 Rubus chingii Hu, namely “Fu-pen-zi” in Chinese, has been used as a functional food in China for a long time.
Rubus chingii Hu, namely “Fu-pen-zi” in Chinese, has been used as a functional food in China for a long time.
Abstract
Rubus chingii Hu, namely “Fu-pen-zi” in Chinese, has been used as a functional food in China for a long time. This study aims to identify its bioactive constituents with antioxidant and anti-tumor properties. R. chingii was extracted with 95% ethanol and then partitioned into four fractions: petroleum ether fraction, ethyl acetate fraction, n-butanol fraction, and water fraction. Results showed that the ethyl acetate fraction had the strongest antioxidant activity and cytotoxicity against human cancer cell lines (HepG-2, Bel-7402, A549 and MCF-7). Therefore, four compounds were isolated from the ethyl acetate fraction, and they were identified as ent-16α,17-dihydroxy-kauran-19-oic acid, tormentic acid, oleanolic acid and β-daucosterol, the first two of which were isolated and identified from R. chingii for the first time. In particular, tormentic acid exhibited excellent cytotoxicity activities against human tumor cell lines. The results obtained in this work might contribute to the understanding of biological activities of R. chingii and further investigation on its potential application is valued for food and drugs.
1. Introduction
Rubus chingii Hu, namely “Fu-pen-zi” in Chinese, is widely distributed in both North and South China.1 Given the advantages of high-nutrition, high-resistance and being pollution-free, the fruit of R. chingii has been used as a health food in China for a long time. Rubus chingii Hu belongs to the family of Rosaceae and has gained more and more attention as a functional fruit in the past decade due to its potential health and economic significance.2 Many bioactivities of R. chingii such as antioxidant, anti-inflammatory, hepatoprotective, and immunomodulatory effects have been reported.3,4
There is increasing interest in R. chingii about its active ingredients and functions because of its applications in tumor chemotherapy and other various pharmacological effects, particularly antioxidant and anti-tumor activities.1R. chingii is still a natural resource that has not been fully utilized. More studies are urgently needed for the isolation and identification of compounds with efficient functions from R. chingii to exploit its health-promoting compounds into new food ingredients or nutraceutical applications.
Studies have shown that R. chingii has antioxidant and anti-tumor effects; however, it is not known which compounds are responsible for these bioactivities. The main purpose of the present study was to isolate the active ingredients by function-guided separation. A crude extract and four fractions (petroleum ether fraction, ethyl acetate fraction, n-butanol fraction, and water fraction) were partitioned from 95% ethanol extract of R. chingii and detected to analyze their activities by the use of total reducing power, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, and cell proliferation assays against human hepatoma cells HepG-2 and Bel-7402, lung cancer cell A549, and breast cancer cell MCF-7.
2. Results and discussion
2.1. Antioxidant activity of the extract and fractions from R. chingii
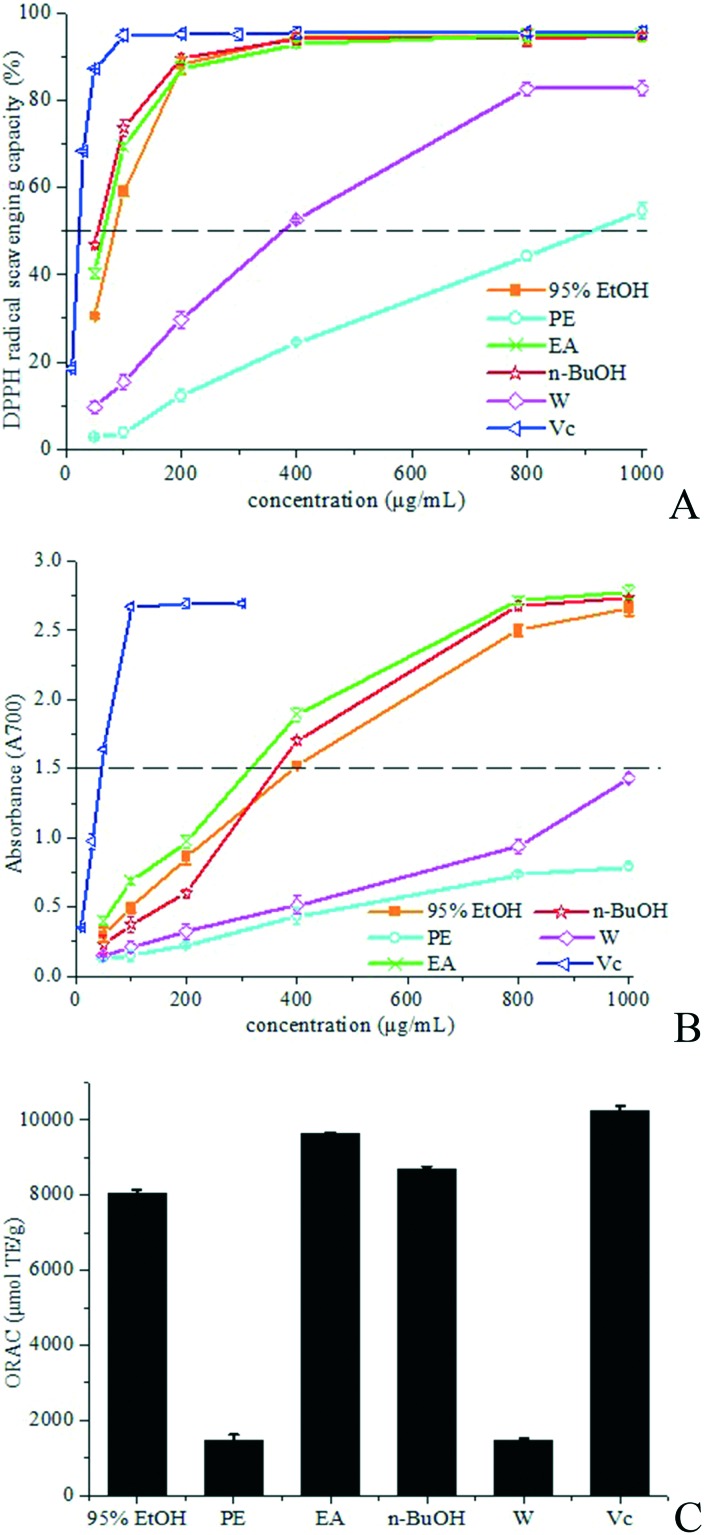
The model of scavenging the stable DPPH radical is a widely used means to determine the free radical scavenging capacity of natural ingredients as well as different plant extracts. The antioxidant ability of a sample can be expressed as its capacity for scavenging the DPPH radical determined by measuring the decrease in absorbance.5,6 Fig. 2A shows the scavenging effect of the samples on the DPPH radical which increased with increasing concentration. The crude 95% EtOH extract, EA fraction and n-BuOH fraction showed excellent DPPH radical-scavenging ability (DPPH radical inhibition rate >94%) at a concentration of 400 μg mL–1, which was very close to Vc at the same concentration. The DPPH radical-scavenging capacity of the PE fraction and W fraction was weaker. The IC50 values (half maximal inhibitory concentration) were determined to quantify the antioxidant ability further. The lowest IC50 is 55.8 ± 2.0 μg mL–1, which is obtained in n-BuOH, indicating the highest DPPH radical scavenging ability among all samples. The IC50 values of EA and 95% EtOH were also low (66.7 ± 1.5 μg mL–1 and 84.0 ± 0.7 μg mL–1, respectively), which were only slightly higher than that of n-butanol fraction. The IC50 values of PE and W are 910.8 ± 1.1 μg mL–1 and 377.5 ± 3.4 μg mL–1, respectively. Thus, the EA fraction and n-BuOH fraction had the most potent antioxidant activity.
Fig. 2. Antioxidant activities. DPPH free radical-scavenging assay (A), reducing power assay (B) and ORAC assay (C). Results are mean ± SD.
Reducing power is related to antioxidant ability and may act as an important index of antioxidant activity.7 Substances with reducing power are electron donors and can reduce the oxidised intermediates of lipid peroxidation processes, thus that they could serve as primary and secondary antioxidants.8Fig. 2B depicts the reducing power of the tested samples. The reducing power is well correlated with increasing concentration. The crude 95% EtOH extract, EA fraction and n-BuOH fraction had stronger reducing power than the PE fraction and W fraction. EA and n-BuOH had remarkable antioxidant capacity, which is in accordance with the results of the DPPH radical scavenging assay.
The ORAC test evaluates the ability of a substance to serve as an antioxidant based on quenching peroxyl radicals (ROO˙), which is assessed by determining the fluorescence decay curve of the substance by comparing with a blank without an antioxidant.9 A higher ORAC value reflects stronger antioxidant activity. The ORAC technique is the only one that combines both time and degree of inhibition into a single magnitude.10 As shown in Fig. 2C, the ORAC value of EA is 9623.0 ± 31.6 μmol TE g–1, which is significantly higher than those of n-BuOH (8689.5 ± 71.4 μmol TE g–1), 95% EtOH (8049.0 ± 85.3 μmol TE g–1), PE (1458.6 ± 164.0 μmol TE g–1) and W (1450.9 ± 86.0 μmol TE g–1), and close to Vc (10229.7 ± 130.6 μmol TE g–1), indicating that the EA fraction and n-BuOH fraction are mainly responsible for the antioxidant capacity of R. chingii.
2.2. Cytotoxicity evaluation of the extract and fractions from R. chingii
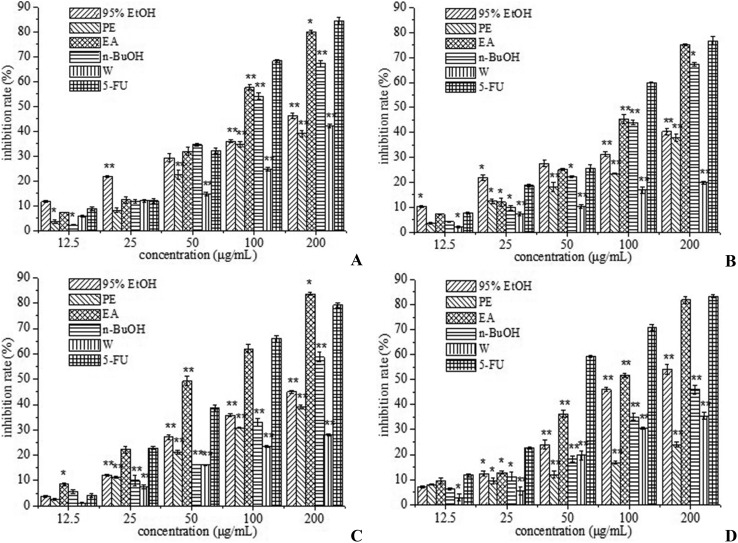
Oxidative stress is the main cause of cancer-associated death, and chemoprevention is considered as the use of natural or synthetic antioxidants such as triterpenoid, flavonoids and sterols to prevent cancer formation or cancer progress.11,12 The proportion of reactive oxygen species (ROS) production generally increased in most diseases and could be adjusted by suitable application of antioxidants.13,14 Antioxidants are considered to be ingredients which can prevent oxidation of easily oxidized substrates and prevent overdose of ROS.14 There is evidence that these molecules can prevent tumor generation and prolong life.15 Treatment with antioxidants is effective in combating tumor cells.16,17 Initially, the antiproliferative activity induced by 95% EtOH, PE, EA, n-BuOH and W extracted from R. chingii was screened in four human cancer cell lines (HepG-2, Bel-7402, A549 and MCF-7 cells) using an MTT reduction assay. All tested cells were treated at various concentrations of the extract and fractions (12.5, 25, 50, 100 and 200 μg mL–1) for 24 h, and the percentage of cell viability is illustrated in Fig. 3A–D. It was found that all of the tested extract and fractions exhibited certain cytotoxicity to these four human cancer cell lines and the higher the crude extract concentrations, the lower the cell viability percentages. 95% EtOH and n-BuOH exhibited moderate cytotoxicity against these four cancer cell lines in a dose-dependent manner (Fig. 3). EA exhibited the strongest cytotoxicity against A549 human cancer cell lines, and the inhibition rate reached 83.6% at the concentration of 200 μg mL–1, which exceeded that of 5-fluorouracil (Fig. 3C). EA also showed higher inhibition rates against HepG-2, Bel-7402, and MCF-7 human cancer cell lines, which were close to that of 5-fluorouracil (Fig. 3). The antiproliferative activity of PE and W was weaker. This outcome suggested that active substances gathered into the EA fraction after the 95% EtOH extract was partitioned into four fractions. Many previous literature reports showed that methanol/ethanol extracts or fractions had good anti-tumor activity. It was reported that the methanol extracts from Salvia menthifolia exhibited anti-proliferative activity against human glioblastoma cell line DBTRG-05MG.18 The ethyl acetate fraction from Polytrichum commune L.ex Hedw also displayed a higher anti-tumor effect against L1210 cells than chloroform and butanol fractions.19 Therefore, the bioactivity-guided separation of R. chingii was designed and four active compounds were isolated from the ethyl acetate fraction.
Fig. 3. Cell proliferation inhibition rate of 95% ethanol extract (95% EtOH), petroleum ether fraction (PE), ethyl acetate fraction (EA), n-butanol fraction (n-BuOH), water fraction (W), and 5-FU (5-fluorouracil) against HepG-2 (A), Bel-7402 (B), A549 (C) and MCF-7 (D) human cancer cells.
2.3. Identification of isolated compounds
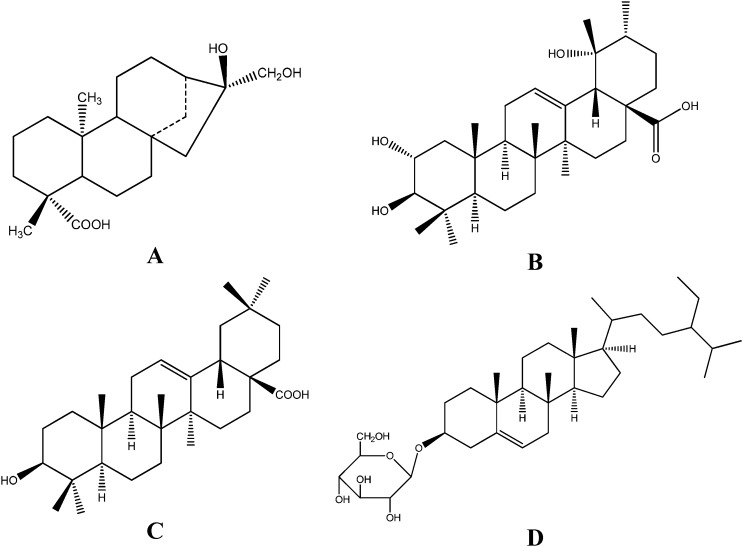
The structure identification of the isolated compounds was carried out via MS, 1H NMR, and 13C NMR analyses and compared with previously reported spectral data; the chemical structures of four compounds were identified and are shown in Fig. 4. The spectroscopic data are described below.
Fig. 4. Compounds and their chemical structures isolated from R. chingii: (A) ent-16α,17-dihydroxy-kauran-19-oic acid; (B) tormentic acid; (C) oleanolic acid; and (D) β-daucosterol.
Compound 1 was obtained as a white powder. The mass spectrometry analysis indicated that the m/z of [M + Na]+ is 359. 1H NMR (400 MHz, DMSO-d6) δ: 0.89 (3H, s, 20-CH3), 1.09 (3H, s, 18-CH3), 3.12 (1H, d, J = 13.5 Hz, 17-CH2), 3.23 (1H, d, J = 13.5 Hz, 17-CH2), 3.90 (1H, s, 16-OH), 4.51 (1H, t, 17-OH), 11.90 (1H, s, 19-COOH). 13C NMR (100 MHz, DMSO-d6) δ: 40.4 (C-1), 18.7 (C-2), 37.7 (C-3), 43.1 (C-4), 56.1 (C-5), 21.6 (C-6), 41.7 (C-7), 42.9 (C-8), 55.9 (C-9), 39.4 (C-10), 18.6 (C-11), 26.7 (C-12), 40.4 (C-13), 37.7 (C-14), 52.3 (C-15), 78.7 (C-16), 69.2 (C-17), 28.7 (C-18), 178.7 (C-19), 15.3 (C-20). By comparison of the above data with the literature,20 compound 1 was identified as ent-16α,17-dihydroxy-kauran-19-oic acid.
Compound 2 was obtained as a white powder. The mass spectrometry analysis indicated that the m/z of [M + Na]+ is 511. 1H NMR (400 MHz, C5D5N) δ: 1.02 (3H, s), 1.11 (3H, s), 1.13 (3H, s), 1.15 (3H, d, J = 6.5 Hz), 1.30 (3H, s), 1.44 (3H, s), 1.74 (3H, s), 3.08 (1H, s, 18-H), 3.41 (1H, d, J = 9.3 Hz, 3-H), 4.13 (1H, m, 2-H), 5.59 (1H, brs, 12-H). 13C NMR (C5D5N) δ: (C-1–30): 48.3, 69.1, 84.3, 40.4, 56.4, 19.5, 34.0, 40.9, 48.4, 39.0, 24.6, 128.4, 140.5, 42.6, 29.9, 26.9, 48.8, 55.1, 73.1, 42.9, 27.4, 39.0, 29.8, 18.2, 17.4, 17.7, 25.2, 181.3, 27.6, 17.3. Compound 2 was identified as tormentic acid according to the literature.21
Compound 3 was obtained as an acicular crystal and its molecular formula was inferred as C30H48O3 from the ESI-MS and NMR data. 1H NMR (400 MHz, C5D5N) δ: 0.89, 0.95, 1.01, 1.02, 1.02, 1.24, 1.28 (3H × 7, s), 3.31 (1H, dd, J = 13.5, 4 Hz, H-18), 3.44 (1H, dd, J = 10.8, 5.0 Hz, H-3α), 5.50 (1H, t, J = 3.0 Hz, H-12). 13C NMR (C5D5N) δ: (C-1–30): 39.0, 28.2, 78.2, 39.5, 55.9, 18.9, 33.4, 39.9, 48.2, 37.5, 23.9, 122.7, 144.9, 42.3, 28.4, 23.9, 46.8, 42.2, 46.6, 31.1, 34.3, 33.3, 28.9, 16.6, 15.7, 17.6, 26.3, 180.3, 33.4, 23.8. Based on the above results and comparison of the NMR and MS data with the literature,22 compound 3 was identified as oleanolic acid.
Compound 4 was obtained as a white amorphous powder and its molecular formula was inferred as C35H60O6 from the ESI-MS and NMR data. 1H NMR (400 MHz, C5D5N) δ: 0.66 (3H, d, J = 4.2 Hz, CH3), 0.93 (3H, s, CH3), 0.87 (3H, s, CH3), 0.88 (3H, s, CH3), 0.92 (3H, s, CH3), 0.85 (3H, s, CH3), 3.94 (1H, m, H-3), 5.36 (1H, s, H-6), 4.59 (1H, d, J = 10.5 Hz, H-1′), 3.75–4.50 (7H, m). 13C NMR (100 MHz, C5D5N) δ: (C-1–29, C-1′-6′): 37.5, 30.3, 78.1, 39.4, 140.9, 121.9, 32.3, 32.0, 50.2, 36.5, 21.4, 39.9, 42.6, 56.9, 24.5, 28.5, 56.3, 12.1, 19.2, 36.9, 19.0, 34.2, 26.3, 46.0, 29.4, 19.5, 20.1, 23.5, 12.0; 102.7, 75.3, 78.7, 71.8, 78.1, 62.9. These ESI-MS, 1H NMR, and 13C NMR data were similar to those in a previous paper,23 therefore, compound 4 was identified as β-daucosterol. Compounds 1 and 2 were isolated and identified from R. chingii for the first time.
2.4. Cytotoxicity evaluation of compounds from R. chingii
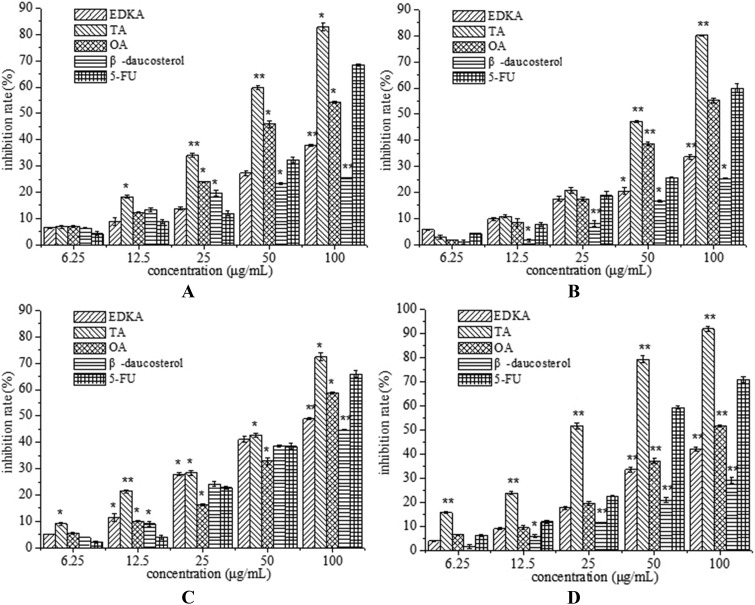
Anticancer activities of the four compounds were evaluated in terms of HepG-2, Bel-7402, A549 and MCF-7 cell proliferation. All of the tested compounds exhibited a dose-dependent effect on HepG-2, Bel-7402, A549 and MCF-7 cell viability and proliferation (Fig. 5). Tormentic acid (TA) showed excellent cytotoxicity against HepG-2, Bel-7402, A549 and MCF-7 cancer cell lines, and the inhibition rate reached 82.81%, 80.18%, 72.41% and 91.84% at the concentration of 100 μg mL–1, respectively, which exceeded those of 5-fluorouracil. The IC50 values of tormentic acid against HepG-2, Bel-7402, A549 and MCF-7 cells are 40.57 μg ml–1, 54.22 μg ml–1, 62.36 μg ml–1, and 24.23 μg ml–1, respectively. Oleanolic acid (OA) exhibited strong cytotoxicity against Bel-7402 cell lines, which was significantly higher than ent-16α,17-dihydroxy-kauran-19-oic acid (EDKA) and β-daucosterol, and its inhibition rate reached 55.2% at the concentration of 100 μg mL–1 and was very close to that of 5-fluorouracil (Fig. 5B). The cell proliferation inhibition rates of OA against HepG-2, A549 and MCF-7 cells were also higher than those of EDKA and β-daucosterol. Studies have reported that both tormentic acid and oleanolic acid exhibited antioxidant activity,24,25 which indicates that there might be a certain connection between antioxidant activity and antiproliferative activity to some extent. The morphological changes of MCF-7 cells after treatment of tormentic acid (TA) for 24 h were examined by phase-contrast microscopy. Tormentic acid is a terpenoid and exhibited the strongest cytotoxicity in our study, thus, the molecular targets and the molecular mechanisms underlying the anti-tumor activities of TA will be the focus of next research.
Fig. 5. Cell proliferation inhibition rate of isolated compounds: ent-16α,17-dihydroxy-kauran-19-oic acid (EDKA), tormentic acid (TA), oleanolic acid (OA), and β-daucosterol against HepG-2 (A), Bel-7402 (B), A549 (C) and MCF-7 (D) human cancer cell lines. Results are mean ± SD. *P < 0.05, **P < 0.01, indicating statistical significance in comparison with the control.
3. Materials and methods
3.1. Plant materials
Dried R. chingii, cultivated in Jiangsu, China, was purchased in Qingping TCM market (Guangzhou China). The plant material was authenticated by South China Botanical Garden, Chinese Academy of Sciences, where voucher specimens (voucher specimen number 50838) were kept. The samples were air-dried under shade for 1 week and pulverized to powder, which were stored in a well-closed container for further use.
3.2. Reagents
The 2,2-diphenyl-1-picryl-hydrazil radical (DPPH) and 3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The solvents used for HPLC (high performance liquid chromatography) were of HPLC grade. All the other chemicals used were of analytical grade.
3.3. Extraction, isolation, and purification
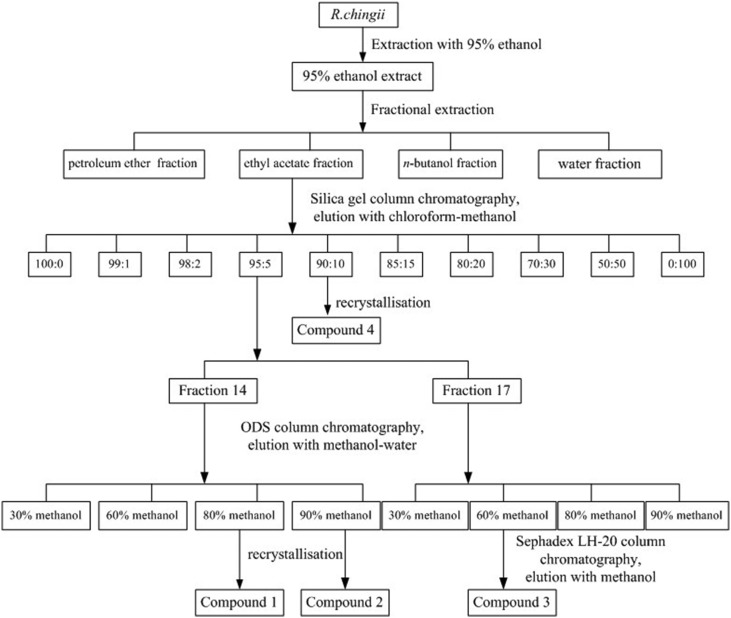
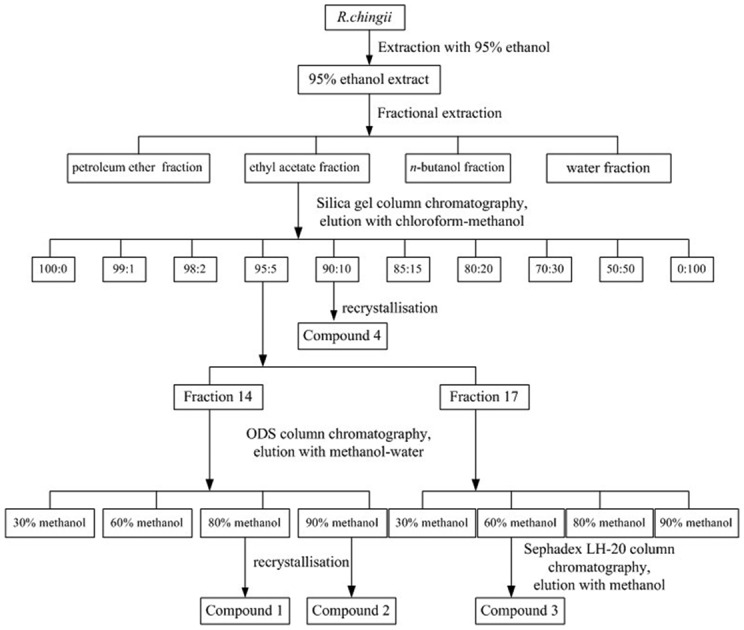
The dried power (5 kg) of R. chingii was exhaustively extracted with 95% (v/v, 5 × 10 L) ethanol by refluxing for 3 h at 80 °C. The supernatant was collected and concentrated under reduced pressure, using a rotatory evaporator, yielding crude 95% ethanol extract (95% EtOH). The extract (680 g; 13.6%) was then suspended in water and successively partitioned with 4 L of petroleum ether, ethyl acetate and n-butanol. The four fractions were evaporated to dryness under reduced pressure to obtain the petroleum ether layer (PE), ethyl acetate layer (EA), n-butanol layer (n-BuOH) and water layer (W), respectively. The process of R. chingii extraction is shown in Fig. 1.
Fig. 1. Extraction and isolation procedure of compounds from the 95% ethanol extract of R. Chingii.
In the bioactivity screening trials, the EA fraction showed better activity among the four fractions. Therefore, the EA fraction was chosen for further isolation (Fig. 1). The EA extract fraction was subjected to column chromatography on a silica gel column (80 × 1200 mm, 200–300 meshes) and eluted with gradient systems of chloroform and methanol (100 : 0, 99 : 1, 98 : 2, 95 : 5, 90 : 10, 85 : 15, 80 : 20, 70 : 30, 50 : 50 and 0 : 100, v/v, each 20 L) to give 44 fractions (100 : 0, fractions 1–2; 99 : 1, fractions 3–7; 98 : 2, fractions 8–12; 95 : 5, fractions 13–17; 90 : 10, fractions 18–22; 85 : 15, fractions 23–27; 80 : 20, fractions 28–32; 70 : 30, fractions 33–37; 50 : 50, fractions 38–42; 0 : 100, fractions 43–44). Each fraction was measured by TLC (thin-layer chromatography) and HPLC (high-performance liquid chromatography) to determine the main compound. Fraction 14 (from the 95 : 5 fraction) was loaded on an ODS column and eluted with a mixture of methanol and water (30, 60, 80, and 90%, v/v, each 2.5 L) to obtain 40 fractions (every 250 mL of the eluant was collected). The 80% methanol fraction (the first two tubes) appears as a crystal, and compound 1 was obtained (147.6 mg). Compound 2 (298.5 mg) was obtained from the 90% methanol fraction (the first five tubes) by recrystallisation. Fraction 17 (from the 95 : 5 fraction) was also loaded on an ODS column and eluted with a mixture of methanol and water (30, 60, 80, and 90%, v/v, each 2.5 L) to obtain 4 fractions. The 60% methanol fraction was further purified by Sephadex LH-20 column chromatography, and compound 3 was obtained (12 mg). A large amount of white particulate matter was precipitated from fraction 19 (from the 90 : 10 fraction) during placement, then compound 4 (322.3 mg) was obtained by recrystallisation. The purity of the four compounds was detected by HPLC, and all were >96%.
3.4. Assays for DPPH radical scavenging
The free radical scavenging capability of the extracts was analysed by the DPPH assay as previously described, with some modifications.26 Briefly, three milliliters of DPPH ethanolic solution was added to a total of 1.0 mL of the sample in ethanol. The mixture was allowed to stand for 30 min in the dark, and the absorbance was monitored at 517 nm. The percentage of DPPH scavenging capacity was determined as DPPH scavenging ability = (Acontrol – Asample/Acontrol) × 100.
3.5. Assays for total reducing power
The reducing power was measured according to a modified method of Pownall, Udenigwe, and Aluko.8 Different concentrations of samples in phosphate buffer (250 μL, 100 mM, pH 6.6) were added to 250 μL of 1% potassium ferricyanide solution at 50 °C for 20 min. After incubation, 250 μL of 10% trichloroacetic acid was mixed with 250 μL of the incubated mixture, 50 μL of 0.1% ferric chloride and 250 μL of distilled water. The sample was additionally incubated for 10 min at room temperature (25 °C) and then the absorbance was recorded at 700 nm. A higher absorbance of the reaction mixture implied a higher reducing power.
3.6. Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was performed according to Garzon et al.,27 with slight modifications. Briefly, 25 μL of the sample was added to a microplate, which also contained a blank (200 μL of PBS, 75 mM, pH 7.1) and a control (25 μL of PBS). Then 150 μL of 40 nmol fluorescein (in PBS) was transferred to the control and sample wells. After incubation (37 °C, 30 min), 25 μL AAPH (153 mmol L–1 in PBS) was added to all of the wells except for the blank. Fluorescence readings were determined every minute for 60 min. Final ORAC values were measured using a regression equation between Trolox concentration and the net area under the curve (AUC) and were expressed as μmol TE (Trolox equivalents) per g of extract.
3.7. Human cell lines and culture
Human hepatoma cells HepG-2 and Bel-7402, lung cancer cell A549, and breast cancer cell MCF-7 were purchased from the cell bank of the Chinese Academy of Sciences (CAS, Shanghai, China). The above human cancer cell lines were cultured in Dulbecco's modified Eagle medium (DMEM), supplied with 10% (v/v) heat-inactivated foetal bovine serum (FBS), penicillin (100 U mL–1) and streptomycin (100 μg mL–1). The cells were maintained at 37 °C with 5% CO2 in a humidified incubator and the medium was changed every 24–48 h.28
3.8. Cell proliferation assay
For in vitro assay, to avoid confluence of the culture during the treatments, human cells were seeded into a 96-well plate at a density of 5 × 104 cells (100 μL) per well. The treatments were started 24 h after seeding to improve environment adaptation of the cells.29,30 The dried samples were firstly dissolved in DMSO and then were diluted in the culture medium into different concentrations. The final concentration of DMSO in the culture medium was maintained at less than 0.1% (v/v) in order to avoid solvent toxicity. One hundred microliters of fresh medium containing various concentrations of samples were added. After incubation for 24 h, the supernatant was discarded and the MTT cytotoxicity test was used to determine the cell proliferation rate.31 The cells were incubated in 200 μL of fresh medium containing 20 μL of MTT (0.5 mg mL–1) solution for 4 h at 37 °C. Metabolically active mitochondrial dehydrogenases convert the tetrazolium salt to insoluble purple formazan crystals at a rate that is proportional to cell viability.32,33 After removal of the supernatant, 150 μL of DMSO was added to each well, to lyse the cells and solubilize the formed formazan crystals. The absorbance was determined at 490 nm after the plates had been shaken for 10 min. The cell proliferation inhibition rate was measured using the following formula: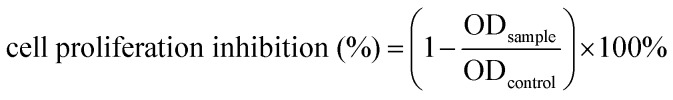
3.9. Statistical analysis
Each trial was performed in triplicate, and the mean values were determined. The data were recorded as means ± standard deviations. The samples were detected in triplicate, and one-way analysis of variance was carried out using SPSS 11.5 software. Significant differences were measured at P < 0.05, and graphs were drawn with Origin 6.0 software.
4. Conclusion
In conclusion, an efficient method for bioassay-guided preparation and isolation was used to identify the antioxidant and antitumor constituents in R. chingii. Chromatographic separation of the ethyl acetate fraction of 95% ethanol extract resulted in the isolation and identification of four compounds, ent-16α,17-dihydroxy-kauran-19-oic acid, tormentic acid, oleanolic acid and β-daucosterol, among which ent-16α,17-dihydroxy-kauran-19-oic acid and tormentic acid were isolated and identified from R. chingii for the first time. Antioxidant and antitumor activities of the crude extract, four fractions, and four isolated compounds were determined. The ethyl acetate fraction exhibited effective antioxidant and antitumor effects. In the evaluation of cytotoxicity against tumor cell lines, tormentic acid showed excellent cytotoxicity against HepG-2, Bel-7402, A549 and MCF-7 cancer cell lines at high concentrations compared with 5-fluorouracil. These outcomes showed that the active substances gathered into the EA fraction after the fractional extraction of 95% EtOH extract into four fractions. Strong cytotoxicity against selected tumour cell lines indicated that tormentic acid warrants further testing as a potential effective nutraceutical compound and chemotherapeutic drug. The outcomes obtained in this study might contribute to the understanding of the bioactivities and further research of R. chingii for food and drug applications.
Conflict of interest
The authors declare no competing interests.
Acknowledgments
This project was supported by the National Natural Foundation of China (grant 31301453).
References
- Zhang T. T., Jiang J. G. Food Anal. Method. 2015;8:937–944. [Google Scholar]
- Han N., Gu Y. H., Ye C., Cao Y., Liu Z. H., Yin J. Food Chem. 2012;132:181–185. doi: 10.1016/j.foodchem.2011.10.051. [DOI] [PubMed] [Google Scholar]
- Ding H. Y. Int. J. Mol. Sci. 2011;12:3941–3949. doi: 10.3390/ijms12063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau M. H., Che C. T., Liang S. M., Kong Y. C., Fong W. P. Life Sci. 2002;72:329–338. doi: 10.1016/s0024-3205(02)02239-7. [DOI] [PubMed] [Google Scholar]
- Malheiro R., Mendes P., Fernandes F., Rodrigues N., Bento A., Pereira J. A. Food Funct. 2014;5:3132–3142. doi: 10.1039/c4fo00560k. [DOI] [PubMed] [Google Scholar]
- Chahdoura H., Barreira J. C. M., Barros L., Santos-Buelga C., Ferreira I. C. F. R., Achour L. Food Funct. 2014;5:2129–2136. doi: 10.1039/c4fo00456f. [DOI] [PubMed] [Google Scholar]
- Torres-Fuentes C., Contreras M. D. M., Reciob I., Alaiza M., Vioquea J. Food Chem. 2015;180:194–202. doi: 10.1016/j.foodchem.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Pownall T. L., Udenigwe C. C., Aluko R. E., Agr J. Food Chem. 2010;58:4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Bogianchini M., Cerezo A. B., Gomis A., López F., García-Parrilla M. C. LWT--Food Sci. Technol. 2011;44:1369–1375. [Google Scholar]
- Fontana A. R., Antoniolli A., Bottini R. J. Agric. Food Chem. 2013;61:8987–9003. doi: 10.1021/jf402586f. [DOI] [PubMed] [Google Scholar]
- Liao W., Lai T., Chen L., Fu J., Sreenivasan S. T., Yu Z., Ren J. J. Agric. Food Chem. 2016;64:1509–1519. doi: 10.1021/acs.jafc.5b04924. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Keaney J. F. Free Radical Biol. Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosaa V., Molinéa T., Somozaa R., Paciuccib R., Kondohc H., LLeonart M. E. Ageing Res. Rev. 2008;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Oh J. Y., Giles N., Landar A., Darley-Usmar V. Biochem. J. 2008;411:297–306. doi: 10.1042/bj20071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P., Jacintho J. D. Curr. Med. Chem. 2001;8:773–796. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- Borrelli A., Schiattarella A., Mancini R., Morelli F., Capasso C., Luca V. D., Gori E., Mancini A. Int. J. Cancer. 2011;128:453–459. doi: 10.1002/ijc.25334. [DOI] [PubMed] [Google Scholar]
- Dhar S. K., Tangpong J., Chaiswing L., Oberley T. D., Clair D. K. S. Cancer Res. 2011;1:6684–6695. doi: 10.1158/0008-5472.CAN-11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore G., Massarelli P., Sajeva M., Franchi G. G. Rev. Bras. Farmacogn. 2012;22:381–388. [Google Scholar]
- Cheng X. X., Xiao Y. P., Wang X. B., Wang P. J. Ethnopharmacol. 2012;143:49–56. doi: 10.1016/j.jep.2012.05.054. [DOI] [PubMed] [Google Scholar]
- Lu H. C., Wang J. X. Zhongyaocai. 2007;23:489–491. [Google Scholar]
- Taniguchia S., Imayoshia Y., Kobayashi E., Takamatsua Y., Itoa H., Hatanoa T. Phytochemistry. 2002;59:315–323. doi: 10.1016/s0031-9422(01)00455-1. [DOI] [PubMed] [Google Scholar]
- Oshima N., Zaima K., Kamakura H., Hamato A., Yamamoto Y., Kang D. H. J. Nat. Med. 2015;69:68–75. doi: 10.1007/s11418-014-0864-6. [DOI] [PubMed] [Google Scholar]
- Lee S., Han S., Kim H. M., Lee J. M., Mok S. Y., Lee S. J. Korean Soc. Appl. Biol. Chem. 2011;54:73–78. [Google Scholar]
- Wei Z. B., Sun J. M., Li P. F., Wang S., Zhang H., Lin Z. Zhongguo Zhong Yao Za Zhi. 2012;37:3591–3594. [PubMed] [Google Scholar]
- Jiang H. B., Dong X. P., Tian R. J., Zhang Z. Q. Huaxi Yaoxue Zazhi. 2015;30:163–164. [Google Scholar]
- Simirgiotis M. J., Schmeda-Hirschmann G. J. Food Compos. Anal. 2010;23:545–553. [Google Scholar]
- Garzon G. A., Manns D. C., Riedl K., Schwartz S. J., Padilla-Zakour O. J. Agric. Food Chem. 2015;63:1803–1811. doi: 10.1021/jf503366c. [DOI] [PubMed] [Google Scholar]
- Zhang T. T., Lu C. L., Jiang J. G. Food Funct. 2014;5:1747–1754. doi: 10.1039/c4fo00169a. [DOI] [PubMed] [Google Scholar]
- Schroeder B. R., Ghare M. I., Bhattacharya C., Paul R., Yu Z., Zaleski P. A., Bozeman T. C., Rishel M. J., Hecht S. M. J. Am. Chem. Soc. 2014;136:13641–13656. doi: 10.1021/ja507255g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. Y., Yang L., Jiang J. G. Food Funct. 2017;8:796–807. doi: 10.1039/c6fo01545j. [DOI] [PubMed] [Google Scholar]
- Huang Y., Yang X., Xu T. R., Kong Q. H., Zhang Y. P., Shen Y. H., Wei Y. L., Wang G. L., Chang K. J. Int. J. Oncol. 2016;49:153–163. doi: 10.3892/ijo.2016.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. Y., Zhang T. T., Zhang W. L., Jiang J. G. Food Funct. 2016;7:4451–4459. doi: 10.1039/c6fo00795c. [DOI] [PubMed] [Google Scholar]
- Liao W., Lu Y., Fu J., Ning Z., Yang J., Ren J. J. Agric. Food Chem. 2015;63:6525–6534. doi: 10.1021/acs.jafc.5b00614. [DOI] [PubMed] [Google Scholar]