Abstract
In the past, radiation therapy was primarily used to control local disease but recent technological advances in accurate, high-dose ionizing radiation (IR) delivery have not only increased local tumor control but in some cases reduced metastatic burden. These “off target” therapeutic effects of IR at non-irradiated tumor sites, also known as abscopal effects, are thought to be mediated by tumor antigen-primed T cells that travel to metastatic sites and promote tumor regression. Similarly, early indications reveal that IR in combination with immune checkpoint inhibitors, such as ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1), can provide superior therapeutic responses. These observations suggest that local radiotherapy results in altered gene expression, exposure of new antigens or cell-death that can interact with immunotherapy. As such, radiotherapy enhancement of immune responses offers a promising synergy with the potential for substantial clinical benefit. This review focuses on the biology that underlies the mechanisms for the interaction between radiation-induced tumor cell death and enhanced immunological response.
Keywords: DNA damage, Ionizing radiation, immunotherapy
Introduction
In non-metastatic cancer patient populations, radiotherapy is a standard of care for curative purposes but is used mainly to relieve symptoms in metastatic cancer. Radiotherapy induces its cytotoxic response by producing DNA double strand breaks (DSBs) (1, 2) that are sensed by protein kinases such as ATM, ATR and DNA-PK (3). While these factors have a direct role in DNA repair they can also activate multiple transcription factors including NF-kB, p53 and MAPK-induced factors (4-6). For example, recent studies have suggested that the macrophage transcriptional response to IR is dependent on a small number of sensors and signaling pathways such as ATM, p53 and reactive oxygen species (ROS) induced transcriptional factor NRF2 (7). Furthermore, following IR exposure DNA can be released into the cytoplasm where it induces an interferon (IFN) response via activation of cytosolic sensory pathway which signals through stimulator of INF signaling (STING) (8).
Earlier studies, primarily preclinical, have shown that radiotherapy immunomodulatory effects can contribute to therapeutic responses (1) as IR, either as a single agent or in combination with immune therapies, can induce phenotypic alterations in cancer cells. Infiltration of T cells into tumors has been shown to be enhanced by local IR (9). Though IR alone can occasionally increase the systemic immune response (10), this can be counterbalanced by increased tumor infiltration of myeloid-derived suppressor cells and regulatory T cells, which can contribute to immune tolerance (11, 12). In addition, the tumor microenvironmental and associated secreted factors can also create an immunosuppressive milieu (13, 14). Despite this, the combination of IR and immunotherapy can elicit a potent systemic immune response (15-17) that may lead to elimination of distant metastatic disease (18, 19). These observations suggest that local radiotherapy results in altered gene expression, exposure of new antigens or cell-death and when combined with immunotherapy, this synergy could produce substantial clinical benefit.
Immune Checkpoints
The field of immune checkpoint therapy is now approaching the same treatment category as surgery, chemotherapy, radiation and targeted therapy for cancer (20). Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are protein receptors expressed on T-cells. Engagement of CTLA-4 and PD-1 with their ligands (B7-1 or B7-2 and B7-H1 or B7-H2 respectively) restricts T-cell activity (1, 21). Anti-CTLA4 or anti-PD1 therapy may work better with tumors having mutational burden as exemplified by melanoma and non-small cell lung cancer, respectively (22, 23). Perhaps not surprising then, patients harboring mutations in genes controlling DNA repair, replication and maintenance of genomic integrity showed greater responses to immune checkpoint inhibition (24). These studies suggest an additional rationale for combining radiotherapy and immunotherapy.
Interaction between Radiation and Immune system
Anti-tumor immunity is a multi-step process that is regulated by multiple signals (25). To initiate the process, tumor antigens activate antigen-presenting cells (dendritic cells-DCs) and these activated DCs migrate to draining lymph nodes. After processing the peptides in the proteasome, DCs present the peptides as part of the major histocompatibility (MHC) molecules to T cells in the context of co-stimulatory signals that result in T cell activation and proliferation. Thereafter, tumor-specific T cells differentiate into effectors and successfully reject tumor cells after overcoming their immune-suppressive niche. Other T cells differentiate into memory T cells, which could protect the host from metastatic disease and suppress tumor recurrence (26). Various immunotherapeutic agents, which are either in development or currently approved act at one or more of the steps of this process. Previous studies have demonstrated that radiotherapy can potentially enhance uptake of tumor antigens by dendritic cells and their subsequent activation, as well as migration of the activated effector T cells back to the tumor (27-29). As discussed before, the main action of radiotherapy is thought to be DNA damage but studies have also shown that local high-dose of IR activates tumor associated dendritic cells which in turn support tumor specific effector CD8+ T cells suggesting that efficacy of radiotherapy efficacy depends on the presence of CD8+ T cells where as CD4+ T cells and macrophages are nonessential (27, 30). One of the primary host defense mechanisms is the recognition and elimination of invading genetic material. Particularly important is the cytosolic nucleic acid sensor cyclic GMP-AMP (cGAMP) synthase (cGAS), which binds to double stranded DNA irrespective of its sequence specificity, activating type-1 interferons and other inflammatory cytokine expression (30-32). cGAS catalyzes the conversion of GTP and ATP into 2′3′-cGAMP that acts as a second messenger to bind and activate endoplasmic reticulum protein STING (stimulator of interferon genes) (33). STING in turn can activate protein kinases IKK and TBK1 further activating transcription factors NF-kB and IRF3, respectively, to induce transcription of type I IFNs and other immune and inflammatory gene products (34).
TREX1 (DNase III) is a 3′→5′ DNA exonuclease that targets both ssDNA as well as dsDNA in the cytoplasm (35) and can remove mismatched 3′ terminal deoxyribonucleotides at DNA strand breaks, suggesting it may serve an editing role in DNA replication or gap filling during DNA repair (36, 37). Loss of Trex 1 in humans has been linked to various autoimmune and inflammatory diseases with a common feature being elevated expression of IFN-stimulated genes due to defective clearing of cytosolic DNA (35, 36). Trex 1-/- deficient mice exhibit inflammatory diseases due to elevated ISG expression. Recent studies have shown that myocarditis and the autoimmune phenotype in Trex 1-/- are rescued by sting deletion, further supporting an important role for DNA sensing pathways play in inflammatory responses (32, 38).
Treatment of tumors that are refractory to immune checkpoint inhibitors with radiotherapy induces cancer cell-intrinsic activation of interferon-beta (IFN-β), mimicking a viral infection, and resulting in recruitment of Batf3-dependent DCs (39). Further, this process is mediated by the accumulation of dsDNA in the cancer cell cytoplasm, which is sensed by cGAS and leads to downstream activation of STING. Ionizing radiation dose and fractionation schedule is critical for IFN-I production. DNA exonuclease TREX-1 is induced in different cancer cells after exposure of cells with 12-18 Gy of ionizing radiation. TREX1 degrades cytoplasmic dsDNA thereby limiting IFN-I activation.
The DNA damage produced by IR and often heightened by DNA repair inhibitors can increase the amount of unrepaired DNA damage. This in turn can create a tumor genomic landscape that parallels the high mutational burdens often observed in tumors. However, it is difficult to substantiate if a qualitative difference in unrepaired DNA damage between radiation alone and radiation with DNA repair inhibitors can be manipulated for priming tumors to immunotherapy. However recent studies suggest that immunotherapeutic response may be more pronounced in tumors that harbor clonal neoantigens rather than sub clonal neoantigens, which are induced by cytotoxic chemotherapy (40). This would dampen the effectiveness of IR to serve as a potent elicitor of a systemic immune response.
Radiotherapy may have immunosuppressive properties due to up-regulation of MHC-1, death receptors, and checkpoint proteins that drive co-inhibitory pathways to evade immune eradication (41). Radiotherapy can also deplete circulating lymphocytes and/or those sequestered in secondary lymphoid organs (42). Furthermore, cytokine TGF-β (which has immunosuppressive activity), is activated by RT in the tumor microenvironment (13, 43, 44) and TGF- β secretion hampers the ability to generate an effective cytotoxic T cell response to tumor antigens (13). Preclinical study data has shown that inhibition of TGF-β before and after RT exposure leads to regression of irradiated tumor and abscopal effects due to priming of T-cells to multiple tumor antigens (13). Specific interventions targeting the unfavorable effect of IR on immune cell/system function could include approaches such as conformal avoidance of lymphocytes in secondary lymphoid organs, delivery of homeostatic cytokines such as interleukin-7 (IL-7) and/or interleukin-15 (IL-15) (that mediate the recovery of CD8+ cytotoxic T cells and CD4+ helper T cells) (45), restitution of lymphocytes with autologous transfusion, inhibition of galectin 1-mediated lymphocyte apoptosis possibly with thiodigalactoside, and blockade of TGF-β or checkpoint signaling. Synergy between focal radiation that serves as an in-situ tumor autovaccination strategy and systemic immunotherapy has been reported in multiple recent anecdotal studies (46-48) as well as in prospective clinical trials (49).
Preclinical studies
Preclinical studies have indicated that radiotherapy exerts its effects through various biological and immunological methods (50) as summarized in the proposed model (Figure 1). Mice treated with hypo-fractionated IR doses (32 Gy in 4 Gy fractions- once weekly) had better survival, tumor control and fewer lung metastases due to higher NK activity as compared to mice receiving conventionally fractionated radiotherapy (60 Gy in 30 daily 2 Gy fractions) (51). In another study, mice that received a 25 Gy single fraction before surgical removal of a tumor had fewer lung metastases compared to mice without IR treatment. The decrease in metastases in irradiated mice was attributed to dendritic cell (DC)-mediated phagocytosis (52). In a mouse model of melanoma, CD8+ T cells mediated the efficacy of high-dose ablative radiotherapy (9).
Figure 1. Combined effect of radiation and immunotherapy.
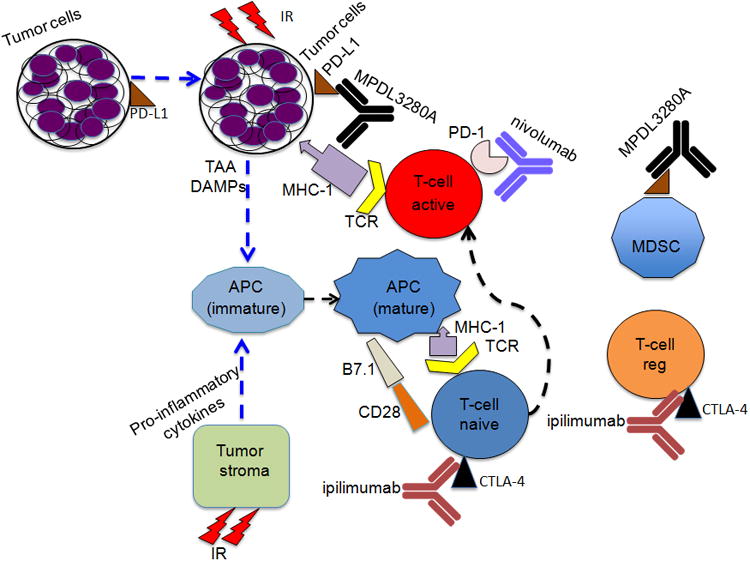
Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are receptors expressed on T-cells and engagement with their ligands (B7-1 or B7-2 and B7-H1 or B7-H2 respectively) restricts T-cell activity. Anti-CTLA-4 antibody and RT in combination induce systemic anti-tumor response in number of carcinomas. PD-1, a cell surface receptor functions as an immune check point and PD-L1 is expressed on a wide variety of cells such as antigen presenting cells, epithelial and endothelial cells. Preclinical studies indicate that combination of RT and Inhibition of PD-1/ or PD-1 ligand increases cytotoxic T cell activity and reduces myeloid-derived suppressor cells. [TCR-T-cell receptor, MHC-1-major histocompatibility complex, APC- Antigen presenting cell, MSDC-myeloid derived suppressor cell, regulatory T-cell (T-cell reg), DAMPs-damage associated molecular patterns (involved in maturation of APCs (dendritic cells), TAA- tumor associated antigens, MPDL3280A- anti-PDL1, Nivolumab –anti-PD1 and ipilimumab- anti-CTL4]. The figure is modified from reference (75).
Furthermore, when radiotherapy is combined with PD-1 inhibitors in an orthotopic glioma xenograft model (53) or xenograft models of breast and colon cancer, control of tumor growth and survival of the mice is improved as compared to radiotherapy or immune checkpoint inhibitors treatment alone (54, 55). When the influence of PD-1 expression on radiotherapy induced anti-tumor response was investigated in wild type PD-1 and PD-1 deficient knock out mice, better survival was observed in PD-1 knock out mice as compared to the wild-type group (1). In the same study, investigators demonstrated that the combination of PD-1 inhibitor and radiation treatment caused synergistic regression of primary tumors in wild type mice, which was not observed with either of the two treatments alone. Such treatment also resulted in a significant response in non-irradiated secondary tumors suggesting an abscopal effect (56).
Similarly, a preclinical study indicated that anti-CTLA-4 antibody and RT in combination induced a systemic anti-tumor response in a poorly immunogenic breast carcinoma relative to anti CTLA-4 alone (57). Furthermore, a patient with metastatic non-small cell carcinoma (NSCLC) treated with ipilimumab and 5 fractions of 6 Gy RT showed a complete response, providing promising evidence of abscopal responses (48). As indicated earlier, programmed death 1 (PD-1) is an inhibitory cell surface receptor which functions as an immune check point and programmed death ligand 1 (PD-L1) is expressed on a wide variety of cells including antigen presenting cells, epithelial and endothelial cells (58). Preclinical studies have shown that the combination of radiotherapy and inhibition of PD-1/ or PD-1 ligand increases cytotoxic T cells activity and reduces myeloid-derived suppressor cells thus further alluding to the synergistic effect with immune checkpoint inhibitors and radiotherapy (54, 55, 59).
Clinical Trials
The combination of immunotherapy and radiotherapy is an active field of clinical investigation and the results have been described in several comprehensive reviews (2, 29, 60). As indicated earlier, CTLA-4 is an inhibitory receptor that suppresses T-cell activation and proliferation (61) that is also expressed on tumor infiltrating regulatory T cells (Tregs) (Figure 1). Antibodies, which selectively target CTLA-4, deplete these cells through antibody dependent cellular cytotoxicity (62). In a clinical study of a patient with metastatic melanoma, the addition of radiotherapy to ipilimumab (a CTLA-4 inhibitor) treatment produced an anti-tumor response in non-irradiated as well as radiated lesions, thus demonstrating an abscopal effect (46).
Multiple clinical investigations have also reported abscopal effects in patients following combined treatment with ipilimumab and RT (47, 63). However, in the absence of a comparator arm where patients do not receive radiation, it is difficult to unequivocally establish whether the responses are due to checkpoint blockade alone or the combination of blockade and radiation. Nonetheless, some studies point to a signficiant contribution of radiation to the abscopal responses observed when radiation is combined with immunotherapy. A retrospective secondary analysis of a Phase 1 trial of pembrolizumab and radiation for non-small cell lung cancer noted that patients who had previously received radiation had a significantly better progression-free survival and overall survival than those who had not received radiation (64).
A Phase 1 clinical trial (NCT01497808) in metastatic melanoma patients treated with 2-3 fractions of radiotherapy (single lung or osseous metastasis) or 6 Gy (subcutaneous or hepatic metastsis) followed by ipilimumab resulted in a partial response in unirriadated lesions in 18% of the patients (65, 66). This response rate is numerically higher than that typically observed with immunotherapy alone, possibly adding credence to the notion that radiation is a key contributor to the responses seen. A Phase 2 trial (NCT02221739) in NSCLC patients treated with ipilimumab and RT supported an in situ vaccacination hypothesis for the interaction with radiation and confirmed the increased activity of the combination in a disease which was not responsive to ipilimumab (anti-CTLA-4) alone (65, 66). A Phase 3 multi-institutional clinical trial (NCT00861614) with castrate resistant prostate cancer with 1 fraction of RT (8 Gy) and ipilimumab for an osseous metastasis did not show any significant improvement in over all survival. However, the combination showed incremental improvement in overall survival in non-visceral metastasis (67). Such studies have delineated clinical activity for PD-1 and PD-L1 targeted therapies against numerous cancers including Hodgkin's lymphoma, non small cell lung cancer, bladder cancer, head and neck and renal cell cancer (68). A Phase 1 clinical trial with anti-PD1 (nivolumab) had similar clinical responses (NCT00730639) (69). A larger Phase 1 trial with anti-PD1 (MK-3475, lambrolizumab) produced a 38% response rate in advanced melanoma patients (NCT01295827) (70). An alternative PD-1 antibody, nivolumab, in a Phase III trial demonstrated an objective response rate of 40% and overall survival rate of 73% compared to a 42% survival rate for patients treated with chemotherapy NCT01721772 (71). On the basis of these promising response rates, Nivolumab received FDA approval for treatment of patients with metastatic melanoma in December of 2014 and for treatment of non-small cell lung carcinoma in March 2015. In addition to predicting clinical efficacy of immune checkpoint blockade (IBD) on tumor immune phenotype, somatic genomic features or the gut micrbiome, a recent study has shown that the patient HLA class I genotype also influences cancer response to IBD. The findings from this study revealed that in two independent melanoma cohorts, patients with the HLA-B44 supertype had extended survival while the HLA-B62 supertype or LOH at HLA-1 was associated with poor outcome. The investigators of the study further suggest that there may be an opportunity for the development of therapeutic vaccines that can potentially target HLA-B44-restricted neoantigens expressed by melanoma (72). Although the extant literature alludes to the promise of radiotherapy-immunotherapy synergy, most of these findings are based on case reports, retrospective reviews, and small non-randomized clinical trials. Emerging data from numerous on-going clinical trials evaluating the combination of radiation and immunotherapy will certainly shed more light on the role of radiation therapy as an immunoadjuvant.
Summary and future prospective
Cancer resistance to chemotherapeutic drugs, radiation and even targeted antibodies impedes the secondary treatment of cancer patients. Somatic mutations in tumor cells hinder their recognition by the immune system and computational analysis of melanoma and NSCLC genome has revealed both have a high somatic mutational load (73). These cancers are classified as “immunogenic” due to their robust response to immunotherapy. In such cancers, ipilimumab and IR exhibit enhanced antitumor activity and abscopal effects (46, 48). To summarize, immunotherapy has opened new avenues to explore the role radiation plays in inducing T-cell responses in the treatment of solid tumors (74). The combination of radiation and immune checkpoint inhibitors (CTLA-4 and PD-1) are promising tools for synergistic improvement in cancer patient outcomes. Moreover, there are many other modalities such as adoptive cell therapies, which may also be enhanced by radiation.
Acknowledgments
We are supported by funds from the Houston Methodist Research Institute and MD Anderson Cancer Center, Houston, TX, the Cancer Prevention and Research Institute (RP150611) and National Institutes of Health grants CA129537 and GM109768.
Financial support: National Institutes of Health grants CA129537 and GM109768
Footnotes
Conflict of interest: Authors declare no conflict of interest.
References
- 1.Mansfield AS, Park SS, Dong H. Synergy of cancer immunotherapy and radiotherapy. Aging (Albany NY) 2015;7(3):144–5. doi: 10.18632/aging.100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123(7):2756–63. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensimon A, Aebersold R, Shiloh Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett. 2011;585(11):1625–39. doi: 10.1016/j.febslet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Gannon HS, Woda BA, Jones SN. ATM phosphorylation of Mdm2 Ser394 regulates the amplitude and duration of the DNA damage response in mice. Cancer Cell. 2012;21(5):668–79. doi: 10.1016/j.ccr.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311(5764):1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 6.Kool J, Hamdi M, Cornelissen-Steijger P, van der Eb AJ, Terleth C, van Dam H. Induction of ATF3 by ionizing radiation is mediated via a signaling pathway that includes ATM, Nibrin1, stress-induced MAPkinases and ATF-2. Oncogene. 2003;22(27):4235–42. doi: 10.1038/sj.onc.1206611. [DOI] [PubMed] [Google Scholar]
- 7.Purbey PK, Scumpia PO, Kim PJ, Tong AJ, Iwamoto KS, McBride WH, et al. Defined Sensing Mechanisms and Signaling Pathways Contribute to the Global Inflammatory Gene Expression Output Elicited by Ionizing Radiation. Immunity. 2017;47(3):421–34 e3. doi: 10.1016/j.immuni.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42(2):332–43. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14(15):4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73(9):2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007;104(21):8959–64. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015;75(11):2232–42. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo P, Bratman SV, Shultz DB, von Eyben R, Chan C, Wang Z, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res. 2014;20(21):5558–69. doi: 10.1158/1078-0432.CCR-14-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–32. [PubMed] [Google Scholar]
- 16.Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther. 2009;11(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Liu L, Yu D, Kandimalla ER, Sun HB, Agrawal S, et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PloS one. 2012;7(5):e38111. doi: 10.1371/journal.pone.0038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acebes-Huerta A, Lorenzo-Herrero S, Folgueras AR, Huergo-Zapico L, Lopez-Larrea C, Lopez-Soto A, et al. Drug-induced hyperploidy stimulates an antitumor NK cell response mediated by NKG2D and DNAM-1 receptors. Oncoimmunology. 2016;5(2):e1074378. doi: 10.1080/2162402X.2015.1074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature reviews Cancer. 2007;7(11):834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189(2):558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 28.Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clin Immunol. 2013;148(1):44–55. doi: 10.1016/j.clim.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao R, Liu Y, Silva-Fernandes A, Fang X, Paulucci-Holthauzen A, Chatterjee A, et al. Inactivation of PNKP by mutant ATXN3 triggers apoptosis by activating the DNA damage-response pathway in SCA3. PLoS Genet. 2015;11(1):e1004834. doi: 10.1371/journal.pgen.1004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–4. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–70. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazur DJ, Perrino FW. Identification and expression of the TREX1 and TREX2 cDNA sequences encoding mammalian 3′-->5′ exonucleases. J Biol Chem. 1999;274(28):19655–60. doi: 10.1074/jbc.274.28.19655. [DOI] [PubMed] [Google Scholar]
- 36.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131(5):873–86. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39(3):482–95. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, et al. Gene-targeted mice lacking the Trex1 (DNase III) 3′-->5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24(15):6719–27. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw. 2015;13(10):1225–31. doi: 10.6004/jnccn.2015.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-beta in breast cancer: too much, too late. Breast Cancer Res. 2009;11(1):202. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93(2):892–9. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, et al. Sustained CD4+ T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3(1):e27357. doi: 10.4161/onci.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–7. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 50.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986–97. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen RN, Hornback NB, Shidnia H, Lu L, Montebello JF, Brahmi Z. A comparison of lung metastases and natural killer cell activity in daily fractions and weekly fractions of radiation therapy on murine B16a melanoma. Radiat Res. 1988;114(2):354–60. [PubMed] [Google Scholar]
- 52.Hassa PO, Hottiger MO. An epigenetic code for DNA damage repair pathways? Biochem Cell Biol. 2005;83(3):270–85. doi: 10.1139/o05-034. [DOI] [PubMed] [Google Scholar]
- 53.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3(6):610–9. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–34. [PubMed] [Google Scholar]
- 58.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–91. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 60.Demaria S, Pilones KA, Vanpouille-Box C, Golden EB, Formenti SC. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat Res. 2014;182(2):170–81. doi: 10.1667/RR13500.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–65. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 62.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4(11):e1046028. doi: 10.1080/2162402X.2015.1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golden EB, Formenti SC. Radiation therapy and immunotherapy: growing pains. Int J Radiat Oncol Biol Phys. 2015;91(2):252–4. doi: 10.1016/j.ijrobp.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gangadhar TC, Salama AK. Clinical applications of PD-1-based therapy: a focus on pembrolizumab (MK-3475) in the management of melanoma and other tumor types. Onco Targets Ther. 2015;8:929–37. doi: 10.2147/OTT.S53164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 72.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2017 doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seyedin SN, Schoenhals JE, Lee DA, Cortez MA, Wang X, Niknam S, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy. 2015;7(9):967–80. doi: 10.2217/imt.15.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buchwald ZSE, JS Immunotherapy and Radiation- A new combined treatment approach for bladder cacer? Bladder Cancer. 2015;1:13. doi: 10.3233/BLC-150014. [DOI] [PMC free article] [PubMed] [Google Scholar]


