Abstract
Neuronal migration defects, including pachygyria, are among the most severe developmental brain defects in humans. Here we identify bi-allelic truncating mutations in CTNNA2, encoding αN-catenin, in patients with a distinct recessive form of pachygyria. CTNNA2 was expressed in human cerebral cortex, and its loss in neurons led to defects in neurite stability and migration. The αN-catenin paralog, αE-catenin, acts as a switch regulating the balance between α-catenin and Arp2/3 actin filament activities1. Loss of αN-catenin did not affect β-catenin signaling, but recombinant αN-catenin interacted with purified actin and repressed ARP2/3 actin-branching activity. The actin-binding domain (ABD) of αN-catenin or ARP2/3 inhibitors rescued the neuronal phenotype associated with CTNNA2 loss, suggesting ARP2/3 de-repression as a potential disease mechanism. Our findings identify CTNNA2 as the first catenin family member with bi-allelic mutations in human, causing a new pachygyria syndrome linked to actin regulation, and uncover a key factor involved in ARP2/3 repression in neurons.
Keywords: alpha-catenin, actin remodeling, Arp2/3, neuronal migration, centrosome, pachygyria, polarity, primary neurite, Wnt signaling, beta-catenin
The Lissencephaly (LIS) spectrum is characterized by defects in the folding pattern of the cerebral cortex, resulting in reduced number or complexity of gyri that characterize the human brain2. LIS encompasses a continuum of malformations ranging from complete agyria to pachygyria, in which gyral formation is diminished but not absent. Patients present clinically with failed motor and cognitive development followed by intractable epilepsy. Most patients are severely intellectually impaired, and are unable to walk or care for themselves.
Projection neurons of the cerebral cortex are born in a zone adjacent to the ventricle, and then migrate to the future six-layered neocortex. Neuronal migration is achieved through a rearrangement of cytoskeletal components in response to extracellular cues, mediated by numerous intracellular signaling pathways3. Coordinated regulation of monomeric (G-actin) and polymerized actin microfilaments (F-actin) is critical for neuronal growth cone morphogenesis, axon pathfinding, and neurite extension during migration4,5. Positive regulators of F-actin assembly include families of nucleating proteins (Formins, Arp2/3) and their co-activators (Rho GTPases, WASP, WAVE), but negative regulators remain elusive, particularly in neurons6.
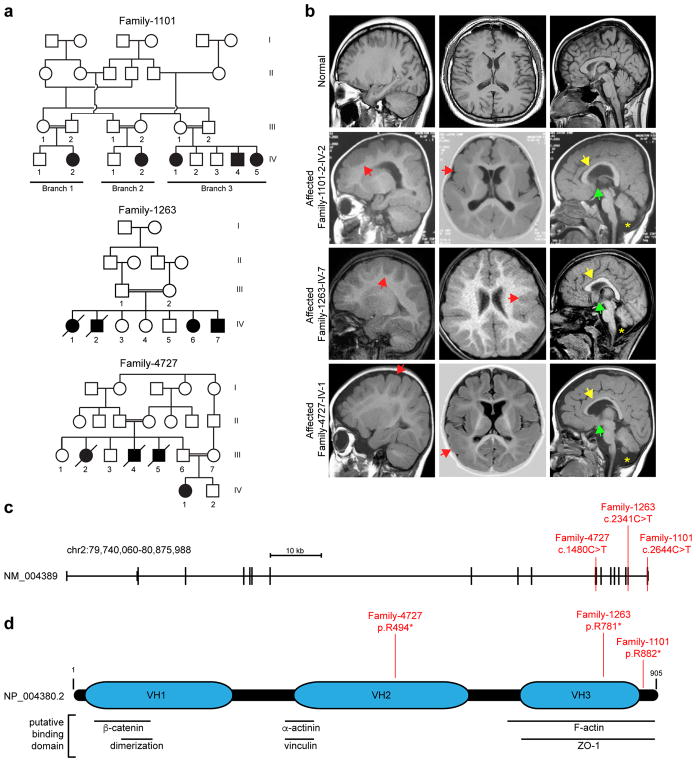
We recruited three families, with seven affected individuals showing neurodevelopmental delay (Fig. 1a, Table 1). All were diagnosed before age two years and displayed acquired microcephaly, hypotonic cerebral palsy, inability to ambulate or speak, and intractable seizures. (Table 1). Magnetic resonance imaging (MRI) demonstrated pachygyria with dramatic cortical gray matter thickening up to 3–4 cm, with paucity of gyri without obvious posterior-anterior gradient or focal dysplasias. There was absent anterior commissure, hypogenesis of the corpus callosum, and cerebellar hypoplasia (Fig. 1b). This phenotype is distinct from LIS1-pachygyria or DCX-pachygyria, which show posterior- or anterior-gradients, respectively. All subjects were enrolled in institutional review board (IRB) approved protocols and provided consent for study. We performed whole exome sequencing7 on at least one member of each family8. We prioritized rare (<0.2% allele frequency) damaging variants (GERP score >4 or SIFT <0.05), and focused on homozygous variants due to parental consanguinity. Parametric linkage analysis from genome-wide SNP arrays and homozygosity mapping9,10 showed identical-by-descent haplotypes (Supplementary Fig. 1a). Aligning variants with corresponding homozygous intervals identified putative nonsense variants in CTNNA2 in all three families (Family 1101, c.2664C>T p.Arg882*; Family 1263, c.2341C>T p.Arg781*; Family 4727, c.1480C>T p.Arg494*) (Fig 1c, d, Supplementary Fig. 1b). The three variants were each observed only heterozygous once in the public databases ExAC and gnomAD. Sanger sequencing confirmed segregation according to a strict recessive mode of inheritance, with full penetrance, in all genetically informative available family members, suggesting that bi-allelic CTNNA2 loss-of-function mutations underlie pachygyria in these patients.
Figure 1. Identification of homozygous truncating CTNNA2 mutations in families with pachygyria.
(a) Pedigrees of three consanguineous families. Parental consanguinity: double bar. Asterisk: sampled individual, Square: male, Circle: female, Filled: affected.
(b) Sagittal, axial, and midline sagittal MRI with symmetrically thickened cortex (red arrowheads) and paucity of cortical gyri, consistent with pachygyria. Patents present with thin corpus callosum (yellow arrowheads), absent anterior commissure (green arrowheads), and fluid cavity as a result of cerebellar hypoplasia (mega cisterna magna, yellow asterisk).
(c) CTNNA2 genomic organization, and location of mutations in Families 1101, 1263 and 4727 in red.
(d) CTNNA2 905 aa polypeptide (Entrez NP_004380.2) with Vinculin Homology domains (VH1-3), and putative protein binding sites. Patient homozygous truncating mutations in red.
Table 1. Clinical Phenotypes.
Patients display acquired microcephaly, hypotonic cerebral palsy, inability to ambulate or speak, and intractable seizures. HC, head circumference; SD, standard deviation below the mean; B/L, bi-lateral; VEP, visual evoked potential; ERG, electroretinogram; EEG, electroencephalogram.
| Family-1101-1-IV-2 | Family 1101-2-IV-2 | Family-1101-3-IV-1 | Family-1101-3-IV-4 | Family-1263-1 | Family-1263-2 | Family-4727-IV-1 | |
|---|---|---|---|---|---|---|---|
| Gender | F | F | F | M | M | F | F |
| Origin | Saudi | Saudi | Saudi | Saudi | Turkish | Turkish | Saudi |
| Weight at birth (kg) | 2.9 | N/A | N/A | N/A | 2.7 | 2.5 | 3.5 |
| Length at birth (cm) | 46 | N/A | N/A | N/A | 49 | 48 | N/A |
| HC at birth (cm) | 35.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC at birth (SD) | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC at last examination (cm) | 50 | 53.5 | 53 | 51 | 44 | 47 | 46.5 |
| HC at last examination (SD) | −4 | −1/0 | −1 | −2/−1 | −5 | −5 | −5 |
| Diagnosis age | 2 yrs | 1.5 yrs | 1 yr | Birth | 30 mos | 9 yrs | 28 mos |
| Intellectual Disability | Severe | Severe | Severe | Severe | Severe | Severe | N/A |
| Development | |||||||
| Gross motor (normal/delayed/absent) | Delayed | Delayed | Delayed | Delayed | Delayed | Absent | Delayed |
| Fine motor (normal/delayed/absent) | Delayed | Delayed | Delayed | Delayed | Delayed | Absent | Delayed |
| Language (normal/delayed/absent) | Single words | Single words | Single words | Absent | Absent | Absent | Absent |
| Social (normal/delayed/absent) | Delayed | Delayed | Delayed | Delayed | Delayed | Absent | Delayed |
| Seizures | |||||||
| Onset | 2–3 yrs | 1.5 yrs | 6 mos | 3 yrs | 8 mos | 6 mos | 6 mos |
| Type | Variable-atonic | Atonic/myoclonic | Variable-atonic | Variable-atonic | Atonic/myoclonic | Atonic/myoclonic | Infantile spasm (hypsarrhythmia) |
| Frequency | 1–2/week | 1–2/week | 1–2/week | 1–2/week | monthly | monthly | N/A |
| Neurological Findings | |||||||
| Hypertonia | − | − | − | − | − | − | + |
| Hypotonia | + | + | − | + | + | + | + (truncal) |
| Deep tendon reflexes | Increased | Increased | − | Increased | Increased | Increased | Increased |
| Spastic tetraplegia | + | − | − | + | − | + | + |
| Ataxia | Nonambulatory | + | + | Nonambulatory | − | Nonambulatory | Nonambulatory |
| Investigations | |||||||
| Metabolic | Normal | Normal | Normal | Normal | N/A | N/A | Negative |
| VEP/ERG | Normal | Normal | N/A | Abnormal | N/A | N/A | N/A |
| EEG | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal |
| MRI | |||||||
| Lissencephaly spectrum | Pachygyria | Pachygyria | Pachygyria | Pachygyria | Pachygyria | Pachygyria | Pachygyria |
| Cerebral mantle thickening (normal 5 mm) | 9–30mm | 12–35mm | 10–30mm | 10–32mm | 12–22mm | 9–24mm | 9–35mm |
| Subcortical band heterotopia | − | − | − | − | − | − | − |
| Corpus callosum | N/A | N/A | thin | thin | thin | thin | thin |
| Cerebellar hypoplasia | Y | Y | Y | Y | Y | Y | Y |
| Brainstem hypoplasia | N | N | N | N | N | N | Y |
| Hydrocephalus | N | N | N | N | N | N/A | N/A |
| White matter paucity | Y | Y | Y | Y | Y | Y | N/A |
| White matter signal | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Miscellaneous | |||||||
| Polyhydramnios | − | − | − | − | − | − | − |
| Lung hypoplasia | − | − | − | − | − | − | − |
| Short stature | − | − | − | − | − | − | − |
| Macrocephaly | − | − | − | − | − | − | − |
| Optic atrophy | − | − | − | − | − | − | − |
| Autistic features | + | + | + | + | + | + | + |
| Dysmorphism | − | − | − | − | B/L pes equinovarus | − | − |
CTNNA2 is the ancestral α-catenin gene and is conserved in all Metazoa, but is predominantly expressed in brain in mammals11. CTNNA1 is the most widely expressed, but is absent from populations of migrating neurons12, whereas CTNNA3 is expressed predominantly in myocardium. We confirmed CTNNA2 expression in human neural tissue (Supplementary Fig. 2a), and found protein co-expression with migration markers Dcx and Tuj1 in murine embryonic day (e) 13.5 brain (Supplementary Fig. 2b). As reported in mouse, a rim of αN-catenin was expressed in the apically localized progenitors of the ventricular zone12. In 20-week gestation human fetal brain αN-catenin was mostly restricted to regions expressing DCX and TUJ1 in developing cortical plate and marginal zone (Supplementary Fig. 2c).
There are two mouse lines harboring loss-of-function mutations of the ortholog to human CTNNA2 (Catna2). Cerebellar deficient folia (cdf) mice have a spontaneous C-terminal deletion13–15, and the conventional knockout removed the first exon16. These mutants share multiple phenotypes including impaired lamination of a subset of Purkinje and hippocampal neurons13–16, hippocampal dendritic spine morphogenesis16,17, axon projections, positioning of subsets of nuclei-specific neurons, and midline axonal crossing18. Of note, many of the phenotypes present in Catna2 mice are shared with CTNNA2 patients, including cerebellar hypoplasia and midline defects, however, neither line showed evidence of an overt neocortical phenotype15. This was not surprising given that mouse models for human cortical migration defects typically show no neocortical defects.
In order to investigate migration in a human model, we generated iPSC and neuronal derivatives from the affected and unaffected member of Family 1263 (1263A and Control, respectively), an individual with LIS due to Miller-Dieker syndrome (MDS, deletion of chromosome 17p11.3) as well as targeted the CTNNA2 gene in the H9 hESC line (herein referred to as CTNNA2KO) 19 (Supplementary Fig. 3). Western blot of the neuronal progenitor cells (NPCs) demonstrated absent αN-catenin protein in the patient and knockout compared to Control (Supplementary Fig. 4).
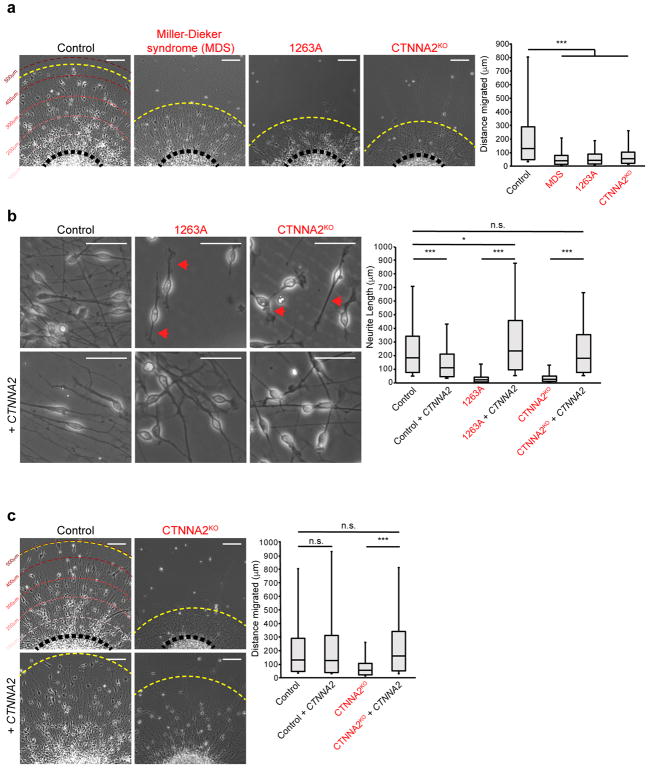
To study migration, we adopted a neurosphere assay20, and measured neuronal cell body distances from the edge after 48 hrs. We first confirmed cells exiting the neurosphere expressed postmitotic neuronal markers and were negative for other lineages (Supplementary Fig. 5a, b). GFAP-positive cells were only observed at the edge of the plated spheres in all of the lines tested (Supplementary Fig. 5a, b). Cell bodies of Control neurons showed a migration front at 514 μm (Fig. 2a, ave. 115 μm, S.D. 97 μm), whereas MDS (ave. 31 μm, S.D. 22 μm, P = 3.28E-34), CTNNA2 patients (ave. 33 μm, S.D. 21 μm, P = 1.19E-27), and CTNNA2KO (ave. 36 μm, S.D. 22 μm, P = 1.01E-18) lines were less than half normal. Consistent with what has been observed in MDS and control cerebral organoid models of neuronal migration21, the distribution of distances of MDS, CTNNA2 patient, and CTNNA2KO exited-neurons was significantly reduced (Fig. 2a, Supplementary Fig. 6, Supplementary Fig. 7). We conclude that loss of CTNNA2 results in a neuronal migration defect in vitro.
Figure 2. Loss of CTNNA2 mirrors Miller-Dieker syndrome (MDS) migration phenotypes in iPSC-derived neurons.
(a) Quantification of neuronal migration from human iPSC-derived neurospheres. MDS-derived neurons do not migrate as far as Control neurons. Migrating neurons from affected member of Family-1263 (1263A) showed reduced migration, similar to CTNNA2KO neurons. The distribution of migrating neurons at right, box plot with top box: Q3, bottom box: Q1, and Median. The whiskers represent the minimum and maximum values observed in the dataset. Repeated in three independent iPSC clones per patient or three CTNNA2KO clones, 828 cells scored. *, significance (see Statistics and Reproducibility) Scale bar 100 μm.
(b) Control neurons show bipolar morphology with long extended primary neurites. CTNNA2 patient and knockout neurons show thickened, short neurites (red arrows), rescued by forced expression of CTNNA2. The distribution of neurite length at right, box plot with top box: Q3, bottom box: Q1, and Median displayed. The whiskers represent the minimum and maximum values observed in the dataset. Repeated in three independent iPSC clones per patient or three CTNNA2KO clones, 552 cells scored. *, significance (see Statistics and Reproducibility). Scale bar 50 μm.
(c) Rescue of neuronal cell body migration distance upon forced expression of CTNNA2 in CTNNA2KO lines. The distribution of migrating neurons at right, box plot with top box: Q3, bottom box: Q1, and Median displayed. The whiskers represent the minimum and maximum values observed in the dataset. Repeated in three CTNNA2KO clones, 532 cells scored. * and ***, significant P (see Statistics and Reproducibility). Scale bar 100 μm.
Time-lapse phase microscopy was performed to study the mechanism of defective migration. Control cells displayed bipolar morphology, with leading neurite length on average 130 μm. CTNNA2-mutant cells showed shortened (ave. length 18 μm) disorganized leading processes, enlarged growth cones, proximal ectopic filopodia and lamellipodia, and failed bipolar morphology (Fig. 2b, Supplementary Movie 1–3). We confirmed that the neurite length and migration defect were due to absence of CTNNA2 using lentiviral transduction rescue with GFP-tagged CTNNA2. Transduced cells had uniform expression of a full-length αN-catenin-GFP at 129 kDa and a processed product at 102 kDa (Supplementary Fig. 8a). Neurite length and migration were largely restored in both patient and CTNNA2KO cells upon CTNNA2-GFP forced-expression (Fig. 2b, c, Supplementary Fig. 6, Supplementary Fig. 7).
Previous studies have shown mouse αE-catenin, encoded by Catna1, is required for preimplantation epithelial integrity22, whereas conditional deletion in embryonic brain disrupts adherens junctions, leading to dysregulated proliferation23. To test whether loss of CTNNA2 affects neuroepithelium polarity, we generated neural rosettes from Control and 1263A iPSCs and used immunostaining to examine apical polarization. Despite the absence of αN-catenin at the apical surface of the rosette, the tight junction marker ZO-1 and the adherens junction markers N-Cadherin and β-Catenin were localized in a polarized fashion near cilia (Supplementary Fig. 9). These results suggest loss of CTNNA2 does not adversely affect apical polarization of neuroepithelial cells in culture.
The presence of a putative β-catenin binding domain suggests αN-catenin loss may alter Wnt signaling24. We therefore compared gene expression profiles between MDS, 1263A and Control NPCs by RNA-sequencing. We found no consistent, differential expression changes, arguing against a major transcriptional effect (Supplementary Fig. 10 a–c). Furthermore, established canonical Wnt target genes were not changed (Supplementary Fig. 10d), suggesting loss of CTNNA2 does not measurably impact Wnt-mediated transcription.
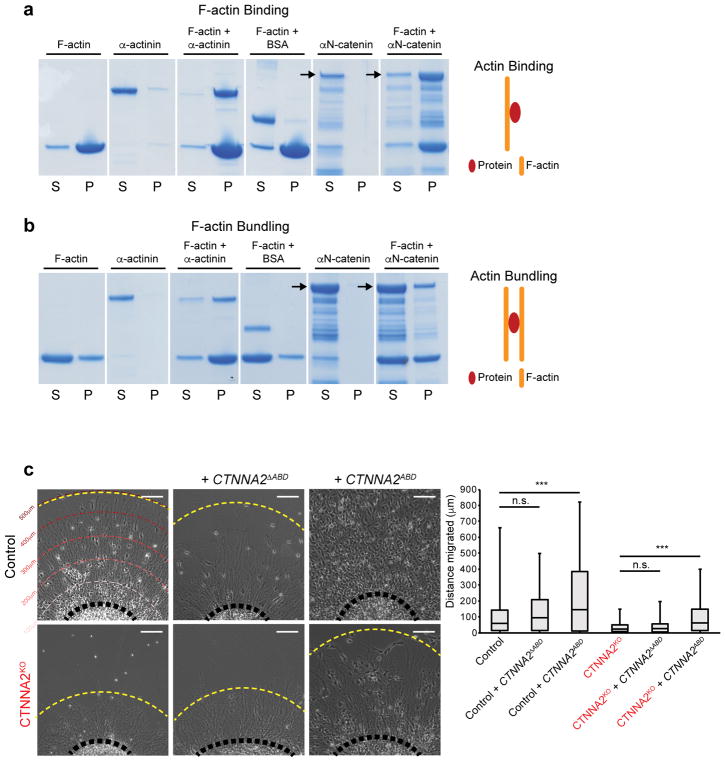
We thus focused on potential αN-catenin regulation of the neuronal cytoskeleton. αN-catenin contains a putative F-actin binding domain (ABD) at the C-terminus. To assess the ability of αN-catenin to directly bind and bundle actin, we performed actin binding and bundling assays in vitro. Similar to the α-actinin control, full-length recombinant αN-catenin co-sedimented with F-actin (Fig. 3a, Supplementary Fig. 11). Full-length αN-catenin appeared to weakly bundle F-actin filaments (Fig. 3b), although more work would be necessary to assess direct bundling activity. There was no noticeable change in the G-actin/F-actin ratio in NPCs from control or affected (Supplementary Fig. 12), arguing against an overall effect on actin stabilization.
Figure 3. Recombinant αN-catenin associates with F-actin, and the actin-binding domain is necessary and sufficient for rescue of neuronal migration.
(a) αN-catenin binds to F-actin in co-sedimentation assays. The majority of F-actin was present in the pellet (P) upon 100,000g spin, and while α-actinin was observed primarily in the supernatant (S) when centrifuged alone, when sedimented with F-actin shifted to P. BSA was found in S even when sedimented with F-actin. αN-catenin was exclusively in S when centrifuged alone, but when sedimented with F-actin the full-length band shifted to P. Repeated in duplicate.
(b) αN-catenin weakly promotes bundling of F-actin in an assembly assay. Upon centrifugation at 10,000g, F-actin was split between S and P. While α-actinin was exclusively in S, when co-pelleted with F-actin promoted a shift of actin to P. BSA showed no bundling activity. Similar to α-actinin, αN-catenin was exclusively in S, but promoted a slight shift of actin to P. Repeated in duplicate.
(c) Migration assay showing rescue of neuronal migration upon forced lentiviral expression of the actin-binding domain of αN-catenin (CTNNA2ABD) but not αN-catenin lacking the actin-binding domain (CTNNA2ΔABD). Quantification shown at right, box plot with top box: Q3, bottom box: Q1, and Median displayed. The whiskers represent the minimum and maximum values observed in the dataset. Repeated in three CTNNA2KO clones, 834 cells scored. ***, significant P (see Statistics and Reproducibility). Scale bar 100 μm.
We next generated lentiviral constructs lacking or containing amino acids 671–905, corresponding to the αN-catenin ABD25, and confirmed that encoded protein was stable in NPCs (Supplementary Fig. 8b, c). CTNNA2ΔABD could not rescue migration defects in the CTNNA2KO neurons, and had no effect on Control cells (Fig. 3c, Supplementary Fig. 6, 7). In contrast, expression of CTNNA2ABD alone mediated rescue of migration in CTNNA2KO, and enhanced migration in Control neurons (Fig. 3c, Supplementary Fig. 6, 7). We conclude the ABD of CTNNA2 is necessary and sufficient for its effect on neuronal migration in CTNNA2-mutant cells.
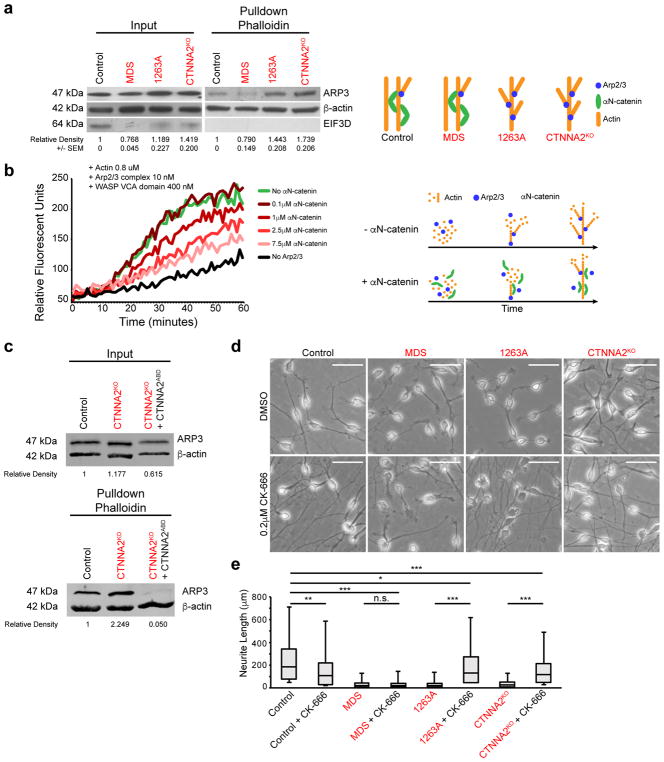
The reduction in leading process stability we observed in CTNNA2-mutant neurons was similar to a phenotype recently described in mouse Arpc2 mutant radial glia26, suggesting αN-catenin might regulate Arp2/3 activities. Recombinant αE-catenin controls actin-filament organization and represses Arp2/3-mediated actin polymerization in vitro25. Thus, we reasoned that the excessive filopodia formation, accompanied by repeated, rapid retraction of the leading process in CTNNA2-mutant neurons, might result from failure to suppress ARP2/3-mediated actin branching. To initiate actin branching, the ARP2/3 complex binds to the side of existing F-actin filaments to nucleate a new filament at a distinctive 70° angle; and consequently ARP2 and ARP3 are incorporated into the microfilament structure27. To test for increased F-actin branching, we assessed the amount of ARP3 protein associated with F-actin filaments. Migrating neurons from the MDS patient showed ARP3 associated with actin to be equal to or less than Control, whereas neurons from 1263A and CTNNA2KO lines showed almost a 50% increase in association (Fig. 4a, c, Supplementary Fig. 13a, b). We conclude that CTNNA2-deficient cells show excessive association between ARP2/3 and actin.
Figure 4. αN-catenin represses Arp2/3-actin association and polymerization.
(a) Elevated association between affinity-purified F-actin and ARP2/3 in CTNNA2-mutant cells. Input: basal expression. F-actin pulldown with phalloidin. Exaggerated ARP3 band in 1263A and knockout lines, compared MDS or Control line. EIF3D (does not bind actin) as a control for pulldown specificity. Cropped images shown. Repeated in triplicate, quantification and SEM below. Schematic depicts model (right).
(b) Actin polymerization assay showing dose-dependent inhibition of Arp2/3 + VCA domain of WASP mediated actin polymerization by αN-catenin, measured by relative fluorescent units. In the absence of Arp2/3 (black) there was minimal polymerization, but when Arp2/3 + VCA was added (green) polymerization increased substantially, which was reversed upon dose escalation of αN-catenin. Repeated in triplicate. Schematic depicts model (right).
(c) Loss of association between F-actin and ARP2/3 in CTNNA2KO cells expressing the actin binding domain (ABD) of αN-catenin. Input: basal expression of ARP3 and Actin. F-actin pulldown with phalloidin. Exaggerated ARP3 band in knockout neurons, and loss of ARP3 in ABD-expressing knockout neurons, compared with band in Control line. Quantification by relative fluorescence of ARP3 to Actin. Cropped images shown. Repeated in duplicate.
(d) ARP2/3 inhibition by CK-666 rescued neurite length defect in CTNNA2 mutant neurons, but not Control and MDS mutant neurons. Scale bar 50 μm.
(e) Quantification of (d) with top and bottom quartiles and Median displayed. Whiskers represent the minimum and maximum values observed in the dataset. Repeated in three independent iPSC clones per patient or three CTNNA2KO clones, 442 cells scored.*, ** and ***, significant P (see Statistics and Reproducibility).
ARP2/3 initiates actin branching in the presence of the Wiskott-Aldrich syndrome family protein (WASP) VCA domain by the nucleation of F-actin filaments. We thus tested whether αN-catenin was sufficient to inhibit the effect of ARP2/3 + VCA on actin polymerization. Recombinant VCA domain of human WASP (400 nM) showed minimal actin polymerizing activity. This was more than doubled upon addition of 10 nM ARP2/3 complex. Increasing concentrations of recombinant αN-catenin resulted in a dosage-dependent inhibition on the ARP2/3 + VCA effect on actin, reduced nearly back to baseline (Fig. 4b). We conclude that αN-catenin is sufficient to suppress the effect of ARP2/3 + VCA on actin polymerization in vitro.
Since αN-catenin repressed ARP2/3 activity in vitro, we next tested whether this activity was mediated by the ABD using CTNNA2KO neurons. We ectopically expressed αN-catenin ABD and assessed the amount of ARP3 associated with actin by Western blot. We observed a near doubling of ARP3 associated with F-actin, whereas expressing the αN-catenin ABD showed potent loss of most ARP3 bound to actin (Fig. 4c, Supplementary Fig. 13b), suggesting the ABD domain of αN-catenin is sufficient to regulate ARP2/3 – actin interaction. We also tested the ability of recombinant αN-catenin ABD to repress ARP2/3-mediated actin polymerization in vitro. The ABD of αN-catenin was sufficient to repress ARP2/3 mediated actin polymerization in a dosage-dependent manner (Supplementary Fig. 13c), albeit to a lesser extent than full-length αN-catenin.
If αN-catenin mediates its effects on neuronal morphology through suppression of ARP2/3, then inhibition of ARP2/3 should at least partially compensate for the loss of αN-catenin in neurons. To test this, we used two different cell-permeable ARP2/3 inhibitors, CK-666 or CK-86928, which inhibit ARP2/3 at different sites. After generating a dose-response curve, we analyzed neurite length in Control, MDS, patient, and CTNNA2KO migrating neurons after 24 hrs. A majority of Control neurons showed long, extended bipolar neurites with ave. length > 100 μm. Both inhibitors showed a negative effect on neurite length in Control neurons, but CTNNA2-mutant cells increased neurite length nearly 10-fold, restoring the distribution to near Control levels (Fig. 4d, Supplementary Fig. 7, 14). MDS neurons showed neurites on average 18 μm in length, without improvement upon ARP2/3 inhibitor treatment. We conclude that ARP2/3 inhibitors can largely rescue the neurite length defect associated with loss of CTNNA2.
In summary, we have identified a new neuronal migration disorder due to bi-allelic truncating mutations in CTNNA2. The involvement of an actin regulator in pachygyria was surprising, given that previously genetic studies focus on microtubules29. Microtubules scaffold the cytoskeleton for the repeated cycles of migration30,31. The main effect of actin appears to be in sensing and responding to extracellular guidance cues through changes in cell morphology, as well as guiding microtubules within the primary neurite32. This is mediated at least in part by calcium influx and enhanced neuronal motility through LIS1-dependent regulation of Rho GTPases33, but also through transmembrane proteins, which can alter cell morphology through effects on actin34.
Actin structure is critically regulated by Arp2/3, which controls the decision to initiate branching. We show Arp2/3 over-activity, as a result of loss of αN-catenin, leads to excessive branching, which impairs neurite growth and stability, possibly by controlling microtubule advance into the growth cone32,35. αN-catenin suppresses actin branching; in vivo this likely occurs by binding F-actin. This activity is mediated by the ABD, which we show competes with ARP2/3 for nucleation sites on F-actin. α-actinin, fascin and αE-catenin can sort actin associated proteins by altering the conformation of actin1,25,36. It will be interesting to determine if αN-catenin also shares this property or if it extends to other ABD containing proteins. Thus, as a more general principle, actin-binding proteins may serve unrecognized roles in actin regulation by controlling F-actin polymerization as well as dictating actin conformation locally within a cell.
Online Methods
Please see the accompanying Life Sciences Reporting Summary for further detailed explanation of experimental design and analysis used in this study.
Patient Recruitment
Patients were enrolled and sampled according to standard local practice in approved human subjects protocols at the respective institutions. Each subject was evaluated by and had MRI images by one of the authors. Excluded were cases with overlapping conditions such as asymmetrical brain dysplasia, primary white matter disease, or evidence of altered metabolism such as elevations in lactate or abnormal peaks on standard clinical serum tandem mass spectroscopy.
Exome Sequencing
For each sample, DNA was extracted from peripheral blood leukocytes by salt extraction. Exon capture was performed with the Agilent SureSelect Human All Exome 50 Mb Kit with paired-end sequencing on an Illumina HiSeq2000 instrument resulting in >94% recovery at > 10x coverage.
Sequences were aligned to the human genome (hg19) with Burrows-Wheeler Aligner (BWA) and variants delineated using the Genome Analysis Toolkit (GATK) software and SAMTools algorithms for both SNPs and insertion/deletion polymorphisms8. Variants were filtered for the criteria: 1] occurring in coding regions and/or splice sites, 2] non-synonymous, 3] found in less than 0.1% frequency in control populations (our in house exome data set of 12,000 individuals, dbSNP and Exome variant server) 4] homozygous in consanguineous families, 5] within linkage intervals or blocks of homozygosity. Variants were ranked by the type of mutation (nonsense/splice/indel > missense), amino acid conservation across species, and damage prediction programs (PolyPhen and Grantham score).
Linkage Analysis
All informative members of Family-1101 were genotyped with the Infinium iSelect24 mapping panel (Center for Inherited Disease Research) and analyzed with easyLINKAGE-Plus software37. Parameters were autosomal recessive with full penetrance and disease allele frequency of 0.001. Genomic regions with LOD scores under -2 were excluded as loci, over 2 were considered as candidate loci, and over 3.3 as statistical evidence for genome-wide significance. Linkage simulations were performed with Allegro 1.2c under the same parameters, with 5,000 markers at average 0.64 cM intervals, codominant allele frequencies, and parametric calculations37.
Tissue Culture
Fibroblasts were generated from unaffected and affected dermal biopsies explants and cultured in MEM (Gibco), supplemented with 20% FBS (Gemini). iPSCs, neural progenitor cells and neurons were obtained as previously described19,38. MDS fibroblasts were obtained from Coriell Biorepository (GM09209) and similarly reprogrammed.
iPSCs were generated as previously described39 using 3 μg expression plasmid mixtures (OCT3/4, SOX2, KLF4, L-MYC, LIN28 and p53 shRNA) electroporated into 6x105 cells, dissociated after 7 d, and 1.5x105 cells re-plated onto 100 mm dishes with 1.5x105 irradiated CF-1 mouse embryonic fibroblasts (MEF) feeder layer. Culture medium was replaced every day with hESC/iPSC medium (DMEM:F12 supplemented with 20% KOSR and 20 ng/ml bFGF (Invitrogen), 1x non-essential amino acids, 110 mM 2-mercaptoethanol. Colonies with healthy appearance (rounded smooth edges) were selected for further cultivation and evaluation. After 3 passages iPSCs cells were transferred to Matrigel (BD Biosciences) coated plates and grown in mTeSR medium (Stem Cell Technologies).
Neural progenitors cells (NPCs) were obtained as previously described40 with embryoid bodies (EBs) formed by mechanical dissociation of cell clusters and plated in suspension in differentiation medium (DMEM:F12, 1x N2, 1 μM Dorsomorphin (Tocris), 2 μM A8301 (Tocris)) and kept shaking at 95 rpm for 7 days, then plated onto Matrigel (BD Biosciences) coated dishes in NPC medium (DMEM:F12, 0.5x N2, 0.5x B27, 20 ng/ml bFGF). Rosettes were visible after 5–7 d, selected with Neural Rosette Selection Media (StemCell Technologies), and NPCs plated onto poly-L-ornithine (PLO)/laminin (Sigma) dishes with NPC medium, which was exchanged every 2 days.
Bright field images were taken on Axiovert.A1, or AxioObserver inverted microscopes (Zeiss), in addition to an EVOS microscope (Life Technologies) and processed with Photoshop CS5 (Adobe Systems). Time-lapse movies were acquired on a Zeiss Axio Observer and compiled with Zeiss Zen software. Fluorescent confocal images of neural rosettes were taken on an LSM 880 (Zeiss) and processed with FIJI/ImageJ software. Images are maximum z-projections of the entire depth of signal for the cell of interest.
CRISPR Targeting
Frame-shifting insertions or deletions (indels), occurring in coding exon 12 of CTNNA2, were generated in H9 hESCs by CRISPR/SpCas9 induced imprecise non-homologous end joining (NHEJ) as previously described41 with CTNNA2 sgRNA-F and CTNNA2 sgRNA-R primers (Supplementary Table 1) into BbsI-digested px330. Individual colonies were isolated after 2 wks, expanded, and genotyped by Sanger sequencing for bi-allelic mutations.
cDNA synthesis and RT-PCR
cDNA synthesis of 1 μg patient RNA was performed with Superscript III First-Strand RT-PCR (Life Technologies). The reaction products were then used for quantitative real-time PCR (qRT-PCR) or cloning. qRT-PCR was performed in triplicate on 10 ng human cDNA for CTNNA2, AXIN2, ID2, LEF1, and GAPDH (see Supplementary Table 1 for primer sequences). CT values were normalized to GAPDH as a loading control and fold change calculated in reference.
Neurosphere Assays
Neurosphere assay methods were modified from previously described protocols42. Three different stem cell lines (clones) per patient or condition were used as biological replicates for each experiment. The iPSC-derived NPCs were dissociated and incubated, shaking at 95 RPM, in NPC media. The following day, the media was changed to DMEM:F12 with 1x N2, 1x B27, shaking at 95 RPM for 12 hrs, the resultant neurospheres plated on PLO/laminin plates, and imaged at 48 hrs to record distance each neuronal cell body traveled from the edge of the neurosphere using FIJI/ImageJ (NIH) per clone. Data from each clone was collected and analyzed together with data from the other 2 clones per patient or condition to facilitate statistical tests.
Neurite Quantification
Three different stem cell lines (clones) per patient or condition were used as biological replicates for each experiment. To assess leading process length, stem cell derived migrating neurons were imaged. The length of the primary neurite from the edge of the cell body to the tip of the growth cone was measured in microns. For ARP2/3 inhibition, 0.2 μM CK-666, 0.2 μM CK-869, or an equivalent dilution of DMSO was added to the neurons 6 hrs after plating28. Neurons were analyzed after 24 hr. Data from each clone was collected and analyzed together with data from the other 2 clones per patient or condition to facilitate statistical tests.
RNA sequencing
Total RNA was extracted from two NPC lines per patient with TRIzol Reagent (Gibco). Full length mRNA was captured by TruSeq Stranded mRNA kit (Illumina) with standard protocol and was submitted for paired-end 100 nucleotide sequencing on MiSeq (Illumina). Roughly 30 million reads per sample were aligned to the 1000 Genomes Project’s version of GRCh37 using standard TopHat2 (http://ccb.jhu.edu/software/tophat/) v2.0.11 with paired-end read options and allowing for intron-spanning reads as defined by transcripts in Illumina iGenomes NCBI build 37.2. The pairwise euclidean distance between all samples using the filtered (median FPKM>1) and log2 transformed gene expression values was calculated, and Pearson’s correlation determined. Differential expression on a gene-based level was tested for with cuffdiff 59 v2.1.1 using default option. Genes reported as significantly differentially expressed between a pair of conditions were determined to have been so based on cuffdiff threshold of a 0.05 false-discovery rate corrected p-value.
Histology
Animal use followed NIH guidelines and was approved by IACUC at UCSD and Rockefeller University. Wild type mice (C57BL/6N) were obtained from Jax and intercrossed to produce embryonic day (e) 13.5 embryos. The embryos were fixed in 4% PFA, cryoprotected then sectioned. Human fetal brain was obtained from the UCSD autopsy service, fixed in 4% PFA and embedded in paraffin. Microtome sections were deparaffinized, blocked with 4% donkey serum, incubated in primary antibody overnight, washed, incubated in secondary antibody for 1 hr, post-fixed in 4% PFA, and counterstained with DAPI or Nissl for imaging.
αN-catenin Lentivirus
Full-length, the actin binding domain (ABD, amino acids 671–905), or the actin binding domain deleted (ΔABD, amino acids 1–670) αN-catenin was cloned into pLVX-AcGFP-N1 vector (Clontech) and co-transfected with psPAX2 and pCMV-VSVg (Addgene) into Lenti-X 293T cells (Clontech) to generate lentivirus. Virus containing media was collected at 66 hrs, concentrated by ultracentrifuge, and stored at −80°C. Patient-derived NPCs were transduced with lentivirus and selected with 1 μg/ml puromycin for 48 hrs before proceeding with experiments.
Recombinant αN-catenin Protein Generation
cDNA encoding full-length αN-catenin was amplified and cloned into bacterial GST expression vector pGEX-6P-1 (GE Healthcare Life Sciences). Recombinant αN-catenin was produced in BL21 cells and affinity purified with anti-GST MagneGST beads (Promega).
Actin Assays
Actin binding and bundling assays were performed according to manufacturer’s recommendations (Cytoskeleton, Inc., BK001). Actin polymerization assays were performed with pyrene actin (Cytoskeleton, Inc., AP05), α-actinin, GST-tagged VCA domain of human WASP protein (#VCG03), and Arp2/3 protein complex from porcine brain (Cytoskeleton, Inc., RP01P) according to manufacturer’s recommendations, with the addition of recombinant αN-catenin. G:F Actin ratios were determined in triplicate from patient-derived NPCs using G-Actin/F-Actin In Vivo Assay Biochem Kit (Cytoskeleton, Inc., BK037). CK-666 and CK-869, block an activating conformational change, binding to different sites28. CK-666 stabilizes the inactive state of the complex, blocking movement of the Arp2 and Arp3 subunits into the activated filament-like (short pitch) conformation, while CK-869 binds to a serendipitous pocket on Arp3 and allosterically destabilizes the short pitch Arp3-Arp2 interface.
F-actin Pulldown
Phalloidin-mediated F-actin pulldown was performed as described43 with Biotin-XX phalloidin (Life Technologies, B7474) then streptavidin conjugated magnetic beads (Pierce Protein Biology), washed 3x, then boiled.
Antibodies
Primary antibodies used for immunocytochemistry or Western blot include mouse anti-GAPDH (Millipore), mouse anti-Nestin (Millipore), mouse anti-Tuj1/βIII-tubulin (Millipore), mouse anti-Tuj1/βIII-tubulin (Santa Cruz Biotenchnology), mouse anti-TRA-1-60 (Millipore), mouse anti-TRA-1-81 (Millipore), goat anti-Lin28a (R&D Systems), rabbit anti-Nanog (Santa Cruz Biotechnology), rabbit anti-Oct3/4 (Santa Cruz Biotechnology), rabbit anti-DCX44, goat anti-DCX (Santa Cruz Biotechnology), goat anti-SOX2 (Santa Cruz Biotechnology), rabbit anti-PAX6 (Covance), mouse anti-MAP2 (Millipore), mouse anti-BLBP (Abcam), rabbit anti-GFAP (Sigma), rabbit anti-OLIG2 (Abcam), mouse anti-β-Catenin (BD), rabbit anti-Arp3 (Santa Cruz Biotechnology), mouse anti-β-actin (Sigma), rabbit anti-EIF3D (Bethyl Labs), rat anti-αN-catenin obtained from the NIH NICHD Developmental Studies Hybridoma Bank (Catalog: NCAT2, deposited by Masatoshi Takeichi and Shinji Hirano). Fluorophore conjugated secondary antibodies were purchased from Invitrogen, and immunohistochemistry was performed with ABC Elite peroxidase staining kit, using ImmpactDAB as a substrate.
Statistics
Neuronal migration and neurite length was graphed in MS Excel as a box plot with quartile 1, median, and quartile 3 displayed. Whiskers represent the maximum and minimum observed values for each comparison group across all replicates. Western blot densitometry and fold change gene expression was plotted as a bar graph with the average of at least 3 replicates displayed, or the values displayed on the figure. Error bars represent the standard error of the mean. Unpaired, 2-tailed student’s t test was used to calculate significance (* P<0.05, ** P<0.001, *** P<0.0001, n.s. not significant.) Fig. 2a: Control vs MDS, P = 3.29x10−34; Control vs 1263A, P = 1.20x10−27; Control vs CTNNA2KO, P = 1.01x10−18. Fig. 2b: Control vs 1263A, P = 2.74x10−26; Control vs CTNNA2KO, P = 4.03x10−57; Control vs Control + CTNNA-GFP, P = 2.64x10−7; 1263A vs 1263A + CTNNA-GFP, P = 4.30x10−26; CTNNA2KO vs CTNNA2KO + CTNNA-GFP, P = 1.66x10−59; Control vs 1263A + CTNNA-GFP, P = 0.04; Control vs CTNNA2KO + CTNNA-GFP, P = 0.73. Fig. 3c: Control vs Control + CTNNA-GFP, P = 0.29; CTNNA2KO vs CTNNA2KO + CTNNA-GFP, P = 1.15x10−21; Control vs CTNNA2KO + CTNNA-GFP, P = 0.10. Fig. 3c: Control vs Control + CTNNAΔABD, P = 0.65; Control vs Control + CTNNAABD, P = 2.68x10−21; CTNNA2KO vs CTNNA2KO + CTNNAΔABD, P = 0.06; CTNNA2KO vs CTNNA2KO + CTNNAABD, P = 1.81x10−30. Fig. 4c: Control vs Control + CK-666, P = 3.37x10−3; MDS vs MDS + CK-666, P = 0.29; 1263A vs 1263A + CK-666, P = 5.75x10−19; CTNNA2KO vs CTNNA2KO + CK-666, P = 3.48x10−52; Control vs MDS + CK-666, P = 2.12x10−53; Control vs 1263A + CK-666, P = 0.03; Control vs CTNNA2KO + CK-666, P = 4.86x10−10. Supplementary Fig. 4: Control vs 1263A, P = 0.003; no detectable protein in CTNNA2KO. Supplementary Fig. 8: AXIN2 Control vs 1263A, P = 0.81; ID2 Control vs 1263A, P = 0.53; LEF1 Control vs 1263A, P = 0.32. Supplementary Fig. 10: Control vs 1263A, P = 0.35. Supplementary Fig. 11: Control vs Control + CK-869, P = 1.88x10−17; MDS vs MDS + CK-869, P = 2.47x10−5; 1263A vs 1263A + CK-869, P = 1.18x10−38; CTNNA2KO vs CTNNA2KO + CK-869, P = 5.47x10−57; Control vs MDS + CK-869, P = 1.47x10−61; Control vs 1263A + CK-869, P = 5.22x10−6; Control vs CTNNA2KO + CK-666, P = 0.26.
Data Availability
The datasets generated and analyzed for the current study are have been deposited in the database Genotypes and Phenotypes (dbGaP) with accession numbers phs000288 and phs000744 and the Gene Expression Omnibus (GEO) repository with accession number GSE72994.
Supplementary Material
Acknowledgments
We thank the patients and their families for participation. We thank A. Wynshaw-Boris for his generous scientific and editorial input. The research was supported by NIH R01NS041537, R01NS048453, R01NS052455, P01HD070494, P30NS047101, Qatar National Research Fund (QNRF) # 6-1463-351, the Simons Foundation Autism Research Initiative (SFARI), and the Howard Hughes Medical Institute (J.G. Gleeson). A.E. Schaffer is a recipient of an A.P. Gianinni Fellowship and an NIH Pathway to Independence Award, R00HD082337. S.T. Baek is supported by a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. We thank the Broad Institute and, the Yale Center for Mendelian Disorders (UMIHG008900 to D. MacArthur and H. Rehm, and UMIHG006504 to R. Lifton and M. Gunel), and the Gregory M. Kiez and Mehmet Kutman Foundation to M.Gunel. We acknowledge M. Gerstein, S. Mane, A. B. Ekici, ad S. Uebe for sequencing support and analysis, the Yale Biomedical High Performance Computing Center for data analysis and storage, the Yale Program on Neurogenetics, and the Yale Center for Human Genetics and Genomics. Exome data has been deposited into dbGaP (phs000288). The authors declare no competing financial interests.
Abbreviations
- CTNNA2
Alpha-N-catenin
- LIS1 or PAFAH1B1
lissencephaly-1
- DCX
doublecortin
- MDS
Miller Dieker syndrome
- iPSC
induced pluripotent stem cell
- NPC
neural progenitor cell
- ABD
actin-binding domain
Footnotes
URLs
NHLBI Exome Sequencing Project (ESP) http://evs.gs.washington.edu/EVS/
SeattleSeq http://snp.gs.washington.edu/SeattleSeqAnnotation137/
OMIM http://www.omim.org/
PolyPhen-2 http://genetics.bwh.harvard.edu/pph2/
NCBI Gene CTNNA2: https://www.ncbi.nlm.nih.gov/gene/1496
Author Contributions
N.A-S., H.Y.A-A., H.K., C.Y., R.O.R., V.S., K.N.J., M.S.Z., S.E., T. B-O., A. Karminejad, H. Kayserili, F.M., M.K., E. Fenercioglu, B.K., H.M., F.I., S.D., Y.A., E. Faqeih, G.M., B.A.B. L.M., I.M., B.S., J.C., W.B.D., M.G. and J.G.G. performed patient recruitment and phenotyping. A.E.S. A.O.C., S.T.B., B.C., D.M., E.C.S., K.B., J.L.S., M.G., and J.G.G. supported sequencing and variant interpretation. A.E.S., N.C., R.W., and R.N. performed the tissue culture experiments. A.E.S. and A. Kalur. generated recombinant proteins and performed the actin assays. A.E.S., M.B. and J.G.G. wrote the manuscript. A.E.S., M.B., and J.G.G. edited the manuscript, and J.G.G. directed the project.
References
- 1.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin is a molecular switch that binds E-Cadherin-β-Catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues Clin Neurosci. 2008;10:47–62. doi: 10.31887/DCNS.2008.10.1/rjleventer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Meyer G, Feldman EL. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem. 2002;83:490–503. doi: 10.1046/j.1471-4159.2002.01185.x. [DOI] [PubMed] [Google Scholar]
- 5.Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- 6.Rocca DL, Martin SP, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nature Cell Biology. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi M, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA. 2009;106:19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magi A, et al. H3M2: detection of runs of homozygosity from whole-exome sequencing data. Bioinformatics. 2014;30:2852–2859. doi: 10.1093/bioinformatics/btu401. [DOI] [PubMed] [Google Scholar]
- 10.Seelow D, Schuelke M, Hildebrandt F, Nurnberg P. HomozygosityMapper--an interactive approach to homozygosity mapping. Nucleic Acids Research. 2009;37:W593–9. doi: 10.1093/nar/gkp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajioka I, Nakajima K. Switching of α-catenin from α-E-catenin in the cortical ventricular zone to α-N-catenin II in the intermediate zone. Developmental Brain Research. 2005;160:106–111. doi: 10.1016/j.devbrainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Stocker AM, Chenn A. Differential expression of α-E-catenin and α-N-catenin in the developing cerebral cortex. Brain Research. 2006;1073–1074:151–158. doi: 10.1016/j.brainres.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Park C, Falls W, Finger JH, Longo-Guess CM, Ackerman SL. Deletion in Catna2, encoding αN-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nature Genetics. 2002;31:279–284. doi: 10.1038/ng908. [DOI] [PubMed] [Google Scholar]
- 14.Park C, Longo CM, Ackerman SL. Genetic and physical mapping of the cerebellar deficient folia (cdf) locus on mouse chromosome 6. Genomics. 2000;69:135–138. doi: 10.1006/geno.2000.6322. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Finger JH, Cooper JA, Ackerman SL. The cerebellar deficient folia (cdf) gene acts intrinsically in Purkinje cell migrations. genesis. 2002;32:32–41. doi: 10.1002/gene.10024. [DOI] [PubMed] [Google Scholar]
- 16.Togashi H, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Chisaka O, van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by α-N-catenin. Nature Neuroscience. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- 18.Uemura M, Takeichi M. α-N-Catenin deficiency causes defects in axon migration and nuclear organization in restricted regions of the mouse brain. Dev Dyn. 2006;235:2559–2566. doi: 10.1002/dvdy.20841. [DOI] [PubMed] [Google Scholar]
- 19.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katakowski M, Zhang Z, deCarvalho AC, Chopp M. EphB2 induces proliferation and promotes a neuronal fate in adult subventricular neural precursor cells. Neurosci Lett. 2005;385:204–209. doi: 10.1016/j.neulet.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 21.Bershteyn M, et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Stem Cell. 2017;20:435–449.e4. doi: 10.1016/j.stem.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres M, et al. An α-E-catenin gene trap mutation defines its function in preimplantation development. PNAS. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lein W-H, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. αE-Catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006:311. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pokutta S, Weis WI. Structure of the dimerization and β-catenin-binding region of α-catenin. Molecular Cell. 2000;5:533–543. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- 25.Hansen SD, et al. E-catenin actin-binding domain alters actin filament conformation and regulates binding of nucleation and disassembly factors. Molecular Biology of the Cell. 2013;24:3710–3720. doi: 10.1091/mbc.E13-07-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P-S, et al. Crucial roles of the Arp2/3 complex during mammalian corticogenesis. Development (Cambridge, England) 2016 doi: 10.1242/dev.130542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouiller I, et al. The structural basis of actin filament branching by the Arp2/3 complex. The Journal of Cell Biology. 2008;180:887–895. doi: 10.1083/jcb.200709092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetrick B, Han MS, Helgeson LA, Nolen BJ. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chemistry & Biology. 2013;20:701–712. doi: 10.1016/j.chembiol.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynshaw-Boris A, Pramparo T, Youn YH, Hirotsune S. Lissencephaly: Mechanistic insights from animal models and potential therapeutic strategies. Semin Cell Dev Biol. 2010;21:823–830. doi: 10.1016/j.semcdb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon HM, Wynshaw-Boris A. Cytoskeleton in action: lissencephaly, a neuronal migration disorder. Wiley Interdiscip Rev Dev Biol. 2013;2:229–245. doi: 10.1002/wdev.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6α signaling controls glial-guided neuronal migration. Nature Neuroscience. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- 32.Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Kholmanskikh SS, Dobrin JS, Wynshaw-Boris A, Letourneau PC, Ross EM. Disregulated RhoGTPases and actin cytoskeleton contribute to the migration defect in Lis1-deficient neurons. The Journal of Neuroscience. 2003;23:8673–8681. doi: 10.1523/JNEUROSCI.23-25-08673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. The Journal of Neuroscience. 2009;29:288–299. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou FQ, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. The Journal of Cell Biology. 2002;157:839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkelman JD, et al. Fascin- and α-Actinin-bundled networks contain intrinsic structural features that drive protein sorting. Current Biology. 2016;26:2697–2706. doi: 10.1016/j.cub.2016.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann K, Lindner TH. easyLINKAGE-Plus--automated linkage analyses using large-scale SNP data. Bioinformatics. 2005;21:3565–3567. doi: 10.1093/bioinformatics/bti571. [DOI] [PubMed] [Google Scholar]
- 38.Lardelli RM, et al. Bi-allelic mutations in the 3′ exonuclease TOE1 cause pontocerebellar hypoplasia and uncover a role in snRNA processing. Nature Genetics. 2017;49:457–464. doi: 10.1038/ng.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okita K, et al. A more efficient method to generate integration-free human iPS cells. Nature Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 40.Marchetto MCN, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youn YH, Pramparo T, Hirotsune S, Wynshaw-Boris A. Distinct dose-dependent cortical neuronal migration and neurite extension defects in Lis1 and Ndel1 mutant mice. The Journal of Neuroscience. 2009;29:15520–15530. doi: 10.1523/JNEUROSCI.4630-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung YG, Wang Y, Einstein DB, Lee PS, Stanley ER. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem. 1998;273:17128–17137. doi: 10.1074/jbc.273.27.17128. [DOI] [PubMed] [Google Scholar]
- 44.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for the current study are have been deposited in the database Genotypes and Phenotypes (dbGaP) with accession numbers phs000288 and phs000744 and the Gene Expression Omnibus (GEO) repository with accession number GSE72994.