Abstract
Objective
Whereas small fiber neuropathy (SFN) is now a recognized part of fibromyalgia (FM), surprisingly little attention has been paid to any findings of large fiber neuropathy (LFN) in this disorder. Since 90% to 95% of FM subjects seen in our outpatient facility routinely undergo EMG and nerve conduction studies (NCS) we elected to retrospectively review the EMG/NCS results garnered from a large cohort of unselected subjects in order to describe the electrodiagnostic features of LFN in FM.
Methods
Records from 100 consecutive, unselected clinic patients meeting the 1990 ACR criteria for FM, who had undergone EMG/NCS, were reviewed. The same electromyographer tested all subjects. After exclusion of FM patients with any other clinically relevant condition that might influence EMG results (e.g., familial neural degenerative conditions, diabetes mellitus, Vitamin B-12 deficiency, etc.) fifty-five FM subjects remained: 29 subjects with “FM Only,” and 26 subjects with FM+Rheumatoid Arthritis (“FM+RA”). All subjects had also undergone ankle area skin biopsy for determination of epidermal nerve fiber density (ENFD). Fourteen other subjects, without FM or RA, examined by the same electromyographer, were used as an EMG/NCS comparison group.
Results
Ninety percent of the “FM Only” subjects demonstrated a demyelinating and/or axonal, sensorimotor polyneuropathy, and 63% had findings of SFN (ENFD ≤7 fibers/mm), suggesting a mixed fiber neuropathy in most. Furthermore, 61% of the “FM Only” subjects showed EMG findings suggestive of non-myotomal lower extremity axonal motor denervation, most likely due to a polyneuropathy, and 41% satisfied published criteria for “possible” chronic inflammatory demyelinating polyneuropathy (CIDP). There was surprisingly little difference in the EMG/NCS findings between the “FM Only” and the “FM+RA” groups. With the exception of carpal tunnel syndrome, our EMG/NCS comparison group showed few to none of these findings.
Conclusion
Our review of the EMG/NCS results, gleaned from the largest FM cohort yet studied with these modalities, shows that electrodiagnostic features of polyneuropathy, muscle denervation, and CIDP are common in FM. Furthermore these electrodiagnostic findings are often seen coincident with SFN, and are not significantly influenced by the presence of RA. These results, particularly when taken as a whole, suggest that EMG/NCS may be clinically useful in detecting LFN in FM and help in better understanding the etiopathogenesis of this painful disorder.
Keywords: Fibromyalgia, electromyography, nerve conduction studies, peripheral neuropathy, CIDP
Introduction
We, and others, have recently shown that FM is highly associated with findings of small fiber neuropathy (SFN), a lesion frequently accompanied by severe peripheral pain (1, 2). Such a SFN is, therefore, an attractive candidate to serve as one of the peripheral nociceptive generators capable of maintaining the putative central (brain and spinal cord) sensitization thought to be integral to the production of FM painful symptomatology (3).
Ironically, the very identification of a SFN in FM has discouraged investigation into the potential contribution of any large fiber neuropathy (LFN) to this disorder. This may be, in part, because a LFN is thought to be uncommon in certain forms of SFN. Grant, for example, states that nerve conduction studies are, “often completely normal in patients with [cryptogenic] SFN (4).” Furthermore, Üçeyler et al. (5) recently conducted limited nerve conduction studies (NCS) on a series of FM subjects in whom they described SFN. Their normal NCS results led them to conclude that there was no evidence for a LFN in FM.
Despite the skepticism regarding any affliction of larger nerves in FM, reports from others, and our own everyday experience, has suggested to us that a significant percentage of FM patients are likely to have a LFN (6, 7). For these reasons we decided to retrospectively review the cumulative electrodiagnostic (EDX) data emanating from a large cohort of our FM patients. Our findings suggest that a LFN is rather common in FM, and likely to be of greater importance to the generation of its symptomatology than currently recognized.
Methods
Setting and patient selection
We retrospectively reviewed clinical and EDX data from 100 consecutive FM subjects seen from June 2011 until December 2013, in a private medical, outpatient setting. All participants met 1990 ACR FM diagnostic criteria, were ≥18 years of age, and had given written consent for the anonymous and Health Insurance Portability and Accountability Act (HIPPA) compliant use of their medical data (8). Our facility’s Institutional Review Board approved the project.
Clinical assessments and demographics
During initial clinical evaluation each of the 100 FM subjects was screened for any disorder known to produce LFN (e.g., diabetes mellitus, Vitamin B-12 deficiency, familial neuropathy, etc.). The laboratory and clinical screening of our FM participants paralleled that used by us previously (9). Only records from those subjects who lacked evidence of any disorder known to be associated with LFN were included in our final FM study cohort. Selection bias was unlikely, as we routinely ask that all of our FM subjects undergo EDX, and skin biopsy for epidermal nerve fiber density (ENFD) determination, if not already performed elsewhere. Consequently, 90% – 95% of our FM subjects undergo EDX testing by our electromyographer (RGG). Only records of EDX examinations performed at our facility were reviewed in this study.
We elected to include records of those FM subjects with concomitant rheumatoid arthritis (RA) in our study, as we have previously found a closer relationship between FM and seronegative RA than is generally appreciated (10). Fifty-five of our original, 100 consecutive FM subjects met our inclusion criteria (i.e., had no other possible explanation for a LFN). Of these, 29 had “FM Only” (27 Caucasian; 23 female; mean age 59, range 21–90 yrs.), and 26 had “FM and Rheumatoid Arthritis” (“FM+RA”) (20 Caucasian; 23 female; mean age 57, range 26–84 yrs.). Six of the 26 “FM+RA” subjects (23%) were positive for IgM rheumatoid factor.
Fourteen other subjects (13 Caucasian; 6 female; mean age 56, range 18–86 yrs.), undergoing routine EDX for a variety of entrapment syndromes (5 subjects) and radiculopathies (9 subjects), constituted a “No FM&No RA” comparison group. None of these EDX comparison subjects had a history of polyarthritis or FM; one with a lumbar radiculopathy, had a diagnosis of non-radiographic axial spondyloarthritis (11).
Electrodiagnostic (EDX) strategy and technique
One electromyographer (RGG) conducted all EDX (EMG/NCS) studies. The electromyographer was not blinded to the diagnosis of FM. EDX examination generally followed the protocol described by Preston and Shapiro (12). This led to NCS results being rendered from 16 nerves in all FM subjects and a minimum of 6 to 8 nerves in our “No FM & No RA” EDX control subjects. EMG examination of at least four muscles was also conducted on all FM subjects, and 10 of 14 EDX controls.
EDX studies were performed with skin temperature ≥30°C recorded at the volar wrist and dorsal leg bilaterally (13). Our electromyographer measured all NCS motor parameters, including distal CMAP duration, by visually identifying the onset of the initial negative deflection and the return to baseline of the last negative deflection (display setting at 2mV/division); cursor placement was adjusted manually, as necessary (14–18). All sensory NCS were measured to the peak of the negative wave.
Our early EDX studies were with a NDI electromyograph (Neurodiagnostics Inc.; Santa Ana, CA, 92707; USA) using filter settings of 2 Hz (low) and 10,000 Hz (high). Subsequent to August 2013, studies were with a LBM 1 electromyograph (Neurodiagnostics Inc.; Santa Ana, CA, 92707; USA) using filter settings of 20 Hz (low) and 10,000 Hz (high). Throughout the study, our definition of temporal dispersion followed that suggested in the 2010 EFNS/PNS criteria set for identifying CIDP; that is, a >30% duration increase between the proximal and distal negative peak CMAP (in 1 nerve for possible, and in > 2 nerves for definite CIDP diagnosis) (17).
During the second portion of our study (when we were using a 20 Hz low filter setting) identification of an abnormal distal CMAP duration followed a previously proposed definition (16). During the early portion of our study (when we were using a 2 Hz low filter setting) identification of an abnormal distal CMAP duration followed the suggestions of Rajabally et al. (15) for low filter settings of ≤10 Hz.
Electromyographic (EMG) studies
Electromyographic studies were with a disposable, 37mm × 27G monopolar exploring needle electrode for muscle potential analysis, and 12mm × 28G needle electrodes for reference and ground components (Chalgren Enterprises, Inc.; Gilroy, CA 95020; USA). Muscles examined in the right upper and lower extremity included: biceps brachii, extensor digitorum communis, quadriceps brachii, and tibialis anterior. Left sided musculature was intentionally left unexamined by EMG in case of a clinical need for muscle biopsy.
Nerve conduction studies (NCS)
Using surface electrodes, NCS followed standard techniques, and included “H” wave reflex measurements of the tibial nerves (including latency in msec and amplitude in mV) (18). The following measurements were also conducted: motor distal latency, conduction velocity [M/sec], amplitude [mV], proximal and distal duration [msec]; sensory (antidromic distal latency, amplitude [μV]); and “F” wave (latency, amplitude) (19). The following nerves were examined bilaterally: median (motor & sensory, and “F” wave), ulnar (motor & sensory, and “F” wave), radial (sensory), peroneal (motor and “F” wave), posterior tibial (motor and “F” wave), and sural (sensory). Polyneuropathy was defined as involvement of two or more nerves with abnormalities in distal latency, conduction velocity, amplitude, or “F” wave, that were not explained by any other cause (e.g., entrapment phenomenon). When there was a significant reduction in motor amplitude, axonopathy was deemed present. Demyelination was deemed present when abnormal prolongation of the distal CMAP duration was observed (16, 17). Temporal dispersion, as a further sign of demyelination, was also reported when detected for motor nerves (16). Median nerves were excluded from these determinations because of the potential for coincident demyelination if carpal tunnel syndrome (CTS) was present (20).
Proximal muscle strength in FM
A composite score of proximal muscle strength, ranging from 0 to 9, was calculated for each subject following our adaption of a well-known modification of the Medical Research Council grading system, as previously described (7, 21). The following muscle groups were examined bilaterally for strength in “FM Only,” and “FM+RA” subjects by two of the authors acting independently (XJC and RGG): shoulder abductors, elbow flexors, hip flexors, and knee flexors. Composite strength scores for both “FM Only,” and “FM + RA” subjects were compared to an expected normal score of 9, and to one another, as a group.
Chronic inflammatory demyelinating polyneuropathy (CIDP) in FM
Any diagnosis of CIDP met criteria suggested by the European Federation of Neurological Societies & American Peripheral Nerve Society, and was rated as being “Possible,” “Probable,” or “Definite” following these guidelines (17).
Two subjects (one “FM Only” and one “FM+RA”) who originally met criteria for “Possible CIDP” were reclassified as non-CIDP subjects due to the potential influence of a change in our low filter settings (see Electrodiagnostic Studies above) on determining prolongation of the distal CMAP duration (14, 15, 17). No other study subject required reclassification.
Small fiber neuropathy (SFN)
An ankle area skin biopsy for determination of epidermal nerve fiber density (ENFD) was conducted by one of the authors (XJC) on all FM subjects in order to assess the presence of any SFN. The technical aspects of these biopsies and their inter-group analyses are described elsewhere (9).
Statistical analysis
Statistical comparisons between groups were performed with Fisher’s exact test, and correlations performed with Spearman’s protocol, with p≤0.05 [2 tailed; p(2)] considered significant. All calculations were performed using public web-based statistical programs.
Results
Polyneuropathy findings in FM
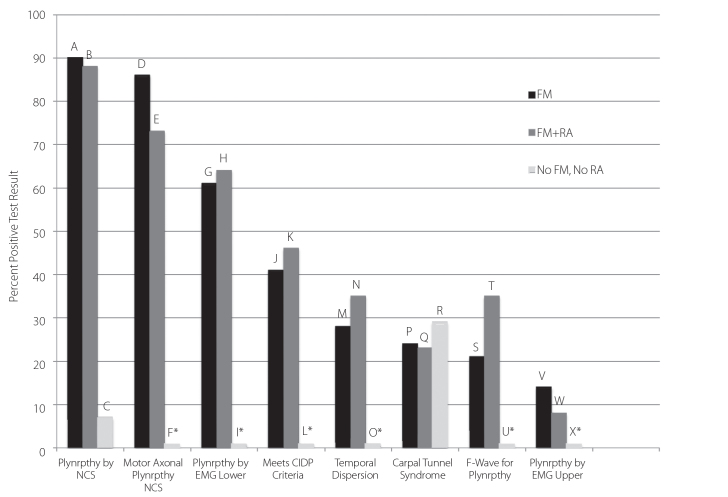
Ninety percent of our 29 “FM Only” subjects had a polyneuropathy, the majority of which were sensorimotor (Figure 1). In comparison, a polyneuropathy was seen in only 7% of control patients; this difference in prevalence was highly significant (p<0.0001). There was no statistically significant difference between the “FM Only” and “FM+RA” groups for the prevalence of polyneuropathy.
Figure 1.
Prevalence of Large Fiber Neuropathic findings detected by EMG & NCS in a cohort of FM patients: A vs. C (p<0.0001), B vs. C (p<0.0001), D vs. F (p<0.0001), E vs. F (p<0.0001), G vs. I (p<0.02), H vs. I (p=0.01), J vs. L (p=0.003), K vs. L (p=0.002), M vs. O (p=0.03), N vs. O (p=0.01), S vs. U (p=0.08), T vs. U (p=0.01). Fisher’s Exact Test (2 tailed). Other comparisons were NS.
*Indicates 1% for illustration purposes; actual value=0%
Nerve conduction abnormalities
Abnormalities in the 29 “FM Only” group included temporal dispersion, prolongation of the distal CMAP duration, abnormal F-wave, or carpal tunnel syndrome in 28%, 10%, 21%, and 24% respectively (Figure 1). There was no statistically significant difference between the “FM Only” and “FM+RA” groups for the prevalence of each of these features. All subjects judged as having prolongation of the distal CMAP duration met strict published criteria for this phenomenon (14–16).
Needle EMG evidence of muscle denervation
Needle EMG examination was successfully carried out in 28 of 29 “FM Only” subjects. Of this group, 17 (61%) showed findings suggestive of non-myotomal lower extremity axonal motor denervation, thought most likely due to a polyneuropathy (Figure 1). Of these 17, motor denervation findings were chronic in 14, and mixed chronic and active in 3. There was no statistically significant difference for the prevalence of muscle denervation between the “FM Only” and “FM+RA” groups.
Proximal muscle strength in FM
A composite proximal muscle strength score was judged weaker, compared to an “ideal normal” score of 9, in 53% of “FM Only” (mean strength score=8.41), and 63% of “FM + RA” (mean=7.89) subjects by the electromyographer (RGG) (7). This strength score was judged as weaker in 75% of “FM Only” (mean=7.59), and 78% of “FM+RA” (mean=7.04) by the clinician (XJC), who saw the subject earlier in their course of treatment than the electromyographer. Although the “FM+RA” generally tested weaker than the “FM Only” subjects, this difference was not found statistically, significantly different in either the electromyographer’s [p(2)=0.74] or clinician’s [p(2)=0.69] assessment. Inter-examiner muscle strength ratings were significantly correlated (r=0.56; p(2)=0.001).
Carpal tunnel syndrome (CTS)
Electrodiagnostic findings of CTS were detected in 24% of “FM Only” subjects (Figure 1). There was no statistically significant difference between the “FM Only” and “FM+RA” groups for the presence of CTS. Twenty-nine percent of our control group (“No FM, No RA”), many of whom were being evaluated for entrapment syndromes, had CTS.
CIDP diagnosis in FM
Electrodiagnostic findings meeting published criteria for, at least, “Possible” CIDP (17) were seen in 41% of “FM Only” subjects, but in no control subjects (p<0.003). There was no statistically significant difference between the “FM Only” and “FM+RA” groups for the presence of, at least, “Possible” CIDP (Figure 1).
Calf skin biopsy findings of small fiber neuropathy
Calf ENFD was reduced to ≤6.5 fibers/mm in 53%, and ≤7.0 fibers/mm in 63% of “FM Only” subjects. There was no statistically significant difference between the “FM Only” and “FM+RA” groups for median calf ENFD values.
Statistical outcomes
Statistical results are indicated in Figure 1. Post hoc Power Calculations for all inter-group comparisons yielding significant statistical results, i.e., p≤0.05, produced an observed power value of approximately 100%. Tabular representation of the prevalence of selected NCS findings in each of our study groups is depicted in Tables 1–3.
Table 1.
Prevalence of NCS abnormalities in FM Only subjects
| Subject | MDL | MCV | M AMP | SDL | SAMP | F | H | DUR | TD | P | TAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 | ||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| 4 | ✓ | ✓ | 2 | ||||||||
| 5 | ✓ | ✓ | ✓ | 4 | |||||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||||
| 7 | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||||
| 9 | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | |||||
| 10 | ✓ | ✓ | ✓ | ✓ | 3 | ||||||
| 11 | 0 | ||||||||||
| 12 | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |||||
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | |||
| 15 | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |||||
| 16 | ✓ | ✓ | ✓ | ✓ | 8 | ||||||
| 17 | ✓ | ✓ | ✓ | ✓ | 6 | ||||||
| 18 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| 19 | ✓ | ✓ | ✓ | ✓ | ✓ | 10 | |||||
| 20 | 0 | ||||||||||
| 21 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| 22 | ✓ | ✓ | ✓ | ✓ | 3 | ||||||
| 23 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | ||
| 24 | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||||
| 25 | ✓ | ✓ | ✓ | ✓ | 3 | ||||||
| 26 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | |||
| 27 | 0 | ||||||||||
| 28 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| 29 | ✓ | ✓ | 4 |
NCS: nerve conduction study; FM: fibromyalgia; ✓: abnormal result; MDL: motor distal latency; MCV: motor conduction velocity;
M AMP: motor amplitude; SDL: sensory distal latency; S AMP: sensory amplitude; F: F-wave; H: H-reflex; DUR: duration; P: polyneuropathy present; TAN: total abnormal nerves
Table 2.
Prevalence of NCS abnormalities in FM + RA subjects
| Subject | MDL | MCV | M AMP | SDL | SAMP | F | H | DUR | TD | P | TAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ✓ | ✓ | ✓ | 2 | |||||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 | ||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| 4 | 0 | ||||||||||
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| 6 | 0 | ||||||||||
| 7 | ✓ | ✓ | ✓ | 5 | |||||||
| 8 | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| 9 | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| 10 | ✓ | ✓ | 2 | ||||||||
| 11 | ✓ | ✓ | ✓ | 3 | |||||||
| 12 | ✓ | ✓ | ✓ | ✓ | 2 | ||||||
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 | ||||
| 14 | ✓ | ✓ | ✓ | ✓ | 8 | ||||||
| 15 | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |||||
| 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||
| 17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| 18 | 0 | ||||||||||
| 19 | ✓ | ✓ | ✓ | 10 | |||||||
| 20 | ✓ | ✓ | ✓ | ✓ | 10 | ||||||
| 21 | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |||||
| 22 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 | ||||
| 23 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 11 | |
| 24 | ✓ | ✓ | 3 | ||||||||
| 25 | ✓ | ✓ | ✓ | ✓ | 6 | ||||||
| 26 | ✓ | ✓ | ✓ | ✓ | 6 |
NCS: nerve conduction study; FM: fibromyalgia; RA: rheumatoid arthritis; ✓: abnormal result; MDL: motor distal latency;
MCV: motor conduction velocity; M AMP: motor amplitude; SDL: sensory distal latency; S AMP: sensory amplitude; F: F-wave; H: H-reflex;
DUR: duration; P: polyneuropathy present; TAN: total abnormal nerves
Table 3.
Prevalence of NCS abnormalities in Control subjects
| Subject | MDL | MCV | M AMP | SDL | SAMP | F | H | DUR | TD | P | TAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | ||||||||||
| 2 | 0 | ||||||||||
| 3 | 0 | ||||||||||
| 4 | ✓ | 1 | |||||||||
| 5 | 0 | ||||||||||
| 6 | 0 | ||||||||||
| 7 | 0 | ||||||||||
| 8 | 0 | ||||||||||
| 9 | ✓ | ✓ | ✓ | 3 | |||||||
| 10 | 0 | ||||||||||
| 11 | 0 | ||||||||||
| 12 | 0 | ||||||||||
| 13 | ✓ | ✓ | ✓ | 3 | |||||||
| 14 | 0 |
NCS: nerve conduction study; ✓: abnormal result; MDL: motor distal latency; MCV: motor conduction velocity; M AMP: motor amplitude;
SDL: sensory distal latency; S AMP: sensory amplitude; F: F-wave; H: H-reflex; DUR: duration; P: polyneuropathy present;
TAN: total abnormal nerves
DUR: duration; P: polyneuropathy present; TAN: total abnormal nerves
Discussion
Our study demonstrates that in a cohort of FM patients, carefully screened to exclude any other cause of LFN, nearly all (90%) had EDX findings of a sensorimotor polyneuropathy and two thirds had skin biopsy findings of a SFN, thus giving most a mixed fiber neuropathy. Their EDX findings were noteworthy for their multiplicity, demyelinating and axonopathic features, and not-infrequent congruence with diagnostic guidelines for CIDP (17).
Detection of such a high prevalence of EMG/NCS abnormalities amongst our “FM Only” cohort (90%) complements the description of polyneuropathy in FM by Ersöz (6), and our previous report of polyneuropathy and CIDP in FM (7). On the other hand, Üçeyler et al. (5) did not find a LFN in FM using a lower extremity protocol confined to NCS examination of only two nerves (unilateral sural and tibial nerves), without any EMG examination. The study of LFN in FM was not the main purpose of their investigation, however, as they were primarily interested in SFN. Furthermore, it is well recognized that a polyneuropathy is more likely to be identified by protocols utilizing more than 2 nerves (22). In our study, we reviewed an EDX experience in FM utilizing four extremity NCS, (i.e., a total of 16 nerves), in addition to the EMG examination of four, right-sided muscles (2 upper and 2 lower extremity).
Moreover, the needle EMG findings in our FM cohorts showed that 61% of the “FM Only” subjects had findings suggestive of lower extremity muscle denervation, a prevalence that contrasted significantly with our controls (p=0.02; Figure 1). Of our “FM Only” subjects, 14% also had needle EMG evidence suggestive of UE denervation, but this prevalence was not statistically different than controls, a finding likely to be due to an insufficient sample size. We also considered these denervation findings implicative of axonal injury (12, 23, 24), probably due - at least in part - to peripheral nerve demyelination, as advanced demyelination may lead to an axonopathy (24).
Significantly, our clinical and electromyographic examiners found that FM, whether partnered with RA or not, was often associated with proximal muscle weakness, a finding frequently seen in LFN (21). A mean proximal muscle strength score for our “FM Only” subjects compared closely to that found in a cohort of FM subjects reported by us previously (7.6 previous cohort vs. 7.59 current cohort), and was weaker than an “ideal” score of 9 in both groups (7). None of our FM subjects were selected for this study based on any pre-study finding of proximal muscle weakness, as this feature was measured only after enrollment. Interestingly, a recent analysis of the 2012 U.S. National Health Interview Survey, assessing the functional capacity of 8446 FM subjects, reported that 39.2% of these FM subjects claimed difficulty or inability to ascend stairs (25).
Our study has a number of inherent strengths. These include describing the largest cohort of FM subjects ever surveyed utilizing EMG/NCS, and providing clinically relevant guidance in the use of EMG/NCS in FM. Nevertheless, we are also aware that any EDX study might undergo a certain degree of scrutiny due to technical considerations. For that reason we used data generated by only one electromyographer (RGG), measured and maintained skin temperature during testing, and followed a standardized protocol for all examinations (12, 13). We also chose a rigorous, newer, criteria set, for our CIDP diagnosis (17). Additionally, when we were required to change our EDX low filter settings once during this 2½ year long experience, potentially influencing a variable that has received some attention recently, we took the precaution of reviewing all of our original CIDP designations (15). As a result, prior to data analyses, we elected to reclassify two subjects as non-CIDP: one “FM Only,” and one “FM+RA.” Finally, the accuracy and consistency of cursor placement for all motor NCS, including during measurement of distal CMAP duration, was optimized by having our elctromyographer (RGG) visually identify the onset of the initial negative deflection for each nerve and manually adjust the cursor setting as required (sensitivity=2 mV/division; see Methods) (14–16).
Although it might be argued that our analysis of retrospectively procured data is in some way inferior to that conducted on “prospectively” obtained data, we doubt that there would have been any significant study difference gained by this latter approach. Nearly all of the FM patients presenting to our facility undergo EDX and skin biopsy evaluation (> 90%; data not shown), thus obviating any propensity toward selection bias.
It might also be reasoned that some sort of blinded reading of the EDX parameters in our subjects would represent a more ideal design, but we think it unlikely that such an unusual approach would be practical. EDX examinations of the extent, and in the detail, we utilized, typically take two or more hours to complete. Social interaction between the examiner and the subject are inevitable during this time, and likely to unmask any blinding. Even having a second, physically and temporally detached, EDX “reader” would not be likely to add greater accuracy than a single, well schooled examiner, since intra-reader agreement is thought to be more reproducible and reliable than inter-reader agreement, at least for radiculopathies (26).
In further assessing the validity of our EDX observations in FM, we also noted that our study findings parallel those expected for biologically important correlates, as inferred, for example, by guidelines proposed in Bradford Hill’s criteria set (27). That is, the association of LFN and FM in our study is strong, consistent, and biologically plausible.
Finally, we were intrigued by certain other aspects of our study results, e.g., the relative lack of significant differences between the EDX findings of our two FM study groups (i.e., “FM Only,” and “FM+RA”), and the relatively low prevalence of rheumatoid factor in our “FM + RA” group (23%), compared to other community RA subjects (28). These findings, in theory, might suggest misclassification of some “FM Only” subjects into the “FM + RA” group. We doubt that there was much misclassification, however. Instead, we suspect that there may be a poorly recognized continuum between the entity known as (isolated) FM and a subset of patients with (seronegative) RA seen within the context of FM (10). Interestingly, an association between FM and seronegative RA has been suggested recently (29). Nonetheless, all of these technical considerations notwithstanding, and taken individually or in their entirety, were not significant enough to influence our conclusions.
We have, then, reported herein readily detectable EDX features of a significant LFN in a substantial portion of FM subjects. Furthermore, we have shown that EDX findings in those with “FM Only” are nearly identical to those seen in a comparison group of “FM+RA,” but differ significantly from those seen in our EDX control subjects. Our study also suggests that proximal muscle weakness is common in FM, and that EMG evidence of lower extremity muscle denervation is frequent in this disorder.
Taken as a whole, these findings may impact the argument regarding whether FM patients have a peripheral neuropathic lesion that is significant to their disorder. Further, they support the concept of a connection between FM and CIDP, at least in a subgroup of these patients. As the immune system is known to be instrumental in the genesis of CIDP, we reason that a subgroup of FM is likely to have a significant immune mediated element to its pathogenesis as well (7, 9, 30). Finally, we believe that our findings may be of considerable interest to students of FM, to those investigating the origin of immune mediated neuropathic pain, and to clinicians who deal with FM on a daily basis.
Footnotes
This study was presented at 79th Annual Scientific Meeting of the American College of Rheumatology, San Francisco, California, USA.
Ethics Committee Approval: Ethics committee approval was received for this study from the our facility’s Institutional Review Board.
Informed Consent: Written informed consent was obtained from subjects who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - X.C; Design - X.C; Supervision - X.C., R.G.; Resources - X.C., R.G; Materials - X.C., R.G; Data Collection and/or Processing - X.C, R.G, E.W; Analysis and/or Interpretation - X.C, R.G. E.W; Literature Search - X.C, R.G; Writing Manuscript - X.C; Critical Review - X.C, R.G, E.W.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This work was supported by the Southern California Fibromyalgia Research & Treatment Center (Northridge, CA, USA, 91325).
References
- 1.Caro XJ, Winter EF. The role and importance of small fiber neuropathy in fibromyalgia pain. Curr Pain Headache Rep. 2015;19:55–62. doi: 10.1007/s11916-015-0527-7. [DOI] [PubMed] [Google Scholar]
- 2.Hovaguimian A, Gibbons CH. Diagnosis and treatment of pain in small fiber neuropathy. Curr Pain Headache Rep. 2011;15:193–200. doi: 10.1007/s11916-011-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states: maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–54. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant IA. Cryptogenic sensory polyneuropathy. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia: Elsevier; 2005. pp. 2321–33. [DOI] [Google Scholar]
- 5.Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–67. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 6.Ersoz M. Nerve conduction tests in patients with fibromyalgia: comparison with normal controls. Rheumatol Int. 2003;23:166–70. doi: 10.1007/s00296-002-0271-2. [DOI] [PubMed] [Google Scholar]
- 7.Caro XJ, Winter EF, Dumas A. A subset of fibromyalgia patients have findings suggestive of chronic inflammatory demyelinating polyneuropathy (CIDP) and appear to respond to IVIg. Rheumatology (Oxford) 2008;47:208–11. doi: 10.1093/rheumatology/kem345. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 9.Caro XJ, Winter EF. Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis Rheumatol. 2014;66:1945–54. doi: 10.1002/art.38662. [DOI] [PubMed] [Google Scholar]
- 10.Caro XJ, Winter EF. Reply to Letter to Editor. Arthritis Rheumatol. 2014;66:3527–8. doi: 10.1002/art.38815. [DOI] [PubMed] [Google Scholar]
- 11.Slobodin G, Eshed I. Non-radiographic axial spondyloarthritis. IMAJ. 2015;17:770–6. [PubMed] [Google Scholar]
- 12.Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. 3rd Ed. Elsevier; New York: 2013. pp. 384–416. [DOI] [Google Scholar]
- 13.Dumitru MD, Amato AA, Zwarts MJ. Nerve conduction studies. In: Dumitru MD, Amato AA, Zwarts MJ, editors. Electrodiagnostic Medicine. Philadelphia: Hanley & Belfus, Inc; 2002. pp. 159–224. [Google Scholar]
- 14.Isose S, Kuwabara S, Kokubun N, Sato Y, Mori M, Shibuya K, et al. Utility of the distal compound muscle action potential duration for diagnosis of demyelinating neuropathies. J Peripher Nerv Syst. 2009;14:151–8. doi: 10.1111/j.1529-8027.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 15.Rajabally YA, Lagarde J, Cassereau J, Viala K, Fournier E, Nicolas G. A European multicentre reappraisal of distal compound muscle action potential duration in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. 2012;19:638–42. doi: 10.1111/j.1468-1331.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 16.Thaisetthawatkul P, Logigian EL, Herrmann DN. Dispersion of the distal compound muscle action potential as a diagnostic criterion for chronic inflammatory demyelinating polyneuropathy. Neurology. 2002;59:1526–32. doi: 10.1212/01.WNL.0000034172.47882.20. [DOI] [PubMed] [Google Scholar]
- 17.Joint Task Force of the EFNS and the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--First Revision. J Peripher Nerv Syst. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 18.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28:144–160. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 19.Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. New York: Oxford University Press; 2013. pp. 49–98.pp. 149–79. [DOI] [Google Scholar]
- 20.Gupta R, Truong L, Bear D, Chafik D, Modafferi E, Hung CT. Shear stress alters the expression of myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) in Schwann cells. J Orthop Res. 2005;23:1232–9. doi: 10.1016/j.orthres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Katirji B. Clinical assessment in neuromuscular disorders. In: Katirji B, Kaminski HJ, Preston DC, Ruff RL, Shapiro BE, editors. Neuromuscular disorders in clinical practice. Boston: Butterworth-Heinemann; 2002. pp. 3–19. [Google Scholar]
- 22.Vo ML, Hanineva A, Chin RL, Carey BT, Latov N, Langsdoef JA. Comparison of 2-limb versus 3-limb electrodiagnostic studies in the evaluation of chronic inflammatory demyelination polyneuropathy. Muscle Nerve. 2015;51:549–53. doi: 10.1002/mus.24424. [DOI] [PubMed] [Google Scholar]
- 23.Bromberg MB. An electrodiagnostic approach to evaluation of peripheral neuropathies. Phys Med Rehabil Clin N Am. 2013;24:153–68. doi: 10.1016/j.pmr.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Dyck PJ, Dyck PJB, Engelstad J. Pathologic alterations of nerves. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Philadelphia: Elsevier Saunders; 2005. pp. 733–830. [DOI] [Google Scholar]
- 25.Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Interview Survey. PLOS One. 2015;10:e0138024. doi: 10.1371/journal.pone.0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanaswami P, Geusbush T, Jones L, Weiss M, Mozaffar T, Gronseth G, et al. Critically re-evaluating a common technique: accuracy, reliability, and confirmation bias of EMG. Neurology. 2016;86:218–23. doi: 10.1212/WNL.0000000000002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machold K. Evaluation and management of early inflammatory polyarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. Philadelphia: Mosby Elsevier; 2015. pp. 785–9. [Google Scholar]
- 29.Doss J, Mo H, Carroll RJ, Crofford LJ, Denny JC. Phenome-Wide Association Study of Rheumatoid Arthritis Subgroups Identifies Association between Seronegative Disease and Fibromyalgia. Arthritis Rheumatol. 2016 Sep 2; doi: 10.1002/art.39851. doi: 10.1002/art.39851. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalakas MC. Autoimmune peripheral neuropathies. In: Rich R, editor. Clinical Immunology. 3rd Ed. Elsevier; New York: 2008. pp. 801–11. [DOI] [Google Scholar]