 Screening of a library of natural derivatives for their virus entry inhibition activity using pseudotype systems shows bornyl ester derivatives containing saturated N-heterocycles exhibiting the highest antiviral activity.
Screening of a library of natural derivatives for their virus entry inhibition activity using pseudotype systems shows bornyl ester derivatives containing saturated N-heterocycles exhibiting the highest antiviral activity.
Abstract
There is currently no approved antiviral therapy for treatment of Marburg virus disease (MVD). Although filovirus infection outbreaks are quite rare, the high mortality rates in such outbreaks make the development of anti-filoviral drugs an important goal of medical chemistry and virology. Here, we performed screening of a large library of natural derivatives for their virus entry inhibition activity using pseudotype systems. The bornyl ester derivatives containing saturated N-heterocycles exhibited the highest antiviral activity. It is supposed that compounds with specific inhibitory activity toward MarV-GP-dependent virus entry will inhibit the rVSIV-ΔG-MarV-GP pseudotype much more efficiently than the control rVSIV-ΔG-G pseudotype. At the same time, the compounds similarly inhibiting both pseudotypes will likely affect rVSIV capsid replication or the cellular mechanisms common to the entry of both viruses. Borneol itself is not active against both pseudotypes and is nontoxic, whereas its derivatives have varying toxicity and antiviral activity. Among low-toxic borneol derivatives, six compounds turned out to be relatively specific inhibitors of MarV-GP-mediated infection (SC > 10). Of them, compound 6 containing a methylpiperidine moiety exhibited the highest virus-specific activity. Notably, the virus-specific activity of this compound is twice as high as that of the reference.
Marburg and Ebola viruses are enveloped negative-stranded RNA viruses that belong to the filovirus family (Latin filum, thread) and cause severe hemorrhagic fever with a high mortality rate in humans and nonhuman primates. The largest filovirus hemorrhagic fever outbreaks were the MVD epidemic in Angola in 2004–2005 and the Ebola virus disease (EVD) epidemic in West Africa in 2014. Both infections have similar clinical manifestations, and their virions are similar in morphology; however, there are differences in their antigenic structure. Humans are infected through contact with body fluids of a virus carrier. Currently, there is no FDA-approved treatment for filovirus fevers, however, a number of candidate drugs have exhibited an anti-filoviral activity in vitro and in vivo in animal models.1 Considerable efforts have been made to create monoclonal antibodies for the treatment of filovirus infections.2,3
There are two main “targets” in therapy for virus infections – the human body and the virus. The first one focuses on using interferon drugs, therapeutic vaccines, and inhibitors of various host proteins.4 The second approach involves direct-acting antivirals agents that inhibit specific viral enzymes (DNA or RNA polymerases, proteases, helicases, neuraminidases). The filoviral life cycle involves several stages: attachment to the target cell, viral entry, capsid uncoating, replication, viral particle assembly, and virion release from the cell.5 Each stage may be a potential target for antiviral drug design. The filovirus entry into the target cell is mediated by the activity of a surface glycoprotein (GP) present on the virion surface in a prefusion conformation, as a trimer consisting of a receptor-binding domain GP1 and a fusion domain GP2.6 During cell entry by endocytosis, virions interact with several cellular factors. GP is converted into its active form inside the endosome due to GP1 domain cleavage by low pH-dependent cellular proteases, cathepsins B and L. Then, processed GP1 interacts with the Niemann–Pick C1 protein (NPC-1), which is an endosomal cholesterol transporter located on the membrane of late endosomes/lysosomes.7 Filoviral entry was shown to depend on NPC-1 expression, but not on NPC-1-dependent lipid transport.8 NPC-1 acts as a membrane receptor for filoviruses, which is required for GP2-dependent fusion of viral and endosomal membranes and release of the viral capsid into the cytoplasm. Furthermore, it was demonstrated that filoviruses require endosomal calcium channels, called two-pore channels (TPCs), to successfully enter the cell, and their inhibitors are able to inhibit filovirus infection.9
Virus entry is an attractive target for therapy because blocking infection at an early stage decreases the virus replication-associated cytopathic effect and reduces the risk of emergence of drug resistance. Therefore, entry inhibitors may play an important role, particularly, in prevention of viral infection. Since cell entry and membrane fusion are controlled by viral surface proteins, the search for entry inhibitors may involve pseudotyped viruses – recombinant, biologically safe viral particles comprising a capsid of one virus and a surface protein of another (“foreign”, often more pathogenic) virus. The use of pseudotypes enables searching for compounds that can specifically inhibit early viral infection steps associated with a “foreign protein”. The biological safety of pseudotypes is related to the fact that they do not form viral progeny in target cells due to targeted pseudotype genome editing. Thus, their application is especially convenient in searching for inhibitors of highly pathogenic viruses because such pseudotypes can be handled in BSL-2 laboratories.
Pseudotyped viruses have been widely used as models for the discovery of virus entry inhibitors.10 The most commonly used pseudotypes are based on the lentivirus capsid or the vesicular stomatitis Indiana virus capsid. Pseudotypes carrying MARV or EBOV surface proteins have been extensively used for the screening of small molecule libraries11,12 as well as for retargeting of currently approved drugs for their use as filovirus entry inhibitors.13,14
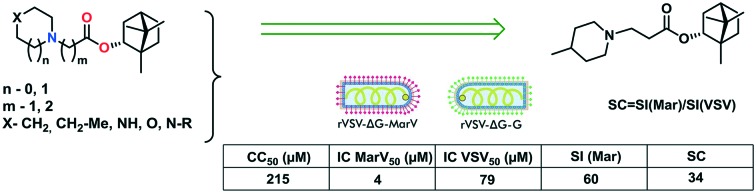
Although filovirus disease outbreaks are quite rare, the high mortality rates in such outbreaks (ranging from 24% to 88% for MVD) make the development of anti-filoviral drugs an important goal of medical chemistry and virology.15 Natural compounds, in particular, monoterpenoids, are known to have a wide spectrum of biological activities, including an antiviral one.16 Here, we screened over 170 natural derivatives in a Marburg glycoprotein-mediated VSIV pseudotype system. The library of natural compounds includes the derivatives of (+)-camphor,17,18 (–)-borneol,19,20 dehydroabietylamine,21,22 para-mentha-1,8-dien-5,6-diol,23–26 (–)-isopulegol,27 deoxycholic acid,28 (+)-usninic acid,29 aminoadamantane30,31 and azaadamantane32 which were previously described by our colleagues. Among the tested derivatives, the bornyl ester containing saturated N-heterocycles exhibited the highest antiviral activity with several hit compounds identified.
Borneol, a bicyclic monoterpenoid alcohol, exists in two enantiomeric forms: d and l. Both borneol forms occur in essential oils of numerous medicinal plants, such as valerian (Valeriana officinalis), chamomile (Matricaria chamomilla), and lavender (Lavandula officinalis). Borneol is widely used in food and drug industries, as well as in traditional oriental medicine. Generally, there are two isomers of borneol: borneol and isoborneol, differing in the hydroxyl group location. These compounds have a wide biological activity spectrum. Recently, isoborneol was found to exhibit an antiviral activity against the herpes simplex virus type 1;33 both borneol enantiomers were found to have a highly efficient positive modulating effect on mammalian GABA (γ-aminobutyric acid) inhibitory neurotransmitter receptors.34
The studied library includes borneol itself as well as two series of N-containing bornyl ester derivatives differing in the length of the aliphatic chain between the nitrogen atom and the ester group. For example, compounds 1, 3, 5, 7, 9, 11, 13, and 15 have one CH2 group, and compounds 2, 4, 6, 8, 10, 12, 14, and 16 have two CH2 groups. Synthesis of the target compounds and investigation of their activity against the influenza virus were described earlier.19
Briefly, the screening strategy was as follows. First, the entire small molecule library was screened for rVSIV-ΔG-MarV-GP pseudotype entry inhibition at the concentration of 25 mg ml–1. Since most of the active compounds were identified among the bornyl ester derivatives containing saturated N-heterocycles, we focused on this class of compounds. In relation to this, we evaluated the toxicity of the prepared compounds toward target HEK293 cells (kindly provided by Prof. R. A. Davey, Texas Biomedical Research Institute) using the MTT assay (see the ESI† for details) and determined their 50% cytotoxic concentration (CC50). On the basis of CC50 values, we selected a range of low-toxic concentrations for each compound to investigate its antiviral activity. Pseudotype preparations having the same recombinant VSIV capsid but differing in surface glycoproteins (GPs), MARV GP (rVSIV-ΔG-MarV-GP) or VSIV glycoprotein G (rVSIV-ΔG-G), were produced according to the previously published procedures (see the ESI†).35 Entry inhibition by the tested compounds was assayed by infecting the target HEK293 cells with both pseudotypes in the presence or absence of the inhibitors and measuring the transduced luciferase signal 24 h post-infection.
For all studied compounds, the half maximal inhibitory concentration (IC50) was determined for both rVSIV-ΔG-MarV-GP and rVSIV-ΔG-G pseudotypes. Further, the selectivity index (SI), a ratio of compound toxicity to inhibitory activity against rVSIV-ΔG-MarV-GP (CC50/ICMarV50), and the inhibitor specificity coefficient (SC), a ratio of half maximal inhibitory concentrations for the two pseudotypes (ICMarV50/ICVSIV50), were calculated for each compound. An ion channel inhibitor, verapamil, was used as a reference compound inhibiting filovirus infection but not affecting VSIV infection at therapeutic concentrations.36 The CC50, ICMarV50, ICVSIV50, SI, and SC values for all tested compounds are presented in Table 1.
Table 1. Specificity and selectivity of borneol ester derivatives as virus entry inhibitors.
| |||||||
| Compound | R | n | CC50 (μM) | ICMarV50 (μM) | ICVSIV50 (μM) | SI | SC |
| (–)-Borneol | — | — | >3000 | 203 | >650 | >14 | >3 |
| 1 |
|
1 | 480 ± 25 | 52 ± 5 | 239 ± 19 | 9 | 5 |
| 2 |
|
2 | 684 ± 67 | 12 ± 1 | 29 ± 6 | 59 | 2 |
| 3 |
|
1 | 321 ± 33 | 86 ± 11 | 102 ± 9 | 4 | 1 |
| 4 |
|
2 | 302 ± 23 | 9 ± 1 | 121 ± 7 | 35 | 14 |
| 5 |
|
1 | 240 ± 18 | 28 ± 2 | 77 ± 16 | 9 | 3 |
| 6 |
|
2 | 215 ± 25 | 4 ± 1 | 79 ± 19 | 60 | 34 |
| 7 |
|
1 | 1279 ± 14 | 263 ± 24 | 267 ± 10 | 5 | 1 |
| 8 |
|
2 | 406 ± 33 | 78 ± 7 | 257 ± 12 | 5 | 3 |
| 9 |
|
1 | 637 ± 18 | 47 ± 3 | 187 ± 24 | 14 | 4 |
| 10 |
|
2 | 421 ± 4 | 19 ± 1 | 318 ± 23 | 20 | 16 |
| 11 |
|
1 | 859 ± 21 | 29 ± 2 | 447 ± 15 | 29 | 15 |
| 12 |
|
2 | 474 ± 29 | 10 ± 2 | 106 ± 5 | 47 | 11 |
| 13 |
|
1 | 361 ± 7 | 20 ± 1 | 69 ± 13 | 18 | 3 |
| 14 |
|
2 | 207 ± 9 | 10 ± 4 | 102 ± 25 | 21 | 10 |
| 15 |
|
1 | 73 ± 5 | 11 ± 1 | 20 ± 1 | 6 | 2 |
| 16 |
|
2 | 86 ± 4 | 6 ± 1 | 48 ± 5 | 15 | 8 |
| Verapamil | — | 280 | 13 ± 1 | >200 | >21 | >15 | |
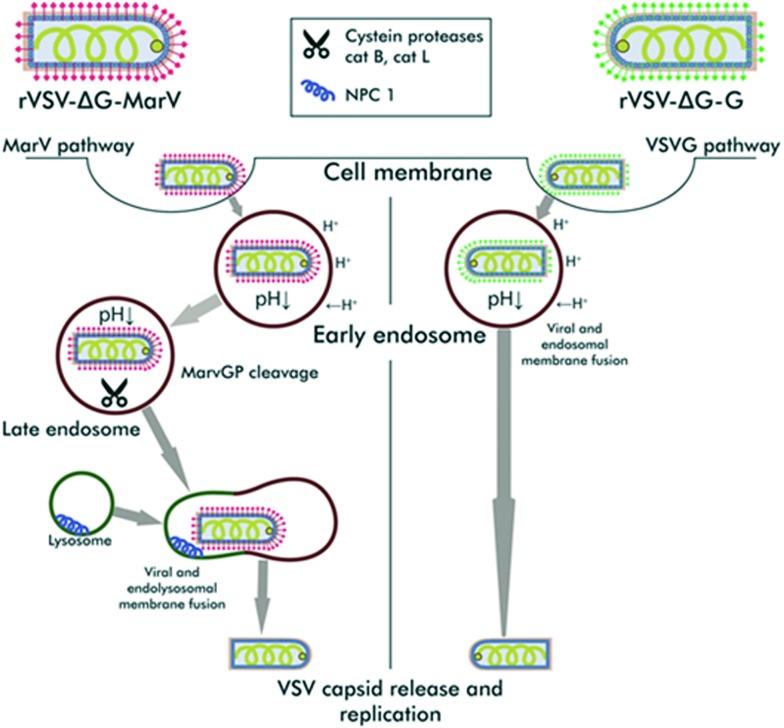
Cell entry is mediated by the interactions of viral glycoproteins with a variety of cellular factors and mechanisms, some of which are employed by many viruses, while others are unique to filoviruses (Fig. 1). A specific filovirus entry inhibitor is expected to affect the interactions of the MARV glycoprotein with cellular factors unique to filoviruses (such as the NPC1 receptor), while the compounds influencing the cell factors common to several viruses will likely be non-specific. Thus, the compounds with specific inhibitory activity toward MarV-GP-dependent entry will inhibit rVSIV-ΔG-MarV-GP much more efficiently than the control rVSIV-ΔG-G pseudotype. At the same time, the compounds similarly inhibiting both pseudotypes will likely affect rVSIV capsid replication or the cellular mechanisms common to the entry of both viruses (composition and properties of cell membranes, endocytosis activity, acidification of the endosome contents, etc.). Inhibition of nonspecific interactions can often be associated with higher toxicity to cells.
Fig. 1. Both pseudotypes enter via endocytosis and initially share the early endocytic pathway. In early endosomes, the VSIV-G pseudotype merges endosomal and viral membranes and releases its capsid into the cytosol. In contrast, the MarV-GP pseudotype continues its traffic along the endosomal pathway and undergoes proteolytic cleavage by cathepsins in late endosomes. After the endolysosomal fusion, the MarV-GP pseudotype binds to its cognate receptor, NPC1, thus, inducing membrane fusion and releasing the capsid into the cytosol. Specific MarV-GP inhibitors are expected to inhibit the stages of pseudovirus entry, which are not shared with the VSIV-G pseudovirus.
We showed that borneol itself is not active against both pseudotypes and is nontoxic, whereas its derivatives have varying toxicity and antiviral activity. The highest toxicity was exhibited by compounds containing an acyclic substituent at a tertiary nitrogen atom: compounds 15 and 16. Among the low-toxic borneol derivatives, six compounds turned out to be relatively specific inhibitors of MarV-GP-mediated infection (SC > 10). Of them, compound 6 containing a methylpiperidine moiety exhibited the highest virus-specific activity. Notably, the virus-specific activity of this compound is twice as high as that of the reference compound (see the ESI† for details). The other compounds, regardless of their toxicity, either inhibited both rVSIV-ΔG-MarV-GP and rVSIV-ΔG-G* pseudotype infections or had no inhibitory properties.
We established some relationships between the structure of the heterocyclic moiety and the compound properties. For example, the presence of the morpholino moiety (compounds 7 and 8) did not have a significant effect on the toxicity of the compounds, and agents 7 and 8 do not exhibit activity against rVSIV-ΔG-MarV-GP. Meanwhile, these compounds were previously shown to exhibit pronounced activity against the influenza virus.19 Borneol derivatives containing the pyrrolidine moiety (1 and 2) exhibit toxicity and antiviral activity against both pseudotypes, i.e. they are non-specific inhibitors. Compounds containing methylpiperazine (9 and 10) and ethylpiperazine (11 and 12) moieties have a high therapeutic index against rVSIV-ΔG-MarV-GP exceeding that of the reference compound. It may be noted that the toxicity of the unsubstituted piperazine derivatives (13 and 14) is generally higher than those of similar substituted compounds.
In addition, we found that the selectivity index of the produced compounds was generally dependent on the length of the aliphatic chain. Compounds with a longer chain length occurred to be more active against rVSIV-ΔG-MarV-GP and have a greater selectivity index. In this case, there was no direct relationship between the length of the linker and compound toxicity. In addition, in piperidine derivatives 3–6, a change in the linker length caused not only an increase in SI but also more than a 10-fold increase in the inhibitory specificity against rVSIV-ΔG-MarV-GP. For example, the ability of compound 6 to inhibit entry of the Marburg virus (ICMarV50, 4 μM) was comparable to that of a well-known filovirus infection inhibitor – the ion channel inhibitor, verapamil (IC50, 13 μM).
Thus, we demonstrated that some N-heterocyclic bornyl esters are able to specifically inhibit MarV entry with the efficiency being comparable to that of the previously described specific filovirus entry inhibitor (verapamil). Most compounds are relatively nontoxic to cells, while those which are toxic should not be considered as clinically relevant inhibitors. Further, our studies should reveal molecular mechanisms responsible for filovirus entry inhibition, and the obtained data may be useful for development of more active and specific borneol-based inhibitors of filovirus infection.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This work was supported by the Russian Foundation Research (N 15-03-00193A). The biological study was supported by the task N 17.5484.2017/BY.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00424a
References
- Kaushik A., Tiwari S., Dev Jayant R., Marty A., Nair M. Biosens. Bioelectron. 2016;75:254. doi: 10.1016/j.bios.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire C., Geisbert J., Borisevich V., Fenton K., Agans K., Flyak A., Deer D., Steinkellner H., Bohorov O., Bohorova N., Goodman C., Hiatt A., Kim D., Pauly M., Velasco J., Whaley K., Crowe Jr. J., Zeitlin L., Geisbert T. Sci. Transl. Med. 2017;9:eaai8711. doi: 10.1126/scitranslmed.aai8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire E. Immunotherapy. 2013;5:1221. doi: 10.2217/imt.13.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Guo J., Du Y., Block T. Emerging Microbes Infect. 2013;2:e77. doi: 10.1038/emi.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Canard B., Decroly E. Antiviral Res. 2017;141:48. doi: 10.1016/j.antiviral.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Martin B., Hoenen T., Canard B., Decroly E. Antiviral Res. 2016;135:1. doi: 10.1016/j.antiviral.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Lennemann N., Maury W. Viruses. 2012;4:258. doi: 10.3390/v4020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M., Misasi J., Ren T., Bruchez A., Lee K., Filone C., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Nature. 2011;477:344. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring G., Rohrmann K., Atenchong N., Mittler E., Becker S., Dahlmann F., Pöhlmann S., Vondran F., David S., Manns M., Ciesek S., von Hahn T. J. Antimicrob. Chemother. 2014;69:2123. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar A., Pessi A., Glickman F., Sengupta U., Briese T., Whitt M., Mathieu C., Horvat B., Moscona A., Porotto M. PLoS One. 2012;7:e30538. doi: 10.1371/journal.pone.0030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantpadma M., Kouznetsova J., Wang H., Huang R., Kolokoltsov A., Guha R., Lindstrom A., Shtanko O., Simeonov A., Maloney D., Maury W., LaCount D., Jadhav A., Daveya R. Antimicrob. Agents Chemother. 2016;60:4471. doi: 10.1128/AAC.00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolina M., Wang J., Caffrey M., Rong L., Wardrop D. J. Med. Chem. 2011;54:765. doi: 10.1021/jm1008715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cui R., Li G., Gao Q., Yuan S., Altmeyer R., Zou G. Antiviral Res. 2016;125:1. doi: 10.1016/j.antiviral.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L., Brannan J., Delos S., Shoemaker C., Stossel A., Lear C., Hoffstrom B., Dewald L., Schornberg K., Scully C., Lehár J., Hensley L., White J., Olinger G. Sci. Transl. Med. 2013;5:190. doi: 10.1126/scitranslmed.3005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.who.int/csr/disease/marburg/en/ .
- Salakhutdinov N., Volcho K., Yarovaya O. Pure Appl. Chem. 2017;89:1105. [Google Scholar]
- Sokolova A., Yarovaya O., Shernyukov A., Gatilov Y., Razumova Y., Zarubaev V., Tretiak T., Kiselev O., Salakhutdinov N. Eur. J. Med. Chem. 2015;105:263. doi: 10.1016/j.ejmech.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Baev D., Shernyukov A., Shtro A., Zarubaev V., Salakhutdinov N. Eur. J. Med. Chem. 2017;127:661. doi: 10.1016/j.ejmech.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Semenova M., Shtro A., Orshanskay Y., Zarubaev V., Salakhutdinov N. Med. Chem. Commun. 2017;8:960. doi: 10.1039/c6md00657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova A., Yarovaya O., Shtro A., Borisova M., Morozova E., Tolstikova T., Zarubaev V., Salakhutdinov N. Chem. Heterocycl. Compd. 2017;53:371. [Google Scholar]
- Kovaleva K., Kononova A., Korobeynikov V., Cheresiz S., Zarubaev V., Shtro A., Orshanskaya Y., Yarovaya O., Pokrovsky A., Salakhutdinov N. Med. Chem. 2016;6:642. [Google Scholar]
- Kovaleva K., Yarovaya O., Shernyukov A., Zarubaev V., Shtro A., Orshanskaya Y., Salakhutdinov N. Chem. Heterocycl. Compd. 2017;53:364. [Google Scholar]
- Il'ina I., Volcho K., Mikhalchenko O., Korchagina D., Salakhutdinov N. Helv. Chim. Acta. 2011;94:502. [Google Scholar]
- Mikhalchenko O., Il'ina I., Pavlova A., Morozova E., Korchagina D., Tolstikova T., Pokushalov E., Volcho K., Salakhutdinov N. Med. Chem. Res. 2013;22:3026. [Google Scholar]
- Mikhalchenko O., Korchagina D., Volcho K., Salakhutdinov N. Helv. Chim. Acta. 2014;97:1406. [Google Scholar]
- Mikhalchenko O., Korchagina D., Volcho K., Salakhutdinov N. Beilstein J. Org. Chem. 2016;12:648. doi: 10.3762/bjoc.12.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazimova E., Pavlova A., Mikhalchenko O., Il'ina I., Korchagina D., Tolstikova T., Volcho K., Salakhutdinov N. Med. Chem. Res. 2016;25:1369. [Google Scholar]
- Popadyuk I., Markov A., Babich V., Salomatina O., Logashenko E., Zenkova M., Salakhutdinov N. Bioorg. Med. Chem. Lett. 2017;27:3755. doi: 10.1016/j.bmcl.2017.06.072. [DOI] [PubMed] [Google Scholar]
- Luzina O., Sokolov D., Komarova N., Salakhutdinov N. Chem. Nat. Compd. 2014;50:266. [Google Scholar]
- Suslov E., Ponomarev K., Rogachev A., Pokrovsky M., Pokrovsky A., Pykhtina M., Beklemishev A., Korchagina D., Volcho K., Salakhutdinov N. Med. Chem. 2015;11:629. doi: 10.2174/1573406411666150518110053. [DOI] [PubMed] [Google Scholar]
- Teplov G., Suslov E., Zarubaev V., Shtro A., Karpinskaya L., Rogachev A., Korchagina D., Volcho K., Salakhutdinov N., Kiselev O. Lett. Drug Des. Discovery. 2013;10:477. [Google Scholar]
- Ponomarev K., Pavlova A., Suslov E., Ardashov O., Korchagina D., Nefedov A., Tolstikova T., Volcho K., Salakhutdinov N. Med. Chem. Res. 2015;24:4146. doi: 10.2174/1573406413666170525124316. [DOI] [PubMed] [Google Scholar]
- Armaka M., Papanikolaou E., Sivropoulou A., Arsenakis M. Antiviral Res. 1999;43:79. doi: 10.1016/s0166-3542(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Granger R., Campbell E., Johnston G. Biochem. Pharmacol. 2005;69:1101. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Whitt M. J. Virol. Methods. 2010;169:365. doi: 10.1016/j.jviromet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Kolokoltsov A., Chen C., Tidwell M., Bauta W., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R. Science. 2015;347:995. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.