Abstract
Introduction
The use of pre-analytical heat treatment (PHT) with the Nijmegen-Bethesda assay (NBA) for inhibitors to factor VIII (FVIII) can remove/destroy infused or endogenous FVIII from patient plasma samples, allowing testing of recently infused patients with haemophilia. Two PHT methods have been described as follows: heating to 56°C for 30 minutes and heating to 58°C for 90 minutes. Data examining the effects of PHT on anti-FVIII IgG4, the antibodies known to correlate most closely with the presence of FVIII inhibitors, are limited.
Aim
To assess the effect of PHT on the levels of detectable anti-FVIII IgG4.
Methods
Nijmegen-Bethesda assay-positive specimens were incubated at 56, 58 or 60°C for 90 minutes, and anti-FVIII IgG4 was measured by fluorescence immunoassay (FLI) at 30-minute intervals. The effects of PHT on the ability of recombinant FVIII (rFVIII) to inhibit detection of patient antibodies by FLI was also examined to assess the stability of rFVIII under the various PHT conditions tested.
Results
Levels of anti-FVIII IgG4 showed little change following incubations at 56°C (mean 101% of original value at 30 minutes and 100% at 60 minutes) but decreased upon exposure to 58°C (mean 85% at 30 minutes and 66% at 60 minutes). In addition, heating to 56°C effectively decreased the ability of rFVIII to block antibody binding compared to unheated rFVIII.
Conclusion
The optimal temperature for PHT in the FVIII NBA is 56°C. Higher temperatures may lead to loss of inhibitory antibodies.
Keywords: complications, factor VIII, factor VIII inhibitor test, haemophilia A, immunology, inhibitor
1 Introduction
The Nijmegen-Bethesda assay (NBA)1 is the preferred method for measurement of inhibitors to factor VIII (FVIII).2 Pre-analytical heat treatment (PHT) of patient specimens has been used in conjunction with the NBA as a means to limit interference by infused or endogenous FVIII in the in vitro clotting reaction utilized by the assay. This process allows more accurate inhibitor testing of patients with haemophilia A (HA) who have been recently infused3 or patients with acquired HA who have endogenous FVIII.4 Allain and Frommel5 originally demonstrated that incubating plasma samples at 56° for 30 minutes dissociated FVIII antibody complexes, allowing measurement of the freed antibodies. This technique has the goal of destabilizing antibody/antigen molecular interactions and/or denaturing the antigen without altering the antibodies, which are stable at that temperature. We recently showed that this method effectively reduced FVIII antigen and activity to undetectable levels in plasma samples from severe and moderate patients with HA who had FVIII infusions within 24 hours prior to blood draw.3 Addition of PHT to the NBA modification used by the Centers for Disease Control and Prevention (CDC-NBA) for FVIII inhibitor testing allowed detection of a higher proportion of the low-titre inhibitors present in patients with haemophilia, particularly in those with a previous history of inhibitor.3 The method also has demonstrated utility for monitoring patients who are receiving immune tolerance induction therapy.6 Other investigators have proposed heating patient plasma to 58°C for 90 minutes,7 and a recent analysis of PHT at 58°C for 90 minutes using specimens from patients with acquired HA reported an increase in the percentage of positive samples as measured by NBA and anti-FVIII enzyme-linked immunosorbent assay in heated vs unheated samples. These results were complicated by the observation that a subset of specimens that tested positive for FVIII antibodies by ELISA without PHT tested negative upon heating.4
Pre-analytical heat treatment of inhibitor specimens for 30 minutes at 56°C is an established, validated technique, but data exploring the effects of a range of PHT temperature and duration on inhibitor detection and on the antibodies that impose inhibition are limited. The goal of this study was to define optimal PHT conditions that limit interference by onboard factor in functional inhibitor assays while maintaining, or increasing, the amount of stable anti-FVIII antibodies in the sample available to impose the inhibitory effects that the assay is designed to measure. With this goal in mind, we examined how heat treatment of plasma over a range of temperatures affects the levels of antifactor VIII IgG4 detectable by fluorescence immunoassay (FLI). Several studies have shown anti-FVIII IgG4 to be the best immunologic indicator of the presence of a functional inhibitor to FVIII.8-11 In addition, we examined the stability of rFVIII under the various PHT conditions tested.
2 Materials and Methods
2.1 Subjects
The specimens used in the study were from a healthy donor and from 8 patients who have HA and were enrolled in either the Haemophilia Inhibitor Research Study conducted at 17 US haemophilia treatment centres between 2006 and 201312 or Community Counts, a public health surveillance programme run by the Division of Blood Disorders at the Centers for Disease Control and Prevention in collaboration with the American Thrombosis and Hemostasis Network and the United States Hemophilia Treatment Center Network (https://www.cdc.gov/ncbddd/hemophilia/communitycounts/about.html). The investigational review boards of the CDC and each participating site approved the protocol, and all participants or parents of minor children gave informed consent.
2.2 Nijmegen-Bethesda assay
Factor VIII inhibitors were measured by the CDC-NBA as previously described,3 including PHT with specimens heated to 56°C for 30 minutes and then centrifuged at 2700 g for 5 minutes at room temperature prior to testing. Imidazole-buffered normal pooled plasma (Precision Biologic, Dartmouth, Nova Scotia, Canada) was used in control and test mixtures and naturally deficient FVIII-deficient plasma (George King Biomedical, Overland Park, KS, USA) in control mixtures and for predilution of specimens >2.0 Nijmegen-Bethesda units (NBU). The threshold for positivity was ≥0.5 NBU.3,13
2.3 Fluorescence immunoassay
The anti-FVIII FLI was performed as previously described.11 Briefly, plasma samples diluted 1:30 in phosphate-buffered saline (PBS) containing 1% dried milk (PBSM) were incubated with SeroMAP beads (Luminex Corporation, Austin, TX) coupled to recombinant FVIII (Kogenate FS; Bayer Healthcare, Tarrytown, NY, USA, or Advate; Shire, Lexington, MA, USA). Anti-FVIII antibodies were detected using serial incubations with biotinylated anti-human IgG4 (ab99818; Abcam, Cambridge, MA, USA) and R-phycoerythrin-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA, USA) using a Bio-Plex 200 suspension array system (Bio-Rad Laboratories, Hercules, CA, USA). Results are expressed as median fluorescence intensity (MFI). The threshold for positivity was set at 2 standard deviations above the mean MFI of the results obtained for healthy donors.
2.4 Effects of heat treatment on levels of detectable anti-FVIII IgG4 in plasma
For stability studies, plasma specimens from a healthy donor and 3 patients with HA who tested positive for an inhibitor by NBA and anti-FVIII IgG4 antibodies by FLI were spun at 20 000 g for 4 minutes and incubated at room temperature or 56, 58 or 60°C for 30, 60 or 90 minutes. After heat treatment, samples were placed on ice for 1 minute, respun, and anti-FVIII IgG4 was measured by FLI. Studies on the effects of heat treatment on rFVIII's ability to act as a competitive inhibitor were performed by pre-incubating 10 μg/mL rFVIII in PBS or PBS alone at room temperature or 56°C for 60 minutes. Heated factor, or buffer control, was added to PBSM to 0.1 μg/mL and incubated with plasma diluted in PBSM 1:30 for 30 minutes. Plasma samples were then combined with rFVIII-conjugated SeroMAP beads, and anti-FVIII IgG4 FLI was performed as described above.
2.5 Statistical methods
Statistical analysis was performed by paired t test using GraphPad Prism (GraphPad Software, San Diego, CA) using a significance level of P = .05.
3 Results and Discussion
The effects of different levels of PHT on free anti-FVIII antibody levels in specimens from one healthy donor and 3 patients with HA are shown in Figure 1. Heating of plasma samples to 56°C for 30 minutes caused a modest increase in detection levels of anti-FVIII IgG4 in patients A and B, while anti-FVIII IgG4 decreased to 91% of baseline in the specimen from patient C. Extending heat treatment to 60 or 90 minutes at 56°C resulted in decreased detectable antibody levels in patients A and B but a negligible impact on detectable anti-FVIII IgG4 levels in patient C. Incubating plasma samples at 58°C for 30 minutes resulted in a decrease in detectable antifactor VIII IgG4 levels in specimens from patients A and B with no effect on antibody levels in patient C's specimen, and all 3 patients had markedly lower detectable IgG4 after 60-and 90-minute incubations at 58°C compared to baseline measurements. 30-, 60-and 90-minute incubations at 60°C resulted in severe reductions in detectable anti-FVIII IgG4 in specimens from all 3 HA patient samples tested (Figure 1).
Figure 1.
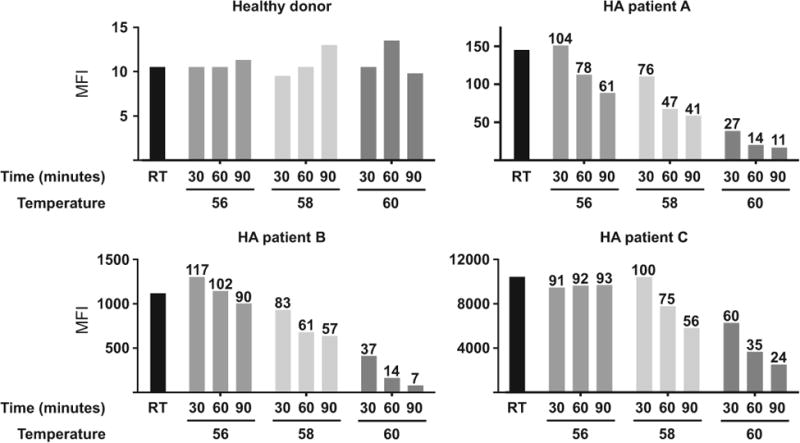
Levels of detectable anti-factor VIII (FVIII) IgG4 by fluorescence immunoassay following heat treatment Median fluorescence intensities (MFI) of anti-FVIII IgG4 in haemophilia A patient plasma samples incubated at room temperature (RT), 56, 58 or 60°C for 30, 60 or 90 min. The per cent of baseline (RT) antifactor VIII IgG4 remaining after incubation is indicated above the bars
Taken together, the data presented in Figure 1 argue that exposure to 60°C or extending incubations beyond 60 minutes at 56 or 58°C are not optimal conditions for maintaining anti-FVIII IgG4 levels. Accordingly, further evaluation of PHT on anti-FVIII IgG4 levels measured in specimens from 5 additional HA patients with inhibitor titres >0.5 NBU focused on PHT at 56 or 58°C for 30 or 60 minutes. Table 1 shows NBA and FLI results on all 8 HA patients studied and the results of heating to 56 or 58°C for 30 or 60 minutes, expressed as percent of the original IgG4 level remaining. Anti-FVIII antibody levels showed no significant change at 56°C but were significantly decreased following 58°C incubations at both 30 minutes (P = .005) and 60 minutes (P = .003). It is notable that the patient specimens with relatively low antibody levels (patients A, B, E and F) exhibited an increased level in detectable anti-FVIII IgG4 following exposure to PHT conditions compared to specimens from patients with relatively high antibody levels (patients C, D, G and H). One possible explanation is that PHT may release a larger proportion antibodies in low-titre specimens from the effects of competitive inhibition by residual onboard FVIII compared to the proportion of antibodies affected in high-titre specimens in which residual FVIII levels are likely insignificant relative to antibody levels.
Table 1. Nijmegen-Bethesda FVIII inhibitor and fluorescence immunoassay (FLI) results for the specimens studied.
| % Remaining at 56°C after | % Remaining at 58°C after | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Specimens | NBU | (MFI) | 30′ | 60′ | 30′ | 60′ |
| Patient A | 1.5 | 169 | 104 | 78 | 76 | 47 |
|
| ||||||
| Patient B | 1.6 | 1919 | 117 | 102 | 83 | 61 |
|
| ||||||
| Patient C | 19.3 | 12 596 | 91 | 92 | 100 | 75 |
|
| ||||||
| Patient D | 1.6 | 16 563 | 91 | 96 | 95 | ND |
|
| ||||||
| Patient E | 1.1 | 303 | 120 | 147 | 93 | 93 |
|
| ||||||
| Patient F | 1.1 | 1208 | 115 | 100 | 76 | 65 |
|
| ||||||
| Patient G | 0.5 | 17 194 | 80 | 93 | 72 | 39 |
|
| ||||||
| Patient H | 5.4 | 22 156 | 92 | 94 | 88 | 79 |
|
| ||||||
| Mean (range) | 101 (80-120) | 100 (78-147) | 85 (72-93)* | 66 (39-93)* | ||
Nijmegen-Bethesda results in Nijmegen-Bethesda units (NBU), baseline anti-FVIII IgG4 FLI results, and per cent (%) of original IgG4 remaining after heating plasma to either 56 or 58°C for 30 or 60 min (′).
MFI, median fluorescence intensity; ND, no data.
Significantly different from original level in paired t test at P < .05.
To assess more directly the heat lability of FVIII, we performed blocking studies to examine the effects of heat treatment on the ability of rFVIII to act as a competitive inhibitor in the anti-FVIII FLI. Plasma samples from 6 inhibitor-positive patients with HA were incubated with buffer or rFVIII that was pre-incubated at room temperature or 56°C for 60 minutes, and anti-FVIII IgG4 was measured by FLI. Unheated rFVIII decreased anti-FVIII IgG4 detection to 11%-89% of levels measured in buffer-treated specimens, demonstrating its ability to compete with the assay's target for antibody binding (Figure 2). By comparison, heated rFVIII was a less effective competitive inhibitor as indicated by antibody detection levels at 59%-90% of the buffer control, thus demonstrating that heating rFVIII to 56°C reduces its capacity to bind anti-FVIII antibodies. Notably, the proportion of antibodies competitively inhibited in the anti-FVIII FLI varies across patient samples. For example, the specimen with the lowest anti-FVIII FLI MFI (patient A) is less efficiently inhibited by the addition of rFVIII compared to specimens from patients who have higher FLI MFIs (patients B, C, E and F). This discrepancy is likely explained by differences in antibody profiles across inhibitor-positive patients, which have been shown to include antibodies of multiple subclasses and affinities.14 High-affinity antibodies, which bind tightly to their target, are more efficiently blocked in competitive inhibition assays compared to low-affinity antibodies because, once bound, high-affinity antibodies are less likely than low-affinity antibodies to disengage the inhibitor and bind the assay target. Accordingly, patient samples that consist of mostly high-affinity antibodies may have less detectable residual antibody binding by FLI in the presence of a competitive inhibitor compared to samples that contain one or more low-affinity antibodies. Patient samples also varied in the degree to which heat treatment decreased competitive inhibition by rFVIII (Figure 2). Differences in the epitope specificity of the anti-FVIII antibody response, which is known to be polyclonal,8,15 likely contribute to this variation. Patient samples that include antibodies with anti-FVIII epitopes that are unaffected by disruptions in secondary, tertiary and/or quaternary structure may retain the capacity to bind FVIII even upon heat denaturation. Antibody titre also plays a role in determining the efficiency of competitive inhibition. For example, the amount of detectable anti-FVIII IgG4 in the specimen from patient D, who had the highest baseline antibody level, was only modestly decreased in the presence of a competitive inhibitor, and the effectiveness of competitive inhibition was only slightly decreased by heating. The results observed from the specimen from patient D are likely explained by (i) the amount of competitive inhibitor used was not sufficient to efficiently compete with the high level of antibodies present (ii) patient D's antibody profile may include low-affinity antibodies, which are less susceptible to efficient competitive inhibition and/or antibodies that recognize epitopes unaffected by heat treatment.
Figure 2.
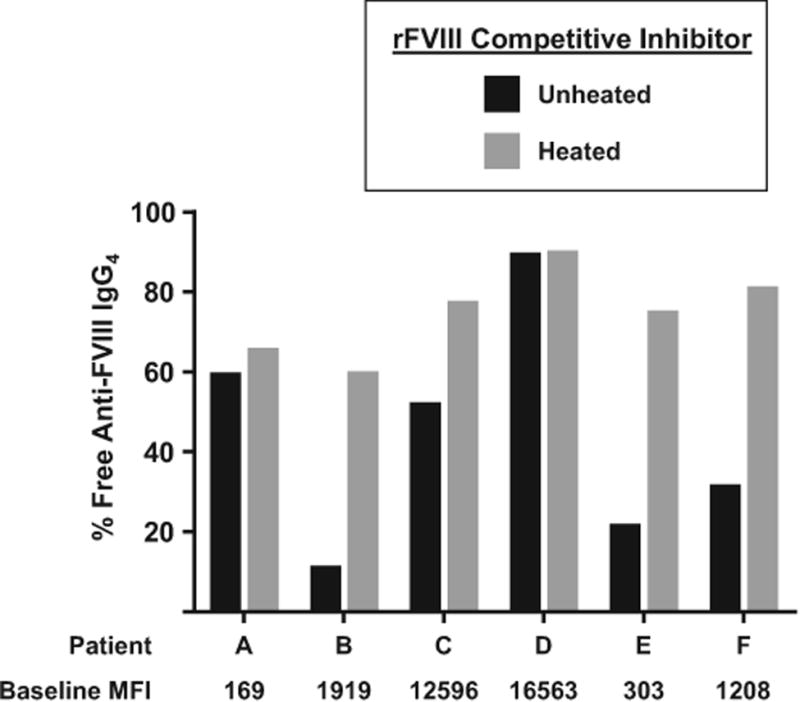
Blocking efficiency of unheated versus heated recombinant (r) factor VIII (FVIII). The percentage of free anti-FVIII IgG4 remaining after incubation of patient plasma with buffer (baseline), rFVIII (black bars) or rFVIII preheated to 56°C for 60 min (grey bars). MFI—median fluorescence intensity
The findings from the current study confirm that PHT decreases the ability of FVIII to react with anti-FVIII antibodies and supports our previous observation that heating to 56°C for 30 minutes decreased both FVIII activity and FVIII antigen to <1 IU/mL.3 Although the number of patient specimens evaluated in the current study is relatively small, the data derived from the study provide compelling evidence that PHT performed above 56°C may reduce the amount of anti-FVIII IgG4 in specimens, which will detrimentally affect the accuracy of inhibitor testing. Taken together, these data demonstrate that 56°C is the optimal temperature for PHT in the NBA for FVIII inhibitors because it minimizes interference by onboard FVIII without decreasing levels of inhibitory antibodies.
Acknowledgments
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Disclosures: The work was supported by the CDC Foundation through grants from Pfizer Pharmaceuticals and Baxter Healthcare. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Author Contributions: B.B. designed and performed the research, analysed the results and wrote the manuscript; C.H.M. analysed results and wrote the manuscript.
References
- 1.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–251. [PubMed] [Google Scholar]
- 2.Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 3.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10:1055–1061. doi: 10.1111/j.1538-7836.2012.04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batty P, Platton S, Bowles L, Pasi KJ, Hart DP. Pre-analytical heat treatment and a FVIII ELISA improve Factor VIII antibody detection in acquired haemophilia A. Br J Haematol. 2014;166:953–956. doi: 10.1111/bjh.12923. [DOI] [PubMed] [Google Scholar]
- 5.Allain JP, Frommel D. Antibodies to factor VIII. I. Variations in stability of antigen-antibody complexes in hemophilia A. Blood. 1973;42:437–444. [PubMed] [Google Scholar]
- 6.de Lima Montalvao SA, Tucunduva AC, de Almeida Sambo AL, De Paula EV, de Souza Medina S, Ozelo MC. Heat treatment of samples improve the performance of the Nijmegen-Bethesda assay in hemophilia A patients undergoing immune tolerance induction. Thromb Res. 2015;136:1280–1284. doi: 10.1016/j.thromres.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Kitchen S, McCraw A, Echenagucia M. Diagnosis of Hemophilia and Other Bleeding Disorders: A Laboratory Manual. 2nd. Montreal, Que: World Federation of Hemophilia; 2010. [Google Scholar]
- 8.Gilles JG, Arnout J, Vermylen J, Saint-Remy JM. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993;82:2452–2461. [PubMed] [Google Scholar]
- 9.Gautier P, Sultan Y, Parquet-Gernez A, Meriane F, Guerois C, Derlon A. Detection and IgG subclass analysis of antibodies to factor VIII in multitransfused haemophiliacs and healthy individuals. Haemophilia. 1996;2:88–94. doi: 10.1111/j.1365-2516.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 10.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121:1039–1048. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 11.Boylan B, Rice AS, Dunn AL, et al. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay. J Thromb Haemost. 2015;13:47–53. doi: 10.1111/jth.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucie JM, Miller CH, Kelly FM, et al. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2013;20:230–237. doi: 10.1111/hae.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CH, Boylan B, Shapiro AD, Lentz SR, Wicklund BM. Hemophilia Inhibitor Research Study I. Limit of detection and threshold for posi-tivity of the centers for disease control and prevention assay for factor VIII inhibitors. J Thromb Haemost. 2017;15:1971–1976. doi: 10.1111/jth.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofbauer CJ, Whelan SF, Hirschler M, et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and non-neutralizing antibodies in humans. Blood. 2015;125:1180–1188. doi: 10.1182/blood-2014-09-598268. [DOI] [PubMed] [Google Scholar]
- 15.Fulcher CA, de Graaf MS, Zimmerman TS. FVIII inhibitor IgG subclass and FVIII polypeptide specificity determined by. Blood. 1987;69:1475–1480. [PubMed] [Google Scholar]


