Abstract
The nature of hippocampal changes in schizophrenia before first treatment, and whether hippocampal subfields are affected by antipsychotic treatment are important questions for schizophrenia research. Forty-one first-episode antipsychotic-naïve acutely ill schizophrenia inpatients had MRI scans before and six weeks after antipsychotic treatment. Thirty-nine matched healthy controls were also scanned, twenty-two of which were scanned a second time six weeks later. Volumes of hippocampal subfields were measured via FreeSurfer v6.0 using a longitudinal analysis pipeline. Before treatment, schizophrenia patients had no significant changes in total hippocampal volume but exhibited significantly greater subfield volumes than controls in bilateral molecular layers of the hippocampus (ML), bilateral granular cell layers of the dentate gyrus (GC-DG), and bilateral cornu ammonis area 4 (CA4). After six weeks of antipsychotic treatment, patients showed volume reductions compared with pretreatment scans in total hippocampus bilaterally, with subfield volume reduction noted in previously enlarged subfields (i.e., bilateral ML, GC-DG and CA4) and in bilateral hippocampal tails, left CA1, CA3, and fimbria. Subfields with volume increases before treatment were reduced to the level of healthy controls (bilateral ML and GC-DG) or near to it (bilateral CA4) after treatment. These results indicate subfield-specific hippocampal hypertrophy prior to treatment, and that these abnormalities were reduced after acute antipsychotic therapy in a dose-related manner together with volume reductions in other areas that were not hypertrophic before treatment.
Keywords: Antipsychotic-naïve schizophrenia, Hippocampal subfields, Antipsychotics, Psychoradiology
Highlights
-
•
Specific hippocampal subfields were enlarged in patients before treatment.
-
•
Volume decrease in regions with dense D2 receptors (CA3–4 and DG) after treatment.
-
•
Most enlarged subfields pretreatment were reduced to normal level after treatment.
-
•
Dosage of antipsychotics was associated with the degree of volume reduction.
1. Introduction
The hippocampus plays well-established roles in perception and memory processes that are abnormal in schizophrenia (Hill et al., 2004; Saykin et al., 1991; Williams et al., 2012; Tamminga et al., 2012). While many neuroimaging and psychoradiology studies (https://radiopaedia.org/articles/psychoradiology) in the schizophrenia literature have focused on measurements of the whole hippocampus, it is anatomically heterogeneous and comprised of distinctive subfields, each with the unique functionality (Kesner and Rolls, 2015). Subfield measurements can be more sensitive to disease processes and treatment effects than whole hippocampal measurements (Wisse et al., 2012; La Joie et al., 2013). For instance, episodic memory deficits have been reported in schizophrenia (Hill et al., 2004; Saykin et al., 1991; Ranganath et al., 2008). This abnormality has been shown to involve disruptions in separate cognitive processes such as conjunctive coding, pattern completion and pattern separation that are supported by different hippocampal subfields (Tamminga et al., 2010). Conjunctive coding depends on CA3-CA3 auto-associative networks and interactions with entorhinal cortex, dentate gyrus (DG), and cornu ammonis area 1 (CA1) (Nakazawa et al., 2002); pattern completion mainly depends on CA3, CA1, the subiculum and their interactions (Nakazawa et al., 2002); and pattern separation is supported by the DG and its interactions with CA3 (Bakker et al., 2008). Because of the high-level of hippocampal neuroplasticity towards memory formation and consolidation (Cramer et al., 2011), these regions (i.e., CA1, CA3 and DG) may be more likely to be affected by pathological processes such as increased metabolic rate, neuroinflammation and oxidative stress in schizophrenia (Bartsch and Wulff, 2015; Li et al., 2015).
These hippocampal subfields can also be sensitive to antipsychotic treatments. The targets of antipsychotic agents, i.e., dopamine D2 receptors, have different expression across subfields, with a relatively dense expression in DG, CA3 and CA4 (Amaral, 1993; Ryoo and Joyce, 1994), suggesting that these subfields may be more likely to be affected by antipsychotic drugs. Therefore, clinical investigations of the structure can benefit from an examination of its separate subfields that include the subiculum, DG and CA fields comprising CA1–4 (Anderson et al., 2006).
Consistent with neuropsychological findings, morphological abnormalities of the hippocampus have been associated with schizophrenia (Nelson et al., 1998; Spoletini et al., 2011; Steen et al., 2006; van Erp et al., 2016; Vita et al., 2006). Although most studies reported local (Rhindress et al., 2017) or whole hippocampal volume deficits in chronic or treated first-episode schizophrenia patients (Steen et al., 2006; Adriano et al., 2012), there have been very few studies (Song et al., 2014; Szeszko et al., 2003; Chen et al., 2014) conducted with antipsychotic-naïve first-episode schizophrenia patients. Thus there is no consensus on whether hippocampal atrophy or hypertrophy is present in the early stage of illness before treatment, especially at the subfield level. Of note, a recent meta-analysis reported increased right hippocampal volumes in high-risk family members of schizophrenia patients (Xiao et al., 2015). Positron emission tomography studies have shown increased metabolic rates in first-episode schizophrenia in the hippocampus compared with healthy controls (Tamminga et al., 2010; Mitelman et al., 2017; Molina et al., 2005), indicating hyperactivity of the hippocampus could result in increased hippocampal volume via blood volume and neuropil changes.
Antipsychotic therapy has shown to impact hippocampus (Ho et al., 2011; Vernon et al., 2011) and antipsychotic dosage has been negatively associated with overall hippocampal volume (Yang et al., 2015). Given the possible opposite effects on hippocampal volume by increased metabolic rates and antipsychotic treatment, data examining hippocampal subfields before and after first acute antipsychotic treatment may provide valuable information about hippocampal abnormalities present at illness onset and effects of antipsychotic medication on this brain structure.
The present study was conducted with first-episode antipsychotic-naïve schizophrenia patients. It was designed: 1) to test for hippocampal subfield abnormalities before treatment in patients relative to healthy controls; and 2) to test for hippocampal changes in patients after acute antipsychotic treatment leveraging a recently improved longitudinal protocol for parcellation and segmentation of the hippocampus (Iglesias et al., 2015) (FreeSurfer v6.0). Since subfields CA1, CA3, CA4, and DG are fundamental to the affected episodic memory in schizophrenia and have prominent dopamine D2 receptor expression (Hill et al., 2004; Saykin et al., 1991; Amaral, 1993; Armstrong et al., 2012), we hypothesized there would be volumetric abnormalities associated with these subfields in untreated patients and that their volumes would be reduced after antipsychotic treatment.
2. Methods and materials
2.1. Participants
43 antipsychotic-naïve first-episode schizophrenia inpatients were recruited at the West China Hospital and 39 matched healthy controls were recruited from local communities (Table 1). Patients were diagnosed using the Structured Clinical Interview for DSM-IV (SCID) at baseline. Patients with other psychotic disorders were not included. We followed up all patients at one year, at which time SCID interviews confirmed pretreatment diagnoses. Symptom severity was investigated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Illness duration at the time of pretreatment studies was determined as time since patients first met diagnostic criteria for schizophrenia.
Table 1.
Demographic and clinical information for study participants.
| Demographic/clinical characters | Patients (n = 41), Mean (SD) |
Controls (n = 39) Mean (SD) |
P-value |
||
|---|---|---|---|---|---|
| Baseline (n = 41) | Follow-up (n = 41) | SZ vs. HC | Baseline vs. Follow-up | ||
| Male/Female | 17/24 | 19/20 | 0.73 | ||
| Age, Years | 23.90 (7.72) | 24.01 (8.18) | 0.89 | ||
| Education, Years | 12.45 (2.90) | 12.65 (3.09) | 0.94 | ||
| Time from onset, Months | 8.99 (12.65) | ||||
| Global assessment of function | 29.12 (9.69) | 53.19 (16.92) | <0.001a | ||
| PANSS Total | 101.10 (18.38) | 67.83 (17.49) | <0.001a | ||
| PANSS Positive | 26.48 (6.62) | 14.00 (4.19) | <0.001a | ||
| PANSS Negative | 18.29 (6.17) | 15.79 (6.19) | <0.001a | ||
| General psychopathology | 48.56 (8.80) | 34.00 (9.49) | <0.001a | ||
| Thought factor | 14.46 (4.21) | 7.90 (3.03) | <0.001a | ||
| Paranoid factor | 10.66 (2.80) | 5.80 (2.11) | <0.001a | ||
| Depression factor | 9.61 (3.83) | 7.54 (3.16) | <0.001a | ||
| Anergia factor | 8.95 (3.78) | 7.98 (3.45) | 0.01 | ||
| Activation factor | 10.10 (3.01) | 5.76 (1.64) | <0.001a | ||
| Dosage of antipsychotics,b mg/d | – | 409.54 (137.69) | |||
Statistical tests performed: group differences in age and education, student's t-test; sex ratio, chi-square test; changes of clinical scores over the course of treatment, paired t-tests.
Abbreviations: PANSS, positive and negative syndrome scale; SD, standard deviation; SZ, schizophrenia patients; HC, healthy controls.
Statistically significant after Bonferroni correction.
Daily dosage of antipsychotic medications in chlorpromazine equivalent during the last 4 weeks of treatment (Andreasen et al., 2010).
Healthy controls had no personal history of Axis I illness, assessed using the SCID, and no first-degree relatives with a known history of psychiatric illness. Patient and healthy control groups were matched for age, sex and years of education, and all participants were right-handed (Table 1). Exclusion criteria for all participants included a history of neurological illness or substance abuse, current pregnancy, MRI contraindications and significant medical illness. MR images of all participants were inspected by an experienced neuroradiologist to exclude subjects with visible brain abnormalities. The study was approved by the research ethics committee of West China Hospital, and all participants provided written informed consent.
At baseline, two patients received clopidogrel (25 and 75 mg/d) for cardiovascular care. None had received previous antipsychotic therapy or treatment with other psychiatric medications. Patients who required benzodiazepines for emergency behavioral control were excluded from the study. All patients were treated with second-generation antipsychotic medications, with drug choice and dose determined by treating physicians. During the 6-week follow-up, except for the two patients taking clopidogrel, none took medications other than antipsychotics. By report of patients, family members and treating physicians, patients took their medications as prescribed by their treating physician. All the patients stuck to the original drugs and no one switched to other antipsychotics. Detailed antipsychotic treatment information is provided in Supplementary Table 1. Drug dose was titrated during the first two weeks and then held constant for four weeks until the follow-up scan. Drug dose in CPZ (chlorpromazine) equivalents during the last 4 weeks of treatment was used in correlational analyses. Patients showed significant clinical improvement after six weeks of antipsychotic treatment (Table 1).
2.2. Data acquisition and hippocampal subfield segmentation
Participants underwent MRI examination on a 3 T scanner (General Electric, Medical Systems, U.S.A.). High-resolution T1 images were obtained using a 3D spoiled gradient-echo sequence with the following parameters: repetition time, 8.5 ms; echo time, 3.93 ms; flip angle, 12°; slice thickness, 1 mm; single shot; field of view, 24 × 24 cm2; and voxel resolution, 0.47 × 0.47 × 1 mm3. 156 axial slices were collected.
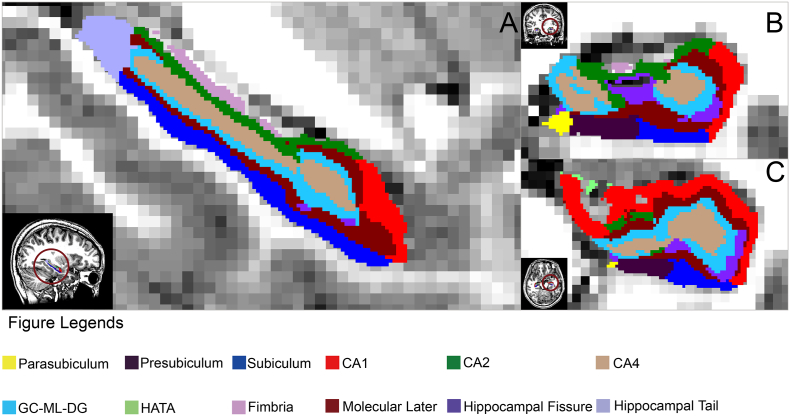
FreeSurfer (version 6.0, http://surfer.nmr.mgh.harvard.edu/) was used to segment the hippocampal subfields (Fig. 1) and measure volumes in a longitudinal analysis protocol. In this framework, an unbiased within-subject template image is created using robust, inverse consistent registration across scanning time points (Reuter et al., 2012). Processing steps, including skull stripping, tissue segmentation, surface reconstruction, registration and parcellation, were initialized with common information from the within-subject template (Reuter et al., 2012). The longitudinal segmentation of hippocampal subfields tool in FreeSurfer was then applied to segment hippocampal subfields using T1 images of the same subject at both baseline and follow-up (if available) (Iglesias et al., 2016). This method uses a subject-specific atlas and treats all time points the same way to avoid processing bias, increasing the robustness of segmentation and measurement of subfield volumes (Iglesias et al., 2016). The volumes of the whole hippocampus as well as twelve hippocampal subfields were extracted for each hemisphere, including the hippocampal tail, subiculum, presubiculum, parasubiculum, CA1, CA3, CA4, hippocampal fissure, granular cells layer of the dentate gyrus (GC-DG), molecular layer of the hippocampus, hippocampus-amygdala transition area (HATA) and fimbria. All segmentation results were visually verified without knowledge of participant characteristics. Two patients were excluded from the study because of visible segmentation errors caused by poor image quality, so forty-one patients and thirty-nine healthy controls were included in statistical analyses.
Fig. 1.
Illustration of hippocampal subfield segmentation by FreeSurfer V6.0.
Hippocampal subfields are shown in sagittal (A), coronal (B) and axial (C) views, respectively for a healthy control subject. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Statistical analyses
Statistical analyses were performed using R software (version 3.3.3, http://www.r-project.com) and SPSS (version 24.0, U.S.A.). Differences in hippocampal subfield volumes between patients and healthy controls at baseline (before treatment for patients) were analyzed using multiple ANOVA analyses for each subfields. In these and all analyses, intracranial volume (ICV), sex and age were treated as covariates and false discovery rate (FDR) correction was applied in multiple comparisons of different subfields. All statistical tests were two-tailed.
All patients were scanned pretreatment and again at six-week follow-up, while only 22 of the 39 control subjects returned for follow-up. Because of this attrition and thus potential bias in the control group at follow-up, analysis of pre-to-post treatment change within the patient group was the primary test for treatment effects. Tests for change over time in controls, which were minimal and are presented as a secondary analysis (Supplementary Table 2). Differences in subfield volumes in patients between baseline and follow-up were tested using a repeated measures ANOVA with the time of assessment and subfields as within-subject factors. To clarify whether hippocampal changes after antipsychotic treatment normalized the structure or increased abnormalities, we analyzed the differences in hippocampal subfield volumes between patients after treatment and healthy controls at baseline using ANOVA analyses. Correlation analysis was performed to test for associations between volume changes and symptoms changes and antipsychotic dose. In all the correlation analyses, ICV, sex and age were treated as covariates. FDR correction for multiple comparisons was also applied to correlational analysis of different subfields.
3. Results
3.1. Hippocampal subfield volume changes in patients before treatment
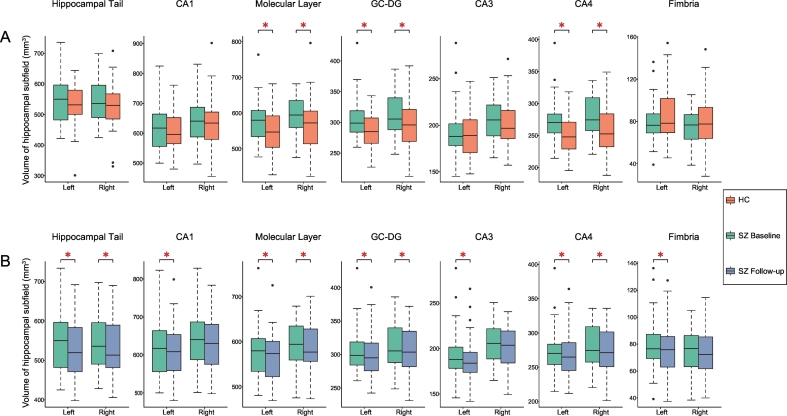
At the whole hippocampus level, there were no significant volume differences between patients and controls (SZ vs. HC, left: 3377 ± 299 mm3 versus 3325 ± 306 mm3, p-value = .089; right: 3420 ± 290 mm3 versus 3370 ± 340 mm3, p-value = .127). However, there were significant differences in subfield volumes between the two groups. ANOVA analyses revealed significantly larger volumes of patients in bilateral ML (left: F = 9.236, p-value = .003; right: F = 6.256, p-value = .015), bilateral GC-DG (left: F = 11.348, p-value = .001; right: F = 9.893, p-value = .002), and bilateral CA4 (left: F = 15.548, p-value = 1.8 × 10−4; right: F = 15.364, p-value = 1.9 × 10−4) compared with healthy controls (Table 2, Fig. 2A).
Table 2.
Difference in subfield volumes between patients (before treatment) and controls.
| Side | Subfield | Volume difference, mm3 |
Std. error | F | P-value |
|---|---|---|---|---|---|
| (SZ - HC) | |||||
| L | Hippocampal Tail | 11.34 | 14.75 | 0.59 | 0.44 |
| L | Subiculum | −8.78 | 9.62 | 0.83483 | 0.36 |
| L | CA1 | 20.20 | 11.91 | 2.88 | 0.09 |
| L | Hippocampal Fissure | 7.24 | 6.81 | 1.13 | 0.29 |
| L | Presubiculum | −5.40 | 6.93 | 0.61 | 0.43 |
| L | Parasubiculum | −2.95 | 2.02 | 2.14 | 0.14 |
| L | Molecular Layer | 31.21 | 10.27 | 9.24 | 0.003a |
| L | GC-DG | 21.01 | 6.23 | 11.35 | 0.001a |
| L | CA3 | 7.28 | 4.68 | 2.42 | 0.12 |
| L | CA4 | 24.98 | 6.33 | 15.55 | 0.0002a |
| L | Fimbria | −3.32 | 4.82 | 0.47 | 0.49 |
| L | HATA | −0.42943 | 1.77 | 0.06 | 0.80 |
| R | Hippocampal Tail | 17.83 | 17.06 | 1.09 | 0.29 |
| R | Subiculum | −7.05 | 8.40 | 0.71 | 0.40 |
| R | CA1 | 15.79 | 14.70 | 1.15 | 0.28 |
| R | Hippocampal Fissure | 7.99 | 6.70 | 1.42 | 0.23 |
| R | Presubiculum | −10.95 | 7.75 | 2.00 | 0.16 |
| R | Parasubiculum | −4.23 | 2.17 | 3.79 | 0.06 |
| R | Molecular Layer | 30.96 | 12.38 | 6.26 | 0.01a |
| R | GC-DG | 21.87 | 6.95 | 9.89 | 0.002a |
| R | CA3 | 8.14 | 5.17 | 2.48 | 0.12 |
| R | CA4 | 26.87 | 6.85 | 15.36 | 0.0002a |
| R | Fimbria | −3.86 | 4.25 | 0.83 | 0.36 |
| R | HATA | −2.52 | 1.64 | 2.37 | 0.12 |
Abbreviations: CA, cornu ammonis; GC-DG, granular cells layer of the dentate gyrus; L, left; R, right; SZ, schizophrenia patients; HATA, hippocampus-amygdala transition area; HC, healthy controls.
The mean difference is statistically significant after false discovery rate correction.
Fig. 2.
Hippocampal subfield volumes before and after acute antipsychotic therapy in schizophrenia patients.
A: Comparison of hippocampal subfield volumes between schizophrenia patients and healthy controls. B: Volume changes after acute antipsychotic therapy in schizophrenia patients. Significant differences after false discovery rate correction are marked with asterisks. Abbreviations: CA, cornu ammonis; GC-DG, granular cells layer of the dentate gyrus; L, left; R, right; SZ, schizophrenia patients; HATA, hippocampus-amygdala transition area; HC, healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Hippocampal subfield volume reductions after treatment
After antipsychotic treatment, patients had significant volume reductions in the left and right whole hippocampus (Left: reduction = 1.71%, p-value = 1.6 × 10−4, Cohen's d = 0.65; Right: reduction = 1.37%, p-value = 1.3 × 10−3, Cohen's d = 0.54). Repeated measures ANOVA revealed a significant time × subfield interaction effect (F (23, 15) = 11.195, p-value = .8 × 10−5, partial η2 = 0.95). Post-hoc analyses with significance determined by ANOVA revealed significant volume reductions in bilateral hippocampal tails (left: F = 8.16, p-value = .007; right: F = 35.12, p-value <.001), left CA1 (F = 13.46, p-value = .001), bilateral ML (left: F = 13.32, p-value = .001; right: F = 8.23, p-value = .007), bilateral GC-DG (left: F = 8.09, p-value = .007; right: F = 7.61, p-value = .009), left CA3 (F = 6.98, p-value = .012), bilateral CA4 (left: F = 6.14, p-value = .018; right: F = 7.25, p-value = .011) and left fimbria (F = 5.57, p-value = .022) after FDR-correction (Table 3, Fig. 2B).
Table 3.
Changes of hippocampal subfield volumes in patients over treatment.
| Side | Subfield | Volume difference, mm3 |
Std. error | F | P-value |
|---|---|---|---|---|---|
| (follow-up - baseline) | |||||
| L | Hippocampal Tail | −9.87 | 3.46 | 8.16 | 0.007a |
| L | Subiculum | −2.97 | 1.69 | 3.09 | 0.09 |
| L | CA1 | −11.12 | 3.03 | 13.46 | 0.001a |
| L | Hippocampal Fissure | −3.97 | 3.27 | 1.48 | 0.23 |
| L | Presubiculum | −3.80 | 2.31 | 2.70 | 0.11 |
| L | Parasubiculum | −0.82 | 0.73 | 1.26 | 0.27 |
| L | Molecular Layer | −10.50 | 2.88 | 13.32 | 0.001a |
| L | GC-DG | −6.13 | 2.16 | 8.09 | 0.007a |
| L | CA3 | −4.36 | 1.65 | 6.98 | 0.012a |
| L | CA4 | −4.67 | 1.88 | 6.14 | 0.018a |
| L | Fimbria | −3.82 | 1.59 | 5.75 | 0.022a |
| L | HATA | −0.651 | 0.67 | 0.95 | 0.34 |
| R | Hippocampal Tail | −12.68 | 2.14 | 35.12 | <0.001a |
| R | Subiculum | 0.429 | 2.49 | 0.03 | 0.86 |
| R | CA1 | −7.64 | 3.29 | 5.39 | 0.03 |
| R | Hippocampal Fissure | −0.68969 | 3.06 | 0.05 | 0.82 |
| R | Presubiculum | −2.70 | 1.44 | 3.51 | 0.07 |
| R | Parasubiculum | −0.49 | 0.582 | 0.71 | 0.41 |
| R | Molecular Layer | −7.62 | 2.66 | 8.23 | 0.007a |
| R | GC-DG | −5.81 | 2.11 | 7.61 | 0.009a |
| R | CA3 | −3.74 | 1.92 | 3.78 | 0.06 |
| R | CA4 | −5.27 | 1.96 | 7.25 | 0.011a |
| R | Fimbria | −1.37 | 1.00 | 1.88 | 0.18 |
| R | HATA | −0.83884 | 0.66 | 1.60 | 0.21 |
Abbreviations: CA, cornu ammonis; GC-DG, granular cells layer of the dentate gyrus; L, left; R, right; HATA, hippocampus-amygdala transition area.
The mean difference is statistically significant after false discovery rate correction.
There were still significant differences in subfield volumes between patients and controls after treatment. ANOVA analyses revealed significantly larger volumes of patients in bilateral CA4 (left: F = 10.68, p-value = .002; right: F = 8.94, p-value = .004) after FDR-correction (Table 4). Other regions that were significantly enlarged before treatment, i.e., bilateral ML and GC-DG, no longer showed larger volumes. No region showed significant volume increase, and no volume declines led to an abnormality in which patient volumes were significantly lower than those of healthy controls.
Table 4.
Difference in subfield volumes between patients (after treatment) and controls.
| Side | Subfield | Volume difference, mm3 |
Std. error | F | P-value |
|---|---|---|---|---|---|
| (SZ - HC) | |||||
| L | Hippocampal Tail | 0.96 | 15.21 | 0.004 | 0.95 |
| L | Subiculum | −11.56 | 9.51 | 1.48 | 0.23 |
| L | CA1 | 8.99 | 12.16 | 0.55 | 0.46 |
| L | Hippocampal Fissure | 3.64 | 6.03 | 0.36 | 0.55 |
| L | Presubiculum | −8.981 | 6.84 | 1.72 | 0.19 |
| L | Parasubiculum | −3.68 | 2.10 | 3.09 | 0.08 |
| L | Molecular Layer | 20.73 | 10.13 | 4.18 | 0.04 |
| L | GC-DG | 15.02 | 6.13 | 6.01 | 0.02 |
| L | CA3 | 2.91 | 4.47 | 0.42 | 0.52 |
| L | CA4 | 20.26 | 6.20 | 10.68 | 0.002a |
| L | Fimbria | −6.96 | 4.96 | 1.97 | 0.17 |
| L | HATA | −1.04 | 1.88 | 0.30 | 0.58 |
| R | Hippocampal Tail | 3.93 | 17.30 | 0.05 | 0.82 |
| R | Subiculum | −7.05 | 8.65 | 0.67 | 0.42 |
| R | CA1 | 7.27 | 14.91 | 0.24 | 0.63 |
| R | Hippocampal Fissure | 7.26 | 6.91 | 1.11 | 0.30 |
| R | Presubiculum | −14.08 | 7.59 | 3.44 | 0.07 |
| R | Parasubiculum | −4.69 | 2.05 | 5.25 | 0.03 |
| R | Molecular Layer | 22.76 | 12.79 | 3.17 | 0.08 |
| R | GC-DG | 15.84 | 7.30 | 4.71 | 0.03 |
| R | CA3 | 4.25 | 5.33 | 0.64 | 0.43 |
| R | CA4 | 21.42 | 7.17 | 8.94 | 0.004a |
| R | Fimbria | −4.80 | 4.35 | 1.22 | 0.27 |
| R | HATA | −3.37 | 1.68 | 4.01 | 0.05 |
Abbreviations: CA, cornu ammonis; GC-DG, granular cells layer of the dentate gyrus; L, left; R, right; SZ, schizophrenia patients; HATA, hippocampus-amygdala transition area; HC, healthy controls.
The mean difference is statistically significant after false discovery rate correction.
3.3. Changes in hippocampal volumes in relation to drug dosage and symptoms
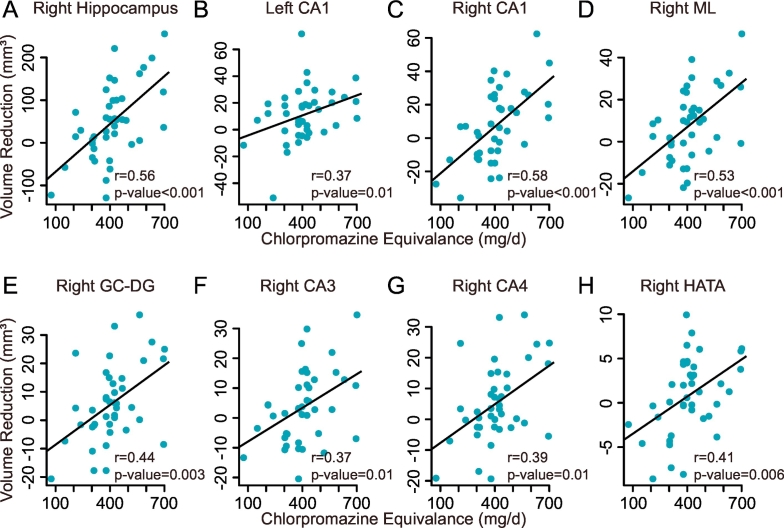
At the whole hippocampus level, volume reduction (baseline – follow-up) in the right whole hippocampus after treatment was positively correlated with the dosage of antipsychotic medication (r = 0.56, p-value = 5.62 × 10−5) (Fig. 3A). This effect was not significant in the left side (r = 0.23, p-value = .16). At the subfield level, volume decrease in the left CA1 (r = 0.37, p-value = .01), the right CA1 (r = 0.58, p-value = 2.06 × 10−5), the right ML (r = 0.53, p-value = 1.9 × 10−4), the right GC-DG (r = 0.44, p-value = .003), the right CA4 (r = 0.39, p-value = .01), and the right HATA (r = 0.41, p-value = .006) were significantly correlated with the dosage after FDR correction (Fig. 3B–H).
Fig. 3.
Association between hippocampal volume reduction and antipsychotic dosage in schizophrenia patients.
A: Positive correlation of the volume reduction in the right hippocampus with the antipsychotic dosage in CPZ equivalents. B-H: Positive correlation of the volume reductions in the left CA1, the right CA1, the right ML, the right GC-DG, the right CA4, and the right HATA with the antipsychotic dosage in CPZ equivalents. Abbreviations: CA, cornu ammonis; GC-DG, granular cells layer of the dentate gyrus; HATA, hippocampus-amygdala transition area; ML, molecular layers.
At baseline, the volume of the right CA3 was positively correlated with higher global assessment of function (GAF) score increases after treatment (follow-up - baseline) (r = 0.46, p-value = .001). Changes in volumes and symptoms over the course of acute treatment were not significantly correlated.
4. Discussion
In the present study, we tested for hippocampal subfield volume changes in antipsychotic-naïve first-episode schizophrenia patients before and after acute antipsychotic treatment. Before treatment, patients showed no differences in hippocampal volume globally but had significantly increased volumes in select subfields including bilateral ML, bilateral CG-DG, and bilateral CA4 compared with healthy controls. After six weeks of antipsychotic treatment, patients exhibited significant bilateral hippocampal volume decrease. While this treatment-related effect appeared to impact many subfields as shown in Table 3 and Fig. 2, it was statistically significant in bilateral hippocampal tails, left CA1, bilateral ML, bilateral GC-DG, left CA3, bilateral CA4 and left fimbria. After treatment, all enlarged subfields except bilateral CA4 were reduced to control levels, and no volume decline left any subfield volume lower than that of healthy controls. Both the pretreatment and after-treatment findings support our hypothesis that subfields fundamental to episodic memory and predominant in dopamine D2 receptor expression are affected by the illness and treatment. Also, we found positive associations between the volume reductions in multiple hippocampal subfields and dosage of antipsychotic medication.
4.1. Hippocampal subfields in antipsychotic-naïve patients before treatment
Our observations of increased hippocampal subfield volumes early in the course of illness before treatment differ from those of some prior studies. One study found no differences from controls except for a reduction in CA1 (Ho et al., 2017), and a second study reported a reduction in nearly all subfields except for the fimbria (Haukvik et al., 2015). However, both study findings may be influenced by a somewhat longer illness duration or prior antipsychotics treatment effects, both of which have been associated with volume reductions in schizophrenia patients previous work (Ho et al., 2011; Vernon et al., 2011). If medications or illness course are associated with reduced hippocampal subfield volumes, this reason might account for why we report increased volumes at first episode before treatment while several studies (Ho et al., 2011; Vernon et al., 2011) of chronically treated patients have reported hippocampal volume decreases. For first-episode antipsychotic-naïve studies (Song et al., 2014; Szeszko et al., 2003; Chen et al., 2014), especially considering subfield volumes, no consensus has yet been reached. Notably, one of our previous meta-analysis (Xiao et al., 2013) found that unaffected high-risk relatives of schizophrenia patients have larger right hippocampal volume compared with healthy controls, consistent with our findings of hippocampal volume increase at illness onset.
There are several potential mechanisms which might contribute to the enlargement of certain hippocampal subfields before treatment and close to illness onset. Neurophysiological hyperactivity of hippocampus during psychosis has been widely reported and correlated with abnormal morphology of the hippocampal formation in schizophrenia patients (Haukvik et al., 2015). Considering the fairly dense dopamine D2 receptor expression and the vulnerability to excitotoxicity of DG/CA4, the increased volumes found in this study during first psychotic episodes and close to illness onset may reflect plastic modifications of hippocampus resulting from activity-dependent increases in neuropil, blood flow and myelination (Tamminga et al., 2010; Amad et al., 2014). The increased volumes in certain subfields might also be explained by neuroinflammation (Giovanoli et al., 2016) or abnormal reinnervation of glial cells (Stevens, 1992). Future studies are needed to replicate findings of the present study and determine their underlying mechanism.
Interestingly, we found a positive correlation between baseline volume of right CA3 and GAF improvement after acute treatment. Another study found that a subgroup of schizophrenia patients with bilateral hippocampal volume increase had a better outcome across clinical, functional and cognitive domains (Lappin et al., 2014). These observations suggest that volume increase in CA3 might play a compensatory or protective role in schizophrenia and can be a potential marker of good prognosis.
4.2. Hippocampal volume reduction after six weeks of antipsychotic therapy
Besides declines in total hippocampal volume bilaterally, significant subfield volume reductions after treatment were observed in all subfields with significantly increased volume before treatment (i.e., bilateral ML, GC-DG and CA4). Volume decrease was also seen in regions that did not show increased volume before treatment (bilateral hippocampal tails, left CA1, CA3, and fimbria).
While the full anatomical and functional effects of antipsychotic treatment on brain remains to be determined, prior studies have shown a decline in cortical thickness and brain volume (Vernon et al., 2011; Konopaske et al., 2008), including changes in the hippocampus (Ho et al., 2011). Acute antipsychotic effects on hippocampal subfields have also been shown in animal studies (Konopaske et al., 2008). For example, olanzapine administration led to a significant volume decrease in the total hippocampus, CA1 and molecular layer of the DG volumes compared with vehicle-treated rats (Boyda et al., 2013), which parallels our findings in schizophrenia patients. Further, using manual segmentation of hippocampal subfields, a recent longitudinal structural MRI study on first-episode patients with different psychotic disorders and limited prior treatment before and after 12 weeks of antipsychotic treatment showed significant volume reductions in the DG and CA4 after treatment (Rhindress et al., 2017). Compared with controls, only bilateral CA4 still showed significantly larger volume in treated patients. Other subfields with enlarged subfields before treatment reduced volumes to control levels and no subfield showed volume loss below the level of healthy controls. In general, alterations before treatment were larger than effects of treatment (see Fig. 2), but changes after treatment were sufficiently consistent to lead to significant treatment effects. We also found significant correlations between antipsychotic dosage and volume reductions in the right hippocampus and several of its subfields. The observed lateralization in correlation analysis might reflect differences of neuroplasticity resulting from antipsychotics between the left and right hippocampus (Koch et al., 2016). While the right lateralization is consistent with the lateralized finding in the meta-analysis of unaffected relatives, confirmation of the laterality effect is needed as well as understanding of its molecular mechanism.
The significantly dose-related hippocampal volume changes in patients after treatment who were typically ill for months and showed robust clinical improvement after treatment, are consistent with the interpretation that the changes observed after antipsychotic treatment are pharmacological effects. There are several possibilities that might explain these findings. One hypothesis is that antipsychotic treatment may have alleviated the pathophysiological processes related to illness onset, resulting in a normalizing reduction in abnormal regional volumes evident before treatment. However, it is also possible that the volume reduction represents an adverse effect of treatment. A detailed examination of associated changes in hippocampal functions could address this point.
The present study has limitations that need consideration. First, treatment dose and drug were not randomly assigned or controlled, so dose correlations must be considered with caution. Second, in a clinical study such as this one, clinical recovery and drug treatment are confounded, so it is not possible to establish that hippocampal changes are direct pharmacological effects. Third, the focus on changes after acute treatment leaves questions about the long-term persistence of treatment-emergent effects on hippocampal anatomy and their neurocognitive implications as issues for future research. Fourth, losing nearly half of the control subjects to follow-up limited the ability to examine group differences in change at follow-up, but as expected, changes in healthy controls over six weeks were minimal.
5. Conclusion
Before treatment, larger volumes of hippocampal subfields were observed in the antipsychotic-naïve schizophrenia patients than healthy controls in bilateral CA4, GC-DG, and molecular layers. After acute antipsychotic treatment, patients showed significant volume declines in bilateral hippocampal tails, left CA1, bilateral ML, bilateral GC-DG, left CA3, bilateral CA4 and left fimbria. All subfields that were enlarged before treatment were reduced to the control levels (bilateral ML and GC-DG) or near to it (bilateral CA4) after treatment. Further studies are needed to replicate findings of the present study and to clarify the neurobiological mechanisms and neurocognitive significance of hippocampal changes associated with illness onset and acute antipsychotic treatment.
Funding
This work was supported by the National Natural Science Foundation [grant numbers 81401477, 81761128023, 81621003, 81030027, 81227002 and 81220108013], and Innovative Research Team in Universities [PCSIRT, grant number IRT16R52] of China.
Disclosures
The authors declare no conflicts of interest.
Contributors
KML and QYG designed the project. WBL, KML, JAS and YC wrote the main manuscript. SL and YX obtained the data. WBL and PJG performed the data analyses. All authors contributed to and approved the final manuscript.
Acknowledgements
We thank the staff of the HMRRC and the participants of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.07.008.
Contributor Information
Kaiming Li, Email: kaiming.li@scu.edu.cn.
Qiyong Gong, Email: qiyonggong@hmrrc.org.cn.
Appendix A. Supplementary data
Supplementary material
References
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Amad A., Cachia A., Gorwood P. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol. Psychiatry. 2014;19:184–191. doi: 10.1038/mp.2012.181. [DOI] [PubMed] [Google Scholar]
- Amaral D.G. Emerging principles of intrinsic hippocampal organization. Curr. Opin. Neurobiol. 1993;3:225–229. doi: 10.1016/0959-4388(93)90214-j. [DOI] [PubMed] [Google Scholar]
- Anderson P., Morris R., Amaral D., Bliss T., O'Keefe J. First ed. Oxford University Press; New York, NY: 2006. The Hippocampus Book. [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K., Williams L.E., Heckers S. Revised associative inference paradigm confirms relational memory impairment in schizophrenia. Neuropsychology. 2012;26:451–458. doi: 10.1037/a0028667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Kirwan C.B., Miller M., Stark C.E. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T., Wulff P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience. 2015;309:1–16. doi: 10.1016/j.neuroscience.2015.07.084. [DOI] [PubMed] [Google Scholar]
- Boyda H.N., Procyshyn R.M., Tse L. Antipsychotic polypharmacy increases metabolic dysregulation in female rats. Exp. Clin. Psychopharmacol. 2013;21:164–171. doi: 10.1037/a0031228. [DOI] [PubMed] [Google Scholar]
- Chen Z., Deng W., Gong Q. Extensive brain structural network abnormality in first-episode treatment-naive patients with schizophrenia: morphometrical and covariation study. Psychol. Med. 2014;44:2489–2501. doi: 10.1017/S003329171300319X. [DOI] [PubMed] [Google Scholar]
- Cramer S.C., Sur M., Dobkin B.H. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp T.G., Hibar D.P., Rasmussen J.M. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. 2016;21:547–553. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S., Engler H., Engler A. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl. Psychiatry. 2016;6:e772. doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik U.K., Westlye L.T., Morch-Johnsen L. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol. Psychiatry. 2015;77:581–588. doi: 10.1016/j.biopsych.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Hill S.K., Beers S.R., Kmiec J.A., Keshavan M.S., Sweeney J.A. Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr. Res. 2004;68:127–136. doi: 10.1016/S0920-9964(03)00125-7. [DOI] [PubMed] [Google Scholar]
- Ho B.C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N.F., Iglesias J.E., Sum M.Y. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol. Psychiatry. 2017;22:142–152. doi: 10.1038/mp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Van Leemput K., Augustinack J. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. NeuroImage. 2016;141:542–555. doi: 10.1016/j.neuroimage.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kesner R.P., Rolls E.T. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci. Biobehav. Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Koch K., Reess T.J., Rus O.G., Zimmer C. Extensive learning is associated with gray matter changes in the right hippocampus. NeuroImage. 2016;125:627–632. doi: 10.1016/j.neuroimage.2015.10.056. [DOI] [PubMed] [Google Scholar]
- Konopaske G.T., Dorph-Petersen K.A., Sweet R.A. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol. Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R., Perrotin A., de La Sayette V. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. Neuroimage Clin. 2013;3:155–162. doi: 10.1016/j.nicl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin J.M., Morgan C., Chalavi S. Bilateral hippocampal increase following first-episode psychosis is associated with good clinical, functional and cognitive outcomes. Psychol. Med. 2014;44:1279–1291. doi: 10.1017/S0033291713001712. [DOI] [PubMed] [Google Scholar]
- Li W., Ghose S., Gleason K. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am. J. Psychiatry. 2015;172:373–382. doi: 10.1176/appi.ajp.2014.14010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman S.A., Bralet M.C., Mehmet Haznedar M. Positron emission tomography assessment of cerebral glucose metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging Behav. 2017;12:532–546. doi: 10.1007/s11682-017-9721-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V., Sanz J., Sarramea F., Benito C., Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J. Psychiatr. Res. 2005;39:117–127. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Quirk M.C., Chitwood R.A. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.D., Saykin A.J., Flashman L.A., Riordan H.J. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch. Gen. Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Minzenberg M.J., Ragland J.D. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol. Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhindress K., Robinson D.G., Gallego J.A. Hippocampal subregion volume changes associated with antipsychotic treatment in first-episode psychosis. Psychol. Med. 2017:1–13. doi: 10.1017/S0033291717000137. [DOI] [PubMed] [Google Scholar]
- Ryoo H.L., Joyce J.N. Loss of dopamine D2 receptors varies along the rostrocaudal axis of the hippocampal complex in Alzheimer's disease. J. Comp. Neurol. 1994;348:94–110. doi: 10.1002/cne.903480105. [DOI] [PubMed] [Google Scholar]
- Saykin A.J., Gur R.C., Gur R.E. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch. Gen. Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Song X., Fan X., Li X. Serum levels of BDNF, folate and homocysteine: in relation to hippocampal volume and psychopathology in drug naive, first episode schizophrenia. Schizophr. Res. 2014;159:51–55. doi: 10.1016/j.schres.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Spoletini I., Cherubini A., Banfi G. Hippocampi, thalami, and accumbens microstructural damage in schizophrenia: a volumetry, diffusivity, and neuropsychological study. Schizophr. Bull. 2011;37:118–130. doi: 10.1093/schbul/sbp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R.G., Mull C., McClure R., Hamer R.M., Lieberman J.A. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stevens J.R. Abnormal reinnervation as a basis for schizophrenia: a hypothesis. Arch. Gen. Psychiatry. 1992;49:238–243. doi: 10.1001/archpsyc.1992.01820030070009. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., Goldberg E., Gunduz-Bruce H. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am. J. Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. Am. J. Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Thomas B.P., Chin R. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr. Res. 2012;138:157–163. doi: 10.1016/j.schres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Vernon A.C., Natesan S., Modo M., Kapur S. Effect of chronic antipsychotic treatment on brain structure: a serial magnetic resonance imaging study with ex vivo and postmortem confirmation. Biol. Psychiatry. 2011;69:936–944. doi: 10.1016/j.biopsych.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Vita A., De Peri L., Silenzi C., Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr. Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Avery S.N., Woolard A.A., Heckers S. Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol. Psychiatry. 2012;71:105–113. doi: 10.1016/j.biopsych.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E., Gerritsen L., Zwanenburg J.J. Subfields of the hippocampal formation at 7 T MRI: in vivo volumetric assessment. NeuroImage. 2012;61:1043–1049. doi: 10.1016/j.neuroimage.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Zhang W., Lui S., Yao L., Gong Q. Similar and different gray matter deficits in schizophrenia patients and their unaffected biological relatives. Front. Psychiatry. 2013;4:150. doi: 10.3389/fpsyt.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Lui S., Deng W. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr. Bull. 2015;41:201–210. doi: 10.1093/schbul/sbt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Wu S., Lu W., Bai Y., Gao H. Brain differences in first-episode schizophrenia treated with quetiapine: a deformation-based morphometric study. Psychopharmacology. 2015;232:369–377. doi: 10.1007/s00213-014-3670-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material