Abstract
Atrial fibrillation (AF) is the most common arrhythmia conferring a fivefold increased risk of stroke. Stroke prevention is the cornerstone of management of patients with AF. Asians have a generally higher incidence of AF-related risks of stroke and bleeding (particularly intracranial bleeding), compared with non-Asians. Despite the well-documented efficacy and relative safety of oral anticoagulation for stroke prevention among Asians, the suboptimal use of oral anticoagulation remains common. The current narrative review aims to provide a summary of the available evidence on stroke prevention among patients with AF focused on the Asia region, regarding stroke and bleeding risk evaluation, the performance of oral anticoagulation, and current use of thromboprophylaxis.
Keywords: Atrial fibrillation, Stroke, Prevention, Anticoagulant agents, Asian continental ancestry group
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia with increasing prevalence worldwide including in Asia.1) The incidence and prevalence of AF increased with population aging.2) By 2050, 2.9 million of people may suffer from AF-associated stroke.3) Overall, AF confers a fivefold increased risk of stroke which is more severe compared with those of other etiologies.4),5),6),7) AF-related stroke often results in up to 20% high-risk of death and approximately 60% of disability.8),9)
Stroke prevention, i.e., oral anticoagulation (OAC), is the cornerstone of management for patients with AF. Therefore, optimizing stroke prevention for patients with AF in Asia has a great impact on the global health burden of this most common arrhythmia.10) Among patients with AF, the risk of stroke is not homogeneous and increases with the presence of more risk factors for stroke. The more common stroke risk factors include age, heart failure (HF), diabetes mellitus, previous stroke/transient ischemic attack (TIA), vascular disease and sex category (female gender), have been used to formulate a simple risk stratification scheme, the congestive HF, hypertension, age ≥75 (2 points), diabetes mellitus, previous stroke/TIA (2 points), vascular disease, age 65–74, sex category (female gender) (CHA2DS2-VASc) score, for predicting individual's risk of stroke.11) Performance of this score has been well-validated in Asians.
Compared to control or placebo, OAC with vitamin K antagonist (VKA) reduces stroke by 64% and all-cause mortality by 26%.12) However, VKA use has significant shortcomings, such as narrow therapeutic range, multiple foods and drugs interaction and the need for monitoring. Further, the performance of VKA is suboptimal in Asians, and is associated with a high-risk of intracranial hemorrhage (ICH), frequently related to poor anticoagulation control (reflected by poorer time in therapeutic range [TTR]) related to use of herbs and dietary habits in Asians.3),13)
Currently, the non-VKA oral anticoagulants (NOACs) have changed the landscape of anticoagulation in patients with AF. The improved efficacy, safety and convenience profiles of NOACs have encouraged the wide use of this anticoagulant worldwide. Also, NOACs are associated with lower risk of bleeding events, especially for ICH, compared with the VKA.14),15),16),17) The recent development of reversal agents for NOACs make it possible to “switch off” the anticoagulation effects of these agents in some urgent situations, such emergent surgery, ICH and other major bleedings. Thus, the multiple benefits of NOAC make them more suitable as an OAC choice for Asians. Indeed, a national cohort study from Korea has shown that Asians benefit more from NOACs compared with non-Asians.18)
Although the provision of stroke prevention has improved among Asians during the past decades, suboptimal OAC use remains common in this region. Multiple factors, including a fear of bleeding events, inconvenience of anticoagulation quality check, lower adherence, etc. have all been related to the underuse of OAC, which contribute to a high-risk of stroke in Asian patients with AF.
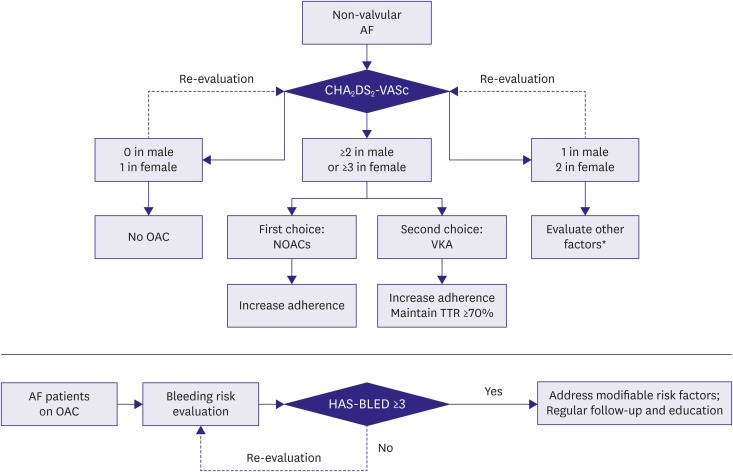
The current narrative review aims to provide a summary of the available evidence on stroke prevention among patients with AF focused on the Asia region, regarding stroke and bleeding risk evaluation, the performance of OAC, and current use of thromboprophylaxis. We also provided a flowchart of decision-making process of stroke prevention in patients with AF from Asia (Figure 1).
Figure 1. Decision-making process of stroke prevention in patients with AF from Asia. The decision-making process includes stroke risk evaluation, OAC choosing and bleeding risk control. Stroke and bleeding risk re-evaluation should be made at each medical contact.
AF = atrial fibrillation; CHA2DS2-VASc = congestive HF, hypertension, age ≥75 (2 points), diabetes mellitus, previous stroke/TIA (2 points), vascular disease, age 65–74, sex category (female gender); HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation; TTR = time in therapeutic range; VKA = vitamin K antagonist.
*Including patients' preference, risk factor severity (such as blood pressure control), bleeding risk.
ATRIAL FIBRILLATION-RELATED STROKE IN ASIAN
The prevalence of AF-related stroke has been reported in Asian cohorts from the recent randomised trials of the NOACs.14),15),16),17) For example, in a subanalysis of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, the absolute annual rate of ischemic stroke was numerically higher in Asians compared with non-Asians (2.05% vs. 1.14% in dabigatran 110 mg group, 1.12% vs. 0.81% in the dabigatran 150 mg group, and 2.02% vs. 0.98% in the warfarin group).19) A subanalysis of the Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF) trial revealed a higher absolute annual rate of ischemic stroke in East Asia (not including Japan) compared with non-East Asia (2.24% vs. 1.60% in warfarin group).20) Also, in the Effective Anticoagulation with factor Xa Next Generation in Atrial Fibrillation (ENGAGE AF) trial, East Asians had a numerically higher annual rate of ischemic stroke compared with non-East Asians (1.31% vs. 0.89% in warfarin group).21) Such differences among Asian populations were again confirmed in the Apixaban versus Warfarin in Patients with Atrial Fibrillation (ARISTOTLE) trial.22)
STROKE RISK STRATIFICATION IN ASIANS
The CHA2DS2-VASc score is widely used in different populations and got recommended by most guidelines regarding stroke risk evaluation in AF.23),24),25),26) The CHA2DS2-VASc score was derived from Western populations (36 countries from Europe), with good accuracy in predicting stroke risk, especially in identifying low-risk patients.11)
Since its original derivation, the CHA2DS2-VASc score has also been validated in Asian populations. For example, in a large cohort study enrolled 186,570 Chinese patients with AF, the CHA2DS2-VASc score outperformed the older CHADS2 score with a better area under curve (AUC) for predicting ischemic stroke (0.698 vs. 0.659, p<0.0001)27). Further, the CHA2DS2-VASc score showed better capability in identifying low-risk patients. Indeed, in patients with a CHADS2 score of 0, the CHA2DS2-VASc score ranged from 0–3 with an annual risk of stroke ranging from 1.15% to 4.47%; of note, among patients with a CHA2DS2-VASc score of 0, the annual stroke risk was around 1.15%.27)
In a meta-analysis including 6 cohort studies of AF patients from Asia regions, absolute event rates were usually lower when patients were categorized as the CHA2DS2-VASc score of 0–1, compared to a CHADS2 score of 0–1.28) This study demonstrated that when compared with the CHA2DS2-VASc score, there was a 1.71-fold elevated stroke risk among patients stratified as “low-risk” using a CHADS2 score of 0, or a 1.40-fold increased with a CHADS2 score of 1.28)
The performance of the CHA2DS2-VASc score in identifying truly low-risk AF patients for stroke was validated in a Korean Nationwide cohort study (n=5,855).29) The CHA2DS2-VASc score had the best sensitivity (98.8% vs. 85.7% in the CHADS2 score and 74.8% in the anticoagulation and risk factor in atrial fibrillation [ATRIA] score) and negative predictive value (98.8% vs. 95.3% for the CHADS2 score and 93.7% for the ATRIA score) for predicting stroke risk during 5 years of follow-up.29) In another Korean Nationwide population-based study (n=10,846), the CHA2DS2-VASc score was superior in identifying truly low-risk patients compared with the CHADS2 score.30)
The CHA2DS2-VASc score also showed better performance than the ATRIA score, in predicting ischemic stroke among AF patients from Taiwan (n=186,570).31) In this study, the CHA2DS2-VASc score had higher c-index than the ATRIA score (0.698 vs. 0.627, Delong test, p<0.0001) with a significantly improved net reclassification index of 11.7% (p<0.0001). Patients stratified as low-risk in the ATRIA score had a CHA2DS2-VASc score ranging from 0 to 7, and annual stroke rates from 1.06% to 13.33%.31)
Different age threshold for the CHA2DS2-VASc score in Asians?
An age ≥65 was set as the threshold of an increased risk of stroke in the original CHA2DS2-VASc score in non-Asians. However, such an age threshold may be different in Asians.
In an observational study from Hong Kong, age ≥50 years was associated with a substantial stroke risk, with an annual ischemic stroke risk of 5.87% in patients aged 50–65 years.32) This result was confirmed in another large Asian nationwide cohort from Taiwan including 186,570 patients with AF, using a cutoff of 50 years, whereby patients could be further stratified into 2 subgroups with different stroke risk (>50 years of age: 1.78%/year vs. <50 years of age: 0.53%/year).33) Therefore, in this Asian cohort study, age at 50–74 was suggested as 1 point in a modified CHA2DS2-VASc score which performed better than the original CHA2DS2-VASc score (AUC, 0.71 [0.70–0.71] vs. 0.69 [0.68–0.69], DeLong test p<0.0001), with an improved net reclassification index of 3.39% [2.16–4.59%].34) Thus, among Asian populations, maybe age ≥50 should be treated as a risk factor and should meet the stroke prevention threshold.
Oral anticoagulation for patients with a CHA2DS2-VASc score of 1 in males and 2 in females from Asia?
For obtaining a net clinical benefit (NCB), the OAC initiation threshold is 1.7% for annual risk of stroke using VKAs and 0.9% for the NOACs.1) Indeed, considering the evidence supporting NCB, patients with a CHA2DS2-VASc score of 1 in males or 2 in females should receive OAC, as is recommended by guidelines.23),24),25),26),35),36)
As demonstrated in an Asian study, in patients with only one non-gender stroke risk factor, OAC may be associated with positive NCB, considering the high stroke risk in these patients (1.96–3.50% in males, 1.91–3.34% in females).31),37) In another study from Taiwan, in patients with AF aged 20 to 49 and with a CHA2DS2-VASc score of 1 in males, the risk of ischemic stroke was 1.30% (0.94-1.71%) per year; which among those with a CHA2DS2-VASc score of 2 in females, this risk was 1.40% (1.11–1.67%).36) For these patients, may be NOAC should be considered for stroke prevention considering the treatment threshold of obtaining a positive NCB at a 0.9% annual risk of stroke.
In clinical work, a comprehensive consideration of OAC is needed for these patients with only one non-major risk factor for stroke. Although the CHA2DS2-VASc score provides a viable approach to evaluate individuals' risk of stroke, however, the stroke risk is not equal for patients for a particular CHA2DS2-VASc score point. For example, well-controlled hypertension may a have lower risk of stroke compared with uncontrolled hypertension. Also, age at 74 would confer higher stroke risk than the age at 65. Therefore, the CHA2DS2-VASc score should be regarded as a simple and practical score for everyday practice, and clinicians still need some degree of individualized decision-making.
Guideline recommendations in Asia
Currently, Asian countries have the 2017 consensus of the Asia Pacific Heart Rhythm Society (APHRS) on stroke prevention in AF.24) In the APHRS consensus document, the CHA2DS2-VASc score was recommended for the evaluation of stroke risk in Asian patients with non-valvular AF. Patients with at least one non-gender stroke risk factor, i.e., CHA2DS2-VASc score ≥1 in males or ≥2 in females, were recommended for OAC use, and NOACs were recommended over VKA.24) In Japan, the 2013 Japan Circulation Society guideline for pharmacotherapy of AF recommend the CHADS2 score for risk stratification, and patients with CHADS2 score ≥2 should consider NOACs use.38) Also, in this guideline, OAC with dabigatran or apixaban should be performed in intermediate-risk patients with a CHADS2 score of 1 (class I). Anticoagulation therapy with rivaroxaban, edoxaban or warfarin should be considered for intermediate-risk patients with a CHADS2 score of 1 (class IIa).38) In the 2016 guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of AF, CHA2DS2-VASc score was recommended to assess stroke risk in nonvalvular AF.39) In patients with a CHA2DS2-VASc score ≥1 (beyond female sex alone), antithrombotic therapy should be considered and NOACs were preferred over VKA.39) In the 2018 Korean Heart Rhythm Society guidelines for stroke prevention therapy, the CHA2DS2-VASc score was recommended for the assessment of stroke risk.40) NOACs or anticoagulation therapy using warfarin should be recommended for antithrombotic therapy when the CHA2DS2-VASc score ≥2.41)
A dynamic evaluation of stroke risk
In the real-world, a patient's stroke risk is not static. Increasing age, newly developed comorbidities, such as diabetes mellitus, HF or vascular diseases, may increase individual's risk of stroke.
In an Asian study including 31,039 patients from Taiwan, the mean baseline CHA2DS2-VASc score increased from 1.29 to 2.31 during follow-up.42) Nearly 60% of patients experienced an increased CHA2DS2-VASc score. Further, the change in risk (referred to as the ‘delta CHA2DS2-VASc score’) performed better in evaluating the stroke risk, when compared with baseline or follow-up CHA2DS2-VASc scores.42) Therefore, a dynamic evaluation of stroke risk is necessary and should be performed at each medical contact.43)
In Korean nationwide population-based study in 2015 (n=276,246), the median age (from 68 to 71 years) and the prevalence of diabetes mellitus and HF in patients with AF has been increased between 2008 and 2015.2) Also, the high-risk population with a CHA2DS2-VASc score of ≥2 increased from 80.2% to 86.8%. The proportion of patient with AF who need to be treated with anticoagulation therapy increased significantly due to aging and increasing comorbidities (i.e., HF and diabetes mellitus).
Another recent nationwide study from Korea (n=167,262) also demonstrated an increasing CHA2DS2-VASc score among patients with AF during 10 years of follow-up. There were 46.6% of ‘low-risk’ patients and 72.0% of ‘intermediate-risk’ patients re-classified to higher stroke risk categories.43)
BLEEDING RISK ASSESSMENT IN THE ASIAN POPULATIONS
It is important to evaluate the risk of bleeding in AF patients taking OAC. Patients with high bleeding risk need more regular review and early follow-up (e.g., 4 weeks rather than 4–6 months), and modifiable bleeding risk factors should be addressed, such as anemia, uncontrolled hypertension, alcohol use, and renal dysfunction.44),45) In terms of bleeding risk prediction, relying on modifiable bleeding risk factors alone is an inferior strategy compared to an established bleeding risk score for risk stratification.46),47),48),48)
Several bleeding risk evaluation tools have been proposed in the last decade.49),50),51),52) In patients with AF from Asia, the hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), elderly, drugs/alcohol concomitantly (HAS-BLED) score was compared with other bleeding risk scoring systems in a large cohort study from Taiwan (n=40,450), whereby the HAS-BLED score outperformed other scoring systems, including the HEMORR2HAGES score, the ATRIA score and the outcomes registry for better informed treatment (ORBIT) score, as evident from the net reclassification index.48)
In a large cohort of Chinese inpatients with AF (n=4,824), the HAS-BLED score showed good performance in predicting major bleeding events (AUC, 0.72 [0.65–0.79]) and ICH (AUC, 0.83 [0.75–0.91]).53) The HAS-BLED score was significantly better in predicting major bleeding events and ICH, compared with the ATRIA and the ORBIT scores (Delong test, p<0.05, respectively). Additionally, the HAS-BLED score resulted in net reclassification improvement of 17.1–65.5% in predicting major bleeding events and 29.5–67.3% in ICH (all p<0.05).53)
Considering the dynamic risk of bleeding, a follow-up HAS-BLED score may be more viable for evaluating individual bleeding risk. In a “real-world” AF cohort study from Taiwan (n=19,566), follow-up HAS-BLED (AUC, 0.63 [0.62–0.64]) and the change in HAS-BLED risk (i.e., ‘delta HAS-BLED score’) (AUC, 0.62 [0.61–0.63]) had higher predictive accuracy of major bleeding compared with the baseline HAS-BLED score (AUC, 0.54 [0.53–0.55]; Delong test, p<0.05 respectively) or simply relying on modifiable bleeding risk factors.54)
In clinical settings, other risk factors may also need consideration for bleeding risk evaluation. For example, in Korean patients with AF taking NOACs (n=1,353), underweight was found to have an increased risk of major bleeding compared with those with normal weight or overweight to obesity.55) Dose reduction in such patients should be considered more in Asian to decrease the risk of bleeding.
EFFECTIVENESS AND SAFETY OF ORAL ANTICOAGULATION IN ASIAN POPULATIONS
The efficiency of OAC in reducing stroke and systemic embolism (SE) has been well identified. Dose-adjusted VKA reduces stroke/SE by 64% and all-cause mortality by 26%, compared with placebo or control.2),3) In patients using VKAs, the TTR >65–70% guarantees an optimal OAC quality and need to be maintained. Nevertheless, poor TTR has been associated with worse outcomes.56),57)
In the last decade, NOAC has superseded traditional VKAs for stroke prevention in patients with nonvalvular AF, given their quick action, less drug and/or food interactions, and lack of necessity for anticoagulation monitoring. The four available NOACs, i.e., dabigatran, rivaroxaban, apixaban and edoxaban, show good efficacy and safety for stroke prevention in AF patients in large randomized trials.14),15),16),17) The good performance of NOACs in randomised trials has been complemented and augmented by so-called ‘real-world’ evidence from large post-marketing observational cohorts.58),59),60) Importantly, all the NOACs were associated with significantly reduced risk (25–82%) for ICH shown in different subgroups of patients.61),62) The efficacy and safety of NOACs among Asian populations with AF have also been demonstrated in subgroup analysis from the major trials (Table 1).14),15),16),17),19),20),21),22),63)
Table 1. Efficacy and safety of NOACs in Asians from the randomised trials.
| Study (No. of Asians) | NOACs | Ischemic stroke | Stroke/SE | Major bleeding | Intracranial hemorrhage | All-cause mortality | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk* | HR | Risk* | HR | Risk* | HR | Risk* | HR | Risk* | HR | ||
| RE-LY19) (n=2,782) | Dabigatran 150 mg | 1.12% vs. 2.02% | 0.55 (0.32–0.95) | 1.39% vs. 3.06% | 0.45 (0.28–0.72) | 2.17% vs. 3.82% | 0.57 (0.38–0.84) | 0.45% vs. 1.10% | 0.40 (0.18–0.92) | 4.01% vs. 5.09% | 0.78 (0.57–1.07) |
| Dabigatran 110 mg | 2.05% vs. 2.02% | 1.01 (0.63–1.61) | 2.50% vs. 3.06% | 0.81 (0.54–1.21) | 2.22% vs. 3.82% | 0.57 (0.39–0.85) | 0.23% vs. 1.10% | 0.20 (0.07–0.60) | 5.01% vs. 5.09% | 0.98 (0.73–1.32) | |
| ROCKET-AF20) (n=932) | Rivaroxaban | 2.12% vs. 2.24% | Not given | 2.63% vs. 3.38% | 0.78 (0.44–1.39) | 3.44% vs. 5.14% | Not given | 0.59% vs. 2.46% | 0.24 (0.08–0.71) | 2.58% vs. 3.57% | 0.73 (0.41–1.27) |
| J-ROCKET AF64) (n=1,278) | Rivaroxaban | Not given | 0.40 (0.17–0.96) | 1.26% vs. 2.61% | 0.49 (0.24–1.00) | 3.00% vs. 3.59% | 0.85 (0.50–1.43) | 0.8% vs. 1.6% | Not given | Not given | Not given |
| ENGAGE AF-TIMI 4821) (n=1,943) | Edoxaban 60 mg | 0.80% vs. 1.31% | 0.64 (0.31–1.32) | 1.34% vs. 2.62% | 0.53 (0.31–0.90) | 2.86% vs. 4.80% | 0.61 (0.41–0.89) | 0.60% vs. 1.92% | 0.31 (0.15–0.66) | 1.73% vs. 2.77% | 0.63 (0.40–0.98) |
| Edoxaban 30 mg | 2.26% vs. 1.31% | 1.77 (1.01–3.10) | 2.52% vs. 2.62% | 0.93 (0.63–1.54) | 1.59% vs. 4.80% | 0.34 (0.21–0.54) | 0.46% vs. 1.92% | 0.24 (0.11–0.56) | 1.84% vs. 2.77% | 0.66 (0.42–1.02) | |
| ARISTOTLE22) (n=1,993) | Apixaban 5 mg | 2.22% vs. 1.90% | 1.17 (0.74–1.85) | 2.52% vs. 3.39% | 0.74 (0.50–1.10) | 2.02% vs. 3.84% | 0.53 (0.35–0.80) | 0.67% vs. 1.88% | 0.36 (0.18–0.71) | 2.86% vs. 2.81% | 1.02 (0.70–1.50) |
Numbers in bold stands for significance.
ARISTOTLE = Apixaban versus Warfarin in Patients with Atrial Fibrillation; ENGAGE AF-TIMI 48 = Effective Anticoagulation with factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48; NOAC = non-vitamin K antagonist oral anticoagulant; RCT = randomized controlled trial; HR = hazard ratio; J-ROCKET AF = Rivaroxaban vs. Warfarin in Japanese Patients with Atrial Fibrillation; RE-LY = Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET-AF = Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation; SE = systemic embolism.
*Annual incidence, NOACs vs. warfarin.
In a subanalysis of the RE-LY study, dabigatran had better (150 mg) or similar (110 mg) efficacy compared with warfarin (Table 1).19) Also, dabigatran 150 mg was associated with a 43% reduced major bleeding, 60% reduced ICH, and 40% reduced total bleeding (hazard ratio [HR], 0.60 [0.51–0.70]) amongst Asian patients. Dabigatran 110 mg was associated with a 43% reduced major bleeding, 80% reduced ICH and 52% reduced total bleeding (HR, 0.48 [0.40–0.56]).19) Further, dabigatran 150 mg in Asians had a greater NCB compared with non-Asians (p interaction, 0.004).19)
In the ROCKET-AF trial, 932 patients with AF were enrolled in East Asia.20) Rivaroxaban had a similar risk of stroke/SE compared with warfarin (HR, 0.78 [0.44–1.39]).20) Also, rivaroxaban had a similar risk of major or nonmajor clinically relevant bleeding risk compared with warfarin (HR, 1.01 [0.79–1.30]).20) In the Rivaroxaban vs. Warfarin in Japanese Patients with Atrial Fibrillation (J-ROCKET AF) trial conducted in Japan (n=1,278), rivaroxaban had similar stroke/SE rates compared with warfarin (Table 1), while the risks of major bleeding and non-major clinically relevant bleeding (HR, 1.20 [0.92–1.56]) were comparable between the rivaroxaban and warfarin groups (Table 1).64)
In a subanalysis of the Effective Anticoagulation with factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial, 1,943 patients with AF were recruited from East Asia, including Japan, China, Taiwan and South Korea.21) Compared with warfarin, edoxaban 60 mg was associated with a 47% reduced risk of stroke/SE21). Edoxaban 30 mg had a similar risk of stroke/SE compared with warfarin. The risk of major bleeding was significantly reduced by edoxaban (39% in edoxaban 60 mg and 66% in edoxaban 30 mg) compared with warfarin (Table 1).21)
In the ARISTOTLE trial, 1,993 patients with AF were from East Asia. Apixaban 5 mg had a similar risk of stroke/SE in East Asians and a 47% significantly reduced risk of major bleeding.22) In addition, a 29% reduced risk of major bleeding or clinical relevant nonmajor bleeding with was observed in apixaban treated group.22)
These trial data are supported by real-world studies. In a real-world study from the Korean National Health Insurance Service database (n=34,833), NOACs (n=11,611) had similar risk of ischemic stroke (HR, 0.98 [0.78–1.22]) and lower risk of ICH (HR, 0.50 [0.36–0.68]) compared with warfarin. All-cause mortality was significantly lower in patients taking dabigatran (HR, 0.52 [0.39–0.68]) or apixaban (HR, 0.32 [0.18–0.53]).18)
The comparison of the performance of NOACs in Asians and non-Asians was also reported in a recent meta-analysis using aggregate data from phase III clinical trials.65) When compared with VKAs, standard-dose NOACs reduced stroke/SE more in Asians than in non-Asians (odds ratio [OR], 0.65 [0.52–0.83] vs. 0.85 [0.77–0.93]; p interaction, 0.045). Standard NOACs were also associated with a 20% reduced all-cause mortality compared with VKAs in Asians (OR, 0.80 [0.65–0.98]).65) Also, standard-dose NOACs had lower major bleeding (OR, 0.57 [0.44–0.74] vs. 0.89 [0.76–1.04]; p interaction, 0.004) and hemorrhagic stroke (OR, 0.32 [0.19–0.52] vs. 0.56[0.44–0.70]; p interaction, 0.046). An increased risk of GI bleeding was only observed in non-Asians (OR, 1.44 [1.12–1.85]) but not in Asians (OR, 0.79 [0.48–1.32]) (p interaction, 0.041).65)
In a retrospective Japan cohort study with propensity-matched comparison (n=11,972 for patients on apixaban and warfarin, respectively), apixaban was associated with significantly lower risk of any bleeding (HR, 0.81 [0.73–0.90]), major bleeding (HR, 0.66 [0.51–0.85]) and stroke/SE (HR, 0.64 [0.48–0.85]) compared with warfarin.66)
In a recent study, including 1,834 patients with non-valvular AF from Korea, dabigatran had similar efficacy in reducing stroke/SE. Dabigatran 110 mg was associated with lower risk of major bleeding (HR, 0.19 [0.07–0.55]). In this study, the number of patients in dabigatran 150 mg was 294 compared with 550 in dabigatran 110 mg group and 990 in warfarin group, which may undermine the safety profile of dabigatran 150 mg.67)
Recently, in a randomized, multicenter study from Korea (n=183), the efficacy and safety of rivaroxaban were compared with warfarin among patients experienced recent mild AF-related stroke within the previous 5 days. During 4 weeks of follow-up, rivaroxaban showed no differences in primary endpoint (composite of new ischemic lesion or new ICH seen on results of magnetic resonance imaging) (relative risk [RR], 0.91 [0.69–1.20]) and new ICH (RR, 1.10 [0.70–1.71]) compared with warfarin.68)
In the real-world setting, choosing the standard or low-dose of NOACs is highly subjective at the level of clinician.69) In the SAKURA AF registry (n=3,266), 20–30% of NOAC users received an inappropriate reduced dose of NOAC.70) However, a reduced dose of NOACs should not be encouraged for general patients. In a meta-analysis of 3,155 Asian patients enrolled from the RE-LY and ENGAGE AF trials, standard-dose NOACs were associated with significantly reduced risk of stroke/SE and ischemic stroke compared with low-dose NOACs (RR, 0.62 [0.45–0.85] for stroke/SE; RR, 0.55 [0.38–0.79] for ischemic stroke); however, rates of major bleeding (RR, 1.31 [0.74–2.33]), ICH (RR, 1.54 [0.72–3.30]), and life-threatening bleeding (RR, 1.49 [0.87–2.55]) with the two dosing regimens were similar.71) Also, only certain clinical risk criteria justify dose reduction.
LEFT ATRIAL APPENDAGE OCCLUSION IN ASIAN POPULATIONS
Left atrial appendage (LAA) has been considered as the major site of clot formation in AF. Occlusion of the LAA was therefore suggested to reduce stroke risk in patients with non-valvular AF.
In the WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) study, the annual primary efficacy event (stroke/SE and cardiovascular death) rate was 3.0% in the left atrial appendage occlusion (LAAO) group (n=463) and 4.9% in the warfarin group (n=244) (RR, 0.62 [0.35–1.25]), the annual primary safety endpoint (major bleeding, pericardial effusion, and device embolization) rate was higher in the LAAO group compared with warfarin group (7.4 per 100 patient-years vs. 4.4 per 100 patient-years; RR, 1.69 [1.01–3.19]).72) In the WATCHMAN Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation vs. Long-term Warfarin Therapy (PREVAIL) study, LAAO was non-inferior to warfarin for ischemic stroke prevention or SE during 18 months of follow-up. The rate of composite of stroke, SE and cardiovascular/unexplained death was 6.4% in the LAAO group vs. 6.3% in the control group (RR, 1.07 [0.57–1.89]).73)
In a patient-level meta-analysis of 5-year outcomes from the PROTECT-AF and PREVAIL studies, LAAO (n=732) was associated with a non-significant increased risk of ischemic stroke/SE (HR, 1.71 [0.94–3.11]), compared with warfarin (n=382). Disabling stroke was lower in the LAAO group (HR, 0.45 [0.21–0.94]). The risk of hemorrhagic stroke was significantly lower in the LAAO group (HR, 0.20 [0.07–0.56]); however, the risks of all-cause major bleeding (including procedure-related) were comparable in the LAAO group and the warfarin group (HR, 0.91 [0.64–1.29]).74)
Currently, there is limited evidence regarding the effectiveness and safety of LAAO in Asians. Previously, a small size report (n=20) showed that the success rate of LAAO was 95% at two Asian centers.75) In this study, one procedure was abandoned because of catheter-related thrombus formation, while one patient experienced coronary artery air embolism. No stroke or death occurred at a mean follow-up of 12.7 months.75)
In a recent registry of patients with AF from Korea (n=142), including 10 patients with and 132 without LAA thrombus, LAAO was performed in multicenter facilities between 2010 to 2016. In this study, 6 patients experienced periprocedural complications. During the mean 23.2±17.5-month follow-up, 7 major adverse cardiac events occurred (1 cardiovascular death, 6 ischemic strokes).76) Another multicenter study in Korea (n=96) demonstrated that during 21.9-month follow-up after LAAO, the incidence of death, stroke, and major bleeding was 5.2%, 4.2%, and 1.0%, respectively.77) On transesophageal echocardiography of 93 patients within 6 months after the procedure, 24 residual leaks were observed (25.8%; 2 mild, 18 moderate, and 4 major).77)
Another single center study from from China included 122 AF patients with CHA2DS2-VASc score ≥1 for LAAO using WATCHMAN device.78) The mean CHA2DS2-VASc score were 4.09 in the primary prevention group and 1.93 in secondary prevention group. The success rate of the procedure was 98.5–100% with a low complication rate (not given). Stroke rates were low in the primary (1.47%) and secondary prevention groups (2.13%).78)
The safety of LAAO procedures is always a major concern. In the PROTECT-AF study, procedural-related adverse events at 7 days after the procedure occurred in 8.7% of patients, including pericardial tamponade requiring intervention in 4.0%.72) In the Amulet Global Observational Registry (n=1,088), 3.2% of patients experienced major procedural complications, including 1.2% pericardial effusion or tamponade, 0.9% vascular complications, 0.2% periprocedural stroke and 0.2% death.79) However, device-related complications may reduce with accumulating operator experience.
When choosing LAAO for patients with AF, the most driven factor would generally be the fear of ICH or reoccurrence of major bleeding. Nevertheless, the NOACs showed a significantly decreased risk of ICH and major bleeding, which may compromise the supporting evidence of choosing LAAO rather than OACs in the general patient with AF. In addition, LAAO cannot eliminate the need for antithrombotic therapy, including a period of peri-procedural anticoagulation and long-term antiplatelet therapy, which is probably no safer than NOACs. Further, LAAO would only aim at reducing the thrombin formation at LAA and has no influence on other clot origins in AF. Also, LAAO induced left atrium size increasing and left ventricular filling pressure need further study.80)
Therefore, LAAO should only be considered in patients with clear absolute contra-indications for long-term OAC.23),24) Also, the performance of LAAO should be compared with NOACs, considering the later showed significant benefits over warfarin.
CURRENT SITUATION OF ORAL ANTICOAGULATION USE IN ASIA
Over the last decade, in Asia-Pacific countries, the utilization rate of VKA has been around 15–20%.81) Such situation has improved recently, with the introduction of NOACs. In the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) registry reported at 2015, OAC use was seen in 57.4% of patients with AF with ≥1 stroke risk factors, including 31.9% on VKAs and 25.5% on NOACs.82) The total OAC use was lower in Asia (55.2%) compared to in Europe (90.1%).83) Amongst those taking OAC, NOACs were used in about 50.2% of patients in Asia, although still lower than other regions, such as North America 66.5%.83)
There are regional differences in OAC use in Asia. In the last decade from 2001 to 2012, in an observational study (n=921) of a relatively rural area, Yunnan Province of southwest China, less than 10% of patients with AF received VKA treatment, which has increased from 0.0% at 2007 to 9.5% at 2012.84) During 2011 to 2014, in Beijing, China, a survey involving 32 hospitals and 7,977 AF patients demonstrated OAC use of 36.5% among those with CHA2DS2-VASc score ≥2, which was increased from 30.2% at 2011 to 57.7% at 2014.85) In a “real-world” observational study from Hong Kong (n=9,727) at 2014, only 19.7% were taking warfarin.86) Also, in the same area, among the elderly patients with AF using the same database (≥80 years, n=2,339), there was only 23.8% received warfarin treatment.81)
A trend study in Korea between 2000 to 2013 showed that 86.1% of patients with AF had CHA2DS2-VASc score ≥2, however, only 39.1% of these patients were taking warfarin.87) In a recent national study of patients with AF from Korea (n=276,246), using the National Health Insurance Service database between 2008 to 2015, most of the patients had CHA2DS2-VASc score ≥2 (78.2% in 2008 and 83.2% in 2015).88) In the whole study population, OAC use increased from 34.7% to 50.6% and NOAC use accounted for 50% of total OAC use.88) In a cross-sectional analysis of Korean adults using the 2015 National Health Insurance Service database (n=41,505,679), OAC utilization among AF patients was lower in the suburban/rural regions compared with that observed in the urban regions (48.2% vs. 51.8%, p<0.001).89) Furthermore, the AF prevalence and income levels showed a J-shaped curve, whereas NOAC tended to be more commonly prescribed in those with higher income groups. In the Comparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry, 6,275 patients with nonvalvular AF were consecutively enrolled between 2016 to 2017 from 10 tertiary hospitals in Korea.90) The mean CHA2DS2-VASc score was 2.7±1.7. OAC was used in 70.1% of patients. Among patients with high stroke risk, OAC rate was 82.7%.90)
OAC use has improved in Japan over the last decade. In the study using Shinken Database, OAC use increased from 40.7% at 2004 to 55.9% at 2012. Among patients with CHADS2 score ≥2, OAC use rate was 64.4% during 2010 to 2012. The use of NOAC also significantly increased, with nearly 60% of anticoagulated patients treated with NOACs at 2010–2012.91) In a recent study from Japan, the Registry of Japanese Patients with Atrial Fibrillation Focused on anticoagulant therapy in New Era (RAFFINE) registry study, including 3,901 patients with AF from 4 university hospitals and 50 general hospitals/clinics from 2013 to 2015, 87.6% of patients received OAC therapy, including 44.6% treated with warfarin, and 43.0% treated with NOACs.92)
TTR CONTROL IN ASIANS ON VITAMIN K ANTAGONISTS
Apart from the rate of OAC use, quality of anticoagulation is important for stroke prevention. The TTR is an essential measurement for anticoagulation quality of VKA.93) Effective anticoagulation when using VKA means TTR ≥65–70%. But TTR can be influenced by multiple factors, which have been summarized as the sex, age, medical history, treatment, tobacco use, race risk (SAMe-TT2R2) score.4) A patient on VKA with SAMe-TT2R2 score >2 tends to have suboptimal TTR, who may need more frequent INR checks and monitoring or a switch to NOAC. Non-Westerns, including Asians, have at least 1 point in the SAMe-TT2R2 score, and tend to have poorer TTR.94),95),96) The SAMe-TT2R2 score has been validated in Asian cohorts97) and was recommended by the APHRS guidelines.24)
Indeed, in a subanalysis of the ENGAGE AF-TIMI 48 trial (n=21,105), the mean TTR was also low (56%) in patients from East Asia, including China, Korea, and Taiwan.63) In a multicenter retrospective observational study in Korea, including 1,230 patients with AF from 16 Korean centers, the mean TTR was 49.1%. None of the 16 centers achieved a mean TTR >60%.98) In Thailand, the mean TTR ranged from 40.1% to 62.7% in a cohort study with 433 AF patients.99) In Malaysia, during a 6 months follow-up with 184 AF patients, the TTR was 65.1% in a professional anticoagulation clinic and 48.3% amongst a ‘general’ medical clinic.100) In Singapore, the mean TTR of AF patients on warfarin was also poor (58%).101)
A low TTR in Asians may explain the suboptimal anticoagulation efficacy of VKAs. Indeed, in a study included 1,034 AF patients in China, warfarin did not show better efficacy in stroke prevention compared with aspirin. On the other hand, because of the relatively higher baseline risk of ICH102) and lower TTR in Asians, the benefits of VKA in Asia were likely to be compromised. However, NOACs were associated with better NCB compared with VKA among Asians.103)
A high rate of aspirin uses in Asia
The sole use of aspirin is highly prevalent in many patients with AF is Asia.103),104) As is shown in phase I of the GLORIA-AF registry, 49.6% of Chinese AF patients received aspirin alone,105) which has decreased to 25.8% at 2015.82) In the Registry on cardiac rhythm disORDers (RecordAF-AP) from eight Asian-Pacific countries, aspirin use was more common than VKAs use (56–66% vs. 35–47%).106) A large cohort study from Taiwan between 2001–2008 also demonstrated a high rate of aspirin use (62%).107) Also, in Hong Kong, a recent real-world data showed that 61% of patients received aspirin alone.108) In Korea, the use of aspirin was high, despite consistently decreased from 48.2% to 31.5%.88)
BARRIERS TO OPTIMISING ANTICOAGULATION USE IN ASIA
Elderly age is a common reason for low OAC use. In a study from China, age ≥75 years was associated with a 74% lower rate of OAC use in patients with AF103); however, a large cohort study (n=25,722) in an Asian population demonstrated that among AF patients age ≥90 years, warfarin was associated with a lower risk of ischemic stroke (HR, 0.69 [0.49–0.96]) and a positive NCB. Further, NOACs were associated with even lower risk of ICH (HR, 0.32 [0.10–0.97]) without difference in ischaemic stroke risk compared to warfarin.109) In a large real-world study using the Korean National Health Insurance Service database, among patients age ≥75 years, NOACs were associated with similar ischemic stroke (HR, 1.10 [0.80–1.49]) but reduced risk of ICH (HR, 0.63 [0.40–0.95]) and all-cause mortality (HR, 0.72 [0.59–0.86]) compared with VKA.18)
A recent meta-regression analysis included 26 studies, exploring the effectiveness and safety of OAC in older patients (≥65 years). Warfarin use was superior to no antithrombotic therapy (RR, 0.59 [0.51–0.76]) and aspirin (RR, 0.44 [0.24–0.64]) for stroke/SE prevention.110) NOACs were superior to warfarin for stroke/SE prevention (HR, 0.81 [0.73–0.89]) and were associated with lower risk of major bleeding (HR, 0.87 [0.77–0.97]).110)
Many clinicians may have a misunderstanding that aspirin can reduce stroke in AF and often underestimated the risk of bleeding related to aspirin. One study from Japan demonstrated the futility of aspirin in reducing stroke risk among low-risk patients.111) In another study from Hong Kong, aspirin showed a non-significant reduction in ischemic strokes, compared with no therapy.86)
Asian populations were associated with higher risk of ICH compared with the Western populations on VKA, which may also related to a low rate of VKA use.3),112) However, such situation could be avoided by using NOACs, which were associated with significantly lower risk of ICH (up to 80%).62) Finally, the inconvenience of INR checks in patients taking VKA and lack of anticoagulation clinics may also induce a high rate of aspirin use in Asia. Low persistence and adherence to VKA therapy have been associated with poor outcomes.113),114)
CONCLUSION
Stroke prevention in Asian patients with AF has many challenges, yet opportunities for improved care are evident. In many countries, OAC use is suboptimal, with high rates of aspirin or non-treatment, and where VKAs are used TTR is poorly managed.115) The NOACs offer an opportunity to improve efforts for stroke prevention, and modelling projections indicate this would have a major impact in reducing stroke and death, with substantial healthcare benefits.116) We should also be aware of the need for a holistic and integrated approach to managing the ‘whole’ AF patient, which should be ‘… as simple as ABC’, that is, Avoid stroke with Anticoagulation, Better symptom management with decisions on rate or rhythm control, and Cardiovascular and comorbidity risk management.117),118) This is relevant since AF independently increases all-cause mortality and strokes only account for 1 in 10 deaths related to AF, while 7 in 10 deaths are cardiovascular.119),120)
Priorities should be to identify the non-treated eligible for stroke prevention, finding those on aspirin and treating them with OAC, and finally identifying those on VKA with poor TTR, and to swop them to NOACs.
Footnotes
Conflict of Interest: EKC receivesresearch grant from Biosense Webster and Daiichi-Sankyo. GYHL isconsultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo.GYHL is also speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. Other authors have no conflict of interest to declare.
- Conceptualization: Lip GYH, Li YG.
- Resources: Choi EK.
- Supervision: Lip GYH, Lee SR.
- Writing - original draft: Li YG.
- Writing - review & editing: Lip GYH.
References
- 1.Chao TF, Liu CJ, Tuan TC, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. 2018;153:453–466. doi: 10.1016/j.chest.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol. 2017;236:226–231. doi: 10.1016/j.ijcard.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 6.Man-Son-Hing M, Laupacis A. Balancing the risks of stroke and upper gastrointestinal tract bleeding in older patients with atrial fibrillation. Arch Intern Med. 2002;162:541–550. doi: 10.1001/archinte.162.5.541. [DOI] [PubMed] [Google Scholar]
- 7.Henninger N, Goddeau RP, Jr, Karmarkar A, Helenius J, McManus DD. Atrial fibrillation is associated with a worse 90-day outcome than other cardioembolic stroke subtypes. Stroke. 2016;47:1486–1492. doi: 10.1161/STROKEAHA.116.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera KS, Vanassche T, Bosch J, et al. Global survey of the frequency of atrial fibrillation-associated stroke: embolic stroke of undetermined source global registry. Stroke. 2016;47:2197–2202. doi: 10.1161/STROKEAHA.116.013378. [DOI] [PubMed] [Google Scholar]
- 9.Tomita H, Hagii J, Metoki N, et al. Impact of sex difference on severity and functional outcome in patients with cardioembolic stroke. J Stroke Cerebrovasc Dis. 2015;24:2613–2618. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Yang PS, Jang E, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018 doi: 10.1136/heartjnl-2017-312930. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 12.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147:590–592. doi: 10.7326/0003-4819-147-8-200710160-00018. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 14.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 17.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 18.Cha MJ, Choi EK, Han KD, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48:3040–3048. doi: 10.1161/STROKEAHA.117.018773. [DOI] [PubMed] [Google Scholar]
- 19.Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 20.Wong KS, Hu DY, Oomman A, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Koretsune Y, Yang Y, et al. Edoxaban vs. warfarin in East Asian patients with atrial fibrillation - an ENGAGE AF-TIMI 48 subanalysis. Circ J. 2016;80:860–869. doi: 10.1253/circj.CJ-15-1082. [DOI] [PubMed] [Google Scholar]
- 22.Goto S, Zhu J, Liu L, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Am Heart J. 2014;168:303–309. doi: 10.1016/j.ahj.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm. 2017;33:345–367. doi: 10.1016/j.joa.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones C, Pollit V, Fitzmaurice D, Cowan C, Guideline Development Group The management of atrial fibrillation: summary of updated NICE guidance. BMJ. 2014;348:g3655. doi: 10.1136/bmj.g3655. [DOI] [PubMed] [Google Scholar]
- 26.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Chao TF, Liu CJ, Tuan TC, et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: which scoring system should be used for Asians? Heart Rhythm. 2016;13:46–53. doi: 10.1016/j.hrthm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Q, Chen S, Senoo K, Proietti M, Hong K, Lip GY. The CHADS2 and CHA2DS2-VASc scores for predicting ischemic stroke among East Asian patients with atrial fibrillation: a systemic review and meta-analysis. Int J Cardiol. 2015;195:237–242. doi: 10.1016/j.ijcard.2015.05.115. [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, Yang PS, Kim D, et al. CHA2DS2-VASc score for identifying truly low-risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48:2984–2990. doi: 10.1161/STROKEAHA.117.018551. [DOI] [PubMed] [Google Scholar]
- 30.Kang SH, Choi EK, Han KD, et al. Risk of ischemic stroke in patients with non-valvular atrial fibrillation not receiving oral anticoagulants - Korean nationwide population-based study. Circ J. 2017;81:1158–1164. doi: 10.1253/circj.CJ-16-1267. [DOI] [PubMed] [Google Scholar]
- 31.Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in ‘low-risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. doi: 10.1016/j.jacc.2014.06.1203. [DOI] [PubMed] [Google Scholar]
- 32.Chan PH, Lau CP, Tse HF, Chiang CE, Siu CW. CHA2DS2-VASc recalibration with an additional age category (50–64 Years) enhances stroke risk stratification in Chinese patients with atrial fibrillation. Can J Cardiol. 2016;32:1381–1387. doi: 10.1016/j.cjca.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. doi: 10.1016/j.jacc.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Chao TF, Lip GY, Liu CJ, et al. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–2469. doi: 10.1161/STROKEAHA.116.013880. [DOI] [PubMed] [Google Scholar]
- 35.Chao TF, Liu CJ, Wang KL, et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–642. doi: 10.1016/j.jacc.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Hung Y, Chao TF, Liu CJ, et al. Is an oral anticoagulant necessary for young atrial fibrillation patients with a CHA2DS2-VASc score of 1 (men) or 2 (women)? J Am Heart Assoc. 2016;5:e003839. doi: 10.1161/JAHA.116.003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013) Circ J. 2014;78:1997–2021. doi: 10.1253/circj.cj-66-0092. [DOI] [PubMed] [Google Scholar]
- 39.Chiang CE, Wu TJ, Ueng KC, et al. 2016 guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. doi: 10.1016/j.jfma.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Jung BC, Kim NH, Nam GB, et al. The Korean Heart Rhythm Society's 2014 statement on antithrombotic therapy for patients with nonvalvular atrial fibrillation: Korean Heart Rhythm Society. Korean Circ J. 2015;45:9–19. doi: 10.4070/kcj.2015.45.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JM, Joung B, Cha MJ, et al. 2018 KHRS guidelines for stroke prevention therapy in Korean patients with nonvalvular atrial fibrillation. Korean J Med. 2018;93:87–109. [Google Scholar]
- 42.Chao TF, Lip GY, Liu CJ, et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 43.Yoon M, Yang PS, Jang E, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost. 2018 doi: 10.1055/s-0038-1651482. [DOI] [PubMed] [Google Scholar]
- 44.Lip GY, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. doi: 10.1111/jth.13386. [DOI] [PubMed] [Google Scholar]
- 45.Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient. 2017;10:17–37. doi: 10.1007/s40271-016-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y, Zhu H, Chen Y, Lip GY. Comparing bleeding risk assessment focused on modifiable risk factors only versus validated bleeding risk scores in atrial fibrillation. Am J Med. 2018;131:185–192. doi: 10.1016/j.amjmed.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Esteve-Pastor MA, Rivera-Caravaca JM, Shantsila A, Roldán V, Lip GY, Marín F. Assessing bleeding risk in atrial fibrillation patients: comparing a bleeding risk score based only on modifiable bleeding risk factors against the HAS-BLED score. The AMADEUS trial. Thromb Haemost. 2017;117:2261–2266. doi: 10.1160/TH17-10-0710. [DOI] [PubMed] [Google Scholar]
- 48.Chao TF, Lip GY, Lin YJ, et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol. 2018;254:157–161. doi: 10.1016/j.ijcard.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Hijazi Z, Oldgren J, Lindbäck J, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–2311. doi: 10.1016/S0140-6736(16)00741-8. [DOI] [PubMed] [Google Scholar]
- 50.O'Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36:3258–3264. doi: 10.1093/eurheartj/ehv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 53.Guo YT, Zhang Y, Shi XM, et al. Assessing bleeding risk in 4824 Asian patients with atrial fibrillation: the Beijing PLA Hospital Atrial Fibrillation Project. Sci Rep. 2016;6:31755. doi: 10.1038/srep31755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao TF, Lip GY, Lin YJ, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768–777. doi: 10.1055/s-0038-1636534. [DOI] [PubMed] [Google Scholar]
- 55.Park CS, Choi EK, Kim HM, et al. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm. 2017;14:501–507. doi: 10.1016/j.hrthm.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 56.Zoppellaro G, Granziera S, Bertozzo G, et al. Consequences of warfarin suspension after major bleeding in very elderly patients with non valvular atrial fibrillation. Thromb Haemost. 2017;117:1828–1830. doi: 10.1160/TH16-11-0846. [DOI] [PubMed] [Google Scholar]
- 57.Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, et al. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb Haemost. 2017;117:1448–1454. doi: 10.1160/TH16-12-0961. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Yang YM, Zhu J, et al. Meta-analysis of efficacy and safety of new oral anticoagulants compared with uninterrupted vitamin K antagonists in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2016;117:926–934. doi: 10.1016/j.amjcard.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Cardoso R, Knijnik L, Bhonsale A, et al. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm. 2018;15:107–115. doi: 10.1016/j.hrthm.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GY. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2017;48:2494–2503. doi: 10.1161/STROKEAHA.117.017549. [DOI] [PubMed] [Google Scholar]
- 61.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 62.Li YG, Pastori D, Lip GY. Fitting the right non-vitamin K antagonist oral anticoagulant to the right patient with non-valvular atrial fibrillation: an evidence-based choice. Ann Med. 2018;50:288–302. doi: 10.1080/07853890.2018.1460489. [DOI] [PubMed] [Google Scholar]
- 63.Shimada YJ, Yamashita T, Koretsune Y, et al. Effects of regional differences in Asia on efficacy and safety of edoxaban compared with warfarin--insights from the ENGAGE AF-TIMI 48 trial. Circ J. 2015;79:2560–2567. doi: 10.1253/circj.CJ-15-0574. [DOI] [PubMed] [Google Scholar]
- 64.Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 65.Wang KL, Lip GY, Lin SJ, Chiang CE. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohsaka S, Katada J, Saito K, Terayama Y. Safety and effectiveness of apixaban in comparison to warfarin in patients with nonvalvular atrial fibrillation: a propensity-matched analysis from Japanese administrative claims data. Curr Med Res Opin. 2018:1–8. doi: 10.1080/03007995.2018.1478282. [DOI] [PubMed] [Google Scholar]
- 67.Lee KH, Park HW, Lee N, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace. 2017;19:iv1–9. doi: 10.1093/europace/eux247. [DOI] [PubMed] [Google Scholar]
- 68.Hong KS, Kwon SU, Lee SH, et al. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol. 2017;74:1206–1215. doi: 10.1001/jamaneurol.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dillinger JG, Aleil B, Cheggour S, et al. Dosing issues with non-vitamin K antagonist oral anticoagulants for the treatment of non-valvular atrial fibrillation: Why we should not underdose our patients. Arch Cardiovasc Dis. 2018;111:85–94. doi: 10.1016/j.acvd.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythm. 2017;33:289–296. doi: 10.1016/j.joa.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang KL, Giugliano RP, Goto S, et al. Standard dose versus low dose non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation: a meta-analysis of contemporary randomized controlled trials. Heart Rhythm. 2016;13:2340–2347. doi: 10.1016/j.hrthm.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Holmes DR, Reddy VY, Turi ZG, et al. PROTECT AF Investigators Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 73.Holmes DR, Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 74.Reddy VY, Doshi SK, Kar S, et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Lam YY, Yip GW, Yu CM, et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. 2012;79:794–800. doi: 10.1002/ccd.23136. [DOI] [PubMed] [Google Scholar]
- 76.Lee OH, Kim JS, Pak HN, et al. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol. 2018;121:1534–1539. doi: 10.1016/j.amjcard.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 77.Kim JS, Lee H, Suh Y, et al. Left atrial appendage occlusion in non-valvular atrial fibrillation in a Korean Multi-Center Registry. Circ J. 2016;80:1123–1130. doi: 10.1253/circj.CJ-15-1134. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y, Zhang Y, Huang W, Huang K, Xu B, Su XI. Primary and secondary stroke prevention using left atrial appendage closure with Watchman devices in atrial fibrillation patients: a single center experience from Mainland China. Pacing Clin Electrophysiol. 2017;40:607–614. doi: 10.1111/pace.13020. [DOI] [PubMed] [Google Scholar]
- 79.Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867–876. doi: 10.4244/EIJ-D-17-00493. [DOI] [PubMed] [Google Scholar]
- 80.Phan QT, Shin SY, Cho IS, et al. Impact of left atrial appendage closure on cardiac functional and structural remodeling: a Difference-in-Difference analysis of propensity score matched samples. Cardiol J. 2018 doi: 10.5603/CJ.a2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siu CW, Tse HF. Net clinical benefit of warfarin therapy in elderly Chinese patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:300–306. doi: 10.1161/CIRCEP.113.000858. [DOI] [PubMed] [Google Scholar]
- 82.Huisman MV, Rothman KJ, Paquette M, et al. Antithrombotic treatment patterns in patients with newly diagnosed nonvalvular atrial fibrillation: the GLORIA-AF Registry, Phase II. Am J Med. 2015;128:1306–1313.e1. doi: 10.1016/j.amjmed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 83.Bassand JP, Accetta G, Camm AJ, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–2889. doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Y, Wang H, Tian Y, Wang Y, Lip GY. Time trends of aspirin and warfarin use on stroke and bleeding events in Chinese patients with new-onset atrial fibrillation. Chest. 2015;148:62–72. doi: 10.1378/chest.14-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang SS, Dong JZ, Ma CS, et al. Current status and time trends of oral anticoagulation use among Chinese patients with nonvalvular atrial fibrillation: the Chinese atrial fibrillation registry study. Stroke. 2016;47:1803–1810. doi: 10.1161/STROKEAHA.116.012988. [DOI] [PubMed] [Google Scholar]
- 86.Siu CW, Lip GY, Lam KF, et al. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. doi: 10.1016/j.hrthm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Son MK, Lim NK, Park HY. Trend of prevalence of atrial fibrillation and use of oral anticoagulation therapy in patients with atrial fibrillation in South Korea (2002–2013) J Epidemiol. 2018;28:81–87. doi: 10.2188/jea.JE20160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GY. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One. 2017;12:e0189495. doi: 10.1371/journal.pone.0189495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SR, Han K, Cha MJ, et al. Prevalence of non-valvular atrial fibrillation based on geographical distribution and socioeconomic status in the entire Korean population. Korean Circ J. 2018;48:e58. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H, Kim TH, Cha MJ, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017;47:877–887. doi: 10.4070/kcj.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki S, Otsuka T, Sagara K, et al. Nine-year trend of anticoagulation use, thromboembolic events, and major bleeding in patients with non-valvular atrial fibrillation - Shinken Database analysis. Circ J. 2016;80:639–649. doi: 10.1253/circj.CJ-15-1237. [DOI] [PubMed] [Google Scholar]
- 92.Miyazaki S, Miyauchi K, Hayashi H, et al. Registry of Japanese patients with atrial fibrillation focused on anticoagulant therapy in the new era: The RAFFINE registry study design and baseline characteristics. J Cardiol. 2018;71:590–596. doi: 10.1016/j.jjcc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 93.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest. 2013;144:1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 94.Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2:e000067. doi: 10.1161/JAHA.112.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 96.Oh S, Goto S, Accetta G, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: Real-world data from the GARFIELD-AF registry. Int J Cardiol. 2016;223:543–547. doi: 10.1016/j.ijcard.2016.08.236. [DOI] [PubMed] [Google Scholar]
- 97.Bernaitis N, Ching CK, Chen L, et al. The sex, age, medical history, treatment, tobacco use, race risk (SAMe TT2R2) score predicts warfarin control in a Singaporean population. J Stroke Cerebrovasc Dis. 2017;26:64–69. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 98.Hong KS, Kim YK, Bae HJ, et al. Quality of anticoagulation with warfarin in Korean patients with atrial fibrillation and prior stroke: a multicenter retrospective observational study. J Clin Neurol. 2017;13:273–280. doi: 10.3988/jcn.2017.13.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saokaew S, Sapoo U, Nathisuwan S, Chaiyakunapruk N, Permsuwan U. Anticoagulation control of pharmacist-managed collaborative care versus usual care in Thailand. Int J Clin Pharm. 2012;34:105–112. doi: 10.1007/s11096-011-9597-8. [DOI] [PubMed] [Google Scholar]
- 100.Thanimalai S, Shafie AA, Hassali MA, Sinnadurai J. Comparing effectiveness of two anticoagulation management models in a Malaysian tertiary hospital. Int J Clin Pharm. 2013;35:736–743. doi: 10.1007/s11096-013-9796-6. [DOI] [PubMed] [Google Scholar]
- 101.Bernaitis N, Ching CK, Teo SC, et al. Factors influencing warfarin control in Australia and Singapore. Thromb Res. 2017;157:120–125. doi: 10.1016/j.thromres.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Yang QD, Niu Q, Zhou YH, et al. Incidence of cerebral hemorrhage in the Changsha community. A prospective study from 1986 to 2000. Cerebrovasc Dis. 2004;17:303–313. doi: 10.1159/000077341. [DOI] [PubMed] [Google Scholar]
- 103.Guo Y, Pisters R, Apostolakis S, et al. Stroke risk and suboptimal thromboprophylaxis in Chinese patients with atrial fibrillation: would the novel oral anticoagulants have an impact? Int J Cardiol. 2013;168:515–522. doi: 10.1016/j.ijcard.2012.09.187. [DOI] [PubMed] [Google Scholar]
- 104.Gamra H, Murin J, Chiang CE, et al. Use of antithrombotics in atrial fibrillation in Africa, Europe, Asia and South America: insights from the International RealiseAF Survey. Arch Cardiovasc Dis. 2014;107:77–87. doi: 10.1016/j.acvd.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Huisman MV, Ma CS, Diener HC, et al. Antithrombotic therapy use in patients with atrial fibrillation before the era of non-vitamin K antagonist oral anticoagulants: the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) Phase I cohort. Europace. 2016;18:1308–1318. doi: 10.1093/europace/euw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amerena J, Chen SA, Sriratanasathavorn C, et al. Insights into management of atrial fibrillation in Asia Pacific gained from baseline data from REgistry on cardiac rhythm disORDers (RecordAF-Asia Pacific [AP]) registry. Am J Cardiol. 2012;109:378–382. doi: 10.1016/j.amjcard.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 107.Chang CH, Yang YH, Chen JH, Lin LJ. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation in Taiwan. Thromb Res. 2014;133:782–789. doi: 10.1016/j.thromres.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 108.Chan EW, Lau WC, Siu CW, et al. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: a population-wide cohort study. Heart Rhythm. 2016;13:1581–1588. doi: 10.1016/j.hrthm.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 109.Chao TF, Liu CJ, Lin YJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation - a nationwide cohort study. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.117.031658. [DOI] [PubMed] [Google Scholar]
- 110.Bai Y, Guo SD, Deng H, et al. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-regression analysis. Age Ageing. 2018;47:9–17. doi: 10.1093/ageing/afx103. [DOI] [PubMed] [Google Scholar]
- 111.Sato H, Ishikawa K, Kitabatake A, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37:447–451. doi: 10.1161/01.STR.0000198839.61112.ee. [DOI] [PubMed] [Google Scholar]
- 112.Yasaka M, Lip GY. Impact of non-vitamin k antagonist oral anticoagulants on intracranial bleeding in Asian patients with non-valvular atrial fibrillation. Circ J. 2014;78:2367–2372. doi: 10.1253/circj.cj-14-0720. [DOI] [PubMed] [Google Scholar]
- 113.Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost. 2016;115:31–39. doi: 10.1160/TH15-04-0350. [DOI] [PubMed] [Google Scholar]
- 114.Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bai Y, Wang YL, Shantsila A, Lip GY. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. 2017;152:810–820. doi: 10.1016/j.chest.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 116.Bai Y, Guo SD, Shantsila A, Lip GY. Modelling projections for the risks related with atrial fibrillation in East Asia: a focus on ischaemic stroke and death. Europace. 2017 doi: 10.1093/europace/eux328. [DOI] [PubMed] [Google Scholar]
- 117.Lip GY. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–628. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 118.Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 119.Fauchier L, Villejoubert O, Clementy N, et al. Causes of death and influencing factors in patients with atrial fibrillation. Am J Med. 2016;129:1278–1287. doi: 10.1016/j.amjmed.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 120.Pokorney SD, Piccini JP, Stevens SR, et al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. 2016;5:e002197. doi: 10.1161/JAHA.115.002197. [DOI] [PMC free article] [PubMed] [Google Scholar]