Introduction
Patient-derived xenografts (PDX) are being increasingly utilized in preclinical oncologic research. Maintaining large colonies of early generation tumor-bearing mice is impractical and cost-prohibitive. Optimal methods for efficient long-term cryopreservation and subsequent reanimation of PDX tumors are critical to any viable PDX program. We sought to compare the performance of “Standard” and “Specialized” cryoprotectant media on various cryopreservation and reanimation outcomes in PDX tumors. Standard (10% DMSO media) and Specialized (Cryostor®) media were compared between overall and matched PDX tumors. Primary outcome was reanimation engraftment efficiency (REE). Secondary outcomes included time-to-tumor-formation (TTF), time-to-harvest (TTH), and potential loss of unique PDX lines. Overall 57 unique PDX tumors underwent 484 reanimation engraftment attempts after previous cryopreservation. There were 10 unique PDX tumors cryopreserved with Standard (71 attempts), 40 with Specialized (272 attempts), and 7 with both (141 attempts). Median frozen time of reanimated tumors was 29 weeks (max.177). Tumor pathology, original primary PDX growth rates, frozen storage times, and number of implantations per PDX model were similar between cryoprotectant groups. Specialized media resulted in superior REE (overall: 82% vs. 39%, p<0.0001; matched: 97% vs. 36%, p<0.0001; >52 weeks cryostorage: 59% vs. 9%, p<0.0001), shorter TTF (overall 24 vs. 54 days, p=0.0051; matched 18 vs. 53 days, p=0.0013) and shorter TTH (overall: 64 vs. 89 days, p=0.009; matched: 47 vs. 88 days, p=0.0005) compared to Standard. Specialized media demonstrated improved REE with extended duration cryostorage (p=0.048) compared to Standard. Potential loss of unique PDX lines was lower with Specialized media (9% vs. 35%, p=0.017). In conclusion, cryopreservation with a specialized cryoprotectant appears superior to traditional laboratory-based media and can be performed with reliable reanimation even after extended cryostorage.
Keywords: patient-derived xenograft, pancreatic cancer, PDX, cryopreservation, reanimation, DMSO, Cryostor, engraftment, cryoprotectant
Introduction
Contemporary translational oncology research requires appropriate preclinical models that can recapitulate the human cancer phenotype. Patient-derived xenografts (PDX) is one such model that are created by implanting freshly obtained tumors from individual patients using either surgical resection specimens or biopsy material into immunocompromised mice and represent a novel approach to the study of human malignancy.1–5 These PDX tumors accurately recapitulate the histologic features of the patient tumors from which they are derived and maintain the biological heterogeneity present in human cancer.1,6,7 Furthermore, they maintain their biological similarity to the original patient tumors from which they were derived in early generation passage and this has been demonstrated via gene expression profiling, copy number variants, single nucleotide polymorphisms, and chromosomal stability, making this model particularly ideal for preclinical studies due to limited genetic drift.1,6–10 The results of in vivo treatment studies in these models have shown improved correlation with actual patient tumor response compared to other traditional methods such as cell-line based or transgenic animal models.2,11–14 Furthermore, PDX models have also shown potential to predict clinical recurrence several months prior to conventional surveillance modalities in patients having undergone curative intent surgery and as such they can be used to predict the natural history of tumor and response to therapy.15–17
Any program that utilizes PDX requires optimized methods to create, maintain, and successfully store such a valuable research platform for future experimentation. Following successful engraftment and establishment of original first generation (F1) tumor bearing mice, PDX tumors can be expanded and amplified into subsequent later generations (F2, F3, etc.) for amplification of primary patient tumor tissue as well as for the purpose of planned formal large scale in vivo treatment investigations. However maintaining a large number of living tumor-bearing mice is not logistically or financially feasible for high-volume programs or labs with limited resources. A method to facilitate the delayed use of previously generated early generation xenografts is cryopreservation.18–21 This process entails freezing biological tissue for extended periods and is made possible with the use of cryoprotectant solutions that facilitate maintenance of cellular viability. Applying this method to PDX tumors allows for long-term storage of a living but frozen biobank of early generation PDX tumors with the ability to thaw and reimplant, or “reanimate”, the tumors at any future time point based on demand. Reliable techniques to cryopreserve, store, and reanimate PDX tumors are a critical component of any successful xenograft program and provide a safeguard against the loss of highly precious and irreplaceable primary patient tumor tissue over the course of time. Prevention of cellular injury during the cryopreservation process is the largest biological obstacle. In order to prevent such freezing injury various cryoprotectants such as glycerol, ethanediol, propanediol and dimethyl sulfoxide (DMSO) have been used for stable freezing and long-term preservation of established cell lines and tissues.22–27 Cryopreservation of primary patient tumor and subsequent PDX has been investigated previously, but the efficacy of reanimation has typically been low, or not reported.23,24,28,29 Cryopreservation of composite tissues, such as PDX tumors, is more complex than for homogenous cell lines as a three-dimensional architecture needs to be maintained during both freezing and thawing. As such, tissues are composed of a variety of cell types with varying degree of vulnerability to the cryopreservation process.30 While the feasibility of cryopreservation is well established, several cryoprotectant agents are utilized, either lab-based or commercially available, for varying applications. As a result, the optimal method of cryopreservation of PDX tissue remains to be elucidated and given the significant amount of resources required for generation of a single patient PDX model, any loss of such would have devastating consequences on the viability of any program. Given the incomplete and variable knowledge regarding the optimal cryoprotectant for PDX, we sought to evaluate a reliable and reproducible method of cryopreservation and reanimation of PDX tumor tissue using pancreatic cancer PDXs.
The most common standard cryopreservation media is a combination of varying cell culture media, serum albumin (10%), and DMSO (10%), which has a composition similar to the extracellular fluid compartment and is used in most laboratories with varying concentrations for cryopreservation of biological tissues.31 Cryostor™ is a specialized commercially available serum and protein-free cryoprotectant solution (10% DMSO) but which has a composition similar to the intracellular fluid compartment.31 This product was designed to mitigate temperature induced cell stress responses by scavenging free radicals, pH buffering, provides oncotic/osmotic support, energy substrates, and ionic concentrations that balance the intracellular state at ultra-low temperature environments (−80°C to −196°C).21,31–33 As this specialized media was specifically developed for the cryopreservation of extremely sensitive and vulnerable cell populations (human embryonic and pluripotent stem cells) we compared cryopreservation and subsequent reanimation engraftment outcomes between standard laboratory cryoprotectant media used in most PDX programs and this specialized media.
Materials and Methods
Original F1 PDX creation/Tumor engraftment
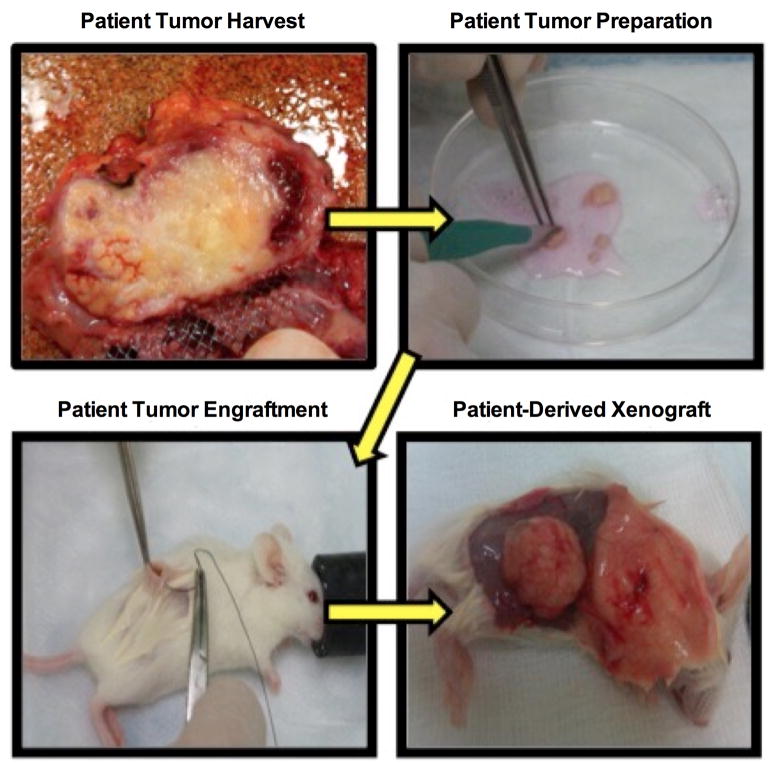
Data was obtained retrospectively from 2 high volume surgeon-directed PDX programs at Mayo Clinic and MD Anderson Cancer Center whose combined engraftment experience over 5 years is in excess of 500 unique cancer patients and subsequent derived PDX models with pancreatic adenocarcinoma representing the most common histologic cancer subtype. Following a protocol approved by the Institutional Review Board (IRB) and the Institutional Animal Care and Use Committee (IACUC) at The University of Texas MD Anderson Cancer Center and Mayo Clinic College of Medicine, patients undergoing curative-intent resection for pancreatic adenocarcinoma or radiologic biopsy of metastatic lesions consented participation for generation of PDX tumors. Using previously reported methods, fresh minimally ischemic (< 30–60 min) tumor tissue was obtained immediately after pathologic examination.3 Once histologic confirmation of cancer had been made pathology, fresh tumor tissue was placed in a 50 ml falcon tube containing 25 ml RPMI media with 1% penicillin/streptomycin. The media-containing tumor was placed on ice and transferred rapidly to an animal procedure room. 6–8 week old female NOD/SCID mice were placed in a mouse induction chamber and anesthetized using 1–3% Isofluorane with oxygen supplementation. The medium from the falcon tube was discarded and the tumor samples were placed on a sterile Petri dish on ice. The tumor sample was sliced into small fragments of 1–2 mm in maximum dimension to allow diffusion and enable viability. 400 μl of Matrigel ® (Corning®) was added to the tumor fragments so that they were fully immersed in order to increase primary engraftment efficiency.34,35 The areas of incision were disinfected with 70–100% ethanol. Incisions were made using sterile scissors just above the hind flank of the mouse and slightly enlarged with blunt dissection to allow a tissue pocket to be created under the hind flank lymphatic fat pad, and a tumor tissue fragment was implanted into the created tissue pocket. The incision was approximated with either interrupted sutures or a bioglue (Vetbond™). If enough primary patient tumor was available, similar primary engraftment was performed on the contralateral side. In general at least 5 mice were engrafted for each unique individual PDX sample to maximize probability of primary (F1) PDX engraftment. PDX growth metrics were recorded and included the time from implantation to the first time a growing tumor is palpated (i.e. 3–4mm) in the mouse and is referred to as the time-to-tumor-formation (TTF).36 (Figure 1) The time from implantation to the harvest of tumor (typically when tumors reach 15mm in greatest dimension) is referred to as the time-to-harvest (TTH). Harvested PDX tumors are then processed with portions sent for histologic confirmation by GI oncologic pathologists. (Figure 2)
Figure 1. PDX creation/Tumor engraftment.
Fresh patient tumor tissue is obtained immediately after pathologic examination and confirmation of cancer and subsequently implanted into NOD/SCID mice.
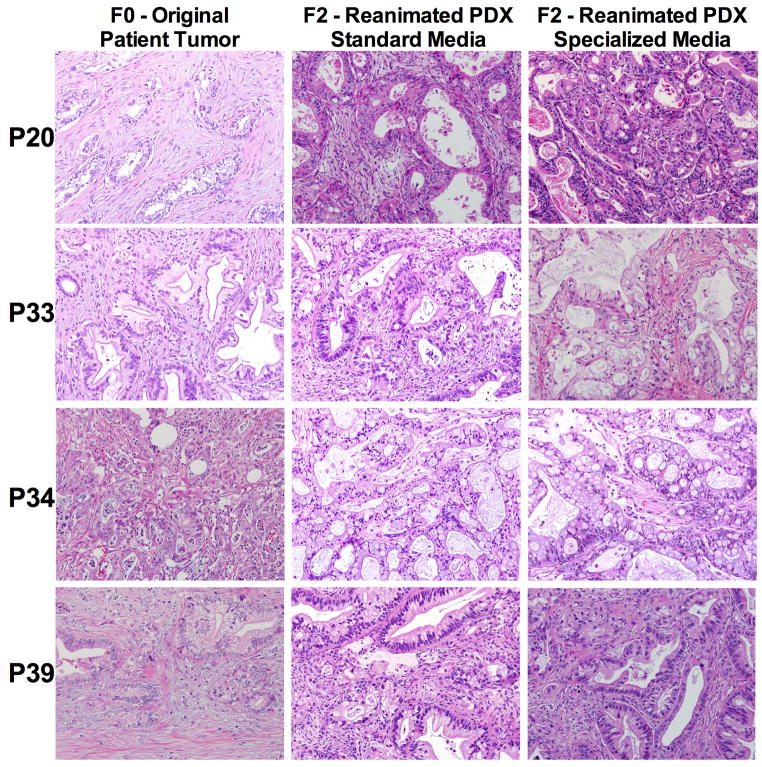
Figure 2. Histology.
H&E histology specimens and comparisons of a set of patient-derived xenografts (PDX) tumors from F0 (Original patient tumor) and the matched F2 (second generation) reanimated PDX tumor grown in standard media and specialized media.
Cryopreservation
At the time of the F1 PDX harvest, engrafted PDX tumors were sliced into 5×2mm pieces with a sterile scalpel blade. This dimension facilitates optimal absorption of cryoprotectant into the tumors, for uniform freezing temperature throughout the sample, and secures enough tumor tissue to subsequently be re-engrafted into 5 mice upon reanimation. The sliced tumors (1–2 slices per vial) were placed in plastic screw top cryopreservation vials (Thermo Scientific) preloaded with 1.5 ml of either Standard cryopreservation media containing 10% Dimethyl sulfoxide (DMSO) with 10% fetal bovine serum and RPMI solution or Specialized (Cryostor™ CS10 - BioLife Solutions, Owego, NY, USA) cryoprotectant alone. These were labeled and entered into an electronic web-based inventory registry according to the PDX ID and generation number (F1, F2 etc.). These vials were placed into a Mr. Frosty™ Freezing container (Thermo Scientific) which is subsequently placed in a −80°C freezer. These freezing containers contain 100% isopropyl alcohol and achieve an ideal rate of cooling near −1°C/min. These freezing chambers are kept in −80°C for at least 24–48hrs before transfer to long-term cold storage.
Thawing/Reanimation
Previously deep frozen cryovials were maintained in liquid N2 or dry ice during transport for thawing and re-engraftment. The thawing is rapid and accomplished with a water bath (25–37°C) or a running water rinse for 30–60 seconds.37 The vial is agitated until almost all ice has thawed inside the vial itself and is then rinsed with 70% ethanol. The contents of the vial are poured onto a Petri dish and the tumor samples are thoroughly rinsed (at least twice) with PBS solution in order to remove all cryoprotectant solvents that may potentially adversely affect engraftment efficacy. The thawed and rinsed cryopreserved tumor samples are implanted into NOD/SCID mice following the methodology described above. Reanimation engraftment efficiency (REE) was recorded as a percentage and defined as: (number of tumors successfully reanimated/number of cryopreserved tumors implanted). TTF and TTH were similarly recorded.
Statistical Analysis
Intergroup comparisons for continuous variables were examined with the student’s t-test (two-tailed) and Welch’s t-test where appropriate. Categorical variables were compared using the Pearson’s chi-square for uniform distribution and with Fischer’s exact test for non-uniform distribution. Analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc) Version 6.01, September 21, 2012 and JMP® 10.0.0 (2012 SAS Institute Inc.). For all comparisons, a p value of <0.05 was considered statistically significant.
Results
Overall 57 unique PDX tumors underwent 484 reanimation engraftment attempts after previous cryopreservation. There were 10 unique PDX tumors cryopreserved with Standard (71 attempts), 40 with Specialized (272 attempts), and 7 with both (141 attempts) cryoprotectants. There were no differences in baseline patient tumor and original F1 PDX growth characteristics in regards to previous neoadjuvant therapy (35.3% vs. 51.1%, p=0.40), poorly differentiated tumor (35.3% vs. 42.6%, p=0.78), median TTF (36 days vs. 25 days, p=0.70), or median TTH (88.5 days vs. 90.5 days, p=0.14) between cryopreservative media cohorts, respectively. (Table 1)
Table 1.
Baseline Patient Tumor and Original (F1) PDX Characteristics and Growth Metrics (TTF and TTH) by Cryopreservation Media.
| Variables | Standard | Specialized | p |
|---|---|---|---|
| Prior Neoadjuvant Therapy | (6/17) 35.3% | (24/47) 51.1% | 0.40 |
| Poorly Differentiated Tumor | (6/17) 35.3% | (20/47) 42.6% | 0.78 |
|
Original (F1) PDX TTF (median), days (IQR) |
36 (18–42.5) | 25.5 (18–42) | 0.70 |
|
Original (F1) PDX TTH (median), days (IQR) |
88.5 (70.8–117) | 90.5 (70–130.5) | 0.14 |
PDX: Patient-Derived Xenograft, F1: First Generation TTF: Time-to-tumor formation, TTH: Time-to-tumor harvest, IQR: Interquartile range
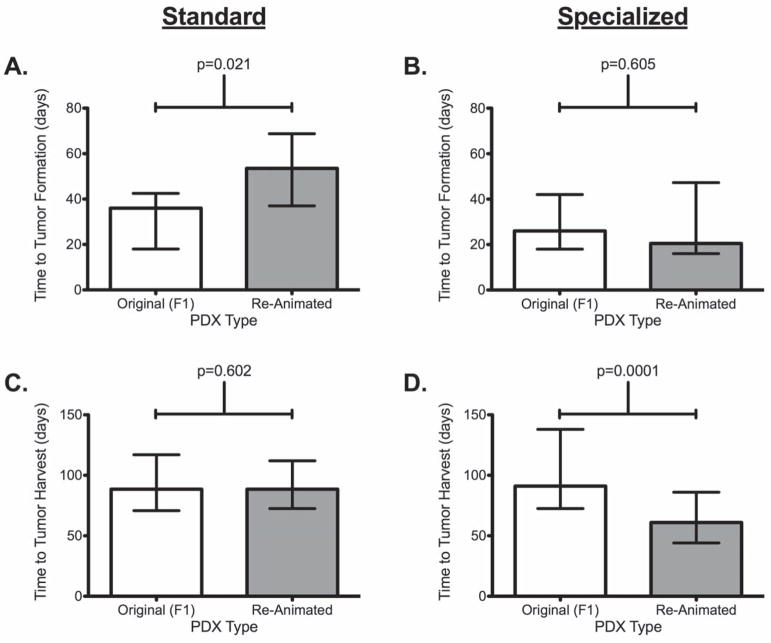
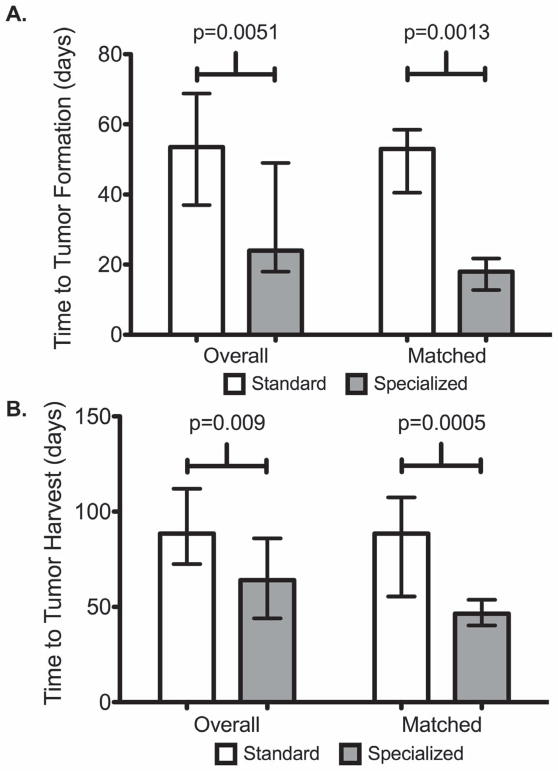
When comparing TTF between original (F1) tumors to reanimated tumors there was a significantly longer median TTF duration (53.5 days vs. 36 days, p=0.021) for tumors cryopreserved with Standard media. When comparing TTH between original (F1) tumors to reanimated tumors there was a significantly shorter median TTH duration (90.5 days vs. 64 days, p = 0.0001) for tumors cryopreserved with Specialized media. (Table 2 and Figure 3) Median duration of cryopreservation was similar between Standard and Specialized media cohorts respectively, for overall as well as for matched cases (21 weeks vs. 29 weeks, p = NS; 25 weeks vs. 37 weeks, p = NS) with the longest duration of successful engraftment after cryopreservation of 73 weeks for Standard and 177 weeks for Specialized. The number of tumors implanted per unique PDX was similar between Standard and Specialized (median 5.0 vs. 5.0, p = NS). In total, 126 (Standard) and 358 (Specialized) cryopreserved tumors underwent reanimation attempts. The median TTF (overall: 54 days vs. 24 days, p=0.0051; matched cases: 53 days vs. 18 days, p=0.0013) and TTH (overall: 89 days vs. 64 days, p=0.009; matched cases: 88 days vs. 47 days, p=0.0005) was significantly longer for Standard versus Specialized. (Table 3 and Figures 4 and 5)
Table 2.
Comparison of growth metrics (TTF and TTH) between Original (F1) PDX tumors and Reanimated PDX Tumors by Cryopreservation Media
| TTF | |||
|---|---|---|---|
| F1 (Original) | Reanimation | p | |
| Standard, days (Median and IQR) | 36 (18–42.5) | 53.5 (37–68.8) | 0.021 |
| Specialized, days (Median and IQR) | 25.5 (18–42) | 24 (18–49) | 0.605 |
| TTH | |||
| F1 (Original) | Reanimation | p | |
| Standard, days (Median and IQR) | 88.5 (70.8–117) | 88.5 (72.5–112) | 0.602 |
| Specialized, days (Median and IQR) | 90.5 (70–130.5) | 64 (44–86) | 0.0001 |
PDX: Patient-Derived Xenograft, F1: First Generation TTF: Time-to-tumor formation, TTH: Time-to-tumor harvest, IQR: Interquartile range
Figure 3. Overall growth metrics comparisons between the original (F1) derived PDX and the reanimated PDX tumors by cryopreservation media. (Shown are median and IQR.).
A. Time to tumor formation (TTF) for F1 and Re-animated tumors from cryopreservation using standard media,
B. Time to tumor formation (TTF) between F1 and Re-animated tumor from cryopreservation in using specialized media
C. Time to Tumor harvest (TTH) between F1 and Re-animated tumor from cryopreservation using standard media
D. Time to tumor harvest (TTH) between F1 and Re-animated tumor from cryopreservation using specialized media.
Table 3.
Overall and Matched Reanimation Engraftment Outcomes by Cryopreservation Media
| Standard Overall PDX = 17 Matched PDX = 7 |
Specialized Overall PDX = 47 Matched PDX = 7 |
p | |
|---|---|---|---|
|
| |||
| Cryopreservation Duration, weeks | |||
| Overall | 21 (11–51) | 29 (11–59) | 0.24 |
| Matched Cases | 25 (15–51) | 37 (25–56) | 0.29 |
|
| |||
| No. Mice Implanted per Unique PDX | |||
| Overall | 5 (5–10) | 5 (4–10) | 0.38 |
| Matched Cases | 5 (3–10) | 5 (4–10) | 0.84 |
|
| |||
| Reanimation TTF, days | |||
| Overall | 54 (37–69) | 24 (18–49) | 0.0051 |
| Matched | 53 (41–59) | 18 (13–22) | 0.0013 |
|
| |||
| Reanimation TTH, days | |||
| Overall | 89 (73–112) | 64 (44–86) | 0.009 |
| Matched | 88 (70–122) | 47 (40–54) | 0.0005 |
|
| |||
| Reanimation Engraftment Efficiency (REE), % (Ratio) | |||
| Overall | 39% (49/126) | 82% (294/358) | <0.0001 |
| Matched | 36% (20/55) | 97% (83/86) | <0.0001 |
| >52 weeks Cryostorage time | 9% (5/37) | 59% (45/70) | <0.0001 |
|
| |||
| Unique PDX Lines Potentially Lost, % (Ratio) | 35% (6/17) | 9% (4/47) | 0.017 |
All values are Median and IQR unless specified.
PDX: Patient-Derived Xenograft, TTF: Time-to-tumor formation, TTH: Time-to-tumor harvest, IQR: Interquartile range
Figure 4. Overall and matched growth metrics by cryopreservation media. Shown are median and IQR.
A. Time to tumor formation (TTF) overall as well as for matched tumors using standard and specialized media
B. Time to tumor harvest (TTH) overall as well as for matched tumors using standard and specialized media.
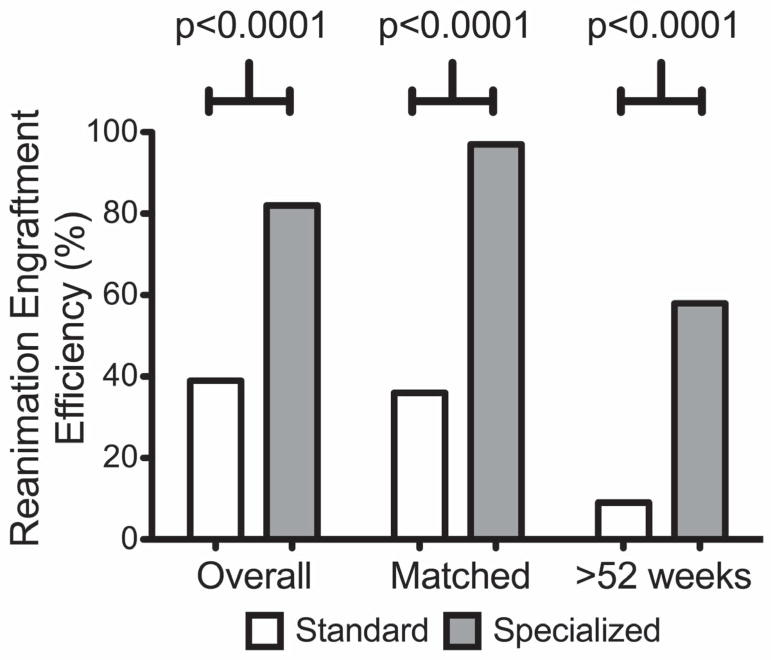
Figure 5. Reanimation Engraftment Efficiency (%).
First set of columns represents the overall Reanimation engraftment efficiency for patient-derived xenografts (PDX) using standard and specialized media. Second set of columns represents reanimation engraftment efficiency between matched tumors using standard and specialized media. The last set of columns represents reanimation engraftment efficiency for extended (>52 wks) cryopreservation using standard and specialized media.
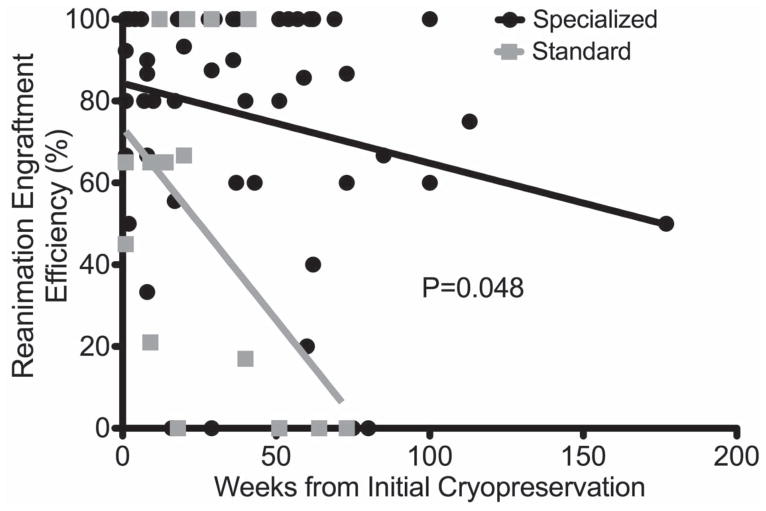
The overall REE was 39% (49/126) for Standard and 82% (294/358) for Specialized and this significantly favored Specialized (p<0.0001). For matched cases REE was 36% (20/55) for Standard and 97% (83/86) for Specialized and this significantly favored Specialized (p<0.0001) media. For those tumors with the longest duration of cryopreservation (>52 weeks frozen time) REE was significantly lower for Standard at 9% (5/37) versus Specialized 59% (45/70) (p<0.0001). A one-way ANCOVA (Analysis of Covariance) was conducted to determine statistically significant differences between REE on the duration of cryopreservation controlling for the cryoprotectant used. Both the type of cryoprotectant (Standard vs. Specialized) and the duration (weeks) of cryopreservation were significant predictors of REE (p<0.0001 and 0.0168, respectively) and while REE decreased for both cryoprotectants as a function of time and duration of cryopreservation, REE remained higher for Specialized media compared to Standard with non-zero engraftment at 3 years with utilization of Specialized media (p=0.048). (Table 2 and Figure 6). As loss of unique PDX models is the most potentially devastating consequence for a program, we assessed for failure of reanimation. Overall when comparing unique PDX lines potentially lost, there were 35% (6/17) Standard cryopreservation failures compared to 8.5% (4/47) Specialized failures (p=0.017).
Figure 6. Reanimation Engraftment Efficiency (%) by duration of cryostorage. Linear regression.
Reanimation Engraftment efficiency as a function of time for standard and specialized media.
Discussion
Cryopreservation refers to freezing biological tissue for long-term storage in such a way that cells remain viable for long durations for subsequent reanimation. Cryopreservation of PDX tumors is essential to maintaining early generation/passage tumors that have minimal biologic deviation from the tumor of origin. The success of the freezing process depends on three critical factors: proper and timely handling of PDX tumor tissues, use of appropriate cryoprotective agents, and controlled rate of freezing and storage at cryogenic temperatures. There are two potential mechanisms for cell injury during freezing.18,37 First, when water freezes in the cell suspending solution, solutes become more concentrated in the remaining liquid phase21,37 that leads to cellular osmotic dehydration of the biological sample and injury/death.38,39 Second, as cells freeze, intracellular ice crystals can form and directly puncture and damage the plasma membrane18,19,24,39–41, In order to prevent such freezing injury various cryoprotectants such as glycerol, ethanediol, propanediol and dimethyl sulfoxide (DMSO) are used for stable freezing and long-term preservation of established cell lines and tissues.18,20,25,40–42 These solutions are non-toxic and easily soluble and are thought to prevent such injury via several proposed mechanisms.18,20,22,25,40–44 They can partially solubilize the cell membrane so that it is less prone to puncture and interrupt the lattice of the ice, so that fewer intracellular crystals form. Cryoprotectants also increase the solute concentration in cells thus lowering the glass transition temperature (for water is −135C) of a solution below which molecular movement ceases and all biological activity is suspended thus preventing actual freezing and the cytosol maintains some flexibility in a glassy phase. Furthermore these solutions displace water molecules and form hydrogen bonds with biological molecules. Hydrogen bonding in aqueous solutions is important for proper protein and DNA function. Thus, as the cryoprotectant replaces the water molecules, the biological material retains its native physiological structure and function, although they are no longer immersed in an aqueous environment. The rate at which cryopreserved tissues are frozen is also a critical component to this process. A freezing rate falling within −0.3 to −1.8C/min is optimal.45 A variety of methods to obtain this rate have been utilized including specifically designed controlled rate freezers, portable freezing containers with or without low-freezing point insulator solutions, and even simple polystyrene boxes found in most labs. Regardless of method, maintaining a rate of cooling very close to −1°C/minute, the optimal rate for cell preservation, is necessary for best long-term reanimation outcomes.
Successful cryopreservation and subsequent reanimation is critically necessary in any high volume PDX program. We have demonstrated that long-term (>3 years) cryopreservation of PDX tumors is feasible and reproducible and that subsequent reanimation can be reliably performed with high efficiency. A specialized and commercially available cryoprotectant specifically formulated for vulnerable cell populations, demonstrated superior performance over standard traditional lab-based DMSO media solution as a cryoprotectant with markedly improved reanimation engraftment efficiency (REE), shorter TTF and TTH, successful reanimation even with extended cryostorage (>52wks), and less risk of potential unique PDX models lost to cellular injury due to the cryopreservation process. The implications of these results are substantial for high volume programs involved in PDX studies or other highly precious and vulnerable cellular and tissue biobanks that require long-term cryopreservation for future reanimation.
Although previous data has demonstrated that derived PDX tumors are remarkably well maintained as they move through subsequent later generations of mice, the potential for new mutations and molecular evolution of these derived tumors does exist.46,47 For this reason, studies involving PDX should optimally be conducted in early generation PDX tumors as these have been demonstrated to have minimal deviation from the original patient tumor.1 Logistically, this poses a problem because maintenance of large numbers of tumor-bearing mice is not practically feasible and continued passage into next generations may alter the biological phenotype which may then further complicate studies as these tumors may deviate from the original patient tumor. Optimal methods of cryopreservation can remedy such issues by allowing safe, replicable, and high efficiency reanimation rates so future studies can be performed on an as needed basis with early generation PDX. Additionally, we have found nearly identical genetic signatures in early generation PDX with and without the use of cryopreservation.48
In addition to the type of cryoprotectant used, there are a number of aspects that may affect the engraftment efficacy after cryopreservation and may have introduced sources of bias to this study. First, tissues may be damaged by ischemia from the time the tumor is removed from its blood supply until they have been cryopreserved. There may have been differences in the ischemic time from harvest until cryopreservation between the two groups. This specific information was not captured during our data collection process and is therefore a potential confounder. If the tumor samples are not thoroughly washed after thawing to ensure removal of the cryoprotectant, this may have affected engraftment rate during reanimation. Tumors that have undergone neoadjuvant therapy prior to surgery and PDX creation may have lower engraftment rates compared to non-treated tumors, however we found no difference in neoadjuvant therapy receipt between the groups. Similarly, high-grade tumors may have higher engraftment rates and shorter TTF and TTH than low-grade tumors, but again there was no difference in the degree of tumor differentiation between groups in this study. When tumors are large, the central portion may outgrow its blood supply and become necrotic. It is therefore critical that viable tissue for cryopreservation is taken from the periphery of the tumor. Another aspect that may introduce bias is differences between the sizes of tumor samples placed in the cryoprotectant-containing vials as this may prevent uniform penetration of the cryoprotectant and may result in inadequate cryopreservation and tissue damage, thus maintaining specific protocols for the cryopreservation process is critical.
Cryopreservation with subsequent reanimation is not only feasible but is also an efficient method to overcome economic and logistic aspects that are otherwise unavoidable in large volume programs. Creation and maintenance of a PDX program is labor intensive and very resource costly. Requisites of a program include high clinical patient volumes of the specific malignant histologic subtype that PDXs are to be created from (in order to better represent patient tumor heterogeneity) as well as active involvement of variety of disciplines including surgical oncologists, pathology staff, veterinary facilities, etc. In addition appropriate CORE facilities including animal veterinary facilities, histologic processing, and downstream molecular analysis of derived PDX tumors are needed. Finally, optimal methods of cryopreservation and reanimation are necessary to continue such programs over the long-term to allow for the creation of a large repertoire and registry of cryopreserved tumors that can be reanimated at a later date for any specific study or experiment. Given these requisites, continued developments in tissue preservation may allow regionalization of PDX programs, facilitating transfer of viable tumors from smaller centers without access to a PDX program but perhaps with different patient characteristics and demographics. This development would allow the PDX model to reach a larger scale and ultimately benefit a greater number of patients.
As our data have demonstrated, PDX tumors can be successfully reanimated after over 3 years in cryopreservation and it appears the use of a specialized commercially available cryoprotectant is superior to standard lab-based cryoprotectant media solutions and our respective laboratories currently utilize such specialized solutions exclusively based on the results of this work. Future studies in this field should aim to determine the upper limit of duration for safe and reliable cryopreservation.
Acknowledgments
Funding/support: The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Dr. Ivanics and in kind material support for Dr. Bergquist. Dr. Bergquist receives salary support from the Mayo Clinic Clinician Investigator Training program. Dr. Fleming’s laboratory is funded by the Skip Viragh Foundation, the Various Donors in Pancreatic Cancer Research Fund, and the Research Animal Support Facility—Houston under NIH/NCI award (P30CA016672). Dr. Rios Perez was also supported in part by National Institutes of Health (NIH) grant T32CA009599. Dr. Truty is supported by Private Benefactor/Development, Departmental/Institutional Support, and Mayo Clinic/Karolinska Institute Grant.
Competing Interest Statement
The authors declare no competing interests
Footnotes
Disclosures: None of the authors have any conflicts of interest.
References
- 1.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12(15):4652–61. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009;85(2):217–221. doi: 10.1038/clpt.2008.200. [DOI] [PubMed] [Google Scholar]
- 3.Kim MP, Evans DB, Wang H, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4(11):1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89(12):5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–50. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hidalgo M, Amant F, Biankin AV, et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73(17):5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosfjord E, Lucas J, Li G, et al. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91(2):135–43. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Williams Sa, Anderson WC, Santaguida MT, et al. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93(9):970–82. doi: 10.1038/labinvest.2013.92. [DOI] [PubMed] [Google Scholar]
- 10.Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314–28. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 11.Hwang CI, Boj SF, Clevers H, et al. Pre-clinical Models of Pancreatic Ductal Adenocarcinoma. J Pathol. 2016;238(2):197–204. doi: 10.1002/path.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voskoglou-nomikos T, Pater JL, Seymour L. Clinical Predictive Value of the in Vitro Cell Line, Human Xenograft, and Mouse Allograft Preclinical Cancer Models Clinical Predictive Value of the in Vitro Cell Line, Human Xenograft, and Mouse Allograft Preclinical Cancer Models. Clin Cancer Res. 2003;9(11):4227–4239. [PubMed] [Google Scholar]
- 13.Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: Correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40(6):802–820. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas RM, Truty MJ, Kim M, et al. The Canary in the Coal Mine: The Growth of Patient-Derived Tumorgrafts in Mice Predicts Clinical Recurrence after Surgical Resection of Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2015;22(6):1884–1892. doi: 10.1245/s10434-014-4241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma-related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17(17):5793–800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–20. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2015;1257:3–19. doi: 10.1007/978-1-4939-2193-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Meryman HT. Cryoprotective agents. Cryobiology. 1971;8(2):173–183. doi: 10.1016/0011-2240(71)90024-1. [DOI] [PubMed] [Google Scholar]
- 20.Mazur P. Cryobiology: The Freezing of Biological Systems. Science. 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 21.Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy. 2006;8(1):57–61. doi: 10.1080/14653240500501021. [DOI] [PubMed] [Google Scholar]
- 22.Thompson M, Nemits M, Ehrhardt R. Rate-controlled Cryopreservation and Thawing of Mammalian Cells. Nature Protocol Exchange. 2011 Mar; [Google Scholar]
- 23.Sorio C, Bonora A, Orlandini S, et al. Successful xenografting of cryopreserved primary pancreatic cancers. Virchows Arch. 2001;438(2):154–158. doi: 10.1007/s004280000343. [DOI] [PubMed] [Google Scholar]
- 24.Linnebacher M, Maletzki C, Ostwald C, et al. Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer. 2010;10:362. doi: 10.1186/1471-2407-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuleshova LL, Wang XW, Wu YN, et al. Vitrification of encapsulated hepatocytes with reduced cooling/warming rates. Cryo Letters. 2004;25(4):241–254. [PubMed] [Google Scholar]
- 26.Polge C. Low-temperature storage of mammalian spermatozoa. Proc R Soc London B Biol Sci. 1957;147(929):498–508. doi: 10.1098/rspb.1957.0068. [DOI] [PubMed] [Google Scholar]
- 27.Isachenko V, Lapidus I, Isachenko E, et al. Human ovarian tissue vitrification versus conventional freezing: Morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138(2):319–327. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 28.Cui JH, Krüger U, Vogel I, et al. Intact tissue of gastrointestinal cancer specimen orthotopically transplanted into nude mice. Hepatogastroenterology. 1998;45(24):2087–2096. [PubMed] [Google Scholar]
- 29.Alkema NG, Tomar T, Duiker EW, et al. Biobanking of patient and patient-derived xenograft ovarian tumour tissue: efficient preservation with low and high fetal calf serum based methods. Sci Rep. 2015;5:14495. doi: 10.1038/srep14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhach J. The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis. 2009;5(3):119–126. doi: 10.4161/org.5.3.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke DM, Yadock DJ, Nicoud IB, et al. Improved post-thaw recovery of peripheral blood stem/progenitor cells using a novel intracellular-like cryopreservation solution. Cytotherapy. 2009;11(4):472–479. doi: 10.1080/14653240902887242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buskirk RG, Van Snyder KK, Baust JG, et al. Cryopreservation: It’s Not Just About Cell Yield. BioProcess Int. 2005;3:64–74. [Google Scholar]
- 33.Sosef MN, Baust JM, Sugimachi K, et al. Cryopreservation of isolated primary rat hepatocytes: enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241(1):125–133. doi: 10.1097/01.sla.0000149303.48692.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corning. Corning® Matrigel® Basement Membrane Matrix Growth Factor Reduced Phenol Red Free, 10 ml vial. Corning. 2013;356231:1–4. [Google Scholar]
- 35.Cassidy JW, Caldas C, Bruna A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Cancer Res. 2015;75(15):2963–2968. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MP, Truty MJ, Choi W, et al. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Ann Surg Oncol. 2012;19(Suppl 3):S395–403. doi: 10.1245/s10434-011-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramachandran H, Laux J, Moldovan I, et al. Optimal Thawing of Cryopreserved Peripheral Blood Mononuclear Cells for Use in High-Throughput Human Immune Monitoring Studies. Cells. 2012;1(3):313–324. doi: 10.3390/cells1030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247(3 Pt 1):C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 39.Pegg DE, Diaper MP. On the mechanism of injury to slowly frozen erythrocytes. Biophys J. 1988;54(3):471–488. doi: 10.1016/S0006-3495(88)82980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 41.Toner M, Cravalho EG, Karel M. Cellular-Response Of Mouse Oocytes To Freezing Stress - Prediction Of Intracellular Ice Formation. J Biomech Eng. 1993;115(2):169–174. doi: 10.1115/1.2894117. [DOI] [PubMed] [Google Scholar]
- 42.Hubel A, Toner M, Cravalho EG, et al. Intracellular ice formation during the freezing of hepatocytes cultured in a double collagen gel. Biotechnol Prog. 1991;7(6):554–559. doi: 10.1021/bp00012a011. [DOI] [PubMed] [Google Scholar]
- 43.Svalgaard JD, Haastrup EK, Reckzeh K, et al. Low-molecular-weight carbohydrate Pentaisomaltose may replace dimethyl sulfoxide as a safer cryoprotectant for cryopreservation of peripheral blood stem cells. Transfusion. 2016;56(5):1088–95. doi: 10.1111/trf.13543. [DOI] [PubMed] [Google Scholar]
- 44.Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J. 2000;41(4):187–196. doi: 10.1093/ilar.41.4.187. [DOI] [PubMed] [Google Scholar]
- 45.Mazur P, Leibo SP, Chu EHY. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- 46.Reyes G, Villanueva A, Garcia C, et al. Orthotopic xenografts of human pancreatic carcinomas acquire genetic aberrations during dissemination in nude mice. Cancer Res. 1996;56(24):5713–5719. [PubMed] [Google Scholar]
- 47.Bocsi J, Zalatnai A. Establishment and long-term xenografting of human pancreatic carcinomas in immunosuppressed mice: Changes and stability in morphology, DNA ploidy and proliferation activity. J Cancer Res Clin Oncol. 1999;125(1):9–19. doi: 10.1007/s004320050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy SJ, Hart SN, Halling GC, et al. Integrated Genomic Analysis of Pancreatic Ductal Adenocarcinomas Reveals Genomic Rearrangement Events as Significant Drivers of Disease. Cancer Res. 2016;76(3):749–762. doi: 10.1158/0008-5472.CAN-15-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]