Abstract
Dual-specificity phosphatase-1 (DUSP1) is an oncogene that is associated with cancer progression following drug resistance. In order to investigate the potential relationship between DUSP1 and apatinib resistance in gastric cancer cells, we preformed many assays to study this problem. DUSP1 gene was detected by RT-qPCR assay, proteins in MAPK pathway were quantified by western blot assay, and CCK-8 assay, flow cytometry and Hoechest 33342 stain were performed to detect the resistance of cells, cell cycles and apoptosis, respectively. Immunohistochemical staining was used to discover the expression of DUSP1 protein in patients' tumor or paratumor tissues. It was found that apatinib (Apa)-resistant gastric cancer (GC) cells showed increased expression of DUSP1, whereas the knockdown of DUSP1 in resistant cells resensitized these cells to Apa. The restored sensitivity to Apa was the result of inactivation of mitogen-activated protein kinase (MAPK) signaling and the induction of apoptosis. The in vitro use of Apa in combination with a DUSP1 inhibitor, triptolide, exerted significant effects on inhibiting the expression of DUSP1, growth inhibition, and apoptosis via the inactivation of MAPK signaling. In patients who did not undergo chemotherapy or targeted therapy, the expression of DUSP1 in adjacent tissues was higher when compared with that observed in tumor tissues. In addition, the expression of DUSP1 was higher in the early stages of GC than in the advanced stages. The expression of DUSP1 in tumor tissues was not associated with the survival rate of the patients. Therefore, increased expression of DUSP1 may be responsible for Apa resistance, and DUSP1 may serve as a biomarker for Apa efficacy. In conclusion, inducing the downregulation of DUSP1 may be a promising strategy to overcome Apa resistance.
Keywords: apatinib, dual-specificity phosphatase-1, gastric cancer, resistance, triptolide
Introduction
Gastric cancer (GC) is a common malignancy, which has been suggested to be the third most common cause of cancer-associated mortality worldwide (1). In general, GC is diagnosed at advanced stages and prognosis is poor. Several drugs are available for the treatment of GC; however, the prognosis for metastatic disease remains unsatisfactory (2,3). The estimated overall survival rate (OS) of patients with metastatic GC that receive conventional chemotherapeutic agents has been reported to be as low as 10% at 5 years (4). However, several innovative, biological therapies have been introduced for the treatment of GC. The trastuzumab for GC (TOGA) trial was the first randomized phase III trial, which demonstrated an advantage in terms of progression-free survival (PFS) and OS rates for patients with positive human epidermal receptor 2 (HER-2) GC tumors (5). Unfortunately, only a small percentage of patients (~20%) are ideal candidates for HER-2-targeted therapy (5–7). In addition, ramucirumab, a human monoclonal antibody directed against vascular endothelial growth factor receptor (VEGFR)-2, which was initially developed for the treatment of human tumors, has shown significant survival benefits as a second line treatment option for patients with metastatic GC who progressed on fluoropyrimidine- or platinum-based first-line chemotherapy (8,9). Other antiangiogenic agents, including bevacizumab, sunitinib and sorafenib, have failed to demonstrate any survival benefit (10). Previous studies have supported the inhibition of angiogenesis in GC (11). Apatinib (Apa), a novel receptor tyrosine kinase inhibitor that selectively targets the intracellular ATP binding site of VEGFR-2 (12,13), has shown promising results in preclinical and clinical trials involving patients with GC. Apa has shown a suitable safety and tolerance profile, and a sufficient treatment efficacy in phase I/II trials. In a phase III trial, Apa prolonged the median OS rates of patients with chemotherapy-refractory metastatic GC by 55 days and the median PFS rate by 25 days, when compared with placebo-treated patients (14).
Dual-specificity phosphatase-1 (DUSP1) was initially identified in cultured murine cells (15,16). DUSP1 has two domains, including an amino terminal non-catalytic domain and a C-terminal catalytic domain, the latter containing the ATPase active site consensus sequence (17,18). Previous studies have shown that DUSP1 regulates MAPK signaling and is involved in cell proliferation, differentiation, transformation, stress responses, inflammation, cycle arrest and apoptosis (19–21). In addition, in vivo studies have demonstrated that DUSP1 inactivates extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 by a dephosphorylation processes (22–25). In several human epithelial tumors, elevated levels of DUSP1 have been reported, including in prostate, colon and bladder cancer (26–28). However, the expression of DUSP1 in tumors progressively decreased with a higher histological grade, indicating that the function and mechanism of DUSP1 in tumors may vary and is complex. In several studies, it has been reported that tumor cell resistance was closely associated with DUSP1, including lung cancer, ovarian cancer, osteosarcoma, breast cancer, hilar cholangiocarcinoma, acute lymphoid system leukemia, prostate cancer and glioma cancer cells (29–38). Upon the expression of DUSP1, the chemotherapeutic resistance of tumor cells is enhanced (31). However, if DUSP1 activity is decreased, the chemotherapeutic resistance of tumor cells reduces, resulting in tumor cells with higher sensitivity (29).
Triptolide, a bioactive ingredient extracted from Tripterygium wilfordii, which is a Chinese medicinal plant, has been reported to exhibit antitumor effects in several types of human cancer (39–41). Triptolide has been used in clinical trials (42) and it is hypothesized is that its antitumor effects are mediated by the suppression of oncoproteins or growth factors, including DUSP1 (43), nuclear factor (NF)-κB (44), and heat shock proteins (HSPs) (45). However, the antitumor effects of triptolide in GC remain to be elucidated.
In the present study, the function and mechanism of DUSP1 in Apa resistance were investigated. It was demonstrated that combined treatment of Apa and triptolide, as protein inhibitors of DUSP1, were able to overcome Apa resistance in GC cells.
Materials and methods
Cell lines
The human MGC803 and MKN45 GC cell lines were purchased from the Cell Bank of Chinese Academy of Science (Beijing, China). High-dose Apa resistant MGC803 cells (MGC803-AR) and MKN45 cells (MKN-45AR) were derived from the MGC803 and MKN45 cells, respectively. In brief, the MGC803 and MKN45 cells were treated with gradually increasing concentrations of Apa (5–80 µM) for 2 months, and were maintained in RPMI medium (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), containing 10% fetal bovine serum (FBS; GE Healthcare Bio-Sciences) and 80 µM Apa for 6 months at 37°C in 5% CO2. Prior to experiments, the cells were cultured in RPMI medium containing 10% FBS for 1 week.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was used to determine the cell proliferation ability of the MGC803, MGC803-AR, MKN45 and MKN45-AR cells. In brief, the cells were seeded into 96-well cell culture plates (Nest Biotechnology Co., Ltd., Wuxi, China) at a density of 5×103 cells/well in 100 µl RPMI medium containing 10% FBS. Following culturing in RPMI medium for 24 h at 37°C, apatinib and triptolide were administrated for 24 h. Subsequently, 10 µl of CCK-8 reagent (Dojindo, Inc., Tokyo, Japan) was added to each well and the cells were incubated for 2 h at 37°C according to the manufacturer's protocol. Subsequently, the absorbance was read at a wavelength of 450 nm using an automated plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The experiments were performed in triplicate.
cDNA synthesis and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Monolayers of MGC803, MGC803-AR, MKN45 and MKN45-AR cells were harvested, cells were lysed, and total RNA was extracted using the TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) method according to the manufacturer's protocol. The total RNA (100 ng) was reverse transcribed into cDNA using a Takara PrimeScript First Strand cDNA Synthesis kit (Takara Bio, Inc., Shiga, Japan). RT-qPCR analysis was performed using the Takara SYBR Premix Ex Taq kit (Takara Bio, Inc.). The sequences of DUSP1 primers were as follows: Forward 5′-ACCACCACCGTGTTCAACTT-3′ and reverse 5′-CTCAAGGAGCATGGAGTCCC-3′. The sequences of ACTB primers were as follows: Forward 5′-CGCCGCCAGCTCACC-3′ and reverse 5′-GACCCATGCCCACCATCAC-3′. Experiments were carried out in triplicate, and the comparative CT was calculated to analyze the expression levels of DUSP1 in GC or ARGC cells (46). ACTB were analyzed as a reference gene for mRNA.
DUSP1 silencing with small interfering (si)RNA
The MGC803-AR and MKN45-AR cells were transiently transfected with DUSP1 siRNA or scrambled siRNA in 6-well plates using TurboFect reagent according to the manufacturer's protocol. The cells were used for experiments at 48 h post-transfection. Cells transfected with scrambled siRNA were used as controls. The siRNA sequences were as follows: DUSP1 siRNA-1, forward 5′-CUAAUCGAGUCAAGCUGGA-3′ and reverse 5′-UCCAGCUUGACUCGAUUAG-3′; DUSP1 siRNA-2, forward 5′-GCUUACCUUAUGAGGACUA-3′ and reverse 5′-UAGUCCUCAUAAGGUAAGC-3′; DUSP1 siRNA-3, forward 5′-CGACGACACAUAUACAUAU-3′ and reverse 5′-AUAUGUAUAUGUGUCGUCG-3′.
Western blot analysis
For western blot analysis, the MGC803, MGC803-AR, MKN45 and MKN45-AR cells were washed in PBS and lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS) containing SigmaFAST™ Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA) for 15 min at 4°C. Cells were removed by scraping and centrifuged at 8,000 × g rotations per minute for 20 min at 4°C. Protein concentration was measured using Bio-Rad DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal quantities of total protein (40 µg) were loaded onto 10% SDS-PAGE gels and separated. The proteins were the transferred onto polyvinylidene fluoride membranes, and the membranes were blocked in TBS-Tween (0.1%)/5% milk for 1 h at room temperature. The membranes were then incubated overnight with primary antibodies directed against DUSP1 (cat. no. Ab61201) from Abcam (Cambridge, UK) and phosphorylated (p)-DUSP1 (cat. no. 2857), JNK (cat. no. 9252), p-JNK (cat. no. 4668), ERK (cat. no. 469), p-ERK (cat. no. 4370), P38 (cat. no. 8690), p-P38 (cat. no. 4511), poly(ADP-ribose) polymerase (PARP) (cat. no. 9664), β-actin (ACTB) (cat. no. 3700) from Cell Signaling Technology, Inc. (Danvers, MA, USA) at 4°C. The primary antibodies were diluted 1:1,000 with universal antibody dilution buffer (Sigma; EMD Millipore, Billerica, MA, USA). The membranes were washed three times for 10 min with TBS-Tween (0.1%) at room temperature, and then incubated with goat anti-rabbit IgG (H+L) secondary antibodies (dilution 1:3,000; cat. no. 70-GAR007; MultiSciences, Co., Ltd., Hangzhou, China) for DUSP1, p-DUSP1, JNK, p-JNK, ERK, p-ERK, P38, p-P38 and PARP, and goat anti-mouse IgG (H+L) secondary antibodies (dilution 1:3,000; cat. no. 70-GAM007; Hangzhou MultiSciences) for ACTB for 1 h at room temperature. The membranes were washed three times for 10 min with TBS-Tween (0.1%) at room temperature. The protein bands were visualized using enhanced chemiluminescence reagents (EMD Millipore). Images were captured and protein levels were quantified using a ChemiDoc™ MP system (Bio-Rad Laboratories).
Cell cycle analyses
The MGC803 and MGC803-AR cells were seeded into 6-well plates at a density of 3×105 cells/well. Following overnight incubation at 37°C, the cells were treated with Apa at concentrations of 5, 10 and 20 µM for 24 h. For the detection of apoptosis, the cells were harvested and washed with PBS, and then stained with Annexin V/PI according to the manufacturer's protocol. The stained cells were analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA). For the analysis of cell cycle distribution, the supernatant was discarded, and the attached cells were harvested and fixed overnight in cold 70% ethanol at −20°C. Cell cycle distribution was evaluated using a flow cytometer provided with the PI staining kit according to the manufacturer's protocol.
Hoechst 33342 staining assay
The MGC803-AR and MKN45-AR cells were seeded into 96-well plates at a density of 1×106 cells/well. The cells were then transfected with siRNA and/or treated with 10 µM Apa or 1 µM triptolide. Following incubation in fresh RPMI medium containing 10% FBS for 6 h, the cells were incubated in 5 µl of Hoechst 33342 (Beyotime Institute of Biotechnology, Shanghai, China) solution per well at 37°C for 10 min, and evaluated using a fluorescence microscope. The nuclei of apoptotic cells exhibited a high level fluorescence, whereas the fluorescence observed in non-apoptotic cells was weak. Images were captured and quantification of apoptotic cells was performed by counting at least 200 cells in four randomly selected fields per well.
Colony formation assays
For the colony formation assay, the cells (3×104 cells/well) were seeded into 6-well plates and were treated with the indicated agents for 5 days. The medium was then replaced with drug-free medium and the cells were incubated for another 10 days. Subsequently, the cells were cultured with 4% paraformaldehyde for 15 min then were stained with crystal violet solution for 30 min before being washed with PBS.
Immunohistochemical (IHC) staining
A total of 101 tumors collected at various departments between January 2009 and March 2011 in Zhejiang Province Tumor Hospital, from patients with newly diagnosed gastric adenocarcinoma, were included in this study. There are 71 male and 30 female patients in this study and the age is from 31 to 80 years with the average age 61 years. All available clinical factors were evaluated. Informed consent was obtained from all patients before testing. The study was approved by the Medical Ethics Committee of Zhejiang Province Tumor Hospital (Hangzhou, China). Tumor specimens were collected to analyze the expression of DUSP1 using IHC staining. These tissues were fixed in 10% formaldehyde and embedded in paraffin. Sections (3–5 µm) were continuously sliced. After dewaxing by xylene, the tissues were dehydrated in 100, 100, 85 and 75% gradient alcohol. Hydrogen peroxide (3%) was applied to repair the antigen. The non-specific staining was blocked by 10% goat serum at room temperature for 30 min. Immunostaining of histological sections was performed using monoclonal antibodies against DUSP1 (dilution 1:1,000; cat. no. Ab61201; Abcam) overnight at 4°C followed by a 30-min incubation with goat anti-rabbit IgG (H&L) Biotin secondary antibody (dilution 1:1,000; cat. no AB97049; BioVision, Inc., Milpitas, CA, USA) and visualization with 3,3′-diaminobenzidine (DAB) for 3 min. Harris hematoxylin was used to stain the nuclei. The sections were left to dehydrate, and sealed with neutral gel. Categorization of the scanned images was performed using density quant software in the Quant Center (3DHistech, Ltd., Budapest, Hungary). A staining intensity score between 0 and 3 was assigned for the intensity of tumor cells (0, no staining; 1, weak staining; 2, intermediate staining; 3, strong staining). A score was assigned by the estimated proportion of positively-stained tumor cells. To assess the average degree of staining within a tumor sample, multiple regions were analyzed, and at least 100 tumor cells were assessed. The cytoplasmic expression was assessed using the H-score system (22). The formula for the H-score was as follows: H-score = ∑ (I × Pi), where I represents the staining intensity and Pi represents the percentage of positively-stained tumor cells, producing a cytoplasmic score ranging between 0 and 300 (47). Scoring was performed independently by two assessors who were not informed of the clinical outcomes.
Statistical analysis
For data analysis, SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) was used. Data are expressed as the mean ± standard deviation. The unpaired t-test was used for comparing data between two groups. Multiple group data and multiple comparisons were analyzed by one-way analysis of variance and the LSD test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of DUSP1 is upregulated in AR gastric cancer cell lines
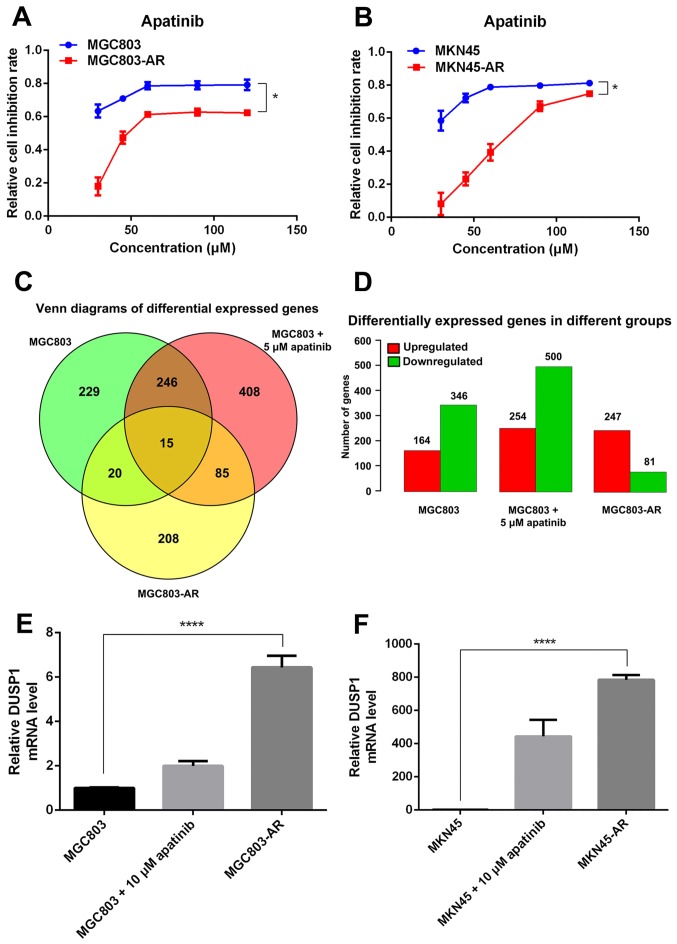
In the present study, two high-dose Apa-resistant GC cell lines were established, MGC803-AR and MKN45-AR, respectively. In brief, the MGC803 and MKN45 cells were treated with 80 µM of Apa for 6 months. To verify the resistance of the MGC803-AR and MKN45-AR cells against Apa, parental cells and AR cells were treated with Apa or culture medium alone (control) for 24 h at 37°C. Cell proliferation was then determined using an CCK-8 assay. As expected, treatment with Apa markedly inhibited the viability of the parental MGC803 and MKN45 cells with IC50 values of 10.30 and 16.23 µM, respectively (Fig. 1A and B). By contrast, the MGC803-AR and MKN45-AR cells were resistant to Apa inhibition with IC50 values of 60.83 and 72.36 µM, respectively (Fig. 1A and B).
Figure 1.
Expression of DUSP1 is upregulated in AR gastric cancer cells. (A) MGC803, MGC803-AR, (B) MKN45 and MKN45-AR cells were treated with various concentrations of Apa and the cell inhibition rate was determined using the Cell Counting Kit-8 assay 24 h following the start of Apa treatment. Data are presented as the mean ± standard deviation (n=5). *P<0.01 between sensitive and resistant cells at all concentrations. (C) MGC803 and MGC803 cells treated with 5 µM Apa for 24 h and MGC803-AR were used for whole genome sequencing, and resulting differentially expressed genes are shown in a Venn diagram. (D) Expression of differentially expressed genes between groups was increased or decreased as indicated. (E) MGC803 and (F) MKN45 cells were incubated with a concentration gradient of Apa for 24 h, then exposed to the corresponding AR cells. Reverse transcription-quantitative polymerase chain reaction analysis was performed to determine mRNA expression levels (n=4; Student's t-test; *P<0.05, ****P<0.0001). DUSP1, dual-specificity phosphatase-1; Apa, apatinib; AR, Apa-resistant.
The Apa-treated cells were divided into three groups, comprising MGC803 cells, MGC803 cells treated with 5 µM Apa for 24 h, and MGC803-AR cells. Venn diagrams indicated that, between the MGC803 and MGC803-AR cells, 20 genes were differentially expressed. The number of differentially expressed genes between the low-dose Apa-treated MGC803 cells and MGC803-AR cells was 85. In addition, the number of genes differentially expressed between these groups was 15 (Fig. 1C and D). As experimental subjects, six of the 15 differentially expressed genes were selected, which included DUSP1, HSPA1A, HSPA1B, serum response factor, tubulin β (TUBB)2A and TUBB4B. RT-qPCR analysis demonstrated that, among these six genes, DUSP1 showed the most marked significant difference; therefore, DUSP1 was selected for further analysis.
To investigate the possible role of DUSP1 in Apa resistance, the mRNA levels of DUSP1 in the MGC803-AR, MKN45-AR, MGC803 and MKN45 cells were evaluated using RT-qPCR analysis. The data indicated that the mRNA expression of DUSP1 was higher in the MGC803-AR and MKN45-AR cells than in the MGC803 and MKN45 cells (Fig. 1E and F).
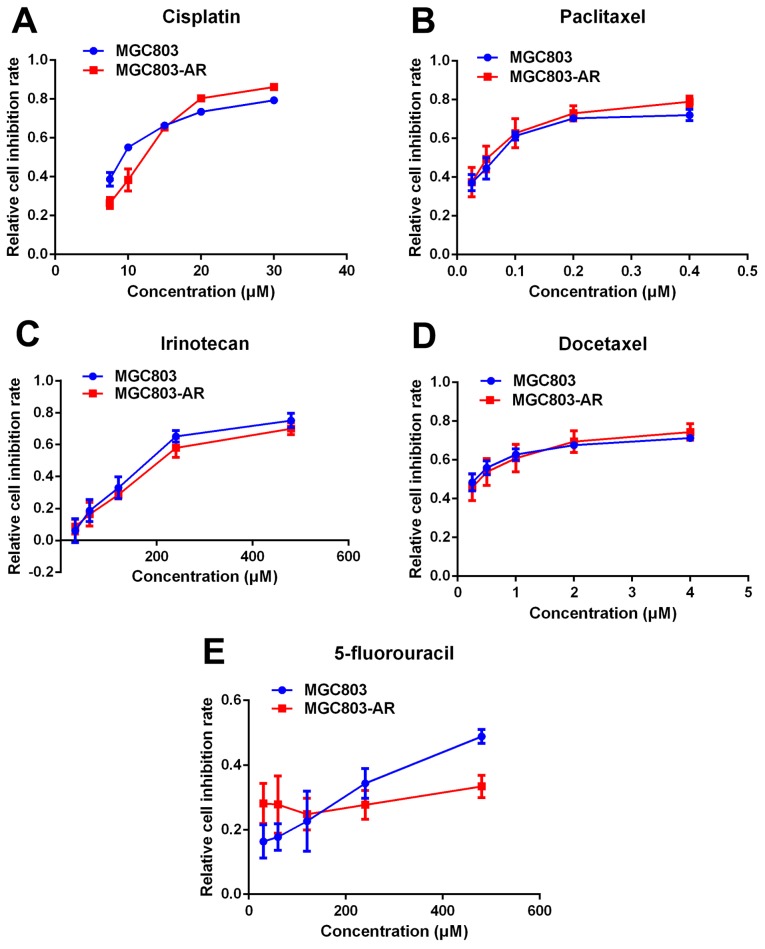
Apatinib resistance does not cause multi-drug resistance of chemotherapy
Previous studies indicated that elevated levels of DUSP1 may be involved in cellular responses to chemotherapy (48). Therefore, it was hypothesized that AR GC cells may be resistant to other drugs used in chemotherapy. In the present study, the effect of five widely used chemotherapeutic agents, cisplatin, paclitaxel, docetaxel, irinotecan and 5-fluorouracil (5-FU), were analyzed in MGC803 and MGC803-AR cells using a CCK-8 assay. The results showed no significant difference in the effects of the five chemotherapeutic agents between the MGC803 and MGC803-AR cells (Fig. 2A-E). Therefore, these findings suggested that Apa resistance in GC cells did not cause multi-drug resistance.
Figure 2.
Apatinib resistance does not lead to multi-drug resistance of chemotherapy. Commonly used chemotherapeutic agents, including (A) cisplatin, (B) paclitaxel, (C) irinotecan, (D) docetaxel and (E) 5-fluorouracil, were administered at different concentrations to MGC803 and MGC803-AR cells. No significant signs of chemotherapeutic drug resistance were observed. AR, apatinib-resistant.
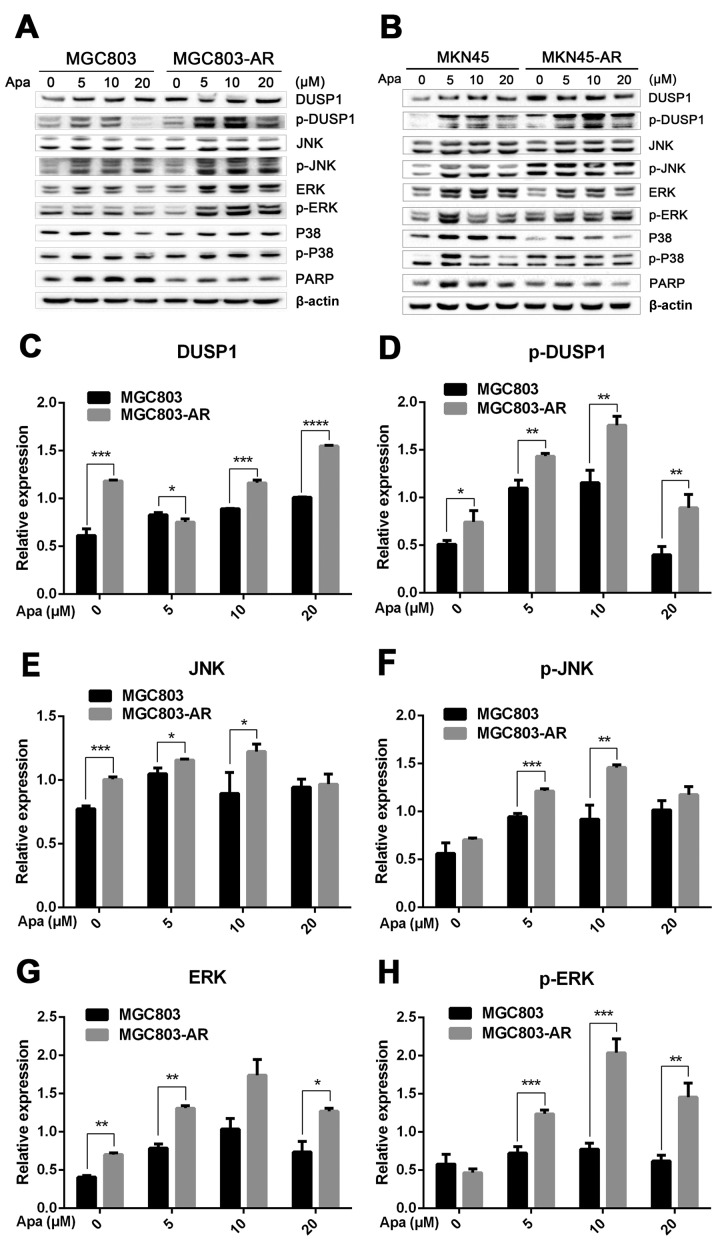
Apatinib resistance in GC cells affects cell cycle, apoptosis and the MAPK signaling pathway
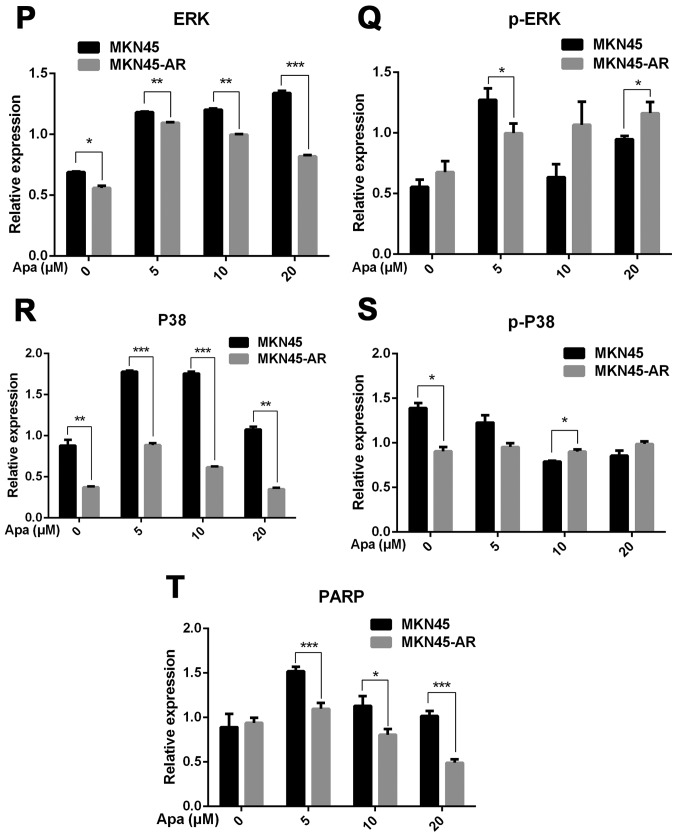
DUSP1 is located at a key position in the MAPK signaling pathway, and directly interacts with downstream proteins, including JNK, ERK and P38 (49). Western blot analysis was performed to investigate whether changes in the mRNA expression of DUSP1 in AR cells affected the expression of these four proteins (Fig. 3A and B).
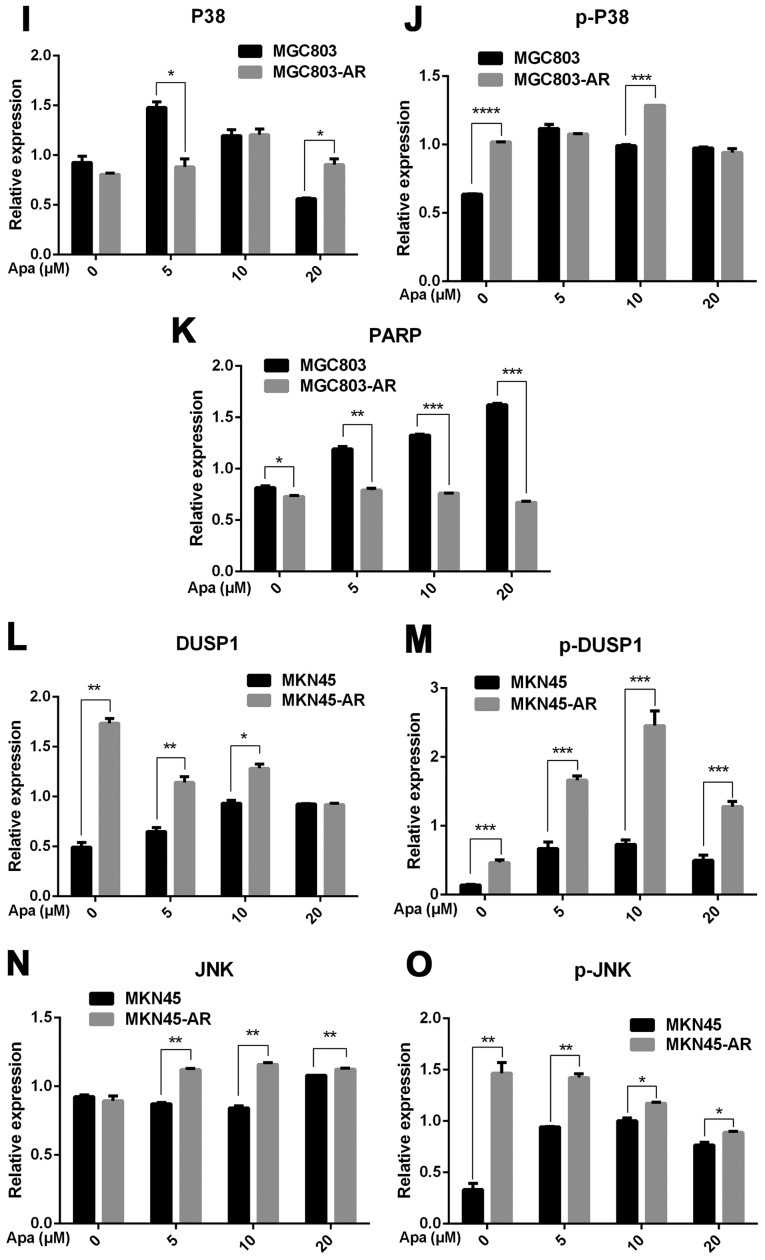
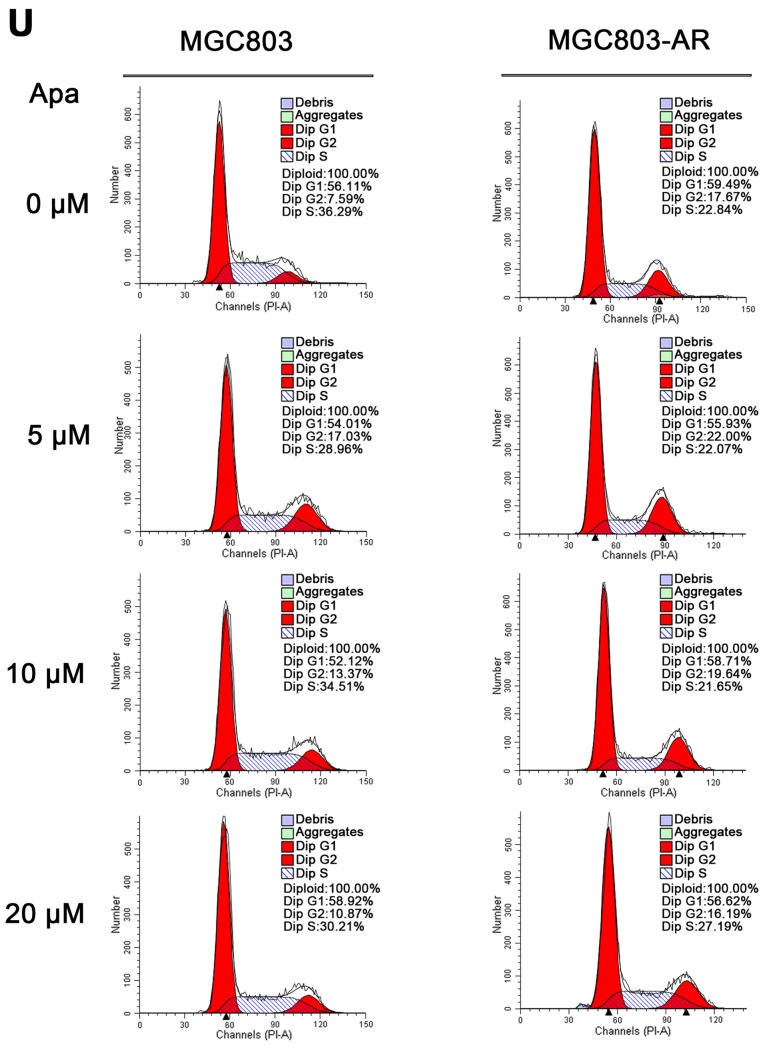
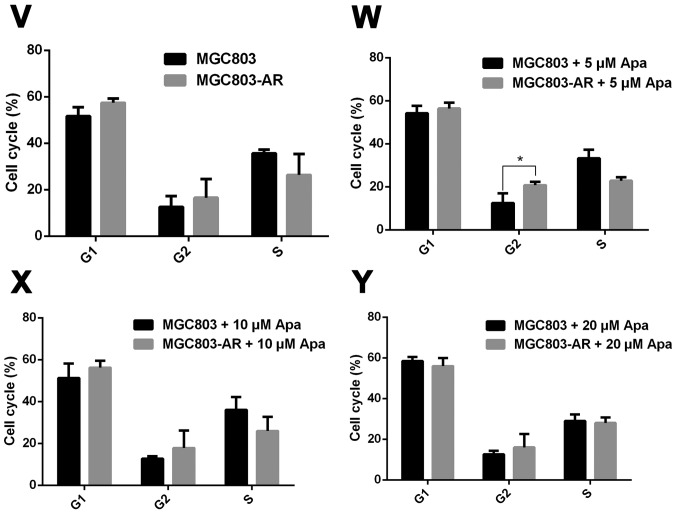
Figure 3.
Apa resistance in gastric cancer cells affect cell cycle, apoptosis and MAPK signaling pathways. (A) MGC803, MGC803-AR, (B) MKN45 and MKN45-AR cells were treated for 24 h with Apa at the indicated concentrations. Total cell lysates were prepared and analyzed by western blot analysis using antibodies directed against MAPK signaling molecules (C) DUSP, (D) p-DUSP, (E) JNK, (F) p-JNK, (G) ERK, (H) p-ERK.. (I) P38, (J) p-P38, and (K) PARP in MGC803, MGC803-AR cells, and (L) DUSP, (M) p-DUSP (N) JNK, (O) p-JNK. (P) ERK, (Q) p-ERK, (R) P38, (S) p-P38, and (T) PARP in MKN45 and MKN45-AR cells. β-actin served as a loading control. Histograms representing the relative quantitative comparison of proteins between parental cells and resistant cells. (U) MGC803 and MGC803-AR cells were treated with Apa for 24 h at indicated concentrations, and cell cycle distribution was analyzed by flow cytometry. Histograms representing the relative cell cycle distribution between sensitive cells and resistant cells at (V) 0, (W) 5, (X) 10 and (Y) 20 µM Apa. Data are presented as the mean ± standard deviation (n=3; Student's t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). Apa, apatinib; AR, Apa-resistant; MAPK, mitogen-activated protein kinase; DUSP, dual-specificity phosphatase-1; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p-, phosphorylated; PARP, poly(ADP-ribose) polymerase.
The results showed that, compared with MGC803 cells, the expression of DUSP1 was significantly increased in MGC803-AR cells (P<0.001). In addition, p-DUSP1 was significantly increased in MGC803-AR cells compared with that in MGC803 cells (P<0.05). The levels of JNK and p-JNK, ERK and p-ERK were all expressed at higher levels in the AR cells (P<0.05), regardless of the concentration of Apa used. In addition, PARP was significantly decreased in the MGC803-AR cells compared with the MGC803 cells (P<0.05; Fig. 3C-K).
When the concentration of Apa increased, the expression of DUSP1 gradually increased in MKN45 cells, whereas that in MKN45-AR cells gradually decreased. In addition, the expression of p-DUSP1 in AR cells was higher than that in parental cells. This was the case at each concentration assessed in the present study. The levels of p-ERK in the MKN45-AR cells gradually increased, whereas levels decreased in MKN45 cells when higher concentrations of Apa were used. At an Apa concentration of 5 µM, the levels of p-ERK in MKN45 cells were significantly higher than those in MKN-45AR cells (P<0.05). However, at 20 µM of Apa, the levels of p-ERK in MKN45-AR cells was significantly higher compared with those in MKN45 cells (P<0.05). At each concentration of Apa examined, the levels of JNK were significantly higher in the MKN45-AR cells compared with those in the MKN45 cells (P<0.01), whereas the levels of PARP were higher in the MKN45 cells than in the MKN45-AR cells (P<0.05; Fig. 3L-T).
As Apa affected the expression of PARP, the cell cycle of MGC803 and MGC803-AR cells were analyzed following treatment with different concentrations of Apa by flow cytometry. As shown in Fig. 3U-Y, the cell populations in the G1 and S phases were decreased, whereas the population of cells in the G2 phase was increased in MGC803-AR cells when compared with that of control cells. In particular, the proportion of MGC803-AR cells in the G2 phase was significantly higher when compared with that of MGC803 cells (P=0.04).
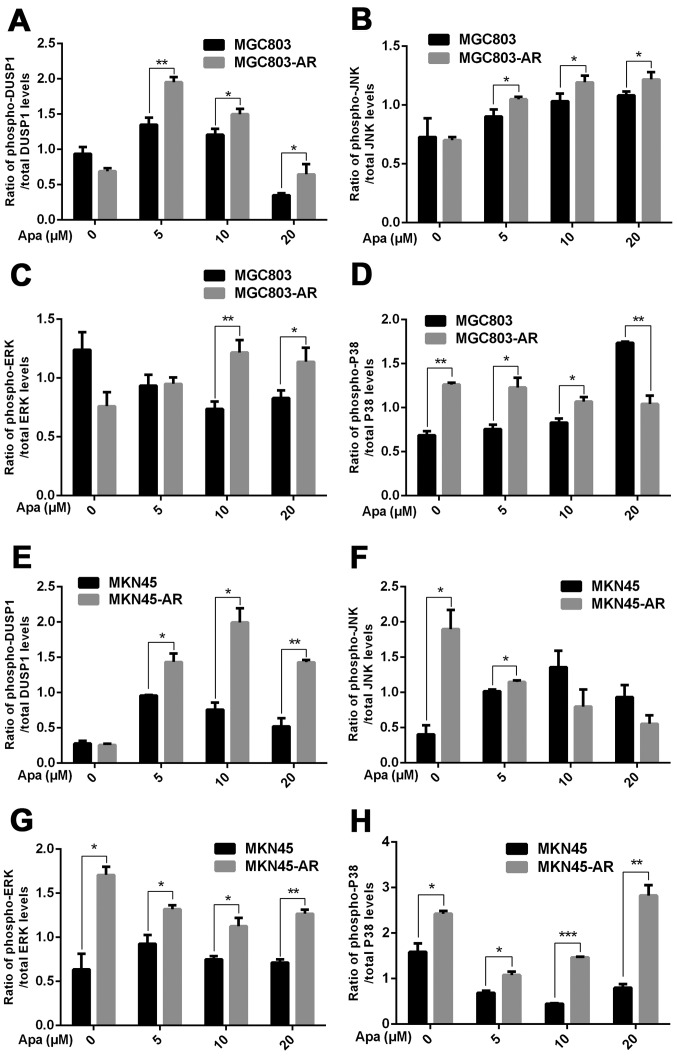
The protein expression of DUSP1 was high in ARGC cells. Due to the overall physiological changes in resistant cells, downstream proteins, including JNK, ERK and P38 were activated (Fig. 4A-H). The protein expression levels of JNK and ERK were higher in AR cells, compared with levels in their corresponding parental cell lines, which may further enhance cellular resistance to Apa. Following Apa treatment, the protein expression of apoptotic PARP in AR cells was lower than that in sensitive cells (P<0.05), indicating that Apa led to a decrease of apoptosis in AR GC cells.
Figure 4.
Ratios of proteins involved in the mitogen-activated protein kinase pathway between AR-resistant and non-resistant gastric cancer cells. Ratios of (A) p-DUSP1/total DUSP1 (B) p-JNK/total JNK, (C) p-ERK/total ERK and (D) p-P38/total P38 in MGC803 and MGC803-AR cells, respectively. Ratios of (E) p-DUSP1/total DUSP1 (F) p-JNK/total JNK, (G) p-ERK/total ERK and (H) p-P38/total P38 in MKN45 and MKN45-AR cells, respectively (n=3; Student's t-test; *P<0.05, **P<0.01, ***P<0.001). AR, apatinib-resistant; p-/phospho, phosphorylated DUSP, dual-specificity phosphatase-1; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p-, phosphorylated; PARP, poly(ADP-ribose) polymerase.
Knockdown of DUSP1 can overcome apatinib resistance
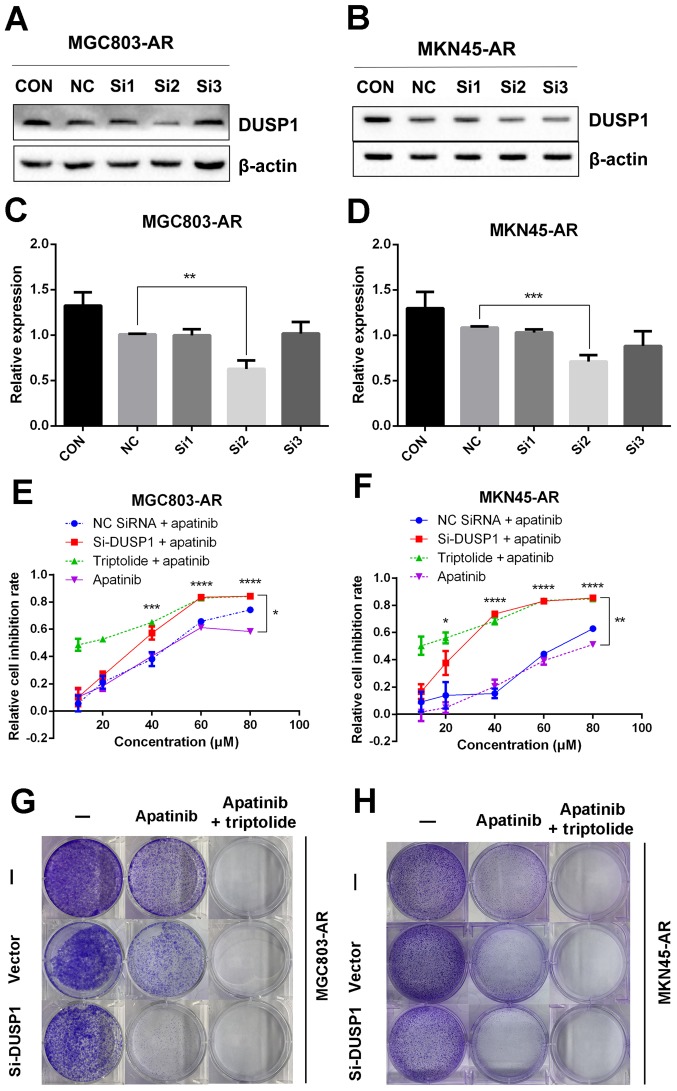
In the present study, it was found that the mRNA and protein levels of DUSP1 were elevated in MGC803-AR and MKN45-AR cells. In previous studies, DUSP1 has been shown to be associated with the resistance of several molecular targeting agents (50,51). Therefore, transient transfection of siRNA targeting DUSP1 was performed in the present study to silence the expression of DUSP1, and changes indicating drug resistance were observed. As shown in Fig. 5A-D, siRNA-2 had the most marked inhibitory effect on DUSP1 protein, therefore, siRNA-2 and the scramble siRNA sequence were selected for further investigation. Following transfection of the corresponding oligos into MGC803-AR and MKN45-AR cells, the inhibition rates of cells treated with Apa were determined using a CCK-8 assay. The knockdown of DUSP1 by siRNA treatment decreased the IC50 values of the MGC803-AR and MKN45-AR cells to 32.19 and 25.18 µM, respectively, indicating that DUSP1 was involved in Apa resistance (Fig. 5E and F). In addition, a colony formation assay showed that the knockdown of DUSP1 decreased the colony-forming ability of cells exposed to Apa (Fig. 5G and H).
Figure 5.
Knockdown of DUSP1 overcomes Apa resistance. (A) MGC803-AR and (B) MKN45-AR cells were treated with siRNA targeting DUSP1 (clones Si1, Si2 and Si3), and expression of DUSP1 was evaluated by western blot analysis. β-actin served as a loading control. Histograms represent the relative quantitative expression in (C) MGC803-AR and (D) MKN45-AR cells. Data are presented as the mean ± standard deviation (n=3; Student's t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). (E) MGC803-AR and (F) MKN45-AR cells were treated with siRNA targeting DUSP1, siRNA control, or triptolide, respectively, and exposed to Apa for 24 h at different concentrations. Cell viability was determined using a Cell Counting Kit-8 assay. The statistical differences between Apa + triptolide and Apa only groups are above the curve; statistical differences between the scramble siRNA NC + Apa and si-DUSP1 + Apa groups are shown to the right of the curve. Data are presented as the mean ± standard deviation (n=5; Student's t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). (G) MGC803-AR and (H) MKN45-AR cells were transiently transfected with a scramble siRNA or DUSP1 siRNA, then treated with 10 µM Apa or 10 µM Apa + 1 µM triptolide for 5 days. Cells were stained with crystal violet and analyzed. Apa, apatinib; AR, Apa-resistant DUSP, dual-specificity phosphatase-1; si, small interfering RNA; CON, control; NC, negative control.
Triptolide combined with apatinib overcomes apatinib resistance by inhibiting MAPK signaling and inducing apoptosis
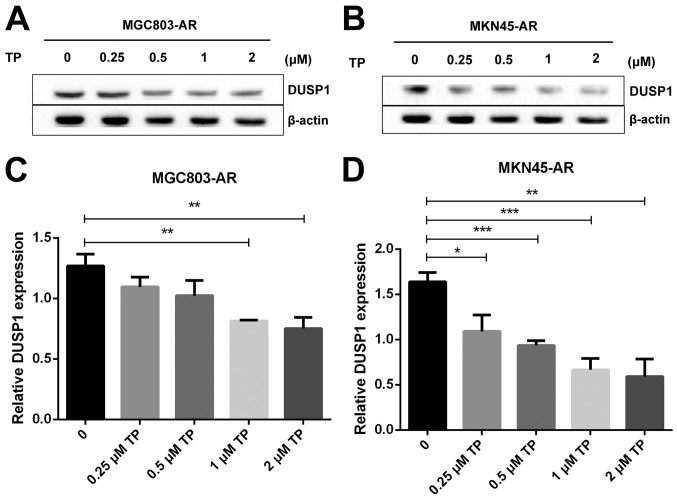
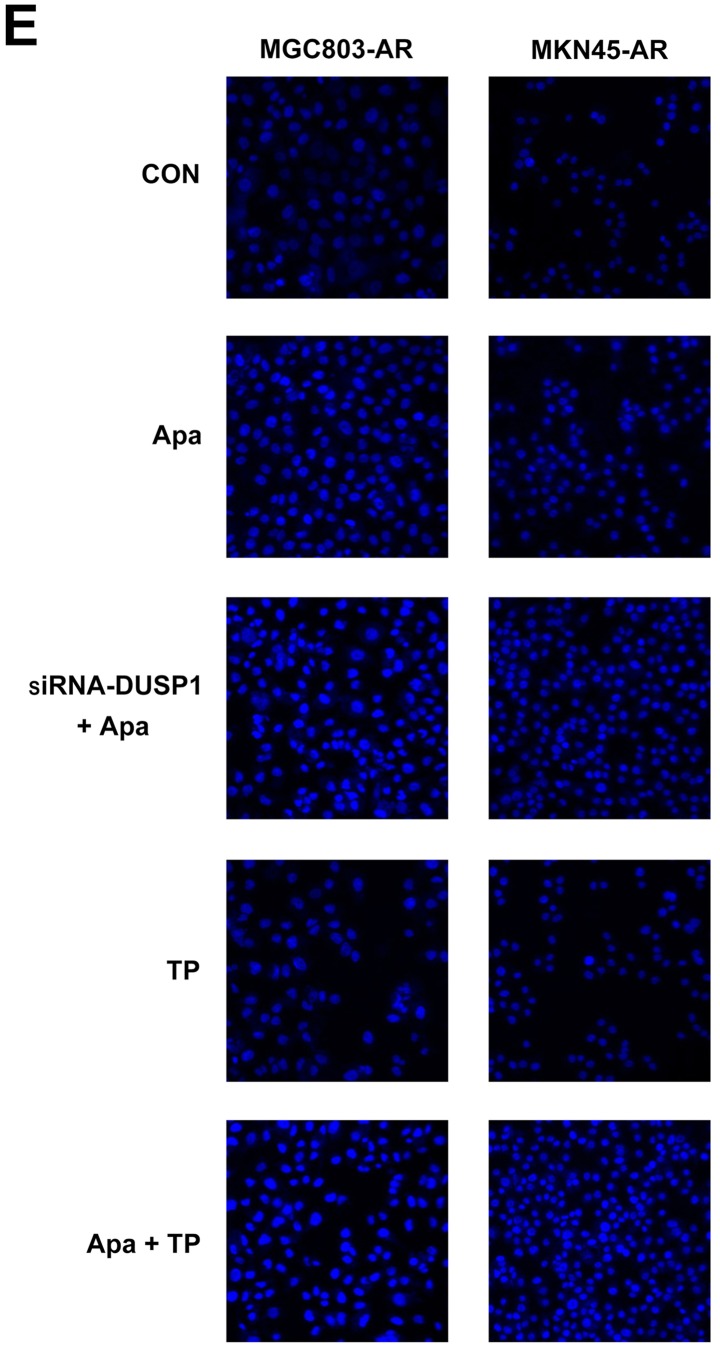
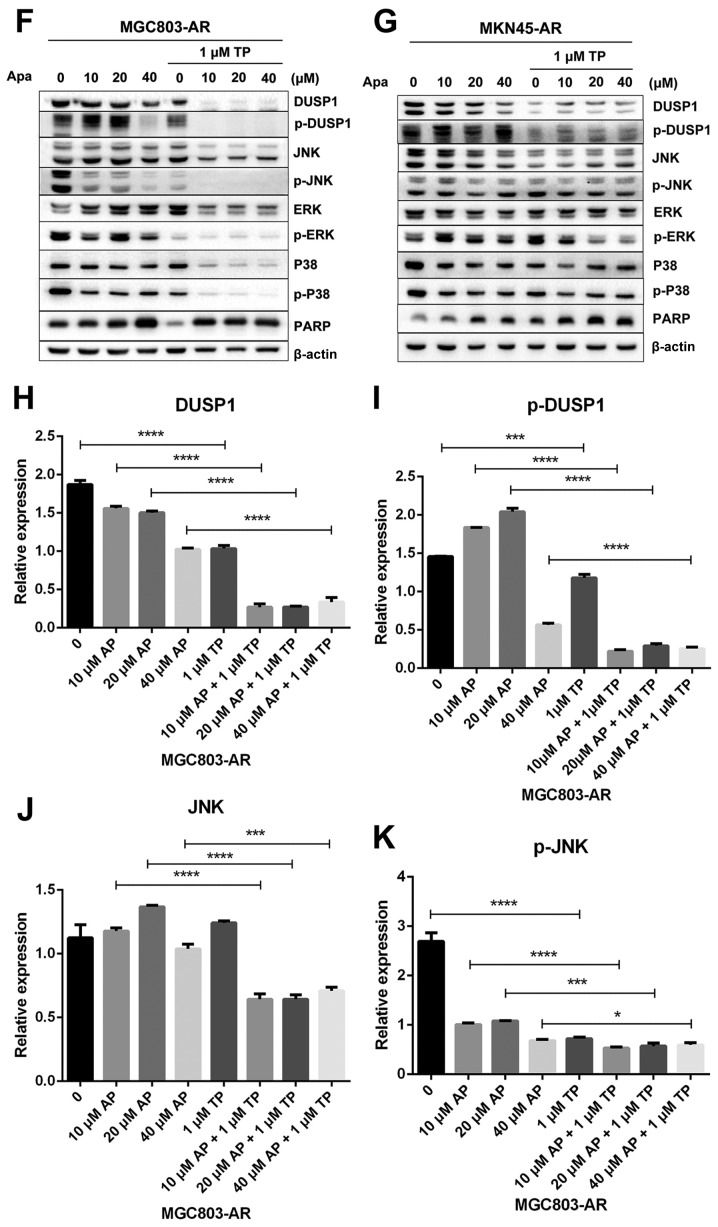
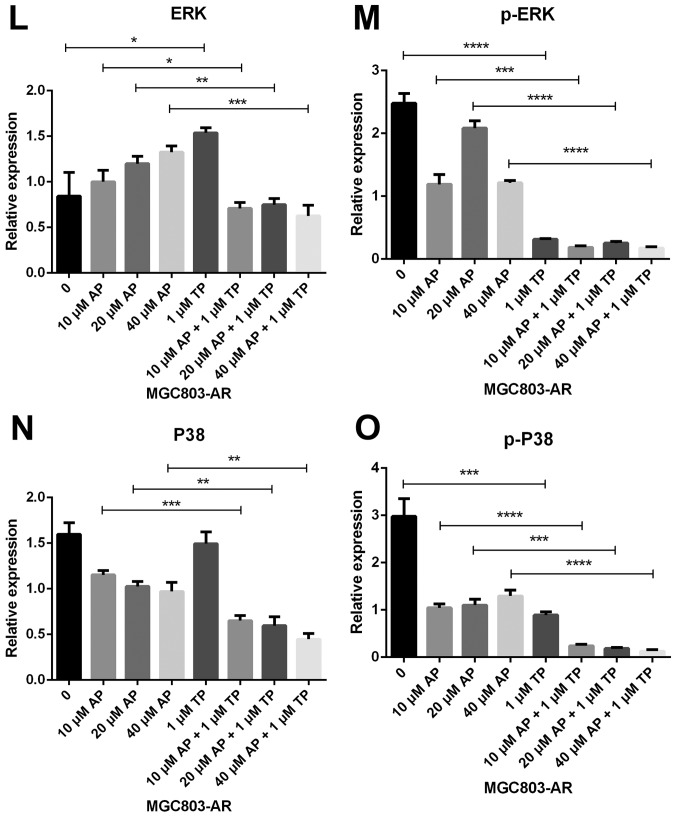
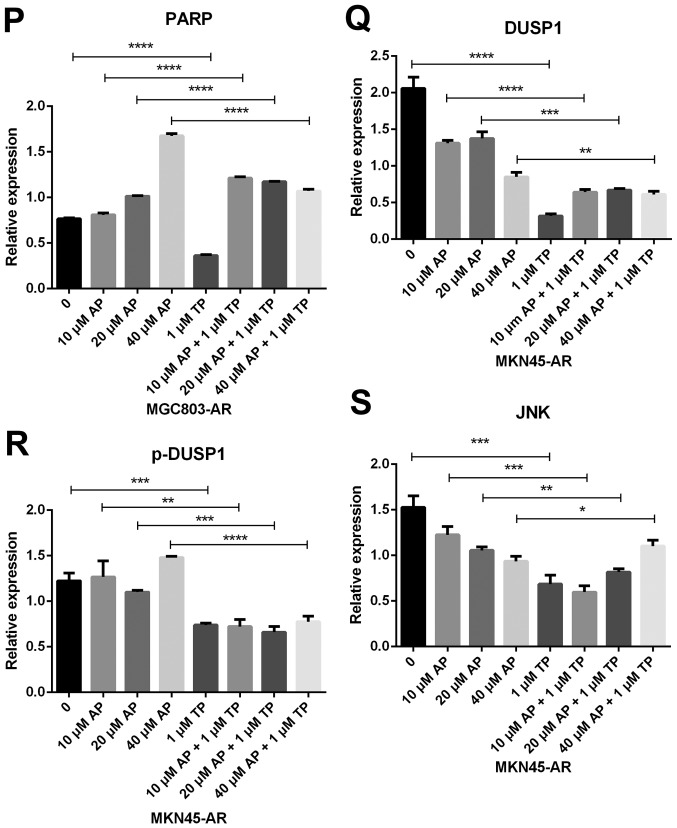
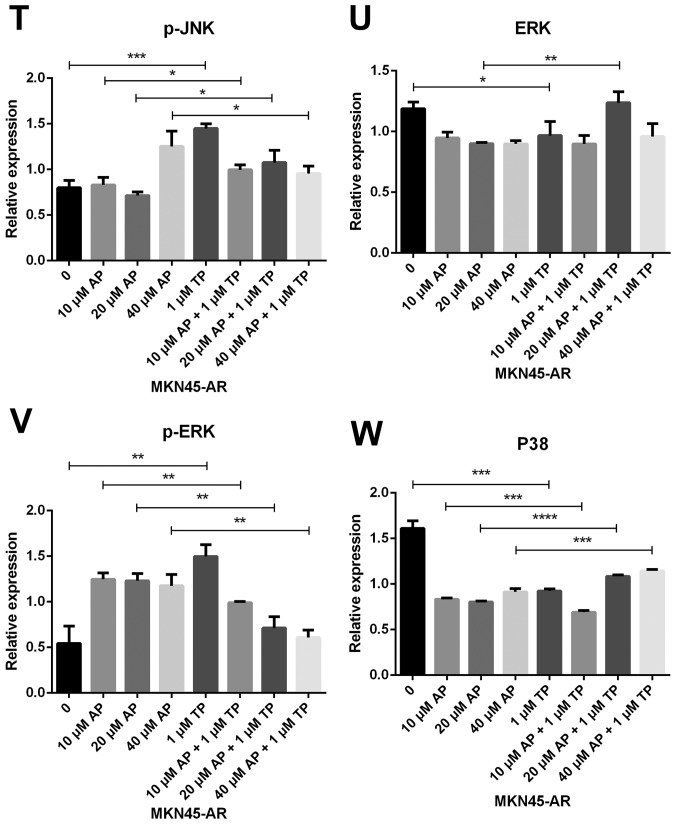
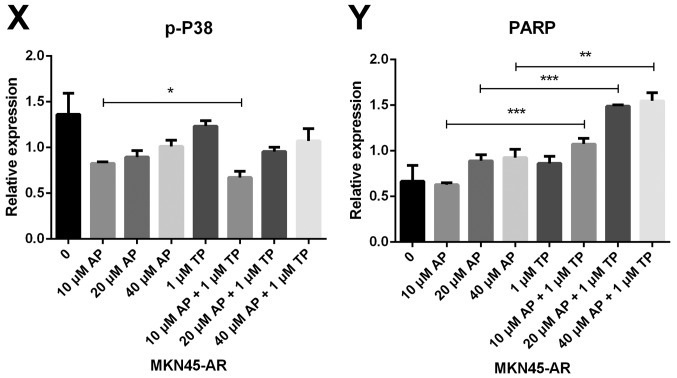
Subsequently, the present study examined the specific inhibition of triptolide on DUSP1 protein. Triptolide, a specific inhibitor of the DUSP1 protein, can arrest the cell cycle without causing cell apoptosis (43,52,53). Therefore, a triptolide concentration gradient was established to identify the optimal concentration of triptolide that inhibits DUSP1 in GC cell lines. In the MGC803-AR cells, 1 µM of triptolide inhibited the expression of DUSP1. In addition, 0.25 µM of triptolide significantly decreased the levels of DUSP1 in MKN45-AR cells. Considering all factors, a concentration of 1 µM triptolide appeared optimal (Fig. 6A-D). To verify whether triptolide treatment combined with Apa can overcome Apa resistance, the cell inhibition rate of triptolide alone or triptolide combined with Apa was determined using a CCK-8 assay (Fig. 5E and F). The results demonstrated that, when 1 µM of triptolide and Apa were administered, the IC50 of the MGC803-AR and MKN45-AR cells was reduced to 13.61 and 12.06 µM, respectively. When Apa was used alone, the IC50 values obtained were 60.83 µM in the MGC803-AR cells and 72.36 µM in the MKN45-AR cells. Subsequently, Hoechst staining was performed to detect cells undergoing apoptosis (Fig. 6E). To identify differences between AR GC cells exposed to Apa following treatment with siRNA-2 or control siRNA, the cells were stained with Hoechst. Within similar-sized fields, and following exposure to 10 µM of Apa, a higher number of apoptotic cells were present in the cells with DUSP1 knockdown, compared with cells in the scramble control group. Together, these findings suggested that the knockdown of DUSP1 resulted in the apoptosis of AR GC cells and reversed Apa resistance. The MGC803-AR cells and MKN45-AR cells showed a weak fluorescence intensity for Hoechst when treated with triptolide alone. These findings were consistent with the findings presented in previous studies in which triptolide affected the cell cycle, but did not affect apoptosis (43,52,53). However, when triptolide and Apa were combined, a significant increase in Hoechst-related fluorescence intensity was found. This observation indicated that triptolide not only inhibited the expression of DUSP1, but also enhanced the effect of Apa on apoptosis. Furthermore, the present study examined the effect of triptolide and Apa treatment on the MAPK signaling pathway (Fig. 6F and G). Compared with Apa treatment alone, the combination of Apa and triptolide significantly inhibited MAPKs. The combined treatment effects include promotion of cell proliferation and survival via the ERK pathway, inhibition of cell apoptosis via the JNK pathway, and regulation of the cell cycle via the P38 pathway (54–60). The levels of DUSP1 were significantly inhibited by combination treatment (Fig. 6F-Y), which may explain why triptolide combined with Apa reversed Apa resistance.
Figure 6.
Triptolide combined with Apa overcomes Apa resistance by inhibiting MAPK signaling and inducing apoptosis. (A) MGC803-AR and (B) MKN45-AR cells were treated with a concentration gradient of triptolide, and expression of DUSP1 was evaluated by western blot analysis using β-actin as a loading control. Histograms show the relative quantitative expression in (C) MGC803-AR and (D) MKN45-AR cells. Data are presented as the mean ± standard deviation (n=3; Student's t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). (E) MGC803-AR and MKN45-AR cells were treated with 10 µM Apa and cells with DUSP1 knockdown were treated with 10 µM Apa or 1 µM triptolide alone or 10 µM Apa + 1 µM triptolide for 6 h. Cells were stained with Hoechst 33342 and images were captured using an Olympus BH-2 fluorescence microscope (magnification, ×40). (F) MGC803-AR and (G) MKN45-AR cells were treated with Apa or Apa + 1 µM triptolide for 24 h at different Apa concentrations. Total cell lysates were prepared and analyzed by western blot analysis using antibodies directed against MAPK signaling molecules (H) DUSP1, (I) p-DUSP1, (J) JNK and (K) p-JNK. (L) ERK, (M) p-ERK, (N) P38, (O) p-P38, (P) PARP in MGC803-AR cells; and (Q) DUSP1, (R) p-DUSP1, (S) JNK. and (T) p-JNK (U) ERK, (V) p-ERK, (W) P38, (X) p-P38, (Y) PARP in MKN45-AR cells. β-actin was a loading control. Data are presented as the mean ± standard deviation (n=3; Student's t-test; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). Apa/AP, apatinib; AR, Apa resistant; MAPK, mitogen-activated protein kinase; p-, phosphorylated DUSP, dual-specificity phosphatase-1; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p-, phosphorylated; PARP, poly(ADP-ribose) polymerase.
Expression of DUSP1 in tissues of patients with GC and the effect of DUSP1 on the prognosis of GC
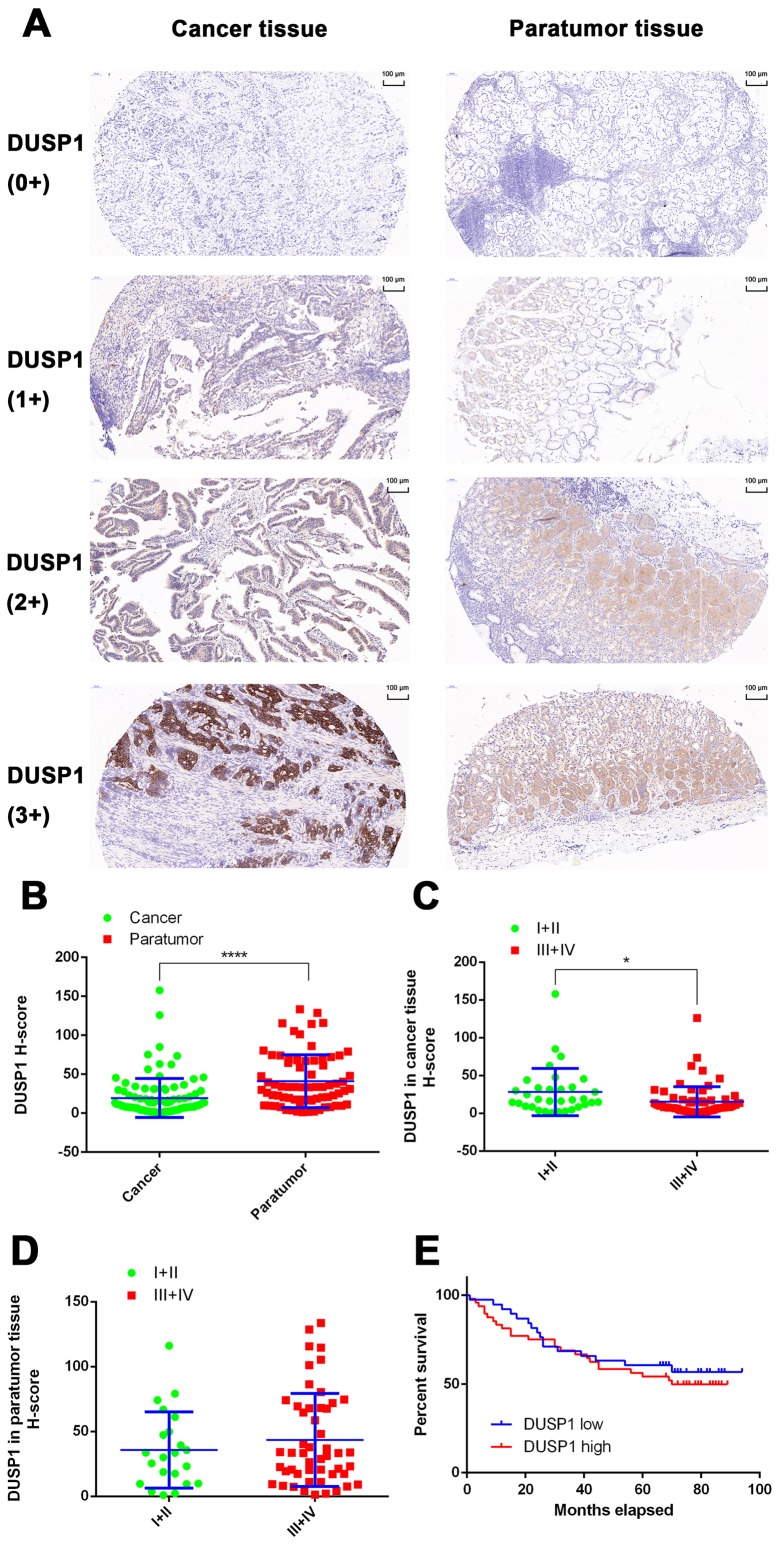
In the present study, the expression of DUSP1 was evaluated in clinical tissue samples from 101 patients with GC. None of the patients had undergone chemotherapy or targeted-drug therapy prior to surgery. In 72 of the 101 patients, both cancerous and paracancerous tissues were collected. In the remaining 29 patients, only cancerous tissue was obtained (Table I). A total of 101 GC tissues and 72 paracancerous tissues were used for IHC experiments and tissues were scanned using the Pannoramic MIDI Tissue Chip Scanner (3DHistech, Ltd.). The GC cells were quantified in tumor tissues, whereas fundic gland cells were quantified in non-tumor tissues. The system automatically identified all dark-brown colored areas in the tissue section as strongly positive, brown-yellow as moderately positive, light-yellow as weak positive, and blue nuclei as negative (Fig. 7A). For statistical analysis, an H-scoring system was used. The results showed that the expression level of DUSP1 in GC tissues was significantly lower than that in adjacent tissues (P<0.0001; Fig. 7B). In addition, it was found that the expression of DUSP1 in cancerous tissue derived from stage I and II patients was higher than that of patients with a GC stage of III and IV (P<0.05; Fig. 7C). In paracancerous tissue, no significant differences were observed between the expression of DUSP1 and tumor stage (Fig. 7D). Finally, the tissue samples were grouped based on the H-Score. A score of >10 points was assigned for high levels of DUSP1, whereas a score of <10 points was assigned for low levels of DUSP1. A total of 39 patients were grouped in the high expression group, whereas the low expression group contained 48 patients. According to the patients' follow-up data, a survival curve was plotted and no statistically significant differences were observed (Fig. 7E). Therefore, it was hypothesized that, in patients who did not receive drugs or surgical treatment, the expression of DUSP1 in cancerous tissue had minimal effect on survival rate or prognosis.
Table I.
Information on patient tissues used for immunohistochemical analysis.
| Tissue samples (n) | ||
|---|---|---|
| Factor | Cancer | Paratumor |
| Patients | 101 | 72 |
| Tumor stage | ||
| I+II | 33 | 22 |
| III+IV | 68 | 50 |
| Follow-up DUSP1 H-score | ||
| High (≥10) | 39 | – |
| Low (<10) | 48 | – |
DUSP1, dual-specificity phosphatase-1.
Figure 7.
Expression of DUSP1 in GC tissues and the effect of DUSP1 on the prognosis of GC. (A) IHC staining of DUSP1 in patients with GC (scale bar, 100 µm). (B) Expression of DUSP1 was lower in tumor tissue than in paracancerous tissue. (C) Expression of DUSP1 was lower in tumor tissue of late stages (III and IV) than tumor tissue of early stages (I and II). (D) Expression of DUSP1 in paracancerous tissue of late stages (III and IV) did not show significant differences with that of paracancerous tissue of early stages (I and II) (*P<0.05, ****P<0.0001). (E) Kaplan-Meier survival plots of patients with GC when grouped by expression of DUSP1. Differences between groups were compared with the log-rank test (n=87). GC, gastric cancer; DUSP1, dual-specificity phosphatase-1; IHC, immunohistochemistry; 0+, no positive staining; 1+, partial weak staining; 2+ weak to moderate staining; 3+, strong staining.
Discussion
VEGFR2-targeted therapy not only represents a novel treatment regimen but also provides novel biologic insight into GC, in which Apa leads to increased PFS and significantly prolonged OS rates in patients with GC (61). However, the emergence of acquired resistance is inevitable and remains a major obstacle. Resistance to Apa suggests the involvement of signaling pathways additional to VEGFR2, including c-Met amplification (62), PI3K catalytic subunit α mutations (63), or BRAF mutations (64). In the present study, two AR GC cell lines were generated, and the role of DUSP1 in the resistance of GC cells to Apa was investigated.
In previous studies, DUSP1 has been reported to be associated with the drug resistance of tumor cells (31,50,51,65–67). In the present study, it was shown that the mRNA expression of DUSP1 was significantly increased in AR GC cells. To further demonstrate whether the overexpression of DUSP1 in AR GC cells resulted in resistance to chemotherapeutic agents, five commonly used chemotherapeutic agents were used to determine the sensitivity of AR GC cells. No significant differences were observed in the sensitivity of these five agents between parental and AR GC cells, suggesting that the resistance of GC cells to Apa does not cause multi-drug resistance to chemotherapy.
To overcome the resistance of GC cells to Apa, two approaches were examined to reverse drug resistance. siRNA transfection technology was used to interfere with the protein expression of DUSP1 in the AR cells, or a specific protein inhibitor of DUSP1, triptolide, was used to inhibit the protein synthesis of DUSP1 in AR cells. The data indicated that, on knocking down the expression of DUSP1 by siRNA, the IC50 of Apa in MGC803-AR cells decreased from 60.83 to 32.19 µM. In addition, in the MKN45-AR cells, the IC50 decreased from 72.34 to 25.18 µM. When the AR GC cells were treated with Apa in combination with triptolide, the expression of DUSP1 decreased further, and the IC50 of Apa in MGC803-AR cells decreased to 13.61 µM. In the MKN45-AR cells, the IC50 decreased to 12.06 µM. Neither were significantly different to the respective parental GC cells. Taken together, a high expression of DUSP1 was required for Apa-resistance in GC cells.
Protein kinase and phosphatase can maintain homeostasis of cellular signaling, including the MAPK signaling pathway. DUSP1, which acts as a phosphatase, can deactivate the MAPKs, ERKs, P38 MAPKs and JNKs by dephosphorylating threonine and tyrosine, which are involved in cellular proliferation, differentiation, and apoptosis, and the progression of tumor carcinogenesis (68). It has been demonstrated that the JNK inhibitor SP-600125 may have antitumor activity in GC cells by inhibiting cell proliferation, promoting apoptosis, or causing cell cycle arrest (54). DUSP1 is known to dephosphorylate ERK (55,69). Activation of the ERK1/2 pathway has been shown to promote cell proliferation (56–60) and result in malignant transformation (70,71). The P38 protein prolongs phosphorylation and induces apoptosis, whereas transient phosphorylation contributes to cell survival (55).
DUSP1 has been reported as a negative regulator of the MAPK signaling pathway, however, in the present study, JNK, ERK, P38 and their corresponding phosphorylated proteins were not significantly decreased due to the high expression and phosphorylation of DUSP1. This may be associated with other regulatory factors of the JNK, ERK and P38 signaling pathways. In addition, the specific MAPK pathway regulated by DUSP1 in conferring drug resistance appears to be dependent on the cell/tissue type in addition to the chemotherapeutic agent used (49). The AR GC cells showed significant phosphorylation of JNK, ERK and P38. A marked anti-apoptotic effect was observed for p-JNK, whereas p-ERK was closely associated with cell proliferation and survival and p-P38 affected the cell cycle. Activation of the JNK, ERK and P38 proteins is likely to further enhance drug resistance. When AR GC cells were treated with a combination of Apa and triptolide, the expression of DUSP1 was downregulated, and phosphorylation of the JNK, ERK and P38 proteins in the MAPK pathway were significantly inhibited, particularly in the MGC803-AR cells, which conferred its proapoptotic and in vivo antitumor activities (72). In the present study, it was demonstrated that DUSP1 was associated with drug resistance. Even when the single factor of DUSP1 in the MAPK pathway was an inhibitor, the overall physiological changes in resistant cells were more marked in changes of the MAPK pathway. This may explain why Apa combined with triptolide reversed drug resistance from the perspective of MAPK signaling pathways. Therefore, the results of the present study confirmed that downregulation of the expression of DUSP1 with triptolide may be a useful strategy to overcome Apa-acquired resistance.
In clinical GC specimens from patients who had not received chemotherapy or targeted drugs, the protein levels of DUSP1 were significantly higher in paracarcinoma tissues than in carcinoma tissues (P<0.0001). In addition, an increase in the expression of DUSP1 was associated with cancer progression, drug resistance and poor prognosis.
In conclusion, DUSP1 may serve as a predictive biomarker for Apa treatment and its increase may be one possible reason for Apa-acquired resistance. Targeting DUSP1 may overcome the impaired efficacy caused by drug resistance and thereby significantly improve the effectiveness of current antitumor drugs. The present study not only demonstrated a novel mechanism for acquired resistance in GC, but also provided an effective combinatorial approach to overcome Apa-acquired resistance.
Acknowledgements
I would like to express my sincere thanks to Professor Juqian Guo for the English language revisions of this manuscript.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81573953), the Program of Zhejiang Provincial TCM Sci-tech Plan (grant no. 2016ZZ012), the Zhejiang Provincial Science and Technology Projects (grant no. 2013C03044-4), the Natural Science Foundation of Zhejiang Province (grant nos. LY16H280011 and LY13H160027) and the Zhejiang Provincial Medical and Healthy Science and Technology Projects (grant nos. WKJ-ZJ-1728, 2016KYB220 and 2017PY009).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FT was the senior author of the study. He participated in every step of the design project and the specific experiment, and was also the writer of this manuscript. ZX also participated in the overall design of this topic and proposed many feasible solutions. JC cultured apatinib-resistant gastric cancer cells. GuowZ participated in the collection of case data. GuodZ participated in IHC-related experiments. HL gave many practical advice and guidance. YW modified the language of the manuscript. LW participated in the analysis and interpretation of IHC results. The XC gave the overall idea of the study and controlled the quality of all the work throughout the entire process. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All procedures performed involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants enrolled in the study. From all participants, tissue samples were included in the sample pool and informed consent was collected from all participants prior to storage of the sample. An ethical review of the sample library has been submitted and the review contains the informed consent of all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev: CD004064. 2010 doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Petrioli R, Francini E, Roviello F, Marrelli D, Fiaschi AI, Laera L, Rossi G, Bianco V, Brozzetti S, Roviello G. Sequential treatment with epirubicin, oxaliplatin and 5FU (EOF) followed by docetaxel, oxaliplatin and 5FU (DOF) in patients with advanced gastric or gastroesophageal cancer: A single-institution experience. Cancer Chemother Pharmacol. 2015;75:941–947. doi: 10.1007/s00280-015-2715-x. [DOI] [PubMed] [Google Scholar]
- 4.Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat Rev. 2014;40:692–700. doi: 10.1016/j.ctrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Meulendijks D, Beerepoot LV, Boot H, de Groot JW, Los M, Boers JE, Vanhoutvin SA, Polee MB, Beeker A, Portielje JE, et al. Trastuzumab and bevacizumab combined with docetaxel, oxaliplatin and capecitabine as first-line treatment of advanced HER2-positive gastric cancer: A multicenter phase II study. Invest New Drugs. 2016;34:119–128. doi: 10.1007/s10637-015-0309-4. [DOI] [PubMed] [Google Scholar]
- 6.de Mello RA, Marques AM, Araujo A. HER2 therapies and gastric cancer: A step forward. World J Gastroenterol. 2013;19:6165–6169. doi: 10.3748/wjg.v19.i37.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA international collaborative analysis. Ann Oncol. 2012;23:2656–2662. doi: 10.1093/annonc/mds104. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 9.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 10.Roviello G, Petrioli R, Marano L, Polom K, Marrelli D, Perrella A, Roviello F. Angiogenesis inhibitors in gastric and gastroesophageal junction cancer. Gastric Cancer. 2016;19:31–41. doi: 10.1007/s10120-015-0537-5. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 14.Geng R, Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother. 2015;16:117–122. doi: 10.1517/14656566.2015.981526. [DOI] [PubMed] [Google Scholar]
- 15.Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kwak SP, Hakes DJ, Martell KJ, Dixon JE. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J Biol Chem. 1994;269:3596–3604. [PubMed] [Google Scholar]
- 18.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 19.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3:Reviews3009. doi: 10.1186/gb-2002-3-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan KL, Broyles SS, Dixon JE. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991;350:359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- 21.Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- 22.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. doi: 10.1096/fasebj.14.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Duff JL, Monia BP, Berk BC. Mitogen-activated protein (MAP) kinase is regulated by the MAP kinase phosphatase (MKP-1) in vascular smooth muscle cells. Effect of actinomycin D and antisense oligonucleotides. J Biol Chem. 1995;270:7161–7166. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- 24.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 25.Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- 26.Bang YJ, Kwon JH, Kang SH, Kim JW, Yang YC. Increased MAPK activity and MKP-1 overexpression in human gastric adenocarcinoma. Biochem Biophys Res Commun. 1998;250:43–47. doi: 10.1006/bbrc.1998.9256. [DOI] [PubMed] [Google Scholar]
- 27.Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork PJ. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149:1553–1564. [PMC free article] [PubMed] [Google Scholar]
- 28.Manzano RG, Montuenga LM, Dayton M, Dent P, Kinoshita I, Vicent S, Gardner GJ, Nguyen P, Choi YH, Trepel J, et al. CL100 expression is down-regulated in advanced epithelial ovarian cancer and its re-expression decreases its malignant potential. Oncogene. 2002;21:4435–4447. doi: 10.1038/sj.onc.1205542. [DOI] [PubMed] [Google Scholar]
- 29.Haagenson KK, Wu GS. The role of MAP kinases and MAP kinase phosphatase-1 in resistance to breast cancer treatment. Cancer Metastasis Rev. 2010;29:143–149. doi: 10.1007/s10555-010-9208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi YY, Small GW, Orlowski RZ. Proteasome inhibitors induce a p38 mitogen-activated protein kinase (MAPK)-dependent anti-apoptotic program involving MAPK phosphatase-1 and Akt in models of breast cancer. Breast Cancer Res Treat. 2006;100:33–47. doi: 10.1007/s10549-006-9232-x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006;66:8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay S, Machado-Pinilla R, Manguan-Garcia C, Belda-Iniesta C, Moratilla C, Cejas P, Fresno-Vara JA, de Castro-Carpeño J, Casado E, Nistal M, et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 2006;25:3335–3345. doi: 10.1038/sj.onc.1209364. [DOI] [PubMed] [Google Scholar]
- 33.Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 34.Abrams MT, Robertson NM, Litwack G, Wickstrom E. Evaluation of glucocorticoid sensitivity in 697 pre-B acute lymphoblastic leukemia cells after overexpression or silencing of MAP kinase phosphatase-1. J Cancer Res Clin Oncol. 2005;131:347–354. doi: 10.1007/s00432-004-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 36.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–237. doi: 10.1016/S0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- 37.Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007;67:4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Zhou JY, Kanakapalli D, Buck S, Wu GS, Ravindranath Y. High level of mitogen-activated protein kinase phosphatase-1 expression is associated with cisplatin resistance in osteosarcoma. Pediatr Blood Cancer. 2008;51:754–759. doi: 10.1002/pbc.21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Zhang H, Liu T, Tian D, Gu L, Zhou M. Triptolide inhibits MDM2 and induces apoptosis in acute lymphoblastic leukemia cells through a p53-independent pathway. Mol Cancer Ther. 2013;12:184–194. doi: 10.1158/1535-7163.MCT-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Yang S, Su Y, Xiao Z, Wang C, Li X, Lin L, Fenton BM, Paoni SF, Ding I, et al. Enhanced antitumor effect of combined triptolide and ionizing radiation. Clin Cancer Res. 2007;13:4891–4899. doi: 10.1158/1078-0432.CCR-07-0416. [DOI] [PubMed] [Google Scholar]
- 41.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM, Saluja AK. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, Ter Steeg J, Brandely M, Puozzo Ch, Verweij J. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer. 2009;45:1764–1772. doi: 10.1016/j.ejca.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Koo HS, Kang SD, Lee JH, Kim NH, Chung HT, Pae HO. Triptolide inhibits the proliferation of immortalized ht22 hippocampal cells via persistent activation of extracellular signal-regulated kinase-1/2 by down-regulating mitogen-activated protein kinase phosphatase-1 expression. J Korean Neurosurg Soc. 2009;46:389–396. doi: 10.3340/jkns.2009.46.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu W, Ou Y, Li Y, Xiao R, Shu M, Zhou Y, Xie J, He S, Qiu P, Yan G. A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-kappa B pathway. Mol Pharmacol. 2009;75:812–819. doi: 10.1124/mol.108.052605. [DOI] [PubMed] [Google Scholar]
- 45.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Yeo W, Chan SL, Mo FK, Chu CM, Hui JW, Tong JH, Chan AW, Koh J, Hui EP, Loong H, et al. Phase I/II study of temsirolimus for patients with unresectable hepatocellular carcinoma (HCC)- a correlative study to explore potential biomarkers for response. BMC Cancer. 2015;15:395. doi: 10.1186/s12885-015-1334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, Lichtenegger W, Dietel M, Hauptmann S. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer. 2002;102:507–513. doi: 10.1002/ijc.10746. [DOI] [PubMed] [Google Scholar]
- 49.Low HB, Zhang Y. Regulatory roles of MAPK phosphatases in cancer. Immune Netw. 2016;16:85–98. doi: 10.4110/in.2016.16.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin YC, Lin YC, Shih JY, Huang WJ, Chao SW, Chang YL, Chen CC. DUSP1 expression induced by HDAC1 inhibition mediates gefitinib sensitivity in non-small cell lung cancers. Clin Cancer Res. 2015;21:428–438. doi: 10.1158/1078-0432.CCR-14-1150. [DOI] [PubMed] [Google Scholar]
- 51.Ma G, Pan Y, Zhou C, Sun R, Bai J, Liu P, Ren Y, He J. Mitogen-activated protein kinase phosphatase 1 is involved in tamoxifen resistance in MCF7 cells. Oncol Rep. 2015;34:2423–2430. doi: 10.3892/or.2015.4244. [DOI] [PubMed] [Google Scholar]
- 52.Donaubauer EM, Law NC, Hunzicker-Dunn ME. Follicle-stimulating hormone (FSH)-dependent regulation of extracellular regulated kinase (ERK) phosphorylation by the mitogen-activated protein (MAP) kinase phosphatase MKP3. J Biol Chem. 2016;291:19701–19712. doi: 10.1074/jbc.M116.733972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulding T, Wu F, McCuaig R, Dunn J, Sutton CR, Hardy K, Tu W, Bullman A, Yip D, Dahlstrom JE, Rao S. Differential roles for DUSP family members in epithelial-to-mesenchymal transition and cancer stem cell regulation in breast cancer. PLoS One. 2016;11:e0148065. doi: 10.1371/journal.pone.0148065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia HH, He H, De Wang J, Gu Q, Lin MC, Zou B, Yu LF, Sun YW, Chan AO, Kung HF, Wong BC. Induction of apoptosis and cell cycle arrest by a specific c-Jun NH2-terminal kinase (JNK) inhibitor, SP-600125, in gastrointestinal cancers. Cancer Lett. 2006;241:268–274. doi: 10.1016/j.canlet.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 55.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 56.Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, Dubinett SM. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 57.Andradas C, Caffarel MM, Perez-Gomez E, Salazar M, Lorente M, Velasco G, Guzmán M, Sánchez C. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene. 2011;30:245–252. doi: 10.1038/onc.2010.402. [DOI] [PubMed] [Google Scholar]
- 58.Kanai M, Konda Y, Nakajima T, Izumi Y, Kanda N, Nanakin A, Kubohara Y, Chiba T. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK-dependent pathway. Oncogene. 2003;22:548–554. doi: 10.1038/sj.onc.1206109. [DOI] [PubMed] [Google Scholar]
- 59.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-κB pathway. Oncotarget. 2014;5:8778–8789. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 61.Roviello G, Ravelli A, Fiaschi AI, Cappelletti MR, Gobbi A, Senti C, Zanotti L, Polom K, Reynolds AR, Fox SB, et al. Apatinib for the treatment of gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:887–892. doi: 10.1080/17474124.2016.1209407. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Wu N, Shen J, Teixido C, Sun X, Lin Z, Qian X, Zou Z, Guan W, Yu L, et al. MET overexpression and amplification define a distinct molecular subgroup for targeted therapies in gastric cancer. Gastric Cancer. 2016;19:778–788. doi: 10.1007/s10120-015-0545-5. [DOI] [PubMed] [Google Scholar]
- 63.Tran P, Nguyen C, Klempner SJ. Targeting the phosphatidylinositol-3-kinase pathway in gastric cancer: Can omics improve outcomes? Int Neurourol J. 2016;20(Suppl 2):S131–S140. doi: 10.5213/inj.1632740.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HS, Kim WH, Kwak Y, Koh J, Bae JM, Kim KM, Chang MS, Han HS, Kim JM, Kim HW, et al. Molecular testing for gastrointestinal cancer. J Pathol Transl Med. 2017;51:103–121. doi: 10.4132/jptm.2017.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candas D, Li JJ. MKP1 mediates resistance to therapy in HER2-positive breast tumors. Mol Cell Oncol. 2015;2:e997518. doi: 10.1080/23723556.2014.997518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang YS, Seok HJ, Jeong EJ, Kim Y, Yun SJ, Min JK, Kim SJ, Kim JS. DUSP1 induces paclitaxel resistance through the regulation of p-glycoprotein expression in human ovarian cancer cells. Biochem Biophys Res Commun. 2016;478:403–409. doi: 10.1016/j.bbrc.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 67.Liu F, Gore AJ, Wilson JL, Korc M. DUSP1 is a novel target for enhancing pancreatic cancer cell sensitivity to gemcitabine. PLoS One. 2014;9:e84982. doi: 10.1371/journal.pone.0084982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 69.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 70.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 71.Webb CP, Van Aelst L, Wigler MH, Vande Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie CQ, Zhou P, Zuo J, Li X, Chen Y, Chen JW. Triptolide exerts pro-apoptotic and cell cycle arrest activity on drug-resistant human lung cancer A549/Taxol cells via modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett. 2016;12:3586–3590. doi: 10.3892/ol.2016.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.