Abstract
Lung disease is a leading cause of morbidity and mortality worldwide. Innate immune responses in the lung play a central role in the pathogenesis of lung disease and the maintenance of lung health, and thus it is crucial to understand factors that regulate them. Hyaluronan is ubiquitous in the lung, and its expression is increased following lung injury and in disease states. Furthermore, hyaladherins like inter-α-inhibitor, tumor necrosis factor-stimulated gene 6, pentraxin 3 and versican are also induced and help form a dynamic hyaluronan matrix in injured lung. This review synthesizes present knowledge about the interactions of hyaluronan and its associated hyaladherins with the lung immune system, and the implications of these interactions for lung biology and disease.
Introduction
Understanding lung biology and immunology has become increasingly important in the past years. Up to 15% of the US population now suffers from asthma and chronic obstructive lung disease (COPD), accounting for an annual total of >16 million lost workdays, 1.5 million hospitalizations and 120,000 deaths, at a cost of >$50 billion [1–4]. COPD is now the third leading cause of death in the US [5]. Globally, COPD and lower respiratory tract infections are among the top 10 leading causes of death across all ages [6]. Infectious lung disease, as well as asthma and COPD, is defined by the immune response to either invading pathogens or inhaled noxious agents such as pollution or cigarette smoke. Thus, the mechanisms that regulate the immune response to pathogens or inhaled noxious agents are of paramount importance in the understanding and treatment of lung disease.
Hyaluronan is ubiquitous in many tissues, including the lung. Recent research has begun to shed light on what appears to be a central role for hyaluronan in the regulation of lung immunity, in response to infections and pollution. In this review, we will synthesize available evidence on hyaluronan-innate immune interactions in the lung, discuss ways to understand the often-conflicting evidence, and point out directions and opportunities for future research. Because there have been several recent reviews on hyaluronan biology, including in the lung [7–12], we will not address this area, but rather discuss lung innate immunity and hyaluronan interactions with it, a subject that has been comparatively less addressed in reviews. We will begin by providing a brief overview of pulmonary innate immunity and its components; we will then discuss interactions of hyaluronan with innate immune receptors, and the variable effects that hyaluronan has on innate immune signaling; we will move on to discuss the interactions of hyaluronan binding proteins, or hyaladherins with cellular and humoral innate immunity, highlighting that hyaladherins truly expand the spectrum of effects of the hyaluronan matrix on the immune system; finally, we will provide a list of still-existing gaps in our knowledge that should be addressed for a more complete picture to emerge. This discussion will hopefully provide the reader with a detailed, but also integrated view of the complex role the hyaluronan matrix plays in the regulation of innate immunity and preservation of lung health.
Lung Innate Immunity: The first line of response to environmental invaders and danger signals
Host mucosal surfaces must maintain an effective barrier between the outside world and the internal host environment. The mucosal interface is a highly regulated checkpoint since it needs to allow a variety of gases, nutrients and cells to transit the barrier in support of normal homeostasis, while retaining the ability to respond to irritants and pathogens. The lung, which is continuously exposed to the external environment, critically regulates these functions. This regulation is physical (epithelial integrity, mucociliary clearance, and cough) and immunologic (cellular and humoral). Balanced and targeted pulmonary immunity is crucial to direct appropriate defense responses while limiting secondary injury. Dysregulated pulmonary immunity leads to either dampened or exacerbated immune function and contributes to the pathogenesis of many common diseases. Therefore, understanding how this system is regulated offers the opportunity to fine-tune lung immunity and maintain appropriate homeostasis.
The pulmonary immune system is divided into innate and adaptive immunity. Innate immunity is a highly evolutionary conserved system that mediates initial responses to pathogens or irritants, promotes clearance, and initiates initial inflammatory cascades. These initial responses are dependent on genetically encoded pattern recognition of pathogens or endogenous motifs, which allows the host to respond to injury without the requirement of prior exposure. Therefore, the innate immune system is always active to respond to pathogens. The adaptive, or acquired immune system, developed in higher-order organisms to allow recognition of prior exposures, to generate targeted and augmented subsequent responses. Adaptive immunity, therefore, provides immunologic memory, allowing the host to adapt and respond to recurrent challenges in the environment. When the immune system functions appropriately, there is an immediate innate response to the initial host-pathogen reaction, which may be followed by development of an adaptive immune response. Communication or cross-talk between the innate and adaptive immune system thus provides the foundation for our current understanding of immunity.
Pulmonary Innate Immunity
The initial mechanisms of pulmonary defense are principally driven by the anatomical structures of the respiratory tract. These are designed to allow gas exchange while protecting against noxious stimuli. Mucociliary clearance removes pathogens and particles deposited in the mucous layer. Pathogens or particles that evade this process, or are too small to be removed in the mucus layer, end up in the alveolus. To maintain the integrity of the lung in this setting, innate immune mechanisms are engaged, and can be divided into cellular and humoral components. The main cellular component involves phagocytes (principally alveolar macrophages) and epithelial cells, which work in concert with lung- and serum-derived humoral factors such as the coagulation and complement systems. The generation of innate immune responses relies on recognition of pathogens via conserved pathogen sequences called pathogen-associated molecular patterns (PAMPs) which are not host-derived. PAMPs include components such as lipopolysaccharide (LPS) of Gram-negative bacteria, lipoteichoic acid of Gram-positive bacteria, β-glucan of fungi, and flagellin of flagellated bacteria [13–15]. These PAMPs interact with soluble factors (both in the airspace and from the circulation) and specific pattern recognition receptors (PPRs) on cell membranes to generate immune responses. Furthermore, initial inflammatory responses also release specific host sequences termed danger-associated molecular peptides (DAMPs). They include extracellular matrix (ECM) components such as hyaluronan, fibronectin and heparin sulphate; stress response elements such as heat-shock proteins (HSPs), nucleic acids and high-mobility group box 1 (HMGB1); and immunomodulary proteins such as surfactant protein A/D and β-defensins [16, 17]. These DAMPs interact with specific PPRs and further regulate initial immune responses by augmenting [18–20] or dampening pulmonary inflammation [21–23] based on local conditions.
Functions of Pattern Recognition Receptors
Given that the innate immune system must respond to a variety of stimuli, it is not surprising that a variety of PPRs are required to recognize PAMPs and DAMPs. The prototypical PPRs are the Toll like receptors (TLR). At this time, 11 TLRs have been identified in humans [24]. Each of these recognize distinct PAMPs to generate immune responses to specific pathogens [25, 26]. Despite this specificity, it has become clear that PPRs have overlapping recognition to individual pathogens. Traditionally, PPRs are restricted to specific cellular compartments. TLR1, 2, 4, 5, 6 and 11 are located on the cell surface, while TLR3, 7, 8, 9 and 10 are in endosomes. Typically, the TLRs based on the cell surface recognize bacterial components, while endosomal TLRs recognize single- and double-stranded RNA and CpG-DNA [27, 28]. Signaling for the TLRs depends on the specific cell type in which it is activated, but generally utilizes two principal pathways, activating either the myeloid differentiation gene 88 (MyD88) [29] or the TIR-domain-containing adaptor-inducing interferon β (TRIF) adaptor proteins [30]. Most TLRs activate MyD88. TRIF signaling is activated by TLR3 [31], and by TLR4 when it is located in the endosome [32], to direct NF-kB activation and activation of the interferon response elements to generate the production of Type I interferons and induction of inducible nitric oxide synthase.
Beyond TLRs, other PPRs regulate innate immune responses. These include the retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), the Nucleotide oligomerization domain (NOD)-like receptors (NLRs) and the C-type lectin receptors (CLRs) [33]. The best characterized pulmonary CLRs are Dectin-1 and -2, which are critical to recognition of fungal infection [34]. Other CLRs in the lung include mannose-binding lectin (MBL) and surfactant proteins. RLRs and NLRs are organized in the cytoplasm. Their function is principally to identify circumstances where pathogens have accessed the cell and are subverting cellular functions to support replication [35–39]. The summation of these PPRs allows for a diversity of innate immune responses to cover a wide variety of pathogens and inflammatory signals.
Pulmonary Cell-Specific Innate Immunity
The diversity of PPRs and their signaling is further regulated by cell-specific expression and functions. In the lung, phagocytes (typically neutrophils and alveolar macrophages) are most frequently associated with innate immunity. However, PPRs are expressed on other cell types including lung structural cells, thus enabling diverse innate immune cellular responses.
Neutrophils are rapidly recruited from circulation in large numbers during acute injury or infection. Neutrophils have a broad array of mechanisms for pathogen clearance/killing including phagocytosis (frequently followed by cell death), secretion of granules, generation of reactive oxygen species, and formation of neutrophil extracellular traps (NETs). Because this neutrophil repertoire can also damage the host, PPRs are expressed on neutrophils to tightly regulate these responses and assure that they are generated against pathogens and not the host (reviewed in [40]). For example, canonical stimulation with TLR2 and TLR4 agonists results in phagocytosis, degranulation, respiratory burst, and migration of neutrophils through the ECM [40, 41]. TLR4, and to a lesser extent TLR2, promote neutrophil survival via NF-kB and MAPKs to prolong their function [42]. Alternatively, TLRs direct NET formation, which is a form of programed cell death where neutrophils extrude their cellular contents to direct further anti-pathogenic functions [43].
Macrophages are critical to pulmonary innate immune responses. Their primary role is to maintain homeostasis and protect delicate pulmonary parenchymal structures. This function requires the ability to dampen immune responses to benign stimuli and yet retain the ability to initiate and propagate pathogen-directed responses. Because of this intricate balancing act, abnormal macrophage function is associated with several chronic pulmonary diseases such as asthma, COPD, cystic fibrosis and pulmonary fibrosis [44]. Macrophages generally express the full complement of PPRs. The diversity of macrophage responses has led to a variety of efforts to characterize their function in vivo [45–47]. Alveolar macrophages (AMs) continuously sample the airspace and phagocytose pathogens or particulates, remove debris and dead cells, clear harmless antigens and perform normal turnover of surfactant. In inflammation, AMs generate reactive oxygen species to kill pathogens and produce both pro-inflammatory and anti-inflammatory cytokines, chemokines and growth factors to coordinate responses with other cells. Beyond release of soluble factors, this coordination includes direct cell-cell contacts, e.g. through the formation of gap junctions with respiratory epithelium after LPS exposure [48]. In addition to AMs, macrophages are also present in the interstitium, termed interstitial macrophages (IMs) [49]. These IMs demonstrate functional diversity to AMs such as increased major histocompatibility class (MHC) II and complement receptor expression, which could suggest specific functions in innate immunity [50]. During inflammation, macrophages derived from monocytes are recruited from the circulation and can exhibit diverse functions in support of inflammation [51, 52] and the development of fibrosis [53]. Therefore, macrophages have an essential and complex role in pulmonary innate immunity.
The identification of PPRs on epithelial cells has led to the understanding of the importance of epithelial innate immunity in support of pathogen clearance (reviewed in [54]). TLR2 and 4 are expressed on epithelium, and upregulated in response to LPS, respiratory syncytial virus, cigarette smoke and Klebsiella pneumoniae [55–58]. Additionally, TLR3 ad RIG-I are both expressed in the epithelium and function to support host-defense against respiratory viruses [59–61]. Signaling in epithelial cells follows similar MyD88 mediated activation of NF-kB and TRIF mediated activation of IFN response elements to produce cytokines and type I IFNs respectively. In addition, PPR signaling generates epithelial-specific defense responses, including secretion of mucins [62, 63] and antimicrobial peptides such as β-defensins [64, 65]. Furthermore, the epithelium supports normal functions of innate immune cells: epithelial GMCSF is required for normal alveolar macrophage function [66–68] and epithelial cytokines recruit neutrophils to sites of lung infection. Much remains to be understood about the complex interactions between pulmonary epithelia and immune cells in the context of innate immunity.
Humoral innate immunity
The humoral arm of the innate immune system consists of a diverse set of soluble factors that recognize pathogens and generate immune responses. The major categories of these include the complement, pentraxins, and collectins [69]. These function as primitive antibody-like factors that act independently but also support or augment cell-mediated innate immune responses.
Complement is a highly conserved network of proteins defined by their ability to rapidly develop immune responses to remove microbial pathogens [70, 71]. In addition to microbial PAMPs, endogenous DAMPs can also activate complement [72]. The activation of the complement cascade is frequently defined by the cleavage of central proteins C3 and C5 into the bioactive intermediates C3a and C5a. Complement activation occurs via three distinct pathways [73]. The classical pathway is activated when C1q binds to an antigen-antibody complex [74]. The lectin pathway is activated when mannose-binding lectin (MBL) binds to pathogenic carbohydrate motifs. The alternative pathway can be activated in overlapping processes, which result in tagging pathogenic cell surfaces with C3b that activates further downstream cascades [70]. The end products generate complement species that support pro-inflammatory signaling (C3a and C5a), cell lysis via the membrane attack complex (C5b-C9) and phagocytosis (via C3b). The coagulation cascade can also support complement activation via the contact system: Factor XII is cleaved to FXIIa by damaged cells, which generates kallikrein from its pro-form. FXIIa, kallikrein, and other contact system intermediates can then directly activate complement [75].
Pentraxins are a superfamily of evolutionarily conserved proteins that recognize and bind pathogens and endogenous ligands to facilitate innate immune responses [76–79]. They are characterized by the presence of a pentraxin domain at the C terminus [80]. Based on the number of additional domains, pentraxins are divided into short and long families. The short pentraxins include C-reactive protein and serum amyloid P protein, which are acute phase reactants produced by the liver and frequently measured as markers of inflammation. Pentraxin 3 (PTX3) is a long pentraxin produced by several cell types and will be discussed in more detail below. Pentraxins are recognized by Fcγ receptors on macrophages and other immune cells to facilitate phagocytosis and clearance [81, 82]. Additionally, PTX3 has binding sites for complement components (C1q and Factor H) [83, 84], and the hyaladherins tumor-necrosis factor α-induced protein 6 (TSG-6) and inter-α-trypsin inhibitor (IαI) [85], suggesting the ability to link changes in ECM with regulation of immune responses (see specific sections below for more detail).
Collectins are a family of proteins which include mannose-binding lectin (MBL) and surfactant proteins–A and –D [80]. MBL is produced in the liver and released in the circulation. SP-A and SP-D are produced by lung epithelium and submucosal cells, although extra-pulmonary production has been described [86–89]. Collectins are defined by a common structure, which includes a C-terminal carbohydrate recognition domain, a short α-helix neck region, a collagenous region, and a cysteine-rich N-terminal region. They form large oligomers that can bind microorganisms and other components through the carbohydrate-binding domain by recognition of pathogenic carbohydrates and lipids. This allows for wide recognition of a variety of bacterial and fungal pathogens and their products, including LPS, peptidoglycan and β-glucan [89]. Collectins also recognize glycoproteins on the surface of the viral envelop and have broad anti-viral activities [90]. Following recognition, collectins interact with cells of the innate immunity through their collagenous tail, resulting in recognition and clearance by phagocytes and activation of the lectin pathway of the complement system [91, 92]. Collectins also recognize damaged cells and facilitate their clearance to maintain homeostasis and resolve inflammation [93].
In summary, lung innate immunity is comprised by a complex system of humoral and cellular components, which serve to protect from invading pathogens, restore homeostasis after lung injury, and maintain lung function. As we will see below, hyaluronan and its associated hyaladherins play a central role in regulating the functions of the pulmonary innate immune system. In the following segments, we will discuss evidence that the hyaluronan matrix can activate, as well as, inhibit innate immune receptors on cells, the complement system and the coagulation cascade. From this discussion, it will become clear that hyaluronan-associated hyaladherins are an integral part of the functions of the hyaluronan matrix on lung immunity, and that hyaluronan and its associated proteins should be viewed as a versatile and adaptable unit supporting the structure and function of the lung. Finally, in every segment we will note the implications of these interactions with lung disease.
Innate immune recognition of hyaluronan – role of toll-like receptors
Hyaluronan activates the innate immune system in several ways: first, hyaluronan can activate TLR signaling, mainly through TLR2 and TLR4. Second, hyaluronan can induce the expression of innate immune receptors, or mobilize them to the cell membrane, and thus prime them for subsequent activation by their cognate ligands. Alternatively, hyaluronan can inhibit TLR signaling, either directly or indirectly. Finally, hyaluronan and innate immune receptors can act as co-factors in the activation of immune pathways via interactions with the collectins.
Activation of lung TLR by hyaluronan
Innate immune activation by hyaluronan involves primarily TLR2 and TLR4. Although the conserved repeating disaccharide structure of hyaluronan fulfills criteria for being a pathogen- or danger-associated molecular pattern, hyaluronan does not share biochemical homology with the respective TLR2 and TLR2 cognate ligands peptidoglycan/lipoteichoic acid and lipopolysaccharide (LPS), and its precise interaction with these receptors is unclear. For many interactions, a signaling complex with CD44 and an innate immune receptor may be necessary, whereby CD44 “captures” the hyaluronan molecule while the TLR is activated. For example, in sterile skin injury, a unique complex of TLR4, MD-2, and CD44 was described, which recognizes hyaluronan [94]. However, TLR4-mediated hyaluronan signaling can also occur in the absence of CD44, even when reasonable precautions were taken to exclude LPS contaminating effects [95]. Therefore, CD44 is not necessarily required for hyaluronan-innate immune interactions. A possible explanation for this observation is that there are many hyaluronan receptors beyond CD44. Thus, if TLRs can interact with hyaluronan in the absence of a hyaladherin remains to be clarified. Additional research will be required to address this question.
The variability in hyaluronan formulation in scientific reports requires consideration when we attempt to interpret the evidence on hyaluronan-innate immune interactions. The major differing characteristic of hyaluronan formulation are the fragment size, and whether individual discrete sizes (usually commercially obtained) or mixtures of sizes (usually generated through degradation in the laboratory) are being used [96]. In general, it appears that hyaluronan of 500 kDa or lower molecular weight (including oligosaccharides) induces TLR-mediated inflammation [95, 97, 98], while hyaluronan of >500 kDa molecular weight is inert in terms of inflammation. A recent report provided some insights into the potential molecular interaction of hyaluronan with TLRs, showing that the N-acetyl groups on hyaluronan are important for TLR4 signaling, while partial de-acetylation or selective butyrylation of hyaluronan created molecules that were either inert, or even anti-inflammatory along the TLR4 axis [99]. High-molecular weight hyaluronan inhibits the pro-inflammatory activity of lower molecular weight hyaluronan [100, 101], but in some contexts can also activate TLR signaling. For example, overexpression of hyaluronan synthase 2 (HAS2), generally thought to express high molecular weight hyaluronan [102, 103], promotes epithelial resiliency to injury through NFκB activation, an effect that requires TLR4 and TLR2 [95]. TLR4 activation through HAS2-generated hyaluronan also supports alveolar progenitor cell renewal, possibly via IL-6 expression [104]. Thus, both higher and lower molecular weight hyaluronans appear able to activate lung TLR signaling, albeit with different effects (inflammation vs. epithelial resilience). The reasons and mechanisms underlying these differences remain unclear.
Increased concentrations of lower molecular weight hyaluronan are found in the lungs in virtually every disease [7, 8] and are generated during acute lung inflammation [97, 100, 101]. TLR induction is also observed in lung diseases like asthma [105], thus creating favorable conditions for TLR-hyaluronan interactions. For example, hyaluronan-induced, TLR4-mediated lung inflammation and hyperresponsiveness is a major pathway after lung exposure to the pollutant gas ozone [97, 100]. Importantly, formation of a hyaluronan matrix through hyaluronan interactions with TSG-6 and inter-α-inhibitor heavy chains are a crucial step in this physiologic response [97, 106]. This suggests that proper conformation or presentation of hyaluronan to TLR4 may be dependent on its incorporation in an ECM milieu (more about this below).
Among all TLRs, TLR4 is the most commonly described receptor that can be activated by hyaluronan. In some instances, TLR2 may contribute to signaling – for example, in murine macrophages, combined TLR2 and TLR4 deficiency abolished the inflammatory response to low molecular weight hyaluronan (as did MyD88 deficiency), while single TLR2 or TLR4 deficiency merely reduced this response [95]. This would suggest that both TLR2 and TLR4 cooperate in hyaluronan signaling, and their pathways converge through the MyD88 adaptor protein. A similar observation was made in another model system, i.e. pro-catabolic responses in mesenchymal cells [107], suggesting that this signaling pathway may be conserved in other organ systems. However, in the lung TLR2 did not appear to be necessary for the protective role of hyaluronan in progenitor cell recovery after injury [104]. Furthermore, depending on the mode of injury, other TLRs may be involved in hyaluronan signaling: we described recently that TLR5 participates in the TLR4 complex in response to in vivo ozone or hyaluronan exposure and modulates the signaling response towards MyD88-mediated cytokine production [108]. Depending on cell type, the downstream signaling pathway after hyaluronan exposure may diverge. For example, murine alveolar macrophages express IFNβ after exposure to hyaluronan fragments; independent of MyD88 but dependent on TLR4 and TRIF [109].
Finally, hyaluronan-TLR interactions may also contribute as a co-factor along other signaling pathways. For example, Foley et al. determined that TLR2, hyaluronan receptor RHAMM (but not CD44) and hyaluronan are required for alveolar macrophage chemotaxis after stimulation by the collectin, surfactant protein A [110]. Thus, it appears that different TLRs and adaptor molecules may participate in hyaluronan signaling depending on injury (and perhaps cell) type. It is likely, that TLR receptor complexes, or converging signaling pathways from other cell receptors, determine the ultimate response to hyaluronan-TLR engagement.
Priming of lung innate and adaptive immunity by hyaluronan
The effect of hyaluronan in lung immunity starts at the level of cell survival. Recently, it was shown that hyaluronan promotes survival and function of alveolar macrophages through CD44 [111]. Although an interaction with a specific TLR was not discussed in that work, TLR4 activation does support macrophage survival [112], and thus it is entirely possible that TLR4-hyaluronan interactions play a role in immune cell survival similar to their effect on epithelial cells [95]. Regardless, hyaluronan supports survival of alveolar macrophages, which is a prerequisite for lung innate immune responses. Beyond this, hyaluronan fragments released during lung injury (e.g. after ozone exposure), induce the mobilization of TLR4 to the cell membrane, and thus prime the lung for a subsequent response to TLR4 activators like lipopolysaccharide [113]. Induction of TLR4 expression by hyaluronan has not been described in the lung, but has been identified in mesenchymal cells [114, 115], and therefore it is possible that in addition to trafficking, TLR4 expression could be increased in lung injury.
Hyaluronan priming of innate and adaptive immunity has been recently described in the solid organ transplant field. Lung allograft rejection is a major cause of morbidity and mortality after lung transplantation [116, 117], and innate immune activation plays a significant role in the pathogenesis of lung allograft rejection [117, 118]. There is significant accumulation of hyaluronan in diseased lung tissue in lung allograft rejection [119–122], which has led scientists to question a potential role of hyaluronan in its pathogenesis. Dendritic cells, which are potent presenters of antigens that induce alloimmunity, can be activated by hyaluronan through TLR4 [123, 124]. Further priming of alloimmunity by hyaluronan-activated dendritic cells requires TLR4 signaling and the presence of TIRAP, a TLR adaptor downstream of TLR2 and TLR4, but not MyD88 or TRIF [122]. Furthermore, neutralization of hyaluronan through administration of peptide pep-1, or removal from the lung though the lymphatic system, improved outcomes in lung rejection [119, 121]. Thus, hyaluronan fragments seem to promote allograft rejection through activation of alloimmunity. This process is clearly TLR dependent. Todd et al. showed that TLR2/4 and MyD88 were necessary for the reduction in immune tolerance and activation of the alloimmune process [120]. Interestingly, in the same report high molecular weight hyaluronan antagonized the lower molecular weight hyaluronan effects [120], suggesting that the signaling process leading to alloimmune activation is size-specific.
Inhibition of lung TLR signaling by hyaluronan
Potential inhibitory effects of hyaluronan on TLR activation in the lung have not been well investigated. We previously demonstrated that ozone-induced airway inflammation and hyperresponsiveness was mediated by low molecular weight hyaluronan and TLR4, and that high molecular weight hyaluronan antagonized the effects of ozone and low molecular weight hyaluronan [97, 100]. This effect was observed in other types of oxidative lung injury as well [101]. Thus, high-molecular weight hyaluronan inhibits TLR4 activation induced by low-molecular weight hyaluronan. This suggests a possible homeostatic effect of native, steady-state hyaluronan in competition with lower molecular weight hyaluronan fragments released during inflammatory conditions.
In LPS-induced TLR4 activation, pre-treatment with hyaluronan (of mixed sizes, up to 500 kDa) inhibited subsequent LPS-induced sepsis in a CD44-dependent manner, possibly through the induction of innate immune suppressor gene A20/Tnfaip3; this induction was both CD44- and TLR4-dependent [125]. In another study, CD44 protected from (high-dose) LPS-induced severe lung inflammation, and was associated with induction of anti-inflammatory molecules IL-1R-associated kinase-M, Toll-interacting protein, and A20 [126]. However, the role of CD44 in LPS-induced lung injury is not entirely resolved, since in another model of (lower dose) LPS-induced milder inflammation, CD44 promoted inflammatory cell influx and inflammatory cytokine expression [127]. The different models utilized, i.e. severe vs. mild lung inflammation, may explain the conflicting results between these two studies.
TLR4 activation by hyaluronan may also lead to inhibition of other innate immune receptors. In murine alveolar macrophages, TLR4 activation by hyaluronan oligosaccharides suppressed subsequent activation of TLR3 by its agonist polyI:C [128]. This trans-inhibitory effect was mediated by A20, similar to the cis-inhibitory effect described above in LPS-induced lung inflammation [125]. The findings of hyaluronan-induced, TLR4-mediated suppression of inflammation in the lung are supported by research in other tissues. In fibroblasts, high molecular weight hyaluronan inhibited TLR4-induced expression of the ligand for the receptor activator of nuclear factor-κB (RANKL) [129], and also inhibited TLR4-dependent expression of cathepsin K and MMP-1, in a manner that was CD44 dependent, and partially ICAM-1 dependent [130]. Furthermore, hyaluronan upregulated IL-1R-associated kinase-M (IRAK-M) to deactivate human monocytes, an effect mediated by CD44 and TLR4 [131].
In summary, available data suggest a multifaceted and diverse or at times conflicting roles of hyaluronan in its interactions with innate immune receptors. Research supports two broad conclusions: first, high molecular hyaluronan antagonizes lower molecular weight hyaluronan in TLR4-mediated inflammation, but can also activate TLR4 signaling in support of cell survival; second, hyaluronan-induced TLR4 signaling may lead to both activation and inhibition of innate immunity. These data strongly suggest that other, unknown co-factors must be involved in hyaluronan-TLR4 interactions to bias responses towards pro- or anti-inflammatory pathways. Receptor clustering is affected by hyaluronan size [132], as is hyaluronan uptake and possible intracellular signaling [133]. It is probable that hyaluronan size, the presence of co-activating or - inhibiting factors, and cell-specific signaling characteristics (i.e. membrane receptors, adaptor molecules and kinases) influence hyaluronan-signaling effects and underscore the versatility of hyaluronan as an immune mediator in the lung.
Humoral innate immunity and hyaluronan: role of matrix hyaladherins in the regulation of lung inflammation
Although often overlooked, humoral innate immunity is a crucial component of the initial response to invading pathogens or sterile tissue injury. The complement and coagulation cascades, in particular, are often the first lines of defense against invading pathogens as immune-activating agents. These factors are in close contact with the ECM, and interactions of matrix components with humoral innate immunity are increasingly recognized. Hyaluronan-binding matrix components are thus an important component of the non-cellular innate immune response.
Inter-α-inhibitor (IαI) and tumor necrosis factor stimulated gene 6 (TSG-6)
IαI and TSG-6 are hyaladherins, which support the deposition of a hyaluronan-based ECM. IαI is a family of composite proteins comprised of a common light chain and several closely related heavy chains (HCs). The light chain consists of a chondroitin 4-sulfate domain and a core protease inhibitory moiety, bikunin, which lends IαI its name. One bikunin molecule is usually linked to 1 or 2 HCs via a unique ester bond, producing pre-α-trypsin inhibitor (Pαl, HC3-bikunin in humans) and lαI (HC1·bikunin-HC2 in humans) [134–136]. HC 1–3 have been well characterized [136] and exhibit considerable sequence homology (40–60% of the amino acid sequence). Typically, they are produced in the liver and coupled with bikunin before release into the circulation, but also can be produced in other organs, including the lung [137]. In lung inflammation, IαI HCs are transferred to hyaluronan, which is catalyzed by tumor necrosis factor-stimulated gene 6 (TSG-6), a 35-kDa secreted ECM hyaladherin. TSG-6, as its name implies, is strongly induced by TNFα, and secreted by a number of immune and structural cells [138, 139]. Thus, during inflammation, induction of TSG-6, and extravasation (as well as local de novo production) of IαI set the stage for interaction with hyaluronan and the deposition of a “pathological” hyaluronan matrix [106, 140, 141].
There are interesting (and very similar) paradoxical effects of TSG-6 and IαI in the pathogenesis of lung inflammation. Both proteins are increased in lung diseases such as asthma [140–143] and cystic fibrosis [144, 145], and after lung injury [101, 106]. Both proteins are necessary for the generation of a pathological hyaluronan matrix, and both are necessary for the development of airway inflammation and hyperresponsiveness in allergic and TLR4-mediated lung injury [100, 101, 106, 141]. On the other hand, both have prominent anti-inflammatory activities in other lung disease models. TSG-6 protects from lung inflammation after endotoxin [146] and bleomycin exposure [147]. In fact, TSG-6 is believed to be the determining factor for beneficial effects of stem cells [148–150] when these are applied with regenerative intent. The ?beneficial? TSG-6 effect may, in some cases, come through downregulation of CD44, i.e. indirectly affecting hyaluronan binding and signaling [151]. IαI binds strongly to coagulation factor IX [152] and is a strong factor XI inhibitor [153]. The bikunin moiety inhibits plasma- and cell-bound plasmin [154], supporting intravascular and tissue injury roles for IαI. Indeed, IαI significantly ameliorates disseminated intravascular coagulation and lung injury in LPS-induced sepsis [155]. Furthermore, IαI inhibits complement activation [156–158] and prevents complement-induced lung injury [156]. IαI interactions with the ECM do not affect its ability to inhibit complement, thus implicating IαI as an important complement inhibitor in local inflammatory processes once enriched in the matrix [158]. Finally, IαI may directly bind invading pathogens. For example, dengue infection leads to severe IαI deficiency, which predicts disease severity [159]. The domain of the dengue viral envelope glycoprotein has been shown to bind to several IαI heavy chains [160]. This binding was attributed to the HC chain gamma-carboxyglutamic (Gla) domain, which also binds other viruses such as adenovirus and HIV [161, 162]. Beyond pathogen binding, shotgun proteomic analysis of histone-spiked plasma showed that histones bind to IαI [163], and can neutralize histone-induced adverse effects [164]. Thus, IαI may sequester infectious and danger-associated molecular patterns in plasma and tissue and thereby inhibit lung inflammation.
Pentraxin-3 (PTX3)
IαI heavy chains in the hyaluronan matrix are crosslinked through PTX3 [165]. PTX3 itself can be induced via TLR activation [166] and has a role in lung injury repair [167], antimicrobial lung defense [168, 169], and allograft survival in lung transplantation [170]. PTX3 in the lung lavage fluid is increased during microbial infections and may protect from the development of bacterial pneumonia [171–175] and invasive aspergillosis in lung transplant patients [176], suggesting that PTX-3 plays a role in microbial defense. In fact, genetic variability in PTX3, leading to decreased PTX3 function, is associated with increased susceptibility to invasive aspergillosis in stem cell transplant patients [168]. PTX3 further contributes to lung health by suppressing the development of allergic lung inflammation [177], ameliorating LPS-induced lung injury (like TSG-6 and IαI) [178], and promoting lung injury repair, fibrin deposition and fibrosis in a lung injury model of acid aspiration [167]. However, there are also reports of adverse PTX3 effects in lung disease. In human lung transplant patients, increased PTX3 levels are associated with graft dysfunction (i.e. ischemia reperfusion injury) [179] and genetic variability in PTX3, leading to increased serum PTX3 levels, predisposes to graft dysfunction [170]. Like IαI, PTX3 can bind viral antigens, such as the influenza hemagglutinin [76]. Also like IαI, PTX3 interacts with the complement, but with conflicting effects on activation [180][181].
How can we synthesize these data? We believe that the conflicting reports on the role of TSG-6, IαI and PTX3 in lung inflammation are explained by the inflammatory mechanism underlying the diseases. I.e. when pathological HA matrix formation is necessary for the development of disease, as is the case with airway hyperresponsiveness after ozone or in asthma [97, 100, 141–143], IαI and TSG-6 promote disease by mediating the formation of pathological hyaluronan matrix. However, in endotoxin lung inflammation, and other non-hyaluronan-mediated processes [146, 148], TSG-6 and IαI-supported hyaluronan matrices may promote an anti-inflammatory role, by providing a lattice for complement- or coagulation factor inhibiting properties. Thus, TSG-6 and IαI are pro-inflammatory in oxidative lung injury and asthma (where hyaluronan is the primary agonist), but anti-inflammatory in LPS, bleomycin, sepsis (where hyaluronan is not the pro-inflammatory agonist). The conflicting activities of PTX3 in lung disease, although slightly different, also harken back to the above-described duality of IαI and TSG-6 effects: PTX-3 protects from lung infection and allergic lung inflammation, but promotes the development of allograft dysfunction, a process in which a pathological hyaluronan matrix has been prominently implicated [119–121]. The interaction of PTX3 with the coagulation system also has interesting parallels to IαI, and suggests that hyaluronan matrix components may play a central role in tissue-based coagulation cascade activation. Moreover, the observation that TSG-6, IαI and PTX3 have very similar effects in similar models of lung injury may suggest that in fact, these are not parallel, but interrelated actions; i.e. we are observing the effects of a hyaluronan-linked IαI/PTX3 matrix facilitated by TSG-6. In aggregate, these data support the hypothesis that a hyaluronan-based matrix, which includes cross-linked PTX3 and IαI heavy chains, modulates innate immune activation in the lung; furthermore, the process of hyaluronan matrix deposition itself (through the involvement of TSG-6 and the release of bikunin) additionally modifies innate immune responses.
Versican
Lung hyaluronan is also decorated by proteoglycans like versican. An excellent review of versican in lung inflammation was published recently [182], thus here we will summarize versican effects specifically on lung innate immune activation. Versican deposition is increased in airways of patients with idiopathic pulmonary fibrosis [183], severe ARDS [184], asthma [185, 186] and in animal asthma- or viral infection models of lung inflammation [187, 188]. Several cell types in the lung are able to express versican. In allergic asthma models and ex vivo examined cells from asthma patients, airway epithelia expressed versican, and may contribute to the subepithelial deposition of versican in asthmatic airways [187].
Undoubtedly, mesenchymal cells are major versican producers, and their versican production is further induced in disease; for example, increased versican production was described in fibroblasts from mild asthmatics [189], moderate to severe COPD [190] and transplanted lungs [191]. The mechanisms of this induction can be innate-immune triggered: versican production by mesenchymal cells was induced by innate immune activation through the TLR3 ligand polyI:C [192]. Mesenchymal versican production modulates immune responses during inflammation. Versican interacts with, and regulates the availability and thus activity of several chemokines [182]. Furthermore, in an in vitro model of mesenchymal-immune cell interaction, mesenchymal-expressed versican supported retention of T-lymphocytes through polyI:C-induced hyaluronan cable-like structures laid down by the mesenchymal cells [192]. Versican also promoted TLR3-dependent lung inflammation [188] via a dual mechanism of enhancing CCL2-mediated chemotaxis and promoting retention of inflammatory cells within hyaluronan cables [188, 192]. Interestingly, in another study by the same group, versican downregulation also promoted retention of immune cells among the hyaluronan cables [193]. The experimental conditions were not identical between these studies; it appears that polyI:C-generated hyaluronan cables and versican generally promote immune cell adhesion, while in the second instance T-lymphocytes led to a degradation of versican in the hyaluronan matrix, but may have promoted other matrix factors that were not examined. These data suggest that we are still missing critical pieces of information about the interaction between hyaluronan, proteoglycans, mesenchymal cells and immune cells during inflammation.
Immune cells can be also induced to express versican, for example via LPS (TLR4) [194] or polyI:C (TLR3) [195] stimulation, in gram-negative bacterial pneumonia [194] or by hypoxia [196]. Versican expression by alveolar macrophages depends on TRIF, but not MyD88 activation, and requires intermediary expression of type I interferons [194]. Interestingly, genetic deficiency in macrophage versican expression exacerbated lung inflammation to instilled polyI:C in vivo, which was associated with decreased macrophage expression of the anti-inflammatory cytokines IL-10 and IFN-β [195]. Thus, versican appears to exhibit anti-inflammatory (through macrophage expression) or pro-inflammatory (through mesenchymal ell expression) properties in lung disease dependent on cell-specific production. Clearly, much remains to be understood about versican biology and its interactions with the innate immune system in lung disease. It is possible, that macrophage-derived versican acts in an autocrine or paracrine fashion, and inhibits macrophage activation via interferon induction, whereas mesenchymal-derived versican acts as a chemotactic and cell-retaining matrix. Furthermore, versican is also a direct TLR agonist, and activates TLR2/6-dependent signaling to induce TNF-α, IL-6 and IL-1β expression in tumor-associated macrophages [197, 198]. It is not clear, however, if versican-TLR interactions involve hyaluronan, a known TLR2 agonist [198]. This interaction adds another layer of complexity in the role of versican in immune cell functions.
Hyaluronan-immune interactions in lung health and disease: Future directions and outstanding questions
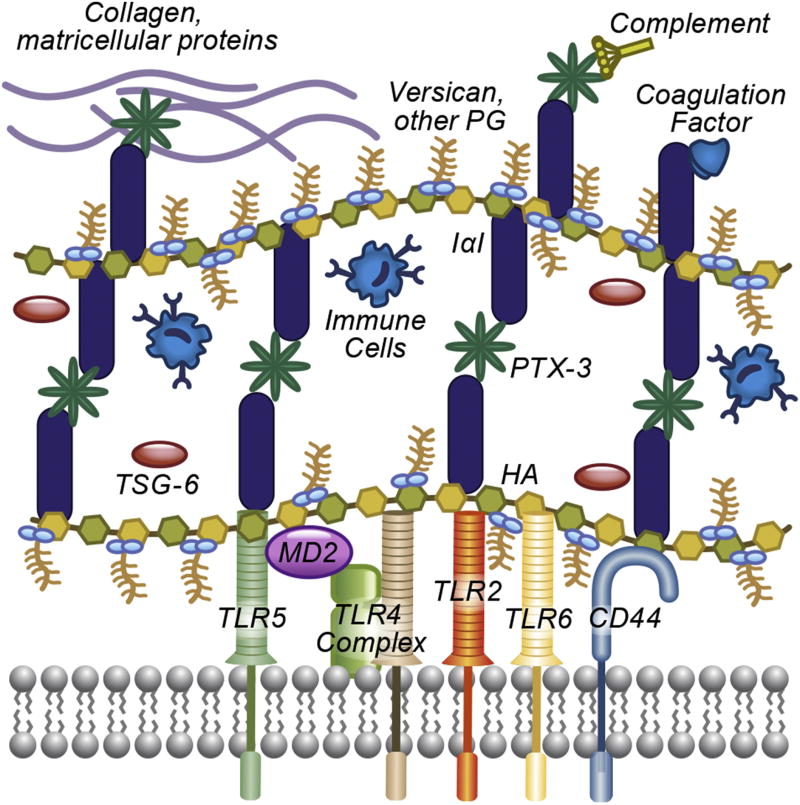
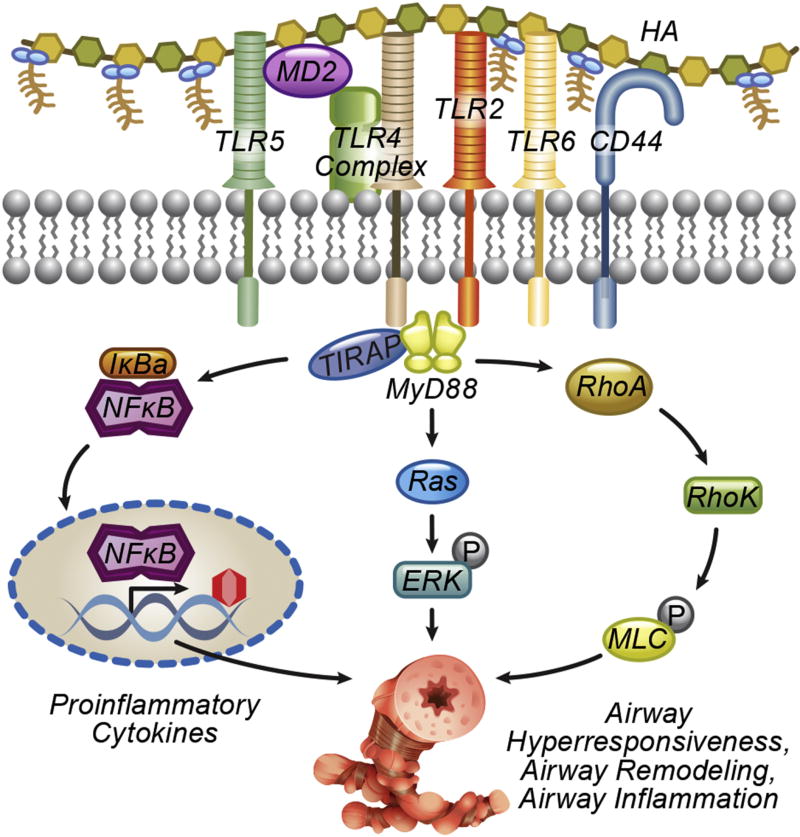
Innate immunity plays a pivotal role in response to environmental threats and tissue injury. The above data highlight the complex role of hyaluronan in modulating the innate response. Hyaluronan can directly activate, prime, or inhibit, pulmonary innate immunity. Moreover, it is becoming increasingly clear that hyaluronan innate immune signaling should be viewed in the context of the ECM, where it interacts with other proteins and proteoglycans, such as inter-α-inhibitor, TSG-6, PTX3 and versican. These factors influence hyaluronan signaling directly, by altering its conformation and availability to receptors, but also greatly expand its signaling repertoire, by enabling interactions with coagulation and complement factors, cytokines and chemokines, and cell receptors (Figure 1). On the cellular side, there is a versatile array of receptor complexes and adaptor molecules, which modulate the cellular response to hyaluronan (Figure 2). Clearly, much remains to be understood about the innate immunology of hyaluronan. Several outstanding questions deserve our attention:
What mechanism underlies the signaling differences of high and lower molecular weight hyaluronan? Does size modulate receptor clustering or affinity, compartmentalization of hyaluronan or interactions with IαI, TSG-6 and versican? Does the hyaluronan size mixture generated in airway disease affect signaling, and is it possible that a dynamic shift in hyaluronan sizes during the response to injury dictates signaling effects?
What is the mechanism of hyaluronan-mediated promotion of cellular homeostasis and survival? We now have evidence that hyaluronan promotes survival of differentiated lung cells, e.g. epithelial [95, 104] and immune cells [111]. The role of innate immunity, mediated through factors such as NFκB [95] and IL-6 [104] has been described, but many gaps remain to be filled. NFκB and IL-6 are commonly induced after TLR4 activation, yet most TLR4 ligands do not promote cell survival. Thus, cofactors or other signaling pathways must be contributing to this effect.
Is there a difference between hyaluronan matrix produced in an autocrine manner, and hyaluronan that the cell encounters as it navigates the extracellular space? The regulatory effects of high molecular weight hyaluronan in epithelia [95, 104] and versican in macrophages [195] were not observed when HAS2 or versican were expressed by fibroblasts [188, 193, 199, 200]. Is cell-associated hyaluronan directly tethered to the cell [201] inducing different signaling pathways than receptor-associated hyaluronan [9]?
Are unique tissue compartments different in their hyaluronan bioactivity? The temporary laying down of hyaluronan and versican matrix after injury has been termed “provisional matrix” [202] and this concept could be expanded to describe a temporarily and spatially highly dynamic modification of hyaluronan, both by expression, degradation and interaction, that guides the immune response and cell fate after injury. In support of this, a recently published proteomic investigation of the ECM after bleomycin lung injury revealed that IαI heavy chains 1–3 peaked 7 days after injury and were in the relatively soluble (i.e. not fibrotic, fraction of the matrix), while heavy chain 5 peaked after day 14 and was found in the insoluble (i.e. fibrotic) fraction [203]. Thus, the hyaluronan matrix becomes a canvas, which can be woven and unraveled, and decorated by ever-changing patterns of hyaladherins to dictate hyaluronan-specific effects and roles after injury.
Are there other classes of effects of hyaluronan on the lung innate immune system? In the gut, oral administration of human milk-derived hyaluronan induces defensins and protects from bacterial colitis [204]. Could hyaluronan expression in the lung have similar effects? Hyaluronan is easily found in the airway lining fluid [144]. Does it have such activity?
Figure 1.
Formation of an extracellular hyaluronan matrix and interactions with the humoral and cellular innate immune system. In lung injury, extracellular hyaluronan is bound to IαI heavy chains through TSG-6, and the matrix is interconnected by heavy chain binding to PTX3. Heavy chains and PTX3 facilitate interactions with humoral innate system such as the complement, coagulation factors, collagen and other matricellular proteins (e.g. fibronectin, vitronectin). Versican finds to hyaluronan and facilitates chemokine and cytokine release. Both versican and IαI participate in the retention of immune cells in the “sticky” hyaluronan matrix. In addition, hyaluronan interacts with innate immune receptors TLR2, TLR4 and TLR5, and versican interacts with TLR2 and TLR4 to activate intracellular signaling. CD44 and other membrane hyaluronan receptors may participate in the signaling cascade.
Figure 2.
Intracellular activation of innate immune signaling by hyaluronan matrix. Activation of innate immune receptors TLR2, 4 and 5 leads to the activation of the MyD88 pathway, resulting in NFκB activation and inflammatory cytokine release (e.g. IL-6, TNFα). Activation of kinases (examples shown: ERK, RhoA, Rho kinase, myosin light chain) lead to additional pro-inflammatory responses, cell migration and contraction. Signaling by intracellular hyaluronan signaling by intracellular receptors or the inflammasome is also possible but less well understood and not shown here.
The above are only some of several outstanding issues that investigators in this field are attempting to answer. Ongoing research in many labs will help address these and other questions and enable us to translate our insights into medical advances. The payback, with regard to improvement in lung health and decreased human morbidity and mortality, will certainly be substantial.
Highlights.
Hyaluronan and lung-related hyaladherins (IαI, TSG-6, PTX3, versican) are highly expressed in lung tissue and respond dynamically to lung injury
Hyaluronan and its hyaladherins activate and modulate the lung innate immune system in response to injury
Hyaluronan-innate immune interactions in the context of lung injury are highly variable and suggest that the hyaluronan matrix is a major regulator of the lung innate immune response.
Acknowledgments
This work was funded partly through the Division of Intramural Research, National Institute of Environmental Health Sciences (to SG) and R01ES027574 (to RMT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SG and RMT contributed to the drafting of this manuscript.
References
- 1.Mannino DM, et al. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 2.Redd SC. Asthma in the United States: burden and current theories. Environ Health Perspect. 2002;110(Suppl 4):557–60. doi: 10.1289/ehp.02110s4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochanek KD, et al. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60(3):1–116. [PubMed] [Google Scholar]
- 4.Ford ES, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 5.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):502–6. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 6.Collaborators, G.B.D.C.o.D. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer ME, et al. The Rise and Fall of Hyaluronan in Respiratory Diseases. Int J Cell Biol. 2015;2015:712507. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garantziotis S, et al. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol. 2016;310(9):L785–95. doi: 10.1152/ajplung.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev. 2016;97:186–203. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 14.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111(7):927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 16.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol. 2013;229(2):145–56. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 17.Sloane JA, et al. A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med. 2010;12(2):149–63. doi: 10.1007/s12017-009-8094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura Y, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 19.Vabulas RM, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 21.Henning LN, et al. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180(12):7847–58. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CF, et al. Surfactant protein D inhibits mite-induced alveolar macrophage and dendritic cell activations through TLR signalling and DC-SIGN expression. Clin Exp Allergy. 2010;40(1):111–22. doi: 10.1111/j.1365-2222.2009.03367.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamazoe M, et al. Pulmonary surfactant protein D inhibits lipopolysaccharide (LPS)-induced inflammatory cell responses by altering LPS binding to its receptors. J Biol Chem. 2008;283(51):35878–88. doi: 10.1074/jbc.M807268200. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97(25):13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brubaker SW, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–90. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexopoulou L, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 28.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 30.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 32.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9(4):361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272(24):6179–217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 34.Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol. 2011;23(8):467–72. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- 35.Seth RB, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Girardin SE, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300(5625):1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 37.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 38.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34(7):317–28. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 42.Sabroe I, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170(10):5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 43.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 44.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 45.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne AJ, et al. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015;70(12):1189–96. doi: 10.1136/thoraxjnl-2015-207020. [DOI] [PubMed] [Google Scholar]
- 47.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17(1):9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 48.Westphalen K, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506(7489):503–6. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu YR, et al. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am J Respir Cell Mol Biol. 2016;54(1):13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibbings SL, et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am J Respir Cell Mol Biol. 2017;57(1):66–76. doi: 10.1165/rcmb.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin KL, et al. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180(4):2562–72. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 52.Mould KJ, et al. Cell Origin Dictates Programming of Resident versus Recruited Macrophages during Acute Lung Injury. Am J Respir Cell Mol Biol. 2017;57(3):294–306. doi: 10.1165/rcmb.2017-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Misharin AV, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017 doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong L, et al. Expression of functional toll-like receptor-2 and-4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31(2):241–5. doi: 10.1165/rcmb.2004-0078OC. [DOI] [PubMed] [Google Scholar]
- 56.Monick MM, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278(52):53035–44. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- 57.Pace E, et al. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology. 2008;124(3):401–11. doi: 10.1111/j.1365-2567.2007.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regueiro V, et al. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect Immun. 2009;77(2):714–24. doi: 10.1128/IAI.00852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Goffic R, et al. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178(6):3368–72. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183(11):6989–97. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groskreutz DJ, et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176(3):1733–40. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 62.Chen R, et al. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324(3):1087–94. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 63.Zhu L, et al. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009;40(5):610–9. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hertz CJ, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171(12):6820–6. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, et al. Airway epithelia regulate expression of human beta-defensin 2 through Toll-like receptor 2. FASEB J. 2003;17(12):1727–9. doi: 10.1096/fj.02-0616fje. [DOI] [PubMed] [Google Scholar]
- 66.Trapnell BC, et al. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol. 2009;21(5):514–21. doi: 10.1016/j.coi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berclaz PY, et al. GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol. 2007;36(1):114–21. doi: 10.1165/rcmb.2006-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210(10):1977–92. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shishido SN, et al. Humoral innate immune response and disease. Clin Immunol. 2012;144(2):142–58. doi: 10.1016/j.clim.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ricklin D, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190(8):3831–8. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol. 2014;772:229–62. doi: 10.1007/978-1-4614-5915-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 74.Elkon KB, Silverman GJ. Naturally occurring autoantibodies to apoptotic cells. Adv Exp Med Biol. 2012;750:14–26. doi: 10.1007/978-1-4614-3461-0_2. [DOI] [PubMed] [Google Scholar]
- 75.Hamad OA, et al. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv Exp Med Biol. 2012;946:185–205. doi: 10.1007/978-1-4614-0106-3_11. [DOI] [PubMed] [Google Scholar]
- 76.Reading PC, et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008;180(5):3391–8. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 77.Garlanda C, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420(6912):182–6. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 78.Diniz SN, et al. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75(4):649–56. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 79.Bozza S, et al. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108(10):3387–96. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 80.Bottazzi B, et al. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–83. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 81.Lu J, et al. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456(7224):989–92. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moalli F, et al. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116(24):5170–80. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 83.Nauta AJ, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33(2):465–73. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 84.Deban L, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol. 2008;181(12):8433–40. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 85.Scarchilli L, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007;282(41):30161–70. doi: 10.1074/jbc.M703738200. [DOI] [PubMed] [Google Scholar]
- 86.Gowdy KM, et al. Novel role for surfactant protein A in gastrointestinal graft-versus-host disease. J Immunol. 2012;188(10):4897–905. doi: 10.4049/jimmunol.1103558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madsen J, et al. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164(11):5866–70. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 88.Han YM, et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol. 2007;211(4):489–96. doi: 10.1002/path.2131. [DOI] [PubMed] [Google Scholar]
- 89.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 90.Leth-Larsen R, et al. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212(3):201–11. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kudo K, et al. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172(12):7592–602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 92.Kuronuma K, et al. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem. 2004;279(20):21421–30. doi: 10.1074/jbc.M312490200. [DOI] [PubMed] [Google Scholar]
- 93.Vandivier RW, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169(7):3978–86. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 94.Taylor KR, et al. Recognition of Hyaluronan Released in Sterile Injury Involves a Unique Receptor Complex Dependent on Toll-like Receptor 4, CD44, and MD-2. J Biol Chem. 2007;282(25):18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 95.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 96.Cyphert JM, Trempus CS, Garantziotis S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garantziotis S, et al. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med. 2010;181(7):666–75. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 98.Voelcker V, et al. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol. 2008;17(2):100–7. doi: 10.1111/j.1600-0625.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 99.Babasola O, et al. Chemically modified N-acylated hyaluronan fragments modulate proinflammatory cytokine production by stimulated human macrophages. J Biol Chem. 2014;289(36):24779–91. doi: 10.1074/jbc.M113.515783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garantziotis S, et al. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284(17):11309–17. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 101.Lazrak A, et al. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308(9):L891–903. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Itano N, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274(35):25085–92. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 103.Koprunner M, Mullegger J, Lepperdinger G. Synthesis of hyaluronan of distinctly different chain length is regulated by differential expression of Xhas1 and 2 during early development of Xenopus laevis. Mech Dev. 2000;90(2):275–8. doi: 10.1016/s0925-4773(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 104.Liang J, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22(11):1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang J, et al. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128(2):403–411. e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stober VP, et al. TNF-stimulated gene 6 promotes formation of hyaluronan-inter-alpha-inhibitor heavy chain complexes necessary for ozone-induced airway hyperresponsiveness. J Biol Chem. 2017 doi: 10.1074/jbc.M116.756627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62(7):2004–12. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson CGSJ, Rice A, Allor J, Cyphert J, Bulek K, Li X, Fessler MB, Garantziotis s. Toll-Like Receptor 5 Modulates Myd88-Dependent Toll-Like Receptor 4 Signaling. Am J Respir Crit Care Med. 2016;193:A2643. [Google Scholar]
- 109.Black KE, et al. Hyaluronan fragments induce IFNbeta via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J Inflamm (Lond) 2013;10(1):23. doi: 10.1186/1476-9255-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foley JP, et al. Toll-like receptor 2 (TLR2), transforming growth factor-beta, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. Journal of Biological Chemistry. 2012;287(44):37406–19. doi: 10.1074/jbc.M112.360982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong Y, et al. The survival of fetal and bone marrow monocyte-derived alveolar macrophages is promoted by CD44 and its interaction with hyaluronan. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.83. [DOI] [PubMed] [Google Scholar]
- 112.Lombardo E, et al. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol. 2007;178(6):3731–9. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 113.Li Z, et al. Hyaluronan fragments contribute to the ozone-primed immune response to lipopolysaccharide. J Immunol. 2010;185(11):6891–8. doi: 10.4049/jimmunol.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 114.Campo GM, et al. Glycosaminoglycans reduced inflammatory response by modulating toll-like receptor-4 in LPS-stimulated chondrocytes. Arch Biochem Biophys. 2009;491(1–2):7–15. doi: 10.1016/j.abb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 115.Campo GM, et al. The stimulation of adenosine 2A receptor reduces inflammatory response in mouse articular chondrocytes treated with hyaluronan oligosaccharides. Matrix Biol. 2012;31(6):338–51. doi: 10.1016/j.matbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Garantziotis S, Palmer SM. Genetics and genomics in human lung transplantation. Expert Rev Respir Med. 2007;1(2):271–8. doi: 10.1586/17476348.1.2.271. [DOI] [PubMed] [Google Scholar]
- 117.Palmer SM, et al. The role of innate immunity in acute allograft rejection after lung transplantation. Am J Respir Crit Care Med. 2003;168(6):628–32. doi: 10.1164/rccm.200303-447OC. Epub 2003 May 28. [DOI] [PubMed] [Google Scholar]
- 118.Garantziotis S, et al. Alloimmune lung injury induced by local innate immune activation through inhaled lipopolysaccharide. Transplantation. 2007;84(8):1012–9. doi: 10.1097/01.tp.0000286040.85007.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stober VP, et al. Bronchial epithelial injury in the context of alloimmunity promotes lymphocytic bronchiolitis through hyaluronan expression. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L1045–55. doi: 10.1152/ajplung.00353.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Todd JL, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189(5):556–66. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui Y, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest. 2015;125(11):4255–68. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tesar BM, et al. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6(11):2622–35. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 123.Termeer C, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Termeer CC, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165(4):1863–70. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 125.Muto J, et al. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009;47(2–3):449–56. doi: 10.1016/j.molimm.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang J, et al. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178(4):2469–75. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 127.Hollingsworth JW, et al. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2007;37(2):248–53. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim MY, Muto J, Gallo RL. Hyaluronic acid oligosaccharides suppress TLR3-dependent cytokine expression in a TLR4-dependent manner. PLoS One. 2013;8(8):e72421. doi: 10.1371/journal.pone.0072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watanabe T, et al. Hyaluronan Inhibits Tlr-4-Dependent RANKL Expression in Human Rheumatoid Arthritis Synovial Fibroblasts. PLoS One. 2016;11(4):e0153142. doi: 10.1371/journal.pone.0153142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hirabara S, et al. Hyaluronan inhibits TLR-4 dependent cathepsin K and matrix metalloproteinase 1 expression in human fibroblasts. Biochem Biophys Res Commun. 2013;430(2):519–22. doi: 10.1016/j.bbrc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 131.del Fresno C, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol. 2005;174(5):3032–40. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]
- 132.Yang C, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287(51):43094–107. doi: 10.1074/jbc.M112.349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hascall VC, et al. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673(1–2):3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Salier JP, et al. The inter-alpha-inhibitor family: from structure to regulation. Biochem J. 1996;315(Pt 1):1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279(37):38079–82. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 136.Zhuo L, Kimata K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res. 2008;49(5):311–20. doi: 10.1080/03008200802325458. [DOI] [PubMed] [Google Scholar]
- 137.Garantziotis S, et al. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med. 2008;178(9):939–47. doi: 10.1164/rccm.200803-386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. Journal of Cell Science. 2003;116(Pt 10):1863–73. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 139.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochemical Society Transactions. 2006;34(Pt 3):446–50. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 140.Lauer ME, et al. Hyaluronan and Its Heavy Chain Modification in Asthma Severity and Experimental Asthma Exacerbation. J Biol Chem. 2015;290(38):23124–34. doi: 10.1074/jbc.M115.663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swaidani S, et al. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem. 2013;288(1):412–22. doi: 10.1074/jbc.M112.389874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng G, et al. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30(2):126–34. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng G, et al. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology. 2013;23(1):43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matuska B, et al. Pathological Hyaluronan Matrices in Cystic Fibrosis Airways and Secretions. Am J Respir Cell Mol Biol. 2016;55(4):576–585. doi: 10.1165/rcmb.2015-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lazrak A, et al. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am J Respir Cell Mol Biol. 2014;50(5):953–62. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danchuk S, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2(3):27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foskett AM, et al. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. 2014;306(2):L120–31. doi: 10.1152/ajplung.00240.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi H, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118(2):330–8. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xie J, et al. Human adipose-derived stem cells ameliorate cigarette smoke-induced murine myelosuppression via secretion of TSG-6. Stem Cells. 2015;33(2):468–78. doi: 10.1002/stem.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kato T, et al. Adipose tissue-derived stem cells suppress acute cellular rejection by TSG-6 and CD44 interaction in rat kidney transplantation. Transplantation. 2014;98(3):277–84. doi: 10.1097/TP.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 152.Winkler CJ, et al. Protein sieving characteristics of sub-20-nm pore size filters at varying ionic strength during nanofiltration of Coagulation Factor IX. Biologicals. 2013;41(3):176–83. doi: 10.1016/j.biologicals.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 153.Delaria KA, et al. Characterization of placental bikunin, a novel human serine protease inhibitor. J Biol Chem. 1997;272(18):12209–14. doi: 10.1074/jbc.272.18.12209. [DOI] [PubMed] [Google Scholar]
- 154.Kobayashi H, et al. Inhibition of the soluble and the tumor cell receptor-bound plasmin by urinary trypsin inhibitor and subsequent effects on tumor cell invasion and metastasis. Cancer Res. 1994;54(3):844–9. [PubMed] [Google Scholar]
- 155.Jourdain M, et al. Effects of inter-alpha-inhibitor in experimental endotoxic shock and disseminated intravascular coagulation. Am J Respir Crit Care Med. 1997;156(6):1825–33. doi: 10.1164/ajrccm.156.6.9611100. [DOI] [PubMed] [Google Scholar]
- 156.Garantziotis S, et al. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol. 2007;179(6):4187–92. doi: 10.4049/jimmunol.179.6.4187. [DOI] [PubMed] [Google Scholar]
- 157.Garantziotis S. Modulation of plasma complement by the initial dose of epirubicin/docetaxel therapy in breast cancer and its predictive value. Br J Cancer. 2011;104(3):542. doi: 10.1038/sj.bjc.6606068. author reply 543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Okroj M, et al. Heavy chains of inter alpha inhibitor (IalphaI) inhibit the human complement system at early stages of the cascade. J Biol Chem. 2012;287(24):20100–10. doi: 10.1074/jbc.M111.324913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koraka P, et al. Plasma levels of inter-alpha inhibitor proteins in children with acute Dengue virus infection. PLoS One. 2010;5(4):e9967. doi: 10.1371/journal.pone.0009967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huerta V, et al. Novel interactions of domain III from the envelope glycoprotein of dengue 2 virus with human plasma proteins. J Proteomics. 2016;131:205–13. doi: 10.1016/j.jprot.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 161.Parker AL, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108(8):2554–61. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 162.Munch J, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131(6):1059–71. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 163.Pemberton AD, Brown JK, Inglis NF. Proteomic identification of interactions between histones and plasma proteins: implications for cytoprotection. Proteomics. 2010;10(7):1484–93. doi: 10.1002/pmic.200900818. [DOI] [PubMed] [Google Scholar]
- 164.Chaaban H, et al. Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015 doi: 10.1182/blood-2014-06-582759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salustri A, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131(7):1577–86. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 166.Balhara J, et al. Pentraxin 3: an immuno-regulator in the lungs. Front Immunol. 2013;4:127. doi: 10.3389/fimmu.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Doni A, et al. An acidic microenvironment sets the humoral pattern recognition molecule PTX3 in a tissue repair mode. J Exp Med. 2015;212(6):905–25. doi: 10.1084/jem.20141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cunha C, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med. 2014;370(5):421–32. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- 169.Jaillon S, et al. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J Immunol. 2013;191(4):1873–82. doi: 10.4049/jimmunol.1201642. [DOI] [PubMed] [Google Scholar]
- 170.Diamond JM, et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186(6):546–52. doi: 10.1164/rccm.201204-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mauri T, et al. Alveolar pentraxin 3 as an early marker of microbiologically confirmed pneumonia: a threshold-finding prospective observational study. Crit Care. 2014;18(5):562. doi: 10.1186/s13054-014-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chiarini M, et al. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun. 2010;11(8):665–70. doi: 10.1038/gene.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moalli F, et al. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol. 2011;186(9):5425–34. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 174.Olesen R, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8(6):456–67. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 175.Soares AC, et al. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8(5):1321–9. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 176.Kabbani D, et al. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J Heart Lung Transplant. 2017;36(9):973–979. doi: 10.1016/j.healun.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 177.Balhara J, et al. Pentraxin 3 deletion aggravates allergic inflammation through a TH17-dominant phenotype and enhanced CD4 T-cell survival. J Allergy Clin Immunol. 2017;139(3):950–963. e9. doi: 10.1016/j.jaci.2016.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han B, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37(2):334–42. doi: 10.1007/s00134-010-2067-2. [DOI] [PubMed] [Google Scholar]
- 179.Diamond JM, et al. Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11(11):2517–22. doi: 10.1111/j.1600-6143.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ma YJ, et al. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J Biol Chem. 2011;286(5):3405–17. doi: 10.1074/jbc.M110.190637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bonavita E, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160(4):700–14. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 182.Wight TN, et al. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol. 2017;312:1–14. doi: 10.1016/j.cellimm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bensadoun ES, et al. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1819–28. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 184.Morales MM, et al. Small airway remodeling in acute respiratory distress syndrome: a study in autopsy lung tissue. Crit Care. 2011;15(1):R4. doi: 10.1186/cc9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lv N, et al. Abdominal and general adiposity and level of asthma control in adults with uncontrolled asthma. Ann Am Thorac Soc. 2014;11(8):1218–24. doi: 10.1513/AnnalsATS.201405-214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Medeiros Matsushita M, et al. Airway proteoglycans are differentially altered in fatal asthma. J Pathol. 2005;207(1):102–10. doi: 10.1002/path.1818. [DOI] [PubMed] [Google Scholar]
- 187.Reeves SR, et al. Subepithelial Accumulation of Versican in a Cockroach Antigen-Induced Murine Model of Allergic Asthma. J Histochem Cytochem. 2016;64(6):364–80. doi: 10.1369/0022155416642989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kang I, et al. Versican Deficiency Significantly Reduces Lung Inflammatory Response Induced by Polyinosine-Polycytidylic Acid Stimulation. J Biol Chem. 2017;292(1):51–63. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Westergren-Thorsson G, et al. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002;34(10):1256–67. doi: 10.1016/s1357-2725(02)00058-4. [DOI] [PubMed] [Google Scholar]
- 190.Zhang J, et al. Pro-inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med. 2012;16(7):1522–32. doi: 10.1111/j.1582-4934.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andersson-Sjoland A, et al. Fibroblast phenotypes and their activity are changed in the wound healing process after lung transplantation. J Heart Lung Transplant. 2011;30(8):945–54. doi: 10.1016/j.healun.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 192.Evanko SP, et al. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31(2):90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gaucherand L, et al. Crosstalk Between T Lymphocytes and Lung Fibroblasts: Generation of a Hyaluronan-Enriched Extracellular Matrix Adhesive for Monocytes. J Cell Biochem. 2017;118(8):2118–2130. doi: 10.1002/jcb.25842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chang MY, et al. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;34:1–12. doi: 10.1016/j.matbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chang MY, et al. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1069–L1086. doi: 10.1152/ajplung.00353.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Asplund A, et al. Hypoxic regulation of secreted proteoglycans in macrophages. Glycobiology. 2010;20(1):33–40. doi: 10.1093/glycob/cwp139. [DOI] [PubMed] [Google Scholar]
- 197.Hope C, et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood. 2014;123(21):3305–15. doi: 10.1182/blood-2014-02-554071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li Y, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208(7):1459–71. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Y, et al. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016;55:35–48. doi: 10.1016/j.matbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Heldin P, Pertoft H. Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Exp Cell Res. 1993;208(2):422–9. doi: 10.1006/excr.1993.1264. [DOI] [PubMed] [Google Scholar]
- 202.Wight TN. Provisional matrix: A role for versican and hyaluronan. Matrix Biol. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schiller HB, et al. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015;11(7):819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hill DR, et al. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem. 2013;288(40):29090–104. doi: 10.1074/jbc.M113.468629. [DOI] [PMC free article] [PubMed] [Google Scholar]