 Preclinical pharmacokinetic, efficacy, and toxicology results are reported for a series of DGAT2 inhibitors for the potential treatment of hypertriglyceridemia.
Preclinical pharmacokinetic, efficacy, and toxicology results are reported for a series of DGAT2 inhibitors for the potential treatment of hypertriglyceridemia.
Abstract
Small molecule DGAT2 inhibitors have shown promise for the treatment of metabolic diseases in preclinical models. Herein, we report the first toxicological evaluation of imidazopyridine-based DGAT2 inhibitors and show that the arteriopathy associated with imidazopyridine 1 can be mitigated with small structural modifications, and is thus not mechanism related.
Introduction
Hypertriglyceridemia is estimated to affect more than one third of adults in the United States.1 Excessive accumulation of triglycerides (TG) has been associated with metabolic disorders such as coronary artery disease, type 2 diabetes mellitus (T2DM) and non-alcoholic steatohepatitis (NASH).2–4 Diacylglycerol acyltransferases 1 and 2 (DGAT1 and DGAT2) catalyze the final step in TG biosynthesis, and their inhibition has been proposed as a therapeutic strategy for the treatment of metabolic disease.5 DGAT1 is abundantly expressed in the intestine while DGAT2 is mainly found in liver and adipose tissue.6–8 A number of selective small molecule inhibitors of DGAT1 have been reported to have an attractive preclinical profile,9–12 however, their clinical development has been hindered by significant gastrointestinal side effects.13,14
Targeted knock-out (KO) of Dgat2 gene in mice has been reported to cause early postnatal death and skin barrier dysfunctions.15 Thus, much of the preclinical characterization of DGAT2 inhibition has been derived from antisense oligonucleotides (ASO). Suppression of the DGAT2 isoform with ASO improved diet-induced hyperlipidemia, hepatic steatosis and insulin resistance in rodents.16–18 A number of small molecule DGAT2 inhibitors from distinct chemical series have been reported in the literature,19–23 and recently our group24 and others25,26 reported in vivo pharmacology results. Treatment of dyslipidemic rodents with the selective DGAT2 inhibitor PF-06424439 (compound 1)§ caused a reduction in plasma cholesterol as well as plasma and hepatic TG levels.24 As for any novel therapeutic mechanism and chemical series, not only efficacy but also safety must be assessed. Reported herein are results of safety studies with compound 1 and close structural analogues.
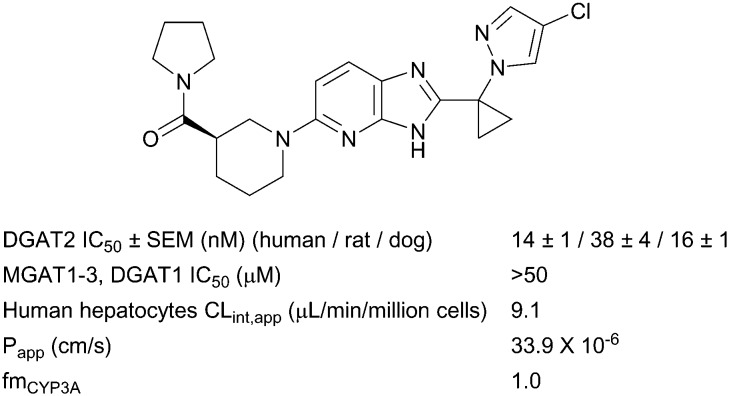
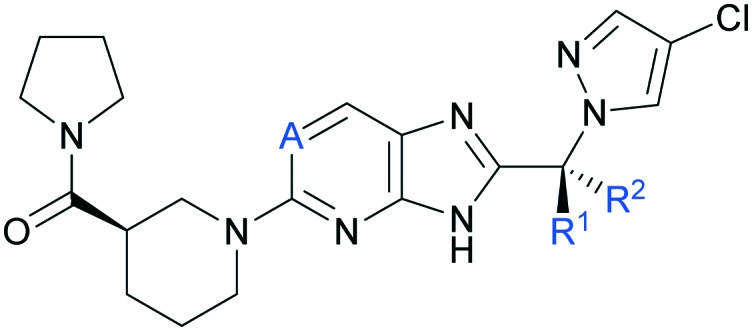
During optimization of the imidazopyridine series, compound 1 was identified as a potent DGAT2 inhibitor with excellent selectivity against functionally related monoacylglycerol acyltransferases 1, 2 and 3 (MGAT1-3) and DGAT1,¶ good metabolic stability and high passive permeability in in vitro assays (Fig. 1).‖ As previously reported, analogues from this series were rapidly cleared via N-glucuronidation of the core, and strategic introduction of a cyclopropyl ring between the two aromatic systems supressed clearance by this pathway.24 This modification provided compound 1 with a moderate rate of clearance in rat and dog, and a pharmacokinetic (PK) profile suitable for in vivo experiments in both species.24 Minimizing glucuronidation of the imidazopyridine core effectively shifted the clearance mechanism towards oxidative metabolism. Metabolism occurred almost exclusively via CYP3A4 (Fig. 1) which was identified as a risk in compound development because drugs that are cleared by a single enzyme pathway are more sensitive to drug–drug interactions (DDI).27,28 No additional selectivity risks were identified in broad panels of in vitro assays (Table S1‡). On the basis of its overall profile,24 compound 1 was nominated as a preclinical candidate (PF-06424439) and was progressed to safety pharmacology and toxicology studies to enable an investigational new drug (IND) application. In one month toxicological studies and cardiovascular safety studies in Beagle dogs, cardiac (coronary artery) and ileum arteriopathy were observed, along with increases in blood pressure, heart rate, and prolongation of QTc interval (vide infra). Arteriopathy is a microscopically observed form of vascular injury and is a significant cause of termination of compounds in nonclinical assessment of safety.29 Arteriopathy cannot be monitored in human clinical studies by noninvasive methods. Multiple mechanisms have been proposed to explain vascular injury,29 however, the mechanism(s) driving these findings with compound 1 is (are) unknown. Due to an insufficient therapeutic index (TI), the development of compound 1 was halted. More data were required to understand whether the toxicological findings were linked to the mechanism, or were specific to the chemical series, or compound 1 only. Comparing toxicological findings with those from a distinct chemotype can help to provide insights on mechanism-based safety and optimization of structurally differentiated chemical series was pursued (data not shown). In addition, within a single chemical series, several examples are reported in the literature where a small structural change provided an improved safety profile.30–32 Based on the desirable physicochemical properties and attractive in vitro profile of compound 1 (Fig. 1), we continued seeking structurally close-in analogues to be evaluated in a 1 month toxicology study in Beagle dogs. Furthermore, while maintaining the positive attributes of compound 1, analogues with a more balanced metabolism profile were sought to avoid the risks associated with an exclusively CYP3A4-driven clearance. In this communication, we report toxicological findings for compound 1, summarize the identification and characterization of analogues with a more balanced metabolic profile compared to compound 1, and report the differentiation of these compounds in in vivo safety studies.
Fig. 1. Summary of in vitro pharmacology and ADME data for compound 1 (PF-06424439).
Results
Synthesis of compounds 1–6
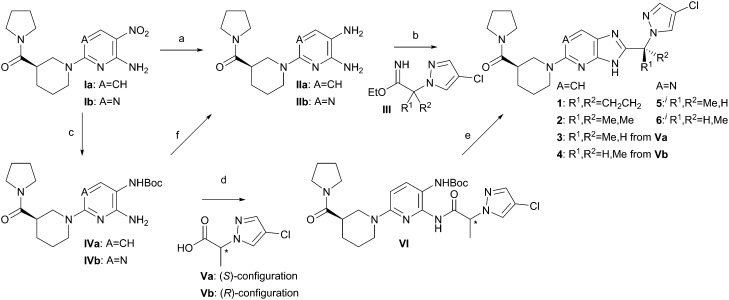
Compounds 1–6 were synthesized as shown in Scheme 1; the initial route to all final compounds involved cyclization of diamine II with imidate III. However, the chiral imidates III (R1 ≠ R2) could only be accessed in racemic form by this route, thus affording diastereomeric mixtures of products at the methyl-bearing stereocenter (compounds 3–6). The diastereomers were readily separated by chiral chromatography, and this method provided efficient access to material for understanding the in vitro and PK properties of these compounds.
Scheme 1. Synthesis of compounds 1–6. (a) H2, Pd/C, EtOH, (HCl); (b) HOAc, EtOH, (Et3N), 70 °C; (c) Boc2O, H2, Pd/C, EtOH; (d) T3P, EtOAc, (A CH); (e) MsOH, CH2Cl2; then NH4OAc; (f) HCl, dioxane. iNote: compounds 5 and 6 are distinct, single diastereomers of undetermined absolute configuration at the R1/R2 stereocenter; one stereochemistry is arbitrarily drawn for clarity.
In working toward safety studies, however, further options for imidazole ring formation were explored. The synthesis of cyclopropyl derivative 1 was previously described.24 An alternative route to 3 and 4 made use of acid V, which was synthesized in high enantiomeric purity by activation and SN2-displacement of methyl lactate by 3-chloro-pyrazole. Hydrogenation of nitro compound I in the presence of Boc2O afforded the amino NHBoc intermediate IV. Pyridine IVa was acylated by acid V using T3P as the coupling reagent to afford amide VI.33 Boc-deprotection was achieved under acidic conditions, and subsequent addition of ammonium acetate to buffer the acidity of the reaction mixture led to cyclized products 3 or 4, with minimal epimerization through the amidation and cyclization steps. The synthesis from single enantiomers of methyl lactate enabled assignment of absolute stereochemistry for compounds 3 and 4. Notably, however, aminopyrimidine IVb was substantially less reactive than aminopyridine IVa, with the result that no amidation conditions for coupling of lactate-derivative V were identified that would have provided an acceptably small level of epimerization. The amino NHBoc-pyrimidine IVb was readily converted to diamine IIb hydrochloride, which reacted with imidate III to afford a diastereomeric mixture of 5 and 6, which was separated by chiral chromatography. For simplicity, the drawn structures of 5 and 6 depict single, arbitrarily drawn diastereomers at the methyl stereocenter, although the absolute configuration at this center was not determined.
To ensure consistent PK properties for safety and efficacy studies, compounds 1, 3, and 5 were isolated as crystalline solids. Compound 1 was most readily isolated as its methanesulfonate salt from a solution in acetonitrile.24 Compound 3 crystallized as a monohydrate monohydrochloride salt from a hydrochloric acid and THF solution. Compound 5 crystallized in a neutral form from a solution in isopropanol.
Optimization of imidazopyridine analogues for a more balanced metabolic profile
As previously described, strategic introduction of a sp3 carbon linker between the two aromatic systems shifted the main clearance pathway from glucuronidation of the imidazopyridine core to CYP3A4-mediated oxidative metabolism (compound 1, Table 1).24 Presumably, this shift results from the steric hindrance caused by the cyclopropyl group adjacent to the site of glucuronidation. To diverge metabolism from CYP3A4-mediated oxidation, modifications of the sp3 linker were evaluated with the goal of restoring some level of glucuronidation while maintaining low rates of oxidative clearance. Replacement of the cyclopropyl ring with dimethyl (2) or either diastereomer of monomethyl (3 and 4) maintained good in vitro potency against DGAT2 (Table 1).
Table 1. In vitro human DGAT2 inhibition and clearance rate in HLM and HLM-UGT assays**.
| |||||
| A | R1/R2 | DGAT2 IC50 (nM) a | HLM (μL min–1 mg–1) | HLM-UGT (μL min–1 mg–1) | |
| 1 | CH | cPr | 17 ± 1 | 23.7 | <1.9 |
| 2 | CH | Me/Me | 31 ± 2 | 35.4 | ND |
| 3 | CH | Me/H | 20 ± 1 | 27.5 | 10.8 |
| 4 | CH | H/Me | 34 ± 10 | 26.0 | 7.6 |
| 5 b | N | Me/H | 26 ± 1 | 23.5 | 20.3 |
| 6 b | N | H/Me | 37 ± 4 | 21.5 | 15.2 |
aDGAT2 potency reported as the geometric mean of at least three replicates ± standard error of the mean (SEM).
bAbsolute configuration of 5 and 6 was not determined.
While an increased rate of oxidative metabolism was observed in the human liver microsome assay (HLM) for dimethyl analogue 2, the monomethyl derivatives 3 and 4 were oxidized at rates comparable to compound 1. In the microsomal assay incubated with UDP-glucuronosyltransferase (UGT) enzyme cofactor UDP-glucuronic acid (HLM-UGT), increased rates of glucuronidation were measured for 3 and 4 (as compared to 1), presumably due to the decreased steric hindrance at the nucleophilic imidazole ring, thus providing a more balanced in vitro metabolic profile compared to 1. Electronic modification of the imidazopyridine core was also pursued as a strategy to influence rates of glucuronidation. Incorporation of a nitrogen atom at the 6-position (imidazopyrimidines 5 and 6) provided comparable potency in the DGAT2 assay with similar low rates of oxidative metabolism and slightly increased rates of glucuronidation in vitro (Table 1). Overall, four analogues were identified that differ from compound 1 by only small structural changes and exhibit good in vitro profiles as defined by potent DGAT2 inhibition, low rates of metabolic clearance and balanced metabolic profile. For the monomethyl analogues (3–6), in vitro properties were similar for both methyl diastereomers. The more potent diastereomer on the imidazopyridine and imidazopyrimidine cores (compounds 3 and 5, respectively) were progressed for advanced profiling and are described in Tables 2 and 3.
Table 2. Summary of in vitro pharmacology and ADME data for compounds 3 and 5.
| Compound |
3 | 5 | |
| DGAT2 IC50 ± SEM (nM) a | Human | 20 ± 1 | 26 ± 1 |
| Rat | 155 ± 10 | 241 ± 18 | |
| Dog | 41 ± 4 | 53 ± 15 | |
| MGAT1-3, DGAT1 IC50 (μM) | >50 b | >50 b | |
| Hepatocytes CLint,app (μL min–1 per million cells) | Human | 16.1 | 13.1 |
| Rat | 12.7 | 12.0 | |
| Dog | 8.4 | <8 | |
| fmUGT/fmCYP c | 0.39/0.61 | 0.52/0.48 | |
| P app (×10–6 cm s–1) d | 13.1 | 16.2 | |
| Thermodynamic solubility e (μg mL–1) | 285 | 53 | |
| f up (rat/dog) f | 0.14/0.12 | 0.18/0.25 | |
aHuman, dog and rat DGAT2 potency reported as the geometric mean of at least three replicates. SEM = standard error of the mean.
bMaximum tested concentration was 50 μM.
cFraction of metabolism of UGT and CYP using microsomal assays.34
dMeasured at pH 7.4.
eMeasured at pH 6.5 for crystalline forms described in the synthesis section.
fUnbound fraction was determined using equilibrium dialysis.35
Table 3. In vivo pharmacokinetic profile of compounds 3 and 5 in dogs a .
| Dose (mg kg–1) | Route c | T 1/2 (h) | CLp (mL min–1 kg–1) | Vdss (L kg–1) | F (%) | |
| 3 | 1 | iv b | 3.2 | 4.0 | 0.54 | NA d |
| 5 | po c | 3.0 | NA d | NA d | 75 | |
| 5 | 1 | iv b | 1.1 | 7.3 | 0.58 | NA d |
| 5 | po c | 3.8 | NA d | NA d | 32 |
aMale Beagle dogs were utilized for PK studies. All data reported here are means of two experiments. The compounds were dosed in the crystalline forms described in the synthesis section.
bVehicle was 10% PEG 200/90% of 12% SBECD in water.
cVehicle was 20% SBECD in 0.5% methylcellulose.
dNA = not applicable.
In the dog DGAT2 enzymatic assay, compounds 3 and 5 showed IC50 values similar to those measured in the human assay. Both compounds also inhibited the rat enzyme, although with decreased potency. At a compound concentration of 10 μM, both compounds were selective for DGAT2 and inactive against broad selectivity panels, including phosphodiesterases (PDE), the hERG channel and functionally related MGAT1-3 and DGAT1 (Tables 2 and S1‡).§ In addition to the balanced metabolic profile (fraction of metabolism (fm) of UGT and CYP),34 compounds 3 and 5 showed good passive permeability and solubility, and reasonable stability in human hepatocytes (Table 2) which prompted their evaluation in in vivo PK studies.¶
In vivo evaluation of pharmacokinetic and pharmacodynamic profile
When dosed intravenously (iv) in dog PK studies, compounds 3 and 5 exhibited moderate clearance which, combined with low volume of distribution, resulted in moderate half-life for both analogues (Table 3). As was the case for oral administration (po) of compound 1, acceptable bioavailability was driven by good permeability and adequate solubility for this series, and the overall PK profiles of 3 and 5 were found to be similar to compound 1,24 albeit with decreased bioavailability. Comparable PK properties were measured in rats for both compounds (Table S2‡).
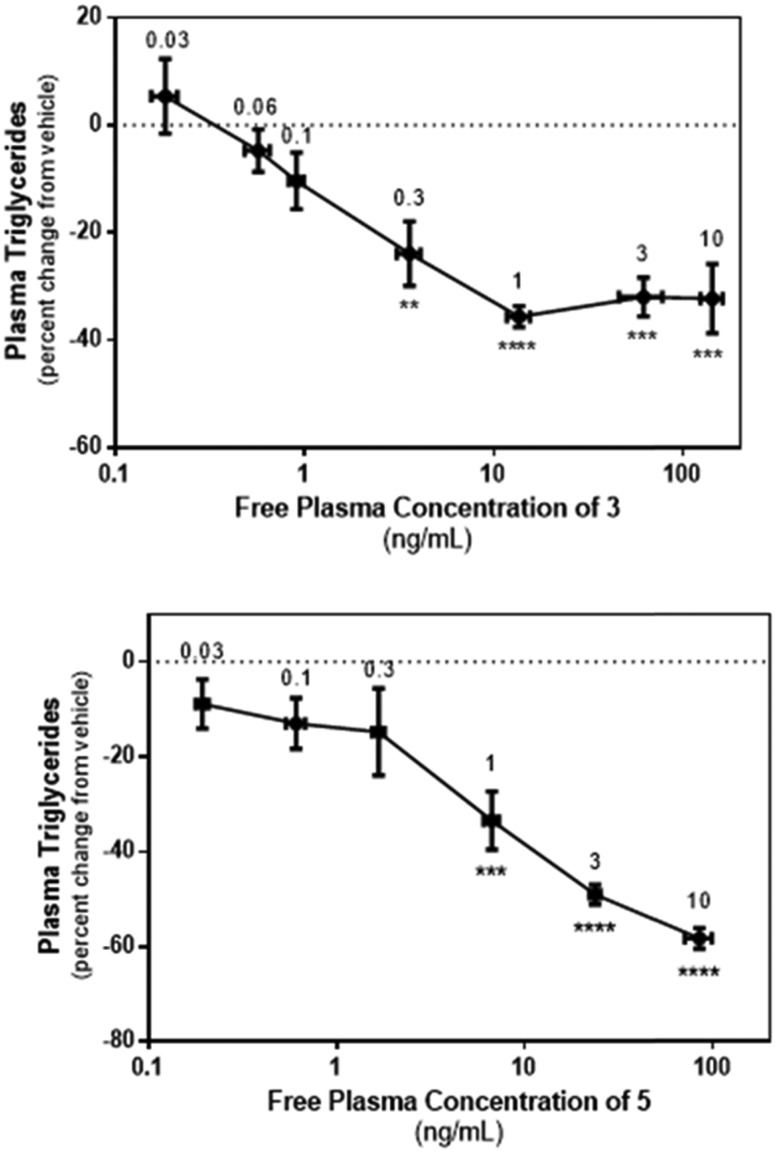
Having demonstrated acceptable in vitro pharmacology and in vivo PK properties, compounds 3 and 5 were advanced to a study in rats to confirm in vivo DGAT2 inhibition by monitoring their effect on circulating TG in rats fed a high-sucrose diet. Prior experience with prototype DGAT2 inhibitors had demonstrated that the acute effects in circulating TG were minimal in rats maintained on a standard laboratory chow and, as a result, the pharmacodynamic effects of compounds 3 and 5 were not assessed in normal rats. The stimulatory effect of high-sucrose diets on hepatic TG synthesis and secretion in rats are well documented and we therefore selected this model in order to maximize the window to assess the pharmacologic response to compounds 3 and 5. In our hands, normal rats exhibited circulating TG levels of ∼65 mg dL–1 whilst high-sucrose fed animals had values of ∼150–330 mg dL–1. As reported in Fig. 2, oral administration of 3 or 5 caused a concentration-dependent reduction in plasma TG, confirming in vivo engagement of the target by both compounds.
Fig. 2. Acute relationship between plasma free level of 3 and 5 and the pharmacodynamic effect (TG lowering). Dose-dependent effects of 3 and 5 on plasma TG levels in sucrose-fed rats. TG levels were determined in blood drawn from the tail vein 2 h after oral administration of the indicated dose of 3, 5 or vehicle (n = 7 or 8 animals per group). Data are expressed as percent change from the vehicle treated group and were analysed using one-way ANOVA followed by Dunnett's multiple comparison test. **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to vehicle treated animals. The values shown adjacent to the individual data points indicate the dose group (mg kg–1).
Evaluation of compounds 1, 3 and 5 in safety pharmacology and toxicology studies
The nonclinical safety of compound 1 was evaluated in Wistar Han rats and Beagle dogs. Initially, 14 day exploratory toxicity studies were conducted to identify the limits of toleration, pharmacokinetics at high doses, and potential target organs. Follow-up 1 month studies were conducted to support potential human clinical studies. The 1 month toxicity studies were designed and conducted in accordance with Good Laboratory Practice and Animal Welfare guidelines. Doses of compound 1 in toxicology studies were selected to explore suitable exposure margins to support early clinical studies to fully evaluate DGAT2 inhibition. These doses attained multiples of the dog in vitro DGAT2 IC50 value, with the lowest dose of 2 mg kg–1 per day achieving 60% average of dog DGAT2 IC50 over the dose interval. See Tables 4–6 for more details about doses and compound exposure. At most doses evaluated in the toxicology studies, decreased plasma concentrations of TG and cholesterol were observed, which is an expected result of DGAT2 inhibition. In the 14 day exploratory rat study (Table 4), the high dose of compound 1 (500 mg kg–1 per day) exceeded the maximum tolerated dose. Key findings included arteriopathy in the kidney and heart, gastric smooth muscle degeneration, gastric ulcers, and foamy macrophage accumulation in the lungs. Since it was thought that there was an adequate exposure multiple relative to the estimated clinically efficacious exposure, the 1 month rat toxicity studies evaluated doses of 15, 20, 30, 60 and 300 mg kg–1 per day. At ≥60 mg kg–1 per day, an increase in incidence and/or severity of microscopic findings in the heart, mainly myocardial necrosis and fibrosis which was consistent with rat cardiomyopathy was observed in male rats (Table 4). In addition, an increase in heart weight was observed in males and females at the same doses. No adverse findings were noted at 30 mg kg–1 per day.
Table 4. Summary of key findings for rat toxicity studies with compound 1.
| Study | 14 day exploratory
a
|
1 month toxicity
a
|
||||
| Dose (po, mg kg–1 per day) | 10 | 100 | 500 | 30 | 60 | 300 |
| C max (nM free) b | 390 | 4650 | 18 800 | 2020 | 5470 | 13 900 |
| Fold rat IC50 at Cmax | 10 | 122 | 495 | 53 | 144 | 366 |
| AUC (ng h mL–1 free) b | 1190 | 25 600 | 88 300 | 4630 | 22 600 | 62 500 |
| Coverage of rat IC50 (%) c | 75 | 98 | >99 | 92 | 98 | >99 |
| Key findings d | None | None | Arteriopathy (kidney, 1/5 M) (heart, 1/5 M), cardiomyocyte vacuolation (1/5 M) | None | ↑ heart weight, myocardial necrosis/fibrosis (1/10 M) | ↑ heart weight, myocardial necrosis/fibrosis (4/10 M, 1/10 F) |
aExploratory groups N = 5/sex/dose, toxicity groups N = 10/sex/dose.
bMean values (N = 3–4/sex/dose for toxicokinetics, male and female systemic values combined).
cAverage coverage (%) above DGAT2 rat IC50 at steady-state over the dosing interval of 24 hours.
dIncidence of finding by sex. M = males, F = females.
Table 5. Summary of key findings for dog toxicity studies with compound 1.
| Study | 14 day exploratory
a
|
1 month toxicity
a
|
|||||
| Dose po (mg kg–1 per day) | 75 | 300 | 2 | 5 | 10 | 50 | 150/100 |
| C max (nM free) b | 6540 | 15 300 | 305 | 1010 | 2030 | 12 700 | 16 300 |
| Fold dog IC50 at Cmax | 409 | 956 | 19 | 63 | 127 | 794 | 1019 |
| AUC (ng h mL–1 free) b | 24 800 | 78 300 | 238 | 1120 | 5280 | 57 500 | 92 600 |
| Coverage of dog IC50 c | 99 | >99 | 60 | 87 | 97 | >99 | >99 |
| Key findings d | None | Unscheduled death, arteriopathy (heart, 1/2) (stomach, 1/2), kidney – mild renal tubular deg./reg. (2/2) and vacuolation (1/2) | Arteriopathy (kidney, 1/3F) | Arteriopathy (esophagus and stomach, 1/3 M) | Arteriopathy (ovary, 1/3F) | Arteriopathy (heart, 1/3 M) (ileum, 1/3F) | Arteriopathy (heart, 2/3F), kidney – mild renal tubular deg./reg. (1/3 M, 1/3F) |
aExploratory groups N = 1–2/sex/dose, toxicity groups N = 3/sex/dose.
bMean values (male and female systemic values combined).
cAverage coverage (%) above DGAT2 dog IC50 at steady-state over the dosing interval of 24 hours.
dIncidence of finding by sex. M = males, F = females. deg./reg. = degeneration/regeneration.
Table 6. Summary of 1 month dog toxicity studies with compounds 3 and 5.
| Compound |
3
|
5
|
||||
| Dose po (mg kg–1 per day) | 3 | 10 | 30 | 3 | 10 | 30 |
| C max (nM free) a | 454 | 1270 | 3760 | 140 | 487 | 1760 |
| Fold dog IC50 at Cmax | 11 | 31 | 92 | 3 | 9 | 33 |
| AUC (ng h mL–1 free) a | 797 | 2680 | 9530 | 149 | 540 | 1560 |
| Coverage of dog IC50 (%) b | 65 | 86 | 96 | 21 | 50 | 74 |
| Key findings c | None | None | None | None | None | None |
aMean values (male and female systemic values combined).
bAverage coverage (%) above DGAT2 dog IC50 at steady-state over the dosing interval of 24 hours.
cToxicity groups N = 2/sex/dose.
In the 14 day exploratory dog study, compound 1 was not tolerated at 300 mg kg–1 per day (moribund euthanasia on day 9). In the kidney, mild degeneration/regeneration of tubular epithelium was noted at 300 mg kg–1 per day along with arteriopathy, in an intramural artery of the heart and in a submucosal artery of the stomach. The maximum tolerated dose was 75 mg kg–1 per day (Table 5). In the first 1 month toxicity dog study, doses of 10, 50 and 150 mg kg–1 per day were evaluated. The 150 mg kg–1 per day dose was not tolerated (adverse clinical observations, decreased body weight and body weight gain, decreased food consumption) and the dogs were given a dosing holiday on study day 10. The dose was decreased to 100 mg kg–1 per day beginning day 11 for the remainder of the study. Subsequently, improvements in clinical condition, body weight gain, and food consumption were observed. Microscopic findings included coronary arteriopathy at 50 and 150/100 mg kg–1 per day and small artery arteriopathy of the ileum at 50 mg kg–1 per day only, and ovary at 10 mg kg–1 per day only. Mild renal tubular degeneration was also noted at 150/100 mg kg–1 per day. Increases in heart rate were also observed at 50 and 150/100 mg kg–1 per day and prolongation of QTc interval occurred at 150/100 mg kg–1 per day on study day 25. A no observed adverse effect level (NOAEL) was not established. Therefore, a second 1 month dog toxicity study was conducted at lower doses (2 and 5 mg kg–1 per day). In the muscularis of the esophagus and stomach in one male at 5 mg kg–1 per day and renal cortex of one female at 2 mg kg–1 per day, there was arteriopathy of single small arteries characterized by minimal fibrinoid necrosis, hemorrhage and inflammation. Overall, arteriopathy was observed at all doses and a NOAEL could not be established for compound 1 within a dose range of 2 to 150 mg kg–1 per day.
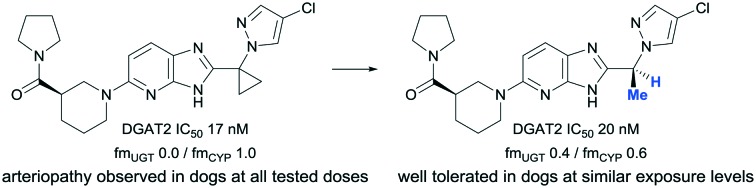
To determine if compounds 3 and 5, closely related analogues of compound 1, show differentiated safety profile, 1 month toxicology studies in dogs were designed. The doses of compounds 3 and 5 were selected to achieve similar ranges of average DGAT2 inhibition as to that of the lower doses of compound 1 (∼60–80% average of the dog enzymatic DGAT2 IC50) and the exposure levels achieved at these doses can be found in Table 6. Although compounds 3 and 5 were structurally very similar to compound 1, they were well tolerated at all doses in the 1 month dog study. Test article-related findings were limited to mild clinical signs (intermittent episodes of emesis), minimal body weight loss, and decreased food consumption, thus establishing NOAEL for both compounds at comparable exposure levels of compound 1, where no NOAEL was achieved.
Evaluation of compounds 1 and 3 in cardiovascular studies
In the single dose cardiovascular (CV) study in Beagle dogs, clinical signs associated with compound 1 were limited to foamy white salivation and multiple bouts of emesis for 2/8 dogs at 5 mg kg–1, 3/8 dogs at 10 mg kg–1, 5/8 dogs at 25 mg kg–1 and all 8 dogs at 75 mg kg–1. At 5 mg kg–1, no changes were noted in blood pressure, heart rate, ECG intervals, left ventricular parameters and activity level. At 10 mg kg–1, threshold increases in systolic pressure (3 mmHg) and heart rate (4–10 bpm) were noted while the CV effects at 25 and 75 mg kg–1 are summarized in Table 7. Oral administration of compound 1 to dogs was associated with increases in systolic blood pressure at doses of 10 mg kg–1 and higher that appeared to align with Cmax but the magnitude of increase was not dose related (5–6 mmHg). Heart rate increased in a dose dependent manner with maximal increases of 10, 20 and 30 bpm at 10, 25 and 75 mg kg–1, respectively.
Table 7. Summary of in vivo cardiovascular studies with compounds 1 and 3 in dogs.
| Compound |
1
|
3
|
||
| Dose (po, mg kg–1) | 25 a | 75 a | 25 b | 50 b |
| C max (nM free) | 4060 | 7510 | 2140 | 4700 |
| Fold dog DGAT2 IC50 | 250 | 470 | 52 | 110 |
| Heart rate (bpm) | ↑6–20 | ↑8–30 | ↑10 | ↑15 |
| Systolic pressure (mmHg) | ↑5 | ↑5 | ↑6 | ↑5 |
| Diastolic pressure (mmHg) | NO c | ↑6 | ↑5 | ↑6 |
a8 animals per dose group.
b3 animals per dose group.
cNO = no observable effect.
Diastolic blood pressure was increased only at 75 mg kg–1 (Table 7). One question posed by the data generated in this study was whether the clinical signs observed (white foamy salivation and multiple bouts of emesis within 4 h of dosing) were contributing partly or wholly to the observed cardiovascular signs and whether this could be mitigated by avoiding gastrointestinal irritation. A follow up study in telemetered dogs administering compound 1 by the intravenous route was conducted to address these questions. A bolus dose of 20 mg kg–1 iv produced a plasma exposure in line with the previous 75 mg kg–1 po dose and the observed CV profile was also similar. A 27 bpm increase in heart rate was evident and clinical signs of foamy salivation and emesis, albeit less pronounced than the po route, indicated a systemic mode of action rather than local gastrointestinal irritation (data not shown). Systolic blood pressure was not increased.
Compound 3 was tested in the same model to determine if the CV signal was evident in a close in analogue. The clinical signs observed during the study were again primarily emesis occurring within the first 4 h after dosing. Oral administration of compound 3 caused increases in systolic and diastolic blood pressure at all dose levels (5, 25 and 50 mg kg–1) during the first 4 hours post dose. These effects were relatively small in magnitude (+4 to +6 mmHg), did not increase with dose level and also occurred during periods of emesis (Table 7). No other effects were observed at 5 mg kg–1. However, at 25 mg kg–1 compound 3 caused an increase in heart rate (+10 bpm for 4 hours post dose) while at 50 mg kg–1, heart rate was further increased (+15 bpm) and persisted for up to 9 hours post dose (Table 7).
Conclusions
While encouraging data emerged from in vivo pharmacology studies with compound 1, dogs treated for one month were observed with multi-organ arteriopathy, increases in blood pressure and heart rate and prolongation of QTc intervals. Based on the desirable physicochemical properties and attractive in vitro profile of compound 1, we sought structurally close-in analogues to evaluate in subsequent toxicology studies in dogs. In this work, we report the identification of analogues 3 and 5 with suitable profiles as defined by good in vitro and in vivo DGAT2 inhibitory potency, outstanding in vitro safety profile, balanced metabolic profile and acceptable dog PK profile. In one month studies in dogs treated with 3 or 5, test article-related findings were limited to emesis, demonstrating that the previously observed arteriopathy was specifically driven by compound 1 and not by DGAT2 inhibition or by the imidazopyridine chemical series. Conversely, both compounds 1 and 3 demonstrated similar CV profiles in telemetry-instrumented dogs. Increases in blood pressure that were small but notable and did not appear to be dose related were observed along with dose- and exposure-dependent increases in heart rate. Differentiated chemical matter can be used to demonstrate that the observed increases in blood pressure and heart rate are associated with the imidazopyridine series but not with the mechanism of action. In line with this, further efforts from our group to optimize a different chemical series to fully elucidate the efficacy and safety of DGAT2 inhibition in human will be published separately. This report provides an example where a small structural modification, replacing a cyclopropyl group with methyl, is sufficient to improve the preclinical safety profile.
Experimental
Synthesis and characterization of compounds 1–6, in vitro selectivity of compounds 1, 3 and 5 as well as detailed methodologies and supporting data for pharmacokinetic, toxicology and cardiovascular experiments are found in the ESI.‡ The acute effect of compounds 3 and 5 on plasma TG was assessed according to the protocol described by Futatsugi.24 All experiments and procedures involving animals were conducted as per the guidelines and protocols reviewed and approved by Pfizer Institutional Animal Care and Use Committee. Detailed protocols for human DGAT2, DGAT1, MGAT1, MGAT2 and MGAT3 as well as dog and rat DGAT2 enzymatic assays were described by Futatsugi.24 Protocols for assessment of metabolic stability in human hepatocytes as well as passive permeability using RRCK membranes were described previously.36,37 Protocols and guidance for assessment of metabolic stability in human liver microsome assays were described previously.38–41
Supplementary Material
Acknowledgments
The authors would like to thank Robert Dullea and David A. Price for help with the manuscript, Jady Rugg, Amanda King-Ahmad and Thomas S. McDonald for bioanalytical analysis, Paul Nkansah, Stephanie Sobotka and Samuel Bell for formulation work, Brandon Pabst, Melissa Martin and Paula Loria for the in vitro assays, Alfin Vaz for biotransformation support and, Andrew J. Jensen and Gregory S. Goeken for their contribution to solid form identification.
Footnotes
†All authors were employed by Pfizer Inc. at the moment the experiments were conducted.
‡Electronic supplementary information (ESI) available: Protocols and supporting data for all experiments and compound preparation. See DOI: 10.1039/c6md00564k
§PF-06424439 (compound 1) is available from Aldrich under # PZ0233.
¶Detailed protocols for human DGAT2, DGAT1, MGAT1, MGAT2 and MGAT3 as well as dog and rat DGAT2 enzymatic assays were described by Futatsugi.24
References
- Ford E. S., Li C., Zhao G., Pearson W. S., Mokdad A. H. Arch. Intern. Med. 2009;169:572. doi: 10.1001/archinternmed.2008.599. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Annu. Rev. Med. 2002;53:319. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- Friedman J. Nature. 2002;415:268. doi: 10.1038/415268a. [DOI] [PubMed] [Google Scholar]
- Schaffer J. E. Curr. Opin. Lipidol. 2003;14:281. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Yen C. L., Stone S. J., Koliwad S., Harris C., Farese Jr. R. V. J. Lipid Res. 2008;49:2283. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Biochem. J. 2013;451:1. doi: 10.1042/BJ20121689. [DOI] [PubMed] [Google Scholar]
- Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese Jr. R. V. J. Biol. Chem. 2001;276:38870. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- Yen C. L., Monetti M., Burri B. J., Farese Jr. R. V. J. Lipid Res. 2005;46:1502. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- DeVita R. J., Pinto S. J. Med. Chem. 2013;56:9820. doi: 10.1021/jm4007033. [DOI] [PubMed] [Google Scholar]
- Naik R., Obiang-Obounou B. W., Kim M., Choi Y., Lee H. S., Lee K. ChemMedChem. 2014;9:2410. doi: 10.1002/cmdc.201402069. [DOI] [PubMed] [Google Scholar]
- Dow R. L. RSC Drug Discovery Ser. 2012;27:215. [Google Scholar]
- Birch A. M., Buckett L. K., Turnbull A. V. Curr. Opin. Drug Discovery Dev. 2010;13:489. [PubMed] [Google Scholar]
- Denison H., Nilsson C., Lofgren L., Himmelmann A., Martensson G., Knutsson M., Al-Shurbaji A., Tornqvist H., Eriksson J. W. Diabetes, Obes. Metab. 2014;16:334. doi: 10.1111/dom.12221. [DOI] [PubMed] [Google Scholar]
- Maciejewski B. S., LaPerle J. L., Chen D., Ghosh A., Zavadoski W. J., McDonald T. S., Manion T. B., Mather D., Patterson T. A., Hanna M., Watkins S., Gibbs E. M., Calle R. A., Steppan C. M. Am. J. Physiol. 2013;304:G958. doi: 10.1152/ajpgi.00384.2012. [DOI] [PubMed] [Google Scholar]
- Stone S. J., Myers H. M., Watkins S. M., Brown B. E., Feingold K. R., Elias P. M., Farese R. V. J. Biol. Chem. 2004;279:11767. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- Choi C. S., Savage D. B., Kulkarni A., Yu X. X., Liu Z. X., Morino K., Kim S., Distefano A., Samuel V. T., Neschen S., Zhang D., Wang A., Zhang X. M., Kahn M., Cline G. W., Pandey S. K., Geisler J. G., Bhanot S., Monia B. P., Shulman G. I. J. Biol. Chem. 2007;282:22678. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- Liu Y., Millar J. S., Cromley D. A., Graham M., Crooke R., Billheimer J. T., Rader D. J. Biochim. Biophys. Acta. 2008;1781:97. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Yu X. X., Murray S. F., Pandey S. K., Booten S. L., Bao D., Song X. Z., Kelly S., Chen S., McKay R., Monia B. P., Bhanot S. Hepatology. 2005;42:362. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- Kim M. O., Lee S. U., Lee H.-J., Choi K., Kim H., Lee S., Oh S.S. J.J., Kim S., Kang J. S., Lee H. S., Kwak Y.-S., Cho S. Biol. Pharm. Bull. 2013;36:1167. doi: 10.1248/bpb.b13-00152. [DOI] [PubMed] [Google Scholar]
- Kim M. O., Lee S., Choi K., Lee S., Kim H., Kang H., Choi M., Kwon E. B., Kang M. J., Kim S., Lee H.-J., Lee H. S., Kwak Y.-S., Cho S. Biol. Pharm. Bull. 2014;37:1655. doi: 10.1248/bpb.b14-00447. [DOI] [PubMed] [Google Scholar]
- Lee K., Kim M., Lee B., Goo J., Kim J., Naik R., Seo J. H., Kim M. O., Byun Y., Song G. Y., Lee H. S., Choi Y. Org. Biomol. Chem. 2013;11:849. doi: 10.1039/c2ob27114a. [DOI] [PubMed] [Google Scholar]
- Song X. S., Zhang J., Chen X., Palyha O., Chung C., Sonatore L. M., Wilsie L., Stout S., McLaren D. G., Taggart A., Imbriglio J. E., Pinto S., Garcia-Calvo M., Addona G. H. J. Biomol. Screening. 2016;21:117. doi: 10.1177/1087057115607463. [DOI] [PubMed] [Google Scholar]
- Wurie H. R., Buckett L., Zammit V. A. FEBS J. 2012;279:3033. doi: 10.1111/j.1742-4658.2012.08684.x. [DOI] [PubMed] [Google Scholar]
- Futatsugi K., Kung D. W., Orr S. T. M., Cabral S., Hepworth D., Aspnes G., Bader S., Bian J., Boehm M., Carpino P. A., Coffey S. B., Dowling M. S., Herr M., Jiao W., Lavergne S. Y., Li Q., Clark R. W., Erion D. M., Kou K., Lee K., Pabst B. A., Perez S. M., Purkal J., Jorgensen C. C., Goosen T. C., Gosset J. R., Niosi M., Pettersen J. C., Pfefferkorn J. A., Ahn K., Goodwin B. J. Med. Chem. 2015;58:7173. doi: 10.1021/acs.jmedchem.5b01006. [DOI] [PubMed] [Google Scholar]
- Imbriglio J. E., Shen D.-M., Liang R., Marby K., You M., Youm H. W., Feng Z., London C., Xiong Y., Tata J., Verras A., Garcia-Calvo M., Song X., Addona G. H., McLaren D. G., He T., Murphy B., Metzger D. E., Salituro G., Deckman D., Chen Q., Jin X., Stout S. J., Wang S.-P., Wilsie L., Palyha O., Han S., Hubbard B. K., Previs S. F., Pinto S., Taggart A. J. Med. Chem. 2015;58:9345. doi: 10.1021/acs.jmedchem.5b01345. [DOI] [PubMed] [Google Scholar]
- Camp N. P. and Naik M., WO2015077299A1, 2015.
- Brown H. S., Ito K., Galetin A., Houston J. B. Br. J. Clin. Pharmacol. 2005;60:508. doi: 10.1111/j.1365-2125.2005.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Lu A. Y. Clin. Pharmacokinet. 1998;35:361. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- Mikaelian I., Cameron M., Dalmas D. A., Enerson B. E., Gonzalez R. J., Guionaud S., Hoffmann P. K., King N. M., Lawton M. P., Scicchitano M. S., Smith H. W., Thomas R. A., Weaver J. L., Zabka T. S. Toxicol. Pathol. 2014;42:635. doi: 10.1177/0192623314525686. [DOI] [PubMed] [Google Scholar]
- Scola P. M., Sun L. Q., Wang A. X., Chen J., Sin N., Venables B. L., Sit S. Y., Chen Y., Cocuzza A., Bilder D. M., D'Andrea S. V., Zheng B., Hewawasam P., Tu Y., Friborg J., Falk P., Hernandez D., Levine S., Chen C., Yu F., Sheaffer A. K., Zhai G., Barry D., Knipe J. O., Han Y. H., Schartman R., Donoso M., Mosure K., Sinz M. W., Zvyaga T., Good A. C., Rajamani R., Kish K., Tredup J., Klei H. E., Gao Q., Mueller L., Colonno R. J., Grasela D. M., Adams S. P., Loy J., Levesque P. C., Sun H., Shi H., Sun L., Warner W., Li D., Zhu J., Meanwell N. A., McPhee F. J. Med. Chem. 2014;57:1730. doi: 10.1021/jm500297k. [DOI] [PubMed] [Google Scholar]
- Olbe L., Carlsson E., Lindberg P. Nat. Rev. Drug Discovery. 2003;2:132. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- Labelle M., Belley M., Gareau Y., Gauthier J. Y., Guay D., Gordon R., Grossman S. G., Jones T. R., Leblanc Y., McAuliffe M., McFarlane C., Masson P., Metters K. M., Ouimet N., Patrick D. H., Piechuta H., Rochette C., Sawyer N., Xiang Y. B., Pickett C. B., Ford-Hutchinson A. W., Zamboni R. J., Young R. N. Bioorg. Med. Chem. Lett. 1995;5:283. [Google Scholar]
- Dunetz J. R., Xiang Y., Baldwin A., Ringling J. Org. Lett. 2011;13:5048. doi: 10.1021/ol201875q. [DOI] [PubMed] [Google Scholar]
- Kilford P. J., Stringer R., Sohal B., Houston J. B., Galetin A. Drug Metab. Dispos. 2009;37:82. doi: 10.1124/dmd.108.023853. [DOI] [PubMed] [Google Scholar]
- Banker M. J., Clark T. H., Williams J. A. J. Pharm. Sci. 2003;92:967. doi: 10.1002/jps.10332. [DOI] [PubMed] [Google Scholar]
- Di L., Keefer C., Scott D. O., Strelevitz T. J., Chang G., Bi Y. A., Lai Y., Duckworth J., Fenner K., Troutman M. D., Obach R. S. Eur. J. Med. Chem. 2012;57:441. doi: 10.1016/j.ejmech.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Di L., Whitney-Pickett C., Umland J. P., Zhang H., Zhang X., Gebhard D. F., Lai Y., Federico 3rd J. J., Davidson R. E., Smith R., Reyner E. L., Lee C., Feng B., Rotter C., Varma M. V., Kempshall S., Fenner K., El-Kattan A. F., Liston T. E., Troutman M. D. J. Pharm. Sci. 2011;100:4974. doi: 10.1002/jps.22674. [DOI] [PubMed] [Google Scholar]
- Obach R. S. Curr. Opin. Drug Discovery Dev. 2001;4:36. [PubMed] [Google Scholar]
- Walsky R. L., Bauman J. N., Bourcier K., Giddens G., Lapham K., Negahban A., Ryder T. F., Obach R. S., Hyland R., Goosen T. C. Drug Metab. Dispos. 2012;40:1051. doi: 10.1124/dmd.111.043117. [DOI] [PubMed] [Google Scholar]
- Gill K. L., Houston J. B., Galetin A. Drug Metab. Dispos. 2012;40:825. doi: 10.1124/dmd.111.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland A., Knights K. M., Mackenzie P. I., Miners J. O. Drug Metab. Dispos. 2008;36:1056. doi: 10.1124/dmd.108.021105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.