Abstract
Stroke continues to be a major cause of morbidity and mortality in atrial fibrillation (AF) patients. Oral anticoagulation (OAC) provides protection against stroke and peripheral embolization in AF but significant proportion of patients could not be started on anticoagulation because of bleeding complications. Left atrial appendage harbors clot in about 90% of nonvalvular AF. The advent of left atrial appendage occlusion (LAAO) techniques has provided these patients with alternative to OAC for stroke prophylaxis. Multiple LAAO devices are currently available with Watchman and Amulet being the most commonly used in clinical practice. Randomized studies are available for Watchman device only. Data on Amplatzer Cardiac Plug, Amulet and Lariat devices are limited by the paucity of randomized data. Long-term data on different LAAO techniques are showing promising results. Device related thrombosis continues to be a serious complication associated with LAAO. Future studies should look into comparative effectiveness between different LAAO techniques, optimal patient selection, risk of complications, and anticoagulant treatment after LAAO. This article aims to provide current available evidence on efficacy and safety of different LAAO devices and future prospective.
Keywords: Left atrial appendage occlusion, Atrial fibrillation, Watchman, Amulet, Lariat
INTRODUCTION
Atrial fibrillation (AF) is a very common medical issue, that is estimated to have affected anywhere from 3 to 6 million Americans.1),2) Projections indicate that the prevalence of AF will triple in the United States by 2050, with similar increases expected in Europe.3) AF significantly increases the risk of ischemic stroke, with varying degree depending on the individual patient's stroke risk factors. Warfarin and novel oral anticoagulants (NOACs) are used to prevent stroke and peripheral embolization in patient with nonvalvular AF. Oral anticoagulation (OAC) decreases the risk of stroke by ≈64%.4) However, a significant percentage of patients could not be started on anticoagulation because of bleeding issues. Elderly patients are more likely to be not started on anticoagulation although studies have indicated that they can also benefit from anticoagulants.5)
Left atrial appendage occlusion (LAAO) was introduced as an alternative for stroke prophylaxis to AF patients who cannot take warfarin or NOACs. Left atrial appendage (LAA) harbors clots in 57% valvular and 90% of nonvalvular AF which is the source of embolic disease.6),7) LAAO is thus an appealing option for patients with nonvalvular AF for stroke prevention.7),8)
LEFT ATRIAL APPENDAGE OCCLUSION DEVICES
Watchman device
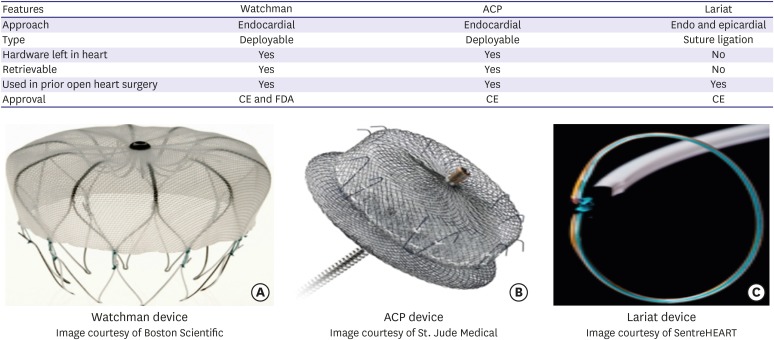
The Watchman device (Boston Scientific, Marlborough, MA, USA) is the most extensively studied and the only device approved by Food and Drug Administration (FDA) in United States (Figure 1). It is a self-expanding nitinol occlusion device and involves catheter-based implantation into left atrium with the guidance of transesophageal echocardiography (TEE). The device is inserted through femoral vein and is passed into left atrium through a small interatrial puncture hole that usually disappears within 6 months. The device has been implanted since 2002 in Europe and 2003 in the United States. U.S. FDA approved LAAO with Watchman to reduce the risk of stroke in patients with nonvalvular AF in March 2015.9) Approval of Watchman was driven by 2 pivotal trials: PROTECT AF and PREVAIL.
Figure 1.
Major features and images of most commonly used LAAO devices.
ACP = Amplatzer Cardiac Plug; CE = Conformite Europeenne; FDA = Food and Drug Administration; LAAO = left atrial appendage occlusion.
In the PROTECT AF non-inferiority trial, 707 patients ≥18 years with nonvalvular AF with CHADS2 risk score of ≥1 were randomized to LAAO with Watchman implantation or dose-adjusted warfarin in 2:1 fashion. The primary efficacy endpoint of stroke, cardiovascular death and systemic embolism was not different between 2 groups (relative risk [RR], 0.62; 95% confidence interval [CI], 0.35–1.25). The probability of non-inferiority of the LAAO was more than 99.9%. Primary safety events including major bleeding, pericardial effusion and device embolization were more frequent in the LAAO group than in the warfarin group (7.4 per 100 patient years, 95% CI, 5.5–9.7 vs. 4.4 per 100 patient-years, 95% CI, 2.5–6.7; RR, 1.69, 1.01–3.19).10)
Compared to the PROTECT AF trial in the follow up PREVAIL trial, 407 patients with higher mean CHADS2 score were randomized in 2:1 ratio to Watchman and warfarin. Designed to show non-inferiority the PREVAIL met the safety endpoint of a composite of cardiac perforation, pericardial effusion with tamponade, ischemic stroke, device embolization, and other vascular complications occurring in the first 7 days after implant. It met non-inferiority for one efficacy endpoint of late-ischemic efficacy endpoint of stroke or systemic embolization >7 days post-randomization.11) Procedural complications decreased from 8.7% in PROTECT AF to 4.2% in PREVAIL (p=0.004).
Since the FDA approval of Watchman, the reported rate of procedure-related complications has been relatively low, including an approximately 1% rate of pericardial tamponade even though 70% of physicians implanting the device were previously inexperienced with the procedure. This initial post-FDA approval U.S. clinical experience with LAAO using the Watchman device in 3,822 consecutive patients implanted by 382 physicians revealed excellent procedural success rate (95.6%) and favorable complication rates with pericardial tamponade, procedure-related stroke, and mortality rates of only approximately 1%, 0.08%, and 0.08%, respectively.12) More recent data from CAP,13) CAP 2,12),14) EWOLUTION15) and UK16) registries show improved procedural safety (Table 1). In addition, the device has been found to result in improved quality of life.17) Cost-effectiveness analysis of LAAO with Watchman device has shown it to be more cost-effective and cost-saving solution than warfarin or newer oral anticoagulants for stroke risk reduction in patients with nonvalvular AF who are at risk of stroke but have contraindications to warfarin.18),19)
Table 1. Major studies for different types of LAAO devices: Watchman.
| PROTECT AF†10) | PREVAIL†11) | CAP registry13) | CAP 2 registry12),14) | EWOLUTION15) | UK registry16) | |
|---|---|---|---|---|---|---|
| Study types | Randomized trial | Randomized trial | Prospective registry | Prospective registry | Prospective registry | Retrospective |
| Population | 463/244 | 269/138 | 460 | 579 | 1,021 | 371 (watchman=234, ACP=129, Lariat=6, Coherex Wavecrest =2) |
| Implantation success rate (%) | 91 | 95.1 | 95 | 94.8 | 98.5 | 92.5 |
| CHA2DS2-Vasc | ≥1* | Mean 3.8* | Mean 2.4 | 2.7 | 4.5 | 4.2 |
| HAS-BLED score | ≥1 in 97% | 2.3 | 3.3 | |||
| Anticoagulation used | Warfarin for 45 days post implantation followed by aspirin and Plavix × 6 months then aspirin alone | Warfarin for 45 days post implantation followed by aspirin and Plavix × 6 months then aspirin alone | Warfarin for 45 days post implantation followed by aspirin and Plavix × 6 months then aspirin alone | Warfarin for 45 days post implantation followed by aspirin and Plavix × 6 months then aspirin alone | 27% treated with OAC, 59% on dual antiplatelets, 7% single APT, 6% without any therapy | OAC-20%, DAPT 50%, OAC plus single APT-20%, single APT-10% |
| Ischemic stroke (%) | 2.2/1.6 | 1.9/0.7 | - | - | - | 0.1 |
| Hemorrhagic stroke (%) | 0.1/1.6 | 0.4/0 | - | - | - | 0.5 |
| Major bleeding (%) | 3.5/4.1 | 0.4/NA | 0.7 | - | 0.7 | 0.5 |
| Pericardial effusion/tamponade (%) | 4.8/0 | 0.4/0 | 2.2 | 2.4 | 0.5 | 0.8 |
| Device embolization (%) | 0.6/0 | 0.7/0 | 0 | 0 | 0.2 | 1.3 |
| Procedure-related stroke (%) | 1/0 | 0.4/0 | 0 | - | - | - |
ACP = Amplatzer Cardiac Plug; APT = antiplatelet therapy; DAPT = dual antiplatelet therapy; LAAO = left atrial appendage occlusion; NA = not available; OAC = oral anticoagulation.
*CHADS2; †Randomized studies, n/n=LAAO/warfarin arms.
Table 3. Major studies for different types of LAAO devices: Lariat.
| Characteristics | Lakkireddy et al.32) | Price et al.34) | Bartus et al.33) |
|---|---|---|---|
| Study type | Prospective registry | Retrospective | Prospective |
| Population | 712 | 154 | 89 |
| Ligation success rate (%) | 98 | 94 | 96 |
| CHA2DS2-Vasc score | 3.9 | 4 | 2.8 |
| HAS-BLED | 3.4 | 3 | 2.4 |
| Anticoagulation used | 21% OAC, 80% on single antiplatelet, 20% on DAPT | 24% OAC, 24% DAPT, aspirin alone 31%, no therapy 21% | 61% on OAC at 1-year follow up |
| Annual ischemic stroke (%) | - | - | - |
| Hemorrhagic stroke (%) | - | - | 1.1 |
| Major bleeding (%) | 0 | 9.1 | - |
| Pericardial effusion/tamponade (%) | - | 10.4 | - |
| Device embolization (%) | - | - | 0 |
| Procedure related stroke (%) | 0 | 0 | 0 |
DAPT = dual antiplatelet therapy; LAAO = left atrial appendage occlusion; OAC = oral anticoagulation.
Amplatzer Cardiac Plug and Amulet
Amplatzer Cardiac Plug (ACP; St. Jude Medical, St. Paul, MN, USA) is a self-expanding double-disc device that is implanted via femoral access into LAA via transseptal puncture (Figure 1). The Amulet device is the second generation of ACP with improvements in the implantation apparatus. Data from large multicenter ACP study consisting of 1,047 patients with an average follow up of 13 months showed annual rate of systemic thromboembolism of 2.3% (59% risk reduction based on patient stroke risk scores) and major bleeding of 2.1% (representing 61% risk reduction).20) Long-term data from cumulative experience of 2 Italian centers obtained from a relatively large cohort treated with LAAO using the ACP device with a follow up period of up to 4 years had demonstrated similar reduced annual rate of systemic thromboembolism and major bleeding (2.5% and 1.3%) respectively.21) A recent global prospective registry of large cohort of AF patients (n=1,088) at high risk for ischemic stroke as well as bleeding, implanted with the Amulet device demonstrated a high implantation success (99.0%) and adequate LAAO in almost all patients who received a device (99.8%). The clinically relevant major adverse events during implantation and subsequent hospitalization was 3.2%.22) Major findings from the other ACP and Amulet registries23),24),25),26),27) are shown in Table 2. Comparative studies have shown similar results obtained with the ACP and Amulet devices in terms of safety, implantation success and appropriate closure of the LAA.28),29)
Table 2. Major studies for different types of LAAO devices: Amplatzer Cardiac Plug and Amulet.
| Characteristics | Tzikas et al.20) | Koskinas et al.25) | Berti et al.24) | López Mínguez et al.27) | Kleinecke et al.26) | Urena et al.23) |
|---|---|---|---|---|---|---|
| Study type/device used | Retrospective/ACP | Prospective/ACP 408, Amulet 92 | Prospective/ACP 91, Amulet 17 | Prospective/ACP | Retrospective/Amulet | Retrospective/ACP |
| Population | 1,047 | 500 | 108 | 167 | 50 | 52 |
| Implantation success rate (%) | 97.3 | 97.8 | 100 | 94.6 | 98 | 98.1 |
| CHA2DS2-Vasc score | 4.5 | 4.3 | 4.3 | 4 | 5.2 | 5 (median) |
| HAS-BLED score | 3.1 | 2.9 | 3.4 | 3 | 3.5 | 4 (median) |
| Anticoagulation used | 16% treated with warfarin, otherwith variable combinations of aspirin, clopidogrel, LMWH | DAPT with aspirin for 5 months plus clopidogrel for 1–6 months | - | DAPT with asprin and clopidogrel. Aspirin for 6–12 months and clopidogrel for 3–6 months | DAPT with clopidogrel × 3 months and aspirin for at least 6 months | DAPT or single APT for 1–6 months followed by single APT |
| Annual Ischemic stroke (%) | 2.3 | - | 2.2 | 3.9 | 6.1 | 1.9 |
| Annual hemorrhagic stroke (%) | 2 | - | 1.1 | - | - | - |
| Major bleeding (%) | 1.2 | 3.2 | 0.9 | 5.7 | 4 | 3.8 |
| Pericardial effusion/tamponade (%) | 1.24 | 3.2* | 2.7 | 1.2 | 4 | 1.9 |
| Device embolization (%) | 0.7 | 2 | 0 | - | 2 | 1.9 |
| Procedure related stroke (%) | 0.8 | 1 | - | 1.2 | - | 0 |
ACP = Amplatzer Cardiac Plug; APT = antiplatelet therapy; DAPT = dual antiplatelet therapy; LAAO = left atrial appendage occlusion; LMWH = low molecular weight heparin.
*Hemodynamically significant pericardial effusion.
Currently, the Watchman and the Amulet are the most commonly implanted devices for catheter-based LAAO, with a higher penetration of the Amulet device within Europe compared with non-European geographies.21) They are increasingly being tried in different subset of population. Recent preliminary study of LAAO with ACP and Watchman has shown promising result in hemodialysis patients with high CHA2DS2-VASCs and HASBLED scores were 4.0 (1.5) and 4.4 (0.9), over short term follow up.30) A recent Korean multicenter registry in nonvalvular AF patients with LAA thrombus found Watchman and ACP devices may be a safe and feasible alternative to anticoagulation in select patients at a high risk of bleeding or contraindication to anticoagulation, or in whom anticoagulation failed to prevent stroke.31)
Lariat device
Alternative epicardial approaches for LAAO have also been studied and are of particular value in patients who cannot tolerate any anticoagulation and do not have suitable LAA anatomy for endocardial occlusion. The procedure is characterized by delivery of a pre-tied suture loop over the LAA by means of guidance from dual-wire access from femoral and epicardial approach. The largest experience is with the LARIAT device (SentreHEART, Redwood, CA, USA) which is a hybrid procedure with transseptal endocardial and pericardial access used to place a ligature around the LAA (Figure 1). Recent studies also suggest LAA as a non-pulmonary vein focus of origin of AF. Epicardial based exclusion procedures can electrically isolate the LAA and there might be a benefit of reducing AF burden in addition to stroke prevention especially in nonparoxysmal AF. The largest prospective registry on Lariat showed the success rate of >95% with low risk of procedure-related mortality (0.14%).32) The most common serious complication was found to be cardiac perforation related to epicardial access which was significantly reduced with the use of micropuncture needle for pericardial access. Similarly, use of micropuncture needle was associated with lower rate of bleeding complications.32) Table 3 show the details of other 2 available large studies33),34) on Lariat device.
OTHER METHODS OF LEFT ATRIAL APPENDAGE OCCLUSION
Articlip system
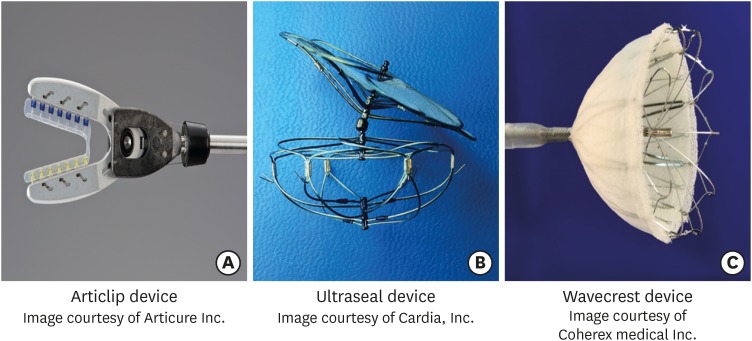
The Atriclip LAA occlusion devices (Articure Inc., Mason, OH, USA) (Figure 2A) have been evaluated for LAA closure at the time of concomitant open heart surgery under direct visualization. High closure success was demonstrated in the Exclusion of the Left Atrial Appendage with a Novel Device: Early Results of a Multicenter Trial (EXCLUDE) trial by TEE or computed tomographic angiography (CTA) at 90-day follow up. With 95.7% implantation success rate the trial met its safety (major bleeding) and efficacy endpoint (ischemic stroke).35)
Figure 2.
Images of (A) Articlip, (B) Ultraseal and (C) Wavecrest devices.
Ultraseal left atrial appendage device
The Ultraseal LAA device (Cardia, Inc., Eagan, MN, USA) is a percutaneous, transcatheter device (Figure 2B) that consists of a distal soft bulb and a proximal sail attached by an articulating joint that allows a high degree of device conformability to the different variations of the LAA anatomy. The device can be retrieved and redeployed multiple times in a single procedure without replacing the device or delivery sheath. Initial experience of Ultraseal device from Canada showed successful implantations in all patients (n=12) without periprocedural complications. No episodes of bleeding, stroke, pericardial effusion or device embolization were noted at 45-day follow up.36)
Wavecrest device
Wavecrest LAAO system (Coherex medical Inc., Salt Lake City, UT, USA) is the latest development in LAAO devices. It consists of a nitinol frame with retractable coils and anchors to enable optimal device positioning (Figure 2C). The device is relatively short and is designed for more proximal deployment in the LAA. Coherex WAVECREST I trial, a prospective observational study to establish the safety and efficacy of Coherex Wavecrest device for LAA closure has been completed and results are awaited.37)
SURGICAL REMOVAL WITH OTHER CARDIAC PROCEDURES
The LAA can also be surgically removed simultaneously with other cardiac procedures. Meta-analysis of 2 randomized trials and five observational studies of surgical LAA closure in the setting of cardiac arterial bypass grafting or mitral valve surgery (n=3,653 patients) suggests that LAA closure is associated with a lower incidence of stroke at 30-day follow up (0.95% vs. 1.9%; odds ratio [OR], 0.46; p=0.005).38)
ANTICOAGULATION MANAGEMENT DURING LEFT ATRIAL APPENDAGE OCCLUSION
Despite these favorable results with LAAO, some serious side effects, such as device-associated strokes, are described. A recently published prospective registry from 8 French centers with 469 patients with AF undergoing LAAO (272 Watchman and 197 Amplatzer devices) with mean follow up of 13 months found an annual incidence of device-related thrombus at 7.2% per year.39) In particular, the first 45 days after implantation are a critical transition period. Anticoagulation with coumadin is warranted for 45 days post-implantation after which complete endothelization of the device is expected.10),40) Once the device is completely endothelized (no gaps around the device larger than 5 mm with communication to the appendage) anticoagulation is stopped and patients are started on aspirin and clopidogrel for 6 months. At 6 months post-implantation, aspirin is recommended indefinitely. However, endothelization might take more time in some patients; consequently they can develop device related thrombus after discontinuation of anticoagulation.41) In PROTECT trial warfarin was prescribed for at least 45 days after successful device implantation. In subsequent studies with Watchman device dual antiplatelet therapy (DAPT) for at least 6 months after implantation was shown to be safe in patients with contraindications for OAC.42),43) DAPT was shown to be safe and effective in one study with regard to the ACP device as well.23) Newer studies are showing the feasibility of NOACs in LAAO. A pilot study from Germany found LAAO with NOACs compared to DAPT shows similar rate of all-cause mortality, major adverse cardiac and cerebrovascular events and major bleeding at 45-day follow up.44) A recently published retrospective multicenter study found the use of NOACs in the post-WATCHMAN period to be feasible alternative with similar rates of bleeding events (0.5% vs. 0.9%, p=0.6) and composite of device-related thrombosis or thromboembolism (1.4% vs. 0.9%, p=1) compared with uninterrupted warfarin.45)
LONG-TERM DATA AND HEAD TO HEAD STUDIES
Patient level meta-analysis from 5-year follow up of PROTECT and PREVAIL trials showed beneficial effect of LAAO watchman device over coumadin for nonvalvular AF with similar stroke reduction but significant reduction in major bleeding including hemorrhagic stroke and mortality.46) Similarly a recently published results of the Iberian registry of LAAO with a total of 598 patients (1,093 patient-years) with a contraindication for anticoagulants and mean follow up of 23 months showed significantly reduced rate of stroke and bleeding events.47) The rate of ischemic stroke was 1.6% (vs. 8.5% expected according to CHA2DS2-VASc; p<0.001); intracranial hemorrhage 0.8%; gastrointestinal bleeding 3.2%; severe bleeding 3.9% (vs. 6.3% expected by HAS-BLED, p=0.002). Further improvement in the outcomes were demonstrated in the subgroup of 176 patients with follow up >24 months (mean follow up 46.6 months, 683 patient-years). LAAO using the ACP device in 134 patients from 2 Italian centers had demonstrated favorable outcome with annual rate of systemic thromboembolism and major bleeding (2.5% and 1.3%) at 4-year follow up.21) A single center study with 10-year experience of LAAO with ACP with a mean follow up of about 3-year showed 7% incidence of safety events. However, the study found excellent intermediate-term outcome once the procedural risk is overcome.48) A recent study involving high risk elderly population (average age 76) with CHA2DS2-VASc of 5 and HAS-BLED of 4 followed for 4 years showed stroke rate of 6.9%. The reported long-term mortality rate was high in the study at 33.7%.49)
Comparative studies between different LAAO devices is sparse. In a multicenter observational study with a total of 479 patients (Watchman=219, Lariat=259) and 12-month of follow up, the Watchman group was found to have statistically higher incidence (21% vs. 13%, p=0.01) and mean leak size (3.10±1.1 mm vs. 2.15±1.4 mm; p<0.001) than did the Lariat group.50) However, there was similar rate of thromboembolism (3.7% vs. 1.6%, p=0.23) and stroke rate (1.3% vs. 1.1%, p=0.99) between Watchman and Lariat.
CURRENT GUIDELINES BY PROFESSIONAL SOCIETIES
The latest American Heart Association/American College of Cardiology guidelines from 2014 suggest that surgical excision of the LAA may be considered in patients undergoing cardiac surgery or thoracoscopic AF surgery (grade IIB).51) However, the guideline does not make any recommendation regarding use of LAAO devices for stroke prevention citing the lack of clinical trials. This guideline was released prior to Watchman's approval by FDA. On the other hand, the current European Society of Cardiology Guidelines recommend that LAAO may be considered for stroke prevention in patients with AF and contraindications for long-term OAC (e.g., those with a previous life-threatening bleed without a reversible cause) (IIb, LOE B) despite the lack of randomized data on safety and efficacy of LAAO in patients with contraindications to OAC.52) We believe that it is time to revise these guidelines to make LAA occlusion a class I indication.
FUTURE STUDIES AND NEWER APPLICATIONS OF LEFT ATRIAL APPENDAGE EXCLUSION
Since the first LAAO device in 2002, rapidly growing studies have supported the strategy and it is gaining popularity. The only randomized controlled trials on LAAO (on Watchman) available until now have excluded patients with contraindications to OAC therapy. However, current data stemming from numerous registry studies may seem to justify LAAO in patients with nonvalvular AF who have contraindications to OAC. On the other hand, evidence for ACP, Amulet and Lariat device are driven by non-randomized retrospective and prospective studies only which has limited its impact. Furthermore, there were several other exclusion criteria in these randomized and non-randomized studies restricting generalization to whole spectrum of AF patients. Thus, randomized studies involving different subsets of AF patients might be warranted in the future.
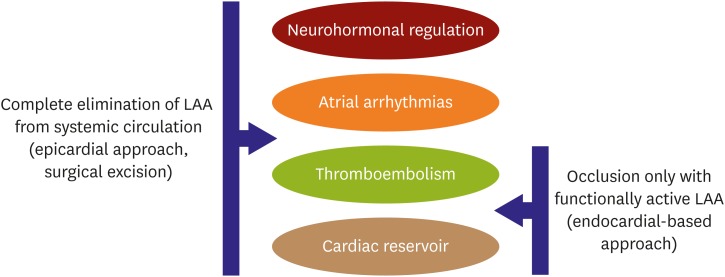
Emerging data highlight the impact of various LAAO approaches in different physiological functions (Figure 3). Although LAA is considered to be a cardiac vestige, its influence on various physiologic aspects of cardiac vascular systems seems to be pretty broad. LAA may have impact on the neurohormonal, left atrial function and even be a potential source of cardiac arrhythmias. Previous surgical data showed that LAA is a rich source of atrial natriuretic peptide (ANP) which is important for fluid and sodium balance in the body. Elimination of the same may result in significant fluid retention. There are also questions whether LAA functions as a reservoir chamber to accommodate volume changes in the human body. Endocardial ablation of the LAA was shown to significantly improve the overall success rates in non-paroxysmal AF patients.
Figure 3.
Physiological impact of different LAAO approaches.
LAA = left atrial appendage; LAAO = left atrial appendage occlusion.
While both endocardial and epicardial approaches reduce stroke risk and improve left atrial reservoir function its role in neurohormonal regulation and arrhythmia burden reduction has been elucidated in studies with epicardial suture delivery approach brain natriuretic peptides (BNPs) and ANP levels were shown to decrease after epicardial LAA isolation.53),54) The impact of such neurohormonal changes in hemodynamics was tested in the LAA HOMEOSTASIS study which demonstrated that epicardial LAAO results in significant decrease in blood pressure at 24 hour and 3 months.55) Similarly, recent studies also suggest LAA as a non-pulmonary vein focus of origin of AF. Epicardial based exclusion procedures can electrically isolate the LAA and there might be a benefit of reducing AF burden in addition to stroke prevention especially in nonparoxysmal AF.56),57),58) The ongoing a MAZE trial (NCT02513797) will give further data regarding the benefit of such a strategy in persistent AF.59) This sets the foundation for a large body of research that needs to happen in this space that was once thought to be irrelevant to cardiac function.
Optimizing safety and efficacy in the real world
LAA has a highly variable anatomical structure and may offer various procedural challenges. Newer studies are showing promising results with increased operator experience and lower rate of complications in the real-world setting. However, comprehensive patient assessment prior to undergoing LAA closure should be encouraged. This should be able to identify patients at high risk of procedural complications and overall prognosis. Besides, future registries and clinical studies should continue to contribute to further design improvement of LAA closure devices.
Data on usefulness of NOACs is sparse. Future studies should look into the role of NOACs in LAAO. Clinical studies should be undertaken not only to compare LAAO versus NOACs but to explore the superiority of different types of NOACs in LAAO for stroke prevention.
There is clear evidence to show the long-term cost-effectiveness of the Watchman device. The LAAO surpasses NOACs in their cost effectiveness after a 4-year mark. Robust clinical and financial models that would help us understand the long-term economic impact of these devices should be undertaken as their use increases.
Currently, there are a few important undergoing studies that can potentially change the practice and utilization of LAAO. The Amplatzer Amulet LAA Occluder trial (Amulet IDE) is a prospective randomized multicenter worldwide trial that started enrolling patients in August 2016, randomizing patients in a 1:1 fashion to either the Amulet device or the Watchman device. The Amulet device will be evaluated for safety and efficacy by demonstrating its performance is non-inferior to Watchman LAA closure device in patients with nonvalvular AF (NCT02879448).60) The primary safety endpoint is a composite of procedure-related complications or all-cause death or major bleeding through 12 months, and the primary efficacy endpoint is a composite of ischemic stroke or systemic embolism through 18 months.
Another important study that is recruiting patient is Interventional Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-risk Patients with Atrial Fibrillation (PRAGUE-17 Study, NCT02426944). This randomized multicenter open label trial is recruiting a total of 400 patients into LAAO (Watchman or Amulet) and NOAC group. The primary endpoint is the combination of stroke, other systemic cardiovascular event, clinically significant bleeding, cardiovascular death or procedure or device-related complications. The trial is expected to be completed by May 2020.61)
A randomized clinical trial Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO; NCT02928497) of patients with nonvalvular AF at increased risk of stroke but who are not candidates for any anticoagulation has been initiated, randomizing patients to either Watchman and aspirin/clopidogrel or conservative medical therapy alone with aspirin and/or clopidogrel. With estimated enrollment of 888 patients the study seeks to evaluate the primary safety endpoint (7-day combined rate of death, ischemic stroke, systemic embolism and complications requiring major cardiovascular or endovascular intervention) and efficacy endpoint (comparison of time to first event of ischemic stroke and systemic embolism).62)
Finally, as more data on LAAO becomes available expert consensus document from professional societies on both sides of the Atlantic is anticipated for best practice guidelines.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lakkireddy D, Park P.
- Data curation: Lakkireddy D, Sharma SP.
- Formal analysis: Sharma SP.
- Methodology: Lakkireddy D, Park P.
- Validation: Lakkireddy D, Park P.
- Writing - original draft: Sharma SP.
- Writing - review & editing: Lakkireddy D, Park P.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62. doi: 10.1002/clc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 6.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 7.Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 8.Onalan O, Crystal E. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke. 2007;38:624–630. doi: 10.1161/01.STR.0000250166.06949.95. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Watchman LAA closure technology -P130013 [Internet] Silver Spring (MD): U.S. Food and Drug Administration; 2015. [cited 2018 May 17]. Available form: https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130013a.pdf. [Google Scholar]
- 10.Holmes DR, Reddy VY, Turi ZG, et al. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 11.Holmes DR, Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VY, Gibson DN, Kar S, et al. Post-approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 14.Holmes DR, Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betts TR, Leo M, Panikker S, et al. Percutaneous left atrial appendage occlusion using different technologies in the United Kingdom: a multicenter registry. Catheter Cardiovasc Interv. 2017;89:484–492. doi: 10.1002/ccd.26782. [DOI] [PubMed] [Google Scholar]
- 17.Alli O, Doshi S, Kar S, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013;61:1790–1798. doi: 10.1016/j.jacc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VY, Akehurst RL, Armstrong SO, et al. Cost effectiveness of left atrial appendage closure with the Watchman device for atrial fibrillation patients with absolute contraindications to warfarin. Europace. 2016;18:979–986. doi: 10.1093/europace/euv412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR., Jr Time to cost-effectiveness following stroke reduction strategies in AF: warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66:2728–2739. doi: 10.1016/j.jacc.2015.09.084. [DOI] [PubMed] [Google Scholar]
- 20.Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 21.Santoro G, Meucci F, Stolcova M, et al. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1188–1194. doi: 10.4244/EIJY14M10_13. [DOI] [PubMed] [Google Scholar]
- 22.Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867–876. doi: 10.4244/EIJ-D-17-00493. [DOI] [PubMed] [Google Scholar]
- 23.Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62:96–102. doi: 10.1016/j.jacc.2013.02.089. [DOI] [PubMed] [Google Scholar]
- 24.Berti S, Pastormerlo LE, Rezzaghi M, et al. Left atrial appendage occlusion in high-risk patients with non-valvular atrial fibrillation. Heart. 2016;102:1969–1973. doi: 10.1136/heartjnl-2015-309150. [DOI] [PubMed] [Google Scholar]
- 25.Koskinas KC, Shakir S, Fankhauser M, et al. Predictors of early (1-week) outcomes following left atrial appendage closure with Amplatzer devices. JACC Cardiovasc Interv. 2016;9:1374–1383. doi: 10.1016/j.jcin.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Kleinecke C, Park JW, Gödde M, Zintl K, Schnupp S, Brachmann J. Twelve-month follow-up of left atrial appendage occlusion with Amplatzer Amulet. Cardiol J. 2017;24:131–138. doi: 10.5603/CJ.a2017.0017. [DOI] [PubMed] [Google Scholar]
- 27.López Mínguez JR, Asensio JM, Gragera JE, et al. Two-year clinical outcome from the Iberian registry patients after left atrial appendage closure. Heart. 2015;101:877–883. doi: 10.1136/heartjnl-2014-306332. [DOI] [PubMed] [Google Scholar]
- 28.Gloekler S, Shakir S, Doblies J, et al. Early results of first versus second generation Amplatzer occluders for left atrial appendage closure in patients with atrial fibrillation. Clin Res Cardiol. 2015;104:656–665. doi: 10.1007/s00392-015-0828-1. [DOI] [PubMed] [Google Scholar]
- 29.Abualsaud A, Freixa X, Tzikas A, et al. Side-by-side comparison of LAA occlusion performance with the Amplatzer cardiac plug and Amplatzer Amulet. J Invasive Cardiol. 2016;28:34–38. [PubMed] [Google Scholar]
- 30.Genovesi S, Slaviero G, Porcu L, et al. Implant success and safety of left atrial appendage occlusion in end stage renal disease patients: peri-procedural outcomes from an Italian dialysis population. Int J Cardiol. 2018;262:38–42. doi: 10.1016/j.ijcard.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 31.Lee OH, Kim JS, Pak HN, et al. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol. 2018;121:1534–1539. doi: 10.1016/j.amjcard.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 32.Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13:1030–1036. doi: 10.1016/j.hrthm.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 34.Price MJ, Gibson DN, Yakubov SJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014;64:565–572. doi: 10.1016/j.jacc.2014.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg . 2011;142:1002–1009. 1009.e1. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 36.Regueiro A, Bernier M, O'Hara G, et al. Left atrial appendage closure: initial experience with the ultraseal device. Catheter Cardiovasc Interv. 2017;90:817–823. doi: 10.1002/ccd.26870. [DOI] [PubMed] [Google Scholar]
- 37.Coherex WAVECREST I left atrial appendage occlusion study [Internet] Bethesda (MD): U.S. National Library of Medicine; 2015. [cited 2018 May 15]. Available form: https://clinicaltrials.gov/ct2/show/NCT02239887?cond=WAVECREST&rank=1. [Google Scholar]
- 38.Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015;47:847–854. doi: 10.1093/ejcts/ezu291. [DOI] [PubMed] [Google Scholar]
- 39.Fauchier L, Cinaud A, Brigadeau F, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–1536. doi: 10.1016/j.jacc.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 40.Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–1495. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Main ML, Fan D, Reddy VY, et al. Assessment of device-related thrombus and associated clinical outcomes with the Watchman left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the PROTECT-AF trial) Am J Cardiol. 2016;117:1127–1134. doi: 10.1016/j.amjcard.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 42.Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology) J Am Coll Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Meincke F, Schmidt-Salzmann M, Kreidel F, Kuck KH, Bergmann MW. New technical and anticoagulation aspects for left atrial appendage closure using the WATCHMAN® device in patients not taking warfarin. EuroIntervention. 2013;9:463–468. doi: 10.4244/EIJV9I4A75. [DOI] [PubMed] [Google Scholar]
- 44.Bösche LI, Afshari F, Schöne D, Ewers A, Mügge A, Gotzmann M. Initial experience with novel oral anticoagulants during the first 45 days after left atrial appendage closure with the Watchman device. Clin Cardiol. 2015;38:720–724. doi: 10.1002/clc.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enomoto Y, Gadiyaram VK, Gianni C, et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017;14:19–24. doi: 10.1016/j.hrthm.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 47.López-Mínguez JR, Nogales-Asensio JM, Infante De Oliveira E, et al. Long-term event reduction after left atrial appendage closure. Results of the Iberian Registry II. Rev Esp Cardiol (Engl Ed) 2018:S1885-5857(18)30112-9. doi: 10.1016/j.rec.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Nietlispach F, Gloekler S, Krause R, et al. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013;82:283–289. doi: 10.1002/ccd.24872. [DOI] [PubMed] [Google Scholar]
- 49.Regueiro A, Cruz-Gonzalez I, Bethencourt A, et al. Long-term outcomes following percutaneous left atrial appendage closure in patients with atrial fibrillation and contraindications to anticoagulation. J Interv Card Electrophysiol. 2018;52:53–59. doi: 10.1007/s10840-018-0356-9. [DOI] [PubMed] [Google Scholar]
- 50.Pillarisetti J, Reddy YM, Gunda S, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm. 2015;12:1501–1507. doi: 10.1016/j.hrthm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 51.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 52.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 53.Cruz-Gonzalez I, Palazuelos Molinero J, Valenzuela M, et al. Brain natriuretic peptide levels variation after left atrial appendage occlusion. Catheter Cardiovasc Interv. 2016;87:E39–43. doi: 10.1002/ccd.25985. [DOI] [PubMed] [Google Scholar]
- 54.Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the Watchman device. J Invasive Cardiol. 2015;27:448–452. [PubMed] [Google Scholar]
- 55.Lakkireddy D, Turagam M, Afzal MR, et al. Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol. 2018;71:135–144. doi: 10.1016/j.jacc.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 56.Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm. 2015;12:52–59. doi: 10.1016/j.hrthm.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 57.Badhwar N, Lakkireddy D, Kawamura M, et al. Sequential percutaneous LAA ligation and pulmonary vein isolation in patients with persistent AF: initial results of a feasibility study. J Cardiovasc Electrophysiol. 2015;26:608–614. doi: 10.1111/jce.12655. [DOI] [PubMed] [Google Scholar]
- 58.Starck CT, Steffel J, Emmert MY, et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012;15:416–418. doi: 10.1093/icvts/ivs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.aMAZE Study: LAA ligation adjunctive to PVI for persistent or longstanding persistent atrial fibrillation (aMAZE) [Internet] Bethesda (MD): U.S. National Library of Medicine; 2018. [cited 2018 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT02513797. [Google Scholar]
- 60.AMPLATZERTM AmuletTM LAA Occluder trial (Amulet IDE) [Internet] Bethesda (MD): U.S. National Library of Medicine; 2018. [cited 2018 May 17]. Available form: https://clinicaltrials.gov/ct2/show/NCT02879448?cond=Amplatzer&draw=2&rank=1. [Google Scholar]
- 61.Left atrial appendage closure vs. novel anticoagulation agents in atrial fibrillation (PRAGUE-17) [Internet] Bethesda (MD): U.S. National Library of Medicine; 2016. [cited 2018 May 17]. Available form: https://clinicaltrials.gov/ct2/show/NCT02426944?cond=PRAGUE+17&rank=1. [Google Scholar]
- 62.Assessment of the WATCHMANTM Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) [Internet] Bethesda (MD): U.S. National Library of Medicine; 2018. [cited 2018 May 17]. Available form: https://clinicaltrials.gov/ct2/show/NCT02928497?cond=ASAP+TOO&rank=1. [Google Scholar]