Abstract
Objective
Conventional treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN) is associated with damage accrual, hence increased morbidity rate. Off-label use of rituximab (RTX) has shown significant promise in this patient group; however, data are still controversial. We aimed to analyze the outcomes of RTX therapy in refractory lupus using a meta-analysis approach.
Methods
Electronic search of the medical literature was conducted using a combination of relevant keywords to retrieve studies on the safety and efficacy of RTX in SLE and LN patients. Results were screened against our inclusion and exclusion criteria and two reviewers independently extracted the data for analysis. Comprehensive meta-analysis software was used to pool the data from individual studies and provide summary effect estimates.
Results
Thirty-one studies that enrolled 1112 patients were finally eligible for the meta-analysis. The overall global, complete, and partial response rates to RTX therapy were 72%, 46%, and 32%, respectively. RTX significantly decreased Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and British Isles Lupus Activity Group (BILAG) scores (p<0.001). Prednisone dose was significantly reduced after RTX treatment in both SLE and LN groups (p<0.001), and proteinuria was lowered in SLE (p<0.001) than in LN patients (p=0.07). Infection and infusion-related reactions were the most common side effects.
Conclusion
RTX therapy in refractory SLE and LN patients proved clinical efficacy and favorable safety outcomes. Larger well-designed randomized clinical trials are warranted.
Keywords: Systemic lupus erythematosus, lupus nephritis, rituximab (RTX), B-cell depletion therapy
Introduction
Systemic lupus erythematosus (SLE) is defined as a systemic autoimmune disorder of idiopathic occurrence. The primary pathogenesis is the overproduction of organ-specific antibodies targeting nuclear antigens, which massively develop immune complex depositions in multiple organs, leading to inflammation and tissue damage (1). One of the major complications and the most common mortality-leading cause, in more than 75% of SLE cases, is lupus nephritis (LN), which causes proteinuria and may progress to end-stage renal failure (2, 3).
Corticosteroids in conjunction with cyclophosphamide or mycophenolate mofetil are the current standard treatment for LN, as they have relatively shown a short-term improvement in disease prognosis (4, 5). Yet, LN resistance to the standard treatment develops rapidly, and the renal response rates at first year reach 50%–80% and then it fails to control the relapse (6, 7). In addition to the toxicity and the fatal infections rising from prolonged use of immunosuppressive agents, the demand for a less toxic, more effective, and fertility-sparing treatment is critical.
Recently, a new medication has been introduced targeting a new member of the immune system, the B cell, usually uncommon to be implicated in autoimmunity (8). However, B lymphocytes are believed to play a principal role in the pathogenesis of SLE, either directly by the production of organ-specific antibodies and cytokines or indirectly by antigen-presenting activity (9). A suggestive chimeric anti-CD20 monoclonal antibody, rituximab (RTX), has been found to suppress immune response with a better efficacy and less toxicity than the standard treatment (10–19). RTX has firstly been approved as a treatment of B-cell lymphomas and then afterward for rheumatoid arthritis and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (20, 21). Recently, clinical trials suggest RTX as a more effective treatment for LN. Nevertheless, its effectiveness is still controversial among studies (22–25), which either demonstrate a trending superiority or noninferiority compared to conventional treatment.
In this systematic review and meta-analysis, we aimed to identify and review clinical trials and observational studies that investigated the effectiveness and safety of rituximab in patients with refractory lupus by analyzing the results from individual studies to create a class one clear evidence.
Methods
Data sources and search terms
Search strategy was designed to identify the full length of publications reporting outcomes of RTX treatment in refractory SLE, refractory LN, or refractory neuropsychiatric SLE (NPSLE) patients. PubMed was searched using Medical subheading (MeSH) using the terms “Rituximab” and “Lupus erythematosus, systemic.” As per this method, RTX is defined as “a murine-derived monoclonal antibody and antineoplastic agent that binds specifically to the CD20 antigen and is used in the treatment of leukemia, lymphoma, and rheumatoid arthritis” with entry terms: CD20 Antibody, Rituximab; Antibody, Rituximab CD20; Rituximab CD20 Antibody; Mabthera; IDEC-C2B8 Antibody; IDEC C2B8 Antibody; IDECC2B8 Antibody; IDEC-C2B8; IDEC C2B8; IDECC2B8; GP2013; or Rituxan. Lupus erythematosus, systemic is defined as “a chronic, relapsing, inflammatory, and often febrile multisystemic disorder of connective tissue, characterized principally by involvement of the skin, joints, kidneys, and serosal membranes; with unknown etiology, but thought to represent a failure of the regulatory mechanisms of the autoimmune system; marked by a wide range of system dysfunctions, an elevated erythrocyte sedimentation rate, and the formation of LE cells in the blood or bone marrow.” The entry terms for SLE were: Systemic Lupus Erythematosus; Lupus Erythematosus Disseminatus; Libman-Sacks Disease; Disease, Libman-Sacks; or Libman Sacks Disease. This MeSH term also included the keywords Lupus Nephritis and Lupus Vasculitis and Central Nervous System. Hence, there was no need for a separate search for LN and NPSLE. Similarly, EMBASE search was conducted using a combination of SLE and rituximab EMTREE terms. Filters were applied to select only English language publications reported on human subjects. Reference lists of the reviews and research articles were manually screened to identify further articles.
Inclusion criteria-retrospective/prospective case series or controlled trials reporting the outcomes of RTX therapy in at least 10 SLE/LN/NPSLE patients’ refractory to traditional therapy. It was also mandatory that the studies reported the score used for measuring the clinical outcomes [SLEDAI (Systemic Lupus Erythematosus Disease Activity Index), BILAG (British Isles Lupus Activity Group), Renal Outcomes].
Exclusion criteria-abstracts, conference proceedings, posters, case reports, reviews, editorials, and non-English publications were excluded. Studies with mixed cohorts or providing insufficient details were not eligible.
Ethics approval was obtained for this study from the institutional review board at college of medicine, Umm Al-Qura University. Disagreements between authors were solved by discussion. Authors of this paper claim no conflict of interest.
Study selection
Duplicate articles were identified and removed. The titles and abstracts of the remaining articles were reviewed by two independent investigators, who were responsible for determining whether the articles were eligible to be included in the study. To address any inconsistencies, the investigators compared lists before they reviewed the full text of the studies identified as eligible. When the final list of articles was complete, a third investigator resolved final discrepancies.
Data extraction and meta-analysis
A standardized custom excel sheet was used to extract all the relevant and specific data on study, patient, intervention, and outcome characteristics. These data were extracted independently by two investigators and compared with resolve discrepancies.
The primary objective was to measure the number of patients showing global response, complete response, and partial response after RTX therapy. The secondary objective was to estimate the change in BILAG or SLEDAI score, proteinuria, and prednisone dose after therapy. Articles qualifying for more than one variable of interest were considered as different data points for each of the variable.
Two meta-analysis models were constructed
Model 1: Pooled estimation of global, complete, and partial response of the patients to RTX therapy.
Model 2: Mean change with statistical significance of SLEDAI/BILAG score, proteinuria, and prednisone dose after RTX therapy.
Publication bias was visualized through funnel plots and quantified with the Egger’s test. A qualitative estimate of statistical heterogeneity between studies was assessed using Cochrane Q. For the chi-square test, p<0.05 was considered statistically significant. In the presence of significance heterogeneity, I2 statistic was used to quantify the level of heterogeneity. I2 was interpreted on the basis of Higgins and Thompson criteria, where 25%, 50%, and 75% correspond, respectively, to low, medium, and high heterogeneity (26). Statistical heterogeneity, forest plot, publication bias, and sensitivity analysis were conducted with Comprehensive Meta-analysis (CMA software) version 3. To accommodate between study heterogeneity, the Dersimonian and Laird random-effects model was used for all of the meta-analysis models (27). Effect size was represented with mean difference (28), which directly reflects the actual difference between the interventions, in all included studies.
Results
Search results
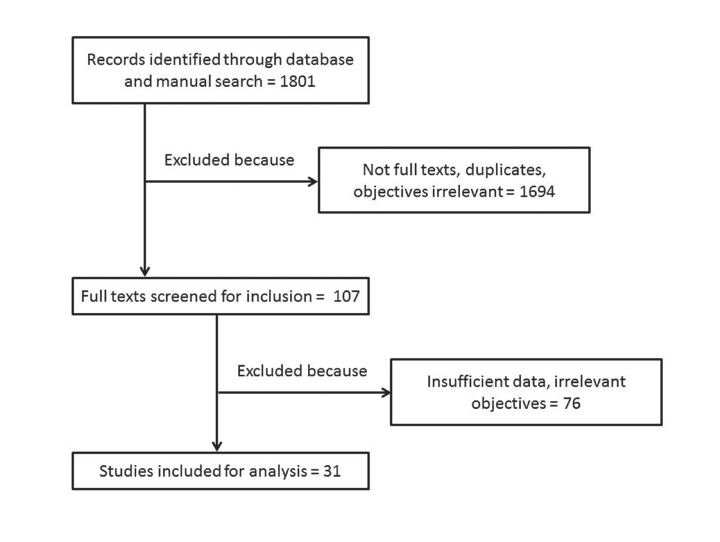
The databases and manual search retrieved 1801 journal articles. Title and abstract of all these articles were screened to eliminate 1694 studies that were duplicates, non-English, meeting abstracts or studied different objectives. In the next phase, 107 article full texts were obtained and screened. Seventy-six of these articles had to be further excluded, as these provided incomplete data or had irrelevant objectives and were unsuitable for meta-analysis. Remaining 31 articles were included for meta-analysis (10–19, 29–49). Flowchart of the studies evaluated is represented in Figure 1.
Figure 1.
PRISMA flow diagram of study selection for meta-analysis
Characteristics of included studies
The included studies consisted of 22 studies that investigated RTX therapy in 866 refractory SLE patients, 10 studies that enrolled 223 refractory LN patients, and one study with 10 NPSLE cases. Sixteen of the eligible studies were retrospective case series, 14 were prospective case series, and two studies were randomized controlled trials. The studies were conducted between 2005 and 2016, and the mean follow-up period was 10.6 months and ranged from 3 to 38 months. The dose of RTX varied among different studies; some investigators used 375 mg/m2 q.i.d., whereas others used 500 mg b.i.d. or 1000 mg b.i.d., 2 weeks apart. Doses of 500 mg q.i.d., 375 mg/m2 b.i.d or q.d., and 750 mg b.i.d. were also infused in other cohorts. Contis et al. used a dose of 375 mg/m2 weekly for 4 weeks or 1 g at day zero and day 15 every 6 months.
Diverse array of adverse events was observed in the included patients. The most common adverse reactions were infection (urinary or respiratory), acute or delayed infusion reactions, sepsis-like syndrome, thrombocytopenia, and serum sickness-like reaction. One patient died from varicella pneumonia, another died from septicemia, and one case caught MRSA. Baseline characteristics of the included studies are summarized in Table 1, and a summary of these findings is shown in Table 2.
Meta-analysis results
Global response
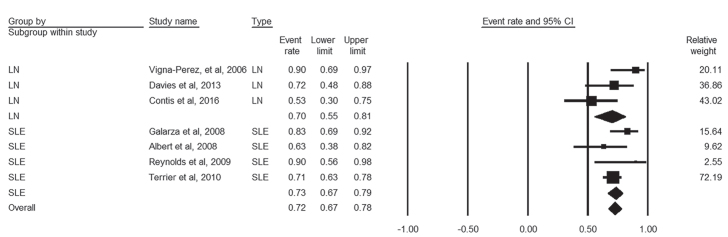
Global response to RTX was reported by three studies that enrolled 57 LN patients and four studies with 206 SLE patients. There was low heterogeneity among these studies (I2=44%, p=0.1). The pooled proportion of global response among LN and SLE patients was 70% (95% CI, 55%–81%) and 73% (95% CI, 67%–78%), respectively, and the overall pooled percent was 72% (95% CI, 67%–78%) (Figure 2).
Figure 2.
Forest plot of global response rate of LN and SLE patients to rituximab therapy
Complete response
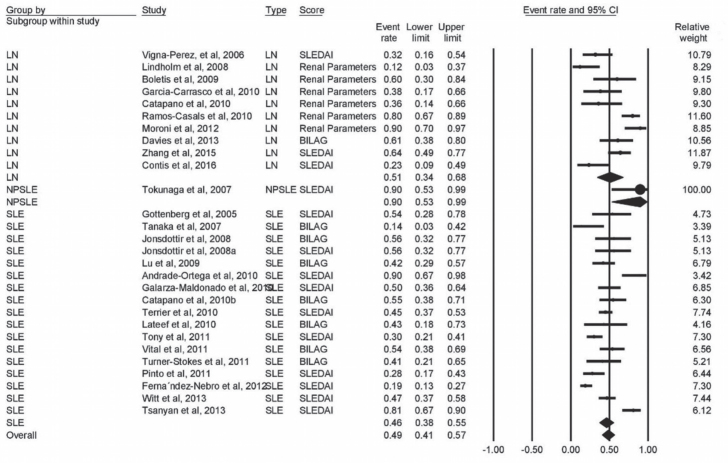
Twenty-eight studies provided data on complete remission; of them, 17 studies (n=773 patients) were on SLE, 10 (n=223 patients) on LN, and one study enrolled 10 patients with NPSLE. The pooled proportion for complete response was 51% (95% CI, 34% to 68%) in LN patients, 90% (95% CI, 53% to 99%) in NPSLE patients, and 46% (95% CI, 38% to 55%) in SLE patients, with overall response rate of 49% (95% CI, 41% to 57%). There was significant heterogeneity among these studies (I2=80%, p<0.001) (Figure 3).
Figure 3.
Forest plot of complete response rate of LN, NPSLE, and SLE patients to rituximab therapy
Partial response
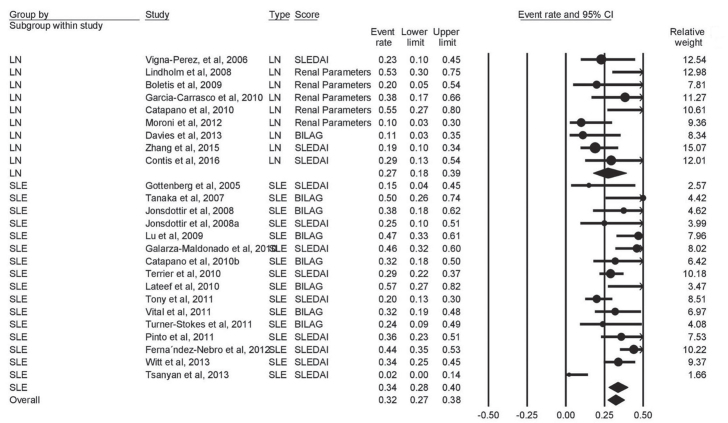
Partial response to RTX was reported by 25 studies (9 on LN and 16 on SLE) that enrolled 928 patients. Moderate heterogeneity was found among the studies (I2=57%, p<0.001). The pooled proportion of patients with partial response to MTX was 27% (95% CI, 18%–39%) and 34% (95% CI, 28%–40%) for LN and SLE, with overall partial response rate of 32% (95% CI, 27%–38%) (Figure 4).
Figure 4.
Forest plot of partial response rate of LN, NPSLE, and SLE patients to rituximab therapy
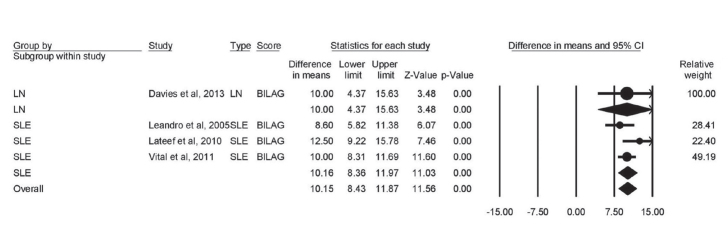
Change in BILAG score
Four studies provided data of BILAG score change from baseline. There was marked heterogeneity among these studies (I2=75%, p<0.001). The BILAG score was significantly reduced in both LN (mean difference=−10; 95% CI [−4.37 to −15.63; p<0.001) and SLE (mean difference=−10.16; 95% CI [−8.36 to −11.97; p<0.001) patients after RTX therapy. The overall score was also significantly lowered (mean difference=−10.15; 95% CI [−8.43 to −11.87; p<0.001) (Figure 5).
Figure 5.
Forest plot of effect of rituximab therapy on BILAG score in LN and SLE patients
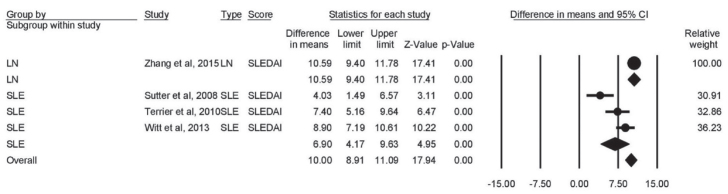
Change in SLEDAI score
The change in SLEDAI score was reported by four heterogeneous studies (I2=87%, p<0.001). SLEDAI score significantly decreased in both LN (mean difference=−10.59; 95% CI [−9.40 to −11.78]; p<0.001) and SLE (mean difference=−6.90; 95% CI [−4.17 to −9.63]; p<0.001) patients after RTX therapy. The overall score was also significantly lowered (mean difference=−10; 95% CI [−8.91 to −11.09]; p<0.001) (Figure 6).
Figure 6.
Forest plot of effect of rituximab therapy on SLEDAI score in LN and SLE patients
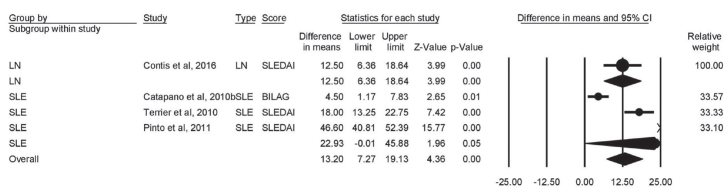
Change in prednisone dose
Five heterogeneous studies provided data on the change from baseline in prednisone dose. The pooled mean difference showed that prednisone dose (mg/d) was significantly decreased in both LN (mean difference=−12.50; 95% CI [−6.36 to −18.64]; p<0.001) and SLE (mean difference=−22.93; 95% CI [−0.01 to −45.88]; p<0.001) patients after RTX therapy. The overall score was also significantly lowered (mean difference=−13.20; 95% CI [−7.27 to −19.13]; p<0.001) (Figure 7).
Figure 7.
Forest plot of effect of rituximab therapy on prednisone dose in LN and SLE patients
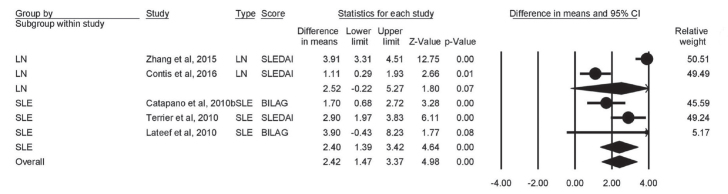
Change in proteinuria
Four studies reported on the change of proteinuria. Proteinuria (g/d) was insignificantly decreased in LN patients (mean difference=−2.52; 95% CI [0.22 to −5.27]; p=0.07). The decline in proteinuria was significant in SLE patients (mean difference=−2.40; 95% CI [−1.39 to −3.42]; p<0.001). The overall score was significantly lowered (mean difference=−2.42; 95% CI [−1.47 to −3.37]; p<0.001) (Figure 8).
Figure 8.
Forest plot of effect of rituximab therapy on proteinuria dose in LN and SLE patients
Discussion
Survival of SLE and LN patients has been markedly improved over the past 50 years mainly due to the use of glucocorticoids and other immunosuppressive agents as well as the introduction of renal dialysis and renal transplant (50). Nevertheless, the accumulation of damage caused by corticosteroid therapy increased morbidity rate among these patients (51). As a result, efforts have been directed to corticosteroid alternatives that can induce and maintain disease remission, attenuate cumulative damage, and improve overall outcomes. These goals have proven particularly challenging for SLE treatment (52).
Rituximab remains a common off-label prescription for the treatment of SLE despite the conflicting evidence from clinical studies (47, 53–56). Thereby, we aimed to generate a robust evidence on the clinical efficacy of RTX in SLE and LN patients, refractory to conventional treatment. Our findings suggest a potential therapeutic efficacy of RTX in both SLE and LN patients. RTX achieved up to 73% global response rate, 51% complete remission, and 34% partial remission in SLE and LN patients. Moreover, it significantly decreased BILAG and SLEDAI scores as well as proteinuria. Additionally, RTX showed a significant corticosteroid sparing effect through marked reduction of prednisone dose in both SLE and LN patients. These effects are consistent with evidence from recent clinical trials (57).
Rituximab displayed promising effects in cases of NPSLE through rapid improvement of cognitive dysfunction, psychosis, and seizures. NPSLE patients on RTX had long-lasting significant reduction of SLEDAI. However, these effects were shown in one study with 10 included patients, so further assessment of the role of RTX in NSPLE in larger studies is warranted.
In terms of adverse reactions, RTX was well tolerated by most of the patients enrolled in the included studies. The most common adverse reactions were infections, acute or delayed infusion reactions, and thrombocytopenia (29, 39, 40, 44, 46, 49). Sepsis-like syndrome and serum sickness-like reaction occasionally occurred in three patients overall (14, 29, 36).
Although not yet authorized for the treatment of SLE and LN, RTX is widely used in these patient groups. Data from Ryden-Aulin et al. (58) study about the off-label use of RTX for SLE in Europe showed that RTX is used in 4% to 20% of SLE patients in Sweden, up to 11% in Spain, and 7% in the U.K. Moreover, adoption of RTX for management of SLE ranged from 1% to 4% in other European countries. The off-label use of RTX in SLE is enabled by its favorable safety profile and the documented benefit that led to its approval by the FDA and the European Medicines Agency for the treatment of rheumatoid arthritis and ANCA-associated vasculitis (20, 21). Clinicians have high expectations for RTX therapy owing to the favorable data from clinical practice and observational studies as well as some promising exploratory outcomes from LUNAR trial, such as potential advantage in African Americans (6, 8, 15, 59). Furthermore, off-label use of RTX is supported by the EULAR and ACR guidelines, which included it as one of the treatment options for patients with refractory LN (60).
B lymphocytes are documented to play a major role in the pathogenesis of SLE (61). Thus, B-cell depletion therapy has gained much interest for management of SLE and LN. RTX is a chimeric monoclonal antibody that binds to its target antigen, CD20, and induces B-cell depletion. CD20 is expressed exclusively on B lymphocytes but not on plasma cells; therefore, treatment with RTX would directly target only CD20+ B cells (62). Isenberg et al. (66) have led the early studies on B-cell depletion therapy with RTX for lupus treatment (50, 63). The first trial of RTX in SLE patients with active disease reported promising clinical efficacy and favorable safety profile (64). This was followed by wide adoption of RTX in clinical practice, and many case reports were published indicating its utility (41, 53). However, unexpectedly, RTX did not meet the primary endpoints in two large trials of non-renal (EXPLORER) and renal (LUNAR) SLE (59, 65). These trials had been later criticized for their poor design, particularly concomitant administration of high doses of corticosteroids, which may have concealed the clinical response attributable to RTX (54, 62, 66, 67).
The establishment of RTX B-cell depletion therapy in SLE by clinical trials has confronted several hurdles including the heterogeneity of the disease and the beneficial effects of background therapy that might mask the added value of short-term RTX treatment (61). Small study size, lack of robust design, and short-term follow-up are further limitations of RTX clinical trials. These “missing pieces in the jigsaw” call for further large well-designed trials with longer follow-up periods given that the data from a long-term follow-up study by Moroni et al. showed promising complete remission after 2 years of follow-up in a significant number of patients (68).
Rituximab can also be used in several hematologic presentations of SLE-like autoimmune hemolytic anemia, immune-mediated thrombocytopenia, macrophage activation syndrome, antiphospholipid syndromes, and other conditions that can be refractory to conventional therapy (69–78). RTX has shown promising results with significant clinical improvement and normalized laboratory parameters. However, all these studies did not qualify inclusion in our analysis due to small sample size.
Our study possesses some limitations. First, there was some heterogeneity in the dose and regimen of RTX in the pooled studies. Second, data were pooled from studies that used different scores (BILAG, SLEDAI, and renal parameters) for the assessment of global complete and partial responses. Finally, some outcomes were provided by few studies (e.g., BILAG and SLEDAI scores in LN patients were reported by one study each).
To recapitulate, our findings demonstrate that RTX treatment achieved significant clinical efficacy and favorable safety profile in SLE and LN patients refractory to conventional treatment. Further large well-designed multicenter randomized controlled trials are warranted to the end of approval of RTX as a standard therapy for lupus.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the institutional review board at Umm Al-Qura University School of Medicine.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.A.; Design - F.A., R.T.; Supervision - H.A.; Resources - R.T., H.E., A.A.; Materials - R.T., H.E., A.A.; Data Colelction and/or Proccessing - R.T., H.E., A.A.; Analysis and/or Interpretation - F.A., E.O.; Literature Search - H.A., F.A., R.T., H.E., A.A., E.O.; Writing Manuscript - F.A., E.O., H.A.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Maidhof W, Hilas O. Lupus: An Overview of the Disease And Management Options. P T. 2012;37:240–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Zhao Y, Zhang J, Lei H, Zhu G, Fu B. Impact analysis of autoantibody level and NR2 antibody level in neuropsychiatric SLE treated by methylprednisolone combined with MTX and DXM intrathecal injection. Cell Biochem Biophys. 2014;70:1005–9. doi: 10.1007/s12013-014-0010-9. [DOI] [PubMed] [Google Scholar]
- 3.Reichert JM. Antibodies to watch in 2014. MAbs. 2014;6:5–14. doi: 10.4161/mabs.29282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn BH, McMahon M, Wilkinson A, Wallace WD, Daikh DI, FitzGerald J, et al. American College of Rheumatology Guidelines for Screening, Case Definition, Treatment and Management of Lupus Nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 7.Sada KE, Makino H. Usefulness of ISN/RPS Classification of Lupus Nephritis. J Korean Med Sci. 2009;24:7–10. doi: 10.3346/jkms.2009.24.S1.S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perosa F, Favoino E, Caragnano MA, Prete M, Dammacco F. CD20: a target antigen for immunotherapy of autoimmune diseases. Autoimmun Rev. 2005;4:526–31. doi: 10.1016/j.autrev.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contis A, Vanquaethem H, Truchetet M-E, Couzi L, Rigothier C, Richez C, et al. Analysis of the effectiveness and safety of rituximab in patients with refractory lupus nephritis: a chart review. Clinical Rheumatol. 2016;35:517–22. doi: 10.1007/s10067-015-3166-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Zhao Z, Hu X. Effect of Rituximab on Serum Levels of Anti-C1q and Antineutrophil Cytoplasmic Autoantibodies in Refractory Severe Lupus Nephritis. Cell Biochem Biophys. 2015;72:197–201. doi: 10.1007/s12013-014-0437-z. [DOI] [PubMed] [Google Scholar]
- 12.Tsanyan Maria E, Soloviev Sergey K, Radenska-Lopovok Stefka G, Torgashina Anna V, Nikolaeva Ekaterina V, Khrennikov Yaroslav B, et al. Clinical And Morphological Improvement Of Lupus Nephritis Treated With Rituximab. Folia Medica. 2014;56:245. doi: 10.1515/folmed-2015-0003. [DOI] [PubMed] [Google Scholar]
- 13.Witt M, Grunke M, Proft F, Baeuerle M, Aringer M, Burmester G, et al. Clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE)-results from a nationwide cohort in Germany (GRAID) Lupus. 2013;22:1142–9. doi: 10.1177/0961203313503912. [DOI] [PubMed] [Google Scholar]
- 14.Davies R, Sangle S, Jordan N, Aslam L, Lewis M, Wedgwood R, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. 2013;22:574–82. doi: 10.1177/0961203313483376. [DOI] [PubMed] [Google Scholar]
- 15.Moroni G, Gallelli B, Sinico RA, Romano G, Sinigaglia L, Messa P. Rituximab versus oral cyclophosphamide for treatment of relapses of proliferative lupus nephritis: a clinical observational study. Ann Rheum Dis. 2012;71:1751–2. doi: 10.1136/annrheumdis-2012-201442. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Nebro A, de la Fuente JM, Carreno L, Izquierdo MG, Tomero E, Rúa-Figueroa I, et al. Multicenter longitudinal study of B-lymphocyte depletion in refractory systemic lupus erythematosus: the LESIMAB study. Lupus. 2012;21:1063–76. doi: 10.1177/0961203312446627. [DOI] [PubMed] [Google Scholar]
- 17.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3038–47. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 18.Turner-Stokes T, Lu TY, Ehrenstein MR, Giles I, Rahman A, Isenberg DA. The efficacy of repeated treatment with B-cell depletion therapy in systemic lupus erythematosus: an evaluation. Rheumatology. 2011;50:1401–8. doi: 10.1093/rheumatology/ker018. [DOI] [PubMed] [Google Scholar]
- 19.Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kotter I, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13:75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Eng J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JC, Szczepański L, Szechiński J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Eng J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 22.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62:2458–66. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 23.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 25.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–90. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 26.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6:107–28. doi: 10.3102/10769986006002107. [DOI] [Google Scholar]
- 29.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis. 2008;67:1724–31. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 30.Andrade-Ortega L, Irazoque-Palazuelos F, López-Villanueva R, Barragán-Navarro Y, Bourget-Pietrasanta F, de los Ángeles Díaz-Ceballos M, et al. Efficacy of rituximab versus cyclophosphamide in lupus patients with severe manifestations. A randomized and multicenter study. Reumatol Clín. 2010;6:250–5. doi: 10.1016/j.reuma.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Boletis JN, Marinaki S, Skalioti C, Lionaki SS, Iniotaki A, Sfikakis PP. Rituximab and mycophenolate mofetil for relapsing proliferative lupus nephritis: a long-term prospective study. Nephrol Dial Transplant. 2009;24:2157–60. doi: 10.1093/ndt/gfp002. [DOI] [PubMed] [Google Scholar]
- 32.Catapano F, Chaudhry AN, Jones RB, Smith KG, Jayne DW. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol DialTransplant. 2010;25:3586–92. doi: 10.1093/ndt/gfq256. [DOI] [PubMed] [Google Scholar]
- 33.Galarza C, Valencia D, Tobón GJ, Zurita L, Mantilla RD, Pineda-Tamayo R, et al. Should rituximab be considered as the first-choice treatment for severe autoimmune rheumatic diseases? Clin Rev Allergy Immunol. 2008;34:124–8. doi: 10.1007/s12016-007-8028-z. [DOI] [PubMed] [Google Scholar]
- 34.Galarza-Maldonado C, Kourilovitch MR, Molineros JE, Cardiel MH, Zurita L, Soroka NF, et al. The administration of low doses of rituximab followed by hydroxychloroquine, prednisone and low doses of mycophenolate mofetil is an effective therapy in Latin American patients with active systemic lupus erythematosus. Autoimmun Rev. 2010;10:108–11. doi: 10.1016/j.autrev.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Carrasco M, Mendoza-Pinto C, Sandoval-Cruz M, Soto-Vega E, Beltran-Castillo A, Jimenez-Hernandez M, et al. Anti-CD20 therapy in patients with refractory systemic lupus erythematosus: a longitudinal analysis of 52 Hispanic patients. Lupus. 2010;19:213–9. doi: 10.1177/0961203309351541. [DOI] [PubMed] [Google Scholar]
- 36.Gottenberg J-E, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64:913–20. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jónsdóttir T, Gunnarsson I, Risselada A, Henriksson EW, Klareskog L, van Vollenhoven RF. Treatment of refractory SLE with rituximab plus cyclophosphamide: clinical effects, serological changes, and predictors of response. Ann Rheum Dis. 2008;67:330–4. doi: 10.1136/ard.2007.079095. [DOI] [PubMed] [Google Scholar]
- 38.Lateef A, Lahiri M, Teng G, Vasoo S. Use of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experience. Lupus. 2010;19:765–70. doi: 10.1177/0961203309358599. [DOI] [PubMed] [Google Scholar]
- 39.Leandro M, Cambridge G, Edwards J, Ehrenstein M, Isenberg D. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology. 2005;44:1542–5. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- 40.Lindholm C, Börjesson-Asp K, Zendjanchi K, Sundqvist A-C, Tarkowski A, Bokarewa M. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. J Rheumatol. 2008;35:826–33. [PubMed] [Google Scholar]
- 41.Lu TYT, Ng KP, Cambridge G, Leandro MJ, Edwards JC, Ehrenstein M, et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at university college london hospital: the first fifty patients. Arthritis Rheum. 2009;61:482–7. doi: 10.1002/art.24341. [DOI] [PubMed] [Google Scholar]
- 42.Pinto L, Velásquez C, Prieto C, Mestra L, Forero E, Márquez J. Rituximab induces a rapid and sustained remission in Colombian patients with severe and refractory systemic lupus erythematosus. Lupus. 2011;20:1219–26. doi: 10.1177/0961203311409273. [DOI] [PubMed] [Google Scholar]
- 43.Ramos-Casals M, Garcia-Hernandez F, De Ramon E, Callejas J, Martínez-Berriotxoa A, Pallarés L, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28:468–76. [PubMed] [Google Scholar]
- 44.Reynolds J, Toescu V, Yee C, Prabu A, Situnayake D, Gordon C. Effects of rituximab on resistant SLE disease including lung involvement. Lupus. 2009;18:67–73. doi: 10.1177/0961203308094653. [DOI] [PubMed] [Google Scholar]
- 45.Sutter JA, Kwan-Morley J, Dunham J, Du Y-Z, Kamoun M, Albert D, et al. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol. 2008;126:282–90. doi: 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Yamamoto K, Takeuchi T, Nishimoto N, Miyasaka N, Sumida T, et al. A multicenter phase I/II trial of rituximab for refractory systemic lupus erythematosus. Mod Rheumatol. 2007;17:191–7. doi: 10.3109/s10165-007-0565-z. [DOI] [PubMed] [Google Scholar]
- 47.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62:2458–66. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 48.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66:470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, Portales-Pérez D, Baranda L, Abud-Mendoza C, et al. Clinical and immunological effects of Rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8:83. doi: 10.1186/ar1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–7. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 51.Urowitz M, Gladman D. How to improve morbidity and mortality in systemic lupus erythematosus. Rheumatology. 2000;39:238–44. doi: 10.1093/rheumatology/39.3.238. [DOI] [PubMed] [Google Scholar]
- 52.Mahieu M, Strand V, Simon L, Lipsky P, Ramsey-Goldman R. A critical review of clinical trials in systemic lupus erythematosus. Lupus. 2016;25:1122–40. doi: 10.1177/0961203316652492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Díaz-Lagares C, Croca S, Sangle S, Vital EM, Catapano F, Martínez-Berriotxoa A, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev. 2012;11:357–64. doi: 10.1016/j.autrev.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Ramos-Casals M, Soto M, Cuadrado M, Khamashta M. Rituximab in systemic lupus erythematosusA systematic review of off-label use in 188 cases. Lupus. 2009;18:767–76. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- 55.Weidenbusch M, Römmele C, Schröttle A, Anders H-J. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28:106–11. doi: 10.1093/ndt/gfs285. [DOI] [PubMed] [Google Scholar]
- 56.Furie R, Looney R, Rovin B, Latinis KM, Appel G, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study. Arthritis Rheum. 2009:60. [Google Scholar]
- 57.Gracia-Tello B, Ezeonyeji A, Isenberg D. The use of rituximab in newly diagnosed patients with systemic lupus erythematosus: long-term steroid saving capacity and clinical effectiveness. Lupus Sccci Med. 2017;4:000182. doi: 10.1136/lupus-2016-000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rydén-Aulin M, Boumpas D, Bultink I, Rubio JLC, Caminal-Montero L, Castro A, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. 2016;3:e000163. doi: 10.1136/lupus-2016-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–26. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 60.Wilhelmus S, Bajema IM, Bertsias GK, Boumpas DT, Gordon C, Lightstone L, et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant. 2016;31:904–13. doi: 10.1093/ndt/gfv102. [DOI] [PubMed] [Google Scholar]
- 61.Mota P, Reddy V, Isenberg D. Improving B-cell depletion in systemic lupus erythematosus and rheumatoid arthritis. Expert Rev Clin Immunol. 2016:1–10. doi: 10.1080/1744666X.2017.1259068. [DOI] [PubMed] [Google Scholar]
- 62.Favas C, Isenberg DA. B-cell-depletion therapy in SLE-what are the current prospects for its acceptance? Nat Rev Rheumatol. 2009;5:711–6. doi: 10.1038/nrrheum.2009.218. [DOI] [PubMed] [Google Scholar]
- 63.Ramos L, Isenberg D. Rituximab: the lupus journey. Curr Treatm Opt in Rheumatol. 2015;1:30–41. doi: 10.1007/s40674-014-0003-2. [DOI] [Google Scholar]
- 64.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheuma. 2004;50:2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 65.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isenberg DA, Merrill JT. Why, why, why de-lupus (does so badly in clinical trials) Expert Rev Clin Immunol. 2016;2:95–98. doi: 10.1586/1744666X.2016.1112270. [DOI] [PubMed] [Google Scholar]
- 67.Ramos-Casals M, Díaz-Lagares C, Khamashta MA. Rituximab and lupus: good in real life, bad in controlled trials. Comment on the article by Lu et al. Arthritis Care Res. 2009;61:1281–2. doi: 10.1002/art.24726. [DOI] [PubMed] [Google Scholar]
- 68.Moroni G, Raffiotta F, Trezzi B, Giglio E, Mezzina N, Del Papa N, et al. Rituximab vs mycophenolate and vs cyclophosphamide pulses for induction therapy of active lupus nephritis: a clinical observational study. Rheumatology. 2014;53:1570–7. doi: 10.1093/rheumatology/ket462. [DOI] [PubMed] [Google Scholar]
- 69.Liu W, Hu Z, Lin S, He J, Zhou Y. Systemic lupus erythematosis with severe aplastic anemia successfully treated with rituximab and antithymocyte globulin. Pak J Med Sci. 2014;30:449. [PMC free article] [PubMed] [Google Scholar]
- 70.Chen H, Zheng W, Su J, Xu D, Wang Q, Leng X, et al. Low-dose rituximab therapy for refractory thrombocytopenia in patients with systemic lupus erythematosus-a prospective pilot study. Rheumatology. 2011;50:1640–4. doi: 10.1093/rheumatology/ker176. [DOI] [PubMed] [Google Scholar]
- 71.Niaz FA, Aleem A. Response to rituximab in a refractory case of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus. Saudi J Kidney Dis Transplant. 2010;21:109. [PubMed] [Google Scholar]
- 72.Gupta R, Ezeonyeji A, Thomas A, Scully M, Ehrenstein M, Isenberg D. A case of pure red cell aplasia and immune thrombocytopenia complicating systemic lupus erythematosus: Responseto rituximab and cyclophosphamide. Lupus. 2011;20:1547–50. doi: 10.1177/0961203311411349. [DOI] [PubMed] [Google Scholar]
- 73.Sardesai VV, Sardesai VR, Agarwal TD. Steroid-resistant autoimmune thrombocytopenia in systemic lupus erythematosus treated with rituximab. Indian J Dermatol. 2015;60:106. doi: 10.4103/0019-5154.147874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jovancevic B, Lindholm C, Pullerits R. Anti B-cell therapy against refractory thrombocytopenia in SLE and MCTD patients: long-term follow-up and review of the literature. Lupus. 2013;22:664–74. doi: 10.1177/0961203313485489. [DOI] [PubMed] [Google Scholar]
- 75.Boyero RG, Esteve EM, Esteve MM, Perseguer MMM, Buades JM, Fabregat JB, et al. Systemic lupus erythematosus and thrombotic thrombocytopenia purpura: a refractory case without lupus activity. Reumatol Clin. 2013;9:373–5. doi: 10.1016/j.reuma.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Bakshi J, Hassan S, D’Cruz D, Chan A. Rituximab therapy in refractory macrophage activation syndrome secondary to systemic lupus erythematosus. Lupus. 2013;22:1544–6. doi: 10.1177/0961203313504634. [DOI] [PubMed] [Google Scholar]
- 77.Rubenstein E, Arkfeld DG, Metyas S, Shinada S, Ehresmann S, Liebman HA. Rituximab treatment for resistant antiphospholipid syndrome. J Rheumatol. 2006;33:355–7. [PubMed] [Google Scholar]
- 78.Iaccarino L, Bartoloni E, Carli L, Ceccarelli F, Conti F, De Vita S, et al. Efficacy and safety of off-label use of rituximab in refractory lupus: data from the Italian Multicentre Registry. Clin Exp Rheumatol. 2014;33:449–56. [PubMed] [Google Scholar]