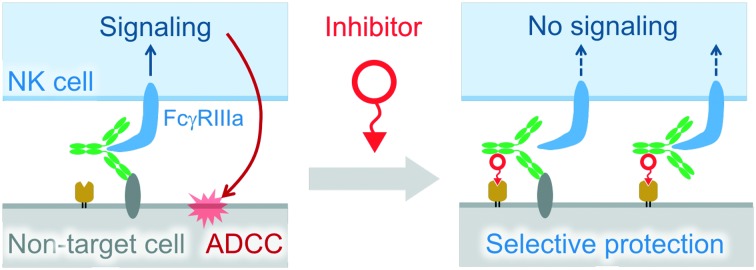
 ADCC is caused by NK cells upon recognition of antigen-bound IgG via FcγRIIIa.
ADCC is caused by NK cells upon recognition of antigen-bound IgG via FcγRIIIa.
Abstract
Antibody-dependent cell-mediated cytotoxicity (ADCC) is caused by natural killer (NK) cells upon recognition of antigen-bound IgG via FcγRIIIa. This mechanism is crucial for cytolysis of pathogen-infected cells and monoclonal antibody (mAb)-mediated elimination of cancer cells. However, there is concern that mAb-based cancer therapy induces ADCC against non-target cells expressing antigens. To date, no strategy has been reported to enhance the selectivity of ADCC to protect non-target cells expressing antigens. Here, we introduce a model inhibitor which specifically blocks ADCC of anti-EGFR mAbs towards EGFR/folate receptor α (FRα) double positive cells. This inhibitor recruits mAbs on the FRα of the cell surface independent of Fab antigen recognition. The resulting ternary and/or quaternary complexes formed on the cell surface suppress signal transduction of FcγRIIIa in NK cells, consequently leading to more specific ADCC.
Natural killer (NK) cells recognize IgGs that bind to antigens on target cells via FcγRIIIa to induce antibody-dependent cell-mediated cytotoxicity (ADCC). Despite the importance of ADCC in biological defense and monoclonal antibody (mAb)-based cancer therapy,1,2 it has the following negative aspects. ADCC caused by autoantibodies is related to progression of autoimmune diseases.3 In addition, a study has indicated that mAbs are potentially harmful by inducing ADCC against non-target cells expressing antigens.4 Glucocorticoids and immunophilin ligands which are used to suppress the infusion reaction of mAb-based therapy could be options to avoid undesired ADCC.5–7 However, these drugs inactivate various kinds of immune cells as well as NK cells, leading to susceptibility to pathogenic infection and reduced anti-cancer effects.7 Thus, specific inhibitors of ADCC for non-target cells which express target antigens are preferable for this application. In our study, we serendipitously found a model inhibitor of ADCC in specific types of cells.
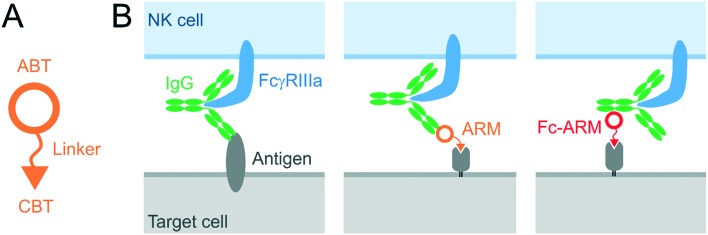
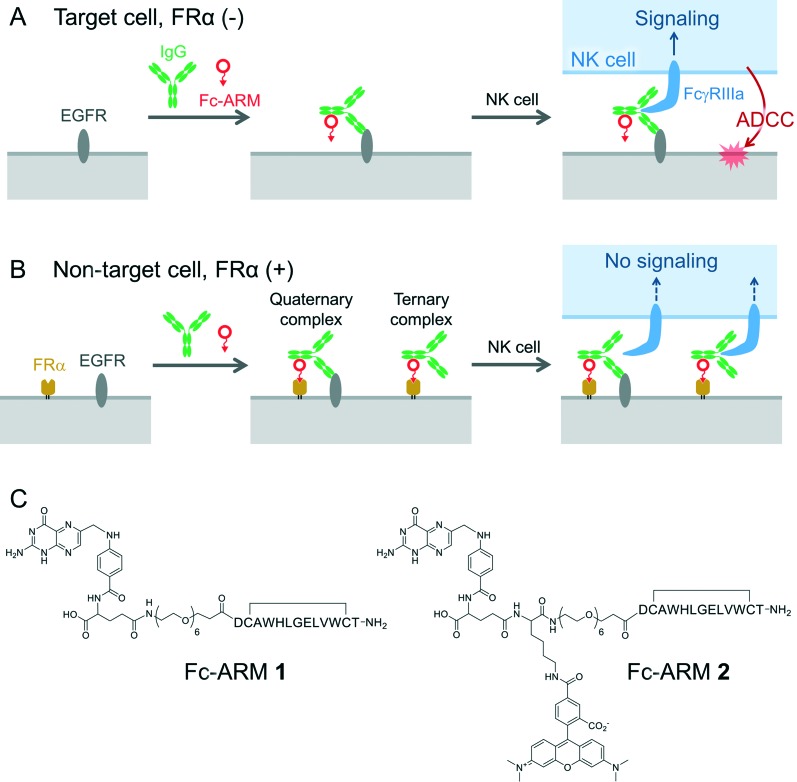
Originally, we intended to develop a new class of antibody-recruiting molecules (ARMs), a bispecific small molecule capable of redirecting antibodies toward target cells to induce ADCC (Fig. 1B, middle panel), which was first defined by Spiegel's group.8,9 An ARM consists of a cell-binding terminus (CBT) and an antibody-binding terminus (ABT) that binds to the Fab of an antibody (Fig. 1A). Different from the original ARM, our ARM was designed to bind to the Fc region of an antibody by employing an Fc-binding cyclic peptide (Fc-III)10 as the ABT (Fig. 1B, right). The binding interface of the Fc with Fc-III does not overlap with that of IgG with FcγRIIIa. Therefore, the recruited IgG should be accessible to FcγRIIIa (Fig. S2†). We named our molecule Fc-ARM. Folate was selected as the CBT of Fc-ARM, which connected to the ABT via an oligoethyleneglycol linker (Fig. 2C). The length of the linker (2.7 nm) is sufficiently longer than the estimated closest distance between the ABT and the CBT (1.1 nm) (Fig. S2†). Fc-ARM was designed to crosslink IgG and folate receptor α (FRα) on the cell surface to potentially generate two kinds of complexes (ternary and quaternary) (Fig. 2B). We found that these complexes could not induce ADCC, but conversely suppressed ADCC if the target cells expressed FRα.
Fig. 1. (A) Schematic representation of the antibody-recruiting molecule (ARM). The ARM consists of a cell-binding terminus (CBT) and an antibody-binding terminus (ABT). (B) Comparison of three kinds of IgG recruitment to a target cell via regular Fab binding to an antigen (left), ARM-mediated recruitment proposed by Spiegel's group (middle), and Fc-ARM-mediated recruitment examined in this study (right).
Fig. 2. Fc-ARM determines either the induction of ADCC in a FRα-negative cell (A) or the specific inhibition of ADCC against FRα-positive cells (B). Chemical structure of Fc-ARMs (C).
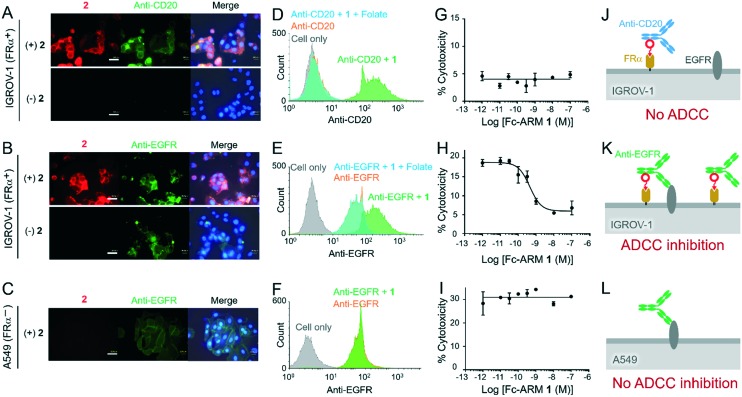
First, we confirmed that Fc-ARM recruited IgG to the cell surface to form the complexes. We used IGROV-1 cells [epidermal growth factor receptor (EGFR)+ FRα+ CD20–]. While the anti-CD20 mAb (ofatumumab) did not bind to the IGROV-1 cells (Fig. 3A, lower panels), the presence of Fc-ARM 2 resulted in accumulation of the anti-CD20 mAb on the cell surface (Fig. 3A, upper panels). Colocalization of mAb fluorescence with Fc-ARM indicated ternary complex formation on the cell surface (Fig. 3J). Flow cytometric analysis also confirmed the recruitment of the anti-CD20 mAb via Fc-ARM 1 (Fig. 3D). The addition of an excess amount of folate resulted in dissociation of the ternary complex from the cell surface (Fig. 3D). Conversely, anti-EGFR mAbs (cetuximab) bound to the IGROV-1 cell surface without Fc-ARM (Fig. 3B, lower panels), while the presence of Fc-ARM resulted in enhanced accumulation of the mAb (Fig. 3E). Next, we added an excess amount of anti-EGFR mAbs (100 nM), which was much higher than the saturation concentration of its binding to IGROV-1 cells (ca. 1 nM, Fig. S3†). Thus, the increased accumulation of anti-EGFR mAbs by the addition of Fc-ARM 1 indicated the presence of the ternary complex independent of Fab-EGFR recognition (Fig. 3K). The addition of an excess amount of folate reduced the accumulation of mAbs (Fig. 3E), indicating the dissociation of the ternary complex from the cell surface. In the case of A549 cells (EGFR+ FRα– CD20–), the presence of Fc-ARM did not change the accumulated amount of anti-EGFR mAbs, because no ternary complex was formed owing to the absence of FRα (Fig. 3C, F and L).
Fig. 3. Accumulation of mAbs on the surface of IGROV-1 and A549 cells was evaluated by fluorescence microscopy (A–C) and flow cytometry (D–F). ADCC inhibition by Fc-ARM was evaluated (G–I). Schematic representation of expected complexes existing on the cell surface (J–L). IGROV-1 was treated with anti-CD mAbs (A, D, G and J) or anti-EGFR mAbs (B, E, H and K), respectively. A549 was treated with anti-EGFR mAbs (C, F, I and L). In the evaluation of ADCC, IGROV-1 cells were treated with various concentrations of Fc-ARM 1 in the presence of 100 nM anti-CD20 mAbs (G) or anti-EGFR mAbs (H) and then mixed with NK cells (effector : target ratio = 8 : 1). A549 cells were treated similarly using the anti-EGFR mAbs (I). Cytotoxicity was calculated by quantitating the amount of LDH released from the targets (n = 3, mean ± SD). The concentrations of Fc-ARM and mAbs are 100 nM and 500 nM under microscopic observation (A–C) and 10 nM and 100 nM in flow cytometry (D–F), respectively. Nuclei were stained using Hoechst33342. Scale bars in the microscopy images are 40 μm.
We next examined ADCC against these two cell lines that were either FRα positive or negative. In this experiment, we used the NK cell line KHYG-1 expressing FcγIIIa (158 V).11 Both IGROV-1 and A549 cells were killed by NK cells via ADCC in the presence of the anti-EGFR mAbs (100 nM). Surprisingly, the ADCC of IGROV-1 cells was suppressed by Fc-ARM 1 in a concentration-dependent manner (Fig. 3H), while Fc-ARM 1 had no effect on the ADCC of A549 cells (Fig. 3I). The IC50 of Fc-ARM 1 was quite small (0.4 nM, Fig. 3H), indicating its excellent inhibitory activity. The inhibitory effect of Fc-ARM 1 was reversed by an excess of competing ligands (folate or Fc-III (Fig. S4†)). Based on these results, it is clear that the inhibition of ADCC by Fc-ARM 1 is specific for FRα-positive cells, and the ternary and/or quaternary complexes formed on the FRα-positive cells are responsible for the inhibition.
In the case of the anti-CD20 mAb, it was recruited on the surface of IGROV-1 cells as the ternary complex by Fc-ARM (Fig. 3A, D and J), but the complex did not induce ADCC (Fig. 3G). This result indicates that the ternary complex cannot activate NK cells through FcγRIIIa. We further examined phosphorylation of extracellular signal-regulated kinase (ERK) resulting from FcγRIIIa signaling12,13 to confirm the inability of the ternary complex to activate NK cells. To isolate the ERK activation of NK cells from that of cancer cells, we used micron-sized particles instead of cancer cells.14 The particle immobilized with IgG (particle a) was used as a model of IgG-bound cells, and the Fc-III-immobilized particle (particle b) mixed with IgG was used as a model of ternary complex-presenting cells. Particle a activated the ERK signaling of NK cells in a dose-dependent manner, while particle b coated with IgG did not (Fig. S5†). These results demonstrate that the ternary complex cannot induce activation signaling via FcγRIIIa.
The reason for the impaired signaling of the ternary complex may be the lack of Fab–antigen interaction to induce a favorable conformational change in the mAb to bind tightly with FcγRIIIa.15 This inability of the ternary complex may be the mechanism of ADCC inhibition observed in Fig. 3H. The other possible mechanism is that the quaternary complex is difficult to be recognized by FcγRIIIa, which is possibly due to steric hindrance because of its crowded structure.
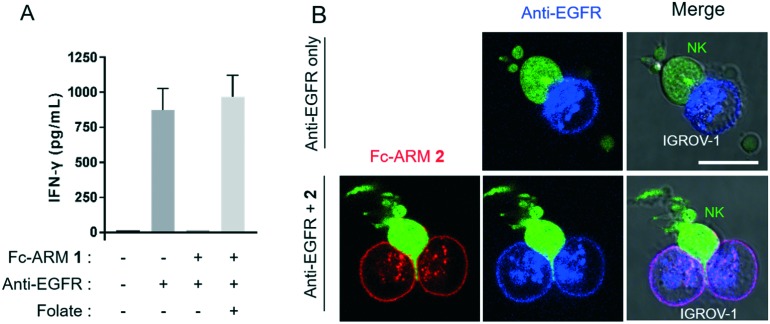
The inhibitory effect of Fc-ARM 1 was further investigated by detecting interferon-γ (IFN-γ) secretion from NK cells, which is also induced by FcγRIIIa signaling.16 As summarized in Fig. 4A, IGROV-1 cells bound with the anti-EGFR mAb induced secretion of IFN-γ from NK cells. However, in the presence of Fc-ARM, secretion of IFN-γ became negligible. The addition of excess folate recovered the secretion of IFN-γ completely. Taken together, these results showed that FcγRIIIa-induced signaling is efficiently suppressed by complex formation with Fc-ARM.
Fig. 4. (A) IFN-γ secretion from NK cells was suppressed by Fc-ARM. IGROV-1 cells coated with the anti-EGFR mAb (100 nM) and Fc-ARM 1 (10 nM) were co-incubated with NK cells (NK : IGROV-1 ratio = 1 : 1). Excess folate (10 μM) was added to suppress the complex formation on the cell surface. The amount of IFN-γ secreted from NK cells was quantitated by ELISA. (B) CLSM images of the recognition of anti-EGFR mAb-coated IGROV-1 cells by NK cells in the presence or absence of Fc-ARM 2. NK cells were transfected with ZsGreen. SeTau-647-labeled anti-EGFR mAb was used. The scale bars are 20 μm.
Finally, we clarified the effect of Fc-ARM from the viewpoint of macroscopic recognition between the effector and target cells. It is known that NK cells tightly adhere to target cells prior to execution of ADCC.17 Then, activating receptors such as FcγRIIIa are concentrated at the adhesion interface. This interface is called the immune synapse, which induces intracellular signaling in NK cells.17Fig. 4B shows fluorescence images of immune synapse formation between NK cells and IGROV-1 cells in the presence of anti-EGFR mAbs. Accumulation of the mAbs at the interface was observed (Fig. 4B, upper panels), which is consistent with the typical observation for ADCC.18 Conversely, in the presence of Fc-ARM, both Fc-ARM and anti-EGFR mAbs were homogeneously distributed on the cell surface and their accumulation was not observed at the adhesion interface (Fig. 4B, lower panels). Taken together, these data indicated that Fc-ARM inhibited the maturation of immune synapse; consequently, NK cells were not activated.
In conclusion, we discovered that Fc-ARM serves as a specific inhibitor of ADCC induced by anti-EGFR mAbs if target cells are EGFR/FRα double positive. This means that Fc-ARM enhanced the selectivity of ADCC, which is desired to reduce adverse effects against non-target cells without secondary risks of pathogen infections or a decrease of therapeutic effects in mAb-based therapy. One of the typical side effects of anti-EGFR mAbs is toxicity to renal tubule epithelium,19 where FRα is also highly expressed.20 Thus, Fc-ARM may be useful to protect renal tubule epithelium from mAb-induced side effects. Further studies about the mechanism, generality, and limitations of this strategy are currently conducted by our group.
Methods
Cell culture
A549 (human lung carcinoma) cells were purchased from Riken. IGROV-1 cells (human ovarian carcinoma) were kindly provided by Dr. Takami Matsuyama. KHYG-1/FcγIIIa-158 V (human NK leukemia) cells were prepared in our previous paper.1 IGROV-1 cells were cultured in folate-free RPMI-1640 culture medium (Invitrogen). A549 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Wako). KHYG-1/FcγIIIa-158 V cells were cultured in RPMI-1640 medium (Nacalai Tesque) containing 10 ng mL–1 recombinant human IL-2 (PeproTech). All culture media contained 10% fetal bovine serum (FBS), 100 U mL–1 penicillin, 100 μg mL–1 streptomycin, and 0.25 μg mL–1 amphotericin B (all from Thermo Fisher Scientific). Cells were harvested in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C.
Fluorescence microscopy to confirm complex formation
IGROV-1 cells or A549 cells were seeded at 1 × 104 cells/well in a folate-free RPMI-1640 culture medium onto a 96-well glass bottom microplate, and incubated for 24 h. The cells were washed twice with 100 μL of DPBS, and then incubated for 30 min at 37 °C with 100 μL of folate-free RPMI-1640 culture medium containing 1% FBS in the presence of 100 nM Fc-ARM 2 and 500 nM fluorescein–anti-EGFR mAbs or fluorescein–anti-CD20 mAbs. For negative control experiments, folic acid and Fc-III were added as competitive inhibitors (final conc.: 10 μM and 200 μM, respectively). After washing and Hoechst 33342 staining of the cells, cellular fluorescence was observed using a BZ-8000 fluorescence microscope (Keyence).
Flow cytometry
IGROV-1 cells or A549 cells were harvested with Accutase (PAN-Biotech) and washed with folate-free RPMI-1640 medium containing 1% FBS. After cell counting, the IGROV-1 cells were re-suspended in folate-free RPMI-1640 medium containing 1% FBS to a density of 5 × 105 cells per mL. A 0.8 mL of this suspension was placed into a microtube. Fc-ARM 1 in medium (1.6 μL, 5 μM conc.) was added and the cells were incubated for 10 min on ice. Anti-EGFR mAbs or anti-CD20 mAbs (0.6 μL, 133 μM conc.) were added and the cells were further incubated for 30 min on ice (final conc. of Fc-ARM 1 and mAbs: 10 nM and 100 nM, respectively). The samples were diluted with DPBS (700 μL) and centrifuged (200 × g). The cells were washed with DPBS (1 mL) twice and suspended in DPBS (0.5 mL). The cells were analyzed using an EC800 cell analyzer (Sony).
Antibody-dependent cellular cytotoxicity (ADCC)
IGROV-1 cells in tissue culture dishes (Greiner Bio-One) were washed in 2 mL DPBS, detached with Accutase and washed with folate-free RPMI-1640 medium containing 1% FBS. After cell counting, IGROV-1 cells were re-suspended in the folate-free RPMI-1640 medium containing 1% FBS diluted to 1 × 105 cells per mL. This suspension was seeded into 96-well U-bottom plates (Greiner Bio-One) at 5000 cells per well (50 μL per well). Cetuximab (100 nM final concentration) or ofatumumab (100 nM final concentration) and Fc-ARM (50 μL, 10 nM final concentration) were added into the wells. The sample plates were incubated at 37 °C under 5% CO2 for 10 min. After incubation, KHYG-1/FcγIIIa-158 V was added (100 μL per well, effector : target ratio = 8 : 1) and the plates were centrifuged (200 × g, 5 min). After incubation at 37 °C under 5% CO2 for 4 h, the plates were centrifuged again (200 × g, 5 min) and 100 μL of the supernatant was transferred to a new 96-well plate (Thermo Fisher Scientific, 260836). 100 μL of the working solution (Cytotoxicity LDH Assay Kit-WST, Dojindo) was added to the wells and incubated at r.t. for 30 min in the dark. 50 μL of the stop solution (Cytotoxicity LDH Assay Kit-WST, Dojindo) was added to the wells, and the absorbance at 490 nm was measured using a Wallac 1420 ARVOsx (PerkinElmer). To achieve maximum release of LDH, target cells were incubated with 200 μL of folate-free RPMI-1640 medium containing 1% FBS and 1% Triton X-100. LDH spontaneous release was achieved by incubating the cells with 200 μL of folate-free RPMI-1640 medium containing 1% FBS. % cytotoxicity was calculated using the following formula: % cytotoxicity = (Abssample – Abseffectorspontaneous – Abstargetspontaneous) × 100/(Abstargetmax – Abstargetspontaneous)
Control experiments were conducted in the absence of Fc-ARM or non-specific antibodies. For competition experiments, Fc-III or folic acid was added simultaneously with mAbs and Fc-ARM (final conc. = 20 μM or 1 μM, respectively).
Enzyme-linked immunosorbent assay
IGROV-1 cells in tissue culture dishes were washed with DPBS (2 mL), detached with Accutase and washed with folate-free RPMI-1640 medium containing 1% FBS. After cell counting, IGROV-1 cells were re-suspended in folate-free RPMI-1640 medium containing 1% FBS diluted to 2 × 106 cells per mL. This suspension was seeded into 96-well U-bottom plates (50 μL per well). Cetuximab (100 nM final concentration) and Fc-ARM (10 nM final concentration) were added into the wells (50 μL per well). The sample plates were incubated at 37 °C under 5% CO2 for 10 min. After incubation, KHYG-1/FcγIIIa-158 V (suspended in folate-free RPMI-1640 medium containing 1% FBS, without rhIL-2) was added (100 μL per well, effector : target ratio = 1 : 1) and the plates were centrifuged (200 × g, 5 min). After 24 hours of incubation (at 37 °C, 5% CO2), the sample plates were centrifuged again. The cell supernatant was collected and human IFN-γ was quantitated by using Human IFN-γ ELISA MAX™ Deluxe (BioLegend).
Fluorescence microscopy to observe cell–cell adhesion
IGROV-1 cells were washed, detached and prepared for imaging by seeding 2000 cells per well into each well of a 96-well glass bottom plate. SeTau-647-Cetuximab (100 nM final concentration) was added to the wells in combination with rhodamine-modified Fc-ARM 2 (final concentration: 10 nM). The sample plates were incubated at 37 °C under 5% CO2 for 30 min. Then, KHYG-1/FcγIIIa-158 V cells were seeded at 6000 cells per well. The sample plates were further incubated for 1 h and the cells were observed by confocal laser scanning microscopy (Zeiss LSM 700).
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We thank Dr. Y. Harada, Dr. Y. Yonemitsu, Dr. Y. Hayakawa, and Dr. J. Hubbell for fruitful discussions. We thank Dr. T. Matsuyama for sharing the IGROV-1 cell line. We also thank Dr. M. Goto and Dr. N. Kamiya for the usage of the flow cytometer and CLSM.
This work was supported by the Challenging Research (project No. 17K19204) of MEXT, Japan. K. Sasaki thanks JSPS (17J05032) for a fellowship.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8md00010g
References
- Grier J. T., Forbes L. R., Monaco-Shawver L., Oshinsky J., Atkinson T. P., Moody C., Pandey R., Campbell K. S., Orange J. S. J. Clin. Invest. 2012;122:3769–3780. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerey C., Huntington N. D., Smyth M. J. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- Schleinitz N., Harle J. Immunology. 2010;131:451–458. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel T. T., Kropshofer H., Singer T., Mitchell J. A., George A. J. T. Nat. Rev. Drug Discovery. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- Rose A. L., Smith B. E., Maloney D. G. Blood. 2002;100:1765–1773. [PubMed] [Google Scholar]
- Shin B. H., Ge S., Mirocha J., Karasyov A., Vo A., Jordan S. C., Toyoda M. Transplantation. 2014;97:294–300. doi: 10.1097/01.TP.0000438636.52085.50. [DOI] [PubMed] [Google Scholar]
- Kumai T., Oikawa K., Aoki N., Kimura S., Harabuchi Y., Kobayashi H. Head Neck. 2016;38:410–416. doi: 10.1002/hed.23906. [DOI] [PubMed] [Google Scholar]
- Murelli R. P., Zhang A. X., Michel J., Jorgensen W. L., Spiegel D. A. J. Am. Chem. Soc. 2009;131:17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnaney P. J., Parker C. G., Zhang A. X., Spiegel D. A. ACS Chem. Biol. 2012;7:1139–1151. doi: 10.1021/cb300119g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano W. L., Ultsch M. H., de Vos A. M., Wells J. A. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Terui Y., Mishima Y., Kuniyoshi R., Matsusaka S., Mikuniya M., Kojima K., Hatake K. Int. Immunol. 2012;24:477–483. doi: 10.1093/intimm/dxs048. [DOI] [PubMed] [Google Scholar]
- Trotta R., a Puorro K., Paroli M., Azzoni L., Abebe B., Eisenlohr L. C., Perussia B. J. Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Kondadasula S. V., Roda J. M., Parihar R., Yu J., Lehman A., Caligiuri M. A., Tridandapani S., Burry R. W., Iii W. E. C. Blood. 2008;111:4173–4184. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano C., Romanelli M., Pighi C., Cimino G., Rago A., Molfetta R., Paolini R., Santoni A. Cancer Res. 2015;75:4097–4109. doi: 10.1158/0008-5472.CAN-15-0781. [DOI] [PubMed] [Google Scholar]
- Woof J. M., Burton D. R. Nat. Rev. Immunol. 2004;4:1–11. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- Roda J. M., Joshi T., Butchar J. P., McAlees J. W., Lehman A., Tridandapani S., Carson 3rd W. E. Clin. Cancer Res. 2007;13:6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- Orange J. S. Nat. Rev. Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlawska-Wasowska K., Ward E., Stevens S., Wang Y., Herbst R., Winter S. S., Wilson B. S. Leukemia. 2013;27:1263–1274. doi: 10.1038/leu.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmai L., Gallieni M., Porta C. J. Nephrol. 2015;28:647–657. doi: 10.1007/s40620-015-0226-9. [DOI] [PubMed] [Google Scholar]
- Elnakat E., Ratnam M. Adv. Drug Delivery Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.