Abstract
Introduction:
Traditional inactivated and protein vaccines generate strong antibodies, but struggle to generate T cell responses. Attenuated pathogen vaccines generate both, but risk of causing the disease they aim to prevent. Newer gene-based vaccines drive both responses and avoid the risk of infection. While these vaccines work well in small animals, they can be weak in humans because they do not replicate antigen genes like more potent replication-competent (RC) vaccines. RC vaccines generate substantially stronger immune responses, but also risk causing their own infections. To circumvent these problems, we developed single-cycle adenovirus (SC-Ad) vectors that amplify vaccine genes, but that avoid the risk of infection. This review will discuss these vectors and their prospects for use as vaccines.
Areas covered:
This review provides a background of different types of vaccines. The benefits of gene-based vaccines and their ability to replicate antigen genes are described. Adenovirus vectors are discussed and compared to other vaccine types. Replication-defective, single-cycle, and replication-competent Ad vaccines are compared.
Expert commentary:
The potential utility of these vaccines are discussed when used against infectious diseases and as cancer vaccines. We propose a move away from replication-defective vaccines towards more robust replication-competent or single-cycle vaccines.
Keywords: Vaccines, gene-based vaccines, adenovirus, replicating
1. Introduction
1.1. Goals for Vaccines.
One simple goal for a vaccine is to produce antibodies that can neutralize pathogens or flag them for destruction by the immune system. A second goal is to drive strong T cell responses to support antibody production and to kill any intracellular viral or bacterial pathogens that have escaped the antibodies.
1.2. Inactivated and Attenuated Whole Pathogen Vaccines.
Towards these goals, vaccines have historically been made first by inactivating the pathogen or attenuating it (1). Inactivated vaccines are generally safe and drive strong antibody responses, but usually drive weak T cell responses. This can be improved by the use of adjuvants, but these can increase vaccine side effects. Attenuated pathogen vaccines are some of our most potent vaccines, since they drive both antibody and cellular immune responses. However, they also run the real risk of causing the disease that they aim to prevent. For example, there are polio virus outbreaks in Africa and other regions that were not caused by wild virus, but were actually caused by the oral polio vaccine (2)
1.3. Recombinant Protein Vaccines.
Proteins from pathogens can be used rather than the pathogen itself. This removes the risk of infection, but provide similar immune responses as inactivated vaccines. Protein vaccines are good at generating antibodies, but usually do not generate robust T cell responses.
1.4. Gene-based Vaccines.
In the late 1980’s and '90s, it became possible to use single genes from pathogens as vaccines that could drive both antibody and T cell responses. These approaches were called various names including: "genetic immunization" (3), "genetic vaccines" (3), "DNA vaccines" (4), "DNA-based vaccines" (5), "polynucleotide vaccines" (6), or "gene-based vaccines" (7).
Gene-based vaccines mimic attenuated vaccines since their delivered DNA or RNA is expressed by host cells to make vaccine antigens intracellularly (8-10). These in situ-produced proteins could be detected by B cells to drive antibody responses. Uniquely, these intracellular proteins could also be displayed on major histocompatibility molecules to drive both CD4 and CD8 T cell to support antibody production and to kill intracellular pathogens. Only one or a few pathogen genes are delivered. Therefore, the pathogen cannot be reconstructed and so there is no risk of infection with gene-based vaccines.
While gene-based vaccines worked well in small animals, they have been less impressive in humans, particularly in terms of driving robust CD8 T cell responses and neutralizing antibodies (11, 12)). Gene delivery and vaccine effects can be improved to some degree with methods like electroporation. However, these approaches have their own problems (e.g. using electrical shock in vaccine recipients).
1.5. Viral Vectors as Gene-based Vaccines.
When naked DNA efficacy did not meet expectations in non-human primates and in humans, many investigators began testing viral gene delivery vectors as vaccines (11, 12). Historically popular viral gene-based vaccine platforms include adenovirus (Ad) and pox virus vectors. More recent interest has been devoted to replication-competent viruses like cytomegalovirus (CMV) and vesicular stomatitis virus (VSV).
1.6. Amplifying Antigen Production and Immune Responses Using Viral Replication.
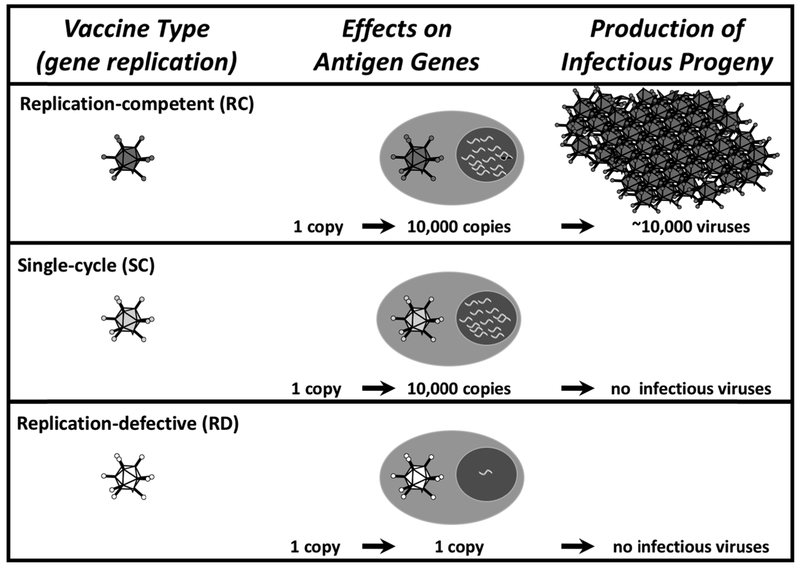
Wild viruses replicate (Fig. 1). One virus or DNA entering a cell delivers one copy of an antigen gene. A wild virus will replicate and amplify its genome up to 10,000 times (13). This not only amplifies its genome, but it also amplifies any antigen genes that it carries (14). These amplified genes can then be further amplified by transcription and translation within that infected cell. This translates into the production of massive amounts of vaccine antigens to more strongly drive T cell and antibody responses (15).
Figure 1:
Effects of Vector Genome Replication on Antigen Gene Amplification and Production of Infectious Progeny Viruses.
A truly wild virus will also replicate new progeny viruses. This can be either good or bad. The good is that these progeny viruses can go forth and infect more cells and further amplify antigen production and vaccine effects (15-17). The bad is that unchecked amplification of progeny viruses can actually cause the disease that the virus originally causes (2). But in this case, we would be intentionally injecting a disease-causing agent into healthy humans.
Plasmid DNA or a replication-defective (RD) vector will also deliver one copy of a vaccine gene into a cell (Fig. 1). However, these non-replicating vectors do not replicate antigen genes and do not amplify antigen production effects (15-17). The good is that RD vaccines are safe. The bad is that they are not very potent.
1.7. The Downside of Replicating Vectors in Humans.
Replication-competent vaccines not only amplify genes and immune responses, but they also produce infectious progeny viruses (Fig. 1). Depending on the virus, this may mediate no side effects or substantial ones. For example, the replication-competent rVSV-ZEBOV vaccine that was used after the Ebola virus outbreak in 2014 was associated with significant side effects including viral replication in peripheral tissues, rash, vasculitis, and dermatitis (18). Viremia was detected in 20% of volunteers receiving low-dose rVSV-ZEBOV (18) and 13.2% and 17.9% reported muscle pain and arthralgia (19).
Replicating CMV vaccines are also not without potential side effects. Human cytomegalovirus infects 60% to 100% of humans (20-22). It is able to establish latency, but is controlled by persistent and robust immune responses. CMV is the “C” in the TORCH anagram used by clinicians to remember which viruses cause congenital anomalies. Approximately 1 in 150 babies are born in the U.S. with congenital CMV infections (23-31)
Approximately, 8,000 of these babies will be born with microcephaly (similar to that caused by Zika virus), hearing or vision loss, seizures, and intellectual disabilities, and 400 may die (24, 25). Attenuated CMV vaccines are potent, but this may be related to their ability to establish latent infections. CMV vaccine safety in humans will likely be determined by which key CMV genes are deleted to attenuate the vaccines and if there is a risk of vertical transmission.
2. Adenovirus (Ad) Vaccines.
2.1. Ad Vaccines against Ad.
Natural “wild” RC-Ad infections are generally transient without lasting consequences (32). In contrast, RC-Ads can be dangerous in immunocompromised individuals and are associated with morbidity and death during stem cell and organ transplantation. For example, in the stem cell transplant patients, the incidence of Ad disease ranges from 3 to 47% (32). Ad infections also occur in liver transplant recipients, pediatric transplant recipients, albeit to a lesser degree than in the stem cell transplantation setting. Interestingly, CMV infections are actually more problematic in solid organ transplant recipients than Ad (33). Since VSV comes from farm animals, it is unclear how it may affect immunocompromised hosts.
Unlike VSV and CMV, “Wild" fully replication-competent Ads have been used as vaccines against respiratory adenovirus infections in nearly a half million military recruits (34). Safety monitoring of 100,000 recruits that received RC-Ad showed no significant greater risk of specified medical events within 6 weeks of vaccination (34). Psoriasis and serum reactions occurred more frequently in the vaccinated group (psoriasis occurred in 21 vs 7 vaccinated vs. control cases and serum reactions in 12 vs 4 vaccinated vs. control cases), but a causal relation of these rare events could not be established (34).
These data are for RC-Ad vector vaccines. Most Ad vector vaccines are E1 deleted RD-Ads that also have deletions in E3 immune evasion genes, making them likely to be safer than wild RC-Ad vector vaccines.
2.2. RD-Ad Vector Vaccines vs. Other Replicating and Non-replicating Vaccines.
Replication-defective Ad (RD-Ad) vectors are one of the most potent gene-based vaccine platforms (11, 35-37). In head to head comparisons, replication-defective RD-Ad vector vaccines are generally more robust than DNA vaccines or vaccinia vaccines (38).
These vaccine comparisons are not usually equal comparisons of different platforms. In most cases, two vectors are not compared based on the number of antigen genes injected. For example, one might compare 2 mg of DNA vaccine to 1011 viral particles of RD-Ad in a human. Under these conditions, 1 mg of DNA would deliver 1014 vaccine genes whereas the RD-Ad vaccine would deliver one thousand times less antigen genes. Of course, vaccine efficacy involves many complicated processes and sometimes unclear efficacy metrics, so relative vaccine efficacy is not measured only on a “per gene” basis. Yet under these apples and oranges comparisons RD-Ads remain more potent.
One indication of RD-Ad vaccine potency can be found in the response to the 2014 Ebola outbreak. Only two gene-based vaccines were tested in humans. One was a replication-competent VSV. The second was a RD-Ad vaccine. Both RD-Ad and VSV mediated significant immune responses (18, 39, 40). After considering phase I trial data VSV-ZEBOV was chosen as the better candidate for phase II and III testing over RD-Ad. This may suggest that VSV is generally a better vaccine than Ad. However, one vector replicates and the other does not. This is therefore a misleading vaccine comparison. A more appropriate one would be RC-Ad vs. VSV.
Similar comparisons are made in discussing promising CMV vaccines for HIV (41). CMV mediates substantial control of SIV in non-human primates (42) and these results are frequently compared in meetings to the results obtained when using plasmid DNA and/or RD-Ad vector vaccines in macaques.
Again, this is a misleading comparison as DNA and RD-Ad have no capacity to amplify SIV antigen genes and spread, whereas CMV replicates SIV antigens and actually becomes a latent virus in the host.
2.3. Head to Head Comparisons of Replicating and Non-Replicating Ad Vector Vaccines.
RD-Ads are potent when compared to other vaccines. However, these comparisons likely under-estimate Ad vaccine potency because RD-Ads the weakest version of all adenovirus vaccines.
This premise is supported by abundant data from Dr. Marjorie Guroff's group, our lab, and others (15-17, 43-55). For example, when chimpanzees were immunized twice with RD-Ads, this generated no detectable antibodies against HIV (15). In contrast, two immunizations with RC-Ads generated significant HIV antibodies. This is notable, since chimps can be nearly as large as humans.
We see similar effects when comparing replicating Ads vs. RD-Ads in mice, hamsters, cotton rats, and in rhesus macaques (16, 17, 55).
2.4. New Single-cycle Ad Vector Vaccines Amplify Antigen Genes without the Risk of Infection.
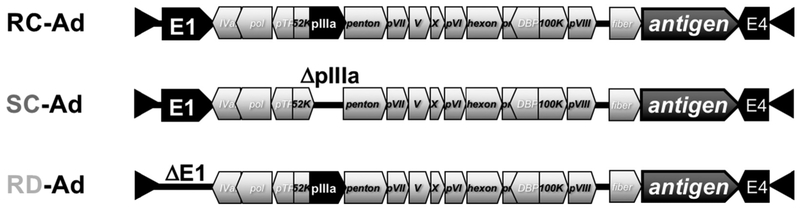
In 2014, we reported the development of a new adenovirus vector called single-cycle Ad (SC-Ad) (14). SC-Ads retain the genes needed to amplify DNA and vaccine antigen genes, but are deleted for viral late genes involved in making functional progeny viruses (Fig. 2). SC-Ad vectors take advantage DNA replication, but avoid the risk of causing frank adenovirus infections (Fig. 1).
Figure 2: Adenovirus Vaccines.
Schematic diagram showing Ad genes key to relevant vaccine functions. RC-, SC-, and RD-A ds all carry most Ad ORFs (not shown). Helper-dependent Ads (HD-Ads) are deleted for all of these adenovir us ORFs.
SC-Ads replicate their genomes and antigen genes as well as RC-Ads (up to 10,000-fold)(14). SC-Ad produces 30 to 300-fold more antigen than RD-Ad (14, 16, 17). This improvement in antigen expression per unit virus by SC-Ad could translate into getting 30-300 times as many vaccine doses out of an SC-Ad GMP production than an RD-Ad production. Alternately, if the same doses are used in humans, SC-Ad should drive markedly stronger immune responses than RD-Ad.
SC-Ads generate more robust and more persistent immune responses than either RD-Ad or RC-Ads (16). For example, after single mucosal intranasal immunization of Ad permissive hamsters, only SC-Ad generated antibodies in vaginal washes that rose over 6 months. RD-Ad did not do this. RC-Ad also surprisingly did not do this. This weak response by RC-Ad may be due to the fact that this nearly wild virus induces stronger antiviral responses than SC-Ad (56)) which may blunt its efficacy in vivo.. Similar effects are seen with single-cycle flavivirus vaccines (57).
After intranasal immunization, SC-Ad expressing influenza hemagglutinin antigen produced markedly higher binding and hemagglutination inhibition (HAI) antibody titers than RD-Ad in Syrian hamsters and cotton rats (17). SC-Ad mediated better protection than RD-Ad in cotton rats after challenge with influenza.
In larger rhesus macaques, SC-Ads generate significant antibodies after single intranasal, sublingual, or intramuscular immunization against antigens from HIV, Ebola, and other sources ((16) and data not shown). These data demonstrate that SC-Ads are reproducibly more potent vaccine platforms that conventional RD-Ad vectors. These data also demonstrate that the platform can be used by intramuscular, nasal, or oral routes of immunization.
To generate SC-Ads, we deleted or selectively repressed several different late genes of the virus to preserve DNA replication, but block production of infectious progeny viruses. Of these, deleting the gene for the viral cement protein pIIIA seems to be optimal, since cells can tolerate expressing this protein better than other viral proteins and sufficiently high amounts can be produced to compliment the deletion. Like RD-Ads, SC-Ads propagate somewhat slower than RC-Ads in normal cells and in first generation 293-IIIA complementing cell lines ((56) and unpublished data). With RD-Ads there is concern with rescuing replication-competent Ad by recombination and capture of E1 from cell lines into the E1-deleted vector (58, 59). This is not a concern with SC-Ads because they already have E1 in their genome. In addition, there is no homology between the pIIIA gene in the cells and the pIIIA flanking region in SC-Ad, so pIIIA cannot be rescued into the viral genome from the cells.
3. Opportunities and Challenges Confronting SC-Ad Translation
3.1. Challenge: Misleading Public Relations (PR) for Ad Vector Vaccines from HIV Trials (The STEP Trial).
For most pathogens, generating antibodies and T cell responses are all beneficial and confer protection. In contrast, generating these responses against pathogens that infect immune cells may actually increase infection rather than protection.
HIV infects CD4 T cells. CD4 T cells are pivotal for the production of antibody and killer T cell responses needed to protect against the virus (60). It is essentially impossible to generate CD4 responses when the immune response is exposed to an active vaccine. Therefore, for HIV, any vaccine may at least transiently generate the very cells that this virus naturally infects (60). This poses a unique safety risk for any HIV vaccine.
RD-Ad vectors expressing HIV antigens were tested in the STEP and HVTN-505 trials. These trials were halted when increases in HIV-1 infections were observed in men with higher anti-Ad5 antibodies before vaccination (61-64). This led to concerns of a special side effect associated with Ad vector vaccines.
3.2. Reality Check: All HIV Vaccines Have the Same Problem.
Emerging data indicate that this side effect is not unique to Ad vector vaccines, but is instead a side effect shared by any HIV vaccine that generates CD4 T cell responses (60).
For example, attenuated SIV and VSV vaccines increase SIV infection in non-human primates (65, 66). Even simple DNA vaccines combined with simple protein vaccines also increases SIV infections (67). Therefore, it is not Ad vector vaccines that have this problem. Any HIV vaccine can have this side effect.
While this might present an intractable problem, this side effect may be a transient phenomenon that peaks soon after immunization (e.g. 2 weeks) and declines with time (65). After this decline, protective immune responses can prevail and the vaccines can protect. A weak vaccine might have the early risk without ever mediating protection. A potent HIV vaccine may run an early risk, but be protective later. If so, it will be key to avoid HIV exposure at times soon after any HIV vaccination. Alternately, if high numbers of protective T cells are produced by a robust vaccine this may mediate protection, whereas a weak T cell response may increase HIV infection.
3.3. Opportunity: Improving Results in the RD-Ad Vaccine Landscape.
As mentioned, RD-Ad vector vaccines are more robust than DNA or vaccinia vaccines (38). RD-Ad vector vaccines have been shown to mediate protection against difficult analogs of HIV in non-human primates (68, 69). The use of RD-Ad (ChAd3-EBO-Z or CAd3-EBO-Z) as one of two Ebola vaccines in accelerated human trials against Ebola virus (18, 39, 40) supports the premise that Ads are robust vaccine platforms. ChAd3-EBO-Z generated significant antibodies and T cells against Ebola with no serious adverse events (18). These immune responses observed in humans would be protective in non-human primates. While immune responses by VSV-EBOV responses were somewhat stronger that ChAd3-EBO-Z, this replication-competent vaccine also generated troubling side effects as discussed above.
3.4. Challenge: Immunity to Adenoviruses.
Neutralizing antibodies against Ads can attenuate their ability to deliver genes (70). Humans may have pre-existing neutralizing antibodies that were produced by natural infection with certain Ad serotypes (71). These antibodies are serotype-specific. There are more than 60 human and many non-human Ad serotypes. So, a person infected with one Ad serotype may only neutralize that serotype and not affect a vaccine made from one of the other 59 serotypes.
Ad neutralizing antibodies are also produced after one inoculation of a given Ad serotype vector into a naive host. These vaccine-induced antibodies can block subsequent re-use of the same Ad serotype in the same person. Because of this, each Ad serotype vaccine be a "one-off" in each vaccinated individual. You might only be able to use it once, but its efficacy may be reduced upon second use. This is not an entirely yes or no issue, since Ads can be effective even in Ad immune animals (72-74). This can also be evaded by changing the route of Ad delivery between boosts (e.g. switching between IM and oral delivery (75)).
3.5. Opportunity: Use Lower Seroprevalence Adenoviruses.
Species C Ads (Ad1, 2, 5, and 6) are some of the most robust Ad vector vaccines when compared to other human Ads and non-human primate Ads (76). Unfortunately, most species C Ads are also prevalent in humans, so many humans are already immune to these most potent vaccines.
For example, Ad5 has seroprevalence of 27% in Texas (77). In contrast, equally robust Ad6 has seroprevalence that is only 3% in Texas (77). Therefore, you could theoretically use lower seroprevalence Ad6 in more Texans than Ad5. Other human Ads like Ad26 and Ad35 have even lower seroprevalence than Ad6 and for this reason are being tested in human trials based to avoid problems with pre-existing immunity (71, 78).
Chimpanzee and other non-human Ads have been championed to avoid immunity against human Ads for clinical trials (79-83). While this makes good sense (assuming vaccinees do not have close contacts with primates…), neutralizing immune responses against chimpanzee Ads can be observed in humans (40). This was a concern regarding ChAd3-EBO-Z vaccine In Ebola vaccine trials. However, when ChAd3 was tested empirically, there was no correlation between pre-existing anti-ChAd3 antibody levels on the production of antibodies or CD4 T cells against Ebola glycoprotein (18). However, there was a correlation to reduced CD8+ T cell responses.
More recent seroprevalence comparisons with sera from volunteers in the U.S. and Europe showed neutralizing antibody (NAb) titers of 38% and 22% against Ad5 and Ad6, respectively (76). Interestingly, 10% of these same volunteers described above had NAb titers above 200 against the more distant chimpanzee derived ChAd3 vaccine (76). Therefore, the rank order of pre-existing antibodies against Ad5, Ad6, and ChAd3 were 38%, 22%, and 10%. Notably, when Ad6 and ChAd3 were used head to head as hepatitis C (HCV) vaccines in humans, both generated robust immune responses (76). This suggests that human and non-humans Ads still have utility even in the face of some level of pre-existing immunity.
3.6. Opportunity: Do Serotype Switching.
To evade vector-induced antibodies, one can use the palette of more than 60 Ad serotypes to “serotype switch” vaccines. In this approach, one serotype is used in the first vaccination and a different serotype is used for subsequent immunizations. Each immunization will generate anti-pathogen responses as well as responses against that one Ad serotype. To avoid this, one simply uses a different Ad serotype for vaccine boost in a vaccine “shell game”. This premise is supported by data from Drs. Barouch and Ertl’s groups, our (55, 73, 74, 80, 81, 84, 85).
RC and SC-Ad generate stronger antibody responses against their target pathogens than RD-Ads. It is therefore not surprising that they also generate stronger antibodies against themselves than RD-Ad. Fortunately, these stronger anti-Ad responses do not create bigger problems for RC or SC-Ad in prime-boost studies. For example, prime-boost with RC-Ad5 and RC-Ad7 strongly boosts anti-HIV responses in chimpanzees (86).
3.7. Opportunity: Most Other Vaccine Platforms Do Not Have the Luxury of Having Many Other Serotypes to Avoid this Problem.
This luxury of many Ad serotypes is not available for some other vaccine vectors. For monotypic viruses like VSV or measles, they can be "one offs", but with no direct palette of serotypes to turn to as a backup. For measles, the problem is compounded by the fact that most humans have been actively immunized against the virus.
VSV or measles virus can made somewhat variable by pseudotyping it with different surface glycoproteins. However, these glycoproteins are frequently the intended target for the vaccine itself. For example, VSV-EBOV displays the Ebola glycoprotein to generate antibodies against the Ebola glycoprotein (87-92). One vaccination with VSV-EBOV will blunt any second use of the same vaccine. If you pseudotype VSV to avoid neutralizing the Ebola glycoprotein, you are no longer vaccinating against Ebola. While this is good from the standpoint of generating the immunity needed, it reduces efficacy if the vaccine needs to be used again. In contrast, Ad is a gene-based vaccine, so it carries the Ebola glycoprotein “hidden” as a gene and not a protein to generate immune responses (11), so productive antibody production against the target glycoprotein has no effect on subsequent rounds of immunization. One can pseudotype VSV with other glycoproteins that are related to similar pathogens like those from Marburg, Sudan, etc., but this is limited subset of shell game proteins when compared to Ad.
3.8. Challenge: The Need to Make Multiple Ad Serotypes Vectors to Avoid Antibodies
It is relatively easy to change the coat of Ads to evade antibodies, so pre-existing immunity to Ads is a minor engineering problem moving forward. The down-side is that one needs to make more than one Ad vaccine to immunize against the same pathogen more than once. This means making more than one GMP vaccine a single disease.
This is less of an issue if your Ad is potent and can mediate protection after a single immunization. Using an RC or SC-Ad rather than RD-Ad reduces or obviates the need for multiple vaccine boosts, so using these replicating vaccines may allow you to make fewer GMP vaccines products for the same indication. If one wants to develop multiple Ad vector vaccines against different pathogens, you will probably need to use a different serotype for each pathogen. For example, use Ad6 for influenza, Ad26 for Ebola, chimpanzee Ad for Zika, etc.
3.9. Opportunity: Shield Ad from Antibodies with Polymers.
Polyethylene glycol (PEG) is an FDA-approved polymer that shields protein therapeutics from proteins including antibodies. PEG and other polymers can be conjugated to the particle surface to shield the virus from neutralizing antibodies (93-95). A potent polymer shield that protects against antibodies without reducing vaccine efficacy may enable the use of one Ad serotype and avoid the need to make multiple serotype platforms.
3.10. Opportunity: Combine Ads with Other Vaccines.
In most cases, gene-based vaccines are used as prime-boost regimens (10, 96). An all-Ad prime boost involves serotype-switching between boosts (80, 81, 84, 85). In contrast, most prime-boost strategies usually change vectors between immunizations. This is observed most frequently in HIV-1 vaccine studies, where it can be difficult to raise protective levels of antibodies against the HIV envelope. Many groups have used plasmid DNA as the priming agent and followed with Ad or another vector (97). One rationale for this is that DNA is relatively weak when compared to viral vectors and the more potent vector is reserved to produce more protein at the end of the process to drive better antibody titers. Another rationale is that DNA has no protein coat and therefore produces no neutralizing antibodies against itself. It can therefore be used for more than one round of immunization without blocking its later uses. Like RD- and RC-Ads, SC-Ads are also useful in combination with DNA. For example, in comparing vaccines against Ebola glycoprotein, SC-Ad was markedly stronger than DNA as priming agents (unpublished data). When both were boosted, SC-Ad then DNA was slightly better than SC-Ad then SC-Ad. In contrast, DNA then DNA was weak and DNA then SC-Ad was intermediate.
Current HIV prime-boost strategies almost always finish immunizations with a protein boost, since one can flood the immune system with more antigen via recombinant protein than by gene-based vaccination (10, 96).
Recent data from Dr. Haigwood’s group suggests that there may even more value in combining the vaccines at the same time in co-immunization strategies (98, 99). When co-immunization and prime-boost strategies are compared head to head, co-immunization increases the speed and level of HIV antibody responses and improves HIV neutralization breadth (98, 99).
There is also good merit in combining two or more robust viral vaccine vectors in prime-boost (10, 96). For example, Ad and vaccinia virus have been combined in a number of studies (38, 100).
3.11. Opportunity: Make Safe Replicating Vaccines.
In the few examples where replicating and non-replicating vaccines have been compared, those that replicate usually win. Therefore, it is likely that non-viral and viral vaccine platforms that have some ability to replicate antigen genes and/or themselves will ultimately be superior in humans over non-replicating vaccines.
The trick is making replicating vaccines safe so that they do not cause infections in vaccinees. Some of the questions that remain include: Will a RC vaccine cause infections in vaccinees? Will a RC vaccine viremic and spread to organs and joints? Will it be shed to family members? Will it infect medical care staff, particularly the nurse who administer hundreds of vaccines?
Adenovirus vaccines have a luxury of safety data since RC-Ads have been administered to a half million military recruits. Interestingly, when live RC-Ad4 and Ad7 were used to vaccinate recruits, they were delivered in oral capsules. This complex delivery system was not used to increase vaccine potency. Rather, they were delivered encased in oral capsules specifically to protect health care personnel and vaccinees from accidental respiratory infections by the Ad vector vaccines themselves [Robert Couch, Baylor College of Medicine, personal communication and (101)].
Therefore, our RC vaccines need to be safe not only for patients, but for health care workers who can potentially be exposed hundreds of times to these vaccines. We do not want vaccine-derived outbreaks like those seen with polio vaccines.
4. Expert Commentary
Single-cycle Ads have as good or better potency as RC-Ads, but without risk of infection in animal models. Until SC-Ad is tested in clinical trials, it is uncertain if this will hold up in humans. All indications are that they will actually be more robust in humans than in the animal models where they have been tested. This is based on the fact that SC-Ad replication is invariably weaker in animal cells than in human cells. For example, SC-Ad does not replicate in most mouse cells, but will replicate its DNA 500-fold in Ad-permissive hamster cells (16). In contrast, human Ads can replicate their genomes up to 10,000-fold in human cells (102). This suggests that promising preclinical data in animals may under-estimate SC-Ad potency in humans.
SC-Ads do not benefit from a second wave of infection like RC-Ads, so they may not be as potent as RC-Ad vector vaccines in humans. While this may be the case, to date we have not observed second waves of RC-Ad propagation in adenovirus-permissive animals like Syrian hamsters. Instead, we see stronger antiviral responses against RC than SC-Ad that may negatively impact RC when compared to SC. It is therefore possible that SC-Ad would be equal to or better than RC-Ad in humans.
While RC vs. SC-Ad is an open question in humans, it is unlikely that this type of comparison will be done in the context of vaccines against infectious agents in the general population. SC-Ad cannot cause uncontrolled adenovirus infections. These are a legitimate risk with RC-Ad vector vaccines. If they are administered intramuscularly, they can leak into the blood and infect the liver. If they are administered intranasally, they risk retrograde transport into the olfactory bulb with potential to drive side effects like Bell’s palsy. It is therefore unlikely that RC-Ads will be tested in the general population unless they are deployed as oral vaccines as in military Ad vaccines.
While RC-Ad vs. SC-Ad may not be directly compared as prophylactic vaccines in humans, it is possible that they may be used as cancer vaccine therapies. While one could use an oncolytic RC-Ad to express these potent immunostimulatory proteins, it should be noted that many cancer patients are immunosuppressed due to the disease or multiple cycles of cytotoxic therapy. Therefore, deploying RC-Ad or another replication-competent virus in these immunosuppressed individuals risks uncontrolled viremia and disease as in immunodeficient transplant recipients. SC-Ad is oncolytic and kills infected cells (56), but that does not spread beyond the first infected cell. Therefore, one argument for using SC-Ad rather than fully RC oncolytic viruses in cancer is that it can still kill cancer cells, liberate cancer antigens, and express immunostimulatory proteins, but that it cannot spread to potentially endanger the patient, health care staff, or family members of the patient.
To summarize, there is good scientific merit for giving Ad vector vaccines the ability to replicate and amplify transgenes. This will make them functionally equivalent to competitor vaccines like VSV and CMV. It will be interesting to perform controlled head to head comparisons of VSV, CMV, and SC or RC-Ad to avoid previous misleading apples and oranges comparisons. There is also a good basis, to restrict replication of replication-competent vectors in settings where they may endanger patients receiving prophylactic vaccines or oncolytic cancer gene therapy.
5. Five-year view
We first reported SC-Ads in 2014, so we are just now moving some of these forward towards clinical translation. While SC-Ad against Ebola appears equal or better than VSV, the absence of a large outbreak makes its testing unlikely within 5 years. In contrast, we have a very robust SC-Ad against Clostridium difficile that may be sufficiently compelling to move rapidly into human testing. Given the quite different cost/benefit ratio of cancer vaccines vs. prophylactic infectious disease vaccines, we may see SC-Ad expressing immunostimulatory genes in humans before it is tested as a vaccine against infectious agents. Given the superior immune responses by RC and SC-Ads when compared to current RD-Ads, we believe moving forward with RD-Ads is not a good strategy. This is not a good strategy particularly when Ad vaccines must compete against replication-competent vectors like VSV and CMV. However, many RD-Ad vaccine clinical trials are in process and cannot be aborted mid-stream. It will therefore take some time to introduce these more robust SC-Ads vaccines in place of RD-Ads in human trials.
6. Key Issues:
Do not be misled by bad public relations for adenovirus vaccines from HIV Vaccine Trials (i.e. The STEP Trial).
Reality check: All HIV vaccines have the same problem.
Pre-existing immunity to certain adenoviruses, but this is easily countered by using lower seroprevalence adenoviruses.
Most other vaccine platforms are “one-offs” that do not have the luxury of having many other serotypes to avoid anti-vector immunity.
Use antigen gene replicating vaccines rather than non-replicating vaccines.
Make these replicating vaccines safe to avoid vaccine-derived infections and epidemics.
For global vaccines, use needle-free vaccines to avoid leaving behind biohazard sharps “landmines”.
Single-cycle adenoviruses may have utility as safe antigen gene replicating vaccines.
Single-cycle adenoviruses may have utility as needle-free mucosal vaccines
Acknowledgments
Funding
The manuscript received funding from the NIH/NIAHD: R01 AI096967, R01 AI136718, and R21 AI130298, the Discovery Translation Fund of Mayo Clinic and the Walter & Lucille Rubin Fund in Infectious Diseases Honoring Michael Camilleri, M.D. at Mayo Clinic.
Footnotes
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
* Of interest
** Of considerable interest
- 1.Lycke N Recent progress in mucosal vaccine development: potential and limitations. Nature reviews Immunology. 2012;12(8):592–605. Epub 2012/07/26. 10.1038/nri3251 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, Gumede N, Pate MA, Abanida EA, Gasasira A, Iber J, Chen Q, Vincent A, Chenoweth P, Henderson E, Wannemuehler K, Naeem A, Umami RN, Nishimura Y, Shimizu H, Baba M, Adeniji A, Williams AJ, Kilpatrick DR, Oberste MS, Wassilak SG, Tomori O, Pallansch MA, Kew O. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. Journal of virology. 2013;87(9):4907–22. Epub 2013/02/15. 10.1128/JVI.02954-12 PubMed PMID: ; PMCID: 3624331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang D, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. [DOI] [PubMed] [Google Scholar]
- 4.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: Protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proceedings of the National Academy of Sciences USA. 1993;90:11478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis HL, Michel M-L, Whalen RG. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Human Molecular Genetics. 1993;2:1847–51. [DOI] [PubMed] [Google Scholar]
- 6.Conry RM, LoBuglio AF, Kantor J, Schlom J, Loechel F, Moore SE, Sumerel LA, Barlow DL, Abrams S, Curiel DT. Immune response to a carcinoembryonic antigen polynucleotide vaccine. Cancer Research. 1994;54:1164–8. [PubMed] [Google Scholar]
- 7.Han R, Cladel NM, Reed CA, Peng X, Christensen ND. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. Journal of virology. 1999;73(8):7039–43. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertl HC, Xiang ZQ. Genetic immunization. Viral Immunology. 1996;9(1):1–9. [DOI] [PubMed] [Google Scholar]
- 9.Chattergoon M, Boyer J, Weiner DB. Genetic immunization: a new era in vaccines and immune therapeutics. FASEB Journal. 1997;11(10):753–63. [DOI] [PubMed] [Google Scholar]
- 10.Barry MA. Genetic Immunization: Applying recombinant DNA technology in the immune system to develop new vaccines. Current Opinion in Drug Discovery and Development. 1999;2(2):118–28. [PubMed] [Google Scholar]
- 11.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17(8):1333–9. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry MA. Recombinant vector vaccines for the prevention and treatment of HIV infection. Drugs of the Future. 2011;36(11):833–45. [Google Scholar]
- 13.Shashkova EV, May SM, Barry MA. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009;Epub Sep 17.PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Crosby CM, Barry MA. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology. 2014;462–463: 158–65. Epub 2014/07/06. 10.1016/j.virol.2014.05.030 PubMed PMID: ; PMCID: PMC4125442. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first proof of principle for using single-cycle adenovirus vectors
- **15.Peng B, Wang LR, Gomez-Roman VR, Davis-Warren A, Montefiori DC, Kalyanaraman VS, Venzon D, Zhao J, Kan E, Rowell TJ, Murthy KK, Srivastava I, Barnett SW, Robert-Guroff M. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. Journal of virology. 2005;79(16):10200–9. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an excellent controlled head to head comparison of eplicating and nonreplicating adenovirus vaccines in non-human primates.
- *16.Crosby CM, Nehete P, Sastry KJ, Barry MA. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. Journal of virology. 2015;89(1):669–75. Epub 2014/10/31. 10.1128/jvi.02184-14 PubMed PMID: ; PMCID: PMC4301142. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper compares replication-defective, single-cycle, and replication-competent adenoviral vector vaccine responses, building on the work of Peng et. al. 2005. This also shows that single-cycle Ads may in some cases mediate better immune responses than fully replication-competent Ads. This observation needs to be explored more fully ideally in humans.
- 17.Crosby CM, Matchett WE, Anguiano-Zarate SS, Parks CA, Weaver EA, Pease LR, Webby RJ, Barry MA. Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. Journal of virology. 2017;91(2). 10.1128/JVI.00720-16 PubMed PMID: ; PMCID: PMC5215357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledgerwood JE, Sullivan NJ, Graham BS. Chimpanzee Adenovirus Vector Ebola Vaccine--Preliminary Report. N Engl J Med. 2015;373(8):776 10.1056/NEJMc1505499 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, Draguez B, Duraffour S, Enwere G, Grais R, Gunther S, Gsell PS, Hossmann S, Watle SV, Konde MK, Keita S, Kone S, Kuisma E, Levine MM, Mandal S, Mauget T, Norheim G, Riveros X, Soumah A, Trelle S, Vicari AS, Rottingen JA, Kieny MP. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389(10068):505–18. 10.1016/S0140-6736(16)32621-6 PubMed PMID: ; PMCID: PMC5364328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008;14(31):4849–60. PubMed PMID: ; PMCID: PMC2739936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcelin JR, Beam E, Razonable RR. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol. 2014;20(31):10658–67. 10.3748/wjg.v20.i31.10658 PubMed PMID: ; PMCID: PMC4138447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atabani SF, Smith C, Atkinson C, Aldridge RW, Rodriguez-Peralvarez M, Rolando N, Harber M, Jones G, O'Riordan A, Burroughs AK, Thorburn D, O'Beirne J, Milne RS, Emery VC, Griffiths PD. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant. 2012;12(9):2457–64. 10.1111/j.1600-6143.2012.04087.x PubMed PMID: ; PMCID: PMC3510308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. 10.1086/652438 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bialas KM, Swamy GK, Permar SR. Perinatal cytomegalovirus and varicella zoster virus infections: epidemiology, prevention, and treatment. Clin Perinatol. 2015;42(1):61–75, viii. 10.1016/j.clp.2014.10.006 PubMed PMID: ; PMCID: PMC4328139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bialas KM, Permar SR. The March towards a Vaccine for Congenital CMV: Rationale and Models. PLoS pathogens. 2016;12(2):e1005355 10.1371/journal.ppat.1005355 PubMed PMID: ; PMCID: PMC4750955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanzieri TM, Chung W, Flores M, Blum P, Caviness AC, Bialek SR, Grosse SD, Miller JA, Demmler-Harrison G, Congenital Cytomegalovirus Longitudinal Study G. Hearing Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics. 2017;139(3). 10.1542/peds.2016-2610 PubMed PMID: ; PMCID: PMC5330400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, Voigt RG, Demmler-Harrison G. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J Perinatol. 2017;37(7):875–80. 10.1038/jp.2017.41 PubMed PMID: ; PMCID: PMC5562509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez AS, Lanzieri TM, Claussen AH, Vinson SS, Turcich MR, Iovino IR, Voigt RG, Caviness AC, Miller JA, Williamson WD, Hales CM, Bialek SR, Demmler-Harrison G, Congenital Cytomegalovirus Longitudinal Study G. Intelligence and Academic Achievement With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics. 2017;140(5). 10.1542/peds.2017-1517 PubMed PMID: ; PMCID: PMC5654402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63. 10.1002/rmv.544 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol. 2008;41(2):57–62. 10.1016/j.jcv.2007.09.004 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Bartlett AW, McMullan B, Rawlinson WD, Palasanthiran P. Hearing and neurodevelopmental outcomes for children with asymptomatic congenital cytomegalovirus infection: A systematic review. Rev Med Virol. 2017. 10.1002/rmv.1938 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43(3):331–9. 10.1086/505498 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, Johannessen I. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013;85(5):893–8. 10.1002/jmv.23539 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Choudhry A, Mathena J, Albano JD, Yacovone M, Collins L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine. 2016;34(38):4558–64. 10.1016/j.vaccine.2016.07.033 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12(5):216–22. Epub 2006/04/20. 10.1016/j.molmed.2006.03.007 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Shiver JW, Emini EA. Recent Advances in the Development of HIV-1 Vaccines Using Replication-Incompetent Adenovirus Vectors. Annu Rev Med. 2004;55:355–72. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Current opinion in HIV and AIDS. 2010;5(5):386–90. 10.1097/COH.0b013e32833cfe4c PubMed PMID: ; PMCID: PMC2967414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–5. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Cohen J Infectious Disease. Ebola vaccines racing forward at record pace. Science. 2014;345(6202):1228–9. Epub 2014/09/13. 10.1126/science.345.6202.1228 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Seto D. Chimpanzee Adenovirus Vector Ebola Vaccine--Preliminary Report. N Engl J Med. 2015;373(8):775–6. 10.1056/NEJMc1505499#SA1PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nature reviews. 2014;12(11):765–71. 10.1038/nrmicro3360 PubMed PMID: ; PMCID: PMC4237164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Lifson JD Jr., Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–7. 10.1038/nature10003 PubMed PMID: ; PMCID: PMC3102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malkevitch N, Patterson LJ, Aldrich K, Richardson E, Alvord WG, Robert-Guroff M. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J Immunol. 2003;170(8):4281–9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Lou Y, Pinczewski J, Malkevitch N, Aldrich K, Kalyanaraman VS, Venzon D, Peng B, Patterson LJ, Edghill-Smith Y, Woodward R, Pavlakis GN, Robert-Guroff M. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine. 2003;21(25–26):4022–35. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, Kalyanaraman VS, Markham PD, Robey FA, Robert-Guroff M. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. Journal of virology. 2004;78(5):2212–21. PubMed PMID : [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao J, Voltan R, Peng B, Davis-Warren A, Kalyanaraman VS, Alvord WG, Aldrich K, Bernasconi D, Butto S, Cafaro A, Ensoli B, Robert-Guroff M. Enhanced cellular immunity to SIV Gag following co-administration of adenoviruses encoding wild-type or mutant HIV Tat and SIV Gag. Virology. 2005;342(1):1–12. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308(1-2):53–67. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Roman VR, Florese RH, Peng B, Montefiori DC, Kalyanaraman VS, Venzon D, Srivastava I, Barnett SW, Robert-Guroff M. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43(3):270–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Roman VR, Grimes GJ, Jr., Potti GK, Peng B, Demberg T, Gravlin L, Treece J, Pal R, Lee EM, Alvord WG, Markham PD, Robert-Guroff M. Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine. 2006;24(23):5064–72. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 50.Peng B, Voltan R, Cristillo AD, Alvord WG, Davis-Warren A, Zhou Q, Murthy KK, Robert-Guroff M. Replicating Ad-recombinants encoding non-myristoylated rather than wild-type HIV Nef elicit enhanced cellular immunity. Aids. 2006;20(17):2149–57. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 51.Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. Journal of virology. 2007;81(7):3414–27. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, Robert-Guroff M. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. Journal of virology. 2009;83(2):791–801. 10.1128/JVI.01672-08 PubMed PMID: ; PMCID: PMC2612365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan C, Marthas M, Miller C, Duerr A, Cheng-Mayer C, Desrosiers R, Flores J, Haigwood N, Hu SL, Johnson RP, Lifson J, Montefiori D, Moore J, Robert-Guroff M, Robinson H, Self S, Corey L. The use of nonhuman primate models in HIV vaccine development. PLoS medicine. 2008;5(8):e173 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demberg T, Robert-Guroff M. Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design. International reviews of immunology. 2009;28(1):20–48. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, Ng P, Sastry KJ, Barry MA. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PloS one. 2009;4(3):e5059 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crosby CM, Barry MA. Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors. Genes (Basel). 2017;8(2). 10.3390/genes8020079 PubMed PMID: ; PMCID: PMC5333068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widman DG, Frolov I, Mason PW. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses. Adv Virus Res. 2008;72:77–126. 10.1016/S0065-3527(08)00402-8 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, Auger C, Cramer SJ, van Ormondt H, van der Eb AJ, Valerio D, Hoeben RC. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther. 1998;9(13):1909–17. 10.1089/hum.1998.9.13-1909 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 59.Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum Gene Ther. 2000;11(15):2105–16. 10.1089/104303400750001417 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- **60.McChesney MB, Miller CJ. New directions for HIV vaccine development from animal models. Current opinion in HIV and AIDS. 2013;8(5):376–81. 10.1097/COH.0b013e328363d3a2 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an important review that in part dubunks the concept that there is a particular problem with adenovirus vaccines with respect to HIV vaccines. Rather, the authors note the unique and problematic interactions between vaccines and activating the target cells for HIV infection.
- 61.STEP study: disappointing, but not a failure. Lancet. 2007;370(9600):1665 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 62.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205(1):7–12. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinbrook R One step forward, two steps back--will there ever be an AIDS vaccine? N Engl J Med. 2007;357(26):2653–5. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 64.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Current opinion in HIV and AIDS.5(5):357–61. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Byrareddy SN, Ayash-Rashkovsky M, Kramer VG, Lee SJ, Correll M, Novembre FJ, Villinger F, Johnson WE, von Gegerfelt A, Felber BK, Ruprecht RM. Live attenuated Rev-independent Nef SIV enhances acquisition of heterologous SIVsmE660 in acutely vaccinated rhesus macaques. PloS one. 2013;8(9):e75556 10.1371/journal.pone.0075556 PubMed PMID: ; PMCID: PMC3787041. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is another important paper with a non-adenovirus vaccine showing that short times after vaccination can increase the chances of lentiviral acquisition, whereas longer times after vaccination can confer vaccine protection.
- 66.Staprans SI, Feinberg MB. The roles of nonhuman primates in the preclinical evaluation of candidate AIDS vaccines. Expert Rev Vaccines. 2004;3(4 Suppl): S5–32. Epub 2004/08/03. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 67.Tenbusch M, Ignatius R, Nchinda G, Trumpfheller C, Salazar AM, Topfer K, Sauermann U, Wagner R, Hannaman D, Tenner-Racz K, Racz P, Stahl-Hennig C, Uberla K. Immunogenicity of DNA vaccines encoding simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PloS one. 2012;7(6):e39038 Epub 2012/06/22. 10.1371/journal.pone.0039038 PubMed PMID: ; PMCID: 3373620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. 10.1038/nature10766 PubMed PMID: ; PMCID: 3271177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng'ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys. Science. 2015. 10.1126/science.aab3886 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morral N, O'Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther. 1997;8(10):1275–86. [DOI] [PubMed] [Google Scholar]
- 71.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81(9):4654–63. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhar D, Spencer JF, Toth K, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. Journal of virology. 2009;83(5):2130–9. 10.1128/JVI.02127-08 PubMed PMID: ; PMCID: PMC2643738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weaver EA, Nehete PN, Nehete BP, Buchl SJ, Palmer D, Montefiori DC, Ng P, Sastry KJ, Barry MA. Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines. Viruses. 2009;1(3):920 Epub 2010/01/29. 10.3390/v1030920 PubMed PMID: ; PMCID: 2811377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weaver EA, Nehete PN, Nehete BP, Yang G, Buchl SJ, Hanley PW, Palmer D, Montefiori DC, Ferrari G, Ng P, Sastry KJ, Barry MA. Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV. PloS one. 2013;8(7):e67574 Epub 2013/07/12. 10.1371/journal.pone.0067574 PubMed PMID: ; PMCID: 3701068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. Journal of virology. 2003;77(20):10780–9. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O'Hara GA, Ewer KJ, Anagnostou N, Bliss C, Hill AV, Traboni C, Klenerman P, Cortese R, Nicosia A. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. 10.1126/scitranslmed.3002925 PubMed PMID: ; PMCID: PMC3627206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998;27(5):1194–200. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 78.Thorner AR, Vogels R, Kaspers J, Weverling GJ, Holterman L, Lemckert AA, Dilraj A, McNally LM, Jeena PM, Jepsen S, Abbink P, Nanda A, Swanson PE, Bates AT, O'Brien KL, Havenga MJ, Goudsmit J, Barouch DH. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. Journal of clinical microbiology. 2006;44(10):3781–3. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. Journal of virology. 2003;77(15):8263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8(+) T Cells to an HIV-1 Antigen through a Prime Boost Regimen with Heterologous E1-Deleted Adenoviral Vaccine Carriers. J Immunol. 2003;171(12):6774–9 [DOI] [PubMed] [Google Scholar]
- 81.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. Journal of virology. 2007;81(12):6594–604. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santra S, Sun Y, Korioth-Schmitz B, Fitzgerald J, Charbonneau C, Santos G, Seaman MS, Ratcliffe SJ, Montefiori DC, Nabel GJ, Ertl HC, Letvin NL. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine. 2009;27(42):5837–45. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tatsis N, Blejer A, Lasaro MO, Hensley SE, Cun A, Tesema L, Li Y, Gao GP, Xiang ZQ, Zhou D, Wilson JM, Ertl HC. A CD46-binding chimpanzee adenovirus vector as a vaccine carrier. Mol Ther. 2007;15(3):608–17. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 84.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172(10):6290–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 85.Lemckert AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, Goudsmit J, Havenga MJ, Barouch DH. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. Journal of virology. 2005;79(15):9694–701. PubMed PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy SC, Chanda PK, Nigida SM, Markham PD Jr., Zolla-Pazner S, Steimer K, Wade M, Reitz MS, Arthur LO Jr., Mizutani S, Davis A, Hung PP, Gallo RC, Eichberg J, Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3(6):651–8. [DOI] [PubMed] [Google Scholar]
- 87.Takada A, Feldmann H, Stroeher U, Bray M, Watanabe S, Ito H, McGregor M, Kawaoka Y. Identification of protective epitopes on ebola virus glycoprotein at the single amino acid level by using recombinant vesicular stomatitis viruses. Journal of virology. 2003;77(2):1069–74. PubMed PMID: : PMCID: PMC140786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. Journal of virology. 2004;78(10):5458–65. PubMed PMID: ; PMCID: PMC400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, Geisbert JB, Leung A, Feldmann F, Hensley LE, Feldmann H, Jones SM. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. Journal of virology. 2008;82(11):5664–8. 10.1128/JVI.00456-08 PubMed PMID: ; PMCID: PMC2395203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Stroher U, Fritz EA, Hensley LE, Jones SM, Feldmann H. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26(52):6894–900. 10.1016/j.vaccine.2008.09.082 PubMed PMID: ; PMCID: PMC3398796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, Paragas J, Matthias L, Smith MA, Jones SM, Hensley LE, Feldmann H, Jahrling PB. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS pathogens. 2008;4(11):e1000225 10.1371/journal.ppat.1000225 PubMed PMID: ; PMCID: PMC2582959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. Journal of virology. 2009;83(14):7296–304. 10.1128/JVI.00561-09 PubMed PMID: ; PMCID: PMC2704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10(8):1349–58. [DOI] [PubMed] [Google Scholar]
- 94.Croyle MA, Chirmule N, Zhang Y, Wilson JM. "Stealth" adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. Journal of virology. 2001;75(10):4792–801. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13(15):1887–900. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 96.Barry MA. Recombinant Vector Vaccines for the Prevention and Treatment of HIV Infection. Drugs of the Future. 2011;36(11):833–45. [Google Scholar]
- 97.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. Journal of virology. 2003;77(11):6305–13. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, Waagmeester G, Sartorius R, D'Apice L, Caivano A, Doria-Rose NA, Malherbe D, Montefiori DC, Barnett S, De Berardinis P, Haigwood NL. Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells. PloS one. 2012;7(2):e31464 10.1371/journal.pone.0031464 PubMed PMID: ; PMCID: PMC3281069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pissani F, Malherbe DC, Schuman JT, Robins H, Park BS, Krebs SJ, Barnett SW, Haigwood NL. Improvement of antibody responses by HIV envelope DNA and protein co-immunization. Vaccine. 2014;32(4):507–13. 10.1016/j.vaccine.2013.11.022 PubMed PMID: ; PMCID: PMC3926420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sridhar S Clinical development of Ebola vaccines. Ther Adv Vaccines. 2015;3(5-6):125–38. 10.1177/2051013615611017 PubMed PMID: ; PMCID: PMC4667768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Couch RB, Chanock RM, Cate TR, Lang DJ, Knight V, Huebner RJ. Immunization with types 4 and 7 adenovirus by selective infection of the intestinal tract. Am Rev Respir Dis. 1963;88:SUPPL 394–403. [DOI] [PubMed] [Google Scholar]
- 102.Turner MA, Middha S, Hofherr SE, Barry MA. Comparison of the Life Cycles of Genetically Distant Species C and Species D Human Adenoviruses Ad6 and Ad26 in Human Cells. Journal of virology. 2015;89(24):12401– 17. 10.1128/JVI.01534-15 PubMed PMID: ; PMCID: PMC466524. [DOI] [PMC free article] [PubMed] [Google Scholar]