Abstract
Background
Obesity is a cancer risk factor. While it does not increase the risk of localized prostate cancer, it raises the risk of the aggressive disease in men of European ancestry. Few studies investigated obesity as a prostate cancer risk factor in men of African ancestry. Findings from those studies were heterogeneous but some reported an association of excess body fatness with aggressive disease.
Methods
We examined the relationship of body mass index (BMI), waist circumference, and waist-hip ratio with prostate cancer in African-American and European-American men in the NCI-Maryland Prostate Cancer Case-Control study consisting of 798 men with incident prostate cancer (402 African-American and 496 European-American) and 1008 population-based controls (474 African-American and 534 European-American). BMI was self-reported. Waist circumference and waist-hip ratio were calculated from measurements at enrollment.
Results
A high BMI either at enrollment or years prior to it was associated with a decreased risk of prostate cancer in African-American men. In contrast, an elevated BMI tended to increase the disease risk in European-American men. Waist circumference was inversely associated with prostate cancer in both African-American and European-American men whereas a high waist-hip ratio did not associate with prostate cancer in African-American men but tended to be associated with advanced/aggressive disease in European-American men.
Conclusions
Our findings reveal an obesity paradox among African-American men in this study population, where a high BMI and waist circumference associated with a decreased disease risk.
Impact
Our observations expand the knowledge of how obesity may affect prostate cancer risks in African-Americans.
Keywords: prostate cancer, body mass index, waist circumference, waist-hip ratio, African-American
Introduction
One of the most prominent cancer health disparities exists in prostate cancer. African American (AA) men have the highest prostate cancer incidence and mortality rates among all population groups in the United States (1–3). Accordingly, prostate cancer is estimated to be a leading cause of cancer deaths for these men. This disparity has been attributed to differences in medical care, tumor growth rates and disease aggressiveness, and to location and histopathological variables of the tumor (1, 4–10). Numerous studies examined the possibility of low penetrance genes contributing to the excessive burden of prostate cancer in AA men. To date, the best characterized risk locus for prostate cancer is located at 8q24. As shown by several studies, this locus confers an increased risk for prostate cancer in men of West African ancestry, when compared with men of European and Asian ancestry (11, 12).
Despite these findings, it is generally thought that modifiable risk factors such as diet and lifestyle account for most prostate cancers globally (13). There is also evidence from migration studies that the environment and adaptation of a Western-type lifestyle modulate prostate cancer risks (14–17). However, other studies including large prospective cohort studies could not confirm possible roles of diet and lifestyle factors in prostate cancer etiology (18, 19). As such the evidence that these factors modify prostate cancer risk is not conclusive. Yet, AA men experience greater age-adjusted prevalence rates of overall [body mass index (BMI) > 30 kg/m2] or class 3 obesity (BMI > 40 kg/m2) compared with European-American (EA) men (20). AA men are also more likely to die from obesity-related conditions such as cardiovascular disease and diabetes. In addition, obesity-derived factors may augment growth and invasion of prostate cancer cells (21). Current research suggests that obesity is only weakly linked to the incidence of localized or low-grade prostate cancer. Instead, it was found to increase the risk of the aggressive disease and prostate cancer mortality (22–28). Observations for men of African ancestry are sparse, and the findings are heterogeneous, although an association of excess body weight with aggressive prostate cancer has been reported (29–35). To address the need for additional studies of the relationship between excess body fatness and prostate cancer risk in AA men, we studied the relationship of BMI, waist circumference and waist-hip ratio with disease development and presentation in the NCI-Maryland Prostate Cancer Case-Control study.
Materials and Methods
The NCI-Maryland Prostate Cancer Case-Control Study has been described elsewhere (36). Briefly, the study was conducted between 2005–2015 and approved by the NCI (protocol# 05-C-N021) and the University of Maryland (protocol #0298229) Institutional Review Boards, and both recruitment and research followed the ethical guidelines set by the Declaration of Helsinki. We obtained informed written consent from all study subjects prior to participation. Eligibility was as follows: Cases with a diagnosis of prostate cancer within the last 2 years prior to recruitment were enrolled from two hospitals, the Baltimore Veterans Affairs Medical Center and the University of Maryland Medical Center. Population-based controls were identified through the Maryland Department of Motor Vehicles from the following areas: Maryland, Washington DC, and the neighboring border counties in Pennsylvania, Delaware, and Virginia, but most of the recruited men resided in four Maryland counties: Anne Arundel, Baltimore City, Baltimore County, and Howard. All men self-reported to be either AA or EA, were between 40 and 90 years old, were born in the United States, spoke English well enough to be interviewed, and were well enough to participate in an in-person interview. A total of 976 cases (489 AA, 487 EA) were recruited. Of these, 823 patients (or 84%) were defined as incident cases because they were recruited into the study within 1 year after the disease diagnosis with an average interval between diagnosis and enrollment into the study of 4.8 months (4.4 months for AA and 5.2 months for EA men). A total of 1,034 population controls (486 AA and 548 EA men) were recruited, as described elsewhere (36). Because of missing data on predictors and covariates, and exclusion of 15 participants with BMI < 18.5 kg/m2 or > 50 kg/m2, the final analytical sample included 798 cases (97% of all incident cases) and 1008 controls (97.5% of all population controls) in the multivariable analysis.
Data collection and disease staging
At enrollment, trained interviewers administered a standardized questionnaire eliciting information on personal medical and cancer history, tobacco use, occupational, family medical history, socioeconomic history, diet, and anthropometric factors. Information on height and weight at various life stages and at enrollment was self-reported. Interviewers measured waist and hip circumference twice, with the final variable being the average. We obtained additional information on cases from pathology reports and medical records [e.g., prostate-specific antigen (PSA) levels at diagnosis, disease stage and grade]. Disease staging was defined according to the 7th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis system for anatomic stage/prognostic groups, and grouped as T1, T2, T3, and T4.
Anthropometric measures
BMI is the most commonly studied anthropometric measure and showed an association with aggressive prostate cancer in previous studies (26, 27). BMI was based on self-reported information and calculated by dividing weight in kilograms by height in meters squared to generate traditional cut-off points of normal weight (BMI of 18.5 to < 25.0, reference), overweight (25.0 to < 30.0), and obese (30.0 to 50.0). Three BMI time points were assessed: BMI at time of enrollment, two years prior to enrollment, and ten years prior to enrollment. Waist circumference was stratified according to health-related thresholds within BMI categories (37), yielding four categories (20.8 – 89.9 [reference], 90 – 99.9, 100 – 109.9, 110 – 197.8). For further validation of our observations, we also stratified waist circumference according to WHO guidelines into ≤ 94 [reference], > 94 – 102 = increased risk of metabolic complications, > 102 = substantially increased risk of metabolic complications) (38). Waist-hip ratio was calculated by dividing the average waist measurement by the average hip measurement and was operationalized into tertiles (< 0.92 [reference], 0.92 to 0.98, > 0.98) based on the waist-hip ratio distribution among the 1008 controls.
Definition of advanced and aggressive disease
Tumor histologic grade was determined according to the Gleason score system and grouped as follows: low-grade (2–7) vs high-grade (8–10). Disease stage at diagnosis was based on information obtained from medical records, with low-stage or localized disease being defined as T1 or T2 and high-stage or advanced disease being defined as T3 or T4. Disease aggressiveness was operationalized as a binary measure where a Gleason score ≤ 7 and a stage I or II was considered non-aggressive, while a Gleason score > 7 or stage III or IV was defined as aggressive.
Covariables
All multivariable models were adjusted for age at enrollment (continuous), education (≤ high school [reference], some college, college, graduate school), diabetes, first degree relative with prostate cancer, and smoking (never [reference], former, current). A never smoker was a man who smoked less than 100 cigarettes in his lifetime according to self-report.
Statistical analysis
Statistical analysis was performed using STATA software, version 13.0 (Stata Corporation). All statistical analysis was two-sided, and a P < .05 was considered statistically significant. We estimated associations between anthropometric variables, prostate cancer, and covariables using student’s t-tests and the chi-square test. For the main analysis, we evaluated whether anthropometric measures were associated with prostate cancer in strata of Gleason score (low or high-grade), stage (low or high-stage) and disease aggressiveness (non-aggressive and aggressive). We calculated odds ratios and 95 percent confidence intervals using multivariable logistic regressions and tested for interactions under a multiplicative model with the log likelihood ratio test. Linear trend analysis was performed using logistic regression models with continuous predictors. In a prior study, we observed that aspirin modified prostate cancer risk in AA men (36). Given these findings, a sensitivity analysis was conducted to determine whether use of aspirin moderated the relationship of BMI and waist circumference with prostate cancer among AA men by stratifying our analysis by aspirin users and non-users. Aspirin use was measured by asking participants the following survey question: Have you taken aspirin or aspirin-containing compounds (such as Bufferin, Anacin, Ascriptin, Excedrin) regularly—at least one pill per week for 2 months during the past 5 years—no, yes, or do not know, as previously described (36).
Results
Participants characteristics
Demographics and the health- and disease-related characteristics of the study population are shown in Table 1. Our analytical sample included 798 incident prostate cancer cases and 1008 population controls with similar proportions of AA (402 and 474, respectively) and EA (396 and 534, respectively) men and information on BMI, waist circumference and waist-hip ratio. Men in the control group were slightly older than cases (AA, 64 vs 63, and EA, 66 vs 65). Cases tended to have a lower education and income level, and a greater proportion of them were smokers and had a family history of prostate cancer, when compared with controls (Table 1). Occurrence of diabetes was not significantly different between cases and controls for either group of men. Aspirin use differed significantly between cases and controls for AA men (42% vs 51.7%, respectively, P < 0.01) but not for EA men (56.3% vs 62%, P = 0.08).
Table 1.
Characteristics of participants in the NCI-Maryland Prostate Case Control Study, N = 1806
| Characteristics | African American | European American | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Casesa | Pb | Control | Casesa | Pb | |
| Patients, No. | 474 | 402 | 534 | 396 | ||
| Demographics | ||||||
| Age, median (IQRc), y | 64 (10) | 63 (10) | 0.001 | 66 (13) | 65 (11) | 0.006 |
| Education, % | 0.000 | 0.011 | ||||
| ≤High school [reference] | 30.0 | 46.5 | 19.3 | 25.0 | ||
| Some college | 29.5 | 37.3 | 22.3 | 27.5 | ||
| College | 21.5 | 11.4 | 28.8 | 24.0 | ||
| Graduate | 19.0 | 4.7 | 29.6 | 23.5 | ||
| Income, % | n = 431 | n = 364 | 0.000 | n = 502 | n = 369 | 0.000 |
| <$30k [reference] | 24.6 | 55.8 | 10.6 | 24.1 | ||
| $30k to $59k | 24.4 | 23.9 | 24.7 | 21.7 | ||
| $60k to $90k | 25.3 | 11.3 | 24.1 | 20.6 | ||
| >$90k | 25.8 | 9.1 | 40.6 | 33.6 | ||
| Marital status, % | 0.000 | 0.008 | ||||
| Never married [reference] | 10.8 | 16.4 | 5.1 | 5.6 | ||
| Married | 70.0 | 42.8 | 81.1 | 73.0 | ||
| Divorced/separated/widowed | 19.2 | 40.8 | 13.9 | 21.5 | ||
| Health related characteristics | ||||||
| Family history of prostate cancerd, % | 6.1 | 11.0 | 0.010 | 7.5 | 12.1 | 0.017 |
| Diabetes, % | 30.6 | 29.6 | 0.751 | 17.8 | 16.4 | 0.582 |
| Smoking statuse, % | 0.000 | 0.032 | ||||
| Never [reference] | 38.0 | 28.9 | 42.0 | 39.9 | ||
| Former | 41.8 | 35.8 | 48.3 | 44.7 | ||
| Current | 20.3 | 35.3 | 9.7 | 15.4 | ||
| Aspirin user, % | 51.7 | 42.0 | 0.004 | 62.0 | 56.3 | 0.081 |
| Anthropometric measures | ||||||
| BMIf at enrollment, % | 0.006 | 0.030 | ||||
| 18.5 to <25.0 [reference] | 18.6 | 25.4 | 27.0 | 20.7 | ||
| 25.0 to 30.0 | 37.8 | 40.6 | 43.3 | 51.3 | ||
| 30.0 to 50.0 | 43.7 | 34.1 | 29.8 | 28.0 | ||
| BMIf 2 years prior to enrollment, % | 0.038 | 0.202 | ||||
| 18.5 to <25.0 [reference] | 19.2 | 24.1 | 25.3 | 21.5 | ||
| 25.0 to 30.0 | 38.0 | 41.0 | 43.6 | 49.2 | ||
| 30.0 to 50.0 | 42.8 | 34.8 | 31.1 | 29.3 | ||
| BMIf 10 years prior to enrollment, % | 0.002 | 0.288 | ||||
| 18.5 to <25.0 [reference] | 21.7 | 32.3 | 26.6 | 25.5 | ||
| 25.0 to 30.0 | 44.7 | 40.1 | 45.9 | 50.8 | ||
| 30.0 to 50.0 | 33.5 | 27.6 | 27.5 | 23.7 | ||
| Waist circumferenceg, cm | 0.000 | 0.000 | ||||
| 20.8 – 89.9 [reference] | 36.7 | 52.0 | 38.0 | 55.8 | ||
| 90 – 99.6 | 18.6 | 13.9 | 16.7 | 13.1 | ||
| 100 – 109.9 | 14.6 | 15.7 | 21.9 | 16.2 | ||
| 110 – 197.8 | 20.2 | 18.4 | 23.4 | 14.9 | ||
| Waist-hip ratioh, % | 0.840 | 0.950 | ||||
| < 0.92 [reference] | 39.9 | 38.3 | 26.4 | 25.5 | ||
| 0.92 to 0.98 | 33.8 | 35.6 | 34.5 | 35.1 | ||
| > 0.98 | 36.4 | 26.1 | 39.1 | 39.9 | ||
| Disease characteristics | ||||||
| PCa Stagei, % | NA | - | NA | - | ||
| T1 | 16.2 | 22.5 | ||||
| T2 | 71.6 | 62.6 | ||||
| T3 | 5.5 | 10.4 | ||||
| T4 | 6.7 | 4.6 | ||||
| PCa Gleason Score, % | NA | - | NA | - | ||
| 0 to 7 (low) | 82.6 | 82.3 | ||||
| 8 to 10 (high) | 17.4 | 17.7 | ||||
| Disease Aggressiveness, % | NA | - | NA | - | ||
| Non-aggressivej | 76.4 | 74.0 | ||||
| Aggressivek | 23.6 | 26.0 | ||||
| PSA, ng/ml, median (IQR) | NA | 6.96 (6.95) | - | NA | 6.04 (4.6) | - |
Cases recruited within 1 year after disease diagnosis with an average interval between diagnosis and enrollment of 4.8 months.
Chi-square analysis comparing cases vs controls.
IQR, interquartile range.
First degree relatives with prostate cancer.
Cigarette smoking.
BMI, body mass index (self-reported weight, calculated as weight in kilograms divided by height in meters squared).
Average waist circumference.
Waist-hip ratio was calculated by dividing average waist circumference by average hip
circumference. Categories based on tertile distribution of population controls.
Pathologically confirmed prostate cancer (PCa) using AJCC 7th edition.
Cases with pathologically confirmed T1 or T2 and Gleason score ≤7.
Cases with pathologically confirmed T3 or T4 or Gleason score >7.
On bivariate analysis, there were no significant differences in the waist-hip ratio measurements between cases and controls (Table 1). Proportionally more cases than controls had a waist circumference in the normal weight range (20.8 to 89.9) for both AA and EA men. In addition, more cases than controls had a BMI in the normal weight range (18.5 to < 25) among AA men while the opposite trend was seen among the EA men. At enrollment, 40.6% and 34.1% of AA cases reported to have a BMI in the overweight (25 to < 30) or obese (≥ 30) weight range, respectively; these proportions were different for controls (37.8% and 43.7%, respectively, P < 0.01). At ten years prior to enrollment into the study, 40.1% and 27.6% of AA cases reported that they had a BMI in the overweight or obese weight range, respectively, compared to controls (44.7% and 33.5%, respectively, P < 0.01). In a correlation analysis of anthropometric measures, we observed strong correlations among our three BMI measures (Spearman’s ρ: 0.77 to 0.91, P < 0.001), and moderate, albeit statistically significant (P < 0.001), correlations between BMI at enrollment and waist circumference (ρ = 0.45) or waist-hip ratio (ρ = 0.38). The relationship between waist circumference and waist-hip ratio was also rather modest (ρ = 0.37, P < 0.001). We did not note significant differences in these correlations by race/ethnicity.
Association of body mass index with prostate cancer
Among AA men, a BMI ≥ 30 at enrollment was inversely associated with prostate cancer risk in all grade (low-grade: odds ratio [OR], 0.59; 95% confidence interval [CI], 0.39–0.91; high-grade: OR, 0.45; 95% CI, 0.22–0.90), stage (low-stage: OR, 0.65; 95% CI, 0.43–0.98; high-stage: OR, 0.26; 95% CI, 0.12–0.59), and disease aggressiveness strata (non-aggressive: OR, 0.62; 95% CI, 0.40–0.96; aggressive: OR, 0.41; 95% CI, 0.22–0.78; Table 2 and Figure 1A). Similar associations were observed when we assessed BMI at two or ten years prior to enrollment (Tables 3 and 4). Within EA men, a BMI in the overweight range was significantly associated with increased odds of low-grade (OR, 1.52; 95% CI, 1.06–2.18), low-stage (OR, 1.44; 95% CI, 1.01–2.06), high-stage (OR, 2.14; 95% CI, 1.03–4.43), and aggressive (OR, 1.86; 95% CI, 1.06–3.26) prostate cancer relative to EA men with a normal weight range BMI (Table 2). At two or ten years prior to enrollment (Tables 3 and 4), there was no BMI-associated disease risk for EA men except for high-grade cancer which showed a positive association with a BMI in the obese weight range at ten years prior to enrollment (OR, 2.28; 95% CI, 1.03–5.04 for BMI ≥ 30 vs < 25; P for trend = 0.04).
Table 2.
Associations between BMI at enrollment and prostate cancer in African-American and European-American men
| Disease Categories | Casesb/Control | BMI At Enrollmenta | P for trende | ||
|---|---|---|---|---|---|
|
| |||||
| 18.5 to <25.0 | 25.0 to <30.0 | 30.0 to 50.0 | |||
|
|
|||||
| OR (95% CI)c,d | |||||
| Low-Grade Cancerf | |||||
| AA | 332/474 | 1 [Reference] | 0.91 (0.61 – 1.37) | 0.59 (0.39 – 0.91) | 0.009 |
| EA | 326/534 | 1 [Reference] | 1.52 (1.06 – 2.18) | 1.17 (0.78 – 1.77) | 0.530 |
| High-Grade Cancerg | |||||
| AA | 70/474 | 1 [Reference] | 0.77 (0.40 – 1.47) | 0.45 (0.22 – 0.90) | 0.022 |
| EA | 70/534 | 1 [Reference] | 1.66 (0.85 – 3.26) | 1.38 (0.64 – 2.97) | 0.457 |
|
| |||||
| Low-Stage Cancerh | |||||
| AA | 353/474 | 1 [Reference] | 0.99 (0.67 – 1.48) | 0.65 (0.43 – 0.98) | 0.020 |
| EA | 337/534 | 1 [Reference] | 1.44 (1.01 – 2.06) | 1.24 (0.83 – 1.85) | 0.358 |
| High-Stage Canceri | |||||
| AA | 49/474 | 1 [Reference] | 0.48 (0.23 – 1.00) | 0.26 (0.12 – 0.59) | 0.001 |
| EA | 59/534 | 1 [Reference] | 2.14 (1.03 – 4.43) | 0.98 (0.41 – 2.38) | 0.917 |
|
| |||||
| Non-Aggressive Cancerj | |||||
| AA | 307/474 | 1 [Reference] | 0.92 (0.61 – 1.40) | 0.62 (0.40 – 0.96) | 0.018 |
| EA | 293/534 | 1 [Reference] | 1.44 (0.99 – 2.10) | 1.21 (0.80 – 1.86) | 0.419 |
| Aggressive Cancerk | |||||
| AA | 95/474 | 1 [Reference] | 0.83 (0.47 – 1.48) | 0.41 (0.22 – 0.78) | 0.004 |
| EA | 103/534 | 1 [Reference] | 1.86 (1.06 – 3.26) | 1.15 (0.59 – 2.22) | 0.758 |
BMI, body mass index (self-reported weight, calculated as weight in kilograms divided by height in meters squared).
Cases recruited within 1 year after disease diagnosis with an average interval between diagnosis and enrollment of 4.8 months.
OR, odds ratio; CI, confidence interval.
Models adjusted for age at recruitment, education, diabetes, family history of prostate cancer, and smoking status.
P for trend tested with BMI as a continuous variable.
Gleason score ≤7.
Gleason score >7.
Cases pathologically confirmed using AJCC 7th edition T1 or T2.
Cases pathologically confirmed using AJCC 7th edition T3 or T4.
Cases with pathologically confirmed T1 or T2 and Gleason score ≤7.
Cases with pathologically confirmed T3 or T4 or Gleason score >7.
Figure 1. Summary odds ratios with 95% confidence intervals for the relationship between anthropometric measures at enrollment and prostate cancer with stratification by race/ethnicity.
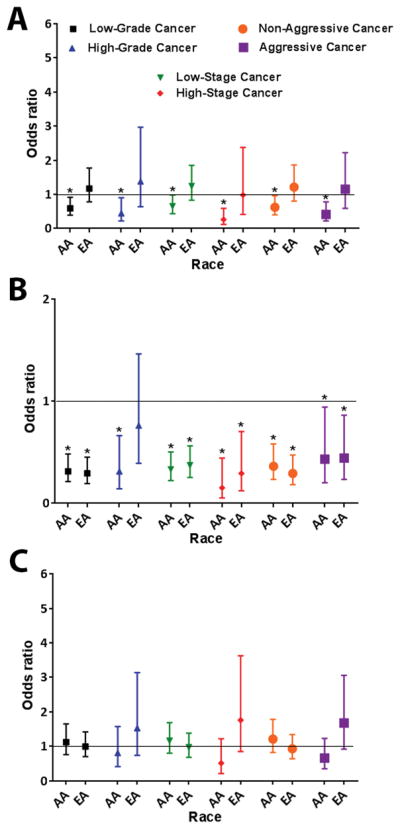
(A) Association of BMI at enrollment with prostate cancer. BMI ≥ 30 vs. < 25 (reference) (B) Association of waist circumference with prostate cancer. Waist circumference ≥ 110 vs. < 90 (reference) (C) Association of waist-hip ratio with prostate cancer. Waist-hip ratio > 0.98 vs. < 0.92 (reference). * Ptrend < 0.05
Table 3.
Associations between BMI at two years prior to enrollment and prostate cancer in African-American and European-American men
| Disease Categories | Casesb/Control | BMI 2 Years Prior To Enrollment | P for trende | ||
|---|---|---|---|---|---|
|
| |||||
| 18.5 to <25.0 | 25.0 to <30.0 | 30.0 to 50.0 | |||
|
|
|||||
| OR (95% CI)c,d | |||||
| Low-Grade Cancerf | |||||
| AA | 332/474 | 1 [Reference] | 1.09 (0.72 – 1.66) | 0.72 (0.47 – 1.12) | 0.071 |
| EA | 326/534 | 1 [Reference] | 1.34 (0.94 – 1.95) | 1.07 (0.71 – 1.62) | 0.829 |
| High-Grade Cancerg | |||||
| AA | 70/474 | 1 [Reference] | 0.81 (0.42 – 1.57) | 0.50 (0.25 – 1.00) | 0.046 |
| EA | 70/534 | 1 [Reference] | 1.21 (0.62 – 2.37) | 1.32 (0.63 – 2.75) | 0.470 |
|
| |||||
| Low-Stage Cancerh | |||||
| AA | 353/474 | 1 [Reference] | 1.08 (0.72 – 1.62) | 0.73 (0.48 – 1.11) | 0.076 |
| EA | 337/534 | 1 [Reference] | 1.33 (0.93 – 1.90) | 1.16 (0.78 – 1.74) | 0.527 |
| High-Stage Canceri | |||||
| AA | 49/474 | 1 [Reference] | 0.74 (0.34 – 1.59) | 0.38 (0.17 – 0.87) | 0.020 |
| EA | 59/534 | 1 [Reference] | 1.36 (0.68 – 2.70) | 0.85 (0.38 – 1.92) | 0.678 |
|
| |||||
| Non-Aggressive Cancerj | |||||
| AA | 307/474 | 1 [Reference] | 1.14 (0.75 – 1.74) | 0.76 (0.49 – 1.18) | 0.114 |
| EA | 293/534 | 1 [Reference] | 1.38 (0.95 – 2.02) | 1.15 (0.75 – 1.77) | 0.590 |
| Aggressive Cancerk | |||||
| AA | 95/474 | 1 [Reference] | 0.79 (0.44 – 1.41) | 0.46 (0.25 – 0.86) | 0.012 |
| EA | 103/534 | 1 [Reference] | 1.20 (0.70 – 2.08) | 1.01 (0.55 – 1.89) | 0.987 |
BMI, body mass index (self-reported weight, calculated as weight in kilograms divided by height in meters squared).
Cases recruited within 1 year after disease diagnosis with an average interval between diagnosis and enrollment of 4.8 months.
OR, odds ratio; CI, confidence interval.
Models adjusted for age at recruitment, education, diabetes, family history of prostate cancer, and smoking status.
P for trend tested with BMI as a continuous variable.
Gleason score ≤7.
Gleason score >7.
Cases pathologically confirmed using AJCC 7th edition T1 or T2.
Cases pathologically confirmed using AJCC 7th edition T3 or T4.
Cases with pathologically confirmed T1 or T2 and Gleason score ≤7.
Cases with pathologically confirmed T3 or T4 or Gleason score >7.
Table 4.
Associations between BMI at ten years prior to enrollment and prostate cancer in African-American and European-American men
| Disease Categories | Casesb/Control | BMI 10 Years Prior To Enrollmenta | P for trende | ||
|---|---|---|---|---|---|
|
| |||||
| 18.5 to <25.0 | 25.0 to <30.0 | 30.0 to 50.0 | |||
| OR (95% CI)c,d | |||||
| Low-Grade Cancerf | |||||
| AA | 332/474 | 1 [Reference] | 0.69 (0.47 – 1.00) | 0.53 (0.35 – 0.80) | 0.002 |
| EA | 326/534 | 1 [Reference] | 1.13 (0.80 – 1.58) | 0.77 (0.50 – 1.18) | 0.276 |
| High-Grade Cancerg | |||||
| AA | 70/474 | 1 [Reference] | 0.82 (0.42 – 1.58) | 0.67 (0.33 – 1.35) | 0.261 |
| EA | 70/534 | 1 [Reference] | 1.64 (0.80 – 3.36) | 2.28 (1.03 – 5.04) | 0.040 |
|
| |||||
| Low-Stage Cancerh | |||||
| AA | 353/474 | 1 [Reference] | 0.72 (0.50 – 1.04) | 0.59 (0.39 – 0.89) | 0.012 |
| EA | 337/534 | 1 [Reference] | 1.20 (0.85 – 1.69) | 1.04 (0.68 – 1.57) | 0.844 |
| High-Stage Canceri | |||||
| AA | 49/474 | 1 [Reference] | 0.60 (0.29 – 1.23) | 0.32 (0.14 – 0.76) | 0.009 |
| EA | 59/534 | 1 [Reference] | 1.09 (0.57 – 2.07) | 0.48 (.20 – 1.19) | 0.165 |
|
| |||||
| Non-Aggressive Cancerj | |||||
| AA | 307/474 | 1 [Reference] | 0.70 (0.48 – 1.03) | 0.54 (0.36 – 0.83) | 0.005 |
| EA | 293/534 | 1 [Reference] | 1.17 (0.82 – 1.67) | 0.89 (0.58 – 1.38) | 0.664 |
| Aggressive Cancerk | |||||
| AA | 95/474 | 1 [Reference] | 0.68 (0.39 – 1.20) | 0.53 (0.28 – 0.98) | 0.045 |
| EA | 103/534 | 1 [Reference] | 1.22 (0.72 – 2.10) | 1.05 (0.55 – 2.02) | 0.852 |
BMI, body mass index (self-reported weight, calculated as weight in kilograms divided by height in meters squared).
Cases recruited within 1 year after disease diagnosis with an average interval between diagnosis and enrollment of 4.8 months.
OR, odds ratio; CI, confidence interval.
Models adjusted for age at recruitment, education, diabetes, family history of prostate cancer, and smoking status.
P for trend tested with BMI as a continuous variable.
Gleason score ≤7.
Gleason score >7.
Cases pathologically confirmed using AJCC 7th edition T1 or T2.
Cases pathologically confirmed using AJCC 7th edition T3 or T4.
Cases with pathologically confirmed T1 or T2 and Gleason score ≤7.
Cases with pathologically confirmed T3 or T4 or Gleason score >7.
Association of waist circumference and waist-hip ratio with prostate cancer
For both AA and EA men, a larger waist circumference at enrollment (≥ 110) was inversely associated with prostate cancer risk when a waist circumference of < 90 cm was used as the reference group (Table 5 and Figure 1B). Controlling for BMI in the analysis did not have a large effect on these findings. For example, among AA men, having a waist circumference ≥ 110 cm significantly decreased the odds of low-grade (OR, 0.36; 95% CI, 0.23–0.47), low-stage (OR, 0.38; 95% CI, 0.24–0.60), high-stage (OR, 0.27; 95% CI, 0.08–0.88), non-aggressive (OR, 0.36; 95% CI, 0.23–0.58), and aggressive cancer (OR, 0.43; 95% CI, 0.20–0.94), even after controlling for BMI (Supplementary Table 1). In contrast to the protective effect of a high waist circumference, an increased waist-hip ratio at enrollment was not significantly associated with odds of prostate cancer among AA and EA men (Supplementary Table 2 and Figure 1C). For EA men, having a waist-hip ratio > 0.98 tended to increase the odds of a high-grade (OR, 1.53; 95% CI, 0.74–3.14), high-stage (OR, 1.76; 95% CI, 0.85–3.63), and aggressive (OR, 1.68; 95% CI, 0.92–3.06) disease (Supplementary Table 2 and Figure 1C).
Table 5.
Associations between waist circumference and prostate cancer in African-American and European-American men
| Disease Categories | Casesb/Control | Waist circumferencea, cm | P for trende | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 20.8 – 89.9 | 90 – 99.6 | 100 – 109.9 | 110 – 197.8 | |||
| OR (95% CI)c,d | ||||||
| Low-Grade Cancerf | ||||||
| AA | 332/474 | 1 [Reference] | 0.42 (0.27 – 0.66) | 0.67 (0.43 – 1.06) | 0.31 (0.21 – 0.48) | 0.000 |
| EA | 326/534 | 1 [Reference] | 0.42 (0.27 – 0.65) | 0.39 (0.26 – 0.58) | 0.29 (0.19 – 0.45) | 0.000 |
| High-Grade Cancerg | ||||||
| AA | 70/474 | 1 [Reference] | 0.43 (0.20 – 0.95) | 0.61 (0.27 – 1.37) | 0.31 (0.14 – 0.66) | 0.003 |
| EA | 70/534 | 1 [Reference] | 0.49 (0.20 – 1.18) | 0.52 (0.25 – 1.08) | 0.76 (0.39 – 1.46) | 0.257 |
|
| ||||||
| Low-Stage Cancerh | ||||||
| AA | 353/474 | 1 [Reference] | 0.45 (0.29 – 0.69) | 0.68 (0.44 – 1.06) | 0.33 (0.22 – 0.50) | 0.000 |
| EA | 337/534 | 1 [Reference] | 0.40 (0.26 – 0.62) | 0.38 (0.25 – 0.57) | 0.37 (0.25 – 0.56) | 0.000 |
| High-Stage Canceri | ||||||
| AA | 49/474 | 1 [Reference] | 0.29 (0.11 – 0.76) | 0.62 (0.26 – 1.49) | 0.15 (0.05 – 0.44) | 0.001 |
| EA | 59/534 | 1 [Reference] | 0.61 (0.28 – 1.33) | 0.57 (0.27 – 1.18) | 0.29 (0.12 – 0.70) | 0.005 |
|
| ||||||
| Non-Aggressive Cancerj | ||||||
| AA | 307/474 | 1 [Reference] | 0.42 (0.26 – 0.66) | 0.67 (0.42 – 1.06) | 0.32 (0.21 – 0.50) | 0.000 |
| EA | 293/534 | 1 [Reference] | 0.41 (0.26 – 0.65) | 0.39 (0.26 – 0.60) | 0.33 (0.21 – 0.51) | 0.000 |
| Aggressive Cancerk | ||||||
| AA | 95/474 | 1 [Reference] | 0.44 (0.22 – 0.87) | 0.70 (0.35 – 1.39) | 0.28 (0.14 – 0.56) | 0.001 |
| EA | 103/534 | 1 [Reference] | 0.48 (0.24 – 0.94) | 0.47 (0.25 – 0.86) | 0.47 (0.26 – 0.85) | 0.004 |
Waist circumference measured twice at enrollment, average score used. Categories based on Arden et al. 2014.
Cases recruited within 1 year after disease diagnosis with an average interval between diagnosis and enrollment of 4.8 months.
OR, odds ratio; CI, confidence interval.
Models adjusted for age at recruitment, education, diabetes, family history of prostate cancer, and smoking status.
P for trend tested with BMI as a continuous variable.
Gleason score ≤7.
Gleason score >7.
Cases pathologically confirmed using AJCC 7th edition T1 or T2.
Cases pathologically confirmed using AJCC 7th edition T3 or T4.
Cases with pathologically confirmed T1 or T2 and Gleason score ≤7.
Cases with pathologically confirmed T3 or T4 or Gleason score >7.
Effect of aspirin use on the relationship between anthropometric measures and prostate cancer in AA men
The results for this sensitivity analysis are shown in Supplementary Table 3, wherein aspirin use modified the relationships between BMI and prostate cancer, but not the relationship between waist circumference and the disease. The aspirin effect was most obvious when a BMI prior to disease diagnosis was assessed (two and ten years prior to enrollment of the cases). For example, in the non-stratified analysis for BMI at ten years prior to enrollment, a BMI in the overweight and obese weight range were both associated with decreased odds of prostate cancer risk compared to a normal weight range BMI (OR, 0.68; 95% CI, 0.48–0.97 and 0.54; 95% CI, 0.37–0.81, respectively). After stratification, this association remained significant only among non-users of aspirin (OR, 0.39; 95% CI, 0.24–0.63 and 0.46; 95% CI, 0.27–0.81, respectively), but not for aspirin users (OR, 1.61; 95% CI, 0.92–2.82 and 0.81; 95% CI, 0.45–1.46, respectively). A test for interaction revealed significant interactions between aspirin and BMI among overweight, but not obese men, when a BMI prior to disease diagnosis was assessed (Pinteraction < 0.01).
Discussion
In this study, we found inverse relationships between two anthropometric measures, BMI and waist circumference, with prostate cancer in AA men that were consistent across strata and time points. Waist-hip ratio did not associate with the disease in these men. The inverse relationship between waist circumference and prostate cancer among these men was also observed when we used WHO guidelines to select health-related cut-off points for waist circumference (Supplementary Table 4). In contrast, a high BMI and waist-hip ratio tended to increase the risk of advanced/aggressive prostate cancer among EA men, although less consistently than the inverse associations in AA men. The positive relationship between an elevated BMI and waist-hip ratio and advanced/aggressive disease in the EA men in our study is consistent with other reports (23, 32, 39, 40). There is also supporting evidence from a meta-analysis that a high BMI increases prostate cancer-specific mortality among these men (25).
Here, we aimed to explore potential differences in the association of body fatness with prostate cancer between AA and EA men. Waist circumference and waist-hip ratio are measures for central body fatness while BMI is a proxy for overall body fatness. The International Agency for Research on Cancer working group on the relationship of body fatness with cancer reported that observations based on BMI were generally consistent with those reported for waist circumference (41). We hypothesized that excess body fatness, measured as BMI, waist circumference, or waist-hip ratio, may partly explain the excessive risk of prostate cancer among AA men. However, in our cohort, AA men with a high BMI and waist circumference were significantly less likely to have prostate cancer than men with body fatness in the normal range. The relationship between BMI and the disease did not change whether we used BMI at time of recruitment or BMI ten years prior to it in our analysis. In two case-control studies of Caribbean men, mostly of African descent, BMI was not a risk factor for prostate cancer (31, 33). Nevertheless, a high waist-hip ratio was positively associated with prostate cancer in both studies, which contrasts with our findings for AA men. Nemesure et al. (33) also found a positive association for waist circumference and concluded that central adiposity may be more predictive of prostate cancer than BMI in men of African descent. From our data, we would not come to the same conclusion. The discrepancies in the observations between our study and the study by Nemesure et al. with African-Barbadian men cannot readily be explained. Few differences in the data analysis approach exist between the two studies. We stratified the waist-hip ratio into tertiles whereas Nemesure et al. used quartiles in their analysis. In addition, the definition of normal range and quartiles for waist circumference was somewhat different between the two studies. Average body mass was higher in our AA population than the African-Barbadian population with mean BMIs of 27.7 versus 25.8 among cases and 29.8 versus 26.0 among the controls. Yet, both are case-control studies of comparable size and used incident cases identified from hospital records and population-based controls. Furthermore, both have a similar age distribution, smoking prevalence, and diabetes history among cases and controls. The two studies also obtained the measurements for waist-hip ratio and waist circumference at time of enrollment using trained personnel, but our study had additional information on BMI for two and ten years prior to recruitment.
Several other studies investigated differences in the association of obesity with prostate cancer risks comparing AA and EA men in the US. Su et al. observed a stronger association of BMI and waist-hip ratio with aggressive prostate cancer in EA than AA men for participants in the North Carolina-Louisiana Prostate Cancer project, a state cancer registry-based study (32). Because AA had more aggressive disease in general, the authors hypothesized that the association between obesity and aggressiveness may not have been as evident in the AA men. Using the same study population, Khan et al. also reported an association of obesity, independent of diabetes, with aggressive disease in EA men, but not AA men (35). Barrington et al. observed a significant association of BMI with prostate cancer and high-grade disease for AA men but not EA men in the Selenium and Vitamin E Cancer Prevention Trial (34). Like Khan et al., this study assessed only BMI as a measure of body fatness. Lastly, analyzing 308 radiotherapy-treated patients, Allott et al. found significant associations for both BMI and waist circumference with high-grade disease among severely obese AA patients (42). In summary, these investigations tended to find an association of increased body fatness with advanced or high-grade prostate cancer in AA men, which contrasts with findings in the present study with unselected patients.
The biological mechanisms through which excess body fatness could influence prostate cancer incidence are complex, potentially explaining differences in findings among observational studies. A gene expression profiling study described effects of obesity on chromatin modifications in prostate tumors (43). If epigenetic differences exist in prostate tumors among patient groups at baseline, as they have been described (44), obesity may have different effects on tumor biology in these patient groups, e.g. AA versus EA men. Others described a significant modifying effect of the TMPRSS2:ERG fusion gene status on the relationship between obesity and aggressive prostate cancer (45). For patients with oncogenic TMPRSS2:ERG fusion gene re-arrangements in their tumors (ERG-positive), obesity increased the risk of lethal prostate cancer while ERG-negative patients did not experience this increased risk associated with obesity. Notably, ERG-negative tumors are more common among AA patients than EA patients (46–48), potentially leading to a reduced risk of lethal disease among obese AA men when compared to EA men. Unfortunately, the ERG tumor status could not be assessed in our study. Lastly, group differences in susceptibility to inflammation have been described, with an increased susceptibility to inflammation among subjects of African ancestry (49). For prostate cancer, a high baseline inflammation based on histologic evaluation of infiltrating leukocytes in the non-cancerous prostate has been linked to a reduced risk of prostate cancer (50). It is possible that AA men in the NCI-Maryland cohort may have had increased baseline inflammation in the non-cancerous prostate that was intensified by excess body fatness, leading to a decreased risk of prostate cancer in this group of patients. This hypothesis is partly supported by our observation that regular aspirin use attenuated the inverse relationship between BMI and prostate cancer in the AA men.
Our study has limitations. In general, a case-control study is potentially influenced by inherent biases. We relied on retrospective, self-reported assessment of height and weight to calculate BMI, which may be affected by a recall bias. However, waist circumference and waist-hip ratio were measured by trained personnel and would not be affected by a recall bias. Moreover, self-reported anthropometric measures were found to be reliable and valid (51). On the other hand, cases were more likely to be current smokers but less likely to have a college or graduate degree than controls. These differences between cases and controls were observed among both AA and EA men. We controlled for these differences in our analysis. As a strength of our study, our estimates for BMI prior to enrollment remained consistent with the findings for BMI at enrollment, diminishing the potential of temporal bias in our analysis.
In summary, we found associations of anthropometric measures with prostate cancer among EA men that were generally consistent with the reported literature. However, we observed an inverse association among AA men, where a high BMI and waist circumference associated with a lower disease occurrence.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute (NCI), Center for Cancer Research (ZIA BC 010499 and ZIA BC 010624).
We would like to thank personnel at the University of Maryland and the Baltimore Veterans Administration Hospital for their contributions with the recruitment of subjects. We would also like to thank Dr. Brid Ryan for critical review of the manuscript.
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- 1.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444–9. doi: 10.1016/j.juro.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Haas GP. Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol. 2014;21:7496–506. [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 4.Jones BA, Liu WL, Araujo AB, Kasl SV, Silvera SN, Soler-Vila H, et al. Explaining the race difference in prostate cancer stage at diagnosis. Cancer Epidemiol Biomarkers Prev. 2008;17:2825–34. doi: 10.1158/1055-9965.EPI-08-0203. [DOI] [PubMed] [Google Scholar]
- 5.Rapiti E, Fioretta G, Schaffar R, Neyroud-Caspar I, Verkooijen HM, Schmidlin F, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009;115:5556–65. doi: 10.1002/cncr.24607. [DOI] [PubMed] [Google Scholar]
- 6.Fedewa SA, Etzioni R, Flanders WD, Jemal A, Ward EM. Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004–2006. Cancer Epidemiol Biomarkers Prev. 2010;19:2437–44. doi: 10.1158/1055-9965.EPI-10-0299. [DOI] [PubMed] [Google Scholar]
- 7.Tewari A, Horninger W, Badani KK, Hasan M, Coon S, Crawford ED, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2005;96:29–33. doi: 10.1111/j.1464-410X.2005.05561.x. [DOI] [PubMed] [Google Scholar]
- 8.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–6. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011;32:1107–21. doi: 10.1093/carcin/bgr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin DN, Starks AM, Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25:235–41. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci USA. 2006;103:14068–73. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins C, Torres JB, Hooker S, Bonilla C, Hernandez W, Candreva A, et al. Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res. 2007;17:1717–22. doi: 10.1101/gr.6782707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–7. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maringe C, Mangtani P, Rachet B, Leon DA, Coleman MP, dos Santos Silva I. Cancer incidence in South Asian migrants to England, 1986–2004: unraveling ethnic from socioeconomic differentials. Int J Cancer. 2013;132:1886–94. doi: 10.1002/ijc.27826. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 17.Hsing AW, Devesa SS. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 18.Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, et al. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur J Cancer. 2016;69:61–9. doi: 10.1016/j.ejca.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75:405–19. doi: 10.1093/nutrit/nux012. [DOI] [PubMed] [Google Scholar]
- 20.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Ishiguro H, Kobayashi N, Hasumi H, Watanabe M, Yao M, et al. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. The Prostate. 2015;75:1009–19. doi: 10.1002/pros.22972. [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 23.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 24.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 27.Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Jr, Freedland SJ. Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol Biomarkers Prev. 2014;23:2936–42. doi: 10.1158/1055-9965.EPI-14-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal AC, Howard LE, Sun SX, Cooperberg MR, Kane CJ, Aronson WJ, et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis. 2017;20:72–8. doi: 10.1038/pcan.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;178:1939–44. doi: 10.1016/j.juro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Jayachandran J, Banez LL, Aronson WJ, Terris MK, Presti JC, Jr, Amling CL, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115:5263–71. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson MD, Walker SP, Simpson CM, McFarlane-Anderson N, Bennett FI, Coard KC, et al. Body size and risk of prostate cancer in Jamaican men. Cancer Causes Control. 2010;21:909–17. doi: 10.1007/s10552-010-9520-y. [DOI] [PubMed] [Google Scholar]
- 32.Su LJ, Arab L, Steck SE, Fontham ET, Schroeder JC, Bensen JT, et al. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol Biomarkers Prev. 2011;20:844–53. doi: 10.1158/1055-9965.EPI-10-0684. [DOI] [PubMed] [Google Scholar]
- 33.Nemesure B, Wu SY, Hennis A, Leske MC Prostate Cancer in a Black Population Study G. Central adiposity and Prostate Cancer in a Black Population. Cancer Epidemiol Biomarkers Prev. 2012;21:851–8. doi: 10.1158/1055-9965.EPI-12-0071. [DOI] [PubMed] [Google Scholar]
- 34.Barrington WE, Schenk JM, Etzioni R, Arnold KB, Neuhouser ML, Thompson IM, Jr, et al. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA Oncol. 2015;1:342–9. doi: 10.1001/jamaoncol.2015.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan S, Cai J, Nielsen ME, Troester MA, Mohler JL, Fontham ET, et al. The association of diabetes and obesity with prostate cancer aggressiveness among Black Americans and White Americans in a population-based study. Cancer Causes Control. 2016;27:1475–85. doi: 10.1007/s10552-016-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith CJ, Dorsey TH, Tang W, Jordan SV, Loffredo CA, Ambs S. Aspirin Use Reduces the Risk of Aggressive Prostate Cancer and Disease Recurrence in African-American Men. Cancer Epidemiol Biomarkers Prev. 2017;26:845–53. doi: 10.1158/1055-9965.EPI-16-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obes Res. 2004;12:1094–103. doi: 10.1038/oby.2004.137. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Report of a WHO Expert Consultation. Geneva: Dec 8–11, 2008. Waist circumference and waist-hip ratio; pp. 1–34. [Google Scholar]
- 39.Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–61. doi: 10.1158/1055-9965.EPI-08-0609. [DOI] [PubMed] [Google Scholar]
- 40.Boehm K, Sun M, Larcher A, Blanc-Lapierre A, Schiffmann J, Graefen M, et al. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: results from the North-American case-control study Prostate Cancer & Environment Study. Urol Oncol. 2015;33:494e1–7. doi: 10.1016/j.urolonc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allott EH, Howard LE, Song HJ, Sourbeer KN, Koontz BF, Salama JK, et al. Racial differences in adipose tissue distribution and risk of aggressive prostate cancer among men undergoing radiotherapy. Cancer Epidemiol Biomarkers Prev. 2014;23:2404–12. doi: 10.1158/1055-9965.EPI-14-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebot EM, Gerke T, Labbe DP, Sinnott JA, Zadra G, Rider JR, et al. Gene expression profiling of prostate tissue identifies chromatin regulation as a potential link between obesity and lethal prostate cancer. Cancer. 2017;123:4130–8. doi: 10.1002/cncr.30831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CJ, Minas TZ, Ambs S. Analysis of Tumor Biology to Advance Cancer Health Disparity Research. Am J Pathol. 2018;188:304–16. doi: 10.1016/j.ajpath.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, et al. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst. 2013;105:1881–90. doi: 10.1093/jnci/djt332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khani F, Mosquera JM, Park K, Blattner M, O’Reilly C, MacDonald TY, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20:4925–34. doi: 10.1158/1078-0432.CCR-13-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faisal FA, Sundi D, Tosoian JJ, Choeurng V, Alshalalfa M, Ross AE, et al. Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur Urol. 2016;70:14–7. doi: 10.1016/j.eururo.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen P, Pfister D, Young D, Petrovics G, Chen Y, Cullen J, et al. Differences in frequency of ERG oncoprotein expression between index tumors of Caucasian and African American patients with prostate cancer. Urology. 2012;80:749–53. doi: 10.1016/j.urology.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pennington R, Gatenbee C, Kennedy B, Harpending H, Cochran G. Group differences in proneness to inflammation. Infect Genet Evol. 2009;9:1371–80. doi: 10.1016/j.meegid.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Moreira DM, Nickel JC, Gerber L, Muller RL, Andriole GL, Castro-Santamaria R, et al. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: results from the REDUCE study. Cancer. 2014;120:190–6. doi: 10.1002/cncr.28349. [DOI] [PubMed] [Google Scholar]
- 51.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


