Supplemental Digital Content is available in the text
Keywords: Alpha7nAChR, CPB, EA, high-mobility group box 1
Abbreviations: α7nAChR-α7, nicotinic acetylcholine receptor, ALI, acute lung injury, BALF, bronchoalveolar lavage fluid, CPB, cardiopulmonary bypass, EA, electroacupuncture, HMGB1, high-mobility group box 1
ABSTRACT
Acute lung injury is a common complication after cardiopulmonary bypass (CPB). α7 Nicotinic acetylcholine receptors (α7nAChR) and α7nAChR-dependent cholinergic signaling are implicated in suppressing the release of high-mobility group box 1 (HMGB1) and reducing the inflammatory response. A previous study has shown the electroacupuncture (EA) pretreatment induces tolerance against lung injury. However, the role of EA in CPB is poorly understood. This study used EA and a rat model of CPB to determine whether EA was associated with CPB-induced lung injury. Rats were treated with EA at “Zusanli (ST36)” and “Feishu (BL13)” acupoints for 5 days before being subjected to CPB. Two hours post-CPB, samples of blood, bronchoalveolar lavage fluid (BALF), and lung tissues were processed for investigations. Our results showed that the expression of α7nAChR in lung tissue was significantly decreased after CPB. EA pretreatment prevented the reduction in the expression of α7nAChR, EA pretreatment reduced lung edema, inhibited inflammatory cytokines release in serum and lung as well as protein concentrations in BALF and HMGB1 release after CPB, and the beneficial effects were attenuated by α-BGT. Our study demonstrates that EA pretreatment plays a protective role in CPB-induced ALI, and inhibits HMGB1 release through α7nAChR activation in rats.
INTRODUCTION
Cardiopulmonary bypass (CPB) is a frequently used technique during heart surgery. However, acute lung injury (ALI) is one of the most severe complications that occurs during and after CPB (1). Approximately 2% to 3% of patients who undergo cardiac surgery suffer the ALI under CPB, resulting in the mortality rates from 15% to 50% (2). However, the exact mechanism of CPB-induced ALI remains uncertain. Therefore, it is of crucial importance to ameliorate ALI after CPB.
The inflammatory response associated with CPB has been considered to contribute to lung injury and postoperative pulmonary dysfunction (3, 4). We and previous studies found that HMGB1, as a key factor, has important functions in mediating the pathology of acute damage and subsequent inflammatory processes in ALI (5, 6). Other investigators have also reported that lung damage could be attenuated by neutralizing HMGB1 with a special antibody in rats (7), which suggests HMGB1 as a therapeutic target for ALI.
The cholinergic anti-inflammatory pathway refers to a physiological mechanism by which the nervous system interacts with the innate immune system to control systemic inflammatory responses (8). Previous study has shown that α7nAChR-dependent cholinergic signaling is implicated in suppressing the release of high-mobility group box 1 (HMGB1), reducing the inflammatory response (9). Our previous work also demonstrated that α7nAChR agonist PNU-282987 attenuated ALI in a CPB model in rats.
Electroacupuncture (EA), as a combination of traditional Chinese medicine and modern technique, has attracted increasing attention. EA has been proven to inhibit systemic inflammatory response in rats with lethal endotoxemia (10). Other researchers have also found that EA protects the brain against transient cerebral ischemic injury, and inhibits HMGB1 release through α7nAChR activation in rats (11). However, it remains unknown whether EA exert the protective effects on the lung after CPB.
Thus, we hypothesized that EA may attenuate ALI after CPB by regulating the expression of α7nAChR, activating the cholinergic anti-inflammatory pathway, which led to the inhibition of HMGB1.
MATERIALS AND METHODS
This study was approved by the Animal Care and Use Committee of Shanghai Jiao Tong University, School of Medicine. All the animal procedures in this study were conducted in accordance with the United States’ National Institutes of Health (NIH) animal care guidelines (Guide for the Care and Use of Laboratory Animals, Department of Health and Human Services, NIH Publication No. 86-23, revised 1985).
Animals and experimental grouping
Male Sprague–Dawley rats, weighing between 400 and 450 g, were provided by the Sino-British SIPPR/BK Lab (Shanghai, China). All the rats were housed under controlled conditions with 12-h light/dark cycle, temperature of 22 ± 2°C, and 60% to 70% humidity, for 1 week before the experiment. The rats were allowed free access to standard rodent diet and water.
Thirty rats were randomly divided into five groups: sham group, CPB group, EA+CPB group, α-BGT+EA+CPB group, and α-BGT+CPB group. The rats in the sham group underwent identical surgery without CPB. Rats in the CPB group received the treatment described below. Rats in the EA+CPB group received EA pretreatment once a day for 5 days and were subjected to CPB at 24 h after the last treatment. Rats in the α-BGT+CPB group received caudal vein injection of α-BGT (1 μg/kg) once a day for 5 days and were subjected to CPB at 24 h after the last injection. Rats in theα-BGT+EA+CPB group received α-BGT (1 μg/kg) at 30 min before EA pretreatment, once a day for 5 days, and were subjected to CPB at 24 h after the end of the last EA pretreatment.
α-Bungarotoxin (α-BGT), as a specific a7nAChR antagonist was purchased from Tocris Company (Bristol, UK) and prepared in 150 mM sodium chloride (NaCl) before use. The dosage was proven to be effective in the previous study (12).
All tissue samples and blood were collected at 2 h after the termination of CPB.
Surgical procedure
The surgical procedures were performed as described in our previous study (6). Briefly, SD rats were anesthetized with 5% isoflurane and given tracheal intubation. Rats were maintained with isoflurane at 1.5% to 2.0%.
For initiation of the extracorporeal circulation, a 22-G catheter (BD Insyte-W) was cannulated in the tail artery and a 5F special catheter (Cordis) was cannulated in the right jugular vein. Continuous blood pressure and arterial blood gases were achieved by cannulation of the left femoral artery. Blood flow was directed from the jugular vein through silicon tubes to the CPB device and back to the corporal circulation via the tail artery. The CPB circuit consists of a venous reservoir (a 5-mL cylinder syringe), a roller pump, and a membrane oxygenator. The circuit was primed with 8 mL of Voluven (hydroxyethyl starch 130/0.4 and sodium chloride injection) and heparin (200 IU/kg). The venous reservoir was placed 35 cm below the heart level and the blood flow was adjusted to 100 mL/kg/min and maintained for 60 min.
Electroacupuncture pretreatment
EA pretreatment was performed as previously described (13) at Zusanli (ST36) acupoint, which is located between the tibia and fibula, laterally to the distal end of the cranial tuberosity of the tibia, approximately 5 mm lateral to the anterior tubercle of the tibia and the Feishu (BL13) acupoint, located between T3 and T4 of the spine approximately 1.5 cm lateral to the midline. All rats received EA pretreatment were anaesthetized with intraperitoneal (ip) 3% Pentobarbital Sodium (0.1 mL/100 g bodyweight). After the animals were anesthetized, the bilateral acupoints of Zusanli (ST36) and Feishu (BL13) were stimulated at a frequency of 2/15 Hz for 30 min by an electronic acupuncture treatment instrument (Model No. SDZ-IIB; Suzhou Medical Appliances Co., Ltd., Suzhou, China) and the intensity was adjusted to induce moderate muscle contraction of the hindlimb. The core temperature of the rats was maintained at 37.0 ± 0.5°C during EA pretreatment.
Lung histology
Changes in the lung tissue were examined morphologically as described previously (6). The right upper lungs of the mice were excised and fixed with 4% paraformaldehyde for 48 h. All tissue samples were embedded in paraffin before cutting into 5 μm sections and then stained with hematoxylin and eosin for microscopic examination. Evaluations were performed by a pathologist blind to experimental groups using an OlympusCH30 microscope.
The severity of lung injury was scored based on the scoring system of the Official American Thoracic Society Workshop Report (14). Details are as follows: (A) neutrophils in the alveolar space, (B) neutrophils in the interstitial space, (C) hyaline membranes, (D) proteinaceous debris filling the airspaces, and (E) alveolar septal thickening. The total score was calculated as follows: score = [(20 × A) + (14 × B) +(7 × C) + (7 × D) + (2 × E)]/ (number of fields × 100).
Measurement of lung W/D ratio, BALF protein contents
The inferior lobe of the right lung was excised and weighed immediately to obtain the wet weight. Then, the tissue was dried in an oven at 60°C for 1 week before weighing to obtain the dry weight. The W/D ratio was calculated by wet weight/dry weight as previously described (12).
To evaluate pulmonary protein infiltration, the protein concentration in BALF was quantified with a BCA Protein Assay Kit (Thermo Scientific).
Immunohistochemistry for α7nAChR
To investigate the differences of α7nAChR in the lung, immunohistochemical staining for α7nAChR was performed using a special rabbit polyclonal antibody. After dewaxing and rehydration, sections were immersed and boiled in TRIS-EDTA buffer for antigen retrieval with a microwave oven, having first blocked endogenous peroxidase by 3% hydrogen peroxide treatment. The tissue sections were then incubated with a 1:500 dilution of the primary antibody α7nAChR overnight at 4°C. Samples were washed with phosphate-buffered saline (PBS) buffer and incubated with a secondary anti-rabbit antibody and processed with an avidin-biotin-immunoperoxidase technique. Finally, the sections were counterstained with Mayer's hematoxylin.
ELISA assay for HMGB1, IL-1β, and TNF-α
The left lobe of the lung was homogenized and centrifuged at 12,000 × g at 4°C for 15 min, and the supernatant was collected and transferred into fresh tubes. The protein content was measured with a BCA Protein Assay Kit (Thermo Scientific). The levels of TNF-α and IL-1β were measured using ELISA kits (R&D system), and HMGB1was measured with a commercial ELISA Kit (Shino-Test, Kanagawa, Japan) after the manufacturer's instructions.
Western blot
Western blot analysis was performed as previously described (6). Thirty microgram of total protein per sample was loaded and the proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel and then transferred to 0.22 μm polyvinylidene fluoride membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 2 h at room temperature and then incubated with alpha7nAChR rabbit antibody (1:1,000; Abcam) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) rabbit antibody (1:5,000; CST) overnight at 4°C. Membranes were washed and then incubated by goat anti-rabbit HRP conjugate secondary antibody (1:5,000; Beyotime) for 1 h at room temperature. The membranes were again washed with TBST and detected by enzymatic chemiluminescence plus Western blot detection system. The quantity analysis of protein was performed by Image Lab (Bio-rad). Protein levels of α7nAChR in lung tissue were normalized against GAPDH levels and expressed as relative fold changes compared with the sham group.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from the lung tissue by using a TRizol reagent (Sigma-Aldrich). cDNA was synthesized using a PrimeScript RT Reagent kit for RT-PCR (TAKARA BIO INC, Japan). qPCR analysis of α7nAChR and GAPDH was performed in the Real-time Detection System Roche LightCycler480 II, Switzerland by ChamQSYBR qPCR Master Mix (Vazyme, Nanjing, China) detection. Equal amounts of RNA (500 ng) were used to prepare cDNA. The primers used were as follows: α7nAChR, sense GGCAAAATGCCTAAGTGGAC, antisense CTTCATGCGCAGAAACCAT; GAPDH, sense AACTTTGGCATTGTGGAAGG, antisense CACATTGGGGGTAGGAACAC. The fold change of the target gene cDNA relative to GAPDH was determined as follows: fold change = 2−ΔΔCt, where ΔΔCt = (Ct Target-Ct GADPH) test − (Ct Target-Ct GADPH) control. GAPDH served as an internal standard control for variations in RT-PCR efficiency.
TUNEL staining of the lung
Apoptosis in the lung tissue was assessed in situ by TUNEL staining as described in our previous study (15). In brief, all the sections were immersed twice in anhydrous ethanol for dehydration, dried, and mounted with neutral gum. Positively stained brown cells as well as other cells in a similar scope were counted under a light microscope (OlympusCH30), and the percentage of apoptotic cells (positively stained brown cells) was calculated.
Data analysis and statistics
Data were expressed as mean ± SD. Analysis was performed using SPSS 19.0 software (IBM, Chicago, Ill). One-way analysis of variance was conducted for multiple comparisons. P < 0.05 was considered statistically significant.
RESULTS
EA pretreatment enhanced α7nAChR in the lung issue after CPB
To investigate the expression of α7nAChR in lung tissue, western blot analysis was used to detect α7nAChR protein expression in the lung tissue at 2 h after operation, respectively (n = 6). Compared with the sham group, the expression of α7nAChR in lung tissue was significantly decreased in the CPB group (P < 0.01). However, there was no significant difference in α7nAChR expression of lung tissue among the sham, EA+CPB and EA+sham groups, which indicated that EA pretreatment may prevent the downregulation of α7nAChR expression (Fig. 1).
Fig. 1.
Expression of α7nAChR protein in the lung tissues of the sham, CPB, EA+CPB, and EA+sham groups.
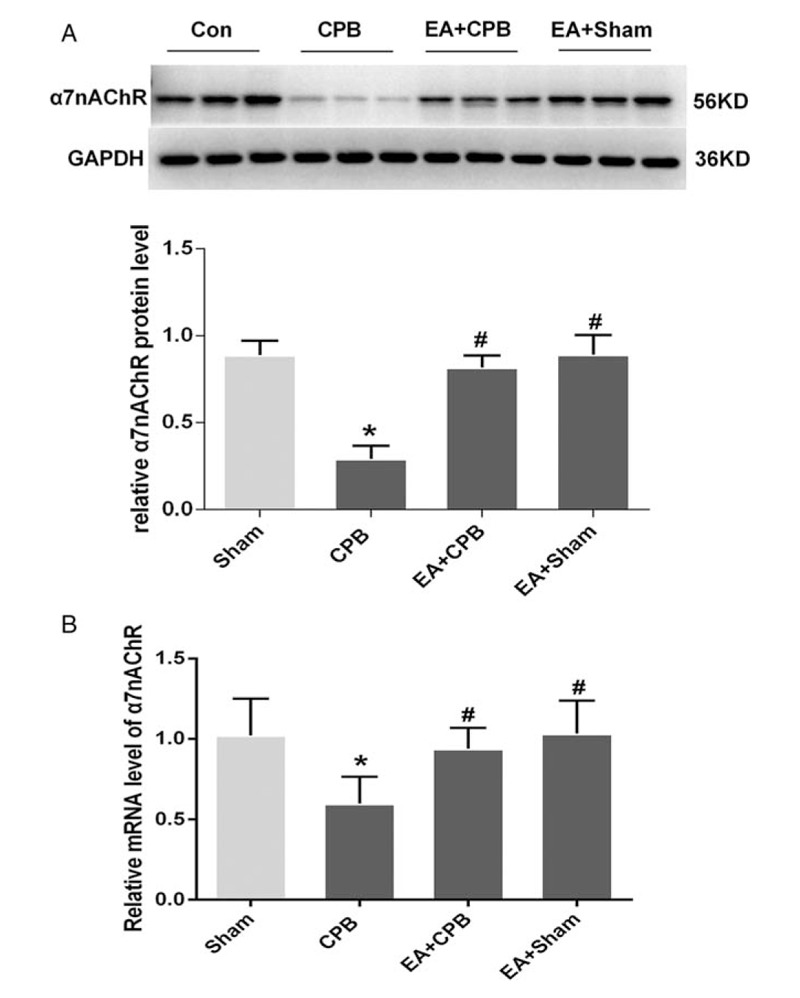
(A) Western blot showing the 56-KD band indicating α7nAChR protein is much lighter in the CPB group than in the sham, EA+CPB, and EA+sham groups. (B) Density of α7nAChR protein relative to that of GAPDH. Density of α7nAChR protein is significantly lower in the CPB group than in the sham, EA+CPB, and EA+ sham groups. Data are means ± SD (n = 6 per group). ∗P < 0.01 vs. sham group, #P < 0.01 vs. CPB group.
To exclude the systematic effects of α-BGT, we also investigated the direct effect of α-BGT on sham rats without CPB. These results were showed in supplementary data. However, there was no significant difference in α7nAChR protein level expression of lung tissue compared with sham group. These results demonstrated α-BGT did not affect the expression of a7nAChR in sham rats.
Levels of α7nAChR in lung tissue were also assessed by immunohistochemistry. In the sham groups, α7nAChR staining in the lung was abundant, whereas the expression of α7nAChR was significantly decreased in the CPB group. And EA pretreatment prevented the downregulation of α7nAChR expression in lung tissue (Fig. 2).
Fig. 2.
Immunohistochemical staining for α7nAChRin lung tissue.
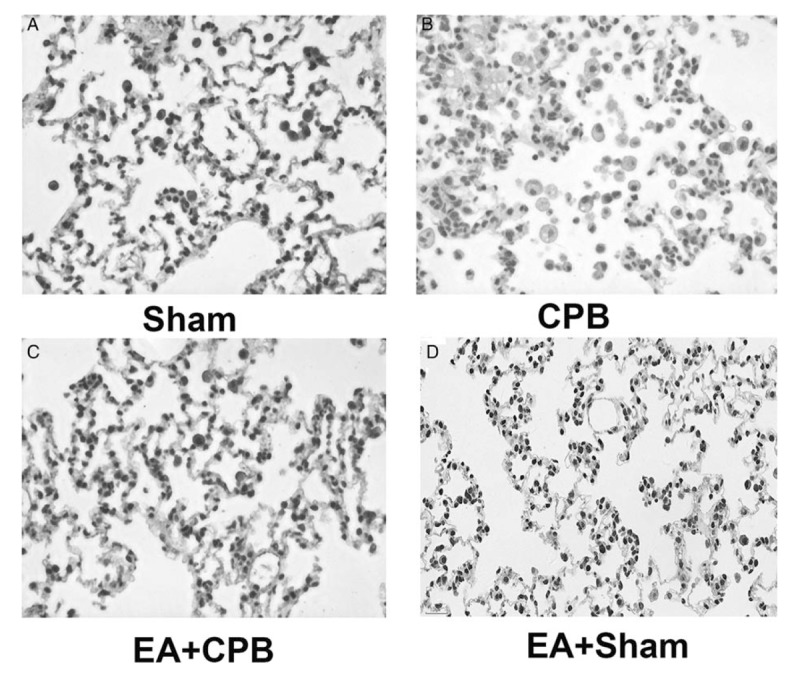
α7nAChR stain was observed in the membranes of the (A) sham group, and α7nAChR was much less abundant in the (B) CPB group. EA pretreatment prevented the downregulation of α7nAChR expression of α7nAChR in lung tissue. Magnification 200×.
We also examined the mRNA levels of α7nAChR in lung tissue, which showed similar results with the protein expression (Fig. 1B). These results suggested that EA pretreatment prevented the downregulation of mRNA levels of α7nAChR in lung tissue after CPB.
EA pretreatment suppressed the apoptosis in the lung tissue after CPB
As shown in Figure 3, little positive TUNEL staining (brown) was detected in lung sections of sham animals, whereas large numbers of TUNEL-positive cells were seen in the rats of the CPB group, a-BGT+EA+CPB and a-BGT+CPB groups. In contrast, only a small amount of TUNEL staining was observed in the EA +CPB group. Quantitative analysis showed that the EA pretreatment significantly reduced the number of TUNEL-positive cells, compared with the CPB, a-BGT+EA+CPB and a-BGT+CPB groups. There was no difference between the CPB, a-BGT+EA+CPB, and a-BGT+CPB groups. These results suggested that EA pretreatment suppressed the apoptosis in the lung after CPB and a-BGT reversed the protective effect of EA pretreatment.
Fig. 3.
Little positive TUNEL staining (brown) was detected in lung sections of sham animals, whereas large numbers of TUNEL-positive cells were seen in the lung in the CPB, a-BGT+EA, and a-BGT+CPB groups.
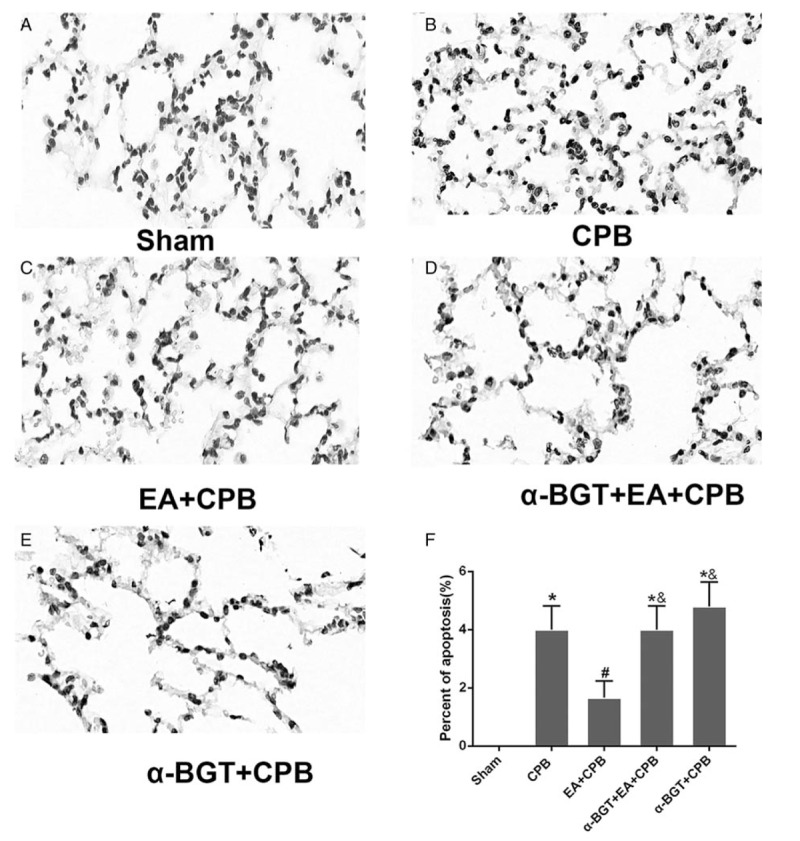
In contrast, only a small amount of TUNEL staining was observed in the EA+CPB and EA+sham groups. Quantitative analysis showed that the EA pretreatment significantly reduced the number of TUNEL-positive cells, compared with the CPB, a-BGT+EA, and a-BGT+CPB groups. There was no difference between the CPB, a-BGT+EA, and a-BGT+CPB groups, suggesting that EA pretreatment alleviates lung apoptosis.
EA pretreatment reduced the morphological inflammatory response of the lung tissue, protein infiltration, and lung water
In the sham group, H&E staining showed intact and very thin alveolar walls (Fig. 4). In the CPB group, acute inflammatory response such as the alveolar wall not being intact and an increase of inflammatory cells was observed in the lung tissue (Fig. 4). However, relatively intact and thin alveolar walls of the lung were observed in the EA+CPB group. The otherthree groups demonstrated similar performance as the CPB group. The lung injury scores also showed that the EA pretreatment significantly decreased the injury after CPB (Fig. 4). The results demonstrated that pretreatment with EA attenuates the severity of the lung injuries after CPB and the protective effects of EA are obviously abated by α-BGT.
Fig. 4.
Effect of EA (A) micrographs of lung H&E staining.
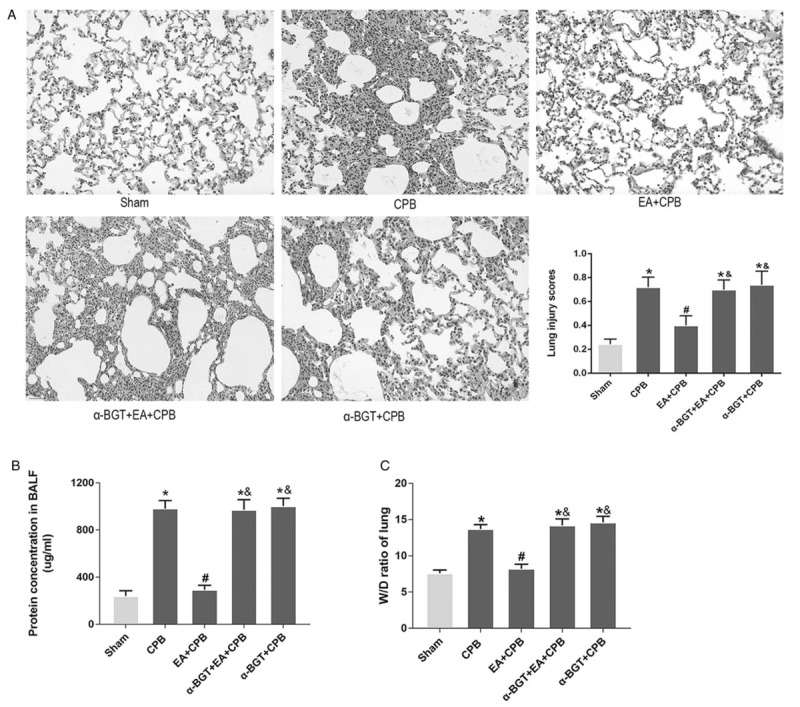
The lung structures of the sham and EA+CPB groups were similar. Lung inflammatory response is very obvious in the CPB group as well as in α-BGT+EA+CPB group and the α-BGT+CPB group. Lung injury scores showed similar results. Magnification 200 × . (B) Protein concentrations in BALF quantified by BCA protein assay. The sham and EA+CPB groups are similar and low. The concentration is significantly higher in the CPB group as well as in the α-BGT+EA+CPB and α-BGT+CPB group. (C) W/D ratios of lung tissue. The W/D ratios of the sham and EA+CPB groups are similarly very low. The W/D ratio in the CPB group is significantly higher than that of the sham and EA+CPB groups, whereas it is similar in the α-BGT+EA+CPB and α-BGT+CPB groups. Data are means ± SD (n = 6 per group). ∗P < 0.01 vs. sham group; #P < 0.01 vs. CPB group; &P < 0.01 vs. EA+CPB group.
The lung W/D ratio and the protein concentration in the BALF were measured to analyze lung edema and vascular permeability. As shown in Figure 4, the lung W/D ratio in the CPB group significantly increased compared with that in the sham group, whereas this increase was significantly suppressed in the EA+CPB group. However, the pretreatment with α-BGT increased the W/D ratio of the lung tissues.
In the CPB group, the BALF protein concentration was significantly enhanced compared with the sham group. By contrast, the EA pretreatment obviously reduced the total BALF protein concentration in the EA+ CPB group. The treatment with α-BGT increased the total protein concentration in the BALF. No significant difference was shown in the lung W/D ratios and protein concentrations in the BALFs of the α-BGT + CPB and CPB groups. These results suggested that EA pretreatment could effectively inhibit CPB-induced lung edema and reduce lung vascular permeability. In addition, the effects of EA pretreatment could be reversed by the α7nAChR antagonist α-BGT.
EA pretreatment decreased inflammation cytokines
In this study, the levels of cytokines TNF-α and IL-1β were examined to further investigate the anti-inflammatory effects of EA pretreatment. As shown in Figure 5, the concentrations of TNF-α and IL-1β significantly increased in the CPB group, when compared with the sham group; however, the pretreatment of EA efficiently suppressed the production of TNF-α and IL-1β. The pretreatment with α-BGT markedly enhanced the levels of TNF-α and IL-1β. No statistically significant difference was observed in the TNF-α or IL-1β concentrations in the lung tissue homogenates of the α-BGT + CPB and CPB groups. The results demonstrated that pretreatment with EA attenuates the TNF-α and IL-1β release in lung tissues and that α-BGT reversed the protective effect.
Fig. 5.
Inflammatory cytokines in serum, lung tissues, and BALF.
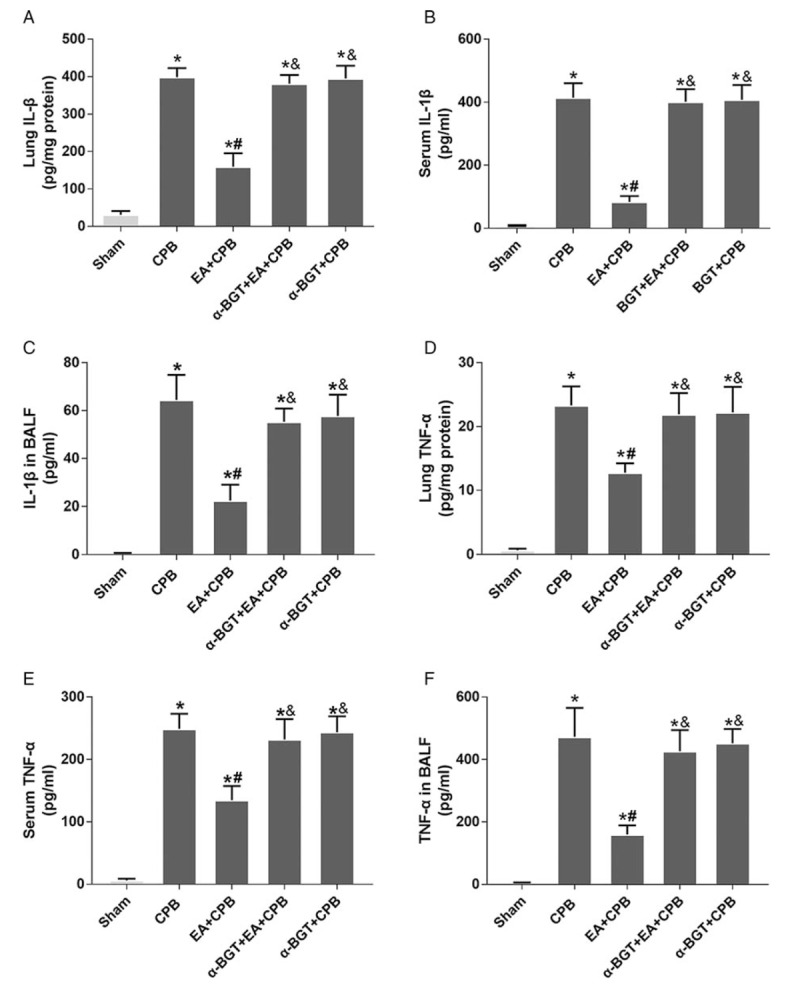
Protein concentrations of IL-1β (A–C) and TNF-α (D–F) were significantly higher in the CPB group compared with the sham group, but significantly lower in the EA+CPB group compared with the CPB group, whereas the α7nAChR antagonist α-BGT attenuated the reduction. Data are means ± SD (n = 6 per group). ∗P < 0.01 vs. sham group, #P < 0.01 vs. CPB group; &P < 0.01 vs. EA+CPB group.
EA pretreatment decreased HMGB1 release
As a key factor to trigger inflammatory response, the levels of HMGB1 were examined by ELISA kit in this study. As shown in Figure 6, the concentrations of HMGB1 significantly increased in the CPB group, when compared with the sham group; however, the pretreatment of EA efficiently suppressed the production of HMGB1. The pretreatment with α-BGT obviously enhanced the levels of HMGB1. No statistically significant difference was observed in the HMGB1 concentrations in the lung tissue homogenates of the α-BGT + CPB and CPB groups. The results suggest that pretreatment with EA attenuates the HMGB1 release in lung tissues and that α-BGT reversed the protective effect.
Fig. 6.
HMGB1 concentrations in serum and BALF.
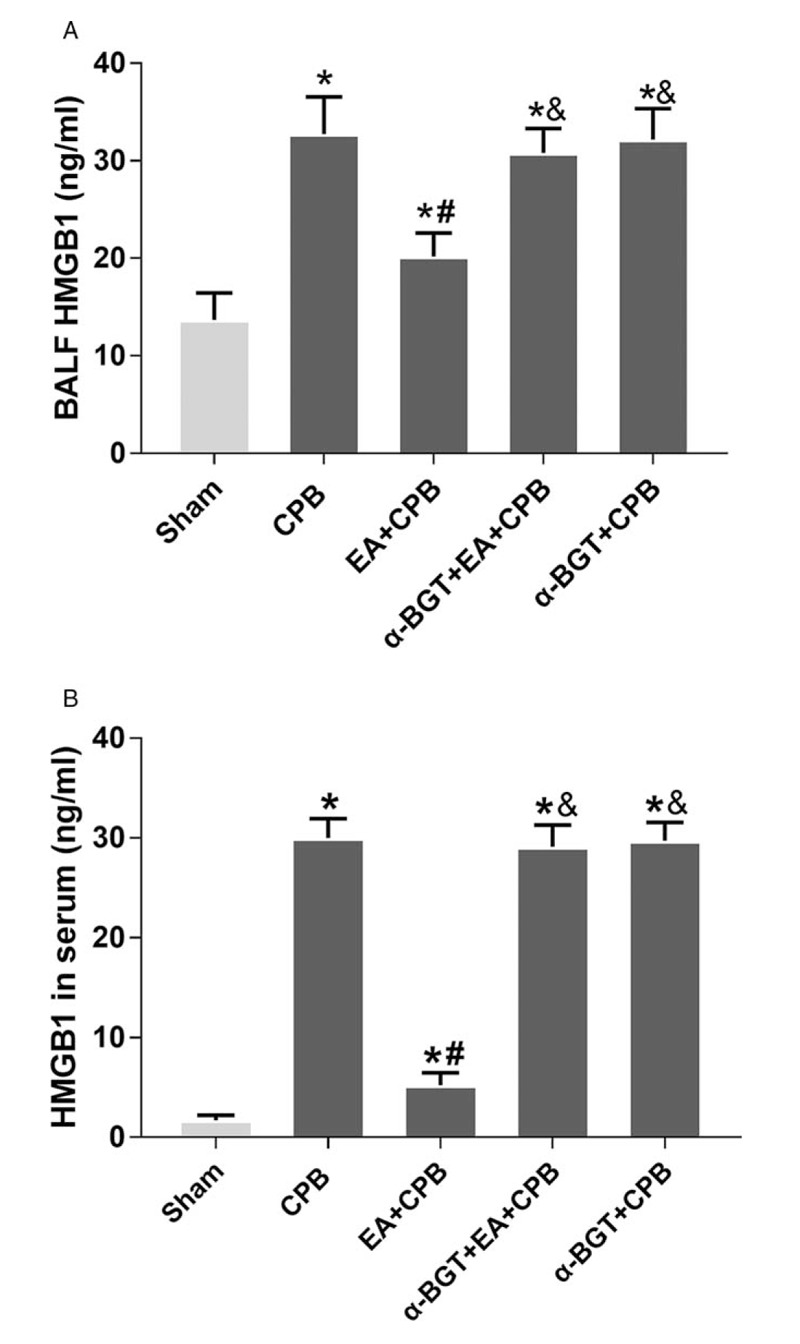
In both serum (A) and BALF (B), HMGB1 concentrations were significantly higher in the CPB group compared with the sham groups. In the EA+CPB group, HMGB1 concentrations were significantly reduced compared with the CPB group, whereas the a7nAChR antagonist a-BGT attenuated the reduction. Data are means ± SD (n = 6 per group). ∗P < 0.01 vs. sham group, #P < 0.01 vs. CPB group; &P < 0.01 vs. EA+CPB group.
DISCUSSION
ALI is one of the most common postoperative pulmonary complications after CPB. Studies of the mechanism of CPB-induced ALI are of crucial importance. The aim of this study was to evaluate the role of EA pretreatment in the process of CPB-induced ALI. In this study, we found that EA pretreatment reduced the morphological inflammatory response of the lung tissue, protein infiltration, and lung water. And the effects of EA pretreatment could be reversed by the α7nAChR antagonist α-BGT, indicating that EA pretreatment might reduce CPB-induced ALI via α7nAChR-dependent cholinergic signaling pathway. In support of this finding, we found that EA pretreatment enhanced α7nAChR in the lung issue after CPB. Mechanistically, we found that the effects of α7nAChR on EA pretreatment were achieved by modulating HMGB1 and downstream TNF-α and IL-1β in the process of CPB-induced ALI. To our knowledge, this is the first study to show that EA pretreatment attenuates CPB-induced ALI via the α7nAChR-HMGB1 signaling pathway.
To investigate whether EA pretreatment can attenuate CPB-induced ALI, we used a classical rat CPB model (3) and EA pretreatment was performed as previously described (13). In the present study, acupoints of ST36 and BL13 were selected. However, other acupoints were also used to treat ALI in rats with acute pancreatitis (16). Therefore, further studies are needed to explore whether other acupoints may exert the protective effects on the lung. The lung histological analysis showed similar results with our previous study (15). Lung tissue after CPB displayed a feature of lung injury, including alveolar septal thickening, interstitial edema, and vascular congestion, as well as a mild neutrophil infiltration. EA pretreatment significantly altered the pathologic changes. In addition, the protein concentration in BALF was considered positively related to pulmonary capillary permeability (17). As shown in the study, the level of protein concentration in BALF was significantly increased after CPB. However, with the pretreatment of EA, it decreased significantly compared with the CPB group. The W/D ratio of lung tissue was measured to analyze the water content of the lung. The W/D ratio of the sham group was low and significantly enhanced after CPB. However, EA pretreatment suppressed the increase in the W/D ratio. As stated above, our findings strongly suggested that EA pretreatment significantly decreased the lung injury after CPB. ALI is a kind of disease with high mortality (2). Therefore, further studies are needed to investigate the changes in survival rate affected by EA pretreatment in future.
It has been well documented that α7nAChR plays an important role in the cholinergic anti-inflammatory pathway, and activation of α7nAChR can inhibit the production of proinflammatory cytokines (18–20). The protective effects of α7AChR in the pathophysiology of organ ischemia–reperfusion such as brain or kidney have been reported by several studies (11, 21), which provides novel opportunities for the treatment of CPB-induced ALI. In our previous study, we also found that α7nAChR expression in the lung tissue after CPB reduced significantly. In addition, PNU-282987, a selective α7nAChR agonist, significantly ameliorated lung inflammation and ALI after CPB, which suggests that the activation of α7nAChR might attenuate CPB-induced ALI (6). However, the role of EA pretreatment on α7AChR expression in the lung after CPB remains unclear. Consistent with previous studies (6), we found that the α7AChR protein expression decreased after 2 h in the CPB groups compared with that of the sham group in the present study. Interestingly, in this study we found that EA pretreatment reversed this reduction in α7AChR expression. Moreover, the beneficial effects of EA pretreatment on lung injury and lung inflammatory response were attenuated when α7nAChR was blocked by α-BGT. These results are consistent with the possibility that α7AChR downregulation plays a role in producing pulmonary injury in CPB, and that EA may have a protective effect on the lung. Our data suggest a new mechanism for the EA pretreatment in CPB-induced ALI—the targeting of α7nAChR. In our study, although we found that the α7AChR protein expression decreased after CPB, the mechanism of downregulation of alpha7nAChR in lung after CPB remains unclear. Dysfunction of a7 nAChRs may have a variety of causes. Dominant negative Dominant kinase A (PKA) abolished 8-Br-cAMP's effect of diminishing α7 nicotinic currents, whereas a constitutively active PKA catalytic subunit decreased α7 currents (22). More interesting, other investigator found EA pretreatment can produce an antiarrhythmic effect in SGIR (simulative global ischemia and reperfusion) rats and PKA is involved in the mediation of the antiarrhythmic effect of EA pretreatment (23). Therefore, we cannot rule out the possibility that PKA is involved in the downregulation of α7nAChR in the lung after CPB and EA pretreatment. Further studies are needed to investigate the possible mechanism.
Apoptosis is known to contribute to cell death and plays an important role in disease state (24), whereas inhibition of apoptosis can reduce organ injury (25). In our study, we found that only a small amount of TUNEL staining was observed in the EA+CPB group, which suggested that EA suppresses the apoptosis of the lung after CPB. However, further studies are needed to investigate the exact mechanism of apoptosis.
Originally, HMGB1 was identified as a nuclear DNA-binding protein, and recognized as a cytokine-like mediator of systemic inflammation recently (9). In the pathophysiology of pulmonary injury, HMGB1 could be released from macrophages and acts as a mediator linking ALI and subsequent inflammatory processes (26–29). Our finding of increased HMGB1 levels in the serum and BALF suggest that exacerbated HMGB1 release may be triggered by CPB and play a role in enhancing the inflammatory response. Consistent with this result, a previous study showed that the serum levels of HMGB1 were significantly elevated in patients undergoing CPB (30), suggesting the potential role of HMGB1 as a therapeutic target for controlling inflammation during CPB. Other investigators have also reported that lung damage could be attenuated by neutralizing of HMGB1 with a special antibody in rats (7), which may further support the views of HMGB1 as a therapeutic target. In our study, we found that EA pretreatment counteracted the increase of HMGB1 levels in serum and BALF after CPB, which indicate that EA pretreatment might attenuate CPB-induced ALI by targeting the release of HMGB1. As we stated above, α7nAChR played an important role in the process of EA pretreatment attenuating CPB-induced ALI. We also found that the effects of EA pretreatment on HMGB1 release were attenuated by α7nAChR blockade. Given that α7nAChR can regulate the release of HMGB1 (31), EA pretreatment may suppress the release of HMGB1 after CPB via the activation of α7nAChR. However, a genetic approach would be a better alternative strategy to strengthen our study, and performing CPB operation on α7nAChR KO mice would provide direct evidence of the role of α7nAChR and the mechanism of EA, which is a limitation of the study.
We further investigated whether HMGB1 release leads to expression of its downstream TNF-α and IL-1β, which are considered to be important in regulating inflammatory response (32). The inflammatory response associated with CPB has been hypothesized to contribute to lung injury and postoperative pulmonary dysfunction (33). In this study, we found that the levels of TNF-α and IL-1β both in the lung tissues and serum as well as in the BALF significantly increased after CPB. EA pretreatment inhibited the release of TNF-α and IL-1β. In addition, α-BGT reversed the protective effect of EA against CPB-induced ALI.
CONCLUSIONS
To conclude, our results suggest that EA pretreatment might play a protective role in CPB-induced ALI, and inhibits HMGB1 release through α7nAChR activation in rats. Furthermore, the results also provide support for the possibility of α7nAChR-mediated anti-inflammatory interventions after CPB.
Supplementary Material
Footnotes
ZW, LH, and HY equally contributed to this work.
This study was supported by grants from the National Natural Science Foundation of China (No. 81400050, 81370178, and 81570068), the Major State Basic Research Development Program of China (973 Program, No. 2013CB531902).
The authors report no conflicts of interest.
REFERENCES
- 1.Clark SC. Lung injury after cardiopulmonary bypass. Perfusion 21:225–228, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F. Incidence and predictors of ARDS after cardiac surgery. Chest 119:884–888, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Kagawa H, Morita K, Nagahori R, Shinohara G, Kinouchi K, Hashimoto K. Prevention of ischemia/reperfusion-induced pulmonary dysfunction after cardiopulmonary bypass with terminal leukocyte-depleted lung reperfusion. J Thorac Cardiovasc Surg 139:174–180, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Schlensak C, Beyersdorf F. Lung injury during CPB: pathomechanisms and clinical relevance. Interact Cardiovasc Thorac Surg 4:381–382, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Rong J, Ye S, Wu ZK, Chen GX, Liang MY, Liu H, Zhang JX, Huang WM. Controlled oxygen reperfusion protects the lung against early ischemia-reperfusion injury in cardiopulmonary bypasses by downregulating high mobility group box 1. Exp Lung Res 38:183–191, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Ge J, Tian J, Yang H, Hou L, Wang Z, He Z, Wang X. Alpha7 nicotine acetylcholine receptor agonist PNU-282987 attenuates acute lung injury in a cardiopulmonary bypass model in rats. Shock 47:474–479, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Yang Z, Gao Y, Xu H, Zheng B, Jiang M, Xu J, He Z, Wang X. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One 8:e64375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallowitsch-Puerta M, Pavlov VA. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci 80:2325–2329, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10:1216–1221, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Song JG, Li HH, Cao YF, Lv X, Zhang P, Li YS, Zheng YJ, Li Q, Yin PH, Song SL, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology 116:406–414, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Wang F, Li X, Yang Q, Li X. Electroacupuncture pretreatment attenuates cerebral ischemic injury through (7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation 9:24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Wang Y, Wang Y, Ning Q, Zhang Y, Gong C, Zhao W, Jing G, Wang Q. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol 35:210–216, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Yu JB, Shi J, Gong LR, Dong SA, Xu Y, Zhang Y, Cao XS, Wu LL. Role of Nrf2/ARE pathway in protective effect of electroacupuncture against endotoxic shock-induced acute lung injury in rabbits. PLoS One 9:e104924, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44:725–738, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma W, Li Z, Lu Z, Tan W, Zhang Z, Li Y, Yang Z, Zhou J, Tang H, Cui H. Protective effects of acupuncture in cardiopulmonary bypass-induced lung injury in rats. Inflammation 40:1275–1284, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Zhu SF, Zhang RR, Zhao XL, Wan MH, Tang WF. Electroacupuncture ameliorates acute lung injury through promoting gastrointestinal motility in rats with acute pancreatitis. Evid Based Complement Alternat Med 2014:943596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YL, Liu YJ, Liu Y, Li XS, Liu SH, Pan YG, Zhang J, Liu Q, Hao YY. Hydroxysafflor yellow A ameliorates lipopolysaccharide-induced acute lung injury in mice via modulating toll-like receptor 4 signaling pathways. Int Immunopharmacol 23:649–657, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, Liu C, Xi T, Su DF, Shen FM. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension 57:298–307, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 265:663–679, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24:1451–1460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komal P, Estakhr J, Kamran M, Renda A, Nashmi R. cAMP-dependent protein kinase inhibits alpha7 nicotinic receptor activity in layer 1 cortical interneurons through activation of D1/D5 dopamine receptors. J Physiol 593:3513–3532, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Zhang L, Wang Y, Lu B, Cui H, Fu W, Wang H, Yu Y, Yu X. Antiarrhythmic effect of acupuncture pretreatment in rats subjected to simulative global ischemia and reperfusion--involvement of adenylate cyclase, protein kinase A, and L-type Ca2+ channel. J Physiol Sci 58:389–396, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21:99–109, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Haque A, Kunimoto F, Narahara H, Okawa M, Hinohara H, Kurabayashi M, Saito S. High mobility group box 1 levels in on and off-pump cardiac surgery patients. Int Heart J 52:170–174, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170:1310–1316, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterenczak KA, Joetzke AE, Willenbrock S, Eberle N, Lange S, Junghanss C, Nolte I, Bullerdiek J, Simon D, Murua Escobar H. High-mobility group B1 (HMGB1) and receptor for advanced glycation end-products (RAGE) expression in canine lymphoma. Anticancer Res 30:5043–5048, 2010. [PubMed] [Google Scholar]
- 30.Zhang Z, Wu Y, Zhao Y, Xiao X, Liu J, Zhou X. Dynamic changes in HMGB1 levels correlate with inflammatory responses during cardiopulmonary bypass. Exp Ther Med 5:1523–1527, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10:1216–1221, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z, Wang X. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab Invest 93:792–800, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 29:163–175, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


