Abstract
Objective
Autonomic dysfunction is a known complication of scleroderma that can affect vascular tone, gastrointestinal (GI) motility, heart rate and blood pressure control. We sought to quantify autonomic symptom burden in scleroderma, and to define the characteristics of patients with scleroderma and autonomic dysfunction.
Methods
Patients with scleroderma were consecutively recruited during routine clinical visits at the Johns Hopkins Scleroderma Center and asked to complete the Composite Autonomic Symptom Score (COMPASS)-31 questionnaire, which is a validated tool to assess symptoms of autonomic dysfunction. We determined the relationship between various clinical and serological features of scleroderma and the total COMPASS-31 scores and domain-specific scores using the student’s t-test or Wilcoxon rank-sum test for dichotomous variables and linear regression analysis for continuous variables.
Results
104 patients with scleroderma completed the COMPASS-31 questionnaire. The mean COMPASS-31 score in this cohort was 24.9 ± 15.5, which is higher than COMPASS-31 scores from previously published healthy controls (8.9 ± 8.7). Compared to patients with mild or absent GI disease, patients with significant GI disease had higher scores across multiple subdomains of the COMPASS-31, including orthostatic intolerance (median 10.0 vs 0, p=0.006) and secretomotor dysfunction (median 6.4 vs 4.3, p=0.03). There was also a dose-response relationship between GI disease severity and autonomic symptom burden.
Conclusions
Symptoms of autonomic dysfunction are common in scleroderma. Patients with more severe GI disease in scleroderma report more symptoms of dysautonomia across multiple facets of the autonomic nervous system.
Keywords: gastrointestinal tract, scleroderma, neurologic manifestations, self-assessment, scleroderma, autonomic dysfunction
INTRODUCTION
Scleroderma is an autoimmune multi-organ disease associated with significant morbidity due to organ dysfunction (1). Pathological studies demonstrate microvascular disease and/or tissue fibrosis involving the skin, lungs, heart, kidney, gastrointestinal (GI) tract, and muscles (2,3). Dysregulation of the autonomic nervous system (dysautonomia) can occur early or late in the disease (4–6). In some cases, dysautonomia results in abnormal vascular tone (7,8), GI dysmotility (9) (10), and dysregulation of heart rate and blood pressure (11).
The mechanisms underlying autonomic dysfunction in scleroderma remain unclear but could be caused by autoimmune damage to nerves, vascular disease, or direct nerve compression from tissue fibrosis (12). Tissue fibrosis is unlikely to be the only cause of autonomic dysfunction in scleroderma, however, because degeneration of the autonomic neural network and ganglion root cells have been observed in areas of unaffected skin (12–14). Autonomic dysfunction is often seen early in the disease course, suggesting that it may play an important pathological role in scleroderma.
Although dysautonomia is recognized to occur in a subset of patients with scleroderma, the prevalence of symptoms related to autonomic dysfunction and the clinical features of scleroderma associated with dysautonomia remain undefined. Understanding the disease features of scleroderma that are associated with dysautonomia may provide important insights into the underlying disease mechanism, and may also improve recognition and diagnosis of this complication in clinical practice.
The Composite Autonomic Symptom Score (COMPASS)-31 is a self-administered 31-item questionnaire that quantifies self-reported autonomic symptoms (15). It is an abbreviated version of the 164-item COMPASS assessment tool (16). The COMPASS-31 was validated in a cohort of patients with small fiber neuropathy (17), and was previously used to quantify self-reported autonomic symptoms in multiple sclerosis (18), diabetic neuropathy (19), and fibromyalgia (20). In the present study, we used the COMPASS-31 to quantify the frequency and features of autonomic symptoms in a cohort of patients with scleroderma.
PATIENTS AND METHODS
Study Population
All patients were seen for routine clinical evaluations at the Johns Hopkins Scleroderma Center between March 2016 and April 2016. Patients were consecutively recruited after their clinical visits if they met either the 2013 ACR/EULAR criteria for scleroderma, the 1980 American College of Rheumatology (ACR) criteria, or had at least three of five features of the CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias). Patients were categorized by extent of skin disease as limited or diffuse scleroderma based on previously established criteria (21)
Healthy Control Population
We compared COMPASS-31 scores in our scleroderma cohort to scores in healthy controls and other populations with autonomic dysfunction in the published literature (17,19,20). The healthy control cohort consisted of 30 female participants who were healthy and free of pain and fatigue (see Vincent et al. (20) for cohort details).
Assessment Tool: COMPASS-31 questionnaire
The COMPASS-31 consists of 31 questions that fall into six domains of dysautonomia: orthostatic intolerance (four items), vasomotor dysfunction (three items), secretomotor dysfunction (four items), gastrointestinal dysfunction (twelve items; includes gastroparesis, constipation and diarrhea), urinary dysfunction (three items), and pupillomotor dysfunction (five items). An answer was scored as zero when it was not assigned a point. A raw domain score was obtained by adding points within each domain together. The total score within each domain was weighted as previously described (15) and then added together to give a total score ranging from 0–100. The maximum weighted scores for each subdomain are as follows: 40 for orthostatic intolerance, 5 for vasomotor dysfunction, 15 for secretomotor dysfunction, 25 for gastrointestinal dysfunction, 10 for urinary dysfunction, and 5 for pupillomotor dysfunction.
Clinical Data Collection
Demographic and clinical data for all study participants were obtained from the Johns Hopkins Scleroderma Center longitudinal database. The Center’s database includes date of birth, sex, race, date of onset of first Raynaud’s and first non-Raynaud’s symptoms, medications, and multiple measures of disease severity and clinical features (see below) obtained at the first clinic visit and at 6 month intervals during follow-up visits. Medications that the patient was taking at the time of completion of the COMPASS-31 questionnaire were used for analysis. Of particular interest was the use of a blood pressure medication or vasodilator, as these medications can cause orthostatic symptoms as a side-effect. We also recorded the patient heart rate by taking the average of the heart rate at the time the COMPASS-31 questionnaire was completed with the heart rates measured at the immediate prior and subsequent clinical visit.
The presence and severity of organ involvement were assessed using the maximum Medsger severity score (22) recorded across all longitudinal visits. Because clinical features of scleroderma are known to change over time, the most extreme data points recorded (max/min, ever/never) were used to fully capture disease manifestations. The degree of skin involvement was scored using the maximum modified Rodnan skin thickness score (MRSS = [range 0–51]). Significant GI disease was defined by a Medsger severity score ≥ 2 (0 = no symptoms; 1 = acid reflux medications or abnormal small bowel series; 2 = high-dose acid reflux medications or antibiotics for bacterial overgrowth; 3 = diagnosis of malabsorptive syndrome or episodes of pseudo-obstruction; 4= dependence on total parenteral nutrition). Severe GI disease was defined as a Medsger severity score of 3 or 4. Significant cardiac disease was defined by a Medsger score ≥ 1, and significant lung disease and Raynaud’s phenomenon were defined by a Medsger score ≥ 2. The presence of myopathy was defined by the presence of muscle weakness (Medsger score ≥ 1 defined by power ≤ 4/5 in the upper or lower extremities) as well as the presence of at least one of the following: elevation in creatinine phosphokinase (CPK), abnormal electromyomyography (EMG), muscle edema visualized on magnetic resonance imaging (MRI), or myopathy confirmed on muscle biopsy. The presence of arthralgia, arthritis, dry eyes or dry mouth, or history of scleroderma renal crisis, digital ulceration/gangrene, and right heart catheterization-confirmed pulmonary arterial hypertension (defined as a mean pulmonary artery pressure ≥ 25 mmHg and pulmonary capillary wedge pressure ≤ 15 mmHg) were also recorded.
Written informed consent was obtained from all patients. This study was approved by the Johns Hopkins Institutional Review Board (approval number 00034985).
Autoantibody Analysis
A commercially available line immunoblot assay (Scleroderma [Nucleoli] Profile Euroline [IgG]; Euroimmun) was used to determine the scleroderma autoantibody profiles for patients with available serum. Assays and analysis were performed by a single operator per the manufacturer’s instructions as previously described (23). Readings defined by the assay as positive or negative were considered as such, and results in the borderline range were considered negative.
Statistical Analysis
COMPASS-31 scores were compared across dichotomous clinical and demographic variables using the student’s t-test for scores that were normally distributed (total COMPASS-31 score, vasomotor dysfunction, gastrointestinal dysfunction, pupillomotor dysfunction) and the Wilcoxon rank-sum test for scores that were not normally distributed (orthostatic intolerance, secretomotor dysfunction and urinary dysfunction). Results are depicted as mean ± standard deviation (SD) or median (interquartile range, IQR). Linear regression analyses were used to determine the relationship between continuous clinical independent variables and COMPASS-31 scores (dependent variable). Multi-variable linear regression models were used to adjust for possible covariates, including age, sex, race, and any variables that are associated with the COMPASS-31 score.
To determine whether the associations between disease features and severity and the total COMPASS-31 score resulted from global autonomic symptoms across multiple sub-domains, we grouped patients by the number of positive subdomains (score > 0 in any category) of the COMPASS-31. We divided the cohort into two groups based on the graphical distribution of the data prior to the analysis: (1) the “global autonomic symptoms” group included all patients with five or more positive subdomains, and (2) the “limited autonomic symptoms” group included patients with fewer than five positive subdomains. Using a χ2 analysis, we determined the relationship between the presence of specific clinical features of scleroderma and global autonomic dysfunction. Multivariable logistic regression models were used to adjust for clinically relevant covariates and potential confounders.
All statistical analyses were conducted using JMP Version 9 (SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and statistical significance was defined as p<0.05 for all analyses.
RESULTS
Patient Characteristics
104 consecutive patients with scleroderma were recruited and completed the COMPASS-31 questionnaire. Demographic information for all study participants is shown in table 1. The mean age was 57 ± SD 12.6 years, 88% of the cohort was female, 74% were Caucasian and 16% were African American. The median disease duration from the onset of Raynaud’s phenomenon was 12.5 (IQR 7–22) years and the median disease duration from the onset of a non-Raynaud’s symptom of scleroderma was 11.0 (IQR 7–18) years. The majority of patients (54%) had limited cutaneous disease and 46% had diffuse cutaneous disease.
Table 1. Demographics and disease characteristics of patients with scleroderma.
Significant Raynaud’s phenomenon (RP), lung disease, and GI disease were defined as a Medsger severity score ≥ 2. Significant heart disease was defined as a Medsger severity score ≥ 1. Right heart catheterization data were available for 20 patients. Pulmonary artery hypertension was defined as a mean pulmonary artery pressure ≥ 25 mmHg and a pulmonary capillary wedge pressure ≤ 15 mmHg. RVSP = right ventricular systolic pressure; FVC = forced vital capacity; DLCO = diffusing capacity of carbon monoxide. SD = standard deviation; IQR = interquartile range
| Age | Age (years), mean (SD) | 57 (12.6) |
| Sex | ||
| Female, n [%] | 92 [88%] | |
| Male, n [%] | 12 [12%] | |
| Race | ||
| Caucasian, n [%] | 77 [74%] | |
| African American, n [%] | 17 [16%] | |
| Scleroderma Type | ||
| Limited, n [%] | 56 [54%] | |
| Diffuse, n [%] | 48 [46%] | |
| Disease duration | ||
| From onset of RP, median (IQR) | 12.5 (7–22) | |
| From onset of non-RP symptom, median (IQR) | 11.0 (7–18) | |
| Number with disease duration < 3 years (from RP) | 12 [12%] | |
| Number with disease duration < 5 years (from RP) | 18 [17%] | |
| Antibody status | ||
| Anti-Centromere | 21/83 [25%) | |
| Anti-Topoisomerase-1 | 31/83 [37%] | |
| Anti-RNA-polymerase III | 19/83 [23%] | |
| Anti-PM-Scl | 12/83 [15%] | |
| Anti-U3-RNP | 8/83 [10%] | |
| Anti-Ro-52 | 19/83 [23%] | |
| Anti-ThTo | 6/83 [7%] | |
| Anti-Ku | 4/83 [5%] | |
| Clinical Features | ||
| Calcinosis, n [%] | 51 [49%] | |
| Arthralgias, n [%] | 92 [88%] | |
| Synovitis, n [%] | 25 [24%] | |
| Digital ulceration or gangrene, n [%] | 27 [26%] | |
| Scleroderma renal crisis, n [%] | 4 [4%] | |
| Myopathy, n [%] | 16 [15%] | |
| Pulmonary arterial hypertension, n [%] | 7 [7%] | |
| Maximum RVSP (mmHg), mean (SD) | 39.6 (12.2) | |
| Modified Rodnan Skin Score, mean (SD) | 13.8 (12.0) | |
| Minimum FVC, mean (SD) | 72.7 (21.1) | |
| Minimum DLCO, mean (SD) | 65.3 (23.7) | |
| Significant Raynaud’s phenomenon, n [%] | 59 [57%] | |
| Significant lung disease, n [%] | 68 [65%] | |
| Significant heart disease, n [%] | 27 [26%] | |
| Significant gastrointestinal disease, n [%] | 64 [62%] |
Patients with scleroderma have high COMPASS-31 scores that are comparable to scores among patients with other disorders known to present with dysautonomia
The mean COMPASS-31 score in our scleroderma cohort was 24.9 ± 15.5. We assessed whether this COMPASS-31 score was similar to COMPASS-31 scores from healthy controls and patients with non-rheumatic diseases that present with dysautonomia (17,19,20) (Table 2). We found that patients with scleroderma had higher COMPASS-31 scores compared to a previously published healthy control population (20) (24.9 ± 15.5 for scleroderma vs 8.9 ± 8.7 for healthy controls), and the differences in scores spanned across multiple domains of autonomic function. Mean COMPASS-31 scores in scleroderma were comparable to other diseases that present with autonomic dysfunction including diabetic polyneuropathy and small-fiber polyneuropathy (17,19).
Table 2.
COMPASS-31 scores and subdomain scores in scleroderma compared to previously published healthy controls (n=30) (20) and patients with other diseases that present with autonomic symptoms, including fibromyalgia (n=30) (20), diabetic polyneuropathy (n=29) (19), and small-fiber polyneuropathy (n=66) (17). Data are presented as mean (standard deviation).
| Subdomain | Scleroderma | Healthy Controls | Fibromyalgia | Diabetic polyneuropathy | Small-fiber polyneuropathy |
|---|---|---|---|---|---|
| Orthostatic | 8.2 (9.3) | 2.9 (6.4) | 9.2 (8.5) | 11.2 (12.4) | 14.0 (12.4) |
| Vasomotor | 2.6 (1.4) | 0.1 (0.3) | 0.8 (1.3) | 1.0 (1.4) | 1.6 (2.1) |
| Secretomotor | 4.8 (3.3) | 1.1 (2.4) | 4.9 (3.2) | 5.5 (3.6) | 5.4 (6.9) |
| Gastrointestinal | 7.7 (4.9) | 3.1 (2.7) | 8.4 (4.4) | 8.1 (4.8) | 6.6 (5.4) |
| Urinary | 0.9 (1.3) | 0.5 (0.9) | 1.8 (1.6) | 1.6 (2.0) | 1.0 (1.6) |
| Pupillomotor | 1.6 (1.1) | 1.2 (0.8) | 2.4 (1.1) | 2.2 (1.3) | 1.7 (1.4) |
| Total COMPASS-31 | 24.9 (15.5) | 8.9 (8.7) | 27.5 (14.2) | 28.9 (19.1) | 30.2 (23.2) |
Given that many patients with scleroderma are treated with vasodilators, which can cause orthostatic symptoms as a side-effect, we examined whether the use of these medications may affect the COMPASS-31 score. We did not find a difference in the mean COMPASS-31 score or orthostatic intolerance score between patients treated with a blood pressure or vasodilator medication (n=67) and those who were untreated (n=37, p>0.05). We next evaluated whether skin fibrosis may directly affect sweat glands and therefore result in more secretomotor dysfunction in scleroderma. We did not find an association between the maximum Modified Rodnan Skin Score (MRSS) and the secretomotor dysfunction score (p>0.05).
COMPASS-31 scores and autoantibody status
Euroimmun immunoblot assays to detect autoantibody profiles were performed on patients with available serum samples (83/104 patients, 80%). Among patients with complete autoantibody data, 21 patients (25%) had anti-CENP antibodies, 31 patients (37%) had anti-Topo-1 antibodies, and 19 patients (23%) had anti-RNA pol 3 antibodies. Patients with anti-CENP antibodies had significantly higher total COMPASS-31 scores compared to anti-CENP-negative patients (30.7 ± 17.9 vs 21.3 ± 13.8, p=0.04). This difference remained after adjustment for age, sex and race (β=4.3 [CI 0.3–8.3], p=0.04), but lost statistical significance after disease duration was added to the model (β=2.6 [CI −1.5–6.6], p=0.21). Higher COMPASS-31 scores among anti-CENP-positive patients was driven by higher scores in vasomotor dysfunction, gastrointestinal dysfunction and secretomotor dysfunction (p<0.05 for all comparisons). There was no difference in COMPASS-31 scores between patients with and without anti-Topo-1 antibodies. Patients with anti-RNA pol 3 antibodies had lower total COMPASS-31 scores (17.9 ± 12.4 vs 25.4 ± 15.8, p=0.04), although this lost significance after adjustment for age, sex and race (β= −3.5 [CI −7.7–0.7], p=0.10) (Table 3).
Table 3.
Mean COMPASS-31 scores by autoantibody for the 83 scleroderma patients with complete Euroimmun autoantibody profiles. Student’s t-test was used to compare COMPASS-31 scores between patients with and without each autoantibody. Multivariable linear regression analysis was used to adjust for age, sex, and race (Model 1) and age, sex, race, and disease duration (Model 2). Data are presented as the β-coefficient and the 95% confidence interval. SD=standard deviation
| Autoantibody Subtype | N [%] | Mean Compass Score (SD) | p-value | Adjusted Model 1: age, sex, race | Adjusted Model 2: Model 1+ disease duration |
|---|---|---|---|---|---|
| Anti-Centromere | |||||
| Positive | 21/83 [25%] | 30.7 (17.9) | P=0.04 | β= 4.3 [0.3–8.3] p=0.04 |
β= 2.6 [−1.5–6.6] p=0.21 |
| Negative | 62/83 [75%] | 21.3 (13.8) | |||
| Anti-Topoisomerase-1 | |||||
| Positive | 31/83 [37%] | 21.2 (13.6) | P=0.24 | β= −2.5 [−6.2–1.2] p=0.19 |
β= −1.7 [−5.3–1.8] p=0.34 |
| Negative | 52/83 [63%] | 25.1 (16.3) | |||
| Anti-RNA polymerase III | |||||
| Positive | 19/83 [23%] | 17.9 (12.4) | P=0.04 | β= −3.5, [−7.7–0.7] p=0.10 |
β= −2.3 [−6.4–1.7] p=0.26 |
| Negative | 64/83 [77%] | 25.4 (15.8) |
COMPASS-31 scores and clinical associations in scleroderma
We found that women with scleroderma had significantly higher mean COMPASS-31 scores than men (26.1 ± 15.1 vs 15.3 ± 16.0, p=0.04). Disease duration was positively associated with total COMPASS-31 score [predicted COMPASS-31 = 0.5*disease duration (years) +18.5, R2=0.07, p<0.01], whereas there was no association between COMPASS-31 score and age (p>0.05). There was no difference in mean COMPASS-31 scores by patient race (Caucasian vs African American) or systemic sclerosis subtype (limited vs. diffuse), and there was no association with resting heart rate. Patients with dry mouth (27.3 ± 15.9 vs 18.3 ± 12.6, p<0.01), arthralgias (26.0 ± 15.7 vs 16.3 ± 11.3, p<0.05), and significant GI disease (29.1 ± 16.6 vs 18.2 ± 10.8, p<0.0001) had higher mean COMPASS-31 scores than patients without these clinical findings.
GI disease in scleroderma is associated with higher COMPASS-31 scores
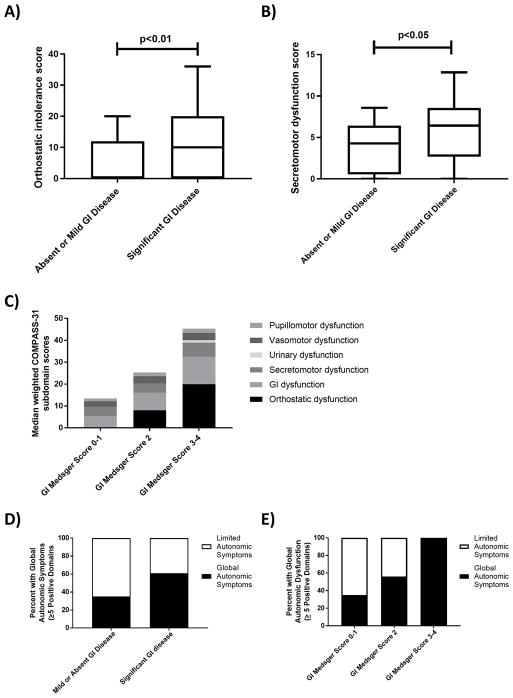
To determine whether GI disease in scleroderma may be part of a dysautonomia syndrome, we investigated whether patients with GI disease had other symptoms of autonomic dysfunction. For this analysis, we compared the COMPASS-31 subdomain scores of patients with significant GI disease to those with absent or mild GI disease. Of our total study cohort, 64 patients (62%) had significant GI disease, which was defined as a Medsger severity score ≥ 2. Compared to patients with mild or absent GI disease, patients with significant GI disease had higher scores in orthostatic intolerance (median 10.0 [IQR 0–20.0] vs 0 [IQR 0–12.0], p=0.006) and secretomotor dysfunction (median 6.4 [IQR 2.7–8.6] vs 4.3 [IQR 0.5–6.4], p=0.03) (see figure 1a–b). There was a higher burden of autonomic gastrointestinal symptoms among patients with more significant GI disease compared to patients with mild or absent GI disease (8.2 ± 4.5 vs 4.8 ± 3.3, p<0.001). We found no relationship between GI disease severity and scores in vasomotor, urinary, or pupillomotor dysfunction. We then constructed a multivariable linear regression model to adjust for potential clinical confounders (age, sex, race, and disease duration). After adjustment for these covariates, the positive association between significant GI disease and total COMPASS-31 score (β=4.8 [CI 1.6–8.1], p=0.004) and orthostatic intolerance score (β=2.4 [CI 0.4–4.4], p=0.02) remained. The association between significant GI disease and secretomotor dysfunction scores remained after adjustment for age, sex and race (β=0.8 [CI 0.1–1.4], p=0.03), but lost statistical significance when disease duration was added to the model (p>0.5).
Figure 1.
Relationship between GI disease severity and autonomic symptoms in patients with scleroderma. Patients with scleroderma and significant gastrointestinal (GI) disease (Medsger severity score ≥ 2) have increased median (A) orthostatic intolerance scores (p<0.01) and (B) secretomotor dysfunction scores (p<0.05) compared to patients with absent or mild GI disease; C) Median COMPASS-31 scores across multiple subdomains increase as GI disease severity increases; D) A higher percent of patients with significant GI disease have global autonomic symptoms (defined as ≥ 5 positive subdomains of the COMPASS-31) compared to patients with mild or absent GI disease (p<0.05); and E) There is a dose-response relationship between GI disease severity and global autonomic symptoms.
We then examined whether a dose-response relationship exists between the severity of GI disease and autonomic symptom burden. We divided our cohort into three groups based on the Medsger GI disease severity score: absent or mild GI disease (Medsger severity score 0–1, n=40), significant GI disease (Medsger severity score of 2, n=57), and severe GI disease (Medsger severity score of 3–4, n=7). We found that autonomic symptom burden across all subdomains of the COMPASS-31 increased as the severity of GI disease increased (see figure 1c).
GI disease in scleroderma is associated with symptoms of global autonomic dysfunction
To further explore the relationship between GI disease in scleroderma and symptoms of global dysautonomia, we divided the cohort into two predefined groups based on the number of positive subdomains (score > 0) within the COMPASS-31. Fifty-three patients (51%) had ≥ 5 positive subdomains and were defined as having “global autonomic symptoms,” and 51 patients (49%) had < 5 positive subdomains and were defined as having “limited autonomic symptoms.” We found that more patients with significant GI disease (Medsger score ≥ 2) had global autonomic symptoms compared to patients with mild or absent GI disease (Medsger score < 2) (61% vs 35%, p= 0.01) (see figure 1d). The association between significant GI disease and the presence of global autonomic symptoms remained after adjusting for age, sex, race and disease duration (OR 3.2 [95% CI 1.3–8.4], p=0.01). We also found that the percent of patients who report symptoms in ≥ 5 subdomains of the COMPASS-31 increases as the severity of GI disease increases (see figure 1e).
Scleroderma patients with myopathy have more symptoms of global autonomic dysfunction
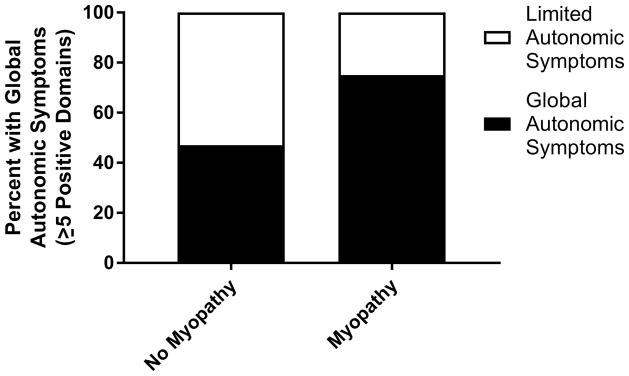
As our previous study identified an association between severe GI disease and myopathy in scleroderma (24), we next explored the relationship between myopathy and symptoms of dysautonomia. We identified higher orthostatic intolerance scores (median 14.0 [IQR 2.0–20.0] vs 0 [IQR 0–16.0], p=0.02) and a trend toward higher total COMPASS-31 scores (32.3 ± 16.4 vs 23.5 ± 15.1, p=0.06) among patients with myopathy compared to patients without myopathy. There was no relationship between the presence of myopathy and subdomain scores in vasomotor, secretomotor, gastrointestinal, urinary or pupillomotor dysfunction (p>0.05). When patients were grouped by the number of positive subdomain scores, more patients with myopathy had global autonomic symptoms (≥ 5 positive subdomains) compared to patients without myopathy (75% vs 47%, p=0.03) (see figure 2). We did not pursue multivariable modeling given the low number of patients with myopathy (n=16).
Figure 2.
Comparison of autonomic symptom burden in scleroderma patients with and without myopathy. Patients with myopathy are more likely to have global autonomic symptoms (defined as ≥ 5 positive domains) compared to patients without myopathy (p<0.05).
DISCUSSION
We administered the COMPASS-31 questionnaire to a well-characterized cohort of patients with scleroderma to quantify symptoms of autonomic dysfunction and to determine clinical risk factors for dysautonomia. To our knowledge, this is the first time that a validated autonomic dysfunction questionnaire has been used to capture global autonomic symptom burden across a large population of patients with scleroderma. Consistent with prior studies (7,9,10), we found that scleroderma patients have a high burden of autonomic symptoms that involve multiple aspects of the autonomic nervous system. Resting heart rate is a poor predictor of autonomic symptom burden in scleroderma, and our findings support the use of patient reported symptom scores in screening all scleroderma patients for autonomic dysfunction.
Among the clinical features of scleroderma that we analyzed, we found a strong association between the presence of GI disease and higher COMPASS-31 scores. We considered whether this finding occurred because questions about GI dysfunction are a component of the COMPASS-31 questionnaire. However, positive responses for GI dysfunction in the questionnaire were not solely responsible for this association. Patients with significant GI disease frequently reported symptoms across multiple facets of the autonomic nervous system (e.g. orthostatic intolerance and secretomotor dysfunction), suggestive of global dysautonomia. Our findings are also consistent with previous studies that suggest that early GI disease in scleroderma may be mediated by dysfunction of the autonomic nervous system (26), and raise the possibility that GI disease in scleroderma may be a cause or consequence of autonomic dysfunction.
Autonomic symptoms are common in other rheumatic diseases associated with GI dysmotility, including sjogren’s syndrome and systemic lupus erythematosus (27–29), suggesting that pathology of the autonomic nervous system may be a common clinical feature among certain patient subsets. While the mechanism of dysautonomia in these patients remains unclear, the cause of autoimmune GI dysmotility in other patient groups, such as those with autoimmune autonomic ganglionopathy, is well-established. In this autoimmune disease, GI dysmotility and other symptoms of autonomic dysfunction result from pathogenic autoantibodies targeting ganglionic acetylcholine receptors (30). More research is needed in scleroderma and other rheumatic diseases to determine if similar pathogenic antibodies targeting components of the autonomic nervous system can be identified.
We observed a trend toward increased global dysautonomia symptoms among scleroderma patients with myopathy. Although these findings require further confirmation because of the low number of patients with myopathy in the cohort, these data are consistent with data from our group and a study by Nishimagi and colleagues which demonstrate an association between myopathy and GI disease in scleroderma (24,31). Future studies are also needed to explore the role of the autonomic nervous system in patients with myopathy and scleroderma.
Notable strengths of our study include the use of a diverse and well-characterized cohort of patients and the use of a validated questionnaire that assesses multiple aspects of the autonomic nervous system. With regard to study limitations, we did not have a healthy control group and therefore compared our findings with other relevant cohorts with COMPASS-31 scores in the literature. It is conceivable that the differences in COMPASS-31 scores observed were unrelated to scleroderma, but instead resulted from demographic differences for which we were unable to control. However, both our scleroderma cohort and the healthy controls were demographically similar, consisting predominately of Caucasian, middle-age women. We also had very few patients with myopathy, so our results regarding these patients are exploratory and need to be repeated with a larger sample size. We were unable to investigate the mental health of participants in our cohort study, although mental health may be impaired in scleroderma (32,33) and has been shown to impact COMPASS-31 scores in other diseases (34). Future studies of autonomic dysfunction in scleroderma should explore what role mental health and fatigue have in mediating the observed associations. Lastly, the COMPASS-31 questionnaire measures symptoms of dysautonomia rather than objective measures of autonomic dysfunction. It can be challenging to distinguish some symptoms of autonomic dysfunction from features of scleroderma itself, particularly in regard to Raynaud’s phenomenon and GI symptoms such as constipation, early satiety and bloating. Although this is an inherent limitation, the substantial overlap in symptoms of scleroderma and global dysautonomia may provide important insights into the role of autonomic dysfunction in the pathogenesis of scleroderma.
Our findings provide important insights into the association between symptoms of dysautonomia and the dysfunction of specific organ systems in patients with scleroderma. Further mechanistic studies investigating the role of autonomic dysfunction in scleroderma, and how it relates to disease biology, are warranted.
Acknowledgments
Funding: This research was supported by the Rheumatology Research Foundation Scientist Development Award, the Jerome L. Greene Foundation (90057213), the Scleroderma Research Foundation (ZM), the Johns Hopkins Clinician Scientist Research Career Development Award (ZM), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (BA, T32AR048522). JWR was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health and Office of Research Development (1R01DK107007-01A1), Department of Veterans Affairs (Biomedical and Laboratory Research Service and Rehabilitation Research and Development, 101RX001030).
References
- 1.Åkesson A, Wollheim FA. Organ manifestations in 100 patients with progressive systemic sclerosis: a comparison between the crest syndrome and diffuse scleroderma. Rheumatology Oxford University Press. 1989;28:281–6. doi: 10.1093/rheumatology/28.4.281. [DOI] [PubMed] [Google Scholar]
- 2.Prescott RJ, Freemont AJ, Jones CJP, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol John Wiley & Sons, Ltd. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Abraham D, Kahaleh B, Jinnin M, Tamaki K. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest American Society for Clinical Investigation. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnex C, Paice E, White AG. Autonomic neuropathy in systemic sclerosis: a case report and evaluation of six patients. Ann Rheum Dis. 1986;45:957–60. doi: 10.1136/ard.45.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessein PH, Gledhill RF. More on autonomic neuropathy in systemic sclerosis. Ann Rheum Dis. 1988;47:261–3. doi: 10.1136/ard.47.3.261-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertinotti L, Bracci S, Nacci F, Colangelo N, Del Rosso A, Casale R, et al. The autonomic nervous system in systemic sclerosis. A review Clin Rheumatol. 2004;23:1–5. doi: 10.1007/s10067-003-0812-4. [DOI] [PubMed] [Google Scholar]
- 7.Kahaleh B, Matucci Cerinic M. Raynaud’s phenomenon and scleroderma dysregulated neuroendothelial control of vascular tone. Arthritis Rheum John Wiley & Sons, Inc. 1995;38:1–4. doi: 10.1002/art.1780380102. [DOI] [PubMed] [Google Scholar]
- 8.Di Franco M, Paradiso M, Riccieri V, Basili S, Mammarella A, Valesini G. Autonomic dysfunction and microvascular damage in systemic sclerosis. Clin Rheumatol Springer-Verlag. 2007;26:1278–83. doi: 10.1007/s10067-006-0492-y. [DOI] [PubMed] [Google Scholar]
- 9.Iovino P, Valentini G, Ciacci C, De Luca A, Tremolaterra F, Sabbatini F, et al. Proximal stomach function in systemic sclerosis: relationship with autonomic nerve function. Dig Dis Sci. 2001;46:723–30. doi: 10.1023/a:1010779729184. [DOI] [PubMed] [Google Scholar]
- 10.Lock G, Straub RH, Zeuner M, Antoniou E, Holstege A, Schölmerich J, et al. Association of autonomic nervous dysfunction and esophageal dysmotility in systemic sclerosis. J Rheumatol. 1998;25:1330–5. [PubMed] [Google Scholar]
- 11.Dessein PH, Joffe BI, Metz RM, Millar DL, Lawson M, Stanwix AE. Autonomic dysfunction in systemic sclerosis: Sympathetic overactivity and instability. Am J Med. 1992;93:143–50. doi: 10.1016/0002-9343(92)90043-b. [DOI] [PubMed] [Google Scholar]
- 12.Cerinic MM, Generini S, Pignone A, Casale R. The nervous system in systemic sclerosis (scleroderma). Clinical features and pathogenetic mechanisms. Rheum Dis Clin North Am. 1996;22:879–92. doi: 10.1016/s0889-857x(05)70306-9. [DOI] [PubMed] [Google Scholar]
- 13.Pawlowski A. The nerve network of the skin in diffuse scleroderma and clinically similar conditions. Arch Dermatol. 1963;88:868–73. doi: 10.1001/archderm.1963.01590240192032. [DOI] [PubMed] [Google Scholar]
- 14.Ormea F. Studio comparative del sistema neuro-vegetativo periferico nella sclerodermia diffusa e nel tessuto connettivale alterato di alcune dermatosi. Dermatology Karger Publishers. 1952;105:8–17. [PubMed] [Google Scholar]
- 15.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87:1196–201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523–8. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 17.Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy. Eur J Neurol. 2015;22:1124–30. doi: 10.1111/ene.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortez MM, Nagi Reddy SK, Goodman B, Carter JL, Wingerchuk DM. Autonomic symptom burden is associated with MS-related fatigue and quality of life. Mult Scler Relat Disord. 2015;4:258–63. doi: 10.1016/j.msard.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Greco C, Di Gennaro F, D’Amato C, Morganti R, Corradini D, Sun A, et al. Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet Med. 2016 doi: 10.1111/dme.13310. [DOI] [PubMed] [Google Scholar]
- 20.Vincent A, Whipple MO, Low PA, Joyner M, Hoskin TL. Patients With Fibromyalgia Have Significant Autonomic Symptoms But Modest Autonomic Dysfunction. PM&R. 2016;8:425–35. doi: 10.1016/j.pmrj.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 22.Medsger TA, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42–6. [PubMed] [Google Scholar]
- 23.Patterson KA, Roberts-Thomson PJ, Lester S, Tan JA, Hakendorf P, Rischmueller M, et al. Interpretation of an Extended Autoantibody Profile in a Well-Characterized Australian Systemic Sclerosis (Scleroderma) Cohort Using Principal Components Analysis. Arthritis Rheumatol. 2015;67:3234–44. doi: 10.1002/art.39316. [DOI] [PubMed] [Google Scholar]
- 24.McMahan ZH, Paik JJ, Wigley FM, Hummers LK. Determining the risk factors and clinical features associated with severe gastrointestinal dysmotility in systemic sclerosis. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23479. [DOI] [PubMed] [Google Scholar]
- 25.Ferri C, Emdin M, Giuggioli D, Carpeggiani C, Maielli M, Varga A, et al. Autonomic dysfunction in systemic sclerosis: time and frequency domain 24 hour heart rate variability analysis. Br J Rheumatol. 1997;36:669–76. doi: 10.1093/rheumatology/36.6.669. [DOI] [PubMed] [Google Scholar]
- 26.Amaral TN, Peres FA, Lapa AT, Marques-Neto JF, Appenzeller S. Neurologic involvement in scleroderma: A systematic review. Semin Arthritis Rheum W B Saunders. 2013;43:335–47. doi: 10.1016/j.semarthrit.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Newton JL, Frith J, Powell D, Hackett K, Wilton K, Bowman S, et al. Autonomic symptoms are common and are associated with overall symptom burden and disease activity in primary Sjögren’s syndrome. Ann Rheum Dis. 2012;71:1973–9. doi: 10.1136/annrheumdis-2011-201009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evrengl H, Dursunoglu D, Cobankara V, Polat B, Seleci D, Kabuku S, et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol Int. 2004;24:198–202. doi: 10.1007/s00296-003-0357-5. [DOI] [PubMed] [Google Scholar]
- 29.Stojanovich L, Milovanovich B, de Luka SR, Popovich-Kuzmanovich D, Bisenich V, Djukanovich B, et al. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjögren syndrome and other autoimmune diseases. Lupus. 2007;16:181–5. doi: 10.1177/0961203306076223. [DOI] [PubMed] [Google Scholar]
- 30.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–55. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 31.Nishimagi E, Tochimoto A, Kawaguchi Y, Satoh T, Kuwana M, Takagi K, et al. Characteristics of patients with early systemic sclerosis and severe gastrointestinal tract involvement. J Rheumatol The Journal of Rheumatology. 2007;34:2050–5. [PubMed] [Google Scholar]
- 32.Mozzetta A, Antinone V, Alfani S, Neri P, Bonda PF, Pasquini P, et al. Mental health in patients with systemic sclerosis: a controlled investigation. J Eur Acad Dermatology Venereol. 2008;22:336–40. doi: 10.1111/j.1468-3083.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 33.Thombs BD, Bassel M, McGuire L, Smith MT, Hudson M, Haythornthwaite JA. A systematic comparison of fatigue levels in systemic sclerosis with general population, cancer and rheumatic disease samples. Rheumatology (Oxford) 2008;47:1559–63. doi: 10.1093/rheumatology/ken331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed J, Derom E, De Wandele I, Rombaut L, Calders P. Autonomic symptoms in patients with moderate and severe chronic obstructive pulmonary disease. Acta Clin Belg. 2017:1–9. doi: 10.1080/17843286.2017.1379255. [DOI] [PubMed] [Google Scholar]