 As part of an ongoing effort to develop new antitubercular and antimicrobial agents, a series of substituted xanthenone derivatives (7a–p) were synthesized.
As part of an ongoing effort to develop new antitubercular and antimicrobial agents, a series of substituted xanthenone derivatives (7a–p) were synthesized.
Abstract
As part of an ongoing effort to develop new antitubercular and antimicrobial agents, a series of substituted xanthenone derivatives (7a–p) were synthesized. Xanthenone derivatives (7a–p) were prepared via a one-pot three-component thermal cyclization reaction of β-naphthol (5), substituted 1-aryl-1H-[1,2,3]triazole-4-carbaldehydes (4a–h), and cyclic-1,3-diones (6a, b) in the presence of a catalytic amount of iodine. The newly synthesized compounds were characterized by IR, NMR, mass spectral data, and elemental analysis. These compounds (4a–h and 7a–p) were screened for in vitro antitubercular activity against the M. tuberculosis H37Rv (ATCC 27294) strain, for antibacterial activity against Gram-positive and Gram-negative strains, and for antifungal activity against a pathogenic strain of fungi. Among the compounds tested, most of them showed good to excellent antimicrobial and antitubercular activity. The active compounds displaying good potency in the MTB were further examined for toxicity in a HEK cell line. In addition, the structure and antitubercular activity relationship were further supported by in silico molecular-docking studies of the active compounds against the pantothenate synthetase (PS) enzyme of M. tuberculosis.
1. Introduction
Tuberculosis (TB) is second only to HIV/AIDS as the greatest killer worldwide due to a single infectious agent. The World Health Organization's (WHO) latest estimates worldwide are that 9.6 million people fell ill with TB in 2014.1 The treatment of TB is a complex process due to several factors, including patients developing resistance to existing drugs, the emergence of multi drug-resistant TB (MDRTB), and the association of human immune deficiency virus (HIV) with TB. Although, there are first-line and second-line TB drugs available, current TB chemotherapy requires lengthy treatment of about 6–9 months for drug-susceptible patients and 18–24 months for drug-resistant patients. To overcome these current barriers, and underling the importance of the discovery process in identifying new drugs development for antitubercular activity, it is important that new drugs inhibit targets different from those of the currently used drugs.
Xanthones are secondary metabolites found in higher plant families, fungi, and lichens.2 This class of compounds exhibits interesting pharmaceutical properties, such as antibacterial, anti-inflammatory, anticancer, and antiviral.3–6 Furthermore, these compounds are also used as dyes,7 as probes for fluorescence-based detection of biomolecules,8 in laser technologies,9 as bactericide agents,10 and as photosensitizers in photodynamic therapy.11
On the other hand, 1,2,3-triazoles are an important class of heterocyclic compounds due to their wide range of applications as pharmaceutical agents. A perusal of the literature shows that triazoles can act as antifungal,12 antibacterial,13 antialergic,14 antiplatelet,15 anti-HIV,16,17 anti-inflammatory,18,19 anticonvulsants,20 β-lactamase inhibitors,21 antiviral,22 and antitubercular agents.23,24
The use of multi component reactions (MCRs) has emerged as an extremely powerful tool in combinatorial chemistry and drug discovery, since it offers significant advantages over conventional linear step synthesis.25 For instance, MCR approaches using a phenolic compound, aldehydes, and 1,3-dicarbonyl compounds under an I2 catalyst are used for the synthesis of xanthones, albeit the result is clearly dependent on the reaction conditions: solvent, temperature, catalyst, concentration of the starting materials, and the functional groups.
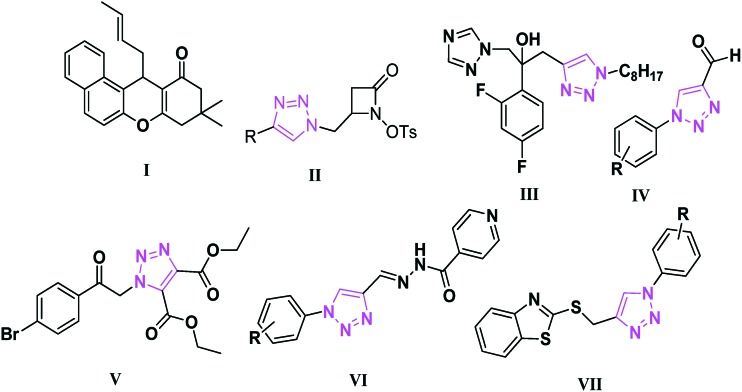
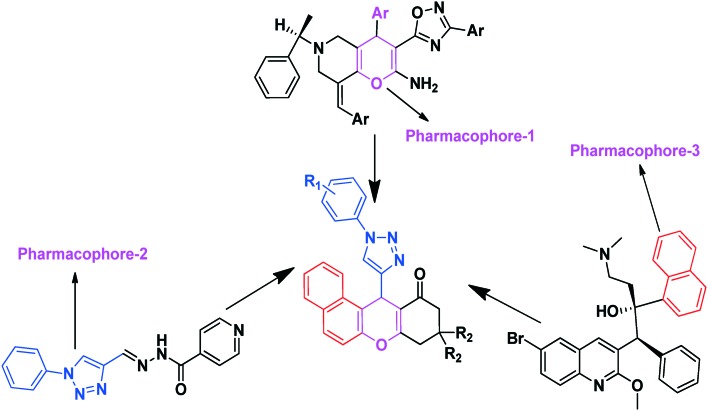
In recent years, 1,2,3-triazole-based antitubercular agents IV–VII (Fig. 1)26 have come to be regarded as a new class of antitubercular agents, providing truly effective lead candidates that are reported to inhibit bacterial growth. Our research group synthesized novel heterocyclic compounds by using green chemistry techniques and then carried out an evaluation of their antimicrobial activity.27–30 Impressed by their broad range of biological activities and in continuation of our ongoing research, we envisaged their integration with them having one molecular platform to generate dual pharmacophore units containing a xanthenone and triazole hybrid framework (Fig. 2). We report herein the design and synthesis of these title compounds by using a one-pot three-component thermal cyclization reaction of β-naphthol (5), substituted 1-aryl-1H-[1,2,3]triazole-4-carbaldehydes (4a–h), and cyclic-1,3-diones (6a, b) and the evaluation of their antimycobacterial and antimicrobial activities.
Fig. 1. I, II. Antimicrobial agents, III. Fluconazole derivative as an antifungal agent, IV–VII 1,2,3-triazole-based antitubercular agents.
Fig. 2. Design strategy of molecules based on TB active compounds.
2. Results and discussion
2.1. Chemistry
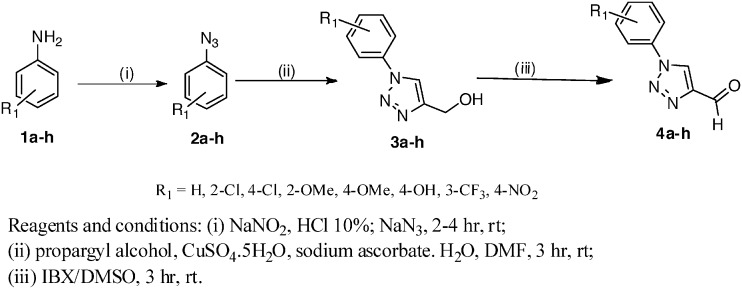
For the preparation of 4-carboxaldehyde-1,2,3-triazoles (4a–h) (Scheme 1), initially, aromatic azides (2a–h) were obtained by a diazotization reaction31 of anilines (1a–h) and further reaction with terminal alkynes to obtain the triazoles (3a–h) by Huisgen 1,3-dipolar cycloaddition reaction.32 4-Carboxaldehyde-1,2,3-triazoles (4a–h) were prepared by the oxidation of compounds (3a–h) using 2-iodoxybenzoic acid (IBX).33
Scheme 1. Synthesis of 1-phenyl-1H-1,2,3-triazole-4-carbaldehydes.
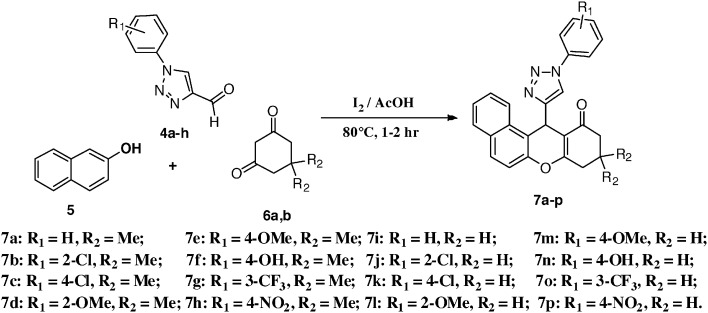
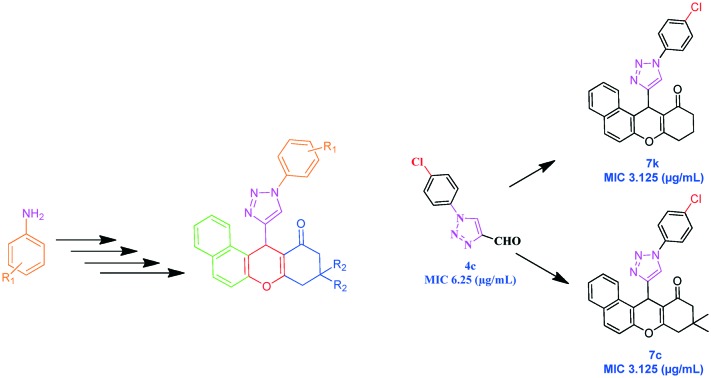
The syntheses of the 1,2,3-triazolo xanthenone derivatives (7a–p) (Scheme 2) were carried out by a three-component condensation of β-naphthol (5), substituted 1-aryl-1H-[1,2,3]triazole-4-carbaldehydes (4a–h), and cyclic-1,3-diones (6a, b) in the presence of I2 in acetic acid. The products were obtained in high yields (85–90%). The newly synthesized compounds were characterized by 1H NMR, 13C NMR, and mass spectral data analysis. In the 1H NMR spectrum of compound 7a, the characteristic singlet for the C12–H proton appeared at δ 6.01. while the C10–He, C10–Ha, and C8–H2 protons appeared at δ 2.69, δ 2.74, and δ 2.37 as a doublet (J = 17.5), doublet (J = 17.5), and singlet, respectively.34 In the 13C-NMR spectrum, the carbonyl carbon appeared at 189.7 ppm, while the C12, C10, C9, and C8 carbons appeared at δ, 50.8, 41.4, 32.4, and 29.2 ppm respectively, and the C9–C[combining low line]H3 carbons appeared at δ, 27.5 and 26.3, respectively. The LC-MS spectrum exhibited the [M + H]+ peak at m/z 422. Thus, on the basis of the above spectral studies, 7a was assigned the structure: 9,9-dimethyl-12-(1-phenyl-1H-[1,2,3]triazol-4-yl)-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one.
Scheme 2. Synthesis of 9,9-dimethyl-12-(1-phenyl-1H-[1,2,3]triazol-4-yl)-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one.
2.2. Biological assay
2.2.1. Antimicrobial activity
The antibacterial activities of all the newly synthesized compounds were evaluated by determination of their minimum inhibitory concentrations (MICs) by using the agar dilution method.35 All the compounds (7a–p) exhibited various levels of inhibitory effects against bacterial strains comprising three Gram-positive bacteria [Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 121) and Bacillus cereus (MTCC 430)] and three Gram-negative bacteria [Escherichia coli (MTCC 1652), Pseudomonas aeruginosa (MTCC 741), Enterobacter aerogenes (MTCC 111)]. The MIC values were compared with the standard drug ciprofloxacin and the results are tabulated in Table 1.
Table 1. Antimicrobial activity (MIC profiles) of the synthesized compounds (7a–p).
| Product | Minimum inhibitory concentrations (μg mL–1) |
|||||||
| Antibacterial activity |
Antifungal activity |
|||||||
| Gram-positive bacteria |
Gram-negative bacteria |
|||||||
| S.aureus | B.subtilis | B.cereus | E. coli | P. aeruginosa | E. aerogenes | A. Niger | C. albicans | |
| 7a | 6.25 | 25 | 12.5 | 12.5 | 25 | 6.25 | 6.25 | 25 |
| 7b | 3.12 | 6.25 | 3.12 | 3.12 | 6.25 | 12.5 | 3.12 | 3.12 |
| 7c | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| 7d | >100 | >100 | 50 | 25 | >100 | >100 | >100 | 25 |
| 7e | 6.25 | 6.25 | 3.12 | 12.5 | 6.25 | 6.25 | 3.12 | 6.25 |
| 7f | 6.25 | 6.25 | 12.5 | 3.12 | 6.25 | 6.25 | 12.5 | 3.12 |
| 7g | 25 | 50 | 12.5 | 100 | 25 | 25 | 12.5 | 100 |
| 7h | 12.5 | 6.25 | 6.25 | 3.12 | 12.5 | 3.12 | 6.25 | 3.12 |
| 7i | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 |
| 7j | >100 | >100 | >100 | 12.5 | 50 | 50 | 12.5 | >100 |
| 7k | 12.5 | 50 | 12.5 | 12.5 | 25 | 12.5 | >100 | >100 |
| 7l | 6.25 | 12.5 | 25 | 12.5 | 6.25 | 6.25 | 12.5 | 6.25 |
| 7m | 3.12 | 12.5 | 3.12 | 3.12 | 6.25 | 6.25 | 3.12 | 3.12 |
| 7n | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 7o | 6.25 | 12.5 | 25 | 12.5 | 6.5 | 12.5 | 50 | 50 |
| 7p | >100 | >100 | >100 | 50 | 50 | >100 | >100 | >100 |
| Stda | ≤6.25 | ≤6.25 | ≤6.25 | ≤6.25 | ≤6.25 | ≤6.25 | Nt | Nt |
| Stdb | Nt | Nt | Nt | Nt | Nt | Nt | ≤3.12 | ≤3.12 |
At a glimpse, all the compounds showed significant antibacterial activity against both Gram-positive and Gram-negative bacteria. Compound 7i exhibited the most potent inhibitory efficiency against all the tested strains (MIC 3.12 μg mL–1) compared to ciprofloxacin. Compounds 7b and 7m, with MIC values of 3.12 μg mL–1, exhibited the most potent inhibitory activity against S. aureus, B. cereus, and E. coli. Moreover, compounds 7e, 7f, and 7h (MIC 3.12 μg mL–1) exhibited potent inhibitory activity against B. cereus and E. coli. Compounds 7a, 7c, 7k, 7l, and 7o were found to exhibit considerable activity against most of the strains. Of the remaining compounds, 7d, 7g, 7j, 7n, and 7p were found to be less active against all the tested strains.
All the compounds (7a–p) were also tested for their in vitro antifungal activity against two fungal strains: Aspergillus niger (MTCC 282) and Candida albicans (MTCC 9062). The standard drug fluconazole was used for comparison of the antifungal activity shown by the compounds and the results were recorded as a MIC in μg mL–1. The results of the antifungal activity of all the compounds are shown in Table 1. All the compounds showed good antifungal activity against the two pathogens (MIC μg mL–1 values in the range of 3.12–100 μg mL–1). Compounds 7b, 7i, and 7m (MIC 3.12 μg mL–1) showed the most potent antifungal activity against the two strains, while compounds 7e (A. niger) and 7f and 7h (C. albicans) (MIC 3.12 μg mL–1) showed good antifungal activity against the corresponding fungi strains. Compounds 7a, 7c, 7f, and 7k exhibited significant activity, while the remaining compounds were less active against the tested fungal strains.
2.3. Antitubercular assay
2.3.1. In vitro MTB screening
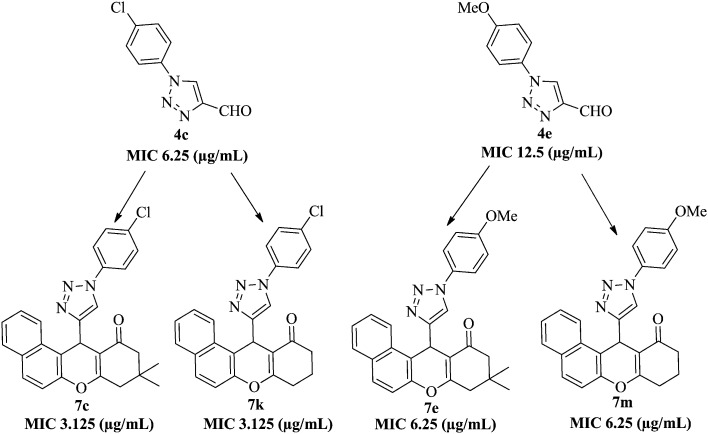
All the compounds were evaluated against M. tuberculosis H37Rv (ATCC 27294) and the principal results are reported in Table 2. The MDR-TB clinical isolate was resistant to isoniazid (INH), rifampicin, ethambutol, pyrazinamide, and ciprofloxacin. The tested compounds showed antimycobacterial activity, with MIC values between 3.125 and ≥25 μg mL–1. Of the various compounds tested, compounds 7c, 7e, 7k, and 7m inhibited mycobacterial growth very effectively compared to others in the series, with MIC values ranging from 3.12 to 6.25 μg mL–1. The highest activity, 3.12 μg mL–1, was registered for compounds 7c and 7k (R1 = 4-Cl, R2 = CH3, H, respectively). It was observed that the presence of the 4-Cl substituent on the R1 position of the 1-phenyl-1H-[1,2,3]triazol-4-yl core brought about an enhancement of the antimycobacterial potency. Moreover, 7e and 7m (R1 = 4-OCH3, R2 = CH3, H, respectively) and 4c showed good activity (MIC 6.25 μg mL–1), while 7a and 7i (R1 = H, R2 = CH3, H, respectively), 7d (R1 = 2-OCH3, R2 = CH3), and 4e showed moderate activity (MIC 12.5 μg mL–1), and the remaining compounds showed poor activity (MIC ≥25 μg mL–1). It can thus be concluded that the newly synthesized compounds 7c and 7k showed a better activity than 4c, while compounds 7e and 7m showed good activity compared to 4e. The comparisions of TB activity among the precursors (4c, 4e) and final compounds (7c, 7e, 7k, 7m) are illustrated in Fig. 3.
Table 2. Antitubercular evaluation of 7a–p against M. tuberculosis H37RV.
| Entry | Product | MIC (μg mL–1) | log p/clog p a | Cytotoxicity in% inhibition at 50 μg m–1 |
| 1 | 4a | 25 | — | — |
| 2 | 4b | 25 | — | — |
| 3 | 4c | 6.25 | — | — |
| 4 | 4d | >25 | — | — |
| 5 | 4e | 12.5 | — | — |
| 6 | 4f | >25 | — | — |
| 7 | 4g | >25 | — | — |
| 8 | 4h | >25 | — | — |
| 9 | 7a | 12.5 | 4.65/5.85 | — |
| 10 | 7b | 25 | 5.21/6.73 | — |
| 11 | 7c | 3.125 | 5.21/6.73 | 18.10 |
| 12 | 7d | 12.5 | 4.53/5.84 | — |
| 13 | 7e | 6.25 | 4.53/5.84 | 21.18 |
| 14 | 7f | >25 | 4.26/5.61 | — |
| 15 | 7g | >25 | 5.57/7.03 | — |
| 16 | 7h | >25 | —/5.96 | — |
| 17 | 7i | 12.5 | 3.85/4.58 | — |
| 18 | 7j | 25 | 4.41/5.46 | — |
| 19 | 7k | 3.125 | 4.41/5.46 | 18.12 |
| 20 | 7l | 25 | 3.73/4.81 | — |
| 21 | 7m | 6.25 | 3.73/4.81 | 22.16 |
| 22 | 7n | >25 | 3.46/4.34 | — |
| 23 | 7o | >25 | 4.77/5.76 | — |
| 24 | 7p | >25 | —/4.69 | — |
| Isoniazid | — | 0.72 | — | — |
| Rifampicin | — | 0.24 | — | — |
| Ethambutol | — | 7.64 | — | — |
| Pyrazinamide | — | 50.77 | — | — |
| Ciprofloxacin | — | 4.71 | — | — |
aCalculated using Chembiodraw 12.0.
Fig. 3. Activity comparision of the intermediates and final compounds.
Liphophilicity has long been recognized as an important factor for the successful passage of drugs through clinical development.36 Generally, calculated log P (clog P) is used for the assessment of liphophilicity and the key events of molecular desolvation, such as transfer from the aqueous phases to the cell membranes and protein binding sites.37 With an evidenced role as a predictor of eventual compound success, the computation of log P (clog P) for liphophilicity is essential for the development of a successful therapeutic compound. To correlate the antitubercular activity of the present series of compounds with respect to liphophilicity, log p/clog p values were calculated using Chembiodraw ultra 12.0. Four potent hybrids 7c, 7e, 7k, and 7m showed log p (clog p) values of 5.21 (6.73), 4.53 (5.84), 4.41 (5.46), and 3.73 (4.81), respectively. It is worth mentioning here that, among all the new compounds examined, 7k bearing a 4-Cl triazolyl xanthenone core was the most optimized analog with the best correlation to liphophilicity, with a log p value <5.0 (Table 2). This result clearly reveals that 4-chloro-phenyl-1,2,3-triazolyl-xanthenone (7k) plays a significant role in controlling liphophilicity and in MTB inhibition activity.
2.3.2. In vitro cytotoxicity screening
As a result, the most active compounds 7e, 7f, 7m, and 7n target and kill MTB to a greater extent compared to the HEK cell line without disordering the immune system and therefore it was justified to consider the further development of the phenanthridinyl piperazine scaffold as a lead candidate to research to attenuate the treatment of this devastating disease, and in line with this, the corresponding results are shown in Table 2.
2.4. Molecular-docking studies
The pantothenate biosynthesis pathway is a potential drug target and is essential for the persistent growth and virulence of MTB. The pantothenate biosynthetic pathway involves four steps catalyzed by enzymes encoded by panB, panC, panD, and panE genes.38 PanC encodes a pantothenate synthetase (PS), which catalyzes the last step of pantothenate biosynthesis, the ATP-dependent condensation of d-pantoate, and b-alanine to form pantothenate.39 Pantothenate is a major precursor of coenzyme A and acyl carrier protein, essential for many intracellular processes, including fatty acid metabolism, cell signaling, and the synthesis of polyketides and non-ribosomal peptides. Microorganisms must synthesize pantothenate, while mammals must obtain it from their diet as its biosynthetic pathway is not present. There is evidence that the pantothenate biosynthesis pathway is a potential drug target and that it is essential for the persistent growth and virulence of MTB, and also that PS is an appropriate target for developing new therapeutics to treat TB.40
While our synthesized compounds are in fact hydrophobic, we hypothesized that these are active against PS inhibitors in which hydrophobic interactions are important for their activity.41 For this we selected PS as a target for docking studies, in order to gain more insight into the interaction between this new series of 1,2,3-triazolyl xanthenone derivatives and pantothenate synthetase. The crystal structure of pantothenate synthetase (pdb id: ; 3IVX)42 was retrieved from the protein data bank. GLIDE 5.6 (ref. 43) was used for the molecular docking. The protein was prepared using the protein preparation wizard in Maestro 9.0, applying the default parameters; a grid was generated around the active site by selecting the cocrystalized ligand. The receptor van der Waals scaling for non-polar atoms was kept at 0.9. The molecules were built using the Maestro build panel and prepared by LigPrep 2.0 application. A low energy conformation of the ligands was selected and docked into the grid generated for the protein using the standard precision (SP) docking mode.44 The dock pose of each ligand was analyzed for interactions with the receptor. The best active molecules showed good interactions with the protein active site. Molecules were deeply embedded into the hydrophobic active site pocket to occupy the same position, with the overlay of the dock poses of 7c, 7e, 7k, and 7m (R- and S-configurations) shown in Fig. 4.
Fig. 4. Dock poses of 7c, 7e, 7k, and 7m (R-enantiomers in plum color and S-enantiomers in yellow color) in the active site of PS. All the aromatic rings are deeply embedded into the active site hydrophobic pocket.
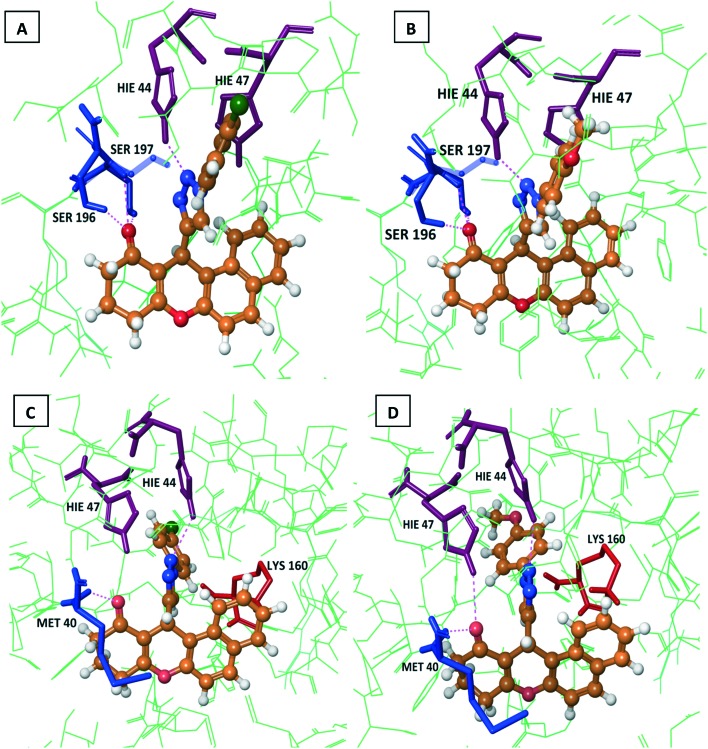
To gain an insight into the structural basis for its activity, the most active compounds 7c, 7e, 7k, and 7m were analyzed in more detail. Docking studies on the R- and S-enantiomeric forms showed dissimilar interactions with the enzyme PS. The R-compounds showed good docking scores and made hydrogen bonding interactions with the relevant amino acid residues, such as MET 40, HIE 44, HIE 47, GLN 72, GLN 164, and LYS 160; whereas the S-compounds showed hydrogen bonding interactions with the HIE 44, HIE 47, GLN 164, LYS 160, SER 196, and SER 197 amino acid residues. In addition to the hydrogen bonding interactions, the compounds were further stabilized by π–π interactions with HIE 44 and HIE 47 and by π–cation interactions with LYS 160. The ligand interaction diagrams, bond lengths, bond angles, dock scores, and emodel energies are provided in the ESI.‡
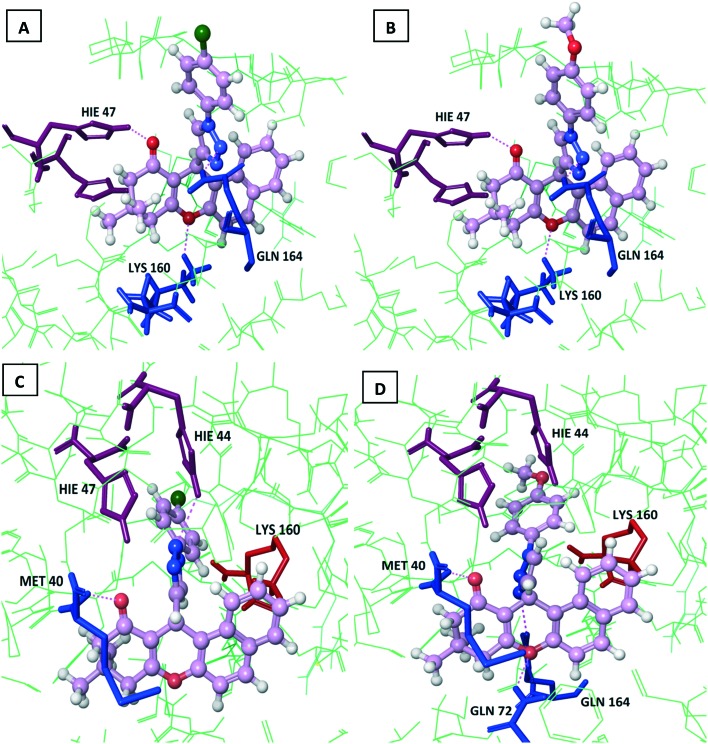
The S-configurations of 7c and 7e showed H-bond interactions with HIE 47 (–CO), LYS 160 (–O–), and GLN 164 (triazole N), as shown in Fig. 5A and B, respectively. The R-configuration of 7c showed H-bond interactions with MET 40 (–CO) and HIE 44 (triazole N), two π–π stacking interactions with HIE 47 (triazole N, benzene ring), and π–cation interaction with LYS 160 (xanthenone benzene ring), as depicted in Fig. 5C. The R-configuration of 7e showed H-bond interactions with MET 40 (–CO), GLN 72 (–O–), and GLN 164 (triazole N), π–π stacking interaction with HIE 44 (benzene ring), and two π–cation interactions with LYS 160 (triazole and xanthenone benzene ring), as represented in Fig. 5D.
Fig. 5. Dock pose diagrams of compounds 7k and 7m. A and B: S-Configurations of 7k and 7m showing H-bond interactions with HIE 44, SER 196, and SER 197, and a π–π stacking interaction with HIE 47. C: R-Configuration of 7k showing H-bond interactions with MET 40 and HIE 44, two π–π stacking interactions with HIE 47, and a π–cation interaction with LYS 160. D: R-Configuration of 7m showing H-bond interactions with MET 40, HIE 44, and HIE 47, a π–π stacking interaction with HIE 44, and three π–cation interactions with LYS 160.
In the S-configurations of 7k and 7m, the structures of triazole N showing an H-bond interaction with HIE 44, –CO showing H-bond interactions with SER 196 and SER 197, the triazole attached to a benzene ring showing π–π stacking interaction with HIE 47 are shown in Fig. 6A and B, respectively. In the R-configuration of 7k, the structures of MET 40 showing an H-bond interactions with –CO and HIE 44 (triazole N), HIE 47 showing two π–π stacking interactions with the triazole ring and triazole attached to the benzene ring, and LYS 160 showing π–cation interaction with the xanthenone benzene ring are depicted in Fig. 6C. The R-configuration of 7m showing H-bond interactions with MET 40 (–CO), HIE 47 (–CO), and HIE 44 (triazole N), π–π stacking interaction with HIE 44 (benzene ring), and three π–cation interactions with LYS 160 (triazole ring and two naphthalene rings) are represented in Fig. 6D.
Fig. 6. Dock pose diagrams of compounds 7c and 7e. A and B: S-Configurations of 7c and 7e showing H-bond interactions with HIE 47, LYS 160, and GLN 164. C: R-Configuration of 7c showing H-bond interactions with MET 40 and HIE 44, two π–π stacking interactions with HIE 47, and a π–cation interaction with LYS 160. D: R-Configuration of 7e showing H-bond interactions with MET 40, GLN 72, and GLN 164, a π–π stacking interaction with HIE 44, and two π–cation interactions with LYS 160.
Analysis of the best active compounds in the active site of panC protein revealed that the hydrophobic interactions (π–π stacking and π–cation) are important for the activity of the compounds. We found that the S-compounds (7k and 7m) with simple hydrogen substitution at the C-9 position showed similar interactions with active site amino acids, like co-crystal ligands; whereas the S-compounds (7c and 7e) with dimethyl groups at the C-9 position flip their orientation in an anticlockwise direction and showed different interactions with the active site protein. This was not observed in the case of the R-compounds (7c, 7e, 7k, and 7m), which showed similar interactions with the active site protein. Hence, the R- and S-enantiomeric forms showed dissimilar interactions with the PS enzyme.
3. Conclusion
In conclusion, we synthesized new 12-(1-phenyl-1H-[1,2,3]triazole-4-yl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives (7a–p) through a three-component one-pot reaction of substituted 1-phenyl-1H-[1,2,3]triazole-4-carbaldehydes, 2-naphthol, and substituted dimedones. The activity of these compounds against the M. tuberculosis strain H37Rv was assessed, whereby compounds 7c, 7e, 7k, and 7m displayed good antitubercular activity. We observed that phenyl triazoles with various substituents exhibited outstandingly different activities. Specifically, the substituent at the 4-position (4-Cl, 4-OMe) on the triazole was much more influential on inhibitory activity than at the other positions, while 1,3 cyclodione containing methyl groups did not affect the activity. An in vitro cytotoxicity study against HEK cell lines at 50 μg mL–1 by MTT assay revealed that the identified hits were found to be less cytotoxic. A molecular-docking study against PS (panC protein) further revealed the favorable interactions of the active molecules with amino acid residues of the enzyme. We found that the R- and S-configuration of compounds 7c, 7e, 7k, and 7m showed different interactions with diverse amino acids of PS, due to their different spatial contact with the target enzyme PS. With new antitubercular agents desperately needed, we believe that the new 12-(1-phenyl-1H-[1,2,3]triazole-4-yl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives (7a–7p) reported in this work offer an interesting potential for further optimization. Further structural modifications of the identified hits are in progress in order to enhance the efficacy of the active compounds against M. tuberculosis.
4. Experimental
4.1. General experimental methods
The melting points were determined in an open glass capillary tube on a Gallen-Kamp MFB-595 apparatus and are reported herein uncorrected. The IR spectra were recorded on a Perkin Elmer FT-IR-8400s, using samples in KBr disks. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance II 400 spectrometer using CDCl3 as the solvent and TMS as the internal standard, with the chemical shifts expressed herein in δ ppm. The mass spectra were recorded on a SHIMADZU LC-MS 2020 mass spectrometer. The progress of the reactions was monitored by TLC (Silica gel, aluminum sheets 60 F254, Merck). Elemental analysis was performed on a Perkin Elmer CHN-2400 analyzer.
4.2. General procedure for the synthesis of 9,9-dimethyl-12-(1-phenyl-1H-[1,2,3]triazol-4-yl)-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7a–p)
A mixture of substituted 1-aryl-1H-[1,2,3]triazole-4-carbaldehydes (4a–h) (1 mmol), β-naphthol (5) (1 mmol), cyclic-1,3-diones (6a, b) (1.2 mmol), and I2 (5 mol%) in acetic acid (2 ml) was heated at 80 °C for 1–2 h. The progress of the reaction was monitored by TLC. Later, the reaction mixture was cooled to room temperature, treated with aqueous Na2S2O3, then extracted with ethyl acetate and the solvent then evaporated off under reduced pressure. The crude product was purified by silica-gel column chromatography using ethyl acetate–hexane (1 : 3; v/v) as the eluent to afford pure products.
4.3. In vitro MTB screening
Two-fold serial dilutions of each test compound/drug were prepared and incorporated into Middle-brook 7H11 agar medium with oleic acid, albumin, dextrose, and catalase (OADC) growth supplement to obtain final concentrations of 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 μg mL–1. An inoculum of M. tuberculosis H37Rv (ATCC 27294) was prepared from fresh Middle-brook 7H11 agar slants with OADC (Difco) growth supplement adjusted to 1 mg mL–1 (wet weight) in Tween 80 (0.05%) saline diluted to 10–2 to give a concentration of ∼107 cfu mL–1. Then, 5 μL of this bacterial suspension was spotted onto 7H11 agar tubes containing different concentrations of the drug as discussed above. The tubes were incubated at 37 °C and the final readings (as MIC in μg mL–1) were determined after 28 days. The MIC is defined as the minimum concentration of compound required to give complete inhibition of the bacterial growth. This method is similar to that recommended by the National Committee for Clinical Laboratory Standards for the determination of MIC in triplicate.
4.4. In vitro cytotoxicity screening
The compounds 7c, 7e, 7k, and 7m displaying good in vitro potency in the MTB MIC were further examined for toxicity in a HEK cell line at the concentration of 50 μg mL–1. After 72 h of exposure, the viability was assessed on the basis of the cellular conversion of MTT into a formazan product using the Promega Cell Titer 96 non-radioactive cell proliferation assay.45
4.4.1. 9,9-Dimethyl-12-(1-phenyl-1H-[1,2,3]triazol-4-yl)-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7a)
Yield 90%, mp: 179–181 °C; Rf = 0.40 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3132, (C O) 1647, (C C)Aromatic 1597, 1508, (C–O–C) 1226 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.10 (s, 3H), 1.16 (s, 3H), 2.37 (s, 2H), 2.58–2.62 (d, J = 17.5 Hz, 1H), 2.69–2.74 (d, J = 17.5 Hz, 1H), 6.01 (s, 1H, C12H), 7.34–7.52 (m, 6H), 7.65–7.68 (m, 2H), 7.79–7.84 (t, J = 9.1 Hz, 2H), 8.01 (s, 1H), 8.03–8.05 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 26.3, (C9–C[combining low line]H3) 27.5, (C9) 29.2, (C8) 32.4, (C10) 41.4, (C12) 50.8, (C7b–Cα) 117.4, (Ar–C[combining low line]) 119.2, (Ar–C[combining low line]) 120.3, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.0, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.0, (Ar–C[combining low line]) 129.5, (Ar–C[combining low line]) 131.4, (C11b–Cβ) 147.9, (C O) 189.7; LC-MS m/z: 422 [M + H]+; anal. calcd for C27H23N3O2: C, 76.94; H, 5.50; N, 9.97. Found: C, 75.39; H, 5.44; N, 9.69.
4.4.2. 12-[1-(2-Chloro-phenyl)-1H-[1,2,3]triazol-4-yl]-9,9-dimethyl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7b)
Yield 86%, mp: 166–168 °C; Rf = 0.42 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3039, (C O) 1640, (C C)Aromatic 1595, 1510, (C–O–C) 1201 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.09 (s, 3H), 1.16 (s, 3H), 2.31–2.34 (d, J = 16.7 Hz, 1H), 2.39–2.42 (d, J = 16.7 Hz, 1H), 2.58–2.61 (d, J = 17.0 Hz, 1H), 2.67–2.69 (d, J = 17.0 Hz, 1H), 6.00 (s, 1H, CH), 7.32–7.58 (m, 7H), 7.78–7.82 (m, 2H), 8.01 (s, 1H, triazole H), 8.07–8.09 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 26.3, (C9–C[combining low line]H3) 27.3, (C9) 29.3, (C8) 32.4, (C10) 41.4, (C12) 50.8, (C7b–Cα) 111.7, (Ar–C[combining low line]) 115.3, (Ar–C[combining low line]) 117.4, (Ar–C[combining low line]) 123.2, (Ar–C[combining low line]) 123.4, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.0, (Ar–C[combining low line]) 127.7, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.1, (Ar–C[combining low line]) 130.3, (Ar–C[combining low line]) 130.6, (Ar–C[combining low line]) 131.4, (Ar–C[combining low line]) 134.9, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 149.5, (C11b–Cβ) 165.5, (C O) 197.0; LC-MS m/z: 456 [M + H]+; anal. calcd for C27H22ClN3O2: C, 71.13; H, 4.86; N, 9.22. Found: C, 70.69; H, 4.56; N, 9.10.
4.4.3. 12-[1(4-Chloro-phenyl)-1H-[1,2,3]triazole-4-yl]-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (7c)
Yield 90%, mp: 173–175 °C; Rf = 0.45 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3147, 3072, (C O) 1647, (C C)Aromatic 1597, 1500, (C–O–C) 1230 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.08 (s, 3H), 1.15 (s, 3H), 2.30–2.34 (d, J = 17.4 Hz, 1H), 2.35–2.37 (d, J = 17.4 Hz, 1H), 2.61–2.63 (d, J = 16.2 Hz, 1H), 2.64–2.68 (d, J = 16.2 Hz, 1H), 5.97 (s, 1H, CH), 7.32–7.47 (m, 5H), 7.59–7.62 (d, J = 8.8 Hz, 2H), 7.80–7.86 (m, 2H), 7.96 (s, 1H, triazole H), 8.02–8.04 (d, J = 8.9 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 26.3, (C9–C[combining low line]H3) 27.5, (C9) 29.2, (C8) 32.4, (C10) 41.4, (C12) 50.8, (C7b–Cα) 111.5, (Ar–C[combining low line]) 115.1, (Ar–C[combining low line]) 117.4, (Ar–C[combining low line]) 119.0, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.1, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.1, (Ar–C[combining low line]) 129.7, (Ar–C[combining low line]) 131.1, (Ar–C[combining low line]) 131.4, (Ar–C[combining low line]) 134.0, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 150.7, (C11b–Cβ) 165.7, (C O) 197.3; LC-MS m/z: 456 [M + H]+; anal. calcd for C27H22ClN3O2: C, 71.13; H, 4.86; N, 9.22. Found: C, 70.68; H, 4.59; N, 9.09.
4.4.4. 12-[1-(2-Methoxy-phenyl)-1H-[1,2,3]triazol-4-yl]-9,9-dimethyl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7d)
Yield 89%, mp: 190–192 °C; Rf = 0.36 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3041, (C O) 1639, (C C)Aromatic 1592, 1516, (C–O–C) 1215 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.07. (s, 3H), 1.11 (s, 3H), 2.19–2.23 (d, J = 16.5 Hz, 1H), 2.39–2.43 (d, J = 16.5 Hz, 1H), 2.63–2.68 (d, J = 17.4 Hz, 1H), 2.72–2.77 (d, J = 17.4 Hz, 1H), 3.79 (s, 3H, OCH3), 5.84 (s, 1H, CH), 7.03–7.07 (t, J = 7.7 Hz, 1H), 7.25–7.27 (d, J = 7.9 Hz, 1H), 7.41–7.49 (m, 4H), 7.50–7.51 (t, J = 7.2 Hz, 1H), 7.57–7.61 (t, J = 8.9 Hz, 2H), 8.34–8.36 (d, J = 8.5 Hz, 1H), 8.41 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 25.6, (C9–C[combining low line]H3) 26.3, (C8) 28.9, (C10) 32.0, (C12) 50.0, (Ar–O–C[combining low line]H3) 56.0, (C7b–Cα) 110.8, (Ar–C[combining low line]) 112.9, (Ar–C[combining low line]) 115.8, (Ar–C[combining low line]) 117.2, (Ar–C[combining low line]) 120.8, (Ar–C[combining low line]) 123.3, (Ar–C[combining low line]) 123.8, (Ar–C[combining low line]) 125.0, (Ar–C[combining low line]) 125.5, (Ar–C[combining low line]) 127.1, (Ar–C[combining low line]) 128.4, (Ar–C[combining low line]) 129.0, (Ar–C[combining low line]) 130.3, (Ar–C[combining low line]) 130.5, (Ar–C[combining low line]) 130.8, (Ar–C[combining low line]) 147.0, (Ar–C[combining low line]) 149.1, (Ar–C[combining low line]) 150.9, (C11b–Cβ) 165.2, (C O) 196.0; LC-MS m/z: 452 [M + H]+; anal. calcd for C28H25N3O3: C, 74.48; H, 5.58; N, 9.31. Found: C, 73.69; H, 5.52; N, 9.21.
4.4.5. 12-[1-(4-Methoxy-phenyl)-1H-[1,2,3]triazol-4-yl]-9,9-dimethyl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7e)
Yield 85%, mp: 212–214 °C; Rf = 0.48 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3174, 3064, (C O) 1651, (C C)Aromatic 1622, 1595, (C–O–C) 1226 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.09. (s, 3H), 1.11 (s, 3H), 1.98–2.01 (d, J = 18.2 Hz, 1H), 2.10–2.13 (d, J = 18.2 Hz, 1H), 2.65–2.67 (d, J = 16.5 Hz, 1H), 2.72–2.75 (d, J = 16.5 Hz, 1H), 3.82 (s, 3H, OCH3), 5.88 (s, 1H, CH), 6.93–6.96 (d, J = 8.2 Hz, 2H), 7.59–7.72 (m, 7H), 8.02–8.06 (d, J = 8.2 Hz, 1H), 8.41 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 25.6, (C9–C[combining low line]H3) 26.3, (C8) 28.9, (C10) 32.0, (C12) 50.1, (Ar–O–C[combining low line]H3) 56.3, (C7b–Cα) 110.8, (Ar–C[combining low line]) 112.8, (Ar–C[combining low line]) 112.9, (Ar–C[combining low line]) 116.2, (Ar–C[combining low line]) 117.2, (Ar–C[combining low line]) 120.8, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 123.7, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 125.4, (Ar–C[combining low line]) 127.1, (Ar–C[combining low line]) 128.5, (Ar–C[combining low line]) 129.0, (Ar–C[combining low line]) 130.3, (Ar–C[combining low line]) 130.6, (Ar–C[combining low line]) 130.8, (Ar–C[combining low line]) 147.0, (Ar–C[combining low line]) 149.5, (Ar–C[combining low line]) 150.9, (C11b–Cβ) 165.1, (C O) 196.2; LC-MS m/z: 452 [M + H]+; anal. calcd for C28H25N3O3: C, 74.48; H, 5.58; N, 9.31. Found: C, 73.65; H, 5.50; N, 9.23.
4.4.6. 12-[1-(4-Hydroxy-phenyl)-1H-[1,2,3]triazol-4-yl]-9,9-dimethyl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7f)
Yield 87%, mp: 158–160 °C; Rf = 0.23 (EtOAc : n-hexane 2 : 3); IR (KBr): (O–H)Aromatic 3445, ( C–H)Aromatic 3043, (C O) 1643, (C C)Aromatic 1617, 1568, (C–O–C) 1192 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.03. (s, 3H), 1.10 (s, 3H), 2.18–2.22 (d, J = 17.5 Hz, 1H), 2.36–2.40 (d, J = 17.5 Hz, 1H), 2.64–2.69 (d, J = 16.5 Hz, 1H), 2.70–2.75 (d, J = 16.5 Hz, 1H), 5.79 (s, 1H, CH), 6.85–6.87 (d, J = 8.7 Hz, 2H), 7.42–7.68 (m, 5H), 7.90–7.94 (t, J = 7.8 Hz, 2H), 8.28–8.30 (d, J = 8.1 Hz, 1H), 8.67 (s, 1H, triazole H), 9.89 (s, 1H, Ar–OH); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 25.7, (C9–C[combining low line]H3) 26.6, (C8) 28.7, (C10) 32.0, (C12) 50.0, (C7b–Cα) 110.7, (Ar–C[combining low line]) 115.6, (Ar–C[combining low line]) 115.9, (Ar–C[combining low line]) 117.2, (Ar–C[combining low line]) 120.0, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 123.1, (Ar–C[combining low line]) 125.0, (Ar–C[combining low line]) 127.2, (Ar–C[combining low line]) 128.5, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.0, (Ar–C[combining low line]) 130.5, (Ar–C[combining low line]) 130.8, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 147.0, (Ar–C[combining low line]) 150.1, (Ar–C[combining low line]) 157.4, (C11b–Cβ) 165.0, (C O) 196.0; LC-MS m/z: 438 [M + H]+; anal. calcd for C27H23N3O3: C, 74.12; H, 5.30; N, 9.60. Found: C, 74.01; H, 5.20; N, 9.51.
4.4.7. 9,9-Dimethyl-12-[1-(3-trifluoromethyl-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7g)
Yield 88%, mp: 195–197 °C; Rf = 0.31 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3040, (C O) 1648, (C C)Aromatic 1619, 1516, (C–O–C) 1210 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.05. (s, 3H), 1.12 (s, 3H), 2.21–2.23 (d, J = 16.3 Hz, 1H), 2.35–2.37 (d, J = 16.3 Hz, 1H), 2.72–2.75 (d, J = 17.5 Hz, 1H), 2.75–2.78 (d, J = 17.5 Hz, 1H), 6.05 (s, 1H, CH), 7.32–7.35 (d, J = 8.2 Hz, 1H), 7.41–7.59 (m, 6H), 7.80–7.84 (t, J = 8.1 Hz, 2H), 8.00–8.02 (d, J = 7.5 Hz, 1H), 8.12 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 25.6, (C9–C[combining low line]H3) 26.5, (C8) 28.7, (C10) 32.1, (C12) 50.8, (C7b–Cα) 110.6, (Ar–C[combining low line]) 115.6, (Ar–C[combining low line]) 115.8, (Ar–C[combining low line]) 117.3, (Ar–C[combining low line]) 120.1, (Ar–C[combining low line]) 121.3, (Ar–C[combining low line]) 123.1, (Ar–C[combining low line]) 125.2, (Ar–C[combining low line]) 127.6, (Ar–C[combining low line]) 128.5, (Ar–C[combining low line]) 128.7, (Ar–C[combining low line]) 129.2, (Ar–C[combining low line]) 130.5, (Ar–C[combining low line]) 130.8, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 146.9, (Ar–C[combining low line]) 150.1, (Ar–C[combining low line]) 157.9, (C11b–Cβ) 165.4, (C O) 197.2; LC-MS m/z: 490 [M + H]+; anal. calcd for C28H22F3N3O2: C, 68.70; H, 4.53; N, 8.58. Found: C, 67.59; H, 4.26; N, 8.46.
4.4.8. 9,9-Dimethyl-12-[1-(4-nitro-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7h)
Yield 85%, mp: 200–202 °C; Rf = 0.32 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3045, (C O) 1640, (C C)Aromatic 1620, 1562, (C–O–C) 1201 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.08. (s, 3H), 1.14 (s, 3H), 2.34–2.36 (d, J = 18.1 Hz, 1H), 2.41–2.44 (d, J = 18.1 Hz, 1H), 2.63–2.66 (d, J = 17.2 Hz, 1H), 2.72–2.77 (d, J = 17.2 Hz, 1H), 6.07 (s, 1H, CH), 7.42–7.58 (m, 5H, Ar–H), 7.58–7.60 (d, J = 8.1 Hz, 2H), 7.81–7.83 (d, J = 8.0 Hz, 2H), 7.98–8.01 (d, J = 7.5 Hz, 1H), 8.12 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9–C[combining low line]H3) 26.3, (C9–C[combining low line]H3) 27.5, (C9) 29.1, (C8) 32.4, (C10) 42.4, (C12) 50.8, (C7b–Cα) 111.5, (Ar–C[combining low line]) 115.0, (Ar–C[combining low line]) 117.4, (Ar–C[combining low line]) 119.0, (Ar–C[combining low line]) 121.5, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.0, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.2, (Ar–C[combining low line]) 131.1, (Ar–C[combining low line]) 134.0, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 150.6, (C11b–Cβ) 165.7, (C O) 197.3; LC-MS m/z: 467 [M + H]+; anal. calcd for C27H22N4O4: C, 69.52; H, 4.75; N, 12.01. Found: C, 68.48; H, 4.52; N, 11.61.
4.4.9. 12-(1-Phenyl-1H-[1,2,3]triazole-4-yl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (7i)
Yield 91%, mp: 172–174 °C; Rf = 0.43 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3040, (C O) 1647, (C C)Aromatic 1596, 1508, (C–O–C) 1221 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.05–2.12 (m, 2H), 2.45–2.52 (m, 2H), 2.71–2.84 (m, 2H), 6.00 (s, 1H, CH), 7.34–7.50 (m, 6H), 7.64–7.68 (d, J = 8.4 Hz, 2H), 7.78–7.83 (t, J = 8.2 Hz, 2H), 8.01–8.03 (m, 2H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.5, (C8) 25.8, (C10) 28.0, (C12) 39.8, (C7b–Cα) 111.5, (Ar–C[combining low line]) 115.4, (Ar–C[combining low line]) 116.5, (Ar–C[combining low line]) 119.5, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 122.5, (Ar–C[combining low line]) 122.9, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 123.4, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 125.1, (Ar–C[combining low line]) 127.6, (Ar–C[combining low line]) 129.1, (Ar–C[combining low line]) 131.1, (Ar–C[combining low line]) 134.2, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 150.8, (C11b–Cβ) 165.8, (C O) 197.2; LC-MS m/z: 394 [M + H]+; anal. calcd for C25H19N3O2: C, 76.32; H, 4.87; N, 10.68. Found: C, 74.98; H, 4.69; N, 9.95.
4.4.10. 12-[1-(2-Chloro-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7j)
Yield 89%, mp: 178–180 °C; Rf = 0.31 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3045, (C O) 1638, (C C)Aromatic 1595, 1505, (C–O–C) 1198 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.01–2.11 (m, 2H), 2.48–2.56 (m, 2H), 2.71–2.86 (m, 2H), 6.01 (s, 1H, CH), 7.32–7.56 (m, 7H), 7.77–7.83 (t, J = 8.1 Hz, 2H), 8.03–8.05 (m, 2H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.4, (C8) 26.4, (C10) 27.7, (C12) 36.9, (C7b–Cα) 112.8, (Ar–C[combining low line]) 115.2, (Ar–C[combining low line]) 117.2, (Ar–C[combining low line]) 123.2, (Ar–C[combining low line]) 123.6, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.0, (Ar–C[combining low line]) 127.7, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.2, (Ar–C[combining low line]) 130.3, (Ar–C[combining low line]) 130.6, (Ar–C[combining low line]) 134.9, (Ar–C[combining low line]) 147.8, (Ar–C[combining low line]) 149.6, (C11b–Cβ) 167.1, (C O) 197.3; LC-MS m/z: 428 [M + H]+; anal. calcd for C25H18ClN3O2: C, 70.18; H, 4.24; N, 9.82. Found: C, 69.89; H, 4.18; N, 9.78.
4.4.11. 12-[1-(4-Chloro-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7k)
Yield 90%, mp: 192–195 °C; Rf = 0.47 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3045, (C O) 1638, (C C)Aromatic 1595, 1506, (C–O–C) 1201 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.10–2.14 (m, 2H), 2.48–2.52 (m, 2H), 2.79–2.89 (m, 2H), 5.98 (s, 1H, CH), 7.26–7.48 (m, 5H), 7.59–7.62 (d, J = 8.4 Hz, 2H), 7.78–7.83 (d, J = 8.4 Hz, 2H), 7.97–8.01 (m, 2H, Ar–H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.5, (C8) 26.4, (C10) 27.7, (C12) 36.9, (C7b–Cα) 112.8, (Ar–C[combining low line]) 115.0, (Ar–C[combining low line]) 117.3, (Ar–C[combining low line]) 119.2, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 127.0, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.2, (Ar–C[combining low line]) 129.7, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 131.4, (Ar–C[combining low line]) 134.0, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 150.9, (C11b–Cβ) 167.2, (C O) 197.3; LC-MS m/z: 428 [M + H]+; anal. calcd for C25H18ClN3O2: C, 70.18; H, 4.24; N, 9.82. Found: C, 69.86; H, 4.14; N, 9.79.
4.4.12. 12-[1-(2-Methoxy-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7l)
Yield 89%, mp: 218–220 °C; Rf = 0.42 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3040, (C O) 1636, (C C)Aromatic 1587, 1505, (C–O–C) 1198 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.04–2.11 (m, 2H), 2.44–2.50 (m, 2H), 2.72–2.80 (m, 2H), 3.85 (s, 3H, –OCH3), 5.99 (s, 1H, CH), 6.99–7.03 (m, 2H), 7.30–7.50 (m, 4H), 7.68–7.82 (m, 3H), 8.06–8.09 (d, J = 8.2 Hz, 1H), 8.15 (s, 1H triazole H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.1, (C8) 25.8, (C10) 27.5, (C12) 37.0, (Ar–O–C[combining low line]H3) 50.9, (C7b–Cα) 110.9, (Ar–C[combining low line]) 115.4, (Ar–C[combining low line]) 116.4, (Ar–C[combining low line]) 118.9, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 122.9, (Ar–C[combining low line]) 123.2, (Ar–C[combining low line]) 123.5, (Ar–C[combining low line]) 123.6, (Ar–C[combining low line]) 123.8, (Ar–C[combining low line]) 124.3, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 125.2, (Ar–C[combining low line]) 127.6, (Ar–C[combining low line]) 129.1, (Ar–C[combining low line]) 131.5, (Ar–C[combining low line]) 134.0, (Ar–C[combining low line]) 147.5, (Ar–C[combining low line]) 150.7, (C11b–Cβ) 165.5, (C O) 197.0; LC-MS m/z: 424 [M + H]+; anal. calcd for C26H21N3O3: C, 73.74; H, 5.00; N, 9.92. Found: C, 71.95; H, 4.84; N, 9.83.
4.4.13. 12-[1-(4-Methoxy-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7m)
Yield 88%, mp: 215–217 °C; Rf = 0.48 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3188, 3070, 2939, (C O) 1649, (C C)Aromatic 1595, 1504, (C–O–C) 1232 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.08–2.15 (m, 2H), 2.35–2.42 (m, 2H), 2.65–2.74 (m, 2H), 3.80 (s, 3H, –OCH3), 6.01 (s, 1H, CH), 7.40–7.45 (m, 7H, Ar–H), 7.77–7.80 (d, J = 8.2 Hz, 2H), 8.03–8.05 (d, J = 8.4 Hz, 1H), 8.12 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.3, (C8) 26.4, (C10) 27.7, (C12) 36.8, (Ar–O–C[combining low line]H3) 51.3, (C7b–Cα) 112.6, (Ar–C[combining low line]) 114.8, (Ar–C[combining low line]) 114.9, (Ar–C[combining low line]) 117.2, (Ar–C[combining low line]) 119.5, (Ar–C[combining low line]) 122.7, (Ar–C[combining low line]) 122.9, (Ar–C[combining low line]) 125.0, (Ar–C[combining low line]) 125.7, (Ar–C[combining low line]) 127.1, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.3, (Ar–C[combining low line]) 130.7, (Ar–C[combining low line]) 131.3, (Ar–C[combining low line]) 137.7 (Ar–C[combining low line]) 147.8, (Ar–C[combining low line]) 151.4, (C11b–Cβ) 167.2, (C O) 197.2; LC-MS m/z: 424 [M + H]+; anal. calcd for C26H21N3O3: C, 73.74; H, 5.00; N, 9.92. Found: C, 71.96; H, 4.86; N, 9.81.
4.4.14. 12-[1-(4-Hydroxy-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7n)
Yield 85%, mp: 171–173 °C; Rf = 0.25 (EtOAc : n-hexane 2 : 3); IR (KBr): (O–H)Aromatic 3440, ( C–H)Aromatic 3042, (C O) 1635, (C C)Aromatic 1590, 1509, (C–O–C) 1197 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.08–2.12 (m, 2H), 2.39–2.42 (m, 2H), 2.70–2.76 (m, 2H), 5.82 (s, 1H, CH), 7.40–7.52 (m, 6H), 7.89–7.92 (d, J = 8.4 Hz, 2H), 8.27–8.29 (d, J = 8.2 Hz, 2H), 8.55 (s, 1H, triazole H), 9.92 (s, 1H, Ar–OH); 13C NMR (100 MHz, CDCl3): δ (C9) 20.5, (C8) 24.5, (C10) 27.9, (C12) 39.5, (C7b–Cα) 119.7, (Ar–C[combining low line]) 115.1, (Ar–C[combining low line]) 117.4, (Ar–C[combining low line]) 119.6, (Ar–C[combining low line]) 121.5, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 128.5, (Ar–C[combining low line]) 129.2, (Ar–C[combining low line]) 129.6, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 131.4, (Ar–C[combining low line]) 134.5, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 151.0, (C11b–Cβ) 167.1, (C O) 197.2; LC-MS m/z: 410 [M + H]+; anal. calcd for C25H19N3O3: C, 73.34; H, 4.68; N, 10.26. Found: C, 72.91; H, 4.49; N, 10.13.
4.4.15. 12-[1-(3-Trifluoromethyl-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7o)
Yield 86%, mp: 162–165 °C; Rf = 0.39 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3040, (C O) 1640, (C C)Aromatic 1590, 1505, (C–O–C) 1193 cm–1; 1H NMR (400 MHz, CDCl3): δ 2.04–2.17 (m, 2H), 2.47–2.51 (m, 2H), 2.72–2.84 (m, 2H), 6.01 (s, 1H, CH), 7.26–7.50 (m, 3H), 7.59–7.64 (m, 2H), 7.79–7.83 (t, J = 8.1 Hz, 2H), 7.89–7.92 (m, 2H), 7.98 (d, J = 8.2 Hz, 1H), 8.01 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.4, (C8) 26.4, (C10) 27.7, (C12) 36.9, (C7b–Cα) 112.8, (Ar–C[combining low line]) 114.9, (Ar–C[combining low line]) 117.1, (Ar–C[combining low line]) 117.3, (Ar–C[combining low line]) 119.3, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 123.4, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 125.0, (Ar–C[combining low line]) 127.1, 1(Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.3, (Ar–C[combining low line]) 130.3, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 131.4, (Ar–C[combining low line]) 137.4, (Ar–C[combining low line]) 147.9, (Ar–C[combining low line]) 151.1, (C11b–Cβ) 167.2, (C O) 197.3; LC-MS m/z: 462 [M + H]+; anal. calcd for C26H18F3N3O2: C, 67.68; H, 3.93; N, 9.11. Found: C, 67.48; H, 3.68; N, 9.01.
4.4.16. 12-[1-(4-Nitro-phenyl)-1H-[1,2,3]triazol-4-yl]-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one (7p)
Yield 86%, mp: 179–182 °C; Rf = 0.33 (EtOAc : n-hexane 2 : 3); IR (KBr): ( C–H)Aromatic 3048, (C O) 1638, (C C)Aromatic 1592, 1507, (C–O–C) 1190 cm–1; 1H NMR (400 MHz, CDCl3): δ 1.98–2.19 (m, 2H), 2.45–2.56 (m, 2H), 2.75–2.83 (m, 2H), 5.91 (s, 1H, CH), 7.32–7.59 (m, 6H, Ar–H), 7.62–7.65 (d, J = 8.2 Hz, 2H), 7.78–7.81 (d, J = 8.4 Hz, 2H), 8.10 (s, 1H, triazole H); 13C NMR (100 MHz, CDCl3): δ (C9) 20.1, (C8) 24.5, (C10) 27.7, (C12) 39.5, (C7b–Cα) 112.8, (Ar–C[combining low line]) 115.0, (Ar–C[combining low line]) 117.3, (Ar–C[combining low line]) 119.2, (Ar–C[combining low line]) 121.4, (Ar–C[combining low line]) 123.0, (Ar–C[combining low line]) 124.9, (Ar–C[combining low line]) 128.6, (Ar–C[combining low line]) 129.0, (Ar–C[combining low line]) 129.7, (Ar–C[combining low line]) 131.0, (Ar–C[combining low line]) 131.2, (Ar–C[combining low line]) 134.0, (Ar–C[combining low line]) 147.8, (Ar–C[combining low line]) 150.9, (C11b–Cβ) 167.2, (C O) 197.3; LC-MS m/z: 439 [M + H]+; anal. calcd for C25H18N4O4: C, 68.49; H, 4.14; N, 12.78. Found: C, 67.94; H, 4.01; N, 11.99.
Acknowledgments
Authors are grateful to The Head, Department of Chemistry, and Director, Central Facilities for Research and Development (CFRD), Osmania University, Hyderabad for providing laboratory facilities. One of the authors, GLG, is grateful to UGC, New Delhi, for an award of JRF.
Supplementary Material
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00593d
References
- World Health Organization, Global Tuberculosis Report, 2015.
- Sethukumar A., Vithya V., Udhaya Kumar C., Arul Prakasam B. J. Mol. Struct. 2012;8:1008. [Google Scholar]
- Peres V., Nagem T. J., Faustino de Oliveira F. Phytochemistry. 2000;55:683. doi: 10.1016/s0031-9422(00)00303-4. [DOI] [PubMed] [Google Scholar]
- Schwaebe M. K., Moran T. J., Whitten J. P. Tetrahedron Lett. 2005;46:827. [Google Scholar]
- Kenji M., Yukihiro A., Hong Y., Kenji O., Tetsuro I., Toshiyuki T., Emi K., Munekazu I., Yoshinori N. Bioorg. Med. Chem. 2004;12:5799. [Google Scholar]
- Mahaburarkam W., Nuangnaowarat W., Taylor W. Phytochemistry. 2006;67:470. doi: 10.1016/j.phytochem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Mukherjee A. K. Biotech. Histochem. 1981;56:83. [Google Scholar]
- Knight C. G., Stephens T. Biochem. J. 1989;258:683. doi: 10.1042/bj2580683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkencioglu O., Talinli N., Akar A. J. Chem. Res. 1995;12:502. [Google Scholar]
- Hideo T. and Teruomi J., JP Pat., 56.005.480, 1981.
- Ion R. M., Planner A., Wiktorowicz K., Frackowiak D. Acta Biochim. Pol. 1998;45:833. [PubMed] [Google Scholar]
- Nilkanth G., Aher V., Pore S., Nripendra N., Kumar A., Shukla P. K., Sharma A., Bhat M. K. Bioorg. Med. Chem. Lett. 2009;19:759. doi: 10.1016/j.bmcl.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Wang X. L., Wan K., Zhou C. H. Eur. J. Med. Chem. 2010;45:4631. doi: 10.1016/j.ejmech.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Buckle D. R., Outred D. R. X., Rockell C. J. M., Smith H., Spicer B. A. J. Med. Chem. 1983;26:251. doi: 10.1021/jm00356a025. [DOI] [PubMed] [Google Scholar]
- Cunha A. C., Figueiredo J. M., Tributino J. L. M., Miranda A. L. P., Castro H. C., Zingali R. B., Fraga C. A. M., de Souza M. C. B. V., Ferreira V. F., Barreiro E. J. Bioorg. Med. Chem. 2003;11:2051. doi: 10.1016/s0968-0896(03)00055-5. [DOI] [PubMed] [Google Scholar]
- Whiting M., Tripp J. C., Lin Y. C., Lindstrom W., Olson A. J., Elder J. H., Sharpless K. B., Fokin V. V. J. Med. Chem. 2006;4:7697. doi: 10.1021/jm060754+. [DOI] [PubMed] [Google Scholar]
- Giffin M. J., Heaslet H., Brik A., Lin Y. C., Cauvi G., Wong C. H., McRee D. E., Elder J. H., Stout C. D., Torbett B. E. J. Med. Chem. 2008;51:6263. doi: 10.1021/jm800149m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi G., Dell'Omodarme G., Ferretti M., Giorgi I., Livi O., Scartoni V. Farmaco. 1990;45:1181. [PubMed] [Google Scholar]
- Biagi G., Livi O., Scartoni V., Lucacchini A., Mazzoni M. R. Farmaco. 1986;41:597. [PubMed] [Google Scholar]
- Kelley J. L., Koble C. S., Davis R. G., McLean E. W., Soroko F. E. B., Cooper R. J. Med. Chem. 1995;38:4131. doi: 10.1021/jm00020a030. [DOI] [PubMed] [Google Scholar]
- Micetich R. G., Maiti S. N., Spevak P., Hall T. W., Yamabe S., Ishida N., Tanaka M., Yamazaki T., Nakai A., Ogawa K. J. Med. Chem. 1987;30:1469. doi: 10.1021/jm00391a032. [DOI] [PubMed] [Google Scholar]
- Alvarez R., Velazquez S., San-Felix A., Aquaro S., De Clercq E., Perno C. F., Karlsson A., Balzarini J., Camarasa M. J. J. Med. Chem. 1994;37:4185. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- Costa M. S., Boechat N., Rangel E. A., Silva F. D. C. D., Souza A. M. T. D., Rodrigues C. R., Castro H. C., Junior I. N., Lourenco M. C. S., Wardell S. M. S. V., Ferreira V. F. Bioorg. Med. Chem. 2006;14:8644. doi: 10.1016/j.bmc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Patpi S. R., Pulipati L., Yogeeswari P., Sriram D., Jain N., Sridhar B., Murthy R., Anjana D. T., Kalivendi S. V., Kantevar S. J. Med. Chem. 2012;55:3911. doi: 10.1021/jm300125e. [DOI] [PubMed] [Google Scholar]
- Handbook of Combinatorial Chemistry, ed. K. C. Nicolaou, R. Hanko and W. Hartwig, Wiley-Germany, 2002, vol. 2, p. 885. [Google Scholar]
- Yempala T., Sridevi J. P., Yogeeswari P., Sriram D., Kantevari S., Patpi S. R., Pulipati L., Yogeeswari P., Sriram D., Jain N., Sridhar B., Murthy R., Anjana Devi T., Kalivendi S. V., Kantevari S., Rangappa S. K., Siddappa A. P., Srinivasa B., Bhari M. N., Surineni G., Yogeeswari P., Sriram D., Kantevari S. Eur. J. Med. Chem. J. Med. Chem. Chem. Biol. Drug Des. Med. Chem. Res. 2014;2012;2015;2015;71558624(4):160. 3911, 410, 1298. doi: 10.1016/j.ejmech.2013.10.082. [DOI] [PubMed] [Google Scholar]
- Ashok D., Mohan Gandhi D., Srinivas G., Vikas Kumar A. Med. Chem. Res. 2014;23:3005. [Google Scholar]
- Ashok D., Hanumantha Rao V., Sreenivas P. Heterocycl. Commun. 2013;19(5):363. [Google Scholar]
- Linga Goud G., Ramesh S., Ashok D., Prabhakar Reddy V. Russ. J. Gen. Chem. 2015;85:673. [Google Scholar]
- Ramesh S., Ashok D., Linga Goud G., Prabhakar Reddy V. Russ. J. Gen. Chem. 2014;84:1608. [Google Scholar]
- Richardson S. K., Jeganathan A., Mani R. S., Haley B. E., Watt D. S., Trusal L. R. Tetrahedron. 1987;43:2925. [Google Scholar]
- Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B. Angew. Chem., Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Karabulut H. R. F., Kac-an M. Turk. J. Chem. 2003;27:713. [Google Scholar]
- Ganesh C. N., Ram Kumar S. S., Singh M. S. Tetrahedron. 2009;65:7129. [Google Scholar]
- Cruickshank R., Duguid J. P. and Marion S. R. H. A., Med. Microbiology, Churchill Livingstone, London, 12th ed, 1975, vol. II, p. 196. [Google Scholar]
- Arnott J. A., Kumar R., Planey S. L. J. Appl. Biopharm. Pharmacokinet. 2013;1:31. [Google Scholar]
- Liu X., Testa B., Fahr A. Pharm. Res. 2011;28:962. doi: 10.1007/s11095-010-0303-7. [DOI] [PubMed] [Google Scholar]
- Gerlier D., Thomasset N. J. Immunol. Methods. 1986;94:57. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- Merkel W. K., Nichols B. P. FEMS Microbiol. Lett. 1996;143:247. doi: 10.1111/j.1574-6968.1996.tb08488.x. [DOI] [PubMed] [Google Scholar]
- Zheng R., Blanchard J. S. Biochemistry. 2001;40:12904. doi: 10.1021/bi011522+. [DOI] [PubMed] [Google Scholar]
- Wang S., Eisenberg D. Protein Sci. 2003;12:1097. doi: 10.1110/ps.0241803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung A. W., Silvestre H. L., Wen S., Ciulli A., Blundell T. L., Abell C. Angew. Chem., Int. Ed. 2009;48:8452. doi: 10.1002/anie.200903821. [DOI] [PubMed] [Google Scholar]
- Schrodinger Suite 2011 Induced Fit Docking protocol, Glide, version 5.6, LLC, New York, NY, 2011.
- Schrodinger Suite 2011 Standard Precision Docking protocol, Glide, version 5.6, LLC, New York, NY, 2011.
- Ganesh S., Brindha P. D., Radhika N., Sridevi J. P., Shalini S., Yogeeswari P., Sriram D. Bioorg. Med. Chem. 2014;22:1938. doi: 10.1016/j.bmc.2014.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.