Abstract
Addiction is characterized as a chronic debilitating disease. One devastating feature of addiction is the susceptibility of relapse (40–60%) after stretches of abstinence. One theory that may account for relapse suggests that drug cues (e.g., paraphernalia) may increase stress hormones, and this may prompt relapse. Repeatedly pairing a neutral cue with a reward is commonly utilized to measure what subjects learn about a cue that is predictive of reward. Research has shown that animals that attend to a cue more than to the reward (sign trackers) may be more vulnerable to drug addiction. Additionally, research has shown that sign tracking is associated with an increase in corticosterone (CORT), a primary stress hormone. PT150 is a novel glucocorticoid receptor antagonist that moderates the release of CORT. In the current experiment, it was hypothesized that subjects given repeated administration of PT150 would reduce sign tracking compared to subjects given placebo. Time spent (sec) near a cue that predicts reward (CS+) served as a measure of sign tracking, and PT150 or placebo was administered following sign tracking. An independent samples t-test revealed that subjects that received PT150 had reduced time spent near the CS+ compared to controls. Given the devastating effects of drug addiction, identification of a potential pharmacological intervention in the reduction of relapse would be of great value. Therefore, future research is needed to validate the use of PT150 in reducing behaviors associated with drug addiction.
Keywords: addiction, corticosterone, stress, Pavlovian conditioning, sign tracking
Decades of substance abuse research have demonstrated the parallels between drug addiction behaviors and behaviors elicited from Pavlovian conditioning (for review, Anselme, 2016; Lamb, Schindler & Pinkston, 2016). For example, in Pavlovian conditioning, environmental cues that become associated with a reward come to elicit approach to the cue following conditioning. When these cues become attractive and motivate actions, they are said to have acquired incentive salience (Meyer, Cogan & Robinson, 2014; Saunders & Robinson, 2013; Robinson & Berridge, 2008). Research conducted with rodents indicates that there are individual differences in the propensity to attribute incentive salience to cues that become associated with reward. For example, when a localizable cue (conditioned stimulus, i.e. CS) becomes associated with the receipt of food reward, for some rodents (sign trackers; STs), the cue itself becomes attractive, eliciting approach and engagement with it (Hearst & Jenkins, 1974). For STs, the CS also serves as a potent conditioned reinforcer (Flagel, Akil & Robinson, 2009; Robinson & Flagel, 2009). For other rodents (goal trackers; GTs), the cue is equally predictive of reward (i.e., it serves as an effective CS), but they instead learn to approach the location of reward delivery, and for these rodents the CS is relatively ineffective as a conditioned reinforcer (Robinson & Flagel, 2009; Yager & Robinson, 2010).
The identification of animals that have the propensity to sign track is important because attribution of incentive salience to cues is associated with addictive behavior. In rats, STs that attribute incentive salience to a cue are more likely to self-administer cocaine compared to GTs (Beckmann, Marusich, Gipson & Bardo, 2011). Additionally, STs show greater cocaine-induced behavioral sensitization (Flagel, et al., 2008), a greater cocaine-induced conditioned place preference (Meyer, Ma, & Robinson, 2012), and have more robust rates of reinstatement to drug-paired cues compared to GTs (Saunders & Robinson, 2010). Overall, these studies show that STs may be more vulnerable to drug addiction.
In rodents, high levels of corticosterone (CORT), a predominant stress hormone, correlate with the propensity to sign track (e.g., Flagel et al., 2008). Additionally, the presentation of a CS that predicts reward increases CORT (Tomie, Silbberman, Williams, & Pohorecky, 2002; Tomie, Toreadp, Yu, and Pohorecky, 2004) and this increase is greater in sign tracking rodents that attribute incentive salience to the cue (Flagel et al., 2008). These findings suggest that CORT may be a potential target for reducing sign tracking behaviors.
It is widely accepted that visual cues in the environment may become associated with drug taking and subsequently, in the absence of drug, cause drug-seeking and ultimately relapse (Childress et al., 1999). Therefore, studying substance abuse with an avian species may be of an additional benefit because birds are primarily visual whereas most rodents largely depend on olfaction (Carsia & Harvey, 2000; Crombag, Badiani, Maren & Robinson, 2000). Japanese quail are a visually-oriented bird species with color vision and high visual acuity (Mills, Crawford, Domjan & Faure, 1997). Numerous drug studies have been conducted with Japanese quail (Levens & Akins, 2004; Geary & Akins, 2007; Akins & Geary 2008; Bolin & Akins, 2012; Gill, Rice & Akins, 2015; Gill, Madison & Akins, 2015). These studies demonstrate that the drug responses in quail are largely conserved relative to rodents.
The HPA axis involves an auto-regulated feedback system that includes 2 types of adrenal steroid receptors, the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (Bachmann, Linthorst, Holsboer & Reul, 2003). This dual receptor system is responsible for homeostasis of the HPA axis. GR antagonists (e.g., PT150) have been shown to increase MR expression (Bachmann, et al., 2003). When the MR/GR balance shifts toward an increase in MRs, there is a decreased corticosterone response to stress (de Kloet, 1991). Therefore, PT150 may be a potential target for reducing the stress hormone corticosterone.
11, 21-Bisphenyl-19-norpregnane derivative (PT150) is a glucocorticoid receptor (GR) antagonist that competes with the binding of CORT in the cytoplasm (Peeters, Ruigt, Craighead & Kitchener, 2008). A similar compound to PT150 is RU486 which is a methylenedioxy phenyl analog of PT150 (Peeters et al., 2008). However, in addition to competing with the GR, RU486 modulates the progesterone receptor (PR) (Gagne, Pons & Philibert, 1985). The compound PT150 is a novel compound in that, unlike RU486, PT150 has minimal effect on the progesterone receptor (PR). PT150 has a 500-fold greater affinity for the GR while RU 486 has a 5.4-fold affinity for the GR over the PR (Gebhard, Van Der Voort, Schuts & Schoonen., 1994).
In the current study, we used a Pavlovian conditioning paradigm that measures individual differences in acquired incentive salience to cues that become associated with reward (Domjan, Lyons, North & Bruell, 1986; Beckmann & Bardo, 2012; Flagel et al., 08). To assess the ability of a GR antagonist to attenuate sign tracking, PT150 was administered after acquisition of sign tracking in male quail. We hypothesized that PT150 would decrease sign tracking compared to placebo following repeated administration.
Methods
Subjects
Twenty-two (N = 22) adult male Japanese quail (Coturnix japonica) were hatched from eggs (Georgia Quail Farm (GQF), Savannah, GA) and were raised in mixed sex groups until approximately 6 weeks of age. Quail were housed in individual wire mesh cages (supplied by GQF Manufacturing, Savannah, GA) and maintained on a 16:8 hr light cycle. At 10 months of age, twenty-two male quail were randomly assigned to one of 2 groups (drug or placebo; ns = 11). The number of subjects per group was chosen based on a power analysis using Gpower (Faul & Erdfelder, 1992; Erdfelder, Faul & Buchner, 1996) with power set at 0.80 and α = 0.05 for two tailed F tests.
All experimental procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky and by the standards outlined in the 8th edition of the Guide for the Care and Use of Laboratory Animals (National Research Council, 2010). These entities adhere to the standards of the APA ethical standards for treatment of animals.
Glucocorticoid receptor antagonist (PT150)
The study was designed to ensure that birds would voluntarily consume PT150 because forced administration (e.g. gavage in liquid vehicle) might have induced stress. Thus, a solid vehicle that birds found highly palatable, peanut butter, was used to assure consumption of the drug. Use of highly palatable solid foods (e.g., Peanut butter and Nutella) are a common means of assuring voluntary oral consumption of pharmacological substances (Goldkuhl, Hau & Abelson, 2010; Berger & deCatanzaro, 2007; Cope, et al., 2005).
Capsules containing drug (150 mg) or placebo were opened and added to Peanut Butter (PB) (150 mg/2g). The mixture was measured out at 0.53 mg/kg, resulting in a 40 mg/kg dose, and was given orally one hour before Pavlovian conditioning on days 11–15. As a control measure, 0.53 mg/kg of PB was provided on Pavlovian conditioning days 1–10. The dose of PT150 was chosen based on previous research with rodents (Sharrett-Field, 2013).
Apparatus
Behavioral testing was conducted in a standard conditioning chamber (Med Associates Inc., Georgia, VT; 22 cm × 19 cm × 13 cm) that had an acrylic hinged loading door, stainless steel side panels, and an acrylic back panel. The chamber was located in a sound- and light- attenuating cabinet equipped with a fan that provided continuous ventilation. A white noise generator provided low-level background noise, and a white house light provided illumination. Two key lights (red and green) were mounted at one end of the chamber 12.7 cm above the metal rod floor, and a food magazine (ENV-205m MED Associates) was located between the key lights. The key lights and food magazine were illuminated with LED lights when activated.
Procedures
Food restriction
During Pavlovian conditioning, food restriction was used to ensure motivation of reward (i.e., grain). Male quail were maintained at 85% body weight, where food was available from 3 PM to 6 PM daily. This body weight matches previously defined avian methods of food deprivation (Duval, Cassey, Miksik, Reynolds & Spencer, 2013a; Duval et al., 2013b; Shousha, Nakahara, Nasu, Sakamoto & Murakami, 2007). Quail were weighed daily, and following the establishment of their mean free-feeding body weight, 18% of their mean body weight was calculated to provide minimum caloric intake in grams of feed to sustain 85% body weight throughout conditioning (e.g., Lejeune & Nagy, 1986).
Conditioning
A Pavlovian-conditioned approach (PCA) procedure was used to measure the attribution of incentive salience to a CS. Before conditioning, birds were shaped with successive approximation until they reliably retrieved grain from the hopper. Shaping was followed by 15 days of Pavlovian conditioning. During conditioning, each trial was composed of presentation of an 8-sec green key light (CS+) followed by immediate 8-sec access to grain (unconditioned stimulus, US) in a food hopper. The pairing of the CS and US was on a variable time interval (VT) of 90 sec resulting in an average of a 30 min session. An 8-sec red key light (CS−) was presented unpaired with the US and used as a control, and thus was not predictive of food. Twenty trials were conducted each day for 15 days for a sum of 300 trials each for CS+ and CS−. Time spent near the CS to both key lights was measured during CS presentation on days 6, 10, 11, and 15. Day 6 was chosen because previous studies have shown that sign tracking is evident by trial 125 of conditioning (e.g., Flagel et al., 2008) which corresponds to day 6 in the current experiment. Day 10 was the last day before PT150 was administered, day 11 was the first day PT150 was administered, and day 15 was the last day PT150 was administered.
Time spent near the CS was scored when subjects had both feet inside the zone (15.24 cm × 7.62 cm) marked off in front of the CS light and were oriented toward the CS. Goal tracking was scored as time spent near the food hopper when subjects had both feet inside of the zone (15.24 cm × 8.89 cm) marked off in front of the hopper. Video recordings were scored by visual observation. Researchers scoring the videos were blind to the treatment condition of the subjects.
Statistical Analysis
Sign and goal tracking
Repeated-measures ANOVAs were conducted with treatment as a between-subjects factor and day (collapsed across trials) as a repeated measure to investigate changes in sign tracking to the CS+ and the CS−. A repeated-measures ANOVA with treatment as a between-subjects factor was conducted for day 10 (the last day before treatment) and day 15 (the last day of treatment) to determine the effects of repeated PT150 administration. A posthoc analysis was conducted on day 15 to examine changes in sign tracking as a result of treatment. To determine the behavioral specificity of PT150, a repeated-measures ANOVA with treatment as a between–subjects factor was conducted on goal tracking for days 10 and 15.
Response bias
A response bias analysis was used to determine the relative amounts of sign and goal tracking and then to investigate whether drug effects were the same regardless of extent of sign tracking. A similar response bias analysis has been used in previous research (e.g., Meyer, Lovi, Saunders, Yager, Flagel, Morrow & Robinson, 2012; Paolone, Angelakos, Meyer, Robinson & martin-Sarter, 2013). Response bias was defined as the difference between time spent near the food hopper and the CS+ light during CS+ presentation. The calculation was expressed as [(time spent at CS+ minus time spent at hopper) / (time spent at CS+ plus time spent at hopper)]. Quail were considered to be expressing a response bias of sign tracking if they obtained scores ranging from +0.4 to +1.0 and were considered to have a response bias of goal tracking if they obtained scores ranging from −0.4 to −1.0. Quail with intermediate scores (ITs) were not considered to be showing a response bias of either sign or goal tracking.
The response bias analysis was conducted on day 10 because it was the last day before treatment.
Difference score
To evaluate the change in sign tracking across days 10 and 15 in relation to the extent of sign tracking (response bias), a difference score was calculated as time spent near the CS+ on day 15 minus time spent sign tracking on day 10 during the CS+ presentation. A positive difference score indicated increased sign tracking from day 10 to day 15, while a negative score difference indicated decreased sign tracking from day 10 to day 15.
To determine whether PT150 had a similar effect regardless of the extent of sign tracking, the relationship between the response bias and the difference score was analyzed with a Pearson’s r correlation with treatment as a factor.
For all statistical analyses, alpha was set at p < 0.05.
Results
Figure 1A shows mean sign tracking across days for each treatment group. A repeated-measures ANOVA revealed a main effect of Day [F(3, 60) = 12.25, p < 0.05], demonstrating that sign tracking (time spent at CS+) increased over conditioning days, regardless of treatment. There was no significant Day × Treatment interaction [F(3, 60) = 2.24], nor a main effect of Treatment [F(1, 20) = 2.6], ps > 0.05.
Figure 1.
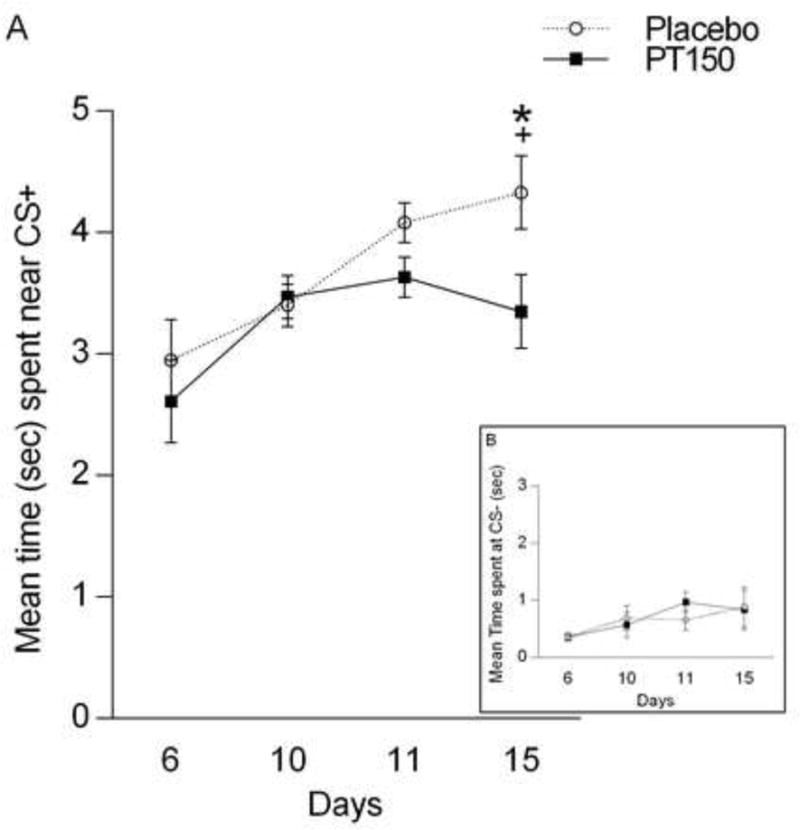
Mean time (sec) near the CS+ (Figure 1A) and CS− (Figure 1B) across days 6, 10, 11, and 15 for placebo and drug treatments. * significant difference between day 10 and 15, + significant difference between treatment groups on day 15.
The repeated-measures ANOVA conducted to investigate the effect of PT150 on sign tracking behavior revealed a Day × Treatment interaction [F(1, 20) = 5.68] and a main effect of Treatment, [F(1, 20) = 5.93], ps < 0.05. A posthoc analysis indicated a significant main effect of Treatment on day 15 [F(1, 20) =5.42, p < 0.05]. Subjects that received PT150 (M = 3.35, SEM = 0.3) spent significantly less time sign tracking than subjects that received the placebo (M = 4.33, SEM = 0.3).
To determine whether PT150 had an effect on time spent near the CS-, a repeated-measures ANOVA was conducted for days 6, 10, 11 and 15 for placebo and drug treatments (see Figure 1B). There was no significant Day × Treatment interaction [F(3, 60) = 2.13], indicating that treatment groups did not change in time spent near the CS- across days. There were also no main effects of Treatment or Day, Fs were between 0.38 to 2.13, ps > 0.05.
To explore the individual differences of sign and goal tracking a response bias was calculated. The analysis showed that 12 out of 22 quail had a response bias toward sign tracking. Ten out of the 22 quail had neither a sign nor goal tracking response bias.
There was no positive correlation between the difference score and the response bias (r = 0.52), nor between treatment groups and the response bias (r = 0.06), ns 22, ps > 0.05. Therefore, the extent of sign tracking did not correlate with the difference score. However, there was a negative correlation between treatment groups and the difference score. Treatment with PT150 was correlated with decreased sign tracking, (r = −0.47, p < 0.05). Thus, regardless of the extent of sign tracking, sign tracking appeared to be associated with treatment effects rather than the degree of the response bias.
To investigate the behavioral specificity of PT150, a repeated-measures ANOVA was conducted on days 10 and 15 with goal tracking as the dependent variable. Results showed that there was no main effect of Day [F(1, 20) = 0.30, p > 0.05], indicating that time spent goal tracking did not change from day 10 to 15. There was also no significant Day × Treatment interaction [F(1, 20) = 0.09, p > 0.05], indicating that the drug did not affect goal tracking. Overall, these results indicate that the behavioral effects of PT150 may have been specific to sign tracking.
Discussion
Similar to rodents, the quail in the current experiment demonstrated an increase in sign tracking toward a cue that had been paired with reward across days (e.g., Flagel, Akil & Robinson, 2009). Most importantly, repeated oral consumption of PT150 resulted in a decrease in sign tracking behavior when compared to placebo. This reduction of sign tracking may have been the result of PT150 blocking the glucocorticoid receptor (GR). Administration of the GR antagonist PT150 has been shown to reduce CORT in rodents that consumed alcohol (Reynolds et al., 2015). This reduction in CORT was thought to occur because PT150 inhibits the nuclear translocation of the GR that indirectly regulates the release of CORT (Peeters, et al., 2008). While the findings of the current experiment suggest that reduced sign tracking may have been a result of a blockade of the GR, it is unknown whether the GR is antagonized in the same manner in birds as in rodents.
There are changes in the brain that are related to sign tracking. For example, repeated activation of the reward pathway results in heightened behavioral responses that have been determined to be a predictor of drug addiction (Tomie, Grimes & Pohorecky, 2008). The hypothalamic-pituitary-adrenal (HPA) axis is involved in the activation and management of stress responses and has been shown to contribute to a heightened reward pathway (Piazza & Moal, 1998). In Pavlovian conditioning, this heightened response (e.g., elevated pecking, sniffing, and licking) is often referred to as sign tracking (e.g., Tomie, et al., 2008). Similar to stress, sign tracking also disrupts the hypothalamic-pituitary-adrenal (HPA) axis and this may result in alteration of the HPA axis’ negative feedback system, (e.g., Flagel et al., 2008; Tomie, et al., 2008). Therefore, in the current study, the reduction in sign tracking may have been associated with dysfunction of the HPA axis.
The current study extends previous research by demonstrating reduced sign tracking as a result of repeated administration of a GR antagonist, suggesting that sign tracking may be associated with an increase in CORT and mediated via the GR.
The link between sign tracking and CORT is particularly interesting in that CORT could be a biomarker for future pharmacological treatments in individuals suffering from substance use disorders. However, further research is needed for validating the reduction of CORT by PT150 and its relationship to sign tracking.
Public Significance Statement.
This is the first research, to date, to explore the effect of pharmacologically manipulating stress hormones on sign tracking, an addiction-like behavior. The main finding is that the GR receptor antagonist PT150 reduced sign tracking. Novel pharmaceutical interventions such as PT150 may stop or reduce the cascade of corticosterone in the presence of addiction-relevant stimuli that may instigate relapse.
Acknowledgments
Disclosures
This research was supported by NIDA of NIH (grant T32DA03520 awarded to Rush; grant R01DA025032 awarded to Akins). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This funding source had no other role beyond financial support.
The authors would like to thank Dr. Lynda Sharrett-Field for guidance in drug administration and measurements, Dr. Joshua Beckman for early stage development of procedures, and Palisades Therapeutics, LLC for the generous gift of PT150.
Footnotes
All authors contributed in a significant way to the manuscript and all authors have read and approved the final manuscript.
All authors have no conflict of interest both real and potential to disclose.
Prior dissemination of the current research ideas and preliminary data analysis were presented as poster presentations. Poster presentations were presented at Behavior, Biology, and Chemistry: Translational Research in Addiction; European Behavior and Psychology Society; and the Pavlovian Society in 2017.
References
- Akins CK, Geary EH. Cocaine-induced behavioral sensitization and conditioning in male Japanese quail. Pharmacology Biochemistry and Behavior. 2008;88(4):432–437. doi: 10.1016/j.pbb.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme P. Motivational control of sign-tracking behaviour: A theoretical framework. Neuroscience & Biobehavioral Reviews. 2016;65:1–20. doi: 10.1016/j.neubiorev.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst AC, Holsboer F, Reul JM. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28(6):1056. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behavioral brain research. 2012;226(1):331–334. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural brain research. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RG, Hancock T. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reproductive Toxicology. 2007;23(2):138–144. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bolin BL, Akins CK. Chronic pre-exposure to methamphetamine following 31 days of withdrawal impairs sexual performance but not sexual conditioning in male Japanese quail. Behavioural processes. 2012;91(2):177–183. doi: 10.1016/j.beproc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsia, Harvey . Adrenals, Chapter 19. In: Johnson AL, Whittow GC, editors. Sturkie’s avian physiology. pp. 489–522. [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. International journal of obesity. 2005;29(6):607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intrquailnous amphetamine. Behavioural brain research. 2000;116(1):1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers in neuroendocrinology. 1991;12(2):95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Domjan M, Lyons R, North NC, Bruell J. Sexual Pavlovian conditioned approach behavior in male Japanese quail (Coturnix coturnix japonica) Journal of Comparative Psychology. 1986;100(4):413. [PubMed] [Google Scholar]
- Duval C, Cassey P, Mikšík I, Reynolds SJ, Spencer KA. Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica) Journal of Experimental Biology. 2013a;216(4):700–708. doi: 10.1242/jeb.077370. [DOI] [PubMed] [Google Scholar]
- Duval C, Cassey P, Lovell PG, Mikšík I, Reynolds SJ, Spencer KA. Eggshell appearance does not signal maternal corticosterone exposure in Japanese quail: an experimental study with brown-spotted eggs. PloS one. 2013b;8(12):e80485. doi: 10.1371/journal.pone.0080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior research methods, instruments, & computers. 1996;28(1):1–11. [Google Scholar]
- Faul F, Erdfelder E. GPOWER: A priori, post-hoc, and compromise power analyses for MS-DOS [Computer software] Bonn, Germany: Bonn University, Department of Psychology; 1992. [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behavioral brain research. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne D, Pons M, Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. Journal of steroid biochemistry. 1985;23(3):247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Gebhard R, Van Der Voort H, Schuts W, Schoonen W. 11, 21-bisphenyl-19-norpregnane derivatives are selective antiglucocorticoids. Bioorganic & Medicinal Chemistry Letters. 1997;7(17):2229–2234. [Google Scholar]
- Geary EH, Akins CK. Cocaine sensitization in male quail: temporal, conditioning, and dose-dependent characteristics. Physiology & Behavior. 2007;90(5):818–824. doi: 10.1016/j.physbeh.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Madison FN, Akins CK. Cocaine-induced sensitization correlates with testosterone in male Japanese quail but not with estradiol in female Japanese quail. Hormones and Behavior. 2015;67:21–27. doi: 10.1016/j.yhbeh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Rice BA, Akins CK. Cocaine induces state-dependent learning of sexual conditioning in male Japanese quail. Physiology & behavior. 2015;138:150–153. doi: 10.1016/j.physbeh.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkuhl R, Hau J, Abelson KS. Effects of voluntarily-ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behavior in permanently catheterized rats. in vivo. 2010;24(2):131–135. [PubMed] [Google Scholar]
- Harvey S, Hall TR. Hormones and stress in birds: activation of the hypothalamo-pituitary-adrenal axis. Progress in clinical and biological research. 1990;342:453. [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. The stimulus-reinforcer relation and directed action. Austin: The Psychonomic Society; 1974. Sign-tracking. Hearst Sign-Tracking: The Stimulus-Reinforcer Relation and Directed Action. [Google Scholar]
- Holmes WN, Phillips JG. The adrenal corticosteroneex of birds. In: Chester-Jones I, Henderson I, editors. General and Comparative Endocrinology of the adrenal cortex. Academic Press; New York: 1976. pp. 293–420. [Google Scholar]
- Lamb RJ, Schindler CW, Pinkston JW. Conditioned stimuli’s role in relapse: preclinical research on Pavlovian-Instrumental Transfer. Psychopharmacology. 2016;233(10):1933–1944. doi: 10.1007/s00213-016-4216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune H, Nagy J. Operant conditioning in the newly hatched quail: fixed interval performance. Behavioral Processes. 1986;12(4):317–325. doi: 10.1016/0376-6357(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Levens N, Akins CK. Chronic cocaine pretreatment facilitates Pavlovian sexual conditioning in male Japanese quail. Pharmacology Biochemistry and Behavior. 2004;79(3):451–457. doi: 10.1016/j.pbb.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one. 2014;9(6):e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one. 2012;7(6):e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012;219(4):999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neuroscience & Biobehavioral Reviews. 1997;21(3):261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press; 2010. [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. Journal of Neuroscience. 2013;33(19):8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters BWMM, Ruigt GSF, Craighead M, Kitchener P. Differential effects of the new glucocorticoid receptor antagonist ORG 34517 and RU486 (mifepristone) on glucocorticoid receptor nuclear translocation in the AtT20 cell line. Annals of the New York Academy of Sciences. 2008;1148(1):536–541. doi: 10.1196/annals.1410.072. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in pharmacological sciences. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Saunders MA, Honoree’ W B, Winchester SR, Elgumati IS, Prendergast MA. Acute oral administration of the novel, competitive and selective glucocorticoid receptor antagonist ORG 34517 reduces the severity of ethanol withdrawal and related hypothalamic–pituitary–adrenal axis activation. Drug and alcohol dependence. 2015;154:100–104. doi: 10.1016/j.drugalcdep.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biological psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neuroscience & Biobehavioral Reviews. 2013;37(9):1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrett-Field L. Characterizing consumption, dependence, and the role of glucocorticoids in an animal model of voluntary ethanol consumption. Doctoral dissertation. 2013 Retrieved from uknowledge.uky.edu.
- Shousha S, Nakahara K, Nasu T, Sakamoto T, Murakami N. Effect of Glucagon-like peptide-1 and-2 on regulation of food intake, body temperature and locomotor activity in the Japanese quail. Neuroscience Letters. 2007;415(2):102–107. doi: 10.1016/j.neulet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacology Biochemistry and Behavior. 2000;65(3):509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Silberman Y, Williams K, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone levels in rats. Pharmacology Biochemistry and Behavior. 2002;72(3):507–513. doi: 10.1016/s0091-3057(01)00781-x. [DOI] [PubMed] [Google Scholar]
- Tomie A, Tirado AD, Yu L, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behavioral brain research. 2004;153(1):97–105. doi: 10.1016/j.bbr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain research reviews. 2008;58(1):121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behavioural brain research. 2010;214(1):30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


