Abstract
Head and neck squamous cell carcinoma (HNSCC) accounts for 300,000 deaths per year worldwide and overall survival rates have shown little improvement over the past three decades. Current treatment methods including surgery, chemotherapy, and radiotherapy leave patients with secondary morbidities. Thus, treatment of HNSCC may benefit from exploration of natural compounds as chemopreventive agents. With excellent safety profiles, reduced toxicities, antioxidant properties, and general acceptance for use as dietary supplements, natural compounds are viewed as a desirable area of investigation for chemoprevention. Though most of the field is early in development, numerous studies display the potential utility of natural compounds against HNSCC. These compounds face additional challenges such as low bioavailability for systemic delivery, potential toxicities when consumed in pharmacological doses, and acquired resistance. However, novel delivery vehicles and synthetic analogs have shown overcome some of these challenges. This review covers eleven promising natural compounds in the chemoprevention of HNSCC including vitamin A, curcumin, isothiocyanate, green tea, luteolin, resveratrol, genistein, lycopene, bitter melon, withaferin A, and guggulsterone. The review discusses the therapeutic potential and associated challenges of these agents in the chemopreventive efforts against HNSCC.
Keywords: head and neck cancer, squamous cell carcinoma, natural compounds, prevention, curcumin, vitamin
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the United States and accounts for more than 300,000 deaths per year worldwide (1). Despite numerous therapies, HNSCC is associated with poor overall survival rates (<50%) that have not improved significantly within the last 30 years.
Exposure to tobacco, alcohol, and numerous other environmental factors can result in field cancerization (1). More recently, HNSCC has been linked to human papilloma virus (HPV) infection. Cessation of exposure to carcinogens is an effective way to decrease incidence of HNSCC. However, this is not always an effective means of prevention as genetic alterations caused by initial carcinogen exposures can ultimately lead to transformation long after cessation (1). Standard therapeutic approaches such as surgical intervention, radiation, and chemotherapy are effective in a limited subgroup of patients and often result in additional morbidities. Therefore, there is a great need for prevention and early diagnosis of high-risk premalignant lesions (2). Chemoprevention is defined as the use of natural or synthetic substances to reverse, suppress, or prevent the initiation, promotion, or progression of cancer (2).
Use of natural compounds for chemoprevention is highly compelling due to their safety, low toxicity, and general acceptance as dietary supplements (3). These natural dietary agents hold great promise for chemopreventive use due to their ability to decrease the occurrence of cancer (3). However, there are many associated challenges with the use of natural compounds. Some have increased toxicities or low bioavailability and may trigger resistance mechanisms, all of which are accentuated by using these compounds in pharmacological doses (2). For chemoprevention to be feasible in premalignant populations, the compound must be well-tolerated and have long-lasting benefit. Moreover, the various signaling pathways contributing to HNSCC tumorigenesis mandate use of compounds with multiple molecular targets (2).
Several approaches including development of synthetic analogs, adding bioadjuvant compounds to conventional therapies, using nanoparticles and other delivery agents to improve bioavailability, increasing solubility using phospholipid complexes, show promise in overcoming these challenges (4–6). In this review, we highlight natural compounds with the most potential as chemoprevention agents against HNSCC (Figure 1).
Figure 1.
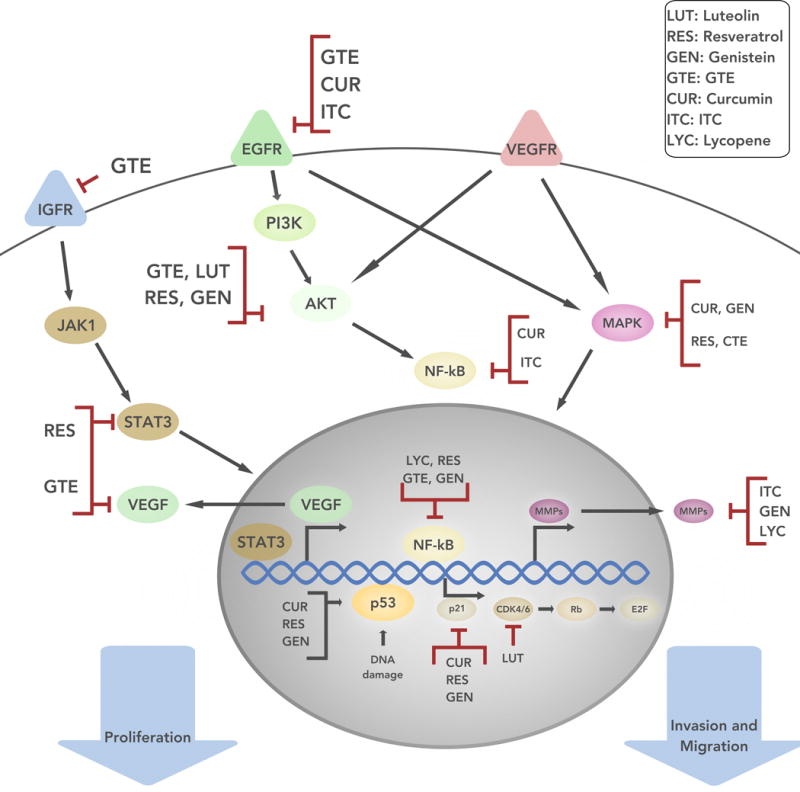
The schematic represents various signaling pathways that occur within tumor cells, which allow for cell growth, proliferation, and invasion. The arrows between the signaling molecules represent progressions in the pathway. For example, IGFR signals through the JAK/STAT3 pathway to allow for cell proliferation. The natural compounds discussed can promote cell apoptosis (Luteolin, Resveratrol) and/or inhibit cell growth, proliferation, and invasion (Luteolin, Resveratrol, Genistein, GTE, Curcumin, ITCs, Lycopene). IGFR = Insulin-like growth factor 1 receptor; JAK1 = Janus kinase 1; STAT3 = signal transducer and activator of transcription 3; EGFR = epidermal growth factor receptor; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT = protein kinase B; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; VEGFR = receptors for vascular endothelial growth factors ; MAPK = mitogen-activated protein kinases; VEGF = vascular endothelial growth factor; p53 = tumor protein p53; p21 = cyclin-dependent kinase inhibitor 1; CDK4/6 = cyclin-dependent kinase 4/6; Rb = retinoblastoma protein; E2F = transcription factor coder for eukaryotes; MMPS = matrix metalloproteinases.
Vitamin A
Vitamin A is a fat-soluble vitamin that is the first and best studied natural chemopreventive compound tested in HNSCC. Analogues of vitamin A, such as retinoids or carotenoids, can be found in liver, fish oil, milk, eggs, and various fruits and vegetables. Several clinical studies have looked at the effect of various vitamin A derivatives on oral premalignancies, second primary tumors, and survival rates (Table 1).
TABLE 1.
Important Natural Forms and Derivatives of Chemopreventive Natural Compounds in HNSCC
Clinical highlights
In 1986, Hong et al. were the first to study isotretinoin (13-cis-retinoic acid), a vitamin A derivative, as a treatment for oral leukoplakia (7). The study showed effectiveness of isoretinoin in decreasing the size of lesions and reversing dysplasia in patients by 67% and 54%, respectively, when compared to placebo. Isoretinoin is also effective in patients with oral premalignancies and in preventing recurrences among HNSCC patients who were disease-free after first-line therapy (surgery, radiotherapy, or both) (8). While there were no differences in recurrence of primary cancers, isoretinoin significantly reduced second primary tumors (SPT) in a dose-dependent manner (9). In each study, isoretinoin induced mild to severe toxicity. Retinyl palmitate and β-carotene are precursors of vitamin A that demonstrated efficacy and minimal toxicity in premalignant lesions (10).
A more recent study compared therapeutic effects of isoretinoin with retinyl palmitate and β-carotene in patients with HNSCC (11). Treatment with isoretinoin had a greater clinical response than the combination of retinyl palmitate with β-carotene or retinyl palmitate alone. However, toxicity remained a significant issue with isoretinoin as six patients left the study from toxicity-related issues. Moreover, the study found no significant association between oral premalignant lesion response and survival.
Synergistic interactions
Isoretinoin in combination with interferon-α and α-tocopherol (vitamin E) demonstrates the most promise in chemoprevention of oral premalignancies (Table 2). Vitamin E decreases isoretinoin toxicity in addition to having possible chemopreventive effects (12). There has been significant response against laryngeal lesions and advanced dysplasia (6). Further, there has been therapeutic benefit from isoretinoin with surgery or radiotherapy in HNSCC patients with advanced disease (13). A follow-up study confirmed the promising results with longer survival rates and fewer incidence of SPT (12). Mild to moderate toxicity, though manageable, was still observed in these patients (12,13).
TABLE 2.
The Most Promising Studies of Chemopreventive Natural Compounds in HNSCC
| Natural Compound | Most Promising Trials | |||
|---|---|---|---|---|
| Type | Compound | Result | Source | |
| Vitamin A | Clinical | Isoretinoin, interferon-α, α-tocopherol | Improved survival rate and lowered secondary primary tumors | (6,12,13) |
| Curcumin | Clinical | Curcumin | Reduced oral premalignant and high risk lesions | (23,24) |
| Isothiocyanate | Pre-clinical | BITC, cisplatin | Increase in apoptosis and decrease in HNSCC cell viability | (31,32) |
| Green Tea | Clinical | GTE, erlotinib | Reduced oral premalignant lesions | (47) |
| Luteolin | Pre-clinical | EGCG, luteolin | Induced apoptosis, inhibited growth of xenograft tumors | (58) |
| Resveratrol | Pre-clinical | Resveratrol | Induced apoptosis | (99) |
| Genistein | Pre-clinical | Cetuximab, genistein | Tumor growth inhibition, apoptosis | (83) |
| Lycopene | Clinical | Lycopene | Reduction in HNSCC | (92) |
| Bitter Melon | Pre-clinical | BME | Inhibition of HNSCC growth in mice | (93,95) |
| Withaferin A | Pre-clinical | Withaferin A | Apoptosis of HNSCC cell lines | (96) |
| Guggulsterone | Pre-clinical | Guggulsterone | Increased efficacy of erlotinib, cetuximab and cisplatin | (97) |
BITC = benzyl isothiocyanate, GTE = green tea extract, EGCG = epigallocatechin-3-gallate, BME = 2-Mercaptoethanol
Future considerations
The use of vitamin A derivatives, such as isoretinoin, has long been proven an effective means of chemoprevention in HNSCC. However, toxicity remains a limitation to the utility of the vitamin A derivatives in patients (Table 3). Use of bioadjuvant therapy, such as vitamin E, may improve associated toxicity, though phase 3 trials are still ongoing (12). Developing novel strategies for delivering these compounds to directly the tumor site could further reduce toxicity in patients.
TABLE 3.
Current Obstacles and Solutions for Chemopreventive Natural Compounds in HNSCC
| Natural Compound | Obstacles | Current Solutions |
Source |
|---|---|---|---|
| Vitamin A | toxicity | In combination, analogs, bioadjuvant | (6–8,11–13) |
| Curcumin | bioavailability | analogs, bioadjuvant, liposomal, nanoparticles, phospholipid complex, in combination | (4,5,15–17,21,23) |
| Isothiocyanate | lack of studies specific to HNSCC | In combination, analogs | (37) |
| Green Tea | bioavailability | In combination, analogs | (1,47,49) |
| Luteolin | bioavailability, lack of studies specific to HNSCC | Nanoparticles, in combination | (100) |
| Resveratrol | bioavailability, lack of studies specific to HNSCC | Nanoparticles, in combination, bioadjuvant | (61,70–72) |
| Genistein | lack of studies specific to HNSCC | In combination | (3,83) |
| Lycopene | lack of studies specific to HNSCC | In combination, bioadjuvant | (3,84) |
| Bitter Melon | lack of studies specific to HNSCC | Early stages of investigation | (93–95) |
| Withaferin A | lack of studies specific to HNSCC | Early stages of investigation | (96) |
| Guggulsterone | lack of studies specific to HNSCC | Early stages of investigation | (97) |
Curcumin
Turmeric, derived from the rhizomes of Curcuma longa, is used as a spice and, particularly in Asia, as a treatment for a number of ailments. Commercially available, turmeric is usually a mixture of three curcuminoids, curcumin (72%), demethoxycurcumin (19%), and bisdemethoxycurcumin (9%). Curcumin, also called diferuloyl methane or bis-a,b-unsaturated b-diketone, is a yellow, hydrophobic polyphenol that has been widely investigated for its antitumor effects in multiple cancer types (2). The major challenge with curcumin lies with its low bioavailability due to limited absorption from the gut.
Several novel packaging strategies including liposomes, polymer nanoparticles, nanogels, and micelles have improved the bioavailability of curcumin (14). Analogs of curcumin also greatly increase the bioavailability, potency, and stability (4,5,15,16). In addition, encapsulation of curcumin and its analogs by lipids and nanoparticles greatly increases bioavailability (17).
Molecular targets and mechanisms of action
Curcumin’s anticancer effects mainly result from its various pro-apoptotic and anti-inflammatory effects. Curcumin induces apoptosis via a number of molecular mechanisms. It causes cell cycle arrest, inhibits proliferation in HNSCC, via upregulation and activation of p53 and p21 (18,19).
Curcumin has anti-inflammatory effects that can work to prevent the development and progression of cancer. Curcumin downregulates nuclear factor-B (NF-κB) and mitogen activated protein kinase (MAPK) pathways, which prevent expression of pro-inflammatory cytokines and cell signaling necessary for proliferation, metastasis, and angiogenesis (19). Specifically, curcumin-mediated NF-κB inhibition mitigates the expression of proinflammatory cytokines IL-6 and IL-8. These cytokines are upregulated in patients with HNSCC compared to controls (20). Moreover, curcumin inhibits tumor growth by targeting the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) (21) (Figure 2).
Figure 2.
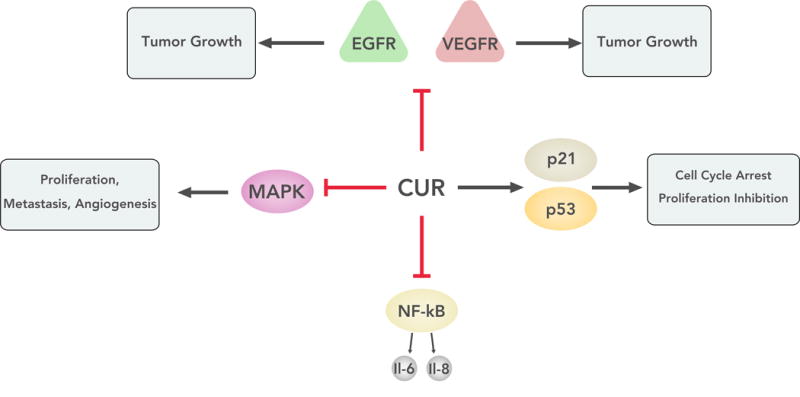
The figure shows the various signaling pathways affected by curcumin. The molecule can act to inhibit a number of pathways that allow for tumor growth and invasion. Curcumin can also lead to upregulation of molecules that lead to cell cycle arrest in tumor cells. EGFR = epidermal growth factor receptor; VEGFR = receptors for vascular endothelial growth factors; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK = mitogen-activated protein kinases; p53 = tumor protein p53; p21 = cyclin-dependent kinase inhibitor 1
Clinical highlights
The first clinical trial investigating turmeric and curcumin as anti-cancerous agents used a one percent curcumin ointment on skin cancer lesions. Reduced pain and lesion size were reported in 10% percent of patients (22). A more recent phase I clinical trial conducted showed histological improvements of precancerous lesions in oral cancer patients upon treatment with curcumin, which was not toxic even at doses of 8000 mg/day (23) (Table 2). However, this trial also clearly showed that curcumin exhibits poor bioavailability, having a mere 1% absorption rate upon oral administration (23). The low plasma and tissue levels of curcumin result from poor absorption due to reduced solubility in water as well as rapid systemic elimination (17).
Synergistic interactions
The chemopreventive effects of curcumin greatly increase when used concomitantly with other natural agents including piperine, genistein, green tea, or embelin. Curcumin also synergistically increased the efficacy of several chemotherapeutic agents including cisplatin, 5-fluorouracil (5-FU), vinca alkaloid, vinorelbine, and gemcitabine (24).
Future considerations
The use of curcumin as a chemopreventive agent in HNSCC is promising due to its effect on multiple molecular pathways essential for cancer progression. Moreover, studies suggest efficacy in combining curcumin with radiation therapy as a first line of treatment in HNSCC (25). Although bioavailability remains a challenge, there are numerous viable approaches to circumvent this problem. Natural derivatives including difluorinated-curcumin (CDF), diphenyl difluoroketone (EF24), and 3, 5-bis (2, 4-difluorobenzylidene)-4-piperidone (DiFiD) also seem to have promise for use in HNSCC as they have been shown to exhibit tumor growth inhibition and increased metabolic stability compared to curcumin in other tumor types (26,27).
Isothiocyanates
Cruciferous vegetables, including broccoli, kale, and watercress, have various anti-cancer properties due to high glucosinolate content. Isothiocyanates (ITCs) are the most potent component of glucosinolates when isolated via enzymatic hydrolysis. Phenethyl isothiocyanate (PEITC), benzyl isothiocyanate (BITC) and sulforaphane are three naturally occurring ITCs that are being studied as chemopreventive agents (28).
Molecular targets and mechanisms of action
ITCs cause apoptosis and prevent activation of the NF-κB signaling pathway via numerous mechanisms. PEITC and sulforaphane inhibit NF-κB transcriptional activity by through multiple methods including preventing dimerization of the molecule and other downstream effects (29). BITC acts to inhibit NF-κB phosphorylation, inducing apoptosis in target cells (30). PEITC also works upstream to decrease EGFR phosphorylation to inactivate NF-κB.
Furthermore, by decreasing EGFR signaling, PEITC can suppress the expression and enzymatic activities of matrix metalloprotease-2 (MMP-2) and MMP-9 (31). MMPs cause extracellular matrix degradation, facilitating tumor metastasis. Both MMP-2 and -9 are expressed in oral cancer and are associated with tumor invasion. BITC induced apoptosis in five HNSCC lines and upregulated anti-proliferative activity in cell lines (32).
Clinical highlights
Preclinical trials showed PEITC reduced growth of oral cancer by inducing apoptosis in cancer cells (33). Furthermore, topical application of ITCs induced strong chemopreventive activity against HNSCC in animal models (34). A more recent study demonstrated that ITCs, specifically BITC, inhibit HNSCC migration and invasion. In addition, BITC increased sensitivity to cisplatin treatment in vitro, enhancing the effects of chemotherapy (35). Sulforaphane down-regulated various MMPs in HNSCC cell lines, which makes it a potential inhibitor of metastasis (28). A pilot clinical trial using broccoli sprout extracts in ten healthy volunteers demonstrated target modulation at low micromolar doses in the oral mucosa on oral administration indicating adequate bioavailability, though further investigation is warranted (36).
Future considerations
ITCs hold great potential as chemopreventive agents due to ease of integration into patient diets. However, it has been difficult for researchers to create formulations of pure ITCs suitable for clinical investigation and small changes in their molecular formulas drastically change the mechanisms of action. As clinical trials continue, use of ITCs as adjuvant therapy to conventional anticancer agents seem especially promising due to the substantial increase in efficacy when used in combination (Table 3).
Green tea
Tea is a widely-consumed beverage rich in substances with antioxidant properties. Studies exist investigating both black and green tea as chemopreventive agents, though green tea shows greater efficacy against multiple types of cancer. The health effects of green tea are mostly associated with its polyphenol (PP) content. PPs are reactive metabolites characterized by several hydroxylated aromatic groups and possess powerful antioxidant activity by scavenging a wide range of reactive oxygen, nitrogen, superoxide anions and metal ions. Green tea extract (GTE) contains four major polyphenols: epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) (38) (Table 1). EGCG is the most abundant polyphenol in green tea and has gained the most attention in anti-carcinogenic studies (2).
Molecular targets and mechanisms of action
EGCG has several mechanisms of action, but its antitumor effects stem primarily from suppression of EGFR phosphorylation (39,40). Up to 90% of HNSCC patients show EGFR overexpression, suggesting GTE could be effective in chemoprevention. EGCG also acts downstream of EGFR to inhibit protein kinase B (Akt) phosphorylation and eventually activation and nuclear translocation of NF-κB (40).
GTE inhibits the vascular endothelial growth factor receptor (VEGFR) activation and VEGF secretion, reducing angiogenesis (40,41). This is a result of inhibition of NF-κB by EGCG (41). EGCG also acts to suppress the MAPK signaling pathway, preventing the transcription of MMPs, specifically MMP-2 and 9, which are expressed in oral cancers (42).
Clinical highlights
Preclinical investigation of GTE chemoprevention started in 1987 with a study that showed topical application of EGCG inhibited tumor formation in mice (43). More recent studies confirm that oral intake of EGCG-rich GTE inhibit cancer development and metastasis in mice models in vitro and in vivo (44). EGCG specifically prevented growth of HNSCC cell lines and enhanced effects of 5-fluorouracil (39).
Several phase I trials show similar antitumor effects in humans (45). According to a phase I trial, a dose of 1.0 g TID, equivalent to 7 or 8 cups of green tea, could safely be consumed by patients (46). In patients with high-risk oral premalignant lesions, high-dose GTE for 12 weeks led to higher clinical response rates but showed no significant improvement in cancer-free survival, indicating the need for further studies. Poor bioavailability was reported in the majority of patients in phase II clinical trials (47) (Table 2). The study also assessed the pharmacokinetics of EGCG in the plasma with 500 or 1000mg/m2 of oral, thrice daily GTE dosing. Free EGCG accounted for only 12-28% of total EGCG possibly due to poor oral absorption of EGCG.
Synergistic interactions
Multiple synergistic interactions involving GTE have been investigated with some continuing to clinical trials. In preclinical studies, GTE increased the efficacy of many compounds including luteolin, genistein, atorvastin, curcumin, erlotinib, sulindac, tamoxifen, celecoxib, cisplatin, adriamycin, dacarbazine, lycopene, and EGFR inhibitors in the inhibition of HNSCC growth (3,40). EGCG also sensitizes multidrug-resistant forms of HNSCC to vincristine sulfate, decreasing tumor growth via down-regulation of VEGF in vitro (48). The combination of erlotinib and GTE created a synergistic effect in mice and is currently being studied in a phase 1 chemoprevention study on patients with premalignant lesions of the head and neck (1).
Future considerations
The use of GTE as a chemopreventive agent in HNSCC seems promising and warrants further studies. There are very few associated toxicities, many synergistic effects, and clear chemopreventive benefit. However, there is a need to improve the bioavailability, standardize the different formulations of GTE, and investigate the effects of greater dosages over longer periods of time (47). Nanoparticle-mediated EGCG delivery enhances bioavailability and reduces toxicity, thus providing an alternate route for EGCG use (49) (Table 3).
Luteolin
Luteolin is flavonoid, a group of PPs that are found in vegetables like cabbage, celery, broccoli, and parsley. Plants rich in flavonoids have been used in traditional East Asian medicine for their anti-inflammatory effects (50). The anti-cancer effects of luteolin are beginning to be investigated, though the exact biological and molecular mechanisms remain somewhat unclear (51).
Molecular targets and mechanisms of action
Luteolin acts to inhibit activation and phosphorylation of Akt, suppressing VEGFR-mediated angiogenesis and downstream activation of NF-κB (52). It is by blocking the NF-κB pathway and subsequent formation of pro-inflammatory cytokines like TNFα and IL-1 that luteolin produces anti-inflammatory effects.
Luteolin causes cell cycle arrest due to decreased CDK4/6 activity. CDK4/6 form a complex with cyclin D1, which is downregulated via suppression of the Akt signaling pathway (53). The Akt pathway plays a major role in cell cycle progression and is often upregulated in many forms of cancer, including HNSCC (54).
Clinical highlights
Preclinical studies showed luteolin causes apoptosis of HNSCC cell lines and inhibits tumor growth in mice (55). A similar study found luteolin decreased both the incidence and tumor volume of lung cancer significantly compared to controls in mice (56). Akt expression was downregulated in a dose-dependent manner in mice. Surprisingly, treated mice showed no decrease in body weight or other cytotoxic effects compared to control mice (55). Delivery of luteolin in nanoparticles significantly inhibited growth of HNSCC tumors in vitro and in vivo and showed increased efficacy compared to free luteolin, which displays poor bioavailability due to poor solubility (57). Despite these encouraging results, no clinical trials have begun to investigate luteolin in humans.
Synergistic Interactions
Luteolin improves the cytotoxic effects of many chemotherapy and chemopreventive treatments including cisplatin, doxorubicin, TNF-α, paclitaxel, and EGCG in various types of cancer (58). Preclinical trials for HNSCC show combination treatment of luteolin with paclitaxel enhanced inhibition of tumor growth compared to paclitaxel alone (59).
Future considerations
Luteolin is a natural agent that still requires investigation to be deemed effective in humans. One major obstacle to the clinical application of luteolin is it’s low systemic bioavailability due to metabolism in the liver and intestine (3). However, the epithelium of the oral cavity can absorb luteolin directly, and delivery with nanoparticles may solve other problems with bioavailability that may arise (60). However, phase I and II trials investigating the role of luteolin in chemoprevention of any cancers have still not begun.
Resveratrol
The compound resveratrol (3,5,4′-trihydroxystilbene) is a naturally occurring phytoalexin found in grapes, peanuts, and mulberries (61). High levels of resveratrol are found in the skin of grapes and in red wine (3). Resveratrol is widely considered the cardioprotective factor in red wine and is being investigated for anti-cancer properties (62).
Molecular targets/mechanisms of action
Resveratrol targets multiple signaling pathways that facilitate cell growth, inflammation, and cell survival. Resveratrol increases expression and activation of p53 and elevation in p21 levels (62). This causes cell cycle arrest and apoptosis in cells. By suppressing phosphorylation of STAT3, resveratrol also inhibits the JAK/STAT pathway of signaling that is constitutively activated in HNSCC cells, causing cell death (63). Furthermore, resveratrol decreases NF-κB activation and nuclear translocation of the p65 subunit in a variety of cell types (62). Resveratrol also acts upstream of NF-κB to suppress Akt activity and signaling (64). In order to reduce inflammation, resveratrol inhibits the MAPK signaling pathway, preventing formation of pro-inflammatory cytokines (18).
Clinical Highlights
Chemopreventive studies involving resveratrol began after 1997 when a study found topical application of the agent prevented tumor formation in mice (65). Subsequent preclinical studies confirmed similar anti-tumor effects in various cancer cell lines, including HNSCC cells (63). One study showed resveratrol inhibited tumor growth in mice, however efficacy of resveratrol in humans proves more complicated to confirm due to poor bioavailability (66). When consumed orally, 70-80% of resveratrol is absorbed by the intestines (67). Further, after administration of 5g daily for 29 days, trans-resveratrol was detected in the plasma at concentrations as high as 4 μM. Mechanisms to increase bioavailability are under investigation and include packaging within nanoparticles and co-administration with curcumin (68,69). In humans, resveratrol toxicity is minimal, and one phase I study showed even amounts as high as 5 g was safe in healthy patients (3).
Synergistic interactions
Resveratrol has many synergistic interactions with chemopreventive compounds including EGCG, vitamin E, genistein, cisplatin, doxorubicin, fluorouracil (FU), paclitaxel, and curcumin (3). In HNSCC, the combination of resveratrol and curcumin was more effective at inhibiting cancer growth in preclinical models than curcumin alone (70). 5-FU and resveratrol also showed a synergistic antitumor effect in HNSCC cell lines (71,72). Resveratrol enhanced sensitivity to chemotherapy and radiotherapy in HNSCC cells (73).
Future considerations
The use of resveratrol shows promise as a chemopreventive agent against HNSCC, especially when used as adjuvant therapy with other chemotherapeutic agents. Clinical trials are currently underway to further investigate the effects of resveratrol in patients, and studies to increase bioavailability may improve efficacy.
Genistein
The natural compound Genistein (4,5,7-trihydroxyisoflavone) is a phytoestrogen abundant in soybeans and other legumes (3,74). There have been no clinical studies that examine the effects of genistein on HNSCC. Epidemiological studies demonstrated efficacy of genistein intake on breast, prostate, and colorectal cancers (75). However, habitual consumption of soy products showed no significant effect on the risk of HNSCC in Chinese adults, though this study had many significant limitations (76).
Molecular targets/mechanisms of action
Several studies revealed the anti-cancer effects of the consumption of soy products. By affecting multiple pathways, genistein has anti-proliferative and anti-angiogenic effects (3). Genistein significantly downregulates MAPK and Akt signaling, inhibiting VEGF-mediated angiogenesis in HNSCC cells (77). It also acts downstream to inhibit NF‐κB and thus cell proliferation (77). Other anti-proliferative effects of genistein result from decreased phosphorylation of the insulin-like growth factor receptor (IGFR) and IGF signaling, inhibiting cell growth (78). Genistein acts to induce cell cycle arrest and apoptosis by activating p53 and increasing p21 expression (79). Secretion of MMP-2 and -9 decreases with genistein, inhibiting tumor invasion and migration capabilities (74).
Clinical Highlights
Despite numerous molecular targets, preclinical trials involving genistein and HNSCC are not conclusive in showing efficacy as a chemopreventive agent. Low concentrations of whole soy protein extract significantly inhibited oral cancer growth in cell lines (80). However, genistein itself varies in its efficacy between studies. One study showed purified genistein had no effect on the tumor growth and metastasis but inhibited tumor invasion (81). A more recent study contradicted previous results, showing genistein inhibited proliferation of HNSCC cell lines (82), thus necessitating further research to confirm genistein efficacy.
Synergistic interactions
As genistein might have its greatest utility in combination, it has many synergistic interactions with chemopreventive agents including EGCG, letrozole, docetaxel, resveratrol, lycopene, vitamin D, tamoxifen, paclitaxel, cisplatin, erlotinib, gemcitabine, doxorubicin, FU, camptothecin, bleomycin, and cetuximab (83). One study combined cetuximab, an anti-EGFR monoclonal antibody, with genistein to evaluate inhibition of the EGFR pathway. Even at low concentrations, the combination resulted in additive growth inhibition and increased apoptosis compared to single agent exposure (83) (Table 2).
Future considerations
Genistein seems to hold its most promise as an adjuvant therapy. However, further preclinical and eventually clinical studies may prove its efficacy as a single agent in chemoprevention of HNSCC.
Lycopene
The natural compound lycopene is a red-colored carotenoid and is predominantly found in tomatoes (3,84). Of the carotenoids, lycopene is the most abundant in fruits and vegetables and the most promising in chemoprevention. Various epidemiological studies show dietary supplementation with lycopene decreases risk of cancer, and effected molecular targets are numerous and diverse (85).
Molecular targets/mechanisms of action
Lycopene acts at multiple points to inhibit VEGF-mediated angiogenesis. Lycopene suppresses Akt activation by preventing phosphorylation of the molecule (86). Further downstream, lycopene directly inhibits the NF-κB signaling pathway by preventing nuclear translocation and NF-κB DNA binding (87). By decreasing plasma levels and activity of MMP-2 and -9, lycopene also acts to inhibit invasion and metastasis (88).
Clinical highlights
Preclinical studies show that lycopene decreased HNSCC cell growth, induced apoptosis in cells, and prevented tumor invasion (89). Lycopene worked to inhibit the Akt signaling pathway in a dose-dependent manner.
When used in humans, lycopene was safe and more effective in the management of oral leukoplakia in patients than placebo (90). The treatment of oral premalignant lesions with lycopene was associated with significant clinical and histological responses compared with placebo or absence of treatment against HNSCC (91). Lycopene significantly reduced multiple forms of HNSCC including laryngeal, oral, and pharyngeal cancer (91) (Table 2). Phase II trials of lycopene have shown effectiveness in decreasing prostate cancer growth, suggesting it may be useful in prevention and treatment in other forms of cancer (92).
Future considerations
Much preclinical and clinical evidence gives promise to the use of lycopene as an effective chemopreventive agent against HNSCC. There is little associated toxicity, and it shows significant response against other forms of cancer. However, the need exists for more mechanistic studies and randomized controlled trials to confirm the benefits of lycopene and its use in routine prevention and management of HNSCC (84) (Table 3).
Bitter Melon
Bitter melon, which is grown primarily in Asia, Africa, and South America, is a plant that has been used in traditional medicine practices, especially for patients with diabetes, and is beginning to be investigated as a chemopreventive agent. Preclinical studies show that bitter melon extract (BME) inhibited HNSCC growth in vitro and in vivo in mice by modulating immune regulatory mechanisms (93–95). Exact molecular mechanisms of action are starting to be elucidated, but no clinical trials investigating anti-tumor effects of bitter melon have begun.
Medicinal Plant Compounds
Numerous medicinal plant-derived compounds are being investigated for their anticancer properties. Such compounds include withaferin A, which is extracted from Vassobia breviflora in Latin America. Preclinical studies show antiproliferative effects of withaferin A on HNSCC cell lines by interacting with Akt and inducing apoptosis (96). Another such compound is guggulsterone, which is widely available and well-tolerated. Guggulsterone enhances efficacy of chemotherapy drugs including erlotinib, cetuximab, and cisplatin by decreasing expression of STAT-3 (97). These compounds are only two examples of extracts from plants that are in studies for HNSCC treatment, though no clinical trials are in effect.
Conclusion
HNSCC claims more than 300,000 lives each year worldwide, and with poor overall survival rates (<50%) using current therapies. Smoking, alcohol use, and HPV-infection are the largest risk factors for HNSCC, thus primary prevention focuses on mitigating risk factors. Even in lieu of improved management of risk factors, HNSCC will remain dangerous through genetic predispositions and prior field cancerization. As treatment with surgery, chemotherapy, and radiotherapy often cause severe morbidity from damage to the upper aerodigestive tract, the prevention and early diagnosis of high-risk premalignant lesions should be emphasized. The use of natural compounds in the chemoprevention of HNSCC shows promise in reversing these abysmal trends.
Natural compounds show promise as chemopreventive agents in HNSCC because of their tolerability, safety, low toxicity, antioxidant properties, and general acceptance as dietary supplements (3). Moreover, many studies have displayed the potential utility of natural compounds against HNSCC.
Depending on the compound, however, specific challenges exist including low bioavailability, high toxicity, and few molecular targets. There are myriad solutions to circumvent these problems including the use of chemical analogs, adjuvant therapies, and nanoparticle delivery mechanisms. Currently, this field remains in its infancy and its biggest challenge is a shortage of research on chemopreventive natural compounds specific to HNSCC. In order to find safe and effective compounds, clinical trials must continue for the different natural agents.
Acknowledgments
This work was supported by the University of Kansas Cancer Center’s CCSG (1-P30-CA168524-02) and Kansas Intellectual and Developmental Disabilities Research Center (NIH U54 HD 090216) at University of Kansas Medical Center. The authors acknowledge artwork by Mr. Phil Shafer and Mr. Levi Arnold, University of Kansas Medical Center.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Saba NF, Haigentz M, Jr, Vermorken JB, Strojan P, Bossi P, Rinaldo A, et al. Prevention of head and neck squamous cell carcinoma: removing the “chemo” from “chemoprevention”. Oral oncology. 2015;51(2):112–8. doi: 10.1016/j.oraloncology.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Rahman MA, Amin AR, Shin DM. Chemopreventive potential of natural compounds in head and neck cancer. Nutrition and cancer. 2010;62(7):973–87. doi: 10.1080/01635581.2010.509538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(16):2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu S, Moore TW, Lin X, Morii N, Mancini A, Howard RB, et al. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integrative biology: quantitative biosciences from nano to macro. 2012;4(6):633–40. doi: 10.1039/c2ib20007d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagliardi S, Ghirmai S, Abel KJ, Lanier M, Gardai SJ, Lee C, et al. Evaluation in vitro of synthetic curcumins as agents promoting monocytic gene expression related to beta-amyloid clearance. Chemical research in toxicology. 2012;25(1):101–12. doi: 10.1021/tx200246t. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitrakopoulou VA, Clayman GL, Shin DM, Myers JN, Gillenwater AM, Goepfert H, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Archives of otolaryngology–head & neck surgery. 1999;125(10):1083–9. doi: 10.1001/archotol.125.10.1083. [DOI] [PubMed] [Google Scholar]
- 7.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. The New England journal of medicine. 1986;315(24):1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. The New England journal of medicine. 1993;328(1):15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 9.Perry CF, Stevens M, Rabie I, Yarker ME, Cochrane J, Perry E, et al. Chemoprevention of head and neck cancer with retinoids: a negative result. Archives of otolaryngology–head & neck surgery. 2005;131(3):198–203. doi: 10.1001/archotol.131.3.198. [DOI] [PubMed] [Google Scholar]
- 10.Garewal HS, Katz RV, Meyskens F, Pitcock J, Morse D, Friedman S, et al. Beta-carotene produces sustained remissions in patients with oral leukoplakia: results of a multicenter prospective trial. Archives of otolaryngology–head & neck surgery. 1999;125(12):1305–10. doi: 10.1001/archotol.125.12.1305. [DOI] [PubMed] [Google Scholar]
- 11.Papadimitrakopoulou VA, Lee JJ, William WN, Jr, Martin JW, Thomas M, Kim ES, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(4):599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seixas-Silva JA, Jr, Richards T, Khuri FR, Wieand HS, Kim E, Murphy B, et al. Phase 2 bioadjuvant study of interferon alfa-2a, isotretinoin, and vitamin E in locally advanced squamous cell carcinoma of the head and neck: long-term follow-up. Archives of otolaryngology–head & neck surgery. 2005;131(4):304–7. doi: 10.1001/archotol.131.4.304. [DOI] [PubMed] [Google Scholar]
- 13.Shin DM, Khuri FR, Murphy B, Garden AS, Clayman G, Francisco M, et al. Combined interferon-alfa, 13-cis-retinoic acid, and alpha-tocopherol in locally advanced head and neck squamous cell carcinoma: novel bioadjuvant phase II trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(12):3010–7. doi: 10.1200/JCO.2001.19.12.3010. [DOI] [PubMed] [Google Scholar]
- 14.Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights into therapeutic activity and anticancer properties of curcumin. Journal of Experimental Pharmacology. 2017;9:31–45. doi: 10.2147/JEP.S70568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo C, Yamakoshi H, Sato A, Nanjo H, Ohori H, Ishioka C, et al. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC pharmacology. 2011;11:4. doi: 10.1186/1471-2210-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(4):1269–77. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 17.Belkacemi A, Doggui S, Dao L, Ramassamy C. Challenges associated with curcumin therapy in Alzheimer disease. Expert reviews in molecular medicine. 2011;13:e34. doi: 10.1017/S1462399411002055. [DOI] [PubMed] [Google Scholar]
- 18.Hu A, Huang J-J, Zhang J-F, Dai W-J, Li R-L, Lu Z-Y, et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget. 2017;8(31):50747–60. doi: 10.18632/oncotarget.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AY-L, Fan C-C, Chen Y-A, Cheng C-W, Sung Y-J, Hsu C-P, et al. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integrative Cancer Therapies. 2015;14(5):484–90. doi: 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AN, Veena MS, Srivatsan ES. Suppression of Interleukin 6 and 8 Production in Head and Neck Cancer Cells With Curcumin via Inhibition of Iκβ Kinase. 2009;135(2):190–7. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, et al. RNA Binding Protein CUGBP2/CELF2 Mediates Curcumin-Induced Mitotic Catastrophe of Pancreatic Cancer Cells. PLOS ONE. 2011;6(2):e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73(1):29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 23.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer research. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 24.Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, et al. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKbeta protein of the NFkappaB pathway. Molecular cancer therapeutics. 2010;9(10):2665–75. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuttle S, Hertan L, Daurio N, Porter S, Kaushick C, Li D, et al. The chemopreventive and clinically used agent curcumin sensitizes HPV (-) but not HPV (+) HNSCC to ionizing radiation, in vitro and in a mouse orthotopic model. Cancer biology & therapy. 2012;13(7):575–84. doi: 10.4161/cbt.19772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy K, Ali S, et al. Fluorocurcumins as Cyclooxygenase-2 Inhibitor: Molecular Docking, Pharmacokinetics and Tissue Distribution in Mice. Pharmaceutical research. 2009;26(11):2438–45. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramaniam D, Nicholes ND, Dhar A, Umar S, Awasthi V, Welch DR, et al. 3, 5-bis (2, 4-difluorobenzylidene)-4-piperidone, a novel compound that affects pancreatic cancer growth and angiogenesis. Molecular cancer therapeutics. 2011;10(11):2146–56. doi: 10.1158/1535-7163.MCT-11-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jee HG, Lee KE, Kim JB, Shin HK, Youn YK. Sulforaphane inhibits oral carcinoma cell migration and invasion in vitro. Phytotherapy research: PTR. 2011;25(11):1623–8. doi: 10.1002/ptr.3397. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Ghosh A, Coyle EM, Lee J, Hahm E-R, Singh SV, et al. Differential Effects of Phenethyl Isothiocyanate and <span class=“sc”>D,L</span>-Sulforaphane on TLR3 Signaling. The Journal of Immunology. 2013;190(8):4400–7. doi: 10.4049/jimmunol.1202093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar G, Tuli HS, Mittal S, Shandilya JK, Tiwari A, Sandhu SS. Isothiocyanates: a class of bioactive metabolites with chemopreventive potential. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(6):4005–16. doi: 10.1007/s13277-015-3391-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya S, Lin YC, et al. Phenethyl isothiocyanate suppresses EGF-stimulated SAS human oral squamous carcinoma cell invasion by targeting EGF receptor signaling. International journal of oncology. 2013;43(2):629–37. doi: 10.3892/ijo.2013.1977. [DOI] [PubMed] [Google Scholar]
- 32.Lui VW, Wentzel AL, Xiao D, Lew KL, Singh SV, Grandis JR. Requirement of a carbon spacer in benzyl isothiocyanate-mediated cytotoxicity and MAPK activation in head and neck squamous cell carcinoma. Carcinogenesis. 2003;24(10):1705–12. doi: 10.1093/carcin/bgg127. [DOI] [PubMed] [Google Scholar]
- 33.Huong LD, Shin JA, Choi ES, Cho NP, Kim HM, Leem DH, et al. β-Phenethyl isothiocyanate induces death receptor 5 to induce apoptosis in human oral cancer cells via p38. Oral Diseases. 2012;18(5):513–9. doi: 10.1111/j.1601-0825.2012.01905.x. [DOI] [PubMed] [Google Scholar]
- 34.Solt DB, Chang K-W, Helenowski I, Rademaker AW. Phenethyl isothiocyanate inhibits nitrosamine carcinogenesis in a model for study of oral cancer chemoprevention. Cancer Letters. 2003;202(2):147–52. doi: 10.1016/j.canlet.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Wolf MA, Claudio PP. Benzyl Isothiocyanate Inhibits HNSCC Cell Migration and Invasion, and Sensitizes HNSCC Cells to Cisplatin. Nutrition and cancer. 2014;66(2):285–94. doi: 10.1080/01635581.2014.868912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauman JE, Zang Y, Sen M, Li C, Wang L, Egner PA, et al. Prevention of Carcinogen-Induced Oral Cancer by Sulforaphane. Cancer prevention research. 2016;9(7):547–57. doi: 10.1158/1940-6207.CAPR-15-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta P, Wright SE, Kim SH, Srivastava SK. Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms. Biochimica et biophysica acta. 2014;1846(2):405–24. doi: 10.1016/j.bbcan.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khuri FR, Shin DM. Head and neck cancer chemoprevention gets a shot in the arm. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(3):345–7. doi: 10.1200/JCO.2007.14.0913. [DOI] [PubMed] [Google Scholar]
- 39.Masuda M, Suzui M, Weinstein IB. Effects of Epigallocatechin-3-gallate on Growth, Epidermal Growth Factor Receptor Signaling Pathways, Gene Expression, and Chemosensitivity in Human Head and Neck Squamous Cell Carcinoma Cell Lines. Clinical Cancer Research. 2001;7(12):4220–9. [PubMed] [Google Scholar]
- 40.Kim JW, Amin AR, Shin DM. Chemoprevention of head and neck cancer with green tea polyphenols. Cancer prevention research. 2010;3(8):900–9. doi: 10.1158/1940-6207.CAPR-09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. Journal of experimental therapeutics & oncology. 2002;2(6):350–9. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 42.Ho Y-C, Yang S-F, Peng C-Y, Chou M-Y, Chang Y-C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. Journal of Oral Pathology & Medicine. 2007;36(10):588–93. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoshizawa S, Horiuchi T, Fujiki H, Yoshida T, Okuda T, Sugimura T. Antitumor promoting activity of (−)-epigallocatechin gallate, the main constituent of “Tannin” in green tea. Phytotherapy Research. 1987;1(1):44–7. doi: 10.1002/ptr.2650010110. [DOI] [Google Scholar]
- 44.Baliga MS, Meleth S, Katiyar SK. Growth Inhibitory and Antimetastatic Effect of Green Tea Polyphenols on Metastasis-Specific Mouse Mammary Carcinoma 4T1 Cells In vitro and In vivo Systems. Clinical Cancer Research. 2005;11(5):1918–27. doi: 10.1158/1078-0432.ccr-04-1976. [DOI] [PubMed] [Google Scholar]
- 45.Shanafelt TD, Lee YK, Call TG, Nowakowski GS, Dingli D, Zent CS, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leukemia Research. 2006;30(6):707–12. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(6):1830–8. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 47.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer prevention research. 2009;2(11):931–41. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y, Chen L, Zhang G, Li L, Liang G. Epigallocatechin-3-gallate sensitizes multidrug-resistant oral squamous carcinoma cells to vincristine sulfate involving angiogenesis inhibition via down-regulating VEGF in vivo. The FASEB Journal. 2017;31(1 Supplement):790–20. [Google Scholar]
- 49.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer research. 2009;69(5):1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y, Shi R, Wang X, Shen H-M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Current cancer drug targets. 2008;8(7):634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Li X, Zhang Y, Luan X. Luteolin induces apoptosis by activating Fas signaling pathway at the receptor level in laryngeal squamous cell line Hep-2 cells. European Archives of Oto-Rhino-Laryngology. 2014;271(6):1653–9. doi: 10.1007/s00405-014-2903-z. [DOI] [PubMed] [Google Scholar]
- 52.Seo Y, Ryu K, Park J, Jeon D-K, Jo S, Lee HK, et al. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLOS ONE. 2017;12(3):e0174935. doi: 10.1371/journal.pone.0174935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ong C-S, Zhou J, Ong C-N, Shen H-M. Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt–GSK-3β–Cyclin D1 pathway. Cancer Letters. 2010;298(2):167–75. doi: 10.1016/j.canlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Chou J, Lin Y-C, Kim J, You L, Xu Z, He B, et al. NASOPHARYNGEAL CARCINOMA—REVIEW OF THE MOLECULAR MECHANISMS OF TUMORIGENESIS. Head & neck. 2008;30(7):946–63. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen P, Zhang J-Y, Sha B-B, Ma Y-E, Hu T, Ma Y-C, et al. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget. 2017;8(16):27471–80. doi: 10.18632/oncotarget.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J-H, Lee E-O, Lee H-J, Ku J-S, Lee M-H, Yang D-C, et al. Caspase Activation and Extracellular Signal-Regulated Kinase/Akt Inhibition Were Involved in Luteolin-Induced Apoptosis in Lewis Lung Carcinoma Cells. Annals of the New York Academy of Sciences. 2007;1095(1):598–611. doi: 10.1196/annals.1397.102_2. [DOI] [PubMed] [Google Scholar]
- 57.Majumdar D, Jung K-H, Zhang H, Nannapaneni S, Wang X, Amin ARMR, et al. Luteolin nanoparticle in chemoprevention – in vitro and in vivo anticancer activity. Cancer prevention research (Philadelphia, Pa) 2014;7(1):65–73. doi: 10.1158/1940-6207.CAPR-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, et al. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: potential role of p53. The Journal of biological chemistry. 2010;285(45):34557–65. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S-F, Yang W-E, Chang H-R, Chu S-C, Hsieh Y-S. Luteolin Induces Apoptosis in Oral Squamous Cancer Cells. Journal of Dental Research. 2008;87(4):401–6. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 60.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. The Journal of nutrition. 2005;135(1):48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer research. 2004;24(5A):2783–840. [PubMed] [Google Scholar]
- 62.AGGARWAL BB, BHARDWAJ A, AGGARWAL RS, SEERAM NP, SHISHODIA S, TAKADA Y. Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies. Anticancer Research. 2004;24(5A):2783–840. [PubMed] [Google Scholar]
- 63.Baek SH, Ko J-H, Lee H, Jung J, Kong M, Lee J-W, et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine. 2016;23(5):566–77. doi: 10.1016/j.phymed.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, et al. Resveratrol Reduces Prostate Cancer Growth and Metastasis by Inhibiting the Akt/MicroRNA-21 Pathway. PLOS ONE. 2012;7(12):e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science. 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 66.Wu S-L, Sun Z-J, Yu L, Meng K-W, Qin X-L, Pan C-E. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World Journal of Gastroenterology: WJG. 2004;10(20):3048–52. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocrine-Related Cancer. 2014;21(3):R209–R25. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geng T, Zhao X, Ma M, Zhu G, Yin L. Resveratrol-Loaded Albumin Nanoparticles with Prolonged Blood Circulation and Improved Biocompatibility for Highly Effective Targeted Pancreatic Tumor Therapy. Nanoscale Research Letters. 2017;12(1):437. doi: 10.1186/s11671-017-2206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ge S, Yin T, Xu B, Gao S, Hu M. Curcumin Affects Phase II Disposition of Resveratrol Through Inhibiting Efflux Transporters MRP2 and BCRP. Pharmaceutical research. 2016;33(3):590–602. doi: 10.1007/s11095-015-1812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masuelli L, Di Stefano E, Fantini M, Mattera R, Benvenuto M, Marzocchella L, et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5(21):10745–62. doi: 10.18632/oncotarget.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heiduschka G, Bigenzahn J, Brunner M, Thurnher D. Resveratrol synergistically enhances the effect of etoposide in HNSCC cell lines. Acta oto-laryngologica. 2014;134(10):1071–8. doi: 10.3109/00016489.2014.888592. [DOI] [PubMed] [Google Scholar]
- 72.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. BioMed research international. 2014;2014:424239. doi: 10.1155/2014/424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikami S, Ota I, Masui T, Itaya-Hironaka A, Shobatake R, Okamoto H, et al. Effect of resveratrol on cancer progression through the REG expression pathway in head and neck cancer cells. Int J Oncol. 2016;49(4):1553–60. doi: 10.3892/ijo.2016.3664. [DOI] [PubMed] [Google Scholar]
- 74.Alhasan SA, Aranha O, Sarkar FH. Genistein elicits pleiotropic molecular effects on head and neck cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7(12):4174–81. [PubMed] [Google Scholar]
- 75.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 76.Liu YT, Fan YY, Xu CH, Lin XL, Lu YK, Zhang XL, et al. Habitual consumption of soy products and risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. PloS one. 2013;8(10):e77822. doi: 10.1371/journal.pone.0077822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, Su H, Li Q, Li J, Zhao Q. Genistein decreases A549 cell viability via inhibition of the PI3K/AKT/HIF1alpha/VEGF and NFkappaB/COX2 signaling pathways. Mol Med Rep. 2017;15(4):2296–302. doi: 10.3892/mmr.2017.6260. [DOI] [PubMed] [Google Scholar]
- 78.Lee J, Ju J, Park S, Hong SJ, Yoon S. Inhibition of IGF-1 signaling by genistein: modulation of E-cadherin expression and downregulation of beta-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr Cancer. 2012;64(1):153–62. doi: 10.1080/01635581.2012.630161. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z, Wang C-Z, Du G-J, Qi L-W, Calway T, He T-C, et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. International Journal of Oncology. 2013;43(1):289–96. doi: 10.3892/ijo.2013.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kingsley K, Truong K, Low E, Hill CK, Chokshi SB, Phipps D, et al. Soy protein extract (SPE) exhibits differential in vitro cell proliferation effects in oral cancer and normal cell lines. Journal of dietary supplements. 2011;8(2):169–88. doi: 10.3109/19390211.2011.571656. [DOI] [PubMed] [Google Scholar]
- 81.Myoung H, Hong SP, Yun PY, Lee JH, Kim MJ. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer science. 2003;94(2):215–20. doi: 10.1111/j.1349-7006.2003.tb01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid Glucosides Are Hydrolyzed and Thus Activated in the Oral Cavity in Humans. The Journal of Nutrition. 2005;135(1):48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SJ, Kim MJ, Kim YK, Kim SM, Park JY, Myoung H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer letters. 2010;292(1):54–63. doi: 10.1016/j.canlet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Lu R, Dan H, Wu R, Meng W, Liu N, Jin X, et al. Lycopene: features and potential significance in the oral cancer and precancerous lesions. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2011;40(5):361–8. doi: 10.1111/j.1600-0714.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang YY, Lu L, Abliz G, Mijit F. Serum carotenoid, retinol and tocopherol concentrations and risk of cervical cancer among Chinese women. Asian Pacific journal of cancer prevention: APJCP. 2015;16(7):2981–6. doi: 10.7314/apjcp.2015.16.7.2981. [DOI] [PubMed] [Google Scholar]
- 86.Tang F-Y, Shih C-J, Cheng L-H, Ho H-J, Chen H-J. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Molecular Nutrition & Food Research. 2008;52(6):646–54. doi: 10.1002/mnfr.200700272. [DOI] [PubMed] [Google Scholar]
- 87.Assar EA, Vidalle MC, Chopra M, Hafizi S. Lycopene acts through inhibition of IκB kinase to suppress NF-κB signaling in human prostate and breast cancer cells. Tumor Biology. 2016;37(7):9375–85. doi: 10.1007/s13277-016-4798-3. [DOI] [PubMed] [Google Scholar]
- 88.Trejo-Solís C, Pedraza-Chaverrí J, Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, Serrano-García N, et al. Multiple Molecular and Cellular Mechanisms of Action of Lycopene in Cancer Inhibition. Evidence-based Complementary and Alternative Medicine: eCAM. 2013;2013:705121. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye M, Wu Q, Zhang M, Huang J. Lycopene inhibits the cell proliferation and invasion of human head and neck squamous cell carcinoma. Molecular Medicine Reports. 2016;14(4):2953–8. doi: 10.3892/mmr.2016.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh M, Krishanappa R, Bagewadi A, Keluskar V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral oncology. 2004;40(6):591–6. doi: 10.1016/j.oraloncology.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. The Cochrane database of systematic reviews. 2006;(4):CD001829. doi: 10.1002/14651858.CD001829.pub3. [DOI] [PubMed] [Google Scholar]
- 92.Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiology Biomarkers & Prevention. 2015;24(7):1003–11. doi: 10.1158/1055-9965.epi-15-0053. [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharya S, Muhammad N, Steele R, Peng G, Ray RB. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget. 2016;7(22):33202–9. doi: 10.18632/oncotarget.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajamoorthi A, Shrivastava S, Steele R, Nerurkar P, Gonzalez JG, Crawford S, et al. Bitter Melon Reduces Head and Neck Squamous Cell Carcinoma Growth by Targeting c-Met Signaling. PLoS ONE. 2013;8(10):e78006. doi: 10.1371/journal.pone.0078006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharya S, Muhammad N, Steele R, Kornbluth J, Ray RB. Bitter Melon Enhances Natural Killer-Mediated Toxicity against Head and Neck Cancer Cells. Cancer Prev Res (Phila) 2017;10(6):337–44. doi: 10.1158/1940-6207.CAPR-17-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samadi AK, Tong X, Mukerji R, Zhang H, Timmermann BN, Cohen MS. Withaferin A, a Cytotoxic Steroid from Vassobia breviflora, Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma. Journal of natural products. 2010;73(9):1476–81. doi: 10.1021/np100112p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leeman-Neill RJ, Wheeler SE, Singh SV, Thomas SM, Seethala RR, Neill DB, et al. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis. 2009;30(11):1848–56. doi: 10.1093/carcin/bgp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. Journal of the National Cancer Institute. 2006;98(7):441–50. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 99.Shrotriya S, Agarwal R, Sclafani RA. A perspective on chemoprevention by resveratrol in head and neck squamous cell carcinoma. Advances in experimental medicine and biology. 2015;815:333–48. doi: 10.1007/978-3-319-09614-8_19. [DOI] [PubMed] [Google Scholar]
- 100.Majumdar D, Jung KH, Zhang H, Nannapaneni S, Wang X, Amin AR, et al. Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer prevention research. 2014;7(1):65–73. doi: 10.1158/1940-6207.CAPR-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]


