Abstract
Obesity increases risk of endometrial cancer through dysregulation of estrogen and insulin signaling. The primary aim of this study was to evaluate the impact of metformin or lifestyle intervention on endometrial proliferation in postmenopausal obese women. Secondary aims included evaluating obesity-related biomarkers and adverse events experienced. Obese, postmenopausal women with pre-diabetes were randomized into four groups for a 16-week intervention using a 2 (metformin 1700 mg/day vs placebo) × 2 (lifestyle intervention vs no lifestyle intervention) factorial design. Pre- and post-intervention endometrial proliferation, anthropometrics, body composition, and serum biomarkers (sex hormones, sex hormone binding globulin, IGF-1, adiponectin, omentin, insulin, glucose, and others) were assessed. Data were analyzed with linear regression models and false-discovery rate correction. Of 576 women approached for the study, 52 attended initial screening, 29 were eligible and randomized, and 26 completed the study. Lifestyle intervention resulted in significant loss of weight (−4.23 kg, p=0.006) and total fat mass (−3.23 kg, p<0.001). Participants receiving metformin lost 3.43 kg of weight (p=0.023), but this was not statistically significant after multiple comparisons adjustment controlling false-discovery rate to 10%. Endometrial proliferation was low at baseline (mean 7.1%) and remained unchanged by 16 weeks, but included substantial variability. Metformin and lifestyle intervention produced minor changes to serum biomarkers. Lifestyle intervention produced the most significant changes in weight and body composition. While it is known that obese postmenopausal women are at increased risk for endometrial cancer, improved biomarkers are needed to stratify risk and test prevention strategies, particularly at the endometrial tissue level.
Keywords: obesity, endometrial cancer, cancer prevention, metformin, lifestyle intervention
INTRODUCTION
It is well known that obesity is associated with increased risk of several types of cancer (1). The strongest link exists between obesity and endometrial cancer (1,2), where overweight and obesity contribute to about 57% of endometrial cancer cases (3–5). In contrast to most other types of cancer, the incidence of endometrial cancer is increasing in the United States as the obesity epidemic continues (6). Further, obesity is associated with an earlier age of onset for endometrial cancer (7). After endometrial cancer diagnosis, obesity continues to negatively influence outcomes, with increased surgical complications (8,9), increased risk of endometrial cancer-related death and increased all-cause mortality (10).
The connection between obesity and endometrial cancer risk is underpinned by the synergistic relationship between inflammation, insulin resistance, and increased bioavailability of estrogen, which has been well-reviewed previously (4,11). In brief, obesity causes macrophage accumulation and increased expression of pro-inflammatory cytokines (12). The increase in pro-inflammatory cytokines contributes to insulin resistance and increased levels of insulin-like growth factor 1 (IGF-1), which leads to the activation of the phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and MAPK signaling pathways, promoting tumorigenesis via cell proliferation and reduction in apoptosis (12). Additionally, this increased inflammation and insulin occurs in tandem with reduced sex hormone binding globulin (SHBG) which results in increased estrogen bioavailability (13). Increased estrogen exposure to the endometrium also may result in DNA damage leading to tumorigenesis (4). Crosstalk between estrogen signaling and the MAPK pathway, as well as other growth factor signaling can further promote cancer growth and progression. Taken together, obesity-induced systemic inflammation, dysregulated hormone signaling, and aberrations in insulin signaling increase endometrial cancer risk.
Interventions to promote weight loss and insulin sensitivity may be advantageous as risk reduction strategies for endometrial cancer. Research has shown that individuals with and without diabetes who have taken metformin have lower endometrial cancer incidence and improved endometrial cancer survival (14–19). Metformin has been shown to inhibit estrogen-mediated endometrial proliferation in an animal model of hyperinsulinemia and insulin resistance (20). Results from “window of opportunity” pre-surgical studies of metformin in endometrial cancer patients show some variation, but standard doses (1500–1700 mg/d) of metformin produced significant reductions in endometrial proliferation (21,22) and lower doses (850 mg/day) decreased serum biomarkers (IGF-1, omentin, insulin, and leptin) and tissue markers of PI3K/AKT signaling (23). Metformin activates AMP kinase (AMPK) and phosphorylates LKB1 tumor suppressor (24,25), thus inhibiting PI3K/AKT/mTOR pathway, a pathway commonly activated in endometrial cancer and other obesity-related cancers (26). Results from metformin studies provide evidence for activation of AMPK and inhibition of mTOR in endometrial cancer cells specifically (27,28), along with inhibited endometrial cell proliferation and epithelial-mesenchymal transitions (EMT), and stimulated apoptosis (29–32).
To date, no studies to our knowledge have assessed the impact of metformin alone and in conjunction with a lifestyle intervention promoting weight loss, on endometrial cancer risk and obesity-related biomarkers of endometrial cancer risk in obese, post-menopausal women with pre-diabetes. The primary aim of the present investigation used a 2 × 2 randomized design to evaluate the independent effects of metformin and a lifestyle intervention on endometrial cancer risk, via assessment of endometrial proliferation. The secondary aim assessed changes in obesity-related biomarkers of endometrial cancer risk, including body composition, anthropometric measurements, and serum biomarkers such as: dehydroepiandrosterone sulfate (DHEA-S), sex hormone binding globulin (SHBG), adiponectin, fasting glucose, fasting insulin, IGF-1, omentin, and others. Completion of these objectives was hampered by significant challenges in recruiting participants for this study, which resulted in stopping the study prior to accruing the planned number of study subjects. Challenges related to recruitment and completion of cancer prevention studies are also discussed further.
MATERIALS AND METHODS
Participants
Participants were recruited following approval by the Institutional Review Board (IRB) at MD Anderson Cancer Center (trial registration ID: NCT01697566). Written informed consent was obtained from all study participants. Patients were included based on the following criteria: female, postmenopausal, body mass index (BMI) ≥ 30 kg/m2, intact uterus, age 50–65 years, Zubrod performance scale 0–1, hemoglobin ≥ 10 g/dL, normal thyroid stimulating hormone (TSH) level, demonstrated hyperinsulinemia (quantitative insulin-sensitivity check index, QUICKI value ≤ 0.357) but not frankly diabetic (fasting blood glucose ≤ 126 mg/dL). Menopause was defined as no menses for 1 year and/or FSH ≥ 25.8 mIU/ml. Exclusion criteria included: receiving hormone treatments within the last 6 months (aromatase inhibitors, Gonadotropin Releasing Hormone agonists, selective estrogen receptor modulators, and hormone replacement therapy) or previous metformin treatment; elevated liver enzyme tests, serum creatinine, or fasting triglyceride levels; prior hysterectomy or endometrial ablation; history of congestive heart failure; prior radiation to the pelvis; previous chemotherapy or current untreated malignancy other than non-melanoma skin cancer. Participants were recruited from the community, Harris Health System, and employees at MD Anderson Cancer Center using various outlets as advertisement. MD Anderson employees were recruited through various internal communications, including websites, fliers, and institutional events. Community recruitment included extensive advertisement at YMCA locations, local events (e.g. Quilt Festival), and the Cancer Prevention Center at MD Anderson, as well as online, print and social media. Additional community outreach was also facilitated through ongoing cohorts developed through the Center for Community-Engaged Translational Research at MD Anderson, which includes church-based cohorts of African-Americans in Houston.
Study design and interventions
This study used a 2 × 2 factorial design to evaluate the independent effects of metformin or a lifestyle intervention on endometrial cancer risk, via assessment of endometrial proliferation (Ki67). The 2×2 factorial design was chosen because of its efficiency for testing the main effects (33), i.e., the marginal or individual effects of metformin and lifestyle interventions, which are the primary interests of this study. The interaction effect between metformin and lifestyle is not the primary interest here as detecting an interaction requires a larger sample size. The original planned design called for 100 total participants, as described further in Statistical Analysis, below. The overall study design and final number of participants are shown in Figure 1. Potentially eligible women were assessed for eligibility during a screening phone call. Upon determining preliminary eligibility, the study nurse obtained medical history and scheduled Screening Visit 1. At Screening Visit 1 (SV1), informed consent was obtained and patients underwent a fasting blood draw, which was used for biomarker measures and clinical lab tests using standard methods for further eligibility screening, including fasting glucose, insulin, thyroid stimulating hormone (TSH), hemoglobin, follicle stimulating hormone (FSH), alanine aminotransferase (ALT), creatinine, and triglycerides. After determining eligibility from SV1 criteria, participants were scheduled for Screening Visit 2 (SV2). On average, SV2 occurred 18 days after the SV1 appointment. The SV2 appointment included an endometrial pipelle biopsy, and additional blood draw for hormone measures, and anthropometric assessments. The endometrial biopsy was reviewed by a pathologist (Dr. Russell R. Broaddus) and if the biopsy revealed endometrial hyperplasia or no endometrial tissue could be obtained, the participant was removed from the study. Participants with abnormal biopsy results were referred for further treatment.
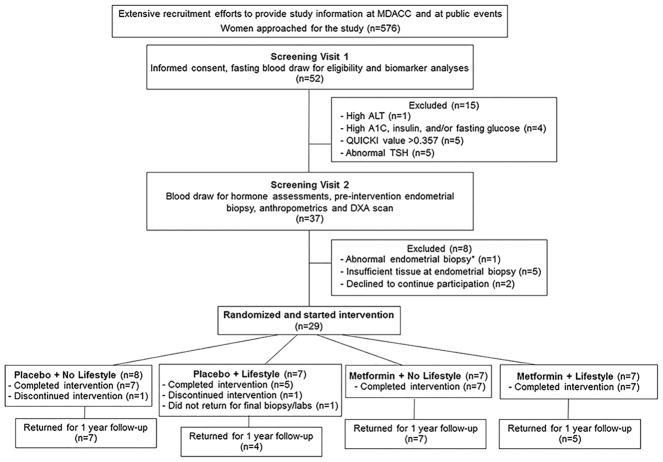
Figure 1.
CONSORT diagram for the study indicating reasons for participant exclusion or ineligibility. *Abnormal endometrial biopsy showed complex atypical hyperplasia (precancerous lesion). ALT, Alanine Aminotransferase; QUICKI, quantitative insulin-sensitivity check index; TSH, thyroid stimulating hormone; DXA, dual energy X-ray absorptiometry.
Eligible participants with a normal endometrial biopsy were randomized to one of four treatment arms for a 16-week intervention: placebo + no lifestyle intervention, placebo + lifestyle intervention, metformin + no lifestyle intervention, or metformin + lifestyle intervention. The study was partially blinded such that the investigator and participants were blinded to treatment of metformin versus placebo, but were aware of whether or not the participant was randomized to a lifestyle intervention. Additional data were collected at 16 weeks (upon completion of intervention) and at a one-year follow-up visit.
Information regarding adverse events was collected by in-person toxicity assessments performed at the end of week 4, 8, 12, and 16, of the 16 week intervention. In addition, phone assessments were conducted periodically during each month on study and 4 weeks after completion of the study.
Lifestyle intervention
The lifestyle intervention was based on the 16-week Diabetes Prevention Program (34) and was delivered as 16 weekly in-person sessions. Participants were provided with individualized calorie goals based on activity level, age, height, and weight, along with a dietary fat intake goal of 25% calories from fat. Participants were instructed to record dietary intake daily, including calculation of total calories and grams of dietary fat consumed, and weigh themselves weekly. Additionally, participants were provided a pedometer to monitor their daily step counts, and worked with study personnel to set up daily step goals. They were also offered the opportunity to participate in two supervised exercise sessions per week.
Metformin treatment
Metformin hydrochloride was provided as 425 mg capsules. Participants in the metformin groups were instructed to increase their daily dose over four weeks in the following manner: one capsule daily during week 1, one capsule twice daily during week 2, one capsule three times daily during week 3, and two capsules twice daily during week 4 (for a total of 1700 mg metformin each day by week 4). This dose matches that used in the Diabetes Prevention Program, which was shown to reduce the incidence of diabetes (34). Adherence to study medication was assessed using pill diaries and pill counts were confirmed by the research nurse.
Weight and body composition
Anthropometrics and body composition measurements were conducted at SV2 (baseline), 16 weeks, and one year follow-up. Weight was measured on a calibrated digital scale; height was measured using a wall-mounted stadiometer. Waist and hip circumference was measured twice following standard procedures (35). Body composition was measured via dual energy x-ray absorptiometry (DXA) scan using Hologic Discovery W QDR DXA system (Waltham, MA).
Immunohistochemistry
Endometrial biopsies were conducted at baseline and 16 weeks. Paraffin-embedded endometrial biopsy tissue sections were used for immunohistochemistry. Antigen retrieval using citrate buffer (pH 6.0) was performed. Sections were incubated in primary antibody against Ki67 (BD Pharmigen, CA) or P27 (R & D System, Minneapolis, MN) at 4°C overnight, followed by incubation with biotinylated anti rabbit and anti-mouse IgG, and streptavidin-HRP (Dako Incorporation). Diaminobenzidine solution (Dako Incorporation) was applied to visualize the complex and sections were counterstained with Mayer’s hematoxylin. The total number of Ki67 staining nuclei was counted and reported as the percentage of positively nuclear stained endometrial cells; procedures consistent with international guidelines for Ki67 assessment were followed (36). P27 loss has been suggested to be a marker of endometrial risk related to obesity and p27 expression was scored as intact, slightly reduced, moderately reduced, or severely reduced, as previously described (37).
Blood-based biomarker analyses
Routine clinical testing procedures were used for estradiol, estrone, adiponectin, IGF-1, DHEA-S, and SHBG assessment. Serum Omentin and FGF21 level were detected with Omentin-1 Human ELISA kit, and Human FGF21 ELISA kit (BioVendor Research and Diagnostic Products, Candler, NC). Serum C-Peptide, Leptin, and Visfatin levels were measured with Human Express Diabetic multiplex (Bio-Rad Laboratories, Hercules, CA). All samples were measured in triplicate.
Sample size and statistical analysis
The primary objective of this study is to evaluate the independent effects of intervention (metformin or lifestyle intervention) on proliferation in the endometrium via Ki-67 staining. The sample size planned for the study was 100, determined to ensure over 95% power to detect an effect size of 2.1 in Ki-67 change between the intervention group and the control for a 2-sided test with 5% statistical significance. The effect size is estimated based on our previous study of endometrial Ki-67 changes following pharmacologic intervention in women at high risk for endometrial cancer (38).
Changes in Ki-67 and all secondary outcomes of interest were analyzed using multiple linear regression models, which generalize the classical ANOVA analysis and contain the latter as a special case when independent variables are categorical (e.g., the indicator of control and intervention arms). Initially models included an interaction term along with main effects; however, if the interaction term was non-significant it was dropped from the model. The methods described by Benjamini and Hochberg (39) were used to control the false discovery rate to 10% in the secondary efficacy analyses objectives; adjusted p-value cutoffs for each analysis are shown as footnotes on each table. Stata v14.2 (College Station, TX) was used for all statistical analysis.
RESULTS
Recruitment, Participation, and Adherence
Recruitment efforts included extensive outreach through events at The University of Texas MD Anderson Cancer Center and public events. Of 576 people approached for the study, 52 women attended the initial screening visit (SV1) to further assess eligibility and 15 were excluded due to abnormal screening blood test results (Figure 1). A total of 37 women attended screening visit 2 (SV2) and 29 women were determined to be eligible and randomized to start the intervention. Of the 8 women who were not randomized, 2 women declined to continue participation, 5 women had insufficient tissue at endometrial biopsy, and one woman had an abnormal endometrial biopsy (complex atypical hyperplasia). Of the 29 women randomized, 26 women completed the study. Two women in the placebo arms discontinued participation. In addition, one woman did not return for the final endometrial biopsy and blood draw at the completion of the intervention, and was therefore excluded from the analyses. Of the 26 women that completed the 16-week intervention, 23 returned for the 1 year follow-up (Figure 1). Adherence was noted to be similar for women on the placebo and metformin arms with 88% and 94% of doses reportedly taken, respectively. Compliance with the lifestyle intervention was similar for participants receiving placebo or metformin. On average, participants receiving lifestyle intervention attended 14 out of 16 intervention sessions for both groups. Participation was also similar for the two weekly optional supervised exercise sessions. On average, women in the placebo + lifestyle group attended 7 optional supervised exercise sessions and women in the metformin + lifestyle group attended 8 optional exercise sessions.
Demographics and clinical characteristics
Overall, demographic variables were well-balanced across the intervention arms and details for each group are shown in Table 1. The mean age for participants was 57.5 years and mean BMI was 39.1 kg/m2. Participants were predominately White (51.7%) or Black/African American (41.4%). Ethnicity was predominantly non-Hispanic (82.8%). Although the majority of participants reported no medical comorbidities, 10 women reported hypertension, 2 women reported hypothyroid, 1 woman reported hypercholesterolemia, and 1 woman reported an asymptomatic heart murmur.
Table 1.
Demographics and clinical characteristics of study participants
| Placebo + No Lifestyle (n = 8) | Placebo + Lifestyle (n = 7) | Metformin + No Lifestyle (n = 7) | Metformin + Lifestyle (n = 7) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Age (years), Mean (SD), range | 60.0 (4.5) | 55–66 | 57.1 (3.3) | 53–61 | 55.8 (5.2) | 50–65 | 57.5 (4.4) | 50–61 | 57.5 (4.4) | 50–66 |
| BMI, Mean (SD), range | 36.7 (5.5) | 29.7–46.6 | 39.7 (5.1) | 33.7–46.5 | 38.3 (5.1) | 32.0–46.6 | 42.2 (6.7) | 32.1–50.7 | 39.1 (5.7) | 29.7–50.7 |
| Race | ||||||||||
| Black | 4 | 50.0 | 2 | 28.6 | 1 | 14.3 | 5 | 71.4 | 12 | 41.4 |
| White | 4 | 50.0 | 4 | 57.1 | 5 | 71.4 | 2 | 28.6 | 15 | 51.7 |
| More than one race | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 1 | 3.4 |
| Other | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 3.4 |
| Ethnicity | ||||||||||
| Hispanic | 2 | 25.0 | 2 | 28.6 | 1 | 14.3 | 0 | 0.0 | 5 | 17.2 |
| Non-Hispanic | 6 | 75.0 | 5 | 71.4 | 6 | 85.7 | 7 | 100.0 | 24 | 82.8 |
| Education | ||||||||||
| Finished High School | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 1 | 3.4 |
| Technical/Vocational degree | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 3.4 |
| Some college or 2 year degree | 5 | 62.5 | 3 | 42.9 | 1 | 14.3 | 3 | 42.9 | 12 | 41.4 |
| Bachelors | 3 | 37.5 | 2 | 28.6 | 4 | 57.1 | 3 | 42.9 | 12 | 41.4 |
| Masters | 0 | 0.0 | 1 | 14.3 | 1 | 14.3 | 1 | 14.3 | 3 | 10.3 |
| Marital Status | ||||||||||
| Single | 1 | 12.5 | 1 | 14.3 | 1 | 14.3 | 1 | 14.3 | 4 | 13.8 |
| Married | 4 | 50.0 | 4 | 57.1 | 4 | 57.1 | 1 | 14.3 | 13 | 44.8 |
| Divorced | 1 | 12.5 | 2 | 28.6 | 2 | 28.6 | 3 | 42.9 | 8 | 27.6 |
| Living with a significant other | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 1 | 3.4 |
| Widow | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 3 | 10.3 |
| Children | ||||||||||
| No | 2 | 25.0 | 1 | 14.3 | 0 | 0.0 | 1 | 14.3 | 4 | 13.8 |
| Yes | 6 | 75.0 | 6 | 85.7 | 7 | 100.0 | 6 | 85.7 | 25 | 86.2 |
| Comorbidities | ||||||||||
| None | 3 | 37.5 | 5 | 71.4 | 4 | 57.1 | 3 | 42.9 | 15 | 51.7 |
| High Cholesterol | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 | 1 | 3.4 |
| Hypertension | 4 | 50.0 | 2 | 28.6 | 1 | 14.3 | 3 | 42.9 | 10 | 34.5 |
| Other+ | 1 | 12.5 | 0 | 0.0 | 2 | 28.6 | 0 | 0.0 | 3 | 10.3 |
Table includes all participants that were randomized and started the intervention.
Comorbidities reported as “Other” were described as hypothyroid (n=2) and asymptomatic heart murmur (n=1).
Weight and body composition
Examining the main effects of the interventions (metformin versus placebo and lifestyle versus no lifestyle intervention), showed that lifestyle intervention had a significant effect on weight (Table 2). At 16 weeks, participants who received lifestyle intervention demonstrated significantly greater reduction in body weight (−4.23 kg, p=0.006) and fat mass (−3.23 kg, p<0.001) compared to those who did not receive lifestyle intervention. The multiple comparison adjusted cutoff for statistically significant p value is 0.00625 for the weight and body composition analysis. There were no other statistically significant changes in body composition variables observed at 16 weeks by metformin intervention status or lifestyle intervention status. Although not statistically significant, changes in lean mass were observed suggesting that participants who took metformin may have lost more lean mass (−1.9 kg, p=0.019). Participants who received metformin also lost total body weight (−3.43 kg, p=0.023), which was not statistically significant. Participants who received lifestyle intervention had lower percent body fat at 16 weeks compared to participants who did not receive lifestyle intervention (−1.4%, p=0.012). In addition, there were no significant changes observed in body weight or body composition by intervention status at one year follow-up (p>0.00625).
Table 2.
Changes in body weight and body composition at 16 weeks and one year follow-up by intervention
| Effect | Mean Change | 95% LB | 95% UB | p-value | |
|---|---|---|---|---|---|
| Weight (kg) | Metformin (ref: No metformin) | −3.43 | −6.34 | −0.53 | 0.023 |
| Lifestyle (ref: no lifestyle) | −4.23 | −7.13 | −1.32 | 0.006 | |
|
| |||||
| BMI (kg/m2) | Metformin | −2.17 | −5.52 | 1.17 | 0.192 |
| Lifestyle | −3.27 | −6.62 | 0.07 | 0.055 | |
|
| |||||
| Fat mass ratio | Metformin | 0.00 | −0.03 | 0.03 | 0.823 |
| Lifestyle | −0.01 | −0.04 | 0.02 | 0.519 | |
|
| |||||
| Visceral Fat Area (cm2) | Metformin | 8.35 | −20.67 | 37.37 | 0.558 |
| Lifestyle | −17.99 | −47.01 | 11.04 | 0.213 | |
|
| |||||
| Total fat mass (kg) | Metformin | −1.60 | −3.24 | 0.04 | 0.056 |
| Lifestyle | −3.23 | −4.88 | −1.58 | <0.001 | |
|
| |||||
| Total lean mass (kg) | Metformin | −1.93 | −3.51 | −0.35 | 0.019 |
| Lifestyle | −0.88 | −2.46 | 0.69 | 0.260 | |
|
| |||||
| Total percent fat (%) | Metformin | 0.02 | −1.01 | 1.06 | 0.961 |
| Lifestyle | −1.37 | −2.40 | −0.33 | 0.012 | |
|
| |||||
| Android Gynoid ratio | Metformin | −0.01 | −0.05 | 0.03 | 0.574 |
| Lifestyle | −0.02 | −0.06 | 0.02 | 0.301 | |
|
| |||||
| Weight (kg) | Metformin | −2.68 | −8.04 | 2.69 | 0.310 |
| Lifestyle | −2.05 | −7.54 | 3.44 | 0.445 | |
|
| |||||
| BMI (kg/m2) | Metformin | −2.12 | −6.30 | 2.06 | 0.302 |
| Lifestyle | −3.15 | −7.42 | 1.13 | 0.140 | |
|
| |||||
| Fat mass ratio | Metformin | 0.00 | −0.04 | 0.04 | 0.991 |
| Lifestyle | 0.01 | −0.03 | 0.05 | 0.725 | |
|
| |||||
| Visceral Fat Area (cm2) | Metformin | 1.89 | −21.69 | 25.46 | 0.869 |
| Lifestyle | −23.34 | −47.47 | 0.80 | 0.057 | |
|
| |||||
| Total fat mass (kg) | Metformin | −1.48 | −6.25 | 3.28 | 0.524 |
| Lifestyle | −1.01 | −5.89 | 3.87 | 0.670 | |
|
| |||||
| Total lean mass (kg) | Metformin | −1.33 | −2.75 | 0.09 | 0.065 |
| Lifestyle | −0.90 | −2.35 | 0.55 | 0.212 | |
|
| |||||
| Total percent fat (%) | Metformin | −0.82 | −3.79 | 2.16 | 0.572 |
| Lifestyle | −1.17 | −4.22 | 1.87 | 0.432 | |
|
| |||||
| Android Gynoid ratio | Metformin | −0.03 | −0.08 | 0.03 | 0.312 |
| Lifestyle | −0.02 | −0.08 | 0.03 | 0.360 | |
Adjusted p-value cutoff for statistical significance = 0.00625. White rows denote changes at 16 weeks compared to baseline; grey rows denote changes at year 1 compared to baseline. Table only includes participants completing the 16 week intervention.
Considering the four intervention groups separately, percent change in body weight from baseline to 16 weeks was reduced in the lifestyle + placebo, metformin + no lifestyle, and metformin + lifestyle groups (−3.9±5.1%, −3.0±2.3%, −7.1±5.2%, respectively). However, percent change in body weight increased slightly in the placebo + no lifestyle group (+0.3±1.6%). At one year follow-up, percent change in body weight was still reduced for the lifestyle + placebo, metformin + no lifestyle, and metformin + lifestyle groups (−3.4±4.9%, −3.1±3.5%, −5.5±13.5%, respectively), and continued to increase slightly in the placebo + no lifestyle group (+0.8±1.0%). There were no statistically significant differences in weight change between the four separate groups at each time point assessed
Biomarker results
Changes in proliferation and blood-based biomarkers by intervention status from baseline to 16 weeks are shown in Table 3. At 16 weeks, there were no significant changes in endometrial proliferation (%Ki-67+) observed by intervention group (Table 3). Assessment of p27 in pre-and post-intervention endometrial biopsies showed no significant changes based on intervention. Overall, p27 expression in pre-intervention biopsies was scored “intact” (7.7%), “slightly reduced” (50%), “moderately reduced” (34.6%), and “severely reduced” (7.7%), while post-intervention biopsies showed “intact” (19.2%), “slightly reduced” (57.7%), “moderately reduced” (15.4%), and “severely reduced” (7.7%). Serum biomarker DHEA-S was increased (+33.67 mcg/dL, p=0.001) by 16 weeks in participants receiving metformin. In addition, we observed reduced hemoglobin A1C (p=0.036) and C-peptide (p=0.027) in participants receiving metformin, but these were not statistically significant after adjusting for multiple comparisons and false-discovery rate correction (adjusted p-value cutoff for biomarker analysis = 0.0025). Biomarker results suggested that participants receiving lifestyle intervention may have lower insulin (p=0.018) and SHBG (p=0.015), but again these were not statistically significant after adjusting for multiple comparisons and false-discovery rate correction.
Table 3.
Changes in endometrial proliferation and blood-based biomarkers at 16 weeks by intervention
| Effect | Mean Change | 95% LB | 95% UB | p-value | |
|---|---|---|---|---|---|
| Ki-67 (%) | Metformin (ref: No metformin) | −2.74 | −8.75 | 3.27 | 0.352 |
| Lifestyle (ref: no lifestyle) | −3.68 | −9.69 | 2.34 | 0.216 | |
|
| |||||
| Estradiol (pg/mL) | Metformin | −0.62 | −6.95 | 5.71 | 0.841 |
| Lifestyle | 0.29 | −6.04 | 6.62 | 0.925 | |
|
| |||||
| Estrone (pg/mL) | Metformin | −1.52 | −8.94 | 5.91 | 0.675 |
| Lifestyle | −0.20 | −7.62 | 7.23 | 0.957 | |
|
| |||||
| DHEA-S (mcg/dL) | Metformin | 33.67 | 16.10 | 51.25 | 0.001 |
| Lifestyle | −6.20 | −23.78 | 11.37 | 0.473 | |
|
| |||||
| SHBG (nmol/L) | Metformin | 5.06 | −2.31 | 12.43 | 0.169 |
| Lifestyle | 9.37 | 1.99 | 16.74 | 0.015 | |
|
| |||||
| Adiponectin (mcg/mL) | Metformin | 0.93 | −0.38 | 2.25 | 0.155 |
| Lifestyle | 0.64 | −0.67 | 1.95 | 0.324 | |
|
| |||||
| IGF-1 (ng/mL) | Metformin | −9.39 | −21.72 | 2.94 | 0.129 |
| Lifestyle | −2.90 | −15.22 | 9.43 | 0.632 | |
|
| |||||
| Omentin (ng/mL) | Metformin | 31.82 | −107.24 | 170.88 | 0.640 |
| Lifestyle | 62.59 | −76.47 | 201.66 | 0.361 | |
|
| |||||
| Insulin (mIU/mL) | Metformin | −2.13 | −10.12 | 5.86 | 0.586 |
| Lifestyle | −9.81 | −17.80 | −1.82 | 0.018 | |
|
| |||||
| Glucose (mg/dL) | Metformin | 2.51 | −3.95 | 8.96 | 0.430 |
| Lifestyle | −1.94 | −8.39 | 4.52 | 0.541 | |
|
| |||||
| FSH (mIU/mL) | Metformin | 0.56 | −4.76 | 5.88 | 0.830 |
| Lifestyle | −0.29 | −5.61 | 5.03 | 0.912 | |
|
| |||||
| Hemoglobin (g/dL) | Metformin | −0.14 | −0.68 | 0.40 | 0.600 |
| Lifestyle | −0.18 | −0.72 | 0.37 | 0.510 | |
|
| |||||
| A1C (%) | Metformin | −0.22 | −0.42 | −0.01 | 0.036 |
| Lifestyle | 0.16 | −0.04 | 0.36 | 0.115 | |
|
| |||||
| Creatine (mg/dL) | Metformin | −0.02 | −0.10 | 0.05 | 0.543 |
| Lifestyle | −0.05 | −0.12 | 0.03 | 0.199 | |
|
| |||||
| ALT (IU/L) | Metformin | −1.36 | −11.34 | 8.61 | 0.780 |
| Lifestyle | −1.35 | −11.33 | 8.63 | 0.782 | |
|
| |||||
| Triglycerides (mg/dL) | Metformin | −2.83 | −30.76 | 25.09 | 0.836 |
| Lifestyle | −21.03 | −48.95 | 6.90 | 0.133 | |
|
| |||||
| FGF21 (pg/mL) | Metformin | 19.70 | −82.62 | 122.01 | 0.694 |
| Lifestyle | −53.16 | −155.48 | 49.15 | 0.294 | |
|
| |||||
| C-peptide (pg/mL) | Metformin | −283.06 | −530.89 | −35.22 | 0.027 |
| Lifestyle | −211.04 | −458.88 | 36.79 | 0.091 | |
|
| |||||
| Leptin (pg/mL) | Metformin | −6746.86 | −15377.00 | 1883.28 | 0.119 |
| Lifestyle | −3741.67 | −12371.81 | 4888.47 | 0.379 | |
|
| |||||
| Visfatin (pg/mL) | Metformin | −70.00 | −1792.21 | 1652.21 | 0.934 |
| Lifestyle | −1167.96 | −2890.17 | 554.25 | 0.174 | |
P-value cutoff for statistical significance = 0.0025. Table only includes participants completing the 16 week intervention. LB, lower bound; UB, upper bound.
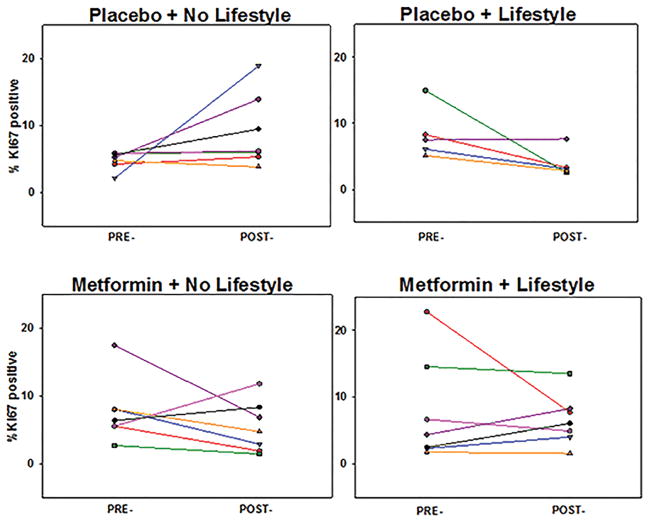
Although the primary objective was to evaluate the independent effects of metformin and lifestyle intervention, further comparisons were evaluated as exploratory analyses. When comparing the 4 treatment groups separately, no significant difference was noted for Ki-67 or other biomarkers when analyzing the groups separately. However, low baseline endometrial proliferation was noted and significant variation in each group is apparent (Figure 2). In addition, the placebo only group (placebo + no lifestyle) was compared separately against the other 3 “active” arms pooled together for Ki-67 and all serum biomarkers. Again, no statistically significant differences were identified after controlling false discovery rate.
Figure 2.
Paired pre- and post-intervention endometrial proliferation by individual treatment group.
Adverse events
Reported adverse events (AEs) by intervention group are listed in Table 4. AEs were grade 1 or 2, most commonly gastrointestinal disturbance (flatulence, diarrhea) and headache. The majority of AEs were reported during the first month of the interventions. At the end of the first month on study, participants receiving placebo reported 14 total AEs (10 in placebo + no lifestyle, 4 in placebo + lifestyle) and participants receiving metformin reported 15 total AEs (10 in metformin + no lifestyle, 5 in metformin + lifestyle). At the end of the first month on study, participants that did not receive lifestyle intervention reported a larger number of AEs, even in the placebo group. Overall, throughout the duration of the intervention, fewer total AEs were reported in the lifestyle arms (17 total AEs in lifestyle arms versus 40 in no lifestyle arms).
Table 4.
Adverse events reported by individual treatment group
| Adverse Event | Placebo + No Lifestyle | Placebo + Lifestyle | Metformin + No Lifestyle | Metformin + Lifestyle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 1 | Month 2 | Month 3 | Month 4 | Month 1 | Month 2 | Month 3 | Month 4 | Month 1 | Month 2 | Month 3 | Month 4 | Month 1 | Month 2 | Month 3 | Month 4 | |
| Blurred vision | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fatigue | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flu like symptoms | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 2 | 1 | 1 | 1 | 0 | 0 | 2 |
| Insomnia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hot flashes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI disturbance | 4 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 5 | 3 | 2 | 1 | 3 | 1 | 1 | 1 |
| Pain | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Muscle Weakness | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||||
| Total | 10 | 3 | 4 | 1 | 4 | 1 | 0 | 1 | 10 | 7 | 3 | 2 | 5 | 1 | 1 | 4 |
All reported adverse events were grade 1 or 2.
DISCUSSION
Given the strong link between obesity and endometrial cancer risk, and the potential for metformin to reduce endometrial cancer risk among obese women, the present investigation sought to evaluate the independent effects of metformin and lifestyle intervention on endometrial cancer risk. Despite achieving weight loss among participants receiving metformin and/or lifestyle intervention, no significant change in endometrial proliferation was observed after the interventions. Low baseline proliferation and substantial variation was noted in endometrial biopsies. Body composition was significantly improved by lifestyle intervention (reduced body weight and total fat mass), while metformin showed only trends toward weight loss and reduced lean mass. The observed significant reductions in body weight and fat mass with lifestyle intervention are consistent with previous investigations comparing lifestyle intervention and/or metformin (34,40). The trend observed for reduction in lean mass among individuals who took metformin is likely related to the greater reductions in total mass observed among participants in both the metformin + no lifestyle and metformin + lifestyle group. Additionally, among blood-based biomarkers associated with endometrial cancer risk, only a statistically significant increase in DHEA-S was observed among participants receiving metformin. Lifestyle intervention showed a trend towards increased SHBG and decreased fasting insulin; while metformin treatment showed a trend towards decreases in hemoglobin A1C and C-peptide. Furthermore, this study demonstrated fewer reported adverse events among individuals who received a lifestyle intervention. Overall, the power to detect statistically significant differences was limited by the small number of participants. Although the small sample size limited power, our analysis still included control of false discovery rate, which was important to reduce the potential for false positive findings given the large number of markers (variables) evaluated. However, these trends are consistent with those reported in the Diabetes Prevention Program, as endogenous sex hormones were previously evaluated at the 1 year follow-up and found that postmenopausal women receiving lifestyle intervention showed increased SHBG, but women receiving metformin did not have significant changes in any hormone markers(41). Previous studies looking specifically at body composition changes in this population within the Diabetes Prevention Program found that loss of visceral adiposity was associated with increased SHBG, but no significant associations were found for changes in DHEA-S or estradiol (42).
Our study sought to evaluate risk reduction in obese postmenopausal women without endometrial pathology. However, it is important to note that of the 32 women who participated in screening visit 2 and had successful endometrial biopsy sampling, one woman was diagnosed with a high-risk precancerous lesion (complex atypical hyperplasia), reiterating the increased cancer risk in this population of obese postmenopausal women. She was referred for further treatment and removed from the study, as an abnormal endometrial biopsy made her ineligible to continue on to the interventions. Although she did not continue on this intervention study, identification of a high-risk cancer precursor and resulting successful treatment was still a positive outcome for this patient.
Although postmenopausal women are at greatest risk for endometrial cancer, postmenopausal status presented two expected, yet significant, limitations in this study, namely low endometrial proliferation and atrophic endometrium. Upon meeting all of the criteria evaluated in screening visit 1, five women could not continue participation due to insufficient tissue obtained from the endometrial biopsy. Low baseline proliferation combined with significant variation across groups will make it difficult to reliably use endometrial proliferation as a readout in postmenopausal women, even when these women have BMI greater than 30 kg/m2. Conducting studies in pre-menopausal obese women would present different challenges due to the difficulty in conducting timed endometrial biopsies because of irregular menstrual cycles and increased anovulatory cycles, in addition to changes in menstrual cycle related to weight loss and metformin use. Using paired pre- and post-intervention evaluation was a significant advantage, but it is likely that changes in proliferation below a certain (yet undetermined) level may not be biologically meaningful. Improved biomarkers of risk will enable future prevention studies by improving identification of participants who are most likely to benefit from risk reduction strategies. To date, endometrial cancer biomarker discovery studies in this population have been limited due to the small amount of tissue obtained in endometrial biopsy sampling. However, current initiatives to create a “Pre-Cancer Genome Atlas” to define the molecular profiles of pre-malignant lesions (43) can provide critical insights that will move this important area of research forward and enable improved prevention studies.
Bariatric surgery has been evaluated as an approach to reduce cancer risk in obese individuals, and provides an important comparison for the results presented here. A study of 9,949 gastric bypass patients in a single practice showed that total cancer incidence was reduced and cancer mortality was reduced in patients that had undergone gastric bypass (44). In addition, they found that cancer incidence was lower for uterine cancer (HR=0.22; 95% confidence interval, 0.13–0.40; p<0.0001). Small studies have evaluated serum biomarkers and endometrial pathology pre- and post-operatively in women undergoing gastric bypass. Argenta et al. found that the prevalence of simple and complex endometrial hyperplasia was reduced following bariatric surgery (45). Although different biomarkers have been evaluated in bariatric surgery studies, significant decreases in insulin, C-peptide, and leptin, as well as increased SHBG and adiponectin were found to be associated with bariatric surgery-induced weight loss (46); yet, steroid hormones remained largely unchanged, except for decreased DHEA-S (47). Importantly, there are substantial differences between the bariatric surgery participants and study characteristics compared to the metformin/lifestyle intervention study described here; the mean age of bariatric surgery study participants was much younger than our study (38 – 44 years), mean BMI was higher (44.9 – 50.9 kg/m2), and mean weight loss was much greater (30.1 – 45.7 kg) at follow-up visits ranging from 6 months – 1 year. It is likely that these differences account for the stronger biomarker changes observed following bariatric surgery-induced weight loss compared to our study.
This study speaks to the substantial challenges in conducting primary prevention studies in obese, post-menopausal women. Although intensive recruitment efforts resulted in 576 people approached for the study, 114 women were not interested, 57 were unable to be contacted after approach, 15 did not respond to contact attempts, 60 women were screened as potentially eligible for participation, and 330 were ineligible. Main reasons for ineligibility included prior hysterectomy or endometrial ablation (n=117), BMI less than 30 kg/m2 (n=47), age outside of the eligible range (n=43), already taking metformin (n=34), patient with diabetes (n=30), not post-menopausal (n=21), history of cancer (n=10), or other (n=28; i.e.- taking hormone replacement therapy, renal insufficiency, metformin allergy, participating in another study, no longer interested after reviewing consent, no health insurance, does not speak English). Women who reported they were uninterested in participation most commonly identified issues related to time constraints, the need to take medication, and concern regarding endometrial biopsies. In seeking obese women with insulin resistance but not frank diabetes, additional participants were deemed ineligible at the screening visits and were excluded from further participation due to abnormal clinical labs (high ALTs, TSH, A1C, insulin, and/or fasting glucose). In addition, some women were shown to lack insulin resistance despite meeting other eligibility criteria. Accrual was ultimately stopped early due to the high number of women deemed ineligible and difficulty identifying sufficient participants that were both eligible and interested in participating. Modifications to prior eligibility criteria (including expanded ranges for age and BMI) were attempted with final criteria reported here, and produced a small number of additional participants. Interestingly, the use of metformin did not result in significant issues for adherence or adverse events, and we didn’t observe significant drop-out for participants taking metformin versus placebo. Our observation of fewer adverse events reported by participants receiving the lifestyle intervention suggests that future chemoprevention studies might consider inclusion of a lifestyle intervention to improve participation and compliance. Experiencing side effects from preventive agents is a major barrier to widespread use of chemopreventive agents and combining a pharmacologic approach with a lifestyle intervention could help patients better manage side effects.
While our goal was to evaluate pharmacologic and lifestyle interventions in obese, postmenopausal women without endometrial lesions, future studies of lifestyle intervention and metformin can be evaluated in women known to be at even higher risk for cancer. For example, women who receive conservative management for complex atypical hyperplasia and low grade, early stage endometrioid endometrial cancer are at high risk of recurrence. Conservative management refers to non-surgical treatment for complex atypical hyperplasia and low grade, early stage endometrioid endometrial cancer, generally consisting of an anti-estrogen treatment such as a progestin intrauterine device or oral progestin therapy. Women typically receive conservative management for two reasons: desire to maintain fertility, or high surgical risk due to multiple medical comorbidities related to obesity. To date, risk reduction studies have primarily focused on additional pharmacologic interventions building on the most commonly used progestins, including metformin in combination with medroxyprogesterone. A recent phase II study of medroxyprogesterone with the addition of metformin for fertility-sparing conservative management showed reduced recurrence of atypical endometrial hyperplasia or grade 1 endometrial cancer (48). Currently, three additional phase II studies are ongoing to evaluate the efficacy of metformin in combination with various forms of progestins (megestrol acetate or levonorgestrel-eluting intrauterine device) in conservative management. Alternatively, interventions in endometrial cancer survivors are likely to identify highly-motivated participants, but will suffer from challenges such as lack of tissue-based evaluation (for survivors post-hysterectomy) and a large number of participants required due to a low percentage that will experience recurrence. As further research is conducted on modified lifestyle interventions, other lifestyle guidelines could be evaluated in the future, such as those focused on reducing total carbohydrate intake.
Taken together, our study shows that lifestyle intervention produced the strongest effects on endometrial cancer risk-related markers (weight loss and total fat mass), as well as trends toward changes in serum markers related to endometrial cancer risk, and fewer adverse events reported. Metformin showed trends toward positive effects on body composition and serum markers. Improved endometrial cancer biomarkers are needed to stratify risk and test prevention strategies, particularly at the endometrial tissue level.
Acknowledgments
This work was supported by the MD Anderson Uterine Cancer SPORE (P50CA098258 supporting M. Yates, Q. Zhang, Y. Yuan, K. Lu, and R. Broaddus) and by the MD Anderson Cancer Center Support Grant (P30CA016672) that supports the Biostatistics Resource Group and PROSPR Shared Resource. A training grant from NIH provided support for A. Coletta (R25CA057730, PI: Shine Chang, PhD). Additional support was provided by MD Anderson’s Center for Energy Balance in Cancer Prevention and Survivorship, the Duncan Family Institute, and the Cancer Prevention and Research Institute of Texas.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer- viewpoint of the IARC working group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 4.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol. 2016;34:4225–30. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Levitt J, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–6. doi: 10.1097/AOG.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 8.Kondalsamy-Chennakesavan S, Janda M, Gebski V, Baker J, Brand A, Hogg R, et al. Risk factors to predict the incidence of surgical adverse events following open or laparoscopic surgery for apparent early stage endometrial cancer: results from a randomised controlled trial. Eur J Cancer. 2012;48:2155–62. doi: 10.1016/j.ejca.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Orekoya O, Samson ME, Trivedi T, Vyas S, Steck SE. The Impact of Obesity on Surgical Outcome in Endometrial Cancer Patients: A Systematic Review. Journal of gynecologic surgery. 2016;32:149–57. doi: 10.1089/gyn.2015.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a Gynecologic Oncology Group study. Cancer. 2006;107:2786–91. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 11.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teoh SL, Das S. Tumour biology of obesity-related cancers: understanding the molecular concept for better diagnosis and treatment. Tumour Biol. 2016;37:14363–80. doi: 10.1007/s13277-016-5357-7. [DOI] [PubMed] [Google Scholar]
- 13.Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2008;66:506–16. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 15.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, et al. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119:555–62. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slomovitz BM, Brown J, Johnston T, Mura D, Levenback CF, Wolf J, et al. A phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. 2011 doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Celestino J, Schmandt R, McCampbell A, Urbauer D, Meyer L, et al. Chemopreventive effects of metformin on obesity-associated endometrial proliferation. Am J Obstet Gynecol. 2013;209:24e1–e12. doi: 10.1016/j.ajog.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laskov I, Drudi L, Beauchamp M, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol Oncol. 2014;134:607–14. doi: 10.1016/j.ygyno.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Sivalingam V, McVey R, Gilmour K, Ali S, Roberts C, Renehan A, et al. A presurgical window-of-opportunity study of metformin in obesity-driven endometrial cancer. Lancet. 2015;26:S90. doi: 10.1016/S0140-6736(15)60405-6. [DOI] [PubMed] [Google Scholar]
- 23.Soliman PT, Zhang Q, Broaddus RR, Westin SN, Iglesias D, Munsell MF, et al. Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer: A window of opportunity study. Gynecol Oncol. 2016;143:466–71. doi: 10.1016/j.ygyno.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods A, Johnstone S, Dickerson K, Leiper F, Fryer L, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Wang Y, Yu L, Hu Q, Ji L, Zhang Y, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–20. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Zou J, Hong L, Luo C, Li Z, Zhu Y, Huang T, et al. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK–FOXO1 signal pathway. Cancer Sci. 2016;107:1806–17. doi: 10.1111/cas.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Barros Machado A, Dos Reis V, Weber S, Jauckus J, Brum I, von Eye Corleta H, et al. Proliferation and metastatic potential of endometrial cancer cells in response to metformin treatment in a high versus normal glucose environment. Oncology letters. 2016;12:3626–32. doi: 10.3892/ol.2016.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laskov I, Abou-Nader P, Amin O, Philip C, Beauchamp M, Yasmeen A, et al. Metformin Increases E-cadherin in Tumors of Diabetic Patients With Endometrial Cancer and Suppresses Epithelial-Mesenchymal Transition in Endometrial Cancer Cell Lines. Int J Gynecol Cancer. 2016;26:1213–21. doi: 10.1097/IGC.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 31.Wallbillich J, Josyula S, Saini U, Zingarelli R, Dorayappan K, Riley M, et al. High Glucose-Mediated STAT3 Activation in Endometrial Cancer Is Inhibited by Metformin: Therapeutic Implications for Endometrial Cancer. PLoS One. 2017;12:e0170318. doi: 10.1371/journal.pone.0170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y, Wang J, Ji M, Yuan Z, Peng Z, Zhang Y, et al. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncology letters. 2014;8:1993–99. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol. 2003;3:26. doi: 10.1186/1471-2288-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pescatello LSAR, Riebe D, Thompson PD, editors. ACSM’s Guidelines for Exercise Testing and Prescription. 9. Baltimore, MD: Lippincott, Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 36.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCampbell AS, Mittelstadt ML, Dere R, Kim S, Zhou L, Djordjevic B, et al. Loss of p27 Associated with Risk for Endometrial Carcinoma Arising in the Setting of Obesity. Current molecular medicine. 2016;16:252–65. doi: 10.2174/1566524016666160225153307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res. 2013;6:774–81. doi: 10.1158/1940-6207.CAPR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 40.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar A, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Kong S, Laughlin GA, Golden SH, Mather KJ, Nan B, et al. Endogenous sex hormone changes in postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab. 2012;97:2853–61. doi: 10.1210/jc.2012-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C, Dabelea D, Kalyani RR, Christophi CA, Bray GA, Pi-Sunyer X, et al. Changes in Visceral Adiposity, Subcutaneous Adiposity, and Sex Hormones in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2017;102:3381–9. doi: 10.1210/jc.2017-00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, et al. The Case for a Pre-Cancer Genome Atlas (PCGA) Cancer Prev Res. 2016;9:119–24. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]
- 44.Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argenta PA, Kassing M, Truskinovsky AM, Svendsen CA. Bariatric surgery and endometrial pathology in asymptomatic morbidly obese women: a prospective, pilot study. BJOG : an international journal of obstetrics and gynaecology. 2013;120:795–800. doi: 10.1111/1471-0528.12100. [DOI] [PubMed] [Google Scholar]
- 46.Linkov F, Goughnour SL, Ma T, Xu Z, Edwards RP, Lokshin AE, et al. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol Oncol. 2017;147:133–8. doi: 10.1016/j.ygyno.2017.07.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modesitt SC, Hallowell PT, Slack-Davis JK, Michalek RD, Atkins KA, Kelley SL, et al. Women at extreme risk for obesity-related carcinogenesis: Baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol Oncol. 2015;138:238–45. doi: 10.1016/j.ygyno.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2016;27:262–6. doi: 10.1093/annonc/mdv539. [DOI] [PubMed] [Google Scholar]