8 ± 3.4 |
| Na (mmol/L) | 135 ± 1 | 137 ± 2 | 135 ± 1 |
| K (mmol/L) | 4.6 ± 0.4 | 4.6 ± 1 | 4.7 ± 0.4 |
Data are expressed as mean ± standard deviation.
Hct indicates hematocrit; MAP, mean arterial pressure; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of carbon dioxide in arterial blood.
Plasma volumes
Average plasma volume at baseline was 51.1 ± 5.3 mL/kg. After the completion of resuscitation plasma volume in the group resuscitated with dextran-70 at a dose of 25 mL/kg had increased more than in the groups resuscitated with dextran-70 at a dose of 15 mL/kg or the albumin group (Fig. 2). No difference in plasma volume expansion between the group resuscitated with dextran-70 at a dose of 15 mL/kg and the albumin group could be detected (difference between means, 2.1 (95% CI [−4.5 to 8.7]). No difference in plasma volumes between any of the groups could be demonstrated at 240 min after resuscitation. At this time point difference in means between the group resuscitated with dextran-70 at a dose of 15 mL/kg and the albumin group was 2.1: (95% CI [−3.2 to 7.5]).
Fig. 2.
Change in plasma volume relative baseline (before the start of hemorrhage) at the completion of resuscitation and 4 h after the completion of resuscitation in animal resuscitated with albumin at a dose of 25 mL/kg (n = 8) or dextran at a dose of 15 mL/kg (n = 8) or 25 mL/kg (n = 6).
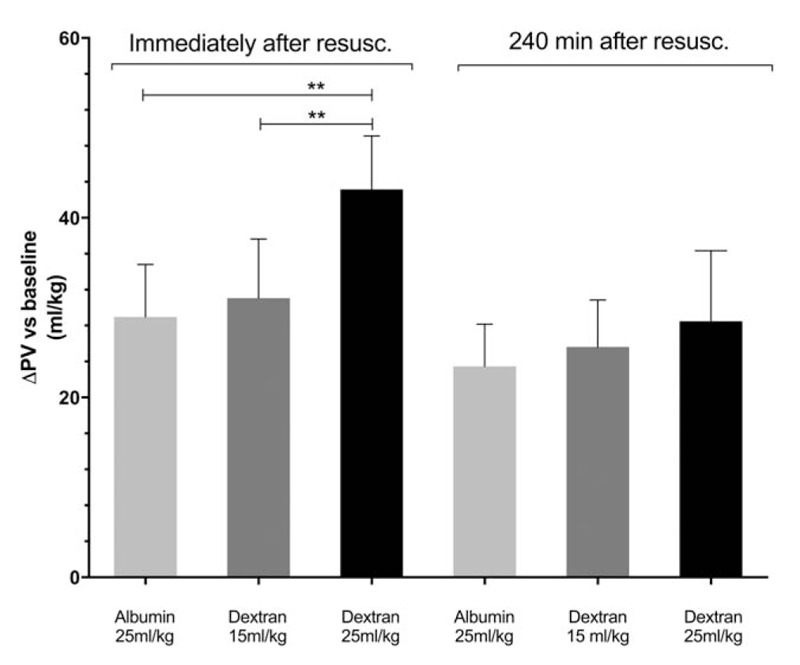
∗∗ = P < 0.01 (one-way ANOVA followed by Turkey's adjustment for multiple comparisons).
Thrombelastography
Baseline TEG parameters were similar in all groups and average R, K, and MA values were 0.48 ± 0.16 min, 0.8 ± 0.0 min, and 71.8 ± 6.8 mm respectively (Fig. 3). No differences in R time could be detected between the groups after resuscitation. Immediately after resuscitation clotting time (K) was longer in the dextran groups. At 240 min after resuscitation K time was longer in the dextran 25 mL/kg group compared with the other groups. No difference between the dextran 15 mL/kg group and the albumin group could be detected at this time point. MA after hemorrhage and resuscitation was decreased compared with baseline in all the groups and was lower in the dextran 25 mL/kg group than in the other groups. The relative reduction in MA at this time point was 13 ± 6.3%, 13 ± 3.2%, and 25 ± 7.7% in the albumin and the dextran 15 mL/kg and 25 mL/kg groups, respectively. At 240 min MA had increased in all groups and at this time point only the dextran 25 mL/kg group differed from the dextran 15 mL/kg group. The relative reduction in MA at this time point was 4.3 ± 8.9%, 5.6 ± 6.4%, and 17 ± 8.1% in the albumin and the dextran 15 mL/kg and 25 mL/kg groups, respectively. Clot lysis could only be detected in 17% of the samples and no differences in lysis could be detected between dextran and albumin resuscitations (data not shown).
Fig. 3.
Reaction time (K, A), speed of clot amplification (R, B) and maximal amplitude (MA, C) as measured using thrombelastography in the albumin and dextran groups at baseline (before the start of hemorrhage) and at the completion of resuscitation and 4 h after the completion of resuscitation with albumin at a dose of 25 mL/kg (n = 8) or dextran at a dose of 15 mL/kg (n = 7) or 25 mL/kg (n = 6).
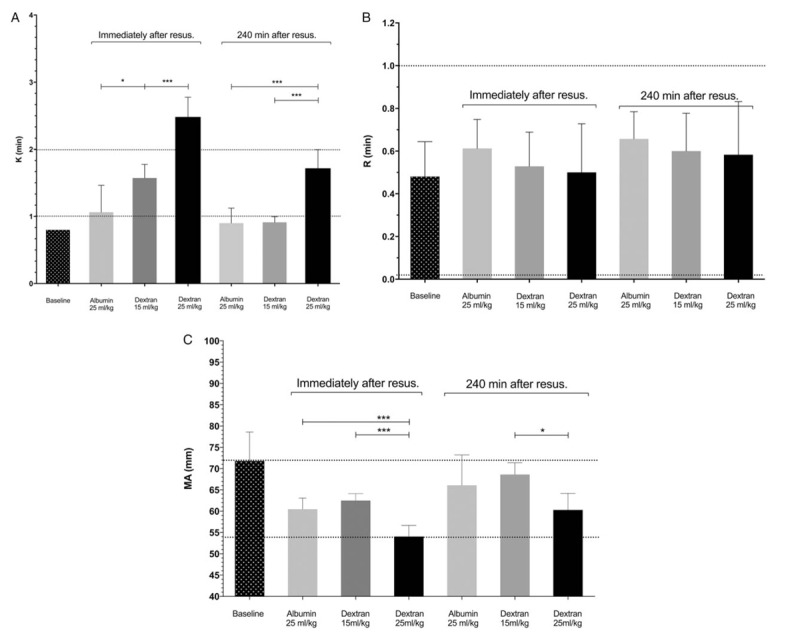
Normal human limits for respective parameter are indicated with the dotted lines (36). ∗ = P < 0.05, ∗∗ = P < 0.01, ∗∗∗ = P < 0.001 (one-way ANOVA followed by Turkey's adjustment for multiple comparisons).
Fibrinogen and von Willebrand factor
After hemorrhage and resuscitation, fibrinogen and vWF Ag plasma concentrations and vWF GP1b activity had decreased compared with baseline in both the dextran 15 ml/kg group and the albumin group (Table 2). No difference in the plasma concentration of fibrinogen or in vWF GP1b activity could be detected between the dextran 15 ml/kg group and albumin group at any of the time points, whereas the vWF Ag plasma concentration was less decreased in dextran 15 ml/kg group compared with the albumin group at both time points.
Table 2.
Fibrinogen, von Willebrand factor function and concentration
| Dextran-70 15 mL/kg (n = 6) | Albumin 25 mL/kg (n = 6) | |
| Baseline | ||
| Fibrinogen (g/L) | 0.85 ± 0.10 | 0.88 ± 0.17 |
| vWF GP1b (kE/L) | 0.70 ± 0.07 | 0.73 ± 0.03 |
| vWF Ag (kE/L) | 0.64 ± 0.05 | 0.71 ± 0.10 |
| At completion of resuscitation | ||
| Fibrinogen delta (%) | −46 ± 4 | −45 ± 4 |
| vWF GP1b delta (%) | −22 ± 3 | −21 ± 4 |
| vWF Ag delta (%) | −25 ± 2* | −30 ± 5 |
| At 240 min after resuscitation | ||
| Fibrinogen delta (%) | −46 ± 6 | −40 ± 6 |
| vWF GP1b delta (%) | −10 ± 5 | −15 ± 8 |
| vWF Ag delta (%) | −20 ± 3† | −28 ± 5 |
Reference intervals using the presently used methods in healthy humans are 2 g/L to 4 g/L for fibrinogen, 0.5 kE/L to 2.0 kE/L for vWF GP1b, and 0.60 kIE/L to 2.73 kIE/L for vWF Ag.
*P < 0.05.
†P < 0.01 versus Alb. (Student t test) Data are expressed as mean ± standard deviation.
GP1b indicates glycoprotein 1b; vWF Ag = von Willebrand factor antigen.
DISCUSSION
Resuscitation with dextran-70 at a dose of 15 mL/kg resulted in a similar plasma volume as 5% albumin at a dose of 25 mL/kg. At these equipotent doses, albumin and dextran had a similar effect on all TEG coagulation parameters except for the clot amplification time (K), which was longer in the dextran group immediately after the completion of resuscitation. The plasma vWF Ag concentration was higher in the dextran group compared with the albumin group, whereas plasma fibrinogen concentration and vWF GP1b activity did not differ between the groups.
Plasma volume measurement using radiolabeled albumin is considered to be gold standard and potential sources of error have been discussed in detail previously and have been found to be small (28). Our finding of similar plasma volumes and a similar precision at baseline as reported previously may further support the reliability of our methodology (29). The finding that the 15 mL/kg dose of dextran-70 resulted in a similar plasma volume expansion as albumin at a dose of 25 mL/kg aligns with previous experimental and human data (10, 28). The result suggests that we reached our objective to compare groups in which dilution of coagulation factors was similar. The higher potency of 6% dextran-70 as a plasma volume expander compared with 5% albumin may be explained both by dextran-70 being a more potent plasma volume expander than albumin and by the higher concentration of the dextran-70 solution.
A dose of dextran-70 in the range of 10 mL/kg to 30 mL/kg is suggested for the treatment of hypovolemic shock in humans. Thus, the dose of dextran-70 in the present study appears to be clinically relevant for the acute treatment of hypovolemia. However, it should be recognized that cumulative doses may be higher in critically ill patients in whom plasma leak occurs over days (16, 17). Our results suggest that the duration of colloid effects on coagulation persists for at least 4 h. At present it is unclear when coagulation is fully normalized. Given that dextran-70 is polydisperse, i.e., consists of molecules with a wide range of sizes, and the long plasma half-life of the larger dextran molecules, it is possible that cumulative dose could influence severity and duration of coagulation effects of dextran-70.
While the present study is the first to compare effects of equipotent doses of albumin and dextran-70 in vivo, several in-vitro studies have diluted human blood with equal volumes of dextran and albumin (30). Most of the in-vitro studies address colloid effects on plasmatic coagulation: as an acquired von Willebrand syndrome and factor VIII decrease, impaired thrombus generation, impaired thrombin–fibrinogen interactions, impaired factor XIII–fibrin polymer interactions, and an enhanced fibrinolytic response (31). The decreased platelet reactivity is referred to a reduced glycoprotein IIb–IIIa availability, decreased platelet aggregability, and adhesion (31).
However as mentioned above, synthetic colloids are polydisperse and the smaller dextran molecules rapidly leave the circulation. In addition, the effects of infused fluids on release and elimination of vWF from the endothelium are not accounted for in-vitro dilution models (32). This means that in-vitro data may not reliably reflect net effects of a synthetic colloid on coagulation (33) and highlights the importance of in-vivo studies. In the present in-vivo study we have shown that the only TEG parameter that differed between the groups after equipotent doses of dextran and albumin was prolonged clot amplification time, also named clot kinetics (K) in TEG terminology in the dextran group. K is a TEG parameter that describes the speed of the initial clot formation with an unclear clinical significance concerning anti-thrombotic effects, whereas the TEG MA, a robust visco-hemostatic parameter, demonstrated a clear dose-dependent decrease. Thus, the present study for the first time suggests that the immediate effects of dextran-70 on coagulation measured with TEG, relative to those of albumin to a large extent, may be referred to differences in dilution of plasma proteins. However, while no differences in TEG parameters could be detected between the lower dextran group and the albumin groups at 4 h it should be noted that both clot amplification and clot strength were reduced at this time point in the high dose dextran group despite similar plasma volumes in all groups. This finding suggests that mechanisms other than plasma dilution are important for coagulation effects of dextran. Our TEG data align with recently published studies on intraoperative effects of dextran-70 or albumin (23, 34) relative those of lactated Ringers solution showing that both colloids mainly affect clot amplification and clot strength.
It has been suggested that fibrinolysis may be enhanced after dextran infusion by increased plasma concentrations of tissue-type plasminogen activator and decreased concentrations of the physiologic inhibitor of fibrinolysis plasminogen activator inhibitor-1 (13–15). No evidence to support increased fibrinolysis was found in our study and fibrinogen concentrations were similar in the dextran group compared with the albumin group.
As described above, dextran administration has been associated with an acquired von Willebrand syndrome (35). Our result showing a 20% to 25% decrease in both vWF Ag concentration and vWF GP1b activity aligns with these findings. Interestingly, our results suggest that albumin impairs vWF GP1b activity to a similar extent as dextran and decrease vWF Ag effect even more than dextran. Studies in human have suggested that effects of dextran on ristocetin cofactor levels (a measure that corresponds to vWF GP1b activity) progressively increase and peak at 6 h after infusion of dextran (35), while our data suggest a peak effect early after resuscitation in our model, possibly reflecting species differences in metabolism of dextran.
Little clinical data is available with regard to potential adverse effects of dextran-70 on bleeding. Two small retrospective studies investigating effects of a change in resuscitation guidelines, which lead to decreased use of dextran, suggested a reduction in serious bleeding episodes (16, 17). In contrast, no effect on number of bleeding episodes could be demonstrated in a larger study, in which sepsis patients resuscitated with dextran-70 at a median dose of 17 mL/kg were propensity score matched to those that received albumin (18). Perioperative effects on coagulation have also been investigated in a small randomized controlled trial, comparing patients resuscitated with dextran to a maximal dose of 25 mL/kg and crystalloids to patients resuscitated with mainly crystalloids. While no significant effect on the primary outcome, blood loss could be detected, more patients in the dextran groups suffered severe bleeding episodes (34). Interestingly, the study suggested that cloth strength is the TEG parameter that best predicts perioperative blood loss and derived a cutoff for a reduction in MA by about 21% to predict significant blood loss. The results raise the question if cloth firmness could be used to tailor resuscitation with colloids to avoid adverse effects on coagulation by colloids in a clinical setting? This may be particularly relevant in patients presenting with acquired coagulation deficits in which the “safe” volume of colloid from a coagulation perspective may be lowered.
Limitations
Based on the rationale that we wanted to avoid hypervolemia while maximizing dose of dextran, transfusion of erythrocytes was withheld although hematocrit after resuscitation was below commonly accepted trigger levels for transfusion and we cannot exclude that this may have influenced our results. Also, even though male guinea pigs have been suggested to have a similar platelet function as humans (25), and that baseline TEG parameters were within or close to normal limits for humans we cannot exclude that species or sex differences may have influenced our results. Moreover, it is unclear to what extent the observed differences in laboratory parameters reflect clinically relevant endpoints such as transfusion requirements.
CONCLUSIONS
In equipotent doses with regard to plasma volume expansion dextran-70 transiently affect clot amplification time more than albumin. The plasma vWF Ag concentration was higher in the dextran group compared with the albumin group, whereas plasma fibrinogen concentration and vWF GP1b activity did not differ between the groups. If confirmed in humans these results indicate that from a coagulation perspective dextran-70 may be a safe alternative albumin at doses up to 15 mL/kg.
Footnotes
Funding: ALF (Swedish government grant), Anna and Edwin Berger Foundation.
The authors report no conflicts of interest.
REFERENCES
- 1.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39 2:259–265, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 43 5:625–632, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 370 15:1412–1421, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 37 1:86–96, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 367 20:1901–1911, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 367 2:124–134, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons RJ, Banghart SB. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol 12 1:11–23, 1967. [DOI] [PubMed] [Google Scholar]
- 8.Arfors KE, Buckley PB. Pharmacological characteristics of artificial colloids. Baillieres Clin Anaesthesiol 11 1:15–47, 1997. [Google Scholar]
- 9.Bohmansson G, Rosenkvist H. Clinical experiences with dextran as a plasma substitute. Acta Chir Scand 94:149–167, 1946. [PubMed] [Google Scholar]
- 10.Lamke LO, Liljedahl SO. Plasma volume changes after infusion of various plasma expanders. Resuscitation 5 2:93–102, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Ljungström KG. Prophylaxis of postoperative thromboembolism with dextran 70: improvements of efficacy and safety. Acta Chir Scand Suppl 514:1–40, 1983. [PubMed] [Google Scholar]
- 12.Aberg M, Hedner U, Bergentz SE. Effect of dextran on factor VIII (antihemophilic factor) and platelet function. Ann Surg 189 2:243–247, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlin G, Karlstrom G, Modig J, Saldeen T. Effect of dextran on fibrinolysis inhibition activity in the blood after major surgery. Acta Anaesthesiol Scand 24 5:375–378, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Carr ME, Gabriel DA. The effect of dextran 70 on the structure of plasma-derived fibrin gels. J Clin Lab Med 96 6:985–993, 1980. [PubMed] [Google Scholar]
- 15.Eriksson M, Saldeen T. Effect of dextran on plasma tissue plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1) during surgery. Acta Anaesthesiol Scand 39 2:163–166, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Hvidt LN, Perner A. High dosage of dextran 70 is associated with severe bleeding in patients admitted to the intensive care unit for septic shock. Dan Med J 59 11:A4531, 2012. [PubMed] [Google Scholar]
- 17.Rasmussen AM, Jakobsen R, Strøm T, Carlsson M, Dahler-Eriksen B, Toft P. More complications in patients with septic shock treated with dextran compared with crystalloids. Dan Med J 62 2:A5018, 2015. [PubMed] [Google Scholar]
- 18.Bentzer P, Broman M, Kander T. Effect of dextran-70 on outcome in severe sepsis; a propensity-score matching study. Scand J Trauma Resusc Emerg Med 25 1:65, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogbill TH, Moore EE, Dunn EL, Cohen RG. Coagulation changes after albumin resuscitation. Crit Care Med 9 1:22–26, 1981. [DOI] [PubMed] [Google Scholar]
- 20.Johnson SD, Lucas CE, Gerrick SJ, Ledgerwood AM, Higgins RF. Altered coagulation after albumin supplements for treatment of oligemic shock. Arch Surg 114 4:379–383, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Statkevicius S, Asgeirsson B, Schött U. Effects of different colloid infusions on ROTEM and Multiplate during elective brain tumour neurosurgery. Perioper Med (Lond) 4:9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen KC, Højskov M, Johansson PI, Kridina I, Kistorp T, Salling L, Nielsen HB, Ruhnau B, Pedersen T, Secher NH. Impact of albumin on coagulation competence and hemorrhage during major surgery: a randomized controlled trial. Medicine (Baltimore) 95 9:e2720, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skhirtladze K, Base EM, Lassnigg A, Kaider A, Linke S, Dworschak M, Hiesmayr MJ. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer's lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth 112 2:255–264, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Kheirabadi BS, Crissey JM, Deguzman R, Perez MR, Cox AB, Dubick MA, Holcomb JB. Effects of synthetic versus natural colloid resuscitation on inducing dilutional coagulopathy and increasing hemorrhage in rabbits. J Trauma 64 5:1218–1228, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Cook NS, Zerwes HG, Tapparelli C, Powling M, Singh J, Metternich R, Hagenbach A. Platelet aggregation and fibrinogen binding in human, rhesus monkey, guinea-pig, hamster and rat blood: activation by ADP and a thrombin receptor peptide and inhibition by glycoprotein IIb/IIIa antagonists. Thromb Haemost 70 3:531–539, 1993. [PubMed] [Google Scholar]
- 26.Voorhees AB, Baker HJ, Pulaski EJ. Reactions of albino rats to injections of dextran. Proc Soc Exp Biol Med 76 2:254–256, 1951. [DOI] [PubMed] [Google Scholar]
- 27.Castaman G, Hillarp A, Goodeve A. Laboratory aspects of von Willebrand disease: test repertoire and options for activity assays and genetic analysis. Haemophilia 20:65–70, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubniks M, Persson J, Grände PO. Comparison of the plasma volume-expanding effects of 6% dextran 70, 5% albumin, and 6% HES 130/0.4 after hemorrhage in the guinea pig. J Trauma 67 6:1200–1204, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Bark BP, Grande PO. Infusion rate and plasma volume expansion of dextran and albumin in the septic guinea pig. Acta Anaesthesiol Scand 58 1:44–51, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Winstedt D, Hanna J, Schött U. Albumin-induced coagulopathy is less severe and more effectively reversed with fibrinogen concentrate than is synthetic colloid-induced coagulopathy. Scand J Clin Lab Invest 73 2:161–169, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Kozek-Langenecker SA. Influence of fluid therapy on the haemostatic system of intensive care patients. Best Pract Res Clin Anaesthesiol 23:225–236, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Collis RE, Collins PW, Gutteridge CN, Kaul A, Newland AC, Williams DM, Webb AR. The effect of hydroxyethyl starch and other plasma volume substitutes on endothelial cell activation: an in vitro study. Intensive Care Med 20 1:37–41, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Coats TJ, Brazil E, Heron M. The effects of commonly used resuscitation fluids on whole blood coagulation. Emerg Med J 23:546–549, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen KC, Hoejskov M, Johansson PI, Kridina I, Kistorp T, Salling L, Nielsen HB, Ruhnau B, Pedersen T, Secher NH. Coagulation competence for predicting perioperative hemorrhage in patients treated with lactated Ringer's vs. Dextran—a randomized controlled trial. BMC Anesthesiol 15:1–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batlle J, del Río F, López Fernández MF, Martín R, López Borrasca A. Effect of dextran on factor VIII/von Willebrand factor structure and function. Thromb Haemost 54 3:697–699, 1985. [PubMed] [Google Scholar]


