Abstract
Selective serotonin reuptake inhibitors (SSRIs) represent a class of pharmaceuticals previously reported in aquatic ecosystems. SSRIs are designed to treat depression and other disorders in humans, but are recognized to elicit a variety of effects on aquatic organisms, ranging from neuroendocrine disruption to behavioral perturbations. However, an understanding of the relationships among mechanistic responses associated with SSRI targets and ecologically important behavioral responses of fish remains elusive. Herein, linking Adverse Outcomes Pathways (AOP) models with internal dosimetry represent potential approaches for developing an understanding of pharmaceutical risks to aquatic life. We selected sertraline as a model SSRI for a 28-d study with adult male fathead minnows. Binding activity of the serotonin reuptake transporter (SERT), previously demonstrated in mammals and fish models to respond to sertraline exposure, was selected as an endpoint associated with therapeutic activity. Shelter-seeking behavior was monitored using digital tracking software to diagnose behavioral abnormalities. Fish plasma levels of sertraline exceeding human therapeutic doses were accurately modeled from external exposure concentrations when pH influences on ionization and log D were considered. We observed statistically significant decreases in binding at the therapeutic target (SERT) and shelter-seeking behavior when fish plasma levels exceeded human therapeutic thresholds. Such observations highlights the strengths of coupling physiologically based pharmacokinetic modeling and AOP approaches and suggest that internal dosimetry should be monitored to advance an understanding of the ecological consequences of SSRI exposure to aquatic vertebrates.
1. INTRODUCTION
Detections of human pharmaceuticals and personal care products in ecosystems and the tissues of aquatic organisms have raised environmental concerns.1 Selective serotonin reuptake inhibitors (SSRI) are a class of pharmaceuticals often detected in municipal wastewater2–4 and have been shown to bioaccumulate in aquatic organisms inhabiting surface waters below discharges.3–9 Widely prescribed to treat depression, anxiety, and other disorders in humans, SSRIs elicit therapeutic effects through increasing serotonergic neurotransmission by blocking reuptake of serotonin by presynaptic serotonin reuptake transporters (SERT) at synapses.10
Serotonergic drug targets are well described in humans and rodents11,12 and appear to be largely conserved among vertebrates.13 For example, Gunnarsson et al.14 reported ~65% ortholog homology between humans and fish for the serotonin receptor and the SERT. Two genes coding for SERT in zebrafish (Danio rerio) were identified by blast search and cloned; these are SERTa and SERTb with 66−69% and 75% sequence homology with human SERT.15 Gould et al.16 used [3H] labeled citalopram to compare the pharmacological profiles of SERT binding sites in whole brain homogenates from fathead minnows (Pimephales promelas), zebrafish, and laboratory rats (Rattus norvegicus), and found similar KD and Bmax values for high-affinity binding sites among the model organisms. The study also demonstrated decreased SERT binding site density in zebrafish following a 21-d dietary exposure to the SSRI sertraline.16 This observation is a critical consideration because a decrease in SERT binding in rodents following chronic administration accompanies therapeutic activity.17
SSRIs trigger a variety of toxicological effects on a range of model aquatic organisms. When standardized endpoints presently used by regulatory agencies are considered (e.g., cladoceran survival, reproduction), individual SSRIs appear to present relatively low ecological risk.2,18 However, sublethal fish responses to SSRIs, such as disruption of behavior,18–25 neuroendocrine function,26–30 and other metabolic pathways,31,32 appear more sensitive and occur at levels far lower than detected by standardized endpoints. Therefore, sublethal fish responses are particularly important for assessing the environmental safety of SSRIs2,21,25 and pharmaceuticals.33,34
Researchers have long recognized the potential applicability of animal behavior as indicators of sublethal stress during laboratory bioassays with fish. Most early studies focused solely on directly quantifiable response measures, such as avoidance, coughs, or body tremors.35 More recently, there has been a transition to identify changes in behaviors that have greater ecologically relevance, such as foraging,36,37 feeding rates,21,25,38 predator –prey interactions,39 reproduction,40 and social hierarchies.41 Further, neuropsychopharmacology, neuropathology, and psychopathology studies commonly employ fish models to understand anxiety, stress, and fear.42,43 SSRIs may elicit physiologically and ecologically important adverse outcomes at subtoxic thresholds of exposure. Therefore, when previous reports of SSRI bioaccumulation in wild fish are considered together with the evolutionary conservation of SSRI targets among vertebrates and available pharmacology and toxicology data, the application of behavior responses is particularly germane for ecological risk assessments (ERA).
In addition to monitoring surface water concentrations of pharmaceuticals to define environmental exposures, it is essential that relationships between internal dosimetry and mechanistic and physiological perturbations are explored more fully so that the wealth of pre-existing mammalian pharmacological safety data may be leveraged to predict thresholds of response in fish.33,44,45 Huggett et al.45 detailed an approach for predicting the partitioning of therapeutics from water to plasma of fish based on physicochemical properties using a model for superhydrophobic chemicals developed by Fitzsimmons et al.46 The researchers further suggested that predicted fish plasma drug concentrations could then be compared to human therapeutic thresholds (e.g., Cmax) to generate an Effect Ratio (ER; ER = human Cmax/predicted fish plasma concentration). The conceptual foundation of this approach is based on receptor homology and other pathway similarities between fish and mammals and, therefore, may be an effective means to identify relative risks among pharmaceuticals.47,48 However, there is little data relating water concentrations of human therapeutics to internal doses in fish, and even less information linking internal dosimetry to specific mechanistic and physiological alterations. A study by Cuklev et al.49 specifically addressed the relationship between blood plasma levels of the pharmaceutical diclofenac in fish and effects on the molecular level. Interestingly, the Cuklev et al.49 study provided evidence supporting assumptions made by Huggett et al.45 as effects were discernible when plasma levels in the fish approached human therapeutic doses.
Recently, Ankley et al.50 proposed the use of Adverse Outcome Pathways (AOP) for use in ERA. AOPs are conceptual frameworks that incorporate existing knowledge about linkages among molecular initiating events (anchor 1) and cascading responses that result in adverse outcomes at higher levels of biological organization used for ERA (anchor 2: individual and population levels).50 Our experiment specifically tested the utility of employing an AOP framework for a model pharmaceutical by examining responses at the therapeutic target level (SERT binding; anchor 1) and a novel, ecologically relevant response at the individual level (shelter-seeking behavior; anchor 2). The objectives of our study were thus 3-fold: (1) to determine if water exposure can be used to predict internal doses of an ionizable weak base SSRI in a fish model when pH is considered; (2) to determine if human therapeutic plasma doses result in decreased SERT binding in fish brain tissue; and (3) to determine if changes in SERT function are associated with quantifiable changes in fish behavior. For this experiment, the SSRI sertraline and adult fathead minnows were selected because previous research identified pH influence on the onset and magnitude of toxicity, including behavioral effects, following sertraline exposure in juvenile fathead minnows.21
2. MATERIALS AND METHODS
2.1. Test Organisms.
Fathead minnows were bred and reared according to an approved Baylor University animal care protocol. Individuals were housed in a flow-through system supplied with aged, dechlorinated tap water at a constant temperature of 25 ± 1 °C under a 16:8 light/dark photoperiod. For the first 30 d following hatch individuals were fed twice daily with newly hatched Artemia spp. and thereafter were fed a mixture of Artemia spp. and certified test-grade flake food. Individuals were aged to 120 d before experiments were initiated. Only adult male fish with pronounced reproductive features were selected for this study.
2.2. Experimental Design.
The experiment was completed in 20-L experimental units (aquariums) filled with 18 L of treatment level solutions. Each exposure aquarium housed 5 adult male P. promelas and had three breeding tiles as shelters, which consisted of 10 cm sections of 3-in PVC cut in half. Dechlorinated tap water was used as the control treatment and the dilution water for each of the 3 sertraline treatment levels. A stock of sertraline hydrochloride (Sigma Aldrich, St. Louis, MO, USA) was prepared and used to create the sertraline concentrations. Treatment levels included the control and three concentrations (3, 10, 30 μg sertraline/L) predicted to result in internal plasma concentrations exceeding the human Cmax of sertraline (142 ng/mL) at pH 8.5 (described further in Section 2.5 below). Each treatment level included 3 replicate experimental units; thus, there were a total of fifteen individual adults per treatment level. Studies for each set of replicates were staggered by one day to accommodate the substantial time associated with completing the behavioral trials.
The study was 28 d in duration and 75% v/v water renewals were performed daily. During the exposure period, organisms were fed certified test-grade flake food daily. All aquaria were housed in a single walk-in incubator set at 25 ± 1 °C with a 16:8 light/dark photoperiod. Temperatures in the aquaria were monitored daily. Water quality parameters, including dissolved oxygen, conductivity, pH, chlorine, and ammonia were measured 3 times a week.
2.3. Behavioral Observations.
To examine P. promelas behavior, we employed the NOLDUS Ethovision XT (http://www.noldus.com/animal-behavior-research/products/ethovision-xt), a software package that allows automated tracking and analysis of animal movement and activity. The system processes video images, has been widely applied to study behavior in mammalian models, and is often employed during drug development. A random number table was used to determine the order in which fish were removed from each treatment level and to determine their position in observation arenas.
A SONY Handycam (HDR-SR11) equipped with a visible light filter (850 nm) was positioned over the center of four observation arenas. Each observation arena consisted of a white plastic container (20 L, 35 cm ×40 cm) that housed one breeding tile for shelter and was filled with 15 L of dechlorinated tap water. The camera and NOLDUS video settings were calibrated to optimize tracking. Two high-powered 3.0-W infrared lights (LEDTRONICS, Inc. R30-123-851-120 AS Manufacturer) were placed below the arenas and positioned to allow for indirect illumination with infrared light. The inside of the support structure was lined with foil and the infrared lights were positioned to allow for consistent, indirect illumination. The infrared lights remained on throughout the duration of each observation period. Two light fixtures fitted with 60-W bulbs were positioned above the arenas, which were either turned on or off to represent light and dark conditions.
One fish was transferred from the exposure system to the observation arena and each NOLDUS trial was initiated within 10 s of the last fish being introduced. After the first 300 s of the trial, the overhead visible lights were remotely switched off, and then turned on again after another 300 s. The process of alternating light conditions was repeated a total of 3 times per observation period. The NOLDUS software was used to analyze the total distance traveled, swim velocity, and overall movement for each fish. The time that the fish spent in the shelter was calculated manually by reviewing digital video files to ensure that the fish was actually inside of the shelter and not merely swimming above it.
Following each completed NOLDUS trial, fish were removed, weighed, length measured, and then euthanized by cervical dislocation. Brain tissues were stored at −80 °C for future analysis, and heparinized hematocrit tubes (StatSpin, Westwood, MA) were used to collect blood. The blood from all fish (n = 5) in a replicate experimental unit (n = 3) was pooled to ensure an adequate volume for analytical measurements. Plasma of the pooled samples was separated at 10 000 rpm for 3 min using a refrigerated microcentrifuge. Plasma was then immediately diluted 1:10 with 100 mM phosphate buffered saline (PBS) with preservatives at pH 7.0, filtered through a 0.22-μm filter (Immunalysis Corporation, Ponoma, CA), and stored at 4 °C.
2.4. Analytical Quantification of Sertraline.
Due to small volumes of plasma in adult fathead minnows, sertraline direct ELISA kits (Immunalysis Corporation, Pomona, CA) were used to quantify plasma sertraline concentrations in control and exposed fish. To ensure that absorbance readings were within the standard curve range developed for the ELISA, 25, 50, and 100 μL of fish plasma were examined in triplicate by ELISA. After incubation, absorbance was measured at 450 and 630 nm using a Bio-Tek ELx 800 microplate reader (Winooski, VT, USA) within 30 min of loading the last sample. Sertraline direct ELISA was also used to confirm nominal concentrations of sertraline in exposure water, which performed consistently with LC-MSMS analyses previously reported from our group.20
2.5. Comparing Measured versus Predicted Fish Plasma Concentrations.
Measured plasma concentrations of sertraline were compared to predictions from a model developed by Huggett et al.45 derived from a plasma bioconcentration model by Fitzsimmons et al.46 with minor modifications to account for pH influences. Sertraline is a weak base with a pKa of 9.47 previously demonstrated to be more toxicity to aquatic life at higher pH.21 Because prior research has shown that site-specific pH affects the ionization, bioavailability, and toxicity of SSRIs,21,51 our group recently proposed accounting for these effects by using log D in model for predicting fish plasma concentrations rather than KOW.1,52 Therefore, log D (3.77 at pH 8.5; ACD/Laboratories, Toronto, ON, Canada) was substituted for the log KOW value in eq 1. The treatment levels of 3, 10, and 30 μg/L were used as the aqueous concentrations in eq 2.45 The human therapeutic plasma concentration (Cmax) was defined as either 142 ng/mL53 or 190 ng/mL (sertraline product registry), each of which were used to calculate ER values (eq 3).45
| (1) |
| (2) |
| (3) |
2.6. Saturation Radioligand Binding to Serotonin Transporters in Whole Brain Homogenates.
SERT saturation binding to [3H] citalopram in membrane homogenate preparations from fathead minnow whole brains was performed as previously described by Gould et al.16 Whole brains from three fish from each replicate were randomly selected and pooled. The remaining two brains were preserved for future studies. Therefore, three homogenates were prepared for each treatment level. Tissue was dispersed at 30 000 rpm for 20 s in 25 mL of 50 mM Tris, 120 mM NaCl, and 5 mM KCl, pH 7.4 at 26 °C buffer using a tissue homogenizer (Polytron 3100, Kinematica, Bohemia, NY). The homogenate was spun for 10 min at 30 600 rpm in a 4 °C centrifuge (Avanti A-J, Beckman-Coulter, Brea, CA). The supernatant was discarded and the pellet was resuspended in 5 mL of buffer on ice using a hand-held tissue homogenizer. Another 20 mL of buffer was added and the homogenate was centrifuged again for 10 min at 30 600 rpm. The final pellet was resuspended to obtain a protein concentration near 1 mg/mL, as determined with Bradford reagent (Sigma, St. Louis, MO) and measured on a spectrophotometer (DU-640, Beckman, Corona CA). The homogenate was incubated at 26 °C for 1 h in buffer at two concentrations of [3H] citalopram, 7 nM and 48 nM, (PerkinElmer, Boston, MA) to examine effects at both KD and Bmax concentrations. These concentrations were chosen based on a KD of 7 nM for [3H] citalopram found in golden shiner minnows in Gould et al.16 At the 48 nM concentration, calculated occupancy at the [3H] citalopram binding sites was 87%. In Gould et al.,16 a KD of 18 nM was also reported for fathead minnow brain homogenate saturation binding to [3H] citalopram when a single site NLR curve fit was used, but it is possible that a second binding site might have contributed to the higher KD found in this species. Therefore, the lower KD estimate determined from the golden shiner was used to capture only the activity of high-affinity SERT-like binding sites.
Nonspecific binding was defined by 50 μM sertraline (Pfizer, Groton, CT). Triplicate tubes were incubated in the presence and absence of sertraline and incubation was terminated by addition of 4 mL of 4 °C pH 7.4 buffer. Labeled homogenates were captured by filtration under vacuum with a tissue harvester (Brandel, Gaithersburg, MD) onto Whatman GF/B filter paper strips (Brandel) presoaked in 5% polyethyleneimine (Sigma). Filters were washed twice more with 4 mL of buffer. [3H] citalopram trapped in membrane tissue on the filters was determined using a liquid scintillation counter (LS 6500, Beckman) with 40% efficiency.
2.7. Statistical Analysis.
A comparison between sertraline water column concentration and sertraline fish plasma concentration was determined using linear regression. SERT binding in adult male Pimephales promelas brain tissue was analyzed by ANOVA with fish plasma concentration as the main experimental factor. Fish behavior (e.g., time in shelter and velocity) was analyzed by 2-factor ANOVA with sertraline concentration (4 levels: 0, 3, 10, 30 μg/L) and lighting condition (2 levels: light, dark) as the factors. For statistical comparisons of fish velocities, mean velocities for each fish were calculated in 15 s intervals. Dunnett’s post hoc comparisons were then completed between exposed groups and the control with α = 0.05. These statistical approaches were used to define no-observable-adverse effect concentration (NOAEC) and lowest-observable-adverse effect concentrations (LOAEC). All statistical analysis was performed with the computer software Statistica 10 (StatSoft, Tulsa, OK). Prior to analysis, data were checked for normality using the Shapiro–Wilk Test and for homogeneity using the Lovene test for homogeneity of variance.
3. RESULTS
3.1. Analytical Quantification of Sertraline.
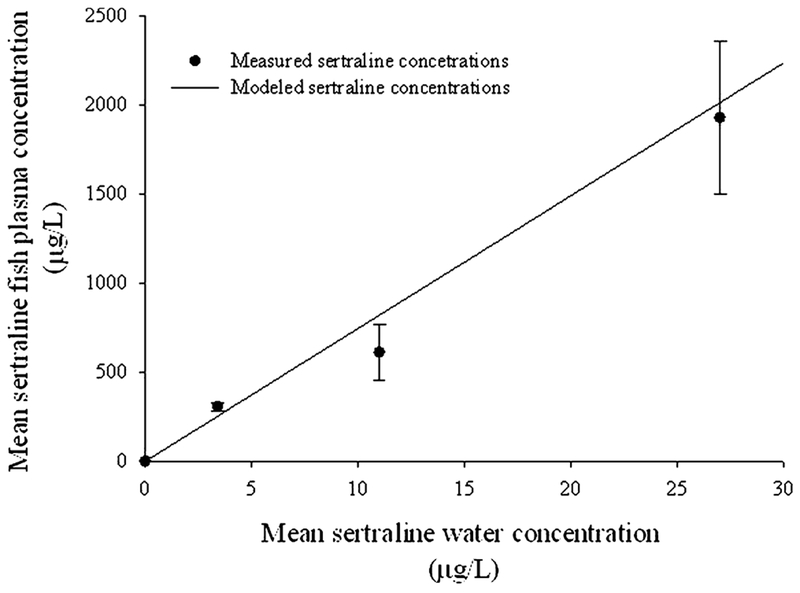
Nominal treatment concentrations for water exposures were very close to those measured and a significant linear dose-dependent relationship between concentrations of sertraline in water and plasma concentration in exposed fish was observed (p = 0.001; Figure 1). Sertraline was not detected in the plasma of control fish but was quantifiable for the other treatment levels (Figure 1). These measured sertraline plasma concentrations were very similar to those predicted by the fish plasma concentration model based on water column exposure concentrations based on eq 2 when log D at exposure pH was considered (Figure 1). Furthermore, the ER values based on the ratio of measured fish plasma levels and human therapeutic plasma doses calculated from eq 3 were all <1, which suggested that biological responses related to therapeutic activity would be observed in exposed fish (Table 1).
Figure 1.
Relationship between mean (n = 3; ± standard deviation), measured (dots), and modeled (solid line) plasma concentration of sertraline in adult male Pimephales promelas following a 28-d study, based on concentrations quantified in the water column. The modeled plasma concentration is a based on an equation described by Fitzsimmons et al.46 in which log D was substituted for log Kow (Berninger et al.52). y = 70.1(x) − 15.3; r2 = 0.98; p = 0.0001.
Table 1.
Modeled and Measured Sertraline Concentrations in the Plasma of Adult Male Pimephales promelas Following 28-d Aqueous Exposures and Associated Effects Ratio Defined As [Fishplasma]:Cmax
| [aqueous] (μg/L) | mean fishplasmaa (μg/L) | effects ratiob |
|---|---|---|
| 3c | 223e | 0.64f–0.85g |
| 10c | 745e | 0.19f–0.26g |
| 30c | 2235e | 0.06f–0.09g |
| 2.8d | 305d | 0.47f–0.62g |
| 9.4d | 610d | 0.23f–0.31g |
| 28.1d | 1927d | 0.07f–0.10g |
[Aqueous] × Pblood:water.
Fishpiasma/Cmax.
Nominal concentration.
Measured concentration.
Modeled concentration.
Cmax 142 μg/L (Thomson 2008).
Cmax 190 μg/L (Product registry).
3.2. SERT Binding.
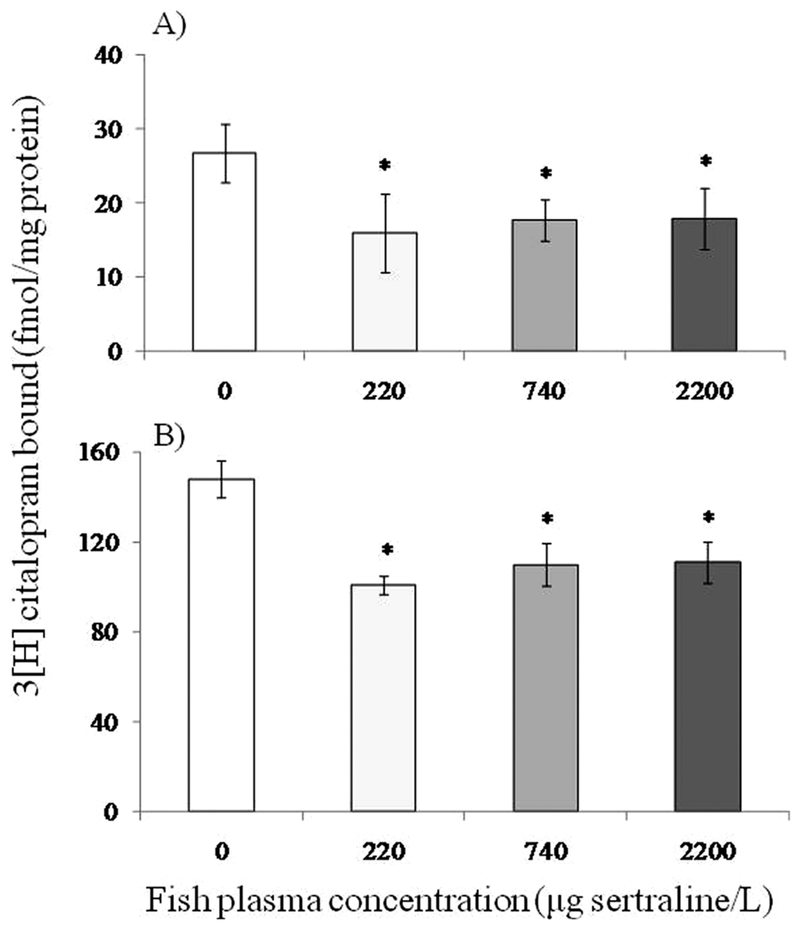
Functional binding changes of the therapeutic target (SERT) were observed in brain tissue of fish with elevated plasma concentrations of sertraline following the 28-d study. Sertraline treatments significantly reduced the Bmax (F[3,8] = 6.9, p = 0.01) and KD (F[3,8] = 4.169, p = 0.04) during [3H] citalopram binding assays. Dunnett’s post hoc analysis indicated that mean SERT binding site densities in fish exposed to all three levels of sertraline were significantly lower than controls for both Bmax and KD (p < 0.05; Figure 2).
Figure 2.
Mean (n = 3; ± standard error) serotonin reuptake transport binding at Bmax (A) and Kd (B) values for adult male Pimephales promelas brain tissue following a 28-d study with a control and three treatment levels of sertraline. *: p < 0.05.
3.3. Behavioral Observations.
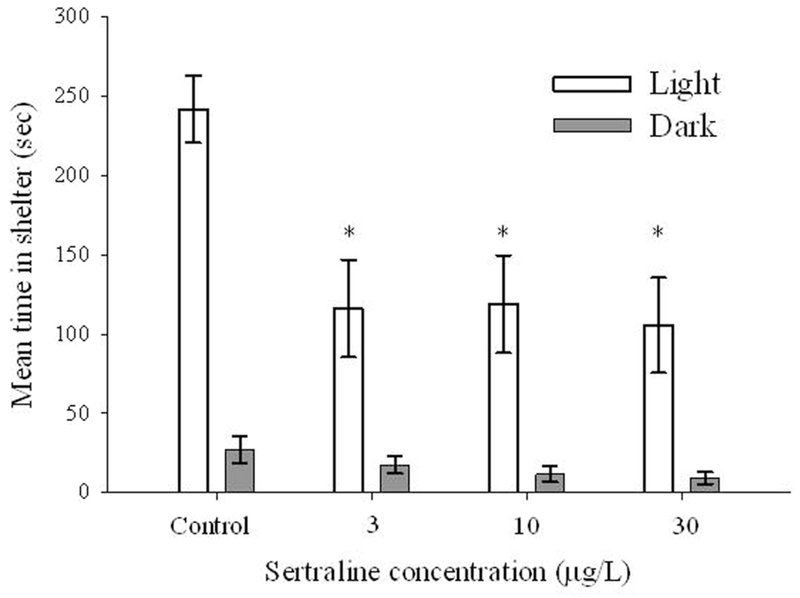
Sertraline concentration and lighting, as well as their interactions, significantly affected the time adult male fathead minnows spent in the shelter. Dunnett’s post hoc analysis indicated that control fish spent significantly greater time in the shelter during light periods compared to the other treatment levels (p < 0.05; Figure 3). Interestingly, during the initial light period when fish were first introduced to the chamber in which behavior was assessed, nearly all organisms sought shelter. For example, control fish spent 258 ± 22 (mean ± SD) s in the shelter, whereas sertraline exposed fish spent between 173 and 203 s (approximately 67–78% of the time that control fish spent in the shelter during the same interval; Figure 3). During the next two light intervals, control fish again spent a substantial amount of time in the shelter (e.g., 217 ± 31 and 239 ± 36 s, respectively), whereas fish exposed to sertraline spent between 18 and 42% less time in the shelters (Figure 3). There was very little difference in the amount of time fish spent in the shelter during the dark periods as individuals for each treatment level only spent between 4 and 33 s per interval (Figure 3).
Figure 3.
Mean (n = 3; ± standard error) time (sec) adult male Pimephales promelas spent in shelters during light or dark conditions following a 28-d study with a control and three treatment levels of sertraline. *: p < 0.05.
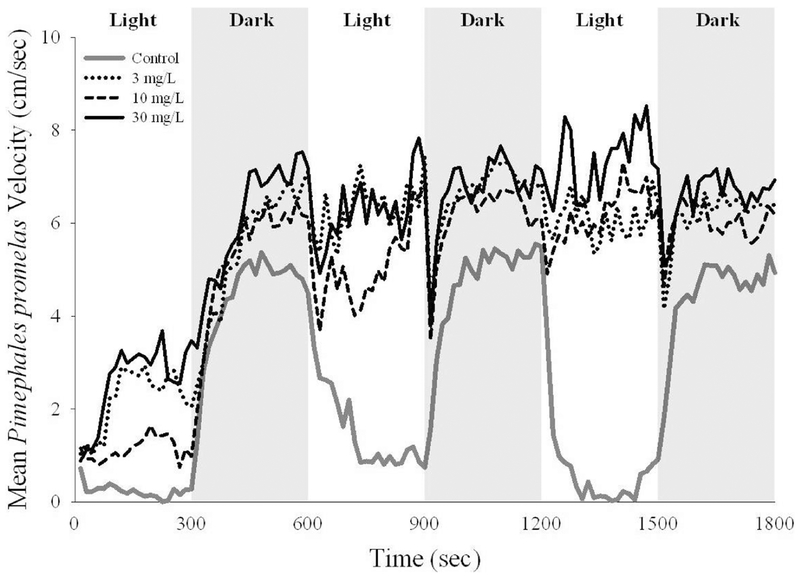
Mean patterns of velocity (cm/sec) for exposed fish also differed from those of control fish during light and dark conditions (Figure 4). NOLDUS data for one trial was not collected because of a recording malfunction; however, data pertaining to the time spent in the shelter was collected by watching the video. Sertraline concentration and lighting, as well as their interactions, had significant effects on the velocity of adult male fathead minnow. The Dunnett’s post hoc analysis indicated that control fish spent swam significantly slower than fish in the sertraline treatment levels (p < 0.05; Figure 4). (See Figures S1–S4 for velocities of individual fish).
Figure 4.
Mean velocities of adult male Pimephales promelas during oscillating periods of light and dark conditions following a 28-d study with sertraline. Each line represents that mean for 13 individual fish to show the general patterns of swim velocities. The swim velocities were calculated using NOLDUS software.
4. DISCUSSION
Behavior can be defined as the visual response of an organism to a culmination of biotic and abiotic stimuli. Such responses to these cues are dependent on physiological (internal) signals and environmental or social (external) factors.54 Contaminant-induced alterations in behaviors may therefore reveal connections among the biochemical, individual, and population levels of biological organization.39 For these reasons, behavioral endpoints may be more robust and comprehensive for ERA than either physiological or biochemical parameters.55 However, because variability of various fish behavioral responses inherently has limited their use in risk assessment, future interlaboratory variability studies coupled with semifield experiments (e.g., with –cosms) are necessary to examine the utility of employing fish shelter-seeking, feeding, and other behaviors as robust measures of effect in risk assessment.
In the present study, exposure to sertraline caused statistically significant changes in shelter-seeking behavior in adult male fathead minnows at water concentrations as low as 3 μg/L, which is lower than a previously reported feeding behavior threshold for juvenile fathead minnows (e.g., 15 μg/L).21 Whereas the effect on shelter-seeking behavior was observed at sertraline concentrations higher than those previously reported in surface waters, total SSRI concentrations below wastewater effluent discharges have been reported as high as 3.2 μg/L.4 Furthermore, fish in aquatic ecosystems may be exposed to SSRIs through both dietary and inhalational routes of exposure. The physicochemical properties of some SSRIs may facilitate their partitioning to aquatic organisms and sediment depending on ambient pH conditions; for example, higher concentrations of SSRIs are found in sediment than in the water column.3 Therefore, these additional routes of exposure may lead to higher internal doses than observed here, emphasizing the importance of considering internal dosimetry when performing ERAs for SSRIs.
The present study provides a novel demonstration of a cascading series of effects in adult male fathead minnows following 28-d water exposure to sertraline that led to internal plasma doses exceeding human therapeutic threshold concentrations (Figure 1). As previously noted, plasma levels of sertraline in fish exposed to all of the treatment levels tested in the present study exceeded therapeutic plasma doses for humans based on the model described by Huggett et al.45 when appropriate modifications for exposure pH and log D were considered.52 In fact, this study supports a growing literature highlighting the importance of accounting for pH during bioaccumulation and toxicity studies of ionizable therapeutics.21,51,52,56–59 Furthermore, all sertraline treatments produced similar physiological and behavioral effects due likely to the maximum stimulation of the drug target and thus lower exposure concentrations may also trigger such effects. The importance of relating internal dose to biological responses is emphasized by Homberg et al.60 who examined the effects of the SSRI citalopram on fish behavior; no effects on aggression in rainbow trout fry or on sexual behavior in male guppies were observed when internal levels were below human therapeutic doses. Therefore, it is important that future research efforts attempt to identify sertraline thresholds at which an external exposure concentration culminates in an internal dose that cause physiological and behavioral effects.
It is also important to note that the exposure concentrations used in our study were well below those previously reported to cause acute mortality21 and; therefore, focused on mode-of-action related effects rather than those associated with gross intoxication resulting from narcosis. The calculated ER values based on predicted and measured fish plasma concentrations were quite similar and all were below 1, suggesting potential hazard associated with therapeutic activity (Table 1). These internal doses resulted in significant modification of binding to the therapeutic target of sertraline (SERT-like binding sites; Figure 2),17 which was also observed in 21-d dietary study with zebrafish exposed to sertraline.16 Whereas our assays demonstrated decreases in SERT-like binding sites, other populations of different transporters may have also been recruited as noted in a review by Daws.61 Furthermore, changes in SERT-like binding sites in our study are more likely attributable to Bmax differences as SERT binding sites may be downregulated with chronic treatment (see 17 for examples). Regardless, decreased SERT-like binding in all sertraline treatments culminated in a potentially ecologically important behavioral change in male fathead minnows (e.g., change in shelter-seeking behavior; Figure 3).
Interestingly, altered fish behavior following sertraline exposure was only apparent when light was present and was not observed in dark conditions. The reduced propensity for exposed fish to seek shelter during light periods suggests that sertraline elicits an anxiolytic effect in teleost fish. Previous researchers have observed that teleosts have natural preferences for dark environments.62,63 Therefore, under the present study conditions, the shelter was the only dark area during light conditions in the observation arena; therefore, unexposed fish apparently retreated to the shelter to reduce anxiety, whereas fish exposed to sertraline appeared to display reduced anxiety and did not exhibit this behavior. Similar patterns of behavior have been observed in rodent models during elevated plus-maze tests designed to measure anxiety. Montgomery64 first used the concept of an elevated plus-maze and observed that rats were less likely to exhibit exploratory behaviors in “open” arms compared to “closed” arms, which was attributed to an avoidance of open alleys due to the fact that they engender higher levels of anxiety. In a review of how the elevated plus X-Maze may be used to study anxiety and anxiety-modulating drugs, Handley and McBlane65 noted that the antianxiety drug diazepam increased the ratio of open/total arm entries, while the pro-anxiety drug picrotoxin diminished this ratio for rats. Consequently, based on observations in the present study, we hypothesize that fish exposed to sertraline displayed reduced levels of anxiety; therefore, fish were more willing to explore the observation arena during both light and dark conditions.
Serotonin is a key neurotransmitter in pathways related to reproduction, stress, appetite regulation, and behavioral functions in fish.26 Several laboratory and field studies have demonstrated that exposure to SSRIs leads to bioconcentration and bioaccumulation of these therapeutics in fish.3–9 Following a 28-d study with the model SSRI sertraline and adult male fathead minnows, we observed significant decreases in the therapeutic target (SERT binding) and shelter-seeking behavior when adult male fish plasma levels exceeded human therapeutic concentrations. Such observations highlight the importance of defining internal dosimetry and employing AOP approaches to advance an understanding of the ecological consequences of pharmaceutical exposure and to reduce uncertainty during future ecological risk assessments involving aquatic vertebrates, particularly for biologically active compounds.
Previous experiments with other fish species have also suggested that SSRIs may induce anxiolytic effects. For example, Egan et al.66 observed reductions in erratic movements and a greater propensity of zebrafish to spend time in the upper half of a swim tank following 14 d of exposure to the SSRI fluoxetine. Painter et al.22 reported a suppression of predator avoidance (C-start) behavior in juvenile fathead minnows exposed to sertraline, other SSRIs, and antidepressants with different modes of action. Previous studies have reported decreased locomotor activity in fish exposed to SSRIs,67,68 which may be the result of an anxiolytic effect. However, our experimental design employed a shelter or “safe haven” for fish, which was not provided in previous experiments performed during light conditions.
For behavior to be used effectively as a tool in ERA, it is essential that observed behavioral modifications be specifically related to survival or ecological fitness.35 Based on the design of this study, we did not directly identify whether the decrease in shelter-seeking behavior by adult male P. promelas exposed to sertraline during light periods was specifically due to anxiolytic effects or another reason (e.g., neuroendocrine change, altered visual perception); however, observation of such a behavioral effect by sertraline exposure likely has direct implications for both survival and fitness of fish. Individuals willing to spend more time away from shelters face greater predation risk and thus their overall survival rate may be reduced. In terms of reproduction, fathead minnows typically spawn and attach adhesive eggs to the underside of aquatic plants, stones, or woody debris.69 Shelter-seeking and nest-guarding behavior in males are intrinsically linked for adult male fathead minnows as establishing and defending territories is important for attracting mates.70 Martinovic et al.40 previously observed that nest-guarding behavior of P. promelas exposed to environmental estrogens ultimately led to complete reproductive failure. Thus, as suggested previously,18,21,23,25 the results of the present study identify the importance of considering important behavioral modifications of fish following SSRI exposure during ERAs.
Supplementary Material
Footnotes
ASSOCIATED CONTENT
Supporting Information
The patterns of swim velocity for individual adult male Pimephales promelas in control and sertraline exposed treatments. This information is available free of charge via the Internet at http://pubs.acs.org
The authors declare no competing financial interest.
REFERENCES
- (1).Daughton CG; Brooks BW Active pharmaceuticals ingredients and aquatic organisms. In Environmental Contaminants in Biota-Interpreting Tissue Concentration, 2nd ed.; CRC Press: Boca Raton, FL: 2011; pp 288–337. [Google Scholar]
- (2).Oakes KD; Coors A; Escher BI; Fenner K; Garric J; Gust M; Knacker T; Küster A; Kussatz C; Metcalfe CD; Monteiro S; Moon TW; Mennigen JA; Parrott J; Péry AR; Ramil M; Roennefahrt I; Tarazona JV; Sánchez-Argüello P; Ternes TA; Trudeau VL; Boucard T; Van Der Kraak GJ; Servos MR Environmental risk assessment for the serotonin re-uptake inhibitor fluoxetine: Case study using the European risk assessment framework. Integr. Environ. Assess. Manage 2010, 6 (1), 524–539. [DOI] [PubMed] [Google Scholar]
- (3).Schultz MM; Furlong ET; Koplin DW; Werner SL; Schoenfuss HL; Barber LB; Blazer VS; Norris DO; Vajda AM Antidepressant pharmaceuticals in two effluent-impacted U.S. streams: Occurrence and fate in water and sediment and selective uptake in fish neural tissue. Environ. Sci. Technol 2010, 44 (6), 1918–1925. [DOI] [PubMed] [Google Scholar]
- (4).Metcalfe CD; Chus S; Judt C; Li H; Oakes KD; Serovs MR; Andrews DM Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Sci. Chem 2010, 29 (1), 79–89. [DOI] [PubMed] [Google Scholar]
- (5).Bringolf RB; Heltsley RM; Newton TJ; Eads CB; Fraley SJ; Shea D; Cope WG Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environ. Toxicol. Chem 2010, 29 (6), 1311–1318. [DOI] [PubMed] [Google Scholar]
- (6).Ramirez AJ; Brain RA; O’Donnel J; Usenko S; Mottaleb MA; Perez-Hurtado P; Dobbins LL; Pitts J; Snyder B; Wathen J; Stahl L; Brooks BW; Chambliss CK Occurrence of pharmaceuticals and personal care products in fish: Results of a national pilot study in the U.S. Environ. Toxicol. Chem 2009, 28 (12), 2587–2597. [DOI] [PubMed] [Google Scholar]
- (7).Ramirez AJ; Mottaleb MA; Brooks BW; Chambliss CK Analysis of pharmaceuticals in fish using liquid chromatographytandem mass spectrometry. Anal. Chem 2007, 79 (8), 3155–3163. [DOI] [PubMed] [Google Scholar]
- (8).Chu S; Metcalfe CD Analysis of paroxetine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatographytandem mass spectrometry. J. Chromatogr. A 2007, 1163 (1–2), 112–118. [DOI] [PubMed] [Google Scholar]
- (9).Brooks BW; Chambliss CK; Stanley JK; Ramirez A; Banks KE; Johnson RD; Lewis RJ Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem 2005, 24 (2), 464–469. [DOI] [PubMed] [Google Scholar]
- (10).Frazer A Serotonergic and noradrenergic reuptake inhibitors: predictions of clinical effects form in vitro potencies. J. Clin. Psychiatry 2001, 62 (12), 16–23. [PubMed] [Google Scholar]
- (11).Owen MJ; Morgan WN; Plott SJ; Nemeroff CB Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J. Pharmacol. Exp. Ther 1997, 283 (3), 1305–1322. [PubMed] [Google Scholar]
- (12).Scheffel U; Kim S; Cline EJ; Kuhar MJ Occupancy of the serotonin transporter by fluoxetine, paroxetine, and sertraline: In vivo studies with [125I]RTI-55. Synapse 1994, 16 (4), 263–268. [DOI] [PubMed] [Google Scholar]
- (13).Dietl M; Palacios JM Receptor Autoradiography as a tool for the study of the phylogeny of the basal ganglia. J. Recept. Signal Transduction 1988, 8 (1–4), 521–532. [DOI] [PubMed] [Google Scholar]
- (14).Gunnarsson L; Jauhiainen A; Kristiansson E; Nerman O; Larsson DGJ Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol 2008, 42 (15), 5807–5813. [DOI] [PubMed] [Google Scholar]
- (15).Wang Y; Takai R; Yoshioka H; Shirabe K Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J. Exp. Med 2006, 208 (3), 267–74. [DOI] [PubMed] [Google Scholar]
- (16).Gould G,G; Brooks BW; Frazer A [3H] citalopram binding to serotonin transporter sites in minnow brains. Basic Clin. Pharmacol 2007, 101 (3), 203–210. [DOI] [PubMed] [Google Scholar]
- (17).Benmansour S; Cecchi M; Morilak DC; Gerhardt GA; Javors MA; Gould GG; Frazer A Effects of chronic antidepressant treatments on serotonin transporter function, density and mRNA level. J. Neurosci 1999, 19 (23), 10494–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Brooks BW; Foran CM; Richards SM; Weston J; Turner PK; Stanley JK; Solomon KR; Slattery M; La Point TW Aquatic ecotoxicology of fluoxetine. Toxicol. Lett 2003, 142 (3), 169–183. [DOI] [PubMed] [Google Scholar]
- (19).McDonald MD; Gonzalez A; Sloman KA Higher levels of aggression are observed in socially dominant toadfish treated with the selective serotonin reuptake inhibitor, fluoxetine. Comp. Biochem. Phys. C 2011, 153 (1), 107–112. [DOI] [PubMed] [Google Scholar]
- (20).Holmberg A; Fogel J; Albertsson E; Fick J; Brown JN; Paxeus N; Forlin L; Johnsson JI; Larsson DGJ Does waterborne citalopram affect the aggressive and sexual behavior of rainbow trout and guppy? J. Hazard. Mater 2011, 187 (1–3), 596–599. [DOI] [PubMed] [Google Scholar]
- (21).Valenti TW; Perez-Hurtado P; Chambliss CK; Brooks BW Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ. Toxicol. Chem 2009, 28 (12), 2685–2694. [DOI] [PubMed] [Google Scholar]
- (22).Painter MM; Buerkley MA; Julius ML; Vajda AM; Norris DO; Barber LB; Furlong ET; Schultz MM; Schoenfuss HL Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem 2009, 28 (12), 2677–2684. [DOI] [PubMed] [Google Scholar]
- (23).Kreke N; Dietrich DR Physiological endpoints for potential SSRI interactions in fish. Crit. Rev. Toxicol 2008, 38 (3), 215–247. [DOI] [PubMed] [Google Scholar]
- (24).Gaworecki KM; Klaine SJ Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine treatment. Aquat. Toxicol 2008, 88 (40), 207–213. [DOI] [PubMed] [Google Scholar]
- (25).Stanley JK; Ramirez AJ; Chambliss CK; Brooks BW Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 2007, 69 (1), 6–16. [DOI] [PubMed] [Google Scholar]
- (26).Mennigen JA; Stroud P; Zamora JM; Moon TW; Trudeau VL Pharmaceuticals as neuroendocrine disruptors: Lessons learned from fish on Prozac. J. Toxicol. Environ. Health B 2011, 14, 387–412. [DOI] [PubMed] [Google Scholar]
- (27).Mennigen JA; Sassine J; Trudeau VL; Moon TW Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol 2010, 100 (1), 128–137. [DOI] [PubMed] [Google Scholar]
- (28).Mennigen JA; Lado WE; Zamora JM; Duarte-Guterman P; Langlois VS; Metcalfe CD; Chang JP; Moon TW; Trudeau VL Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish Carassius auratus. Aquat. Toxicol 2010, 100 (4), 354–364. [DOI] [PubMed] [Google Scholar]
- (29).Lister A; Regan C; Van Zwol J; Van Der Kraak G Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: A mechanistic evaluation. Aquat. Toxicol 2009, 95 (4), 320–329. [DOI] [PubMed] [Google Scholar]
- (30).Foran CM; Weston J; Slattery M; Brooks BW; Huggett DB Reproductive assessment of Japanese medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch. Environ. Contam. Toxicol 2004, 46 (4), 511–517. [DOI] [PubMed] [Google Scholar]
- (31).Lajeunesse A; Gagnon C; Gagne F; Louis S; Cejka P; Sauve S Distribution of antidepressants and metabolites in brook trout exposed to municipal wastewaters before and after ozone treatment – Evidence of biological effects. Chemosphere 2011, 83 (4), 564–571. [DOI] [PubMed] [Google Scholar]
- (32).Morando MB; Medeiros LR; McDonald MD Fluoxetine treatment affects nitrogen waste excretion and osmoregulation in a marine teleost fish. Aquat. Toxicol 2009, 95 (2), 164–171. [DOI] [PubMed] [Google Scholar]
- (33).Ankley GT; Brooks BW; Huggett DB; Sumpter JP Repeating history: Pharmaceuticals in the environment. Environ. Sci. Technol 2007, 41 (24), 8211–8217. [DOI] [PubMed] [Google Scholar]
- (34).Brooks BW; Huggett DB; Boxall ABA Pharmaceuticals and personal care products: Research needs for the next decade. Environ. Toxicol. Chem 2009, 28 (12), 2469–2472. [DOI] [PubMed] [Google Scholar]
- (35).Scott GR; Sloman KA The effects of environmental pollutants on complex fish behavior: Integrating behavioral and physiological indicators of toxicity. Aquat. Toxicol 2004, 68 (4), 369–392. [DOI] [PubMed] [Google Scholar]
- (36).Kasumyan AO Effects of chemical pollutants on foraging behavior and sensitivity of fish to food stimuli. J. Ichthyol 2001, 41 (1), 76–87. [Google Scholar]
- (37).Sandheinrich MB; Atchison GJ Sublethal toxicant effects on fish foraging behavior: Empirical vs. mechanistic approaches. Environ. Toxicol. Chem 1990, 9 (1), 107–119. [Google Scholar]
- (38).Grippo MA; Heath AG The effect of mercury on the feeding behavior of fathead minnows (Pimephales promelas). Ecotoxicol. Environ. Safe 2003, 55 (20), 187–198. [DOI] [PubMed] [Google Scholar]
- (39).Weis JS; Samson J; Zhou T; Skurnick J; Weis P Evaluating prey capture by larval mummichogs (Fundulus heteroclitus) as a potential biomarker for contaminants. Mar. Environ. Res 2003, 55 (1), 27–38. [DOI] [PubMed] [Google Scholar]
- (40).Martinovic D; Hogarth WT; Jones RE; Sorensen PW Environmental estrogens suppress hormones, behavior, and reproductive fitness in male fathead minnows. Environ. Toxicol. Chem 2007, 26 (2), 271–278. [DOI] [PubMed] [Google Scholar]
- (41).Perreault HAN; Semsar K; Godwin J Fluoxetine treatment decreases territorial aggression in coral reef fish. Physiol. Behav 2003, 79 (4–5), 719–724. [DOI] [PubMed] [Google Scholar]
- (42).Blaxter JHS; Hallers-Tjabbes CCT The effect of pollutants on sensory systems and behaviour of aquatic animals. Neth. J. Aquat. Ecol 1992, 26 (1), 43–58. [Google Scholar]
- (43).Baraban SC; Taylor MR; Castro PA; Baier H Pentylenetetrazole induced changes in zebrafish behavior, neural activity, and c-fos expression. Neuroscience 2005, 131 (3), 759–768. [DOI] [PubMed] [Google Scholar]
- (44).Berninger JP; Brooks BW Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicol. Lett 2010, 193 (1), 69–78. [DOI] [PubMed] [Google Scholar]
- (45).Huggett DB; Cook JC; Ericson JF; Williams RT A theoretical model for utilizing mammalian pharmacological and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk. Assess 2003, 9 (7), 1789–1799. [Google Scholar]
- (46).Fitzsimmons PN; Fernadez JD; Hoffman AD; Butterworth BC; Nichols JW Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol 2001, 5 (1–2), 23–34. [DOI] [PubMed] [Google Scholar]
- (47).Fick J; Lindberg RH; Parkkonen J; Arvidsson B; Tysklind M; Larsson DGJ Therapeutic levels of levonorgestrel detected in blood plasma of fish: Results from screening rainbow trout exposed to treated sewage effluents. Environ. Sci. Technol 2010, 44 (7), 2661–2666. [DOI] [PubMed] [Google Scholar]
- (48).Fick J; Lindberg RH; Tysklind M; Larsson DGJ Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol 2010, 58 (3), 516–523. [DOI] [PubMed] [Google Scholar]
- (49).Cuklev F; Kristiansson E; Fick J; Asker N; Forlin L; Larsson DGJ Diclofenac in fish: Blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ. Toxicol. Chem 2011, 30 (9), 2126–2134. [DOI] [PubMed] [Google Scholar]
- (50).Ankley GT; Bennett RS; Erickson RJ; Hoff DJ; Horning MW; Johnson RD; Mount DR; Nichols JW; Russom CL; Schmieder PK; Serrano JA; Tietge JE; Villeneuve DL Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem 2010, 29 (3), 730–741. [DOI] [PubMed] [Google Scholar]
- (51).Nakamura Y; Yamamoto H; Sekizawa J; Kondo T; Hirai N; Tatarazako N The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 2008, 70 (5), 865–873. [DOI] [PubMed] [Google Scholar]
- (52).Berninger JP; Du B; Conners KA; Eytcheson SA; Kolkmeier MA; Prosser KN; Valenti TW; Chambliss CK; Brooks BW Effects of the antihistamine diphenhydramine on selected aquatic organisms. Environ. Toxicol. Chem 2011, 30 (9), 2065–2072. [DOI] [PubMed] [Google Scholar]
- (53).Thomson PDR Physicians’ Desk Reference, 62nd ed.; Thomson P.D.R.: Montvale, NJ, 2008; 3482 pp. [Google Scholar]
- (54).Gernhardt A Aquatic behavioral ecotoxicology-Prospects and limitations. Hum. Ecol. Risk. Assess 2007, 13 (3), 481–491. [Google Scholar]
- (55).Peakall DB Disrupted patterns of behavior in natural populations as an index of ecotoxicity. Environ. Health Perspect 1996, 104 (Suppl. 2), 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Rendal C; Kusk KO; Trapp S Optimal choice of pH for toxicity and bioaccumulation studies of ionizing organic chemicals. Environ. Toxicol. Chem 2011, 30, 2395–2406. [DOI] [PubMed] [Google Scholar]
- (57).Valenti TW; Taylor JT; Back JA; King RS; Brooks BW Influence of drought and total phosphorus on diel pH in wadeable streams: Implications for ecological risk assessment of ionizable contaminants. Integr. Environ. Assess. Manage 2011, 7, 636–647. [DOI] [PubMed] [Google Scholar]
- (58).Meredith-Williams M; Carter LJ; Fussell R; Raffaelli D; Ashauer R; Boxall ABA Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut, 2012, in press. [DOI] [PubMed] [Google Scholar]
- (59).Brausch JM; Connors KA; Brooks BW; Rand GM Human pharmaceuticals in the aquatic environment: A critical review of recent toxicological studies and considerations for toxicity testing. Rev. Environ. Contam. Toxicol 2012, 218, in press. [DOI] [PubMed] [Google Scholar]
- (60).Homlberg A; Fogel J; Albertsson E; Fick J; Brown JN; Paxeus N; Forlin L; Johnsson JI; Larsson DGJ Does waterborne citalopram affect the aggressive and sexual behaviour of rainbow trout and guppy? J. Hazard. Mater 2011, 187, 596–599. [DOI] [PubMed] [Google Scholar]
- (61).Daws LC Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressants. Pharmacol. Ther 2009, 121 (1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Maximino C; de Brito M; de Moraes FD; de Oliveira FVC A comparative analysis of the preference for dark environments in five teleosts. Int. J. Comp. Psych 2007, 20, 351–367. [Google Scholar]
- (63).Serra EL; Medhalha CC; Mattioli R Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res 1999, 32 (12), 1551–1553. [DOI] [PubMed] [Google Scholar]
- (64).Montgomery KC The relationship between fear induced by novel stimulation and exploratory behavior. J. Comp. Physiol. Psychol 1955, 48 (4), 254–260. [DOI] [PubMed] [Google Scholar]
- (65).Handley SL; McBlane JW An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J. Pharmacol. Toxicol. Methods 1993, 29 (3), 129–138. [DOI] [PubMed] [Google Scholar]
- (66).Egan RJ; Bergner CL; Hart PC; Cachat JM; Canavello PR; Elegante MF; Elkhayat SI; Bartels BK; Tien AK; Tien DH; Mohnot S; Beeson E; Glasgow E; Amri H; Zukowska Z; Kalueff AV Understanding behavioural and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain. Res 2009, 205 (1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Henry TB; Black MC Acute and chronic toxicity of fluoxetine (Selective serotonin reuptake inhibitor) in western mosquitofish. Arch. Environ. Contam. Toxicol 2008, 54, 325–330. [DOI] [PubMed] [Google Scholar]
- (68).Clements S; Schreck CB Chronic administration of fluoxetine alters locomotor behavior, but does not potentiate the locomotor stimulating effects of CRH in juvenile Chinnok salmon (Oncorhynchus tshawytscha). Comp. Biochem. Physiol 2007, 147 (1), 43–49. [DOI] [PubMed] [Google Scholar]
- (69).Nelson JS; Paetz MJ The Fishes of Alberta; University of Alberta Press: Edmonton, Alberta, Canada, 1992. [Google Scholar]
- (70).Jamieson I Do female fish prefer to spawn in nests with eggs for reasons of mate choice copying or egg survival? Am. Nat 1995, 145 (5), 824–832. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.