ABSTRACT
It is unclear whether the superiority of eccentric over concentric training on neuromuscular improvements is due to higher torque (mechanical loading) achievable during eccentric contractions or due to resulting greater total work.
Purpose
This study aimed to examine neuromuscular adaptations after maximal eccentric versus concentric training matched for total work.
Methods
Twelve males conducted single-joint isokinetic (180°·s−1) maximal eccentric contractions of the knee extensors in one leg (ECC-leg) and concentric in the other (CON-leg), 6 sets per session (3–5 sets in the initial 1–3 sessions), 2 sessions per week for 10 wk. The preceding leg performed 10 repetitions per set. The following leg conducted the equivalent volume of work. In addition to peak torque during training, agonist EMG and MRI-based anatomical cross-sectional area (ACSA) and transverse relaxation time (T2) at midthigh as reflective of neural drive, hypertrophy, and edema, respectively, were assessed weekly throughout the training period and pre- and posttraining. Whole muscle volume was also measured pre- and posttraining.
Results
Torque and EMG (in trained contraction conditions) significantly increased in both legs after week 1 (W1) and week 4 (W4), respectively, with a greater degree for ECC-leg (torque +76%, EMG +73%: posttraining) than CON-leg (+28%, +20%). ACSA significantly increased after W4 in ECC-leg only (+4%: posttraining), without T2 changes throughout. Muscle volume also increased in ECC-leg only (+4%). Multiple regression analysis revealed that changes (%Δ) in EMG solely explained 53%–80% and 30%–56% of the total variance in %Δtorque through training in ECC-leg and CON-leg, respectively, with small contributions (+13%–18%) of %ΔACSA for both legs.
Conclusion
Eccentric training induces greater neuromuscular changes than concentric training even when matched for total work, whereas most of the strength gains during 10-wk training are attributable to the increased neural drive.
Key Words: KNEE EXTENSION TORQUE, NEURAL DRIVE, HYPERTROPHY, EDEMA, WEEKLY ASSESSMENT
It is well documented that eccentric (ECC) training can produce greater muscle hypertrophy than concentric (CON) training (1–3). This superiority of ECC training is suggested to be mainly attributable to the higher torque (i.e., mechanical loading) achievable during ECC contractions (4,5). Indeed, it is known that higher (>20%) torque can be produced during ECC than CON contractions (5), and that mechanical loading is one of the most important training variables for muscle hypertrophy (6). However, almost all of the previous studies that compared these training modes have used fixed numbers of repetitions and sets in their training programs, resulting in greater total work for ECC training (5). Because total work also influences training-induced muscle hypertrophy (6), it is poorly understood whether the superiority of ECC training is due to the higher mechanical loading or due to the resulting greater total work.
Moore et al. (7), to the author’s knowledge, is the only study that explored the effects of work-matched maximal ECC versus CON training on muscle size. In their study, participants conducted 9-wk isokinetic (45°·s−1) training of the elbow flexors, in which one arm performed 2–6 sets of 10 repetitions of maximal ECC contractions, followed by the other arm performing the equivalent volume of work by maximal CON contractions (7). It is worth noting that a greater number of repetitions (+~40%) were required for the CON arm to match the total work. Interestingly, training-induced changes in muscle anatomical cross-sectional area (ACSA) did not significantly differ between the arms (ECC +6.5% vs CON +4.6%), suggesting that the mechanical loading is not a key factor for muscle hypertrophy when total work is matched.
Although the finding of Moore et al. (7) is to be commended, there are methodological considerations to be explored. First, the velocity used in their training was relatively slow (45°·s−1). It is known that maximum torque decreases hyperbolically with increasing velocity during CON contractions, while it increases with increasing velocity until a certain point (e.g., ~200°·s−1 for the knee extensors [8]) during ECC contractions. Thus, to accentuate the difference in the mechanical loading between the training modes, the velocity should be relatively fast (e.g., 150°·s−1−200°·s−1). Second, Moore et al. (7) trained upper limb muscles. Training-induced hypertrophy is reported to be often less in lower limb muscles, particularly the antigravity muscles such as the quadriceps femoris (9). A plausible explanation is that these muscles are more habitually activated during daily activities than upper limb muscles, and thus respond less to a given stimulus (10). In this sense, the higher mechanical loading that is only achievable during ECC contractions would be a key stimulus for promoting muscle hypertrophy in the lower limb muscles.
Regardless of contractions modes, it remains unclear when the “true” hypertrophy occurs after resistance training. Recent studies have found significant increases in ACSA after 3–4 wk of quadriceps resistance training (11–13). However, the early phase of increases in ACSA may be influenced by muscle edema (swelling) and may not necessarily reflect the true hypertrophy (11,12). Muscle edema can be noninvasively evaluated by transverse relaxation time (T2)–weighted MRI, which provides information on water content of muscle tissue given as a T2 value (14–16). Thus, this technique allows for quantification of changes in water content as well as ACSA of muscles induced by acute and repeated exercise.
Finally, resistance training is known to produce changes in the nervous system (i.e., neural adaptation) as well, which largely contributes to the training-induced strength gain particularly in the early phase of training (10,17). Previous studies have reported that the neural drive, assessed as surface EMG or interpolated twitch technique, is lower during ECC than CON contractions in untrained individuals, although the exerted torque is much higher (18,19). This is suggested to be due to a neural regulatory mechanism that limits the discharge rate of motor units during maximal ECC contractions, the dominant mechanism of which remains unknown (20). Nevertheless, it is generally agreed that such inhibition can be downregulated or removed by resistance training (18,20). On the basis of this, it is envisaged that the neural changes would be also greater after ECC than CON training.
The purpose of this study was therefore to examine the neural and hypertrophic adaptations after ECC versus CON training matched for total work. To this end, we designed a within-subject comparison model of 10-wk isokinetic resistance training of the knee extensors using a fast velocity (180°·s−1), with weekly assessments of strength, neural drive, muscle size, and edema. We hypothesized that ECC training induces greater neural and hypertrophic changes than CON training even when matched for work.
METHODS
Participants
This study was approved by the Ethics Committee of the Waseda University (2016-048) and was consistent with institutional ethical requirements for human experimentation in accordance with the Declaration of Helsinki. Similarly to Moore et al. (7), we estimated the sample size based on changes in muscle ACSA as these are typically of lower magnitude than those of strength and/or EMG after training (5,21). Using an α-level of 0.05, a power (1 − β) of 0.80, and changes in muscle ACSA at midthigh of 7.3% ± 4.5% (effect size = 1.6) after 12 wk of quadriceps resistance training with whey protein ingestion (22), which was also taken in this study (detailed in the Training section), it was calculated that six participants would be needed to detect a significant change in muscle ACSA (G*Power; Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany). Because the training period of this study was 10 wk (which would result in less change than that of the 12-wk training study [22]), and considering potential dropouts during the intervention, we recruited 12 young males (age = 25.6 ± 3.9 yr, height = 1.69 ± 0.04 m, body mass = 63.5 ± 9.1 kg). Before any measurements, participants visited the laboratory and were fully informed about the procedures and possible risks involved as well as the purpose of the study, and written informed consent was obtained. All participants underwent a familiarization session, where all of the voluntary contraction tasks involved in this study (explained below) were performed. All participants were right leg dominant, and one of their legs was assigned to ECC (ECC-leg) and the other CON (CON-leg) training, which was counterbalanced between the right and left legs among participants. All participants were healthy and physically active, but none had been competitive athletes, or involved in any type of systematic (≥30 min·d−1, ≥2 d·wk−1) training program in the past 12 months before the onset of the study. Participants were instructed to avoid any intensive and unfamiliar physical activities within 2 d before the pretest and throughout the experimental period.
Overview
A schematic illustration of the experimental design is shown in Figure 1. Participants conducted training 2 sessions a week, separated by at least 2 d, for 10 wk. Work and repetitions performed by each leg during training were recorded at all sessions. Torque and EMG in the trained contraction condition were measured at pre- and posttests and at all sessions for torque and at every other session (i.e., at sessions 1, 3, 5, …, 19) for EMG. MRI scans for assessing ACSA and T2 were conducted at pre- and posttests and between sessions in each week (i.e., between sessions 1 and 2, 3 and 4, …, 19 and 20), at least 2 d apart from the previous training session. Pre- and posttests were conducted 2–5 d before and after the first and last training sessions, respectively, and included assessments of whole muscle volume of the quadriceps femoris, muscle compound action potential (M-wave), and evoked torque by octet contractions (detailed below), in addition to the torque, EMG, ACSA, and T2 measurements.
FIGURE 1.
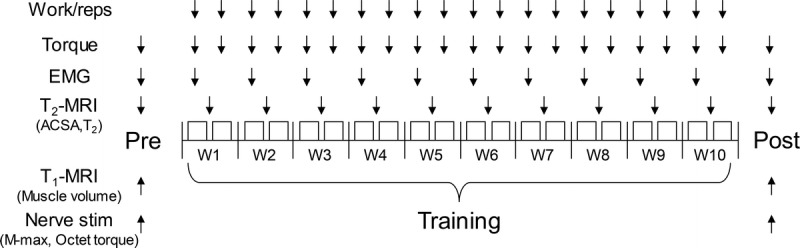
Experimental design. Arrows indicate measurement timing of each variable. Reps, repetitions; M-max, maximal muscle compound action potential; Nerve Stim, peripheral (femoral) nerve stimulation; T1, longitudinal relaxation time; T2, transverse relaxation time; W, week.
Training
Training was conducted by using an isokinetic dynamometer (CON-TREX; CMV AG, Dübendorf, Switzerland). Participants sat in an adjustable chair with the hip joint at 90° (anatomical position = 0°), and the torso and hips were held tightly in the seat using adjustable lap belts to prevent extraneous movement during training. The dynamometer setting (i.e., height and length of the seat and crank) was kept the same throughout the study for each participant. The knee extension/flexion attachment on the dynamometer was set so that the range of motion of the knee joint was from 20° to 90°. Each training session commenced with warm-up consisting of four to six submaximal to near maximal contractions (~50%–90% effort) with the assigned contraction mode. After the warm-up, participants performed maximal ECC or CON contractions at 180°·s−1, 10 repetitions per set, for 6 sets. Eight-second rests were taken in between repetitions, during which the leg was passively (automatically) returned to the start position by the dynamometer at 20°·s−1 (~5 s) followed by a static rest (~3 s). Two-minute rests were taken in between sets, during which participants rested statically with the knee joint angle at approximately the middle of the range. To minimize exercise-induced muscle damage, which often occurs when unaccustomed ECC exercise is performed (16), the number of the sets in the initial training phase was gradually increased from 3, 4, and then 5 sets at the first, second, and third sessions, respectively, and 6 sets were performed at the fourth session and thereafter. After training one leg, participants performed the equivalent volume of work per set in the contralateral leg with the assigned contraction mode. The preceding leg was counterbalanced in the first training session among participants, and it was switched every session for each participant. Torque signal was recorded at a sampling rate of 2000 Hz using a 16-bit A/D converter (PowerLab 16s; ADInstruments, Sydney, Australia) and stored on a personal computer running data acquisition/analysis software (LabChart version 8; ADInstruments). Visual feedback of the torque signal and verbal encouragement (i.e., countdown from 3, 2, 1, and then go) was given to the participants to promote the maximal effort for each contraction through all training sessions. After each session, participants ingested 30 g of whey protein to enhance postexercise muscle protein synthesis (7,11). It is worth noting that whey protein intake augments muscle hypertrophy regardless of contraction modes used in training (22).
To examine the influence of fatigue within a session, the average peak torque per set was calculated for each of the 6 sets for all training sessions (except for the first three sessions that conducted 3–5 sets only) and averaged across sessions for each leg. In addition, fatigue index was calculated as the average peak torque of the final set/the average peak torque of the first set × 100 (modified from [23]) for each leg. Peak torque data at EMG-measured sessions (i.e., sessions 1, 3, 5, …, 19) were used for further analysis to allow for a comparison between changes in these variables through the training period (explained in the EMG and Statistical analysis sections). Work and repetitions were summed among sessions in each week, and weekly data were used for further analysis.
Voluntary and evoked torque measurements at pre- and posttests
Using the same isokinetic dynamometer and posture as for the training, maximal torque of the knee extensors in the trained contraction condition for each leg was measured. After the warm-up as described earlier, maximal ECC or CON contractions at 180°·s−1 were performed twice for each leg with ≥30 s of rest. If the difference in the peak torque between the two trials was ≥10%, additional trials were conducted until the closest two peak torques were <10%. The highest peak torque was adopted for further analysis. Maximal voluntary isometric knee flexion (~5 s) was also performed twice with the same rest period and criteria as above for the purpose of normalization of antagonist EMG during contractions of the knee extensors (detailed in the EMG section). In addition, to measure the M-wave and evoked torque of the knee extensors, the femoral nerve was stimulated by using a constant-current stimulator (DS7AH; Digitimer, Welwyn Garden City, UK) with the hip and knee joints both kept at 90°. Disposable cathode (2 × 2 cm) and anode (4 × 5 cm) electrodes were attached to the skin over the femoral nerve in the femoral triangle and over the greater trochanter, respectively. Cathode location was determined by delivering single electrical impulses (square wave pulses of 0.2-ms duration, ≥5 s apart) to identify the position that elicited the greatest submaximum twitch response. The current intensity was then progressively increased until plateaus in peak twitch torque and peak-to-peak M-wave amplitude were reached. Then three supramaximal twitch and maximal M-wave (M-max) responses were evoked (15 s apart) at +50% current intensity to ensure supramaximal stimulation. The three M-max responses were averaged for each muscle and used for EMG normalization (detailed in the EMG section). Then, octet contractions (eight impulses at 300 Hz) were evoked at progressive currents (≥15 s apart) until a plateau in the peak torque was achieved. Further three discrete pulse trains (≥15 s apart) at +20% current intensity were delivered to evoke maximum contractions. Peak torque in each of the three trials was averaged for analysis. Because of the discomfort and concomitant muscle activation during the stimulation, two participants were excluded from the analysis.
EMG
EMG was recorded at every other training session and at pre- and posttests using a wireless EMG system (Trigno; Delsys, Boston, MA). After skin preparation (shaving, abrading, and cleansing ethanol), surface EMG electrodes were placed over the belly of the rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), and biceps femoris (BF) at 50% (RF, VL, and BF) and 80% (VM) of the thigh length (distance between the greater trochanter and lateral femoral condyle) and parallel to the presumed orientation of the muscle fibers (modified from [24]). The electrode positions were marked on the skin using indelible ink and marked repeatedly throughout the study period so that the same electrode positioning was achieved. EMG signals were amplified (×300) and band-pass filtered (20–450 Hz) at source and sampled at 2000 Hz via the same A/D converter and computer software as for the torque signal to enable data synchronization. In offline analysis, EMG signals were corrected for the 48-ms delay inherent in the Trigno EMG system. From the contraction in which the highest peak torque occurred in each of the EMG-measured sessions, root mean square EMG in the 30°–80° range of knee joint excursion was calculated to allow for a comparison of EMG activities between the contraction modes (18) (Fig. 2). Raw EMG data were used for the time course change analysis (i.e., pretraining, during, and posttraining) for both of the agonist EMG (AGO-EMG) and antagonist EMG (ANT-EMG). In addition, for each of the pre- and posttests, AGO-EMG was normalized to M-max for each of the three quadriceps muscles and then averaged across the three muscles to provide a whole quadriceps value (AGO-EMG/M-max [%]) (25). Similarly, ANT-EMG during knee extension was normalized to maximal BF EMG amplitude during isometric knee flexion (500-ms window centered on the time at which peak torque occurred) for each of the pre- and posttests and expressed as a coactivation level (ANT-EMG/BF-max [%]) (26).
FIGURE 2.
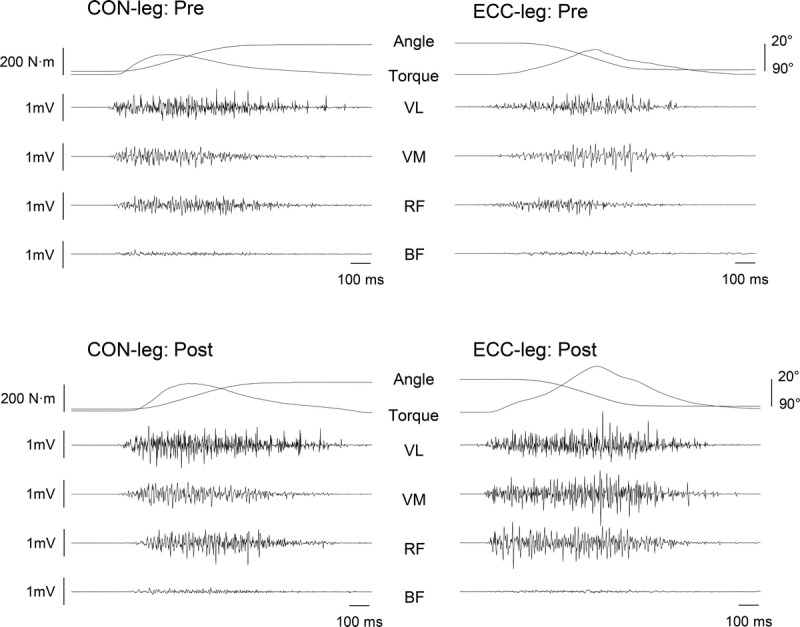
Example data of torque, joint angle, and EMG during concentric (left) and eccentric (right) contractions pretraining (top) and posttraining (bottom). CON-leg, concentrically trained leg; ECC-leg, eccentrically trained leg.
MRI
At pre- and posttests and weekly throughout the training period, T2-weighted MRI (echo times, 25, 50, 75, and 100 ms; slice thickness, 1.5 cm; gap, 1 cm) for each thigh in the transverse plane were recorded using an eight-channel body array coil (GE Medical Systems, Chicago, IL). Before the scanning, oil capsules were put as markers at 50% and 80% of the thigh length on the skin surface at the lateral side for both legs (at pre- and posttests only). Participants lay supine with their legs fully extended and muscles relaxed in a magnet bore (Signa EXCITE 1.5T, GE Medical Systems). To obtain images reproducibly throughout the study, a specific slice was always set using the same anatomical marker. Namely, the most proximal slice was always set at the proximal edge of the femoral head, and the nearest slice to the 50% marker in the pretraining scanning was used for analysis throughout for each participant (15,16). Images were analyzed using Osirix software (version 8; Pixmeo, Geneva, Switzerland). Regions of interest (ROI) were drawn by manually tracing the border of the ACSA of each of the quadriceps femoris muscles. Care was taken to exclude visible adipose and connective tissue incursions. ACSA values of the four muscles were summed to provide a quadriceps ACSA. T2 relaxation time was calculated from the same ROI as for the ACSA of each muscle, by least-squares analysis fitting the signal intensity at each of the four echo times (n × 25 ms: 25, 50, 75, and 100 ms) to a monoexponential decay using the following equation:
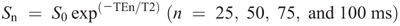
where TE is echo time, S0 is signal intensity at 0 ms, and Sn is signal intensity at TEn. T2 values were averaged across the four muscles to provide a quadriceps T2 value.
In addition to the T2-weighted MRI, T1-weighted MRI values (echo times, 5 ms; slice thickness, 4 mm; gap, 0 mm) of the whole thigh for both legs were also obtained from two or three (depending on participants’ body size) overlapping blocks at pre- and posttests. The oil capsule markers allowed alignment of the blocks during the analysis. The ROI values of each of the quadriceps muscles were manually outlined in every third image (i.e., every 12 mm) from the most proximal to the most distal image in which the muscle is visible, and ACSA values for the skipped images were estimated based on a linear relation between the images in which ACSA values were outlined. The volume of each muscle was determined by summing all ACSA values multiplied by the slice thickness (4 mm), and the total quadriceps volume was calculated by summing the individual muscle volumes. During the MRI analysis, the investigators were blinded to the training modes and measurement timing.
Reproducibility of the measurements
Day-to-day (separated by 1–3 d) reproducibility of the measurements was examined on seven participants (14 legs). Paired t-tests revealed no significant difference between days in all variables. The coefficient of variation (CV) and the intraclass correlation coefficient for each measurement variable were as follows: eccentric peak torque, 5.9% ± 4.8%, 0.917; eccentric AGO-EMG, 11.0% ± 7.1%, 0.701; eccentric AGO-EMG/Mmax, 13.4% ± 8.7%, 0.806; eccentric ANT-EMG, 29.9% ± 22.0%, 0.412; eccentric ANT-EMG/BF-max, 31.0% ± 18.0%, 0.478; concentric peak torque, 6.9% ± 5.8%, 0.857; concentric AGO-EMG, 14.2% ± 9.0%, 0.618; concentric AGO-EMG/M-max, 12.8% ± 10.4%, 0.765; concentric ANT-EMG, 30.2% ± 23.1%; 0.492; concentric ANT-EMG/BF-max, 28.4% ± 29.3%, 0.561; octet torque, 3.7% ± 2.7%, 0.861; ACSA, 1.1% ± 0.9%, 0.993; T2, 2.2% ± 1.8%, 0.403. CV was low for voluntary and evoked torque but relatively high for EMG, particularly ANT-EMG (both raw and normalized data), and those of ACSA and T2 were very low. Intraclass correlation coefficient values were interpreted as excellent, 0.80–1.00; good, 0.60–0.80; and poor, <0.60. All variables ranged from good to excellent (0.618–0.993), except for T2 (0.403) and ANT-EMG (0.412–0.561), which would be due to its relatively low interindividual variability (14) and high CV of this measurement (24), respectively.
Statistical analysis
Descriptive data are presented as mean ± SD. All data were analyzed using SPSS software (version 23.0; IBM Corp., NY). Statistical significance was set at P < 0.05. A paired t-test was used for a comparison between legs for the following variables: fatigue index, total work, and total repetitions. A two-way repeated-measures ANOVA was used for a comparison of the following variables: the average peak torque per set (6 sets × 2 legs); work and repetitions through training (10 time points × 2 legs); peak torque, AGO-EMG, ANT-EMG, ACSA, and T2 from pre- through posttraining (12 time points × 2 legs); AGO-EMG/M-max, ANT-EMG/BF-max, muscle volume, and evoked torque (2 time points × 2 legs). When significant main effects or interactions were found, a paired t-test (when comparing 2 means) or a Tukey test (when comparing >2 means) was used as a post hoc test, where appropriate. In addition, to examine the difference between legs in the degree of training-induced changes in peak torque, AGO-EMG, AGO-EMG/M-max, ACSA, and muscle volume, percentage changes (%Δ) from pre- to posttraining were calculated for these variables and were compared by a paired t-test between legs. As indices of effect size, Cohen’s d (for a post hoc test) and partial η2 (for ANOVA) were also calculated. Sphericity was checked by Mauchly’s test in ANOVA, and P values were modified with Greenhouse–Geisser correction when necessary.
To describe relationships of individual %Δtorque with %ΔAGO-EMG and %ΔACSA, Pearson’s correlations were calculated for each time point from week 1 (W1) through posttraining. Finally, a stepwise multiple linear regression analysis was conducted to create a predictive model of %Δtorque, with %ΔAGO-EMG, %ΔACSA, and pretraining torque as independent variables (21), at each time point from W1 through posttraining.
RESULTS
Average peak torque per set, fatigue index, work, and repetition during training
All participants, except for one who missed one session (non-EMG measurement session), completed all of the 20 training sessions. The average peak torque per set (averaged across sessions) was as follows: ECC-leg set 1, 214.3 ± 52.6 N·m; set 2, 227.0 ± 59.6 N·m; set 3, 230.1 ± 57.2 N·m; set 4, 230.8 ± 57.0 N·m; set 5, 229.6 ± 58.5 N·m; set 6, 229.9 ± 59.9 N·m, and CON-leg set 1, 145.6 ± 30.3 N·m; set 2, 151.7 ± 31.5 N·m; set 3, 154.2 ± 32.2 N·m; set 4, 154.7 ± 31.9 N·m; set 5, 154.7 ± 32.1 N·m; set 6, 154.5 ± 31.8 N·m. A two-way ANOVA found a main effect of set (P < 0.001, partial η2 = 0.588) and leg (P = 0.001, partial η2 = 0.648), without their interaction (P = 0.270, partial η2 = 0.11). Combining data of both legs, a Tukey test indicated that set 1 was lower than the other sets (P < 0.001, d = 0.22–0.30), without any differences among the rest (P ≥ 0.38, d ≤ 0.08), for both legs. Fatigue index was 107.2% ± 2.5% for ECC-leg and 106.2% ± 4.2% for CON-leg, and it did not differ between legs (P = 0.703, d = 0.016).
Work and repetition performed through training are shown in Figure 3. Work had a significant main effect of time (P < 0.001, partial η2 = 0.86), without a main effect of leg (P = 0.248, partial η2 = 0.12) or their interaction (P = 0.113, partial η2 = 0.13), indicating no difference between legs throughout the training period (Fig. 3A). Total work also did not differ between legs (ECC-leg vs CON-leg, 186.0 ± 39.2 vs 183.7 ± 39.1 kJ, P = 0.248, d = 0.06). A significant leg–time interaction (P < 0.001, partial η2 = 0.67) was found in the repetition (Fig. 3B), which was significantly less in ECC-leg than CON-leg at all weeks (P ≤ 0.017, d = 1.39–6.35) except for W1 (P = 0.421, d = 0.44). Total repetition was also less in ECC-leg than CON-leg (985.3 ± 38.3 vs 1358.2 ± 70.1, P < 0.001, d = 6.60).
FIGURE 3.
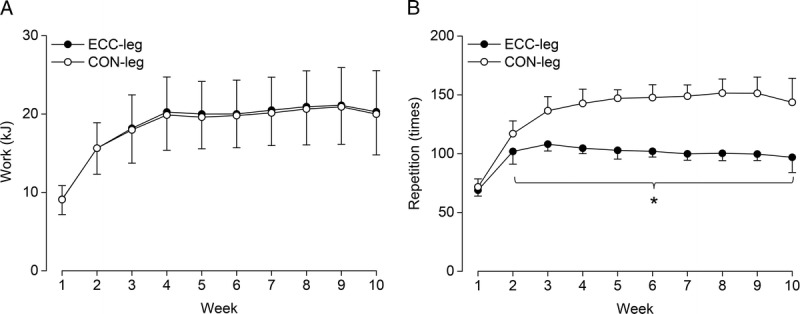
Work (A) and repetition (B) through training. Values are presented as mean ± SD. Note that the values are reported weekly (the sum of two sessions). *Significant difference between legs at P < 0.05. CON-leg, concentrically trained leg; ECC-leg, eccentrically trained leg.
Time course changes in peak torque, EMG, ACSA, and T2
Figure 4 shows changes in peak torque, AGO-EMG, ACSA, and T2 pretraining, during, and posttraining. Peak torque had a significant leg–time interaction (P < 0.001, partial η2 = 0.60) (Fig. 4A). A Tukey test found significant increases from pretraining at W1 and thereafter for both ECC-leg (P ≤ 0.011, d = 0.76–2.22) and CON-leg (P < 0.001, d = 0.72–1.58). Significant increases were also found at W3 or thereafter compared with all or some of the initial 3 wk for both ECC-leg (P ≤ 0.33, d = 0.66–1.37) and CON-leg (P ≤ 0.038, d = 0.42–0.85). Torque was always higher for ECC-leg than CON-leg (P ≤ 0.018, d = 0.75–2.06), with 22% and 62% differences at pre- and posttraining, respectively. %Δtorque from pre- to posttraining was significantly (P < 0.001, d = 1.43) greater for ECC-leg (+76% ± 43%) than CON-leg (+28% ± 20%).
FIGURE 4.

Absolute changes in peak torque (A), agonist EMG amplitude (AGO-EMG, B), ACSA (C), and transverse relaxation time (T2, D). Values are presented mean ± SD. The numbers 0, 1, 2, and 3 indicate a significant increase from pretraining (0), week 1, 2, 3, respectively. *Significant difference between legs at P < 0.05. CON-leg, concentrically trained leg; ECC-leg, eccentrically trained leg.
AGO-EMG had a significant main effect of time (P < 0.001, partial η2 = 0.39), without a main effect of leg (P = 0.521, partial η2 = 0.04) or their interaction (P = 0.143, partial η2 = 0.15) (Fig. 4B). Combining data from both legs, a Tukey test found significant increases from pretraining at W4 and thereafter (P ≤ 0.006, d = 0.12–0.79). Significant increases were also found at W5, W7–W10, posttraining from W1, and at posttraining from W2 to W3 (P ≤ 0.040, d = 0.43–0.63). %ΔAGO-EMG from pre- to posttraining was significantly (P = 0.015, d = 1.20) greater for ECC-leg (+73% ± 58%) than CON-leg (+20% ± 24%). ANT-EMG did not find a significant main effect of time (P = 0.676, partial η2 = 0.05) or leg (P = 0.315, partial η2 = 0.09) or their interaction (P = 0.254, partial η2 = 0.11), indicating no changes in antagonist activity during either ECC (0.046 ± 0.061 mV, averaged across all time points) or CON (0.038 ± 0.034 mV) contractions of the knee extensors.
ACSA had a significant leg–time interaction (P < 0.001, partial η2 = 0.54) (Fig. 4C). A Tukey test found significant increases from pretraining at W4 and thereafter in ECC-leg (P ≤ 0.031, d = 0.10–0.20) but not in CON-leg (P = 0.182, partial η2 = 0.11). ACSA was greater for ECC-leg than CON-leg at W9–W10 (P = 0.024–0.029, d = 0.10–0.14), with a tendency at posttraining (P = 0.059, d = 0.13). %ΔACSA from pre- to posttraining was significantly (P < 0.001, d = 1.49) greater for ECC-leg (+4% ± 3%) than CON-leg (+0% ± 2%).
T2 had a significant main effect of time (P = 0.003, partial η2 = 0.20), without a main effect of leg (P = 0.453, partial η2 = 0.05) or their interaction (P = 0.395, partial η2 = 0.09) (Fig. 4D). However, a Tukey test with the pooled data did not find a significance in the T2 changes (P ≥ 0.135, d ≤ 0.46).
Pre- and posttraining changes in normalized EMG, muscle volume, and evoked torque
Figure 5 shows changes in AGO-EMG/M-max and muscle volume pre- and posttraining. AGO-EMG/M-max had a significant main effect of time (P = 0.001, partial η2 = 0.627) without a main effect of leg (P = 0.231, partial η2 = 0.128) or their interaction (P = 0.130, partial η2 = 0.196), indicating that both legs increased AGO-EMG/M-max after the training (Fig. 5A). %ΔAGO-EMG/M-max from pre- to posttraining was significantly (P = 0.027, d = 0.88) greater for ECC-leg than CON-leg (Fig. 5B). ANT-EMG/BF-max did not find a significant main effect of time (P = 0.544, partial η2 = 0.021) or leg (P = 0.438, partial η2 = 0.031), or their interaction (P = 0.463, partial η2 = 0.033), indicating no changes for both ECC-leg (22.3% ± 16.1% at pretraining, 19.5% ± 18.2% at posttraining) and CON-leg (18.7% ± 17.1%, 21.2% ± 23.0%).
FIGURE 5.
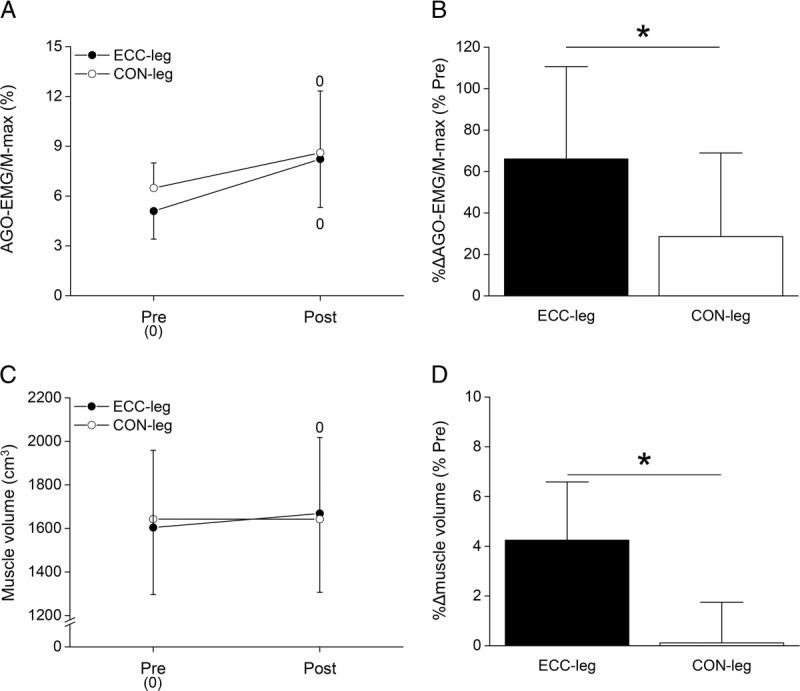
Absolute and percentage (%Δ) changes in agonist EMG amplitude normalized to maximal muscle compound action potential (AGO-EMG/M-max, A and B) and those of muscle volume (C and D) pre- and posttraining. Values are presented as mean ± SD. The number 0 indicates a significant increase from pretraining (0). *Significant difference between legs at P < 0.05. CON-leg, concentrically trained leg; ECC-leg, eccentrically trained leg.
Muscle volume had a significant leg–time interaction (P < 0.001, partial η2 = 0.83). A paired t-test found a significant increase in ECC-leg but not in CON-leg (Fig. 5C). %Δmuscle volume from pre- to posttraining was significantly (P < 0.001, d = 2.05) greater for ECC-leg than CON-leg (Fig. 5D).
Changes in evoked torque did not show a significant main effect of time (P = 0.631, partial η2 = 0.03) or leg (P = 0.208, partial η2 = 0.17), or their interaction (P = 0.833, partial η2 = 0.01), indicating no changes for both ECC-leg (112.8 ± 27.6 N·m at pretraining, 114.7 ± 22.3 N·m at posttraining) and CON-leg (108.1 ± 15.5 N·m, 108.6 ± 23.0 N·m).
Relationship of %Δtorque with %ΔAGO-EMG and %ΔACSA through training
Significant correlations were found between %Δtorque and %ΔAGO-EMG at all weeks and posttraining in ECC-leg (r = 0.572–0.821, P ≤ 0.004). CON-leg also had significant correlations in these changes (r = 0.367–0.602, P ≤ 0.037), but it was relatively sporadic (i.e., significant at W1–W2, W4, W8, and posttraining) compared with those of ECC-leg. No significant correlations were found between %Δtorque and %ΔACSA in both legs at all time points, except for at W4 in CON-leg (r = 0.602, P = 0.003). Multiple regression analysis revealed that %ΔAGO-EMG solely explained 53%–80% and 30%–56% of the total variance in %Δtorque through the training period in ECC-leg and CON-leg (Fig. 6A and B), respectively, with small (+13%–18%) and sporadic contributions of %ΔACSA for both legs.
FIGURE 6.
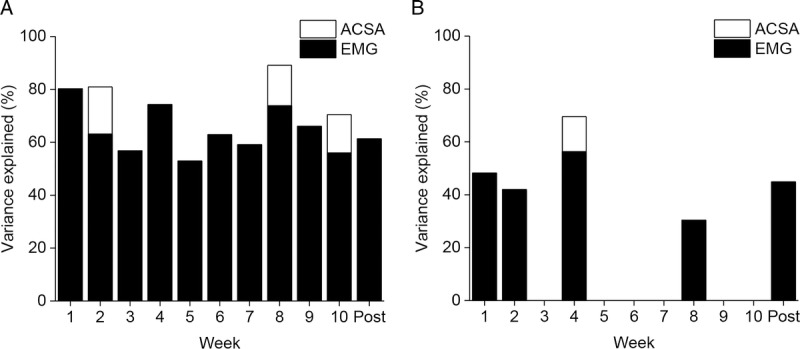
Determinants of changes in torque throughout the training period for eccentrically trained leg (ECC-leg, A) and concentrically trained leg (CON-leg, B). Predictor variables that independently explained a significant proportion of the total variance assessed with stepwise multiple linear regressions are shown. No data at some time points for CON-leg indicate that multiple regression analysis did not find any significant predictor variables at these time points.
DISCUSSION
The main findings of this study were that 1) even when matched for total work, ECC training was more effective in inducing hypertrophy than CON training; 2) significant hypertrophy was observed as early as 4 wk of ECC training without an indication of muscle edema; and 3) neural adaptation was also greater for ECC than CON training, which explained the majority of strength gains in both training modes throughout the training period. These results supported our hypothesis and suggest that ECC training induces greater neural and hypertrophic changes than CON training even when matched for total work, but most of the strength gains during this relatively early phase (10 wk) of training are attributable to the increased neural drive.
Both legs significantly increased the peak torque as the training progressed (Fig. 4A), with a greater degree for ECC-leg than CON-leg. Consequently, this made it necessary for CON-leg to perform more repetitions than ECC-leg after W2 to match the total work (Fig. 3B), which well agrees with Moore et al. (7) who used a similar approach. Previous studies have reported increases of 36%–86% in ECC strength and 13%–44% in CON strength after 10- to 12-wk quadriceps isokinetic maximal ECC and CON training, respectively, using 30°·s−1−90°·s−1 velocities (1,23,27–30). The corresponding changes observed in this study, in which the training was performed at 180°·s−1, were 76% and 28% at posttraining, which are within the ranges shown above. Thus, it appears that typical strength improvements after ECC and CON training were induced in this study as well by this relatively high velocity training.
Accompanying the torque changes, AGO-EMG significantly increased in both legs after W4 (Fig. 4B), with a greater degree for ECC-leg than CON-leg (+73% and +20% at posttraining, respectively). %ΔAGO-EMG well aligned with %Δtorque, showing high correlations between them throughout the training period in both legs but particularly in ECC-leg (r = 0.572–0.821). Furthermore, multiple regression analysis revealed that %ΔAGO-EMG solely explained 53%–80% and 30%–56% of the total variance in %Δtorque for ECC-leg and CON-leg, respectively, throughout the training period (Fig. 6). The higher explained values for ECC-leg than CON-leg are likely due to the greater changes in both torque and AGO-EMG in ECC-leg than CON-leg. These results support the concept that strength gains in an early phase of resistance training are mainly derived from neural adaptations (10,17) and add that its degree largely varies with contraction modes used in training (i.e., ECC > CON).
The greater changes in AGO-EMG and torque for ECC-leg would be due to a reduced pretraining ability to maximally activate all motor units during ECC compared with CON contractions (18,19). Indeed, both raw AGO-EMG and normalized AGO-EMG (to M-max) at pretraining tended to be lower for ECC-leg than CON-leg (i.e., raw, −19%, P = 0.08 by t-test, Fig. 4B; normalized, −21%, P = 0.03, Fig. 5A), whereas the interaction (time–leg) did not reach a significance (P = 0.14 and 0.13) in the two-way ANOVA. It would have been ideal if M-max or EMG during maximal isometric contractions had been measured throughout the training period for the purpose of normalization to reduce the interindividual and spatial variabilities. However, we did not do so because it may affect the magnitude of training effects for muscle strength (especially by maximal isometric contractions) and also was infeasible time wise. Including the interpolated twitch technique (ITT) at least at pre- and posttests may have provided better representation of voluntary activation (19). In addition, given the relationship between type II fiber distribution/area and postactivation potentiation (PAP) (31), together with the propensity for the ECC contractions to recruit these muscle fibers (1), it would have been interesting to examine if postactivation potentiation is increased after ECC training. In this study, ITT was not included because we found it difficult to conduct ITT during fast dynamic contractions (as performed in this study) with a reasonable reproducibility in our pilot study, as well as due to time restrictions. Future studies should be directed toward adopting various approaches, including but not limited to ITT to substantiate the above issue.
No change was found in ANT-EMG in both legs (for both the raw and the normalized data). This is in line with previous studies (18,23,30), which conducted maximal ECC or CON training in the knee extensors. Although reduced ANT-EMG can theoretically increase the torque output and thus can be taken as a neural adaptation to resistance training (10), studies often report no change in ANT-EMG after resistance training (18,19,23,26,30). The poor reliability of this measurement (24) may hinder the detection of a significant change (the interday CV was ~30% in this study). Even when a significant decrease was found in ANT-EMG after training (21), its significance or contribution to the training-induced strength gain has been regarded as negligible (21). Overall, we consider that ANT-EMG did not show a meaningful change in this training intervention.
Significant increases in ACSA occurred at W4 and thereafter in ECC-leg without any changes in T2 throughout (Fig. 4CD). This suggests that muscle edema derived from muscle damage did not occur throughout the training period. Stock et al. (32) recently reported a significant increase in muscle thickness after 3 wk of CON-only resistance training of the elbow flexors, without an indication of muscle edema (assessed as ultrasound echo intensity) and soreness. Although it is controversial whether muscle damage promotes muscle hypertrophy in resistance training, a recent review (33) suggests otherwise. However, it should be reminded that muscle edema (assessed as either T2 or ultrasound echo intensity) is not a direct measure of muscle damage (11,15,16,32). Thus, caution is needed when interpreting the results. Taken together, these suggest that muscle hypertrophy could occur as early as 3–4 wk (depending on muscle groups) without an influence of muscle edema, but the occurrence of muscle damage cannot be ruled out and therefore its potential role remains to be clarified.
By contrast, it is somewhat surprising that muscle size did not change in CON-leg. Previous studies (1,23,27–29) have reported some degree of hypertrophy after isokinetic CON training of this muscle group, although it could be less than those after ECC training. A possible explanation is that by choosing a fast training velocity (180°·s−1), total time under tension and thus total work may have been too small to induce a significant amount of hypertrophy compared with the previous studies mentioned above that used slower velocities (30°·s−1−90°·s−1). It is also possible that the training frequency of 2 sessions per week was not sufficient. However, we chose the fast velocity based on our specific purpose of accentuating the difference in the mechanical loading between the contraction modes as explained earlier, and several studies have reported significant hypertrophy after resistance training performed 2 sessions per week in untrained individuals (6). Most importantly, significant hypertrophy was observed in ECC-leg, but not CON-leg, even with the total work achieved in this study. Therefore, we consider that the main purpose of this study was accomplished by our approach. It is beyond the scope of this study to discuss what training velocity or frequency is most effective for hypertrophy, and it would probably vary between contraction modes and/or muscle groups (7). Further research is needed to clarify these issues.
Another factor that could affect the training response is fatigue during training, especially for CON-leg, which performed a greater number of repetitions. In this study, however, the average peak torque per set did not differ among the sets from the second to sixth, whereas that of the first set was lower than those of the rest, for both legs. As a result, fatigue index (first vs final set) indicated no fatigue, or rather an increase in torque, at the final set compared with the first for both legs. These indicate that the magnitude of the training-induced fatigue was minimal and similar between legs and also suggest that participants were not able to elicit maximal recruitment at the first set, although systematic warm-up and verbal encouragement were always provided throughout the training period. A similar phenomenon has been reported in a previous study (23) conducting maximal ECC training. This may be at least partly due to a psychological factor that limits maximal effort in the initial phase of a training session, which would be likely inherent in a training program that requires maximal effort in every contraction. This aspect should be taken into account, and more thorough feedback and encouragement should be provided in future studies.
Evoked torque as reflective of the contractile property did not change in both legs. This was also unexpected, especially for ECC-leg, because this leg showed a significant increase in muscle size. This may be due to a combination of the small changes in muscle size and a measurement error of evoked torque. It is reported that training-induced changes in evoked torque (+7%) were similar to those of muscle volume (+8%) (25). The interday CV of evoked torque measurement was 3.7% ± 2.7% in this study, which is almost the same as the changes (+4%) in muscle size in ECC-leg. Thus, it is possible that the degree of hypertrophy observed in this study was not large enough to induce significant changes in the muscle contractile property. Although this issue warrants more research, no changes in the contractile property would further suggest substantial contributions of neural factors to the training-induced strength gains.
We chose a within-subject design to minimize potential effects of interindividual hypertrophic responses to resistance training, which is inherent with a between-subject design (34). However, we are aware of a potential confounding factor of within-subject model when interpreting strength changes, namely, the cross-education effect where training-induced strength improvement transfers to the contralateral homologous muscle (35). Unfortunately, it is impossible to distinguish the potential influence of the cross education from the observed strength changes, which may have been less than the current results if one participant had performed one training mode only (i.e., between-subject design). It is worth noting that some studies (36,37) reported greater cross-education effect after ECC than CON training, suggesting that the difference in the strength gains between ECC-leg and CON-leg might have been even larger if trained solely. By contrast, because previous studies have only focused on the cross-education effect on the “untrained” contralateral limb, it is unclear whether the cross education provides the “additional” strength improvement if the contralateral limb is also being trained (as conducted in this study), the effect of which is suggested to be small if any (38). In fact, as mentioned earlier, the strength improvements observed in this study were similar to those of previous studies (1,23,27–30), which all used a between-subject model. Thus, we consider that the cross education would not have substantially influenced our results. Importantly, because the cross education is purely attributable to neural factors (35), it would not influence our main finding on the different hypertrophic responses between training modes, as well as the fact that most of the strength gains were explained by the neural than hypertrophic changes. Nevertheless, more research using both within- and between-subject models is needed to establish practicality for athletes and training practitioners.
In relation with the point above, it should be remarked that isokinetic training was used in this study because this modality favors the precise control of exercise velocity, range of motion, and work, which were all essential in our approach. However, Guilhem et al. (39) suggested that neuromuscular adaptations may differ after isokinetic versus isotonic training; the latter would produce a greater strength gain. This notion was supported by their later study (40) directly comparing these training modes by matching total work, showing greater changes in strength and muscle size after isotonic training. Thus, together with the potential influence of training velocity discussed earlier, it is important to note that the findings obtained here may not necessarily replicate those using other training protocols (e.g., slow isotonic ECC vs CON training). Finally, this study did not consider a potential influence of the difference between the contraction modes in the behavior of the muscle–tendon unit, as well as its training-induced changes (if any), which may provide more detailed mechanisms as to how strength gains can be achieved by these training modes. More research is needed to comprehensively understand how strength is improved through ECC and CON training.
In summary, this study revealed that ECC training was more effective in inducing hypertrophy than CON training even when matched for total work. Furthermore, the weekly assessments allowed us to observe that significant hypertrophy can occur as early as 4 wk of training without an indication of muscle edema. Finally, the majority of strength gains through 10-wk training were attributable to the changes in the neural drive for both ECC and CON training, with a small contribution of the changes in muscle size for both legs. Although longer-term (e.g., >6 months) effects of these training modalities are unknown and thus warrant further research, our results indicate that when ECC and CON training are performed at a relatively high velocity, the higher torque (mechanical loading) that is achievable during ECC contractions, rather than total work, is indeed a key stimulus for promoting muscle hypertrophy in the knee extensors. The current results would also help us better understand how strength gains can be achieved in this relatively early phase (≤10 wk) of both ECC and CON training.
Acknowledgments
This work was supported by a Grant-in-Aid for Japan Society for the Promotion of Science Research Fellow (15J03228). The authors thank all the participants for their time and effort.
The authors declare that there is no conflict of interest, that no companies or manufacturers will benefit from the results of the study, and that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Hortobagyi T, Dempsey L, Fraser D, et al. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol. 2000;524(Pt 1):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vikne H, Refsnes PE, Ekmark M, Medbo JI, Gundersen V, Gundersen K. Muscular performance after concentric and eccentric exercise in trained men. Med Sci Sports Exerc. 2006;38(10):1770–81. [DOI] [PubMed] [Google Scholar]
- 3.LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Lindstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther. 2003;33(10):557–71. [DOI] [PubMed] [Google Scholar]
- 4.Ashida Y, Himori K, Tatebayashi D, Yamada R, Ogasawara R, Yamada T. Effects of contraction mode and stimulation frequency on electrical stimulation-induced skeletal muscle hypertrophy. J Appl Physiol (1985). 2018;124(2):341–8. [DOI] [PubMed] [Google Scholar]
- 5.Roig M, O’Brien K, Kirk G, et al. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43(8):556–68. [DOI] [PubMed] [Google Scholar]
- 6.American College of Sports Medicine. American College of Sports Medicine Position Stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 7.Moore DR, Young M, Phillips SM. Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur J Appl Physiol. 2012;112(4):1587–92. [DOI] [PubMed] [Google Scholar]
- 8.Pain MT, Young F, Kim J, Forrester SE. The torque-velocity relationship in large human muscles: maximum voluntary versus electrically stimulated behaviour. J Biomech. 2013;46(4):645–50. [DOI] [PubMed] [Google Scholar]
- 9.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 2000;81(3):174–80. [DOI] [PubMed] [Google Scholar]
- 10.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–68. [DOI] [PubMed] [Google Scholar]
- 11.Damas F, Phillips SM, Lixandrão ME, et al. Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol. 2016;116(1):49–56. [DOI] [PubMed] [Google Scholar]
- 12.DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR., 2nd An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol. 2011;111(11):2785–90. [DOI] [PubMed] [Google Scholar]
- 13.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985). 2007;102(1):368–73. [DOI] [PubMed] [Google Scholar]
- 14.Foure A, Duhamel G, Wegrzyk J, et al. Heterogeneity of muscle damage induced by electrostimulation: a multimodal MRI study. Med Sci Sports Exerc. 2015;47(1):166–75. [DOI] [PubMed] [Google Scholar]
- 15.Maeo S, Ando Y, Kanehisa H, Kawakami Y. Localization of damage in the human leg muscles induced by downhill running. Sci Rep. 2017;7(1):5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeo S, Saito A, Otsuka S, Shan X, Kanehisa H, Kawakami Y. Localization of muscle damage within the quadriceps femoris induced by different types of eccentric exercises. Scand J Med Sci Sports. 2018;28(1):95–106. [DOI] [PubMed] [Google Scholar]
- 17.Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58(3):115–30. [PubMed] [Google Scholar]
- 18.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Halkjaer-Kristensen J, Dyhre-Poulsen P. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol (1985). 2000;89(6):2249–57. [DOI] [PubMed] [Google Scholar]
- 19.Duclay J, Martin A, Robbe A, Pousson M. Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc. 2008;40(4):722–34. [DOI] [PubMed] [Google Scholar]
- 20.Duchateau J, Enoka RM. Neural control of lengthening contractions. J Exp Biol. 2016;219(Pt 2):197–204. [DOI] [PubMed] [Google Scholar]
- 21.Balshaw TG, Massey GJ, Maden-Wilkinson TM, et al. Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol. 2017;117(4):631–40. [DOI] [PubMed] [Google Scholar]
- 22.Farup J, Rahbek SK, Vendelbo MH, et al. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. 2014;24(5):788–98. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol (1985). 1996;80(3):765–72. [DOI] [PubMed] [Google Scholar]
- 24.Tillin NA, Pain MT, Folland JP. Short-term unilateral resistance training affects the agonist–antagonist but not the force-agonist activation relationship. Muscle Nerve. 2011;43(3):375–84. [DOI] [PubMed] [Google Scholar]
- 25.Balshaw TG, Massey GJ, Maden-Wilkinson TM, Tillin NA, Folland JP. Training-specific functional, neural, and hypertrophic adaptations to explosive- vs. sustained-contraction strength training. J Appl Physiol (1985). 2016;120(11):1364–73. [DOI] [PubMed] [Google Scholar]
- 26.Maeo S, Yoshitake Y, Takai Y, Fukunaga T, Kanehisa H. Neuromuscular adaptations following 12-week maximal voluntary co-contraction training. Eur J Appl Physiol. 2014;114(4):663–73. [DOI] [PubMed] [Google Scholar]
- 27.Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol (1985). 2007;103(5):1565–75. [DOI] [PubMed] [Google Scholar]
- 28.Higbie EJ, Cureton KJ, Warren GL, 3rd, Prior BM. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol (1985). 1996;81(5):2173–81. [DOI] [PubMed] [Google Scholar]
- 29.Seger JY, Arvidsson B, Thorstensson A. Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur J Appl Physiol Occup Physiol. 1998;79(1):49–57. [DOI] [PubMed] [Google Scholar]
- 30.Seger JY, Thorstensson A. Effects of eccentric versus concentric training on thigh muscle strength and EMG. Int J Sports Med. 2005;26(1):45–52. [DOI] [PubMed] [Google Scholar]
- 31.Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J Appl Physiol (1985). 2000;88(6):2131–7. [DOI] [PubMed] [Google Scholar]
- 32.Stock MS, Mota JA, DeFranco RN, et al. The time course of short-term hypertrophy in the absence of eccentric muscle damage. Eur J Appl Physiol. 2017;117(5):989–1004. [DOI] [PubMed] [Google Scholar]
- 33.Damas F, Libardi CA, Ugrinowitsch C. The development of skeletal muscle hypertrophy through resistance training: the role of muscle damage and muscle protein synthesis. Eur J Appl Physiol. 2018;118(3):485–500. [DOI] [PubMed] [Google Scholar]
- 34.Hubal MJ, Gordish-Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37(6):964–72. [PubMed] [Google Scholar]
- 35.Farthing JP, Zehr EP. Restoring symmetry: clinical applications of cross-education. Exerc Sport Sci Rev. 2014;42(2):70–5. [DOI] [PubMed] [Google Scholar]
- 36.Hortobagyi T, Lambert NJ, Hill JP. Greater cross education following training with muscle lengthening than shortening. Med Sci Sports Exerc. 1997;29(1):107–12. [DOI] [PubMed] [Google Scholar]
- 37.Kidgell DJ, Frazer AK, Daly RM, et al. Increased cross-education of muscle strength and reduced corticospinal inhibition following eccentric strength training. Neuroscience. 2015;300:566–75. [DOI] [PubMed] [Google Scholar]
- 38.Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol (1985). 2006;101(5):1514–22. [DOI] [PubMed] [Google Scholar]
- 39.Guilhem G, Cornu C, Guevel A. Neuromuscular and muscle–tendon system adaptations to isotonic and isokinetic eccentric exercise. Ann Phys Rehabil Med. 2010;53(5):319–41. [DOI] [PubMed] [Google Scholar]
- 40.Guilhem G, Cornu C, Maffiuletti NA, Guevel A. Neuromuscular adaptations to isoload versus isokinetic eccentric resistance training. Med Sci Sports Exerc. 2013;45(2):326–35. [DOI] [PubMed] [Google Scholar]


